Abstract
Inflammation can regulate hepatic drug metabolism enzymes and transporters. The impact of inflammation on renal drug transporters remains to be elucidated. We aimed to quantify the effect of inflammation (caused by acute pyelonephritis) on the in vivo activity of renal OAT1/3, using the probe drug furosemide. Pregnant women (second or third trimester) received a single oral dose of furosemide 40 mg during acute pyelonephritis (Phase 1; n = 7) and after its resolution (Phase 2; n = 7; by treatment with intravenous cefuroxime 750 mg TID for 3–7 days), separated by 10 to 14 days. The IL-6, IFN-γ, TNF-α, MCP-1, and C-reactive protein plasma concentrations were higher in Phase I vs. Phase II. The pregnant women had a lower geometric mean [CV%] furosemide CLsecretion (3.9 [43.4] vs. 6.7 [43.8] L/h) and formation clearance to the glucuronide (1.1 [85.9] vs. 2.3 [64.1] L/h) in Phase 1 vs. Phase 2. Inflammation reduced the in vivo activity of renal OAT1/3 (mediating furosemide CLsecretion) and UGT1A9/1A1 (mediating the formation of furosemide glucuronide) by approximately 40% and 54%, respectively, presumably by elevating the plasma cytokine concentrations. The dosing regimens of narrow therapeutic window OAT drug substrates may need to be adjusted during inflammatory conditions.
1. Introduction
Substantial evidence has indicated that inflammation plays a crucial role in the regulation of drug-metabolizing enzymes and transporters (DMET). In vitro and animal studies have demonstrated that increased concentrations of multiple cytokines, such as interleukin (IL) 6, tumor necrosis (TNF) α, and interferon (IFN) γ, can alter the expression and/or activity of DMET [1,2,3]. However, these studies have not mimicked the physiological inflammatory conditions in humans, making it difficult to translate the findings to changes in the in vivo pharmacokinetics (PK) of drugs in humans. Studies from this research group have reported that systemic inflammation resulting from rheumatoid arthritis, systemic lupus erythematosus, visceral leishmaniasis, and chronic hepatitis C alters the activity of CYP enzymes and hepatic/intestinal drug transporters [4,5,6,7,8]. However, to date, have been no studies on the impact of inflammation on the in vivo activities of the renal transporters, especially during pregnancy.
Pregnant women are a population that has been historically excluded from clinical research, even though 200 million pregnancies occur annually worldwide [9]. Recent studies have recommended the inclusion of pregnant women in clinical studies, either for the study of new drugs or the optimization of pharmacotherapy in this population [9,10,11,12]. Pregnant women are affected by diseases, such as infections, diabetes, and gestational hypertension. For example, urinary tract infections (UTIs) are the most common bacterial infections during pregnancy [13]. UTIs are defined as the presence and replication of bacteria in the urinary tract, leading to tissue damage in the urinary system. Acute pyelonephritis stands out among severe cases of UTIs, caused mainly by the intestinal flora Gram-negative bacteria Escherichia coli, but also by the bacteria of the Enterobacter and Proteus and the Gram-positive bacteria of the genus Streptococcus group B [13,14]. Despite a relatively low prevalence during pregnancy (1–2%), acute pyelonephritis is one of the most common causes of prenatal hospitalization, which can lead to maternal–fetal death and is the primary cause of septic shock in pregnant women [13]. The treatment of acute pyelonephritis requires hospitalization and the use of antibiotics to resolve the condition and prevent its progression to septicemia [13]. Acute pyelonephritis results in inflammation caused by pro-inflammatory cytokines. For example, in non-pregnant women with acute pyelonephritis, the plasma concentrations of the pro-inflammatory cytokines IL-6 and IL-8, TNF-α, and protein C reactive (CRP) [15,16,17] can reach from 2 to 75 times the concentrations in healthy pregnant and non-pregnant women [15,16,17].
Furosemide is a relatively old drug that was approved by the Food and Drug Administration (FDA) in 1966 and has been used as a diuretic since then. In the presence of the anti-gout drug probenecid, a known tubular drug secretion inhibitor, furosemide’s plasma exposure increases, while its total clearance (CL) and renal clearance (CLrenal) are decreased [18]. Subsequent studies in human primary cells such as HEK293 have confirmed and characterized furosemide as a substrate for the OAT1 and OAT3 transporters, with affinity constant values (Km) of 38.9 and 21.5 μM, respectively [19]. Recently, furosemide has been used as part of cocktails of probe drugs to evaluate the in vivo activities of the renal transporters OAT1 and OAT3 in drug–drug and drug–disease interactions [20,21]. Thus, the primary goal of this study was to study the impact of inflammation caused by acute pyelonephritis (in pregnant women) on the renal secretion clearance of furosemide. Since UGT1A9 and UGT1A1 mediate the formation of furosemide glucuronide [22,23], which is excreted unchanged in the urine, our secondary goal was to evaluate the effect of inflammation on the in vivo activities of these two enzymes.
2. Materials and Methods
2.1. Clinical Study
The research protocols were conducted according to the guidelines of the Declaration of Helsinki and were approved by the Research Ethics Committee of the School of Medicine of Ribeirão Preto from the University of São Paulo (HCFMRP-USP) and the Brazilian Registry of Clinical Trials (ReBEC, http://www.ensaiosclinicos.gov.br, accessed on 1 October 2023) under the ID number RBR-4npsyxz. All the participants received a detailed explanation about the purpose of the study, its duration, the procedures, and the possible risks involved. Informed consent was obtained from all the subjects involved in the study. The participants were free to refuse to participate or withdraw their consent at any stage of the research, without penalty or prejudice to their care and/or treatment.
Pregnant women aged over 18 years diagnosed with acute pyelonephritis with indications for treatment with antibiotics were investigated. The acute pyelonephritis diagnosis was based on clinical (costovertebral angle tenderness, fever, and general malaise) and laboratory (pyuria, positive nitrite in urine, and urine culture showing at least 10,000 colony-forming units) exams. The participants were excluded from the study if they presented at least one of the following conditions: chronic renal failure, hypertensive syndromes (chronic arterial hypertension and/or pre-eclampsia), chronic fetal distress, or other inflammatory conditions. The participants were excluded from the protocol if they used drugs that inhibit OAT 1/3.
