Recrystallization Mediates the Gelation of Amorphous Drugs: The Case of Acemetacin
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Amorphous ACM
2.3. Dissolution Tests
2.3.1. Dissolution under Non-Sink Conditions
2.3.2. Intrinsic Dissolution Rate
2.4. Solid-State Characterization
2.4.1. Polarizing Light Microscopy
2.4.2. Differential Scanning Calorimetry
2.4.3. X-Ray Powder Diffractometry
2.4.4. Fourier-Transform Infrared Spectroscopy
2.5. Influencing Factors of Amorphous ACM Gelation
2.6. Texture Profile Analysis
3. Results and Discussion
3.1. Dissolution Behaviors of Crystalline and Amorphous ACM
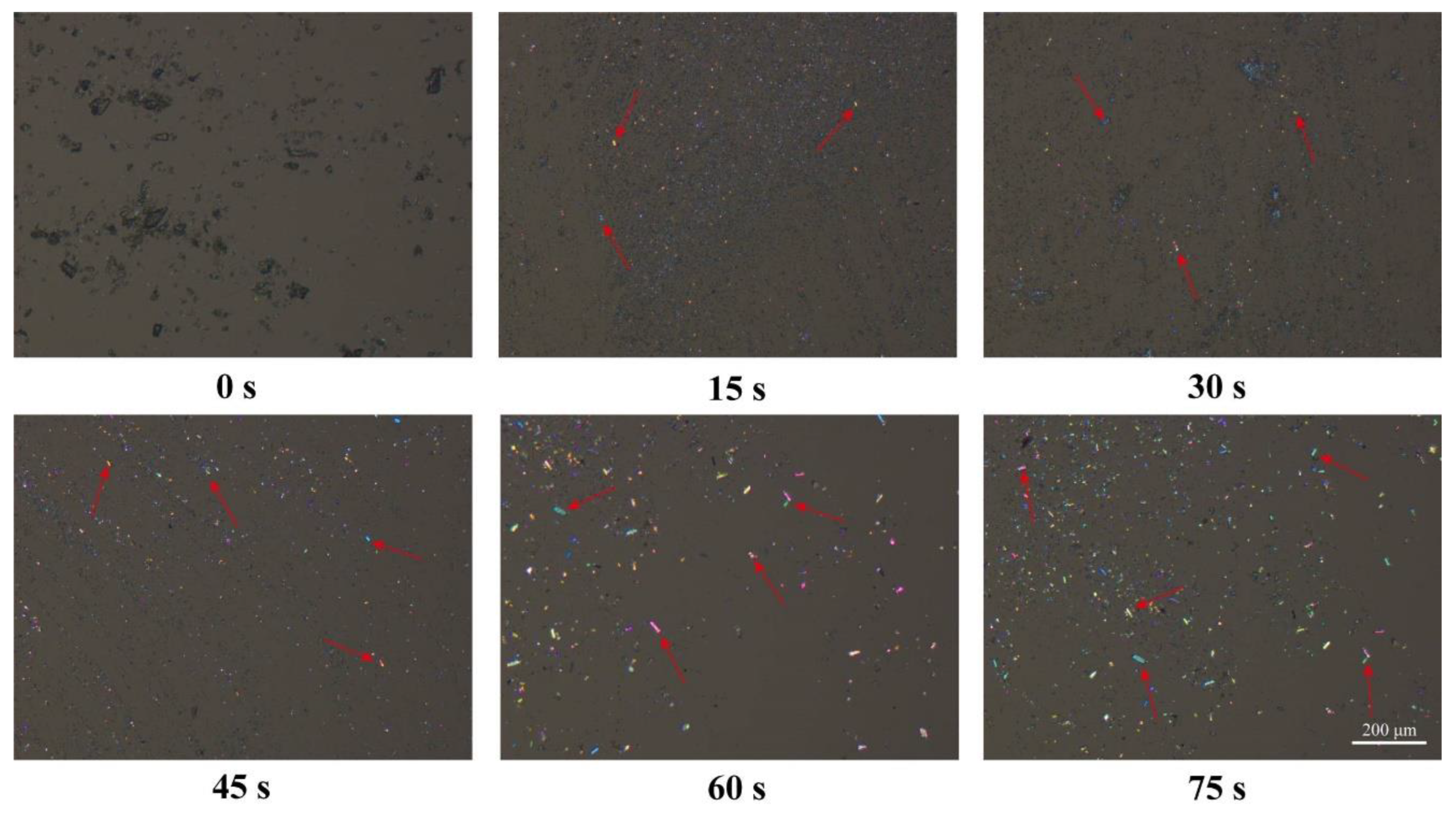
3.2. PLM
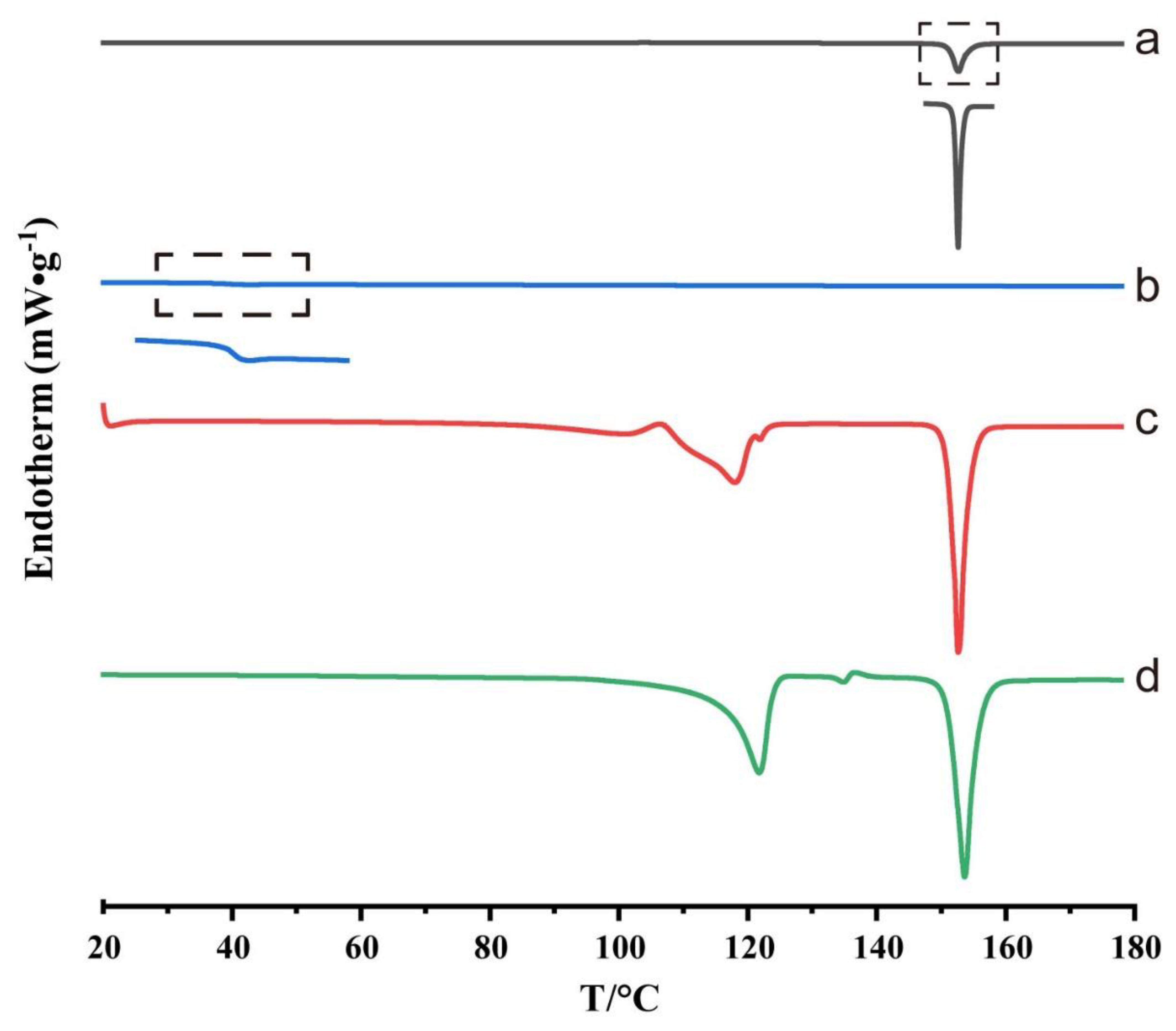
3.3. DSC
3.4. XRPD
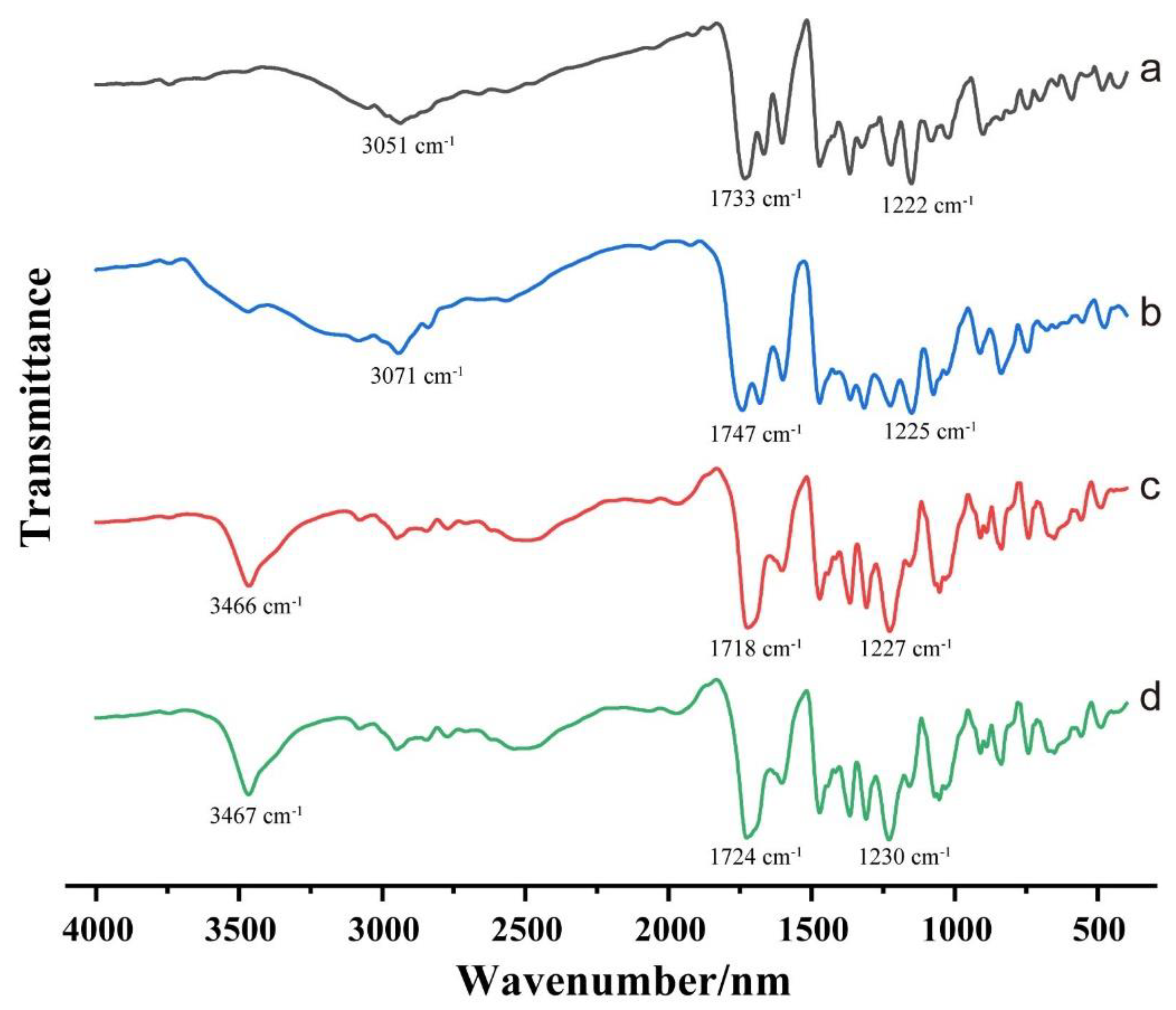
3.5. FTIR
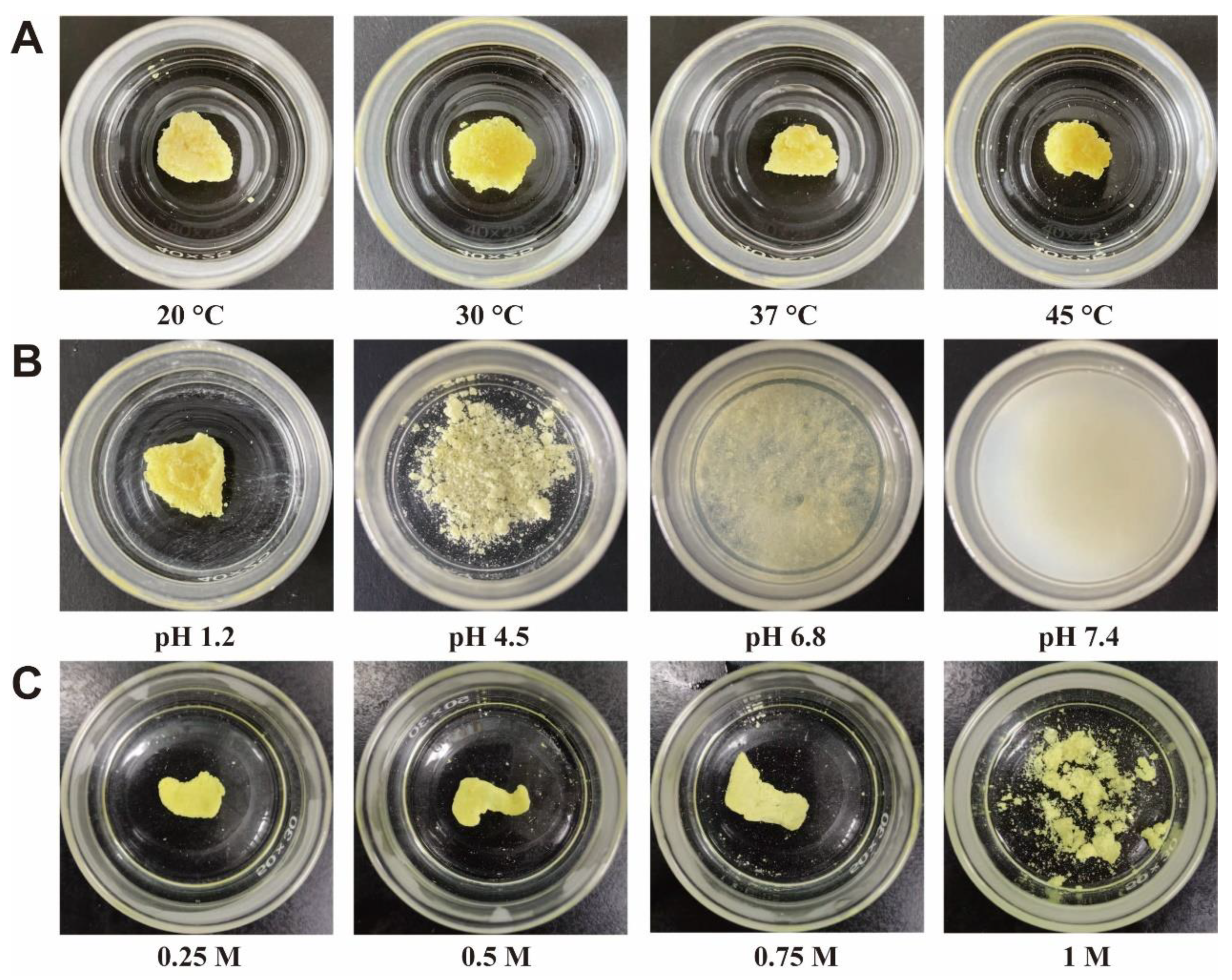
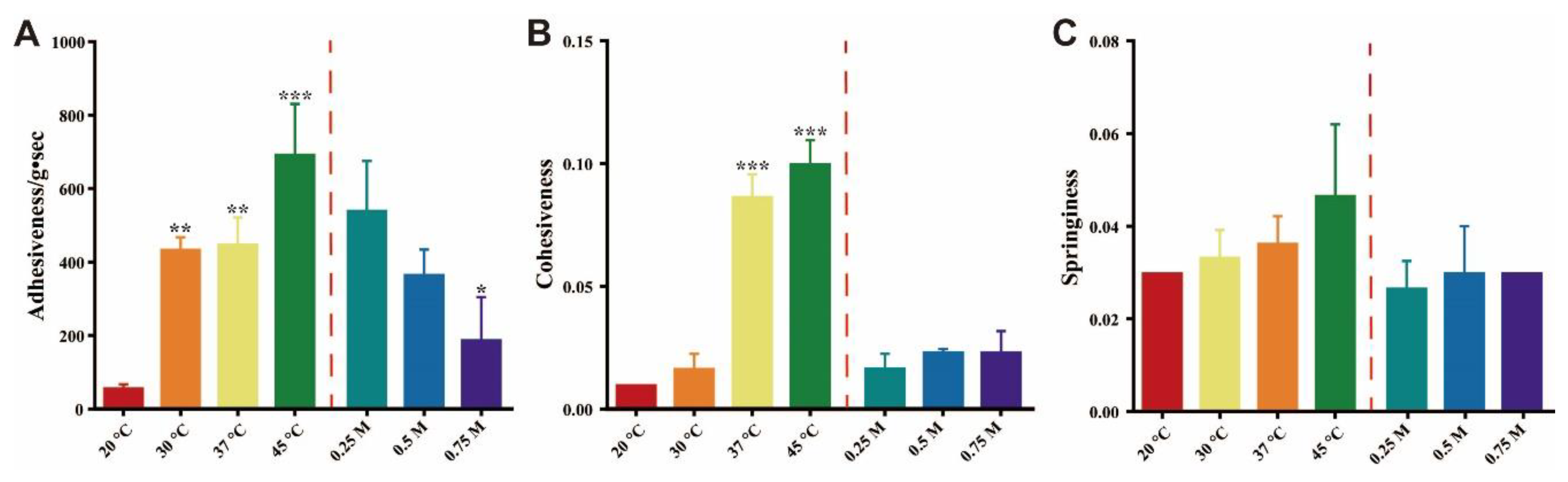
3.6. Influencing Factors of Amorphous ACM Gelation
3.7. Texture Profile Analysis
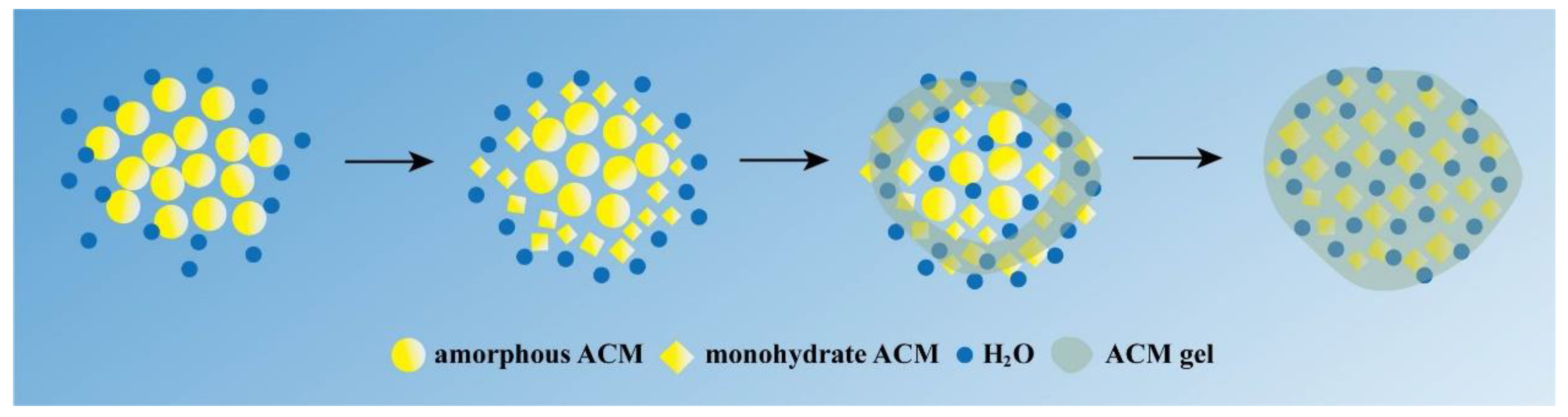
3.8. Gelation Mechanism Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Di, L.; Fish, P.V.; Mano, T. Bridging solubility between drug discovery and development. Drug Discov. Today 2012, 17, 486–495. [Google Scholar] [CrossRef] [PubMed]
- Abbasi, S.; Higashino, H.; Sato, Y.; Minami, K.; Kataoka, M.; Yamashita, S.; Harashima, H. Maximizing the Oral Bioavailability of Poorly Water-Soluble Drugs Using Novel Oil-Like Materials in Lipid-Based Formulations. Mol. Pharm. 2021, 18, 3281–3289. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.W.; Xing, H.J.; Zhao, Y.; Ma, Z.G. Pharmaceutical Dispersion Techniques for Dissolution and Bioavailability Enhancement of Poorly Water-Soluble Drugs. Pharmaceutics 2018, 10, 74. [Google Scholar] [CrossRef] [PubMed]
- Thakuria, R.; Delori, A.; Jones, W.; Lipert, M.P.; Roy, L.; Rodríguez-Hornedo, N. Pharmaceutical cocrystals and poorly soluble drugs. Int. J. Pharm. 2013, 453, 101–125. [Google Scholar] [CrossRef] [PubMed]
- Descamps, M.; Dudognon, E. Crystallization from the Amorphous State: Nucleation–Growth Decoupling, Polymorphism Interplay, and the Role of Interfaces. J. Pharm. Sci. 2014, 103, 2615–2628. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.H.; Sun, C.S.; Hao, Y.R.; Jiang, T.Y.; Zheng, L.; Wang, S.L. Mechanism of Dissolution Enhancement and Bioavailability of Poorly Water Soluble Celecoxib by Preparing Stable Amorphous Nanoparticles. J. Pharm. Pharm. Sci. 2010, 13, 589–606. [Google Scholar] [CrossRef]