The clinical protocol was divided into 2 phases (Figure 1). In Phase 1, after the patient’s diagnosis and the indication of antibiotic treatment by the medical team, 2 mL of heparinized blood containing EDTA was collected to quantify the plasma concentration of cytokines. Anthropometric, biochemical, and hematological assessments were routinely performed by the local hospital, and such data were subsequently accessed via electronic medical records. After the administration of the first dose of antibiotic (intravenous cefuroxime, 750 mg, TID), the pregnant women received a single oral dose of 40 mg of furosemide with 200 mL of water. Serial blood samples were collected before and after the administration of furosemide at 30 min, 1; 1.5; 2; 4; 6; 8; 10; 12; 16; and 24 h [24]. The blood samples were centrifuged, and plasma was stored at −80 °C. Urine was collected over 0–24 h, the pH was immediately adjusted to 4–5 to avoid the hydrolysis of the furosemide glucuronide [25], and the volume was measured. Aliquots (10 mL) of urine were separated and stored at −80 °C. According to the local hospital protocol, after continued treatment with intravenous cefuroxime (TID for 3 to 7 days) and after showing improvement in the clinical condition, the pregnant women were discharged from the hospital and continued the treatment of oral cefuroxime (250 mg, TID) for 10–14 days.
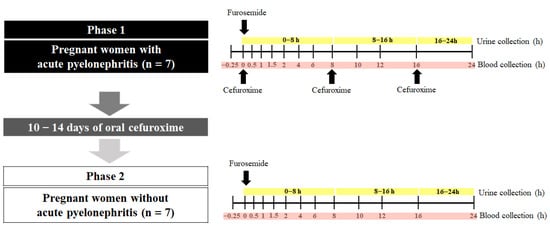
Figure 1.
The in vivo impact of inflammation on the activity of the renal transporters OAT1/3 was quantified using a paired study design. Pregnant women diagnosed with (Phase 1) and without acute pyelonephritis (Phase 2) received a single dose of furosemide (40 mg, PO). Plasma and urine samples were collected (0–24 h). During Phase 1, acute pyelonephritis was treated with intravenous cefuroxime TID over 24 h. Phase 2 was conducted after pyelonephritis was resolved by 10–14 days of treatment with cefuroxime (250 mg/TID).
After the end of the cefuroxime treatment, the resolution of acute pyelonephritis was confirmed, and the second phase of the protocol was carried out within the shortest possible time so that the pregnant women were in the same trimester of pregnancy as they were in Phase 1. In Phase 2, the pregnant women received a single oral dose of 40 mg of furosemide with 200 mL of water. Similar to Phase I, blood and urine samples were collected to determine the furosemide pharmacokinetics for the quantification of plasma cytokines and biochemical and hematological assessments.
2.2. Power Analysis
The sample size was calculated based on the furosemide pharmacokinetics in healthy volunteers administered with a single dose (40 mg, PO) of furosemide [26]. This calculation indicated that, to observe a difference of at least 40% in the renal secretion clearance (CLsecretion) of the furosemide at p < 0.05 and power >80%, 7 participants would need to be studied in a pairwise fashion.
2.3. Analyses of Furosemide and Furosemide-Glucuronidein Plasma, Urine, and Plasma Ultrafiltrate
Furosemide and its glucuronide metabolite (FUR-GLU) concentrations in the plasma, urine, and the ultrafiltrate (from protein binding studies) were quantified using liquid chromatography coupled to tandem mass spectrometry (LC/MS), as developed and validated by us [27]. Briefly, 50 µL of plasma, urine, or ultrafiltrate were used for the analyses. The plasma samples were analyzed by acidified liquid–liquid extraction, while the urine and plasma ultrafiltrate were simply diluted with the mobile phase. The ultrafiltrate was obtained after centrifuging 200 µL of plasma through the Centrifree® Ultrafiltration Device (Millipore Corp., Carrigtwohill, Ireland) as follows. The samples were centrifuged at 1875× g for 40 min in a centrifuge with a fixed-angle rotor (angle of 36°) (Model NT 825, Nova Técnica, Piracicaba, Brazil). The calibration lines of the total and unbound furosemide analysis were linear in the ranges of 0.50–2.500 and 0.125–250 ng/mL, respectively. Additionally, the calibration lines for the furosemide and FUR-GLU in the urine were linear in the range of 50–20,000 ng/mL. The coefficients of variation and the relative standard errors of the standard curve and quality control samples were lower than 15%.
2.4. Quantification of Plasma Cytokine Concentrations
The blood samples of all the participants enrolled in this study were stored at 4 °C and centrifuged (2500× g, 10 min, 4 °C) within 2 h of collection. The harvested plasma samples were stored at −80 °C until analysis. A broad panel of cytokines was evaluated, including IFN-γ, IL-1β, IL-2, IL-6, IL-8, IL-10, IL-12p40, IL-12p70, TNF-α, monocyte chemoattractant protein (MCP) 1, and CRP. Fifty microliters of undiluted, freshly thawed plasma and twenty-five microliters of freshly thawed plasma diluted at 1:40,000 were used for the cytokine and CRP analyses, respectively. These samples were analyzed using a 96-well plate assay, as per the manufacturer’s instructions, using the Luminex® xMAP® magnetic bead platform (Milliplex Map Human Cytokine Panel; Millipore, Billerica, MA, USA). Standards provided by the manufacturer were assayed in duplicates to generate calibration lines in the range from 3.2 to 10,000 pg/mL for each cytokine and from 0.01 to 50 ng/mL. The coefficients of variation and the relative standard errors of the standard curve and quality control samples were <15%.
2.5. Pharmacokinetic Analyses
The furosemide pharmacokinetic parameters were estimated by non-compartmental analyses using Phoenix WinNonlin®, version 8.3.4.295 (Certara USA, Inc., Princeton, NJ, USA, EUA). The parameters, maximum plasma concentration (Cmax), and time to Cmax (Tmax) were documented. The area under the plasma concentration–time curve (AUC) was calculated using the linear trapezoidal rule and extrapolated to infinite by Clast/Kel, where Clast is the last predicted plasma concentration based on the terminal elimination rate (Kel) estimated from the log-linear regression of the last four data points. The unbound fraction of furosemide (fu) in the plasma was determined by the ratio of the unbound plasma concentration and the total plasma concentration in the Cmax samples (furosemide plasma protein binding has been documented to be concentration-independent) [28]. The furosemide oral clearance (CL/F) was estimated as CL/F = dose/AUC and the renal clearance (CLrenal) was estimated as CLrenal = Ae/AUC0–24h, where Ae is the amount of furosemide excreted unchanged into the urine over 24 h (the half-life of furosemide in our study and others was 3–5 h [24,26]). The CLsecretion was estimated as CLsecretion = CLrenal − fu × creatinine clearance (CrCL), where the CrCL was estimated using the Cockcroft–Gault equation and the participant’s actual body weight [29], a recommended approach to evaluating CrCL in pregnant women. The non-renal clearance (CL/Fnon-renal) was estimated as CL/Fnon-renal = CL/F – CLrenal. Finally, the formation clearance to the metabolite FUR-GLU (CLformation, FUR-GLU) was estimated as the AeFUR-GLU/AUC0–24, furosemide, where the AeFUR-GLU is the amount of furosemide excreted as FUR-GLU (i.e., the total amount of FUR-GLU recovered in the urine multiplied by the ratio of furosemide/FUR-GLU molecular weight). This estimation assumes that, over 24 h, most, if not all, of the metabolite formed in the body is recovered in the urine, with minimal non-renal excretion or sequential metabolism.
2.6. Statistical Analyses
The normality of the log-transformed data was accessed using the Shapiro–Wilk statistical test. The normally distributed parameters were compared using the Student’s t-test and are shown as geometric means and 90% confidence intervals, whereas the non-normally distributed parameters were compared using the Wilcoxon test and are shown as medians (interquartile ranges) [30]. In addition, the 90% confidence interval of the ratio (presence vs. absence of acute pyelonephritis) of the geometric means of the furosemide CLrenal, CLsecretion, CLformation, FUR-GLU was computed. If this 90% confidence interval fell within the 0.8–1.25 range (i.e., the bioequivalence range), the groups were considered to be not significantly different [20]. The statistical analyses were performed using the software R (https://www.r-proje ct.org/, accessed on 1 October 2023) version 4.2.0.
3. Results
Seven pregnant women treated for acute pyelonephritis participated in both Phase I and II of the study. Though an additional three women participated in Phase 1, they did not participate in Phase II. Since our goal was paired comparison, they were excluded from all the data analyses. Most of the participants were in their third trimester (five out of seven; see Table 1 for the pregnant women’s demographic, biochemical, and hematological parameters). Higher median concentrations of CRP and plasma cytokines were observed during Phase I when compared to Phase II for IL-6, IFN-γ, TNF-α, and MCP-1, but not for the other cytokines (Table 2).

Table 1.
Clinical characteristics of the pregnant women investigated in the presence (Phase 1) and absence (Phase 2) of acute pyelonephritis.

Table 2.
Plasma CRP and cytokines’ concentrations in the presence (Phase 1) and absence (Phase 2) of acute pyelonephritis in pregnant women.
The geometric means of CLrenal (4.2 vs. 6.9 L/h), CLsecretion (3.9 vs. 6.7 L/h), and CLformation, FUR-GLU (1.1 vs. 2.3 L/h) were significantly lower in Phase 1 when compared to Phase 2 (Table 3; Figure 2). This conclusion was confirmed when the Phase 2/Phase 1 geometric mean ratios of these parameters and their 90% confidence intervals were examined. None of the 90% confidence intervals fell within the bioequivalence threshold of 0.8–1.25 (Figure 3). In contrast, the Tmax, Cmax, AUC0–24, AUC0–∞, CL/F, fu, Ae, and CL/Fnon-renal were not significantly different between Phase 1 and Phase 2 (Table 3). These results did not differ if the three pregnant women, previously excluded from the analyses, were included and the data were analyzed using an unpaired approach (data not shown).

Table 3.
Furosemide (40 mg, PO) pharmacokinetic parameters in the presence (Phase 1) and absence (Phase 2) of acute pyelonephritis in pregnant women.
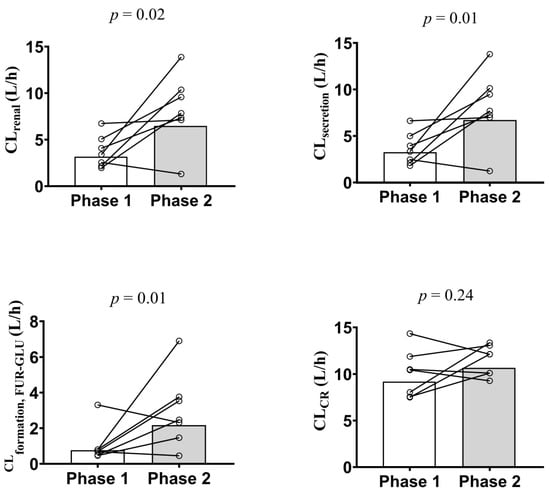
Figure 2.
Furosemide renal (CLrenal; top left panel) and secretion (CLsecretion; top right panel) clearances, furosemide glucuronide formation clearance (CLformation, FUR-GLU; bottom left panel), and creatinine clearance (CLCr; bottom right panel) estimation in the presence (Phase 1) and absence (Phase 2) of acute pyelonephritis in 7 pregnant women after a single furosemide dose (40 mg, PO). Data are presented for each individual and the bars represent the geometric mean. The difference in the indicated parameter between Phase 1 and Phase 2 was evaluated using the paired Student’s t-test (p > 0.05). CLCr was estimated using the Cockcroft–Gault equation and the participant’s actual body weight.
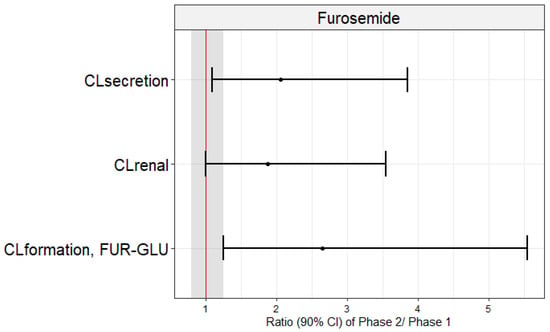
Figure 3.