- Wlodarski, K.; Sawicki, W.; Paluch, K.; Tajber, L.; Grembecka, M.; Hawelek, L.; Wojnarowska, Z.; Grzybowska, K.; Talik, E.; Paluch, M. The influence of amorphization methods on the apparent solubility and dissolution rate of tadalafil. Eur. J. Pharm. Sci. 2014, 62, 132–140. [Google Scholar] [CrossRef]
- Dhumal, R.S.; Biradar, S.V.; Yamamura, S.; Paradkar, A.R.; York, P. Preparation of amorphous cefuroxime axetil nanoparticles by sonoprecipitation for enhancement of bioavailability. Eur. J. Pharm. Biopharm. 2008, 70, 109–115. [Google Scholar] [CrossRef]
- Qian, S.; Wang, S.; Li, Z.; Wang, X.; Ma, D.; Liang, S.; Gao, Y.; Zhang, J.; Wei, Y. Charge-assisted bond N + H mediates the gelation of amorphous lurasidone hydrochloride during dissolution. Int. J. Pharm. 2017, 518, 335–341. [Google Scholar] [CrossRef]
- Han, J.; Wei, Y.; Li, L.; Song, Y.; Pang, Z.; Qian, S.; Zhang, J.; Gao, Y.; Heng, W. Gelation Elimination and Crystallization Inhibition by Co-Amorphous Strategy for Amorphous Curcumin. J. Pharm. Sci. 2022, 112, 182–194. [Google Scholar] [CrossRef]
- Heng, W.; Wei, Y.; Xue, Y.; Cheng, H.; Zhang, L.; Zhang, J.; Gao, Y.; Qian, S. Gel Formation Induced Slow Dissolution of Amorphous Indomethacin. Pharm. Res. 2019, 36, 159. [Google Scholar] [CrossRef]
- Pang, Z.T.; Wei, Y.F.; Wang, N.N.; Zhang, J.J.; Gao, Y.; Qian, S. Gel formation of puerarin and mechanistic study during its cooling process. Int. J. Pharm. 2018, 548, 625–635. [Google Scholar] [CrossRef]
- Sanphui, P.; Bolla, G.; Nangia, A.; Chernyshev, V. Acemetacin cocrystals and salts: Structure solution from powder X-ray data and form selection of the piperazine salt. IUCrJ 2014, 1, 136–150. [Google Scholar] [CrossRef]
- Babu, N.J.; Nangia, A. Solubility Advantage of Amorphous Drugs and Pharmaceutical Cocrystals. Cryst. Growth Des. 2011, 11, 2662–2679. [Google Scholar] [CrossRef]
- Heng, W.; Su, M.; Cheng, H.; Shen, P.; Liang, S.; Zhang, L.; Wei, Y.; Gao, Y.; Zhang, J.; Qian, S. Incorporation of Complexation into a Coamorphous System Dramatically Enhances Dissolution and Eliminates Gelation of Amorphous Lurasidone Hydrochloride. Mol. Pharm. 2019, 17, 84–97. [Google Scholar] [CrossRef]
- Löbmann, K.; Flouda, K.; Qiu, D.; Tsolakou, T.; Wang, W.; Rades, T. The Influence of Pressure on the Intrinsic Dissolution Rate of Amorphous Indomethacin. Pharmaceutics 2014, 6, 481–493. [Google Scholar] [CrossRef]
- Wei, Y.; Zhou, S.; Hao, T.; Zhang, J.; Gao, Y.; Qian, S. Further enhanced dissolution and oral bioavailability of docetaxel by coamorphization with a natural P-gp inhibitor myricetin. Eur. J. Pharm. Sci. 2019, 129, 21–30. [Google Scholar] [CrossRef]