Geometric mean ratios (dots) and confidence intervals of 90% (lines) of furosemide renal (CLrenal) and secretion (CLsecretion) clearances and furosemide glucuronide formation clearances (CLformation, FUR-GLU) in pregnant women in the presence (Phase 1) and absence (Phase 2) of acute pyelonephritis. These mean ratios did not fall within the bioequivalence range (0.80–1.25; shaded area).
4. Discussion
This study reported, for the first time, reductions in the in vivo activities of the renal transporters OAT1/3 (~40%) and UGT1A9/1A1 (~50%) due to systemic inflammation caused by acute pyelonephritis. The advantage of using acute pyelonephritis as a model for infection is that the infection can be resolved by a short course of cephalosporin (usually cefuroxime). Thus, this allowed us to study the impact of inflammation on the renal OATs in the presence and absence of acute pyelonephritis, where the same subjects acted as their own controls. We chose to study the OAT1/3 transporters because they are involved in the renal secretion of many drugs used to treat a variety of infections that result in inflammation (e.g., pyelonephritis, sepsis, and hepatitis). Additionally, the paired study minimized the important interindividual variability in the plasma cytokine concentrations [32]. This paired design allowed us to have sufficient power to determine a significant difference in the furosemide CLsecretion with only seven subjects. We chose to use furosemide as a probe OAT1/3 drug because most (~65–85%) of an intravenous dose of furosemide is renally eliminated by the uptake transporters OAT1/3 and efflux transporter MRP4 [33]. A smaller fraction (~35%) [34] is metabolized (likely in the liver and kidneys) into glucuronide by the UGT1A9 isoform and, to a lesser extent, by 1A1 [23]. Less than 12% of the drug is excreted unchanged in feces [35].
Our primary endpoint was CLsecretion, rather than other systemic parameters such CL/F, AUC, or Cmax, as these can be influenced by absorption (potentially modulated by intestinal OATP2B1, BCRP, and MRP4 [34]) and metabolic processes. We and others [26,34,36] interpreted the furosemide CLsecretion to reflect the in vivo activity of the renal OAT1/3 transporters. This interpretation assumes that the OAT1/3-mediated active secretion is the only rate-determining step in the furosemide CLsecretion and CLrenal, since the latter approximates the former. Additionally, furosemide has been documented not to be an OAT2 substrate [37]. Since furosemide exhibits CLsecretion and CLrenal that are much smaller than the renal blood flow (Qrenal; approximately 1.2 L/min) and its blood-to-plasma partition (B/P) value is 0.6, possible changes in Qrenal due to acute pyelonephritis can be disregarded as a confounding factor in the interpretation of the data. Additionally, acute pyelonephritis did not affect the fu of the furosemide in the plasma. Thereby, we can conclude that the renal OAT 1/3 activity was reduced by inflammation, as evidenced by the lower CLsecretion (~43%) and CLrenal (~38.5%) in Phase 1 vs. Phase 2 (Table 3; Figure 2 and Figure 3). The furosemide CLsecretion was estimated by its filtration CL, which, in turn, was estimated by CrCL. Though creatinine CL is routinely used to estimate GFR, it is also partially secreted by OAT2 [38]. However, we observed no change in CrCL (Table 1; Figure 2), indicating that inflammation resulting from acute pyelonephritis reduces the tubular secretion of furosemide by OAT1/3 rather than glomerular filtration.
The UGT1A9/1A1 activity was also reduced by inflammation, as evidenced by a decrease in the CLformation, FUR-GLU (~54%). In contrast, inflammation did not affect the other furosemide pharmacokinetic parameters (Table 3; Supplement furosemide). The lack of change in the AUC, Cmax, or Tmax suggested that the rate and extent of furosemide absorption were not affected by the inflammation; thus, the activities of the intestinal OATP2B1, BCRP, and MRP4, did not appear to be affected by the inflammation. Moreover, the inflammation did not appear to affect the biliary clearance of the furosemide.
Inflammation is an important component of a range of clinical conditions such as bacterial, viral, fungal, and protozoal infections, chronic diseases such as type 2 diabetes mellitus, neoplasms, and autoimmune diseases such as rheumatoid arthritis and systemic lupus erythematosus [21,39,40]. Chronic or acute inflammation can result in changes in pharmacokinetics, resulting in variability in the efficacy and toxicity of drugs [21,39,41]. For example, the total clearance of meropenem was reduced by ~30–40% in critically ill patients and CRP was identified as a covariate for this reduction [42]. Meropenem is primarily eliminated renally (70%) by OAT1/3 and multidrug resistance-associated protein (MRP)4 [42,43]. Similarly, a higher plasma exposure (~70%) to the immunosuppressive mycophenolate mofetil [44,45] (primarily metabolized by hepatic UGT1A9) was observed in transplanted patients with a cytomegalovirus infection, an inflammatory viral disease [46]. However, the literature lacks in vivo studies that have characterized the activity of the renal drug transporters in inflammatory conditions. Limited data have shown a reduction in OAT1/3 mRNA (and other renal transporters) in an experimental rat inflammation model generated with lipopolysaccharide or polyinosinic:polycytidylic [47,48].
When compared to Phase II, the Phase I participants showed higher median values of some plasma cytokines such as IL-6 (97-fold), IFN-γ (6-fold), CRP (11-fold), MCP-1 (2-fold), and TNF-α (2-fold) (Table 2). Higher or similar fold-plasma concentrations (4 to 75-fold) of IL-6 have been observed in non-pregnant women with acute pyelonephritis vs. patients with asymptomatic bacteriuria, after acute pyelonephritis treatment or healthy volunteers [15,16,17]. Yet, in those studies, the CRP and TNF-α values were similar (11.2 mg/dL and 35.0 pg/mL, respectively) in the patients before and 24 h after the acute pyelonephritis treatment to those observed in Phase 1 of our study [17]. Additionally, we reported, for the first time, that the MCP-1 plasma concentrations were elevated during acute pyelonephritis, reaching similar values to those observed in critically ill COVID-19 patients. MCP-1 is also relevant in other infectious/inflammatory diseases such as tuberculosis, inflammatory bowel disease, and rheumatoid arthritis [49]. The elevated plasma concentrations of CRP and the cytokines evaluated in the present study have also been observed in other inflammatory conditions, such as rheumatoid arthritis, systemic lupus erythematosus, visceral leishmaniasis, and COVID-19 [4,6,8,50].
The cytokines IL-6, TNF-α, and IL-1β have been associated with changes in DMET expression and activity in in vitro studies. However, these have all focused on the transporters expressed in human hepatocytes. Plated human hepatocytes treated with from 100 to 10,000 pg/mL of IL-6 (a concentration range that includes the highest concentrations observed in this study) for from 8 to 48 h resulted in the reduced expression of the mRNA of several transporters, such as P-gp, MRP2, BCRP, Na+-taurocholate co-transporting polypeptide (NTCP), organic anion transporting polypeptide (OATP)2B1, OATP1B1, OATP1B3, organic cation transport (OCT) 1, and OAT 2 [2,51,52]. To date, there have been no in vitro studies that have characterized the activities or expressions of the renal transporters in the presence of cytokines. Nevertheless, we interpret the impact of pyelonephritis on the reduced renal OAT1/3 activity as being due to the elevation in the plasma cytokine concentrations reported here.
This study has some limitations. First, we assumed that the effect of acute pyelonephritis on the furosemide CLsecretion was caused solely by the resulting inflammation, leading to elevations in the plasma cytokine concentrations. We found no significant correlations between the cytokine plasma concentrations and the furosemide PK parameters (data not shown), probably because of the inherent variability in cytokines’ plasma concentrations and the small sample size of the study. However, we cannot discount the possibility that other physiological changes caused by the disease (or the disease–pregnancy interaction) also contributed to the observed change. Second, we assumed that the furosemide CLsecretion was not rate-determined by MRP4. If it was, it is possible that the inflammation reduced the activity of one or some combination of the three renal transporters (OAT1/3 and MRP4). Third, we assumed that the administration of cefuroxime during Phase 1 did not affect the furosemide CLsecretion. Cefuroxime, a cephalosporin antibiotic, may be an OAT1/3 substrate [53,54]. Even if it is an OAT1/3 substrate, based on the following data, we do not believe that the plasma concentrations of cefuroxime observed in the study inhibited OAT1/3. The plasma Cmax of cefuroxime observed in this study (unbound geometric mean and [CV%] of 28.81 [30.97]) mg/L) was lower than the reported cefuroxime’s IC50 (250 mg/L) to inhibit OATs [55]. Finally, no drug–drug interaction was observed when the intravenous cefuroxime (1.5 g) was administered simultaneously with the known OAT substrate, NXY-059 [56].
5. Conclusions
In conclusion, the data from this paired study showed that systemic inflammation, due to a bacterial infection caused by acute pyelonephritis, reduced the in vivo activity of the renal transporters OAT1/3 and renal UGT1A9/1A1 in pregnant women by approximately 40% and 50%, respectively. This magnitude of change would necessitate adjustment in the dosing regimen of drugs that have a narrow therapeutic window and are predominately cleared by OAT1/3 and/or UGT1A9/1A1.
Author Contributions
J.R.d.L.B.: wrote the manuscript, designed the research, performed the research, and analyzed the data. P.P.d.S.M.: performed the research. G.D.: designed and performed the research. J.D.U.: reviewed and edited the manuscript. V.L.L.: wrote the manuscript, designed the research, analyzed the data, reviewed and edited the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the São Paulo Research Foundation (FAPESP) grant numbers 2018/05616-3, 2019/03429-4 and 2021/10292-5, the Coordination for the Improvement of Higher Education Personnel (CAPES, Brazil, Finance Code 001), the Brazilian National Council for Scientific and Technological Development (CNPq) and in part by NIH Grant R01HD102786.
Institutional Review Board Statement
The research protocols were conducted according to the guidelines of the Declaration of Helsinki and approved by the Research Ethics Committee of the School of Medicine of Ribeirão Preto from the University of São Paulo (HCFMRP-USP), CAAE: 06827219.50000.5403 and 06827219.5.3001.5440 and by the Brazilian Registry of Clinical Trials (ReBEC, http://www.ensaiosclinicos.gov.br, accessed on 1 October 2023) under ID number RBR-4npsyxz.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Data are available on reasonable request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Morgan, E.T. Impact of Infectious and Inflammatory Disease on Cytochrome P450-Mediated Drug Metabolism and Pharmacokinetics. Clin. Pharmacol. Ther. 2009, 85, 434–438. [Google Scholar] [CrossRef]
- Fardel, O.; Le Vée, M. Regulation of Human Hepatic Drug Transporter Expression by Pro-Inflammatory Cytokines. Expert. Opin. Drug Metab. Toxicol. 2009, 5, 1469–1481. [Google Scholar] [CrossRef]