- Yoneda, M.; Ohkawa, Y.; Watanabe, Y.; Ogawa, M.; Nagai, H. [Polymorphism of acemetacin, a new antiinflammatory drug (author’s transl)]. Yakugaku Zasshi J. Pharm. Soc. Jpn. 1981, 101, 939–944. [Google Scholar] [CrossRef]
- Heinz, A.; Strachan, C.J.; Gordon, K.C.; Rades, T. Analysis of solid-state transformations of pharmaceutical compounds using vibrational spectroscopy. J. Pharm. Pharmacol. 2010, 61, 971–988. [Google Scholar] [CrossRef]
- Gelbrich, T.; Haddow, M.F.; Griesser, U.J. Acemetacin monohydrate. Acta Crystallogr. Sect. C 2007, 63, o451–o453. [Google Scholar] [CrossRef]
- Yoshioka, M.; Hancock, B.C.; Zografi, G. Crystallization of Indomethacin from the Amorphous State below and above Its Glass Transition Temperature. J. Pharm. Sci. 1994, 83, 1700–1705. [Google Scholar] [CrossRef] [PubMed]
- Heng, W.; Wei, Y.; Zhou, S.; Ma, D.; Gao, Y.; Zhang, J.; Qian, S. Effects of Temperature and Ionic Strength of Dissolution Medium on the Gelation of Amorphous Lurasidone Hydrochloride. Pharm. Res. 2019, 36, 72. [Google Scholar] [CrossRef] [PubMed]
- Matos, C.; Chaimovich, H.; Lima, J.; Cuccovia, I.; Reis, S. Effect of Liposomes on the Rate of Alkaline Hydrolysis of Indomethacin and Acemetacin. J. Pharm. Sci. 2001, 90, 298–309. [Google Scholar] [CrossRef] [PubMed]
- Collins, K. Charge density-dependent strength of hydration and biological structure. Biophys. J. 1997, 72, 65–76. [Google Scholar] [CrossRef] [PubMed]
- Adhikari, B.; Howes, T.; Bhandari, B.R.; Truong, V. Stickiness in foods: A review of mechanisms and test methods. Int. J. Food Prop. 2001, 4, 1–33. [Google Scholar] [CrossRef]
- Xiong, W.; Ren, C.; Jin, W.; Tian, J.; Wang, Y.; Shah, B.R.; Li, J.; Li, B. Ovalbumin-chitosan complex coacervation: Phase behavior, thermodynamic and rheological properties. Food Hydrocoll. 2016, 61, 895–902. [Google Scholar] [CrossRef]
- Yuan, C.; Du, L.; Zhang, G.; Jin, Z.; Liu, H. Influence of cyclodextrins on texture behavior and freeze-thaw stability of kappa-carrageenan gel. Food Chem. 2016, 210, 600–605. [Google Scholar] [CrossRef]
- Newman, A.; Zografi, G. What We Need to Know about Solid-State Isothermal Crystallization of Organic Molecules from the Amorphous State below the Glass Transition Temperature. Mol. Pharm. 2020, 17, 1761–1777. [Google Scholar] [CrossRef]
- Flory, P.J. Introductory lecture. Faraday Discuss. Chem. Soc. 1974, 57, 7–18. [Google Scholar] [CrossRef]
- Zhang, J.-Y.; Zeng, L.-H.; Feng, J. Dynamic covalent gels assembled from small molecules: From discrete gelators to dynamic covalent polymers. Chin. Chem. Lett. 2017, 28, 168–183. [Google Scholar] [CrossRef]