- Klöditz, K.; Tewolde, E.; Nordling, Å.; Ingelman-Sundberg, M. Mechanistic, Functional and Clinical Aspects of pro-Inflammatory Cytokine Mediated Regulation of ADME Gene Expression in 3D Human Liver Spheroids. Clin. Pharmacol. Ther. 2023, 114, 673–685. [Google Scholar] [CrossRef]
- Lanchote, V.L.; Almeida, R.; Barral, A.; Barral-Netto, M.; Marques, M.P.; Moraes, N.V.; Da Silva, A.M.; Souza, T.M.V.; Suarez-Kurtz, G. Impact of Visceral Leishmaniasis and Curative Chemotherapy on Cytochrome P450 Activity in Brazilian Patients. Br. J. Clin. Pharmacol. 2015, 80, 1160–1168. [Google Scholar] [CrossRef]
- Pippa, L.F.; Vieira, C.P.; Caris, J.A.; Rocha, A.; Garcia, C.P.; Rezende, R.E.F.; Lanchote, V.L. Clinical Treatment for Hepatitis C Reverses CYP2C19 Inhibition. Br. J. Clin. Pharmacol. 2021, 87, 4013–4019. [Google Scholar] [CrossRef]
- Caris, J.A.; de Lima Benzi, J.R.; de Souza, F.F.L.; de Oliveira, R.D.R.; Donadi, E.A.; Lanchote, V.L. Rheumatoid Arthritis Downregulates the Drug Transporter OATP1B1: Fluvastatin as a Probe. Eur. J. Pharm. Sci. 2020, 146, 105264. [Google Scholar] [CrossRef]
- Pippa, L.F.; Vieira, C.P.; Caris, J.A.; Rocha, A.; Marques, M.P.; Garcia, C.P.; Rezende, R.E.F.; Lanchote, V.L. Effect of Chronic Hepatitis C on the Activity of the Membrane Transporters P-gp and OATP1B1/BCRP on Patients with Different Stages of Hepatic Fibrosis. Clin. Pharmacol. Ther. 2023, 114, 173–181. [Google Scholar] [CrossRef] [PubMed]
- Cestari, R.N.; de Oliveira, R.D.R.; de Souza, F.F.L.; Pippa, L.F.; Nardotto, G.H.B.; Rocha, A.; Donadi, E.A.; Lanchote, V.L. Systemic Lupus Erythematosus Activity Affects the Sinusoidal Uptake Transporter OATP1B1 Evaluated by the Pharmacokinetics of Atorvastatin. Clin. Transl. Sci. 2020, 13, 1227–1235. [Google Scholar] [CrossRef] [PubMed]
- Quinney, S.K.; Bonate, P.L. A Pharmacometrician’s Role in Enhancing Medication Use in Pregnancy and Lactation. J. Pharmacokinet. Pharmacodyn. 2020, 47, 267–269. [Google Scholar] [CrossRef]
- Kazma, J.M.; van den Anker, J.; Allegaert, K.; Dallmann, A.; Ahmadzia, H.K. Anatomical and Physiological Alterations of Pregnancy. J. Pharmacokinet. Pharmacodyn. 2020, 47, 271–285. [Google Scholar] [CrossRef] [PubMed]
- Eke, A.C.; Olagunju, A.; Momper, J.; Penazzato, M.; Abrams, E.J.; Best, B.M.; Capparelli, E.V.; Bekker, A.; Belew, Y.; Kiser, J.J.; et al. Optimizing Pharmacology Studies in Pregnant and Lactating Women Using Lessons From HIV: A Consensus Statement. Clin. Pharmacol. Ther. 2021, 110, 36–48. [Google Scholar] [CrossRef] [PubMed]
- Abduljalil, K.; Ning, J.; Pansari, A.; Pan, X.; Jamei, M. Prediction of Maternal and Fetoplacental Concentrations of Cefazolin, Cefuroxime, and Amoxicillin during Pregnancy Using Bottom-Up Physiologically Based Pharmacokinetic Models. Drug Metab. Dispos. 2022, 50, 386–400. [Google Scholar] [CrossRef]
- Saleh, P.; Noshad, H.; Mallah, F.; Ramouz, A. Acute Pyelonephritis in Pregnancy and the Outcomes in Pregnant Patients. Arch. Clin. Infect. Dis. 2015, 10, e28886. [Google Scholar] [CrossRef]
- Jim, B.; Garovic, V.D. Acute Kidney Injury in Pregnancy. Semin. Nephrol. 2017, 37, 378–385. [Google Scholar] [CrossRef]
- Hedges, S.; Stenqvist, K.; Lidin-Janson, G.; Martinell, J.; Sandberg, T.; Svanborg, C. Comparison of Urine and Serum Concentrations of Interleukin-6 in Women with Acute Pyelonephritis or Asymtomatic Bacteriuria. J. Infect. Dis. 1992, 166, 653–659. [Google Scholar] [CrossRef] [PubMed]
- Jacobson, S.H.; Hylander, B.; Wretlind, B.; Brauner, A. Interleukin-6 and Interleukin-8 in Serum and Urine in Patients with Acute Pyelonephritis in Relation to Bacterial-Virulence-Associated Traits and Renal Function. Nephron 1994, 67, 172–179. [Google Scholar] [CrossRef] [PubMed]
- Horcajada, J.P.; Velasco, M.; Filella, X.; Alvarez, L.; De Làzzari, E.; Marín, J.L.; Collvinent, B.; Smithson, A.; Martínez, J.A.; Noguero, M.; et al. Evaluation of Inflammatory and Renal-Injury Markers in Women Treated with Antibiotics for Acute Pyelonephritis Caused by Escherichia Coli. Clin. Diagn. Lab. Immunol. 2004, 11, 142–146. [Google Scholar] [CrossRef]
- Vree, T.B.; Van Den Biggelaar-Martea, M.; Verwey-van Wissen, C.P. Probenecid Inhibits the Renal Clearance of Frusemide and Its Acyl Glucuronide. Br. J. Clin. Pharmacol. 1995, 39, 692–695. [Google Scholar] [PubMed]
- Ebner, T.; Ishiguro, N.; Taub, M.E. The Use of Transporter Probe Drug Cocktails for the Assessment of Transporter-Based Drug-Drug Interactions in a Clinical Setting—Proposal of a Four Component Transporter Cocktail. J. Pharm. Sci. 2015, 104, 3220–3228. [Google Scholar] [CrossRef]
- Stopfer, P.; Giessmann, T.; Hohl, K.; Sharma, A.; Ishiguro, N.; Taub, M.E.; Zimdahl-Gelling, H.; Gansser, D.; Wein, M.; Ebner, T.; et al. Pharmacokinetic Evaluation of a Drug Transporter Cocktail Consisting of Digoxin, Furosemide, Metformin, and Rosuvastatin. Clin. Pharmacol. Ther. 2016, 100, 259–267. [Google Scholar] [CrossRef]