- Ouyang, J.; Wang, J.; Huang, X.; Gao, Y.; Bao, Y.; Wang, Y.; Yin, Q.; Hao, H. Gel Formation and Phase Transformation during the Crystallization of Valnemulin Hydrogen Tartrate. Ind. Eng. Chem. Res. 2014, 53, 16859–16863. [Google Scholar] [CrossRef]
- Pereira, A.; Duarte, H.; Nosrati, P.; Gubitosi, M.; Gentile, L.; Romano, A.; Medronho, B.; Olsson, U. Cellulose gelation in NaOH solutions is due to cellulose crystallization. Cellulose 2018, 25, 3205–3210. [Google Scholar] [CrossRef]
- Luo, F.-L.; Zhang, X.-Q.; Ning, W.; Wang, D.-J. Investigation on the gelation behavior of biodegradable poly(butylene succinate) during isothermal crystallization process. Chin. J. Polym. Sci. 2011, 29, 251–258. [Google Scholar] [CrossRef]
- Yin, Y.; Gao, Z.; Bao, Y.; Hou, B.; Hao, H.; Liu, D.; Wang, Y. Gelation Phenomenon during Antisolvent Crystallization of Cefotaxime Sodium. Ind. Eng. Chem. Res. 2014, 53, 1286–1292. [Google Scholar] [CrossRef]
- Masuda, K.; Tabata, S.; Sakata, Y.; Hayase, T.; Yonemochi, E.; Terada, K. Comparison of Molecular Mobility in the Glassy State Between Amorphous Indomethacin and Salicin Based on Spin-Lattice Relaxation Times. Pharm. Res. 2005, 22, 797–805. [Google Scholar] [CrossRef]
- Babu, J.S.; Mondal, C.; Sengupta, S.; Karmakar, S. Excess vibrational density of states and the brittle to ductile transition in crystalline and amorphous solids. Soft Matter 2016, 12, 1210–1218. [Google Scholar] [CrossRef]
- Han, J.; Li, L.; Pang, Z.; Su, M.; He, X.; Qian, S.; Zhang, J.; Gao, Y.; Wei, Y. Mechanistic insight into gel-induced aggregation of amorphous curcumin during dissolution process. Eur. J. Pharm. Sci. 2022, 170, 106083. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Teng, M.; Li, J.; Li, Z.; Zhang, G.; Zhao, P.; Fu, Q. Recrystallization Mediates the Gelation of Amorphous Drugs: The Case of Acemetacin. Pharmaceutics 2023, 15, 219. https://doi.org/10.3390/pharmaceutics15010219
Teng M, Li J, Li Z, Zhang G, Zhao P, Fu Q. Recrystallization Mediates the Gelation of Amorphous Drugs: The Case of Acemetacin. Pharmaceutics. 2023; 15(1):219. https://doi.org/10.3390/pharmaceutics15010219
Chicago/Turabian StyleTeng, Manlin, Jianfeng Li, Zhaohua Li, Guangshuai Zhang, Peixu Zhao, and Qiang Fu. 2023. "Recrystallization Mediates the Gelation of Amorphous Drugs: The Case of Acemetacin" Pharmaceutics 15, no. 1: 219. https://doi.org/10.3390/pharmaceutics15010219
APA StyleTeng, M., Li, J., Li, Z., Zhang, G., Zhao, P., & Fu, Q. (2023). Recrystallization Mediates the Gelation of Amorphous Drugs: The Case of Acemetacin. Pharmaceutics, 15(1), 219. https://doi.org/10.3390/pharmaceutics15010219