- Evers, R.; Piquette-Miller, M.; Polli, J.W.; Russel, F.G.M.; Sprowl, J.A.; Tohyama, K.; Ware, J.A.; de Wildt, S.N.; Xie, W.; Brouwer, K.L.R. Disease-Associated Changes in Drug Transporters May Impact the Pharmacokinetics and/or Toxicity of Drugs: A White Paper from the International Transporter Consortium. Clin. Pharmacol. Ther. 2018, 104, 900–915. [Google Scholar] [CrossRef] [PubMed]
- Hammarlund-Udenaes, M.; Benet, L.Z. Furosemide Pharmacokinetics and Pharmacodynamics in Health and Disease-An Update. J. Pharmacokinet. Biopharm. 1989, 17, 1–46. [Google Scholar] [CrossRef] [PubMed]
- Kerdpin, O.; Knights, K.M.; Elliot, D.J.; Miners, J.O. In Vitro Characterisation of Human Renal and Hepatic Frusemide Glucuronidation and Identification of the UDP-Glucuronosyltransferase Enzymes Involved in This Pathway. Biochem. Pharmacol. 2008, 76, 249–257. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, P.V.B.; de Lima Moreira, F.; de Lima Benzi, J.R.; Duarte, G.; Lanchote, V.L. A Pilot Study of the Maternal-Fetal Pharmacokinetics of Furosemide in Plasma, Urine, and Amniotic Fluid of Hypertensive Parturient Women Under Cesarean Section. J. Clin. Pharmacol. 2020, 60, 1655–1661. [Google Scholar] [CrossRef]
- Mizuma, T.; McDonagh, A.F.; Lin, E.T.; Benet, L.Z. Photoinduced Covalent Binding of Frusemide and Frusemide Glucuronide to Human Serum Albumin. Br. J. Clin. Pharmacol. 1999, 48, 79–87. [Google Scholar] [CrossRef]
- Shen, H.; Holenarsipur, V.K.; Mariappan, T.T.; Drexler, D.M.; Cantone, J.L.; Rajanna, P.; Gautam, S.S.; Zhang, Y.; Gan, J.; Shipkova, P.A.; et al. Evidence for the Validity of Pyridoxic Acid (PDA) as a Plasma-Based Endogenous Probe for OAT1 and OAT3 Function in Healthy Subjects. J. Pharmacol. Exp. Ther. 2019, 368, 136–145. [Google Scholar] [CrossRef]
- De Lima Benzi, J.R.; Rocha, A.; Colombari, J.C.; Pego, A.M.G.; dos Santos Melli, P.P.; Duarte, G.; Lanchote, V.L. Determination of Furosemide and Its Glucuronide Metabolite in Plasma, Plasma Ultrafiltrate and Urine by HPLC-MS/MS with Application to Secretion and Metabolite Formation Clearances in Non-Pregnant and Pregnant Women. J. Pharm. Biomed. Anal. 2023, 235, 115635. [Google Scholar] [CrossRef]
- Klinkmann, G.; Klammt, S.; Jäschke, M.; Henschel, J.; Gloger, M.; Reuter, D.A.; Mitzner, S. Impact of Albumin Binding Function on Pharmacokinetics and Pharmacodynamics of Furosemide. Medicina 2022, 58, 1780. [Google Scholar] [CrossRef]
- Zaghloul, D.E.; Ryu, R.; Kestenbaum, B.; Smith, C.; Fay, E.; Hebert, M.F. Renal Function Estimating Equations Performance during Pregnancy and Postpartum. Pharmacotherapy 2023, 43, 359–372. [Google Scholar] [CrossRef]
- Statistical Guide for Clinical Pharmacology Therapeutics. Clin. Pharmacol. Ther. 2010, 88, 150–152. [CrossRef]
- Abbassi-Ghanavati, M.; Greer, L.G.; Cunningham, F.G. Pregnancy and Laboratory Studies A Reference Table for Clinicians. Obstet. Gynecol. 2009, 114, 1326–1357. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.O.; Kim, H.S.; Youn, J.C.; Shin, E.C.; Park, S. Serum Cytokine Profiles in Healthy Young and Elderly Population Assessed Using Multiplexed Bead-Based Immunoassays. J. Transl. Med. 2011, 9, 113. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, M.; Kusuhara, H.; Adachi, M.; Schuetz, J.D.; Takeuchi, K.; Sugiyama, Y. Multidrug Resistance-Associated Protein 4 Is Involved in the Urinary Excretion of Hydrochlorothiazide and Furosemide. J. Am. Soc. Nephrol. 2007, 18, 37–45. [Google Scholar] [CrossRef]
- Chapa, R.; Li, C.Y.; Basit, A.; Thakur, A.; Ladumor, M.K.; Sharma, S.; Singh, S.; Selen, A.; Prasad, B. Contribution of Uptake and Efflux Transporters to Oral Pharmacokinetics of Furosemide. ACS Omega 2020, 5, 32939–32950. [Google Scholar] [CrossRef]
- Prandota, J.; Witkowska, M.; Man, I. Pharmacokinetics and Metabolism of Furosemide in Man. Eur. J. Drug Metab. Pharmacokinet. 1976, 4, 177–181. [Google Scholar] [CrossRef]
- Mathialagan, S.; Feng, B.; Rodrigues, A.D.; Varma, M.V.S. Drug-Drug Interactions Involving Renal OCT2/MATE Transporters: Clinical Risk Assessment May Require Endogenous Biomarker-Informed Approach. Clin. Pharmacol. Ther. 2021, 110, 855–859. [Google Scholar] [CrossRef]
- Hasannejad, H.; Takeda, M.; Taki, K.; Shin, H.J.; Babu, E.; Jutabha, P.; Khamdang, S.; Aleboyeh, M.; Onozato, M.L.; Tojo, A.; et al. Interactions of Human Organic Anion Transporters with Diuretics. J. Pharmacol. Exp. Ther. 2004, 308, 1021–1029. [Google Scholar] [CrossRef]
- Lepist, E.I.; Zhang, X.; Hao, J.; Huang, J.; Kosaka, A.; Birkus, G.; Murray, B.P.; Bannister, R.; Cihlar, T.; Huang, Y.; et al. Contribution of the Organic Anion Transporter OAT2 to the Renal Active Tubular Secretion of Creatinine and Mechanism for Serum Creatinine Elevations Caused by Cobicistat. Kidney Int. 2014, 86, 350–357. [Google Scholar] [CrossRef] [PubMed]
- Lenoir, C.; Rollason, V.; Desmeules, J.A.; Samer, C.F. Influence of Inflammation on Cytochromes P450 Activity in Adults: A Systematic Review of the Literature. Front. Pharmacol. 2021, 12, 733935. [Google Scholar] [CrossRef]
- Stanke-Labesque, F.; Gautier-Veyret, E.; Chhun, S.; Guilhaumou, R. Inflammation Is a Major Regulator of Drug Metabolizing Enzymes and Transporters: Consequences for the Personalization of Drug Treatment. Pharmacol. Ther. 2020, 215, 107627. [Google Scholar] [CrossRef] [PubMed]
- Cressman, A.M.; Petrovic, V.; Piquette-Miller, M. Inflammation-Mediated Changes in Drug Transporter Expression/Activity: Implications for Therapeutic Drug Response. Expert. Rev. Clin. Pharmacol. 2012, 5, 69–89. [Google Scholar] [CrossRef] [PubMed]
- Shibayama, T.; Sugiyama, D.; Kamiyama, E.; Tokui, T.; Hirota, T.; Ikeda, T. Characterization of CS-023 (RO4908463), a Novel Parenteral Carbapenem Antibiotic, and Meropenem as Substrates of Human Renal Transporters. Drug Metab. Pharmacokinet. 2007, 22, 41–47. [Google Scholar] [CrossRef]
- Akanuma, S.I.; Uchida, Y.; Ohtsuki, S.; Kamiie, J.I.; Tachikawa, M.; Terasaki, T.; Hosoya, K.I. Molecular-Weight-Dependent, Anionic-Substrate-Preferential Transport of β-Lactam Antibiotics via Multidrug Resistance-Associated Protein 4. Drug Metab. Pharmacokinet. 2011, 26, 602–611. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, M.; Wakabayashi, Y.; Higuchi, A.; Kadotani, Y.; Ogino, S.; Ushigome, H.; Akioka, K.; Kaihara, S.; Yoshimura, N. Therapeutic Drug Monitoring of Mycophenolic Acid in Renal Transplant Recipients. Transplant. Proc. 2005, 37, 859–860. [Google Scholar] [CrossRef] [PubMed]
- Sommerer, C.; Müller-Krebs, S.; Schaier, M.; Glander, P.; Budde, K.; Schwenger, V.; Mikus, G.; Zeier, M. Pharmacokinetic and Pharmacodynamic Analysis of Enteric-Coated Mycophenolate Sodium: Limited Sampling Strategies and Clinical Outcome in Renal Transplant Patients. Br. J. Clin. Pharmacol. 2010, 69, 346–357. [Google Scholar] [CrossRef] [PubMed]
- García-Torre, A.; Bueno-García, E.; López-Martínez, R.; Rioseras, B.; Díaz-Molina, B.; Lambert, J.L.; Quirós, C.; Alonso-Álvarez, S.; Alonso-Arias, R.; Moro-García, M.A. CMV Infection Is Directly Related to the Inflammatory Status in Chronic Heart Failure Patients. Front. Immunol. 2021, 12, 687582. [Google Scholar] [CrossRef]
- Pour, N.K.; McColl, E.R.; Piquette-Miller, M. Impact of Viral Inflammation on the Expression of Renal Drug Transporters in Pregnant Rats. Pharmaceutics 2019, 11, 624. [Google Scholar] [CrossRef]
- Höcherl, K.; Schmidt, C.; Bucher, M. COX-2 Inhibition Attenuates Endotoxin-Induced Downregulation of Organic Anion Transporters in the Rat Renal Cortex. Kidney Int. 2009, 75, 373–380. [Google Scholar] [CrossRef]
- Deshmane, S.L.; Kremlev, S.; Amini, S.; Sawaya, B.E. Monocyte Chemoattractant Protein-1 (MCP-1): An Overview. J. Interferon Cytokine Res. 2009, 29, 313–325. [Google Scholar] [CrossRef]
- Lenoir, C.; Terrier, J.; Gloor, Y.; Curtin, F.; Rollason, V.; Desmeules, J.A.; Daali, Y.; Reny, J.L.; Samer, C.F. Impact of SARS-CoV-2 Infection (COVID-19) on Cytochromes P450 Activity Assessed by the Geneva Cocktail. Clin. Pharmacol. Ther. 2021, 110, 1358–1367. [Google Scholar] [CrossRef]
- Le Vee, M.; Lecureur, V.; Stieger, B.; Fardel, O. Regulation of Drug Transporter Expression in Human Hepatocytes Exposed to the Proinflammatory Cytokines Tumor Necrosis Factor-α or Interleukin-6. Drug Metab. Dispos. 2009, 37, 685–693. [Google Scholar] [CrossRef]
- Le Vee, M.; Jouan, E.; Stieger, B.; Lecureur, V.; Fardel, O. Regulation of Drug Transporter Expression by Oncostatin M in Human Hepatocytes. Biochem. Pharmacol. 2011, 82, 304–311. [Google Scholar] [CrossRef]
- Coppola, P.; Kerwash, E.; Cole, S. The Use of Pregnancy Physiologically Based Pharmacokinetic Modeling for Renally Cleared Drugs. J. Clin. Pharmacol. 2022, 62, S129–S139. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Olaleye, O.E.; Yu, X.; Jia, W.; Yang, J.; Lu, C.; Liu, S.; Yu, J.; Duan, X.; Wang, Y.; et al. Supporting Information for High Degree of Pharmacokinetic Compatibility Exists between the Five-Herb Medicine XueBiJing and Antibiotics Comedicated in Sepsis Care. Acta Pharm. Sin. B 2019, 9, 1035–1049. [Google Scholar] [CrossRef] [PubMed]
- Verhagen, C.A.; Mattie, H.; Van Strijen, E. The Renal Clearance of Cefuroxime and Ceftazidime and the Effect of Probenecid on Their Tubular Excretion. Br. J. Clin. Pharmac. 1994, 37, 193–197. [Google Scholar] [CrossRef] [PubMed]
- Kågedal, M.; Nilsson, D.; Huledal, G.; Reinholdsson, I.; Cheng, Y.F.; Åsenblad, N.; Pekar, D.; Borgå, O. A Study of Organic Acid Transporter-Mediated Pharmacokinetic Interaction between NXY-059 and Cefuroxime. J. Clin. Pharmacol. 2007, 47, 1043–1048. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).