Magnetic Platelets as a Platform for Drug Delivery and Cell Trapping
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Citrate-Stabilized Magnetite Nanoparticles
2.3. Synthesis of FITC-Labeled and TRITC-Labeled BSA
2.4. Synthesis of Magnetite Nanoparticles Conjugated with BSA, BSA (FITC) or BSA (TRITC)
2.5. Determination of Size and -Potential of FeNP-BSA(FITC)
2.6. Platelet Investigation
2.6.1. Separation of Platelets from Whole Blood
2.6.2. Labeling of Platelets with Magnetic Particles
2.6.3. Magnetic Separation of Magnetite Labeled Platelets
2.6.4. Magnetite-Rich Platelet Staining
2.6.5. Platelet Aggregation Activity after Labeling
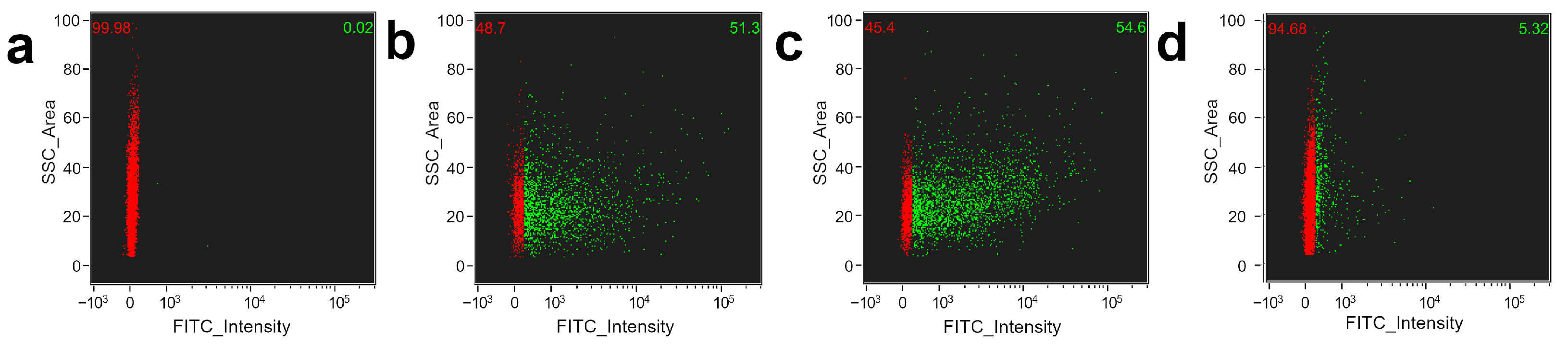
2.6.6. Flow Cytometry
2.7. Isolation of PBMC from Whole Blood
2.8. Cell Culturing
2.8.1. Cell Interaction Study
2.8.2. Confocal Laser Scanning Microscopy
2.9. Cell Samples Preparation for SEM and TEM Morphological Study
2.9.1. Scanning Electron Microscopy
2.9.2. Transmitted Electron Microscopy
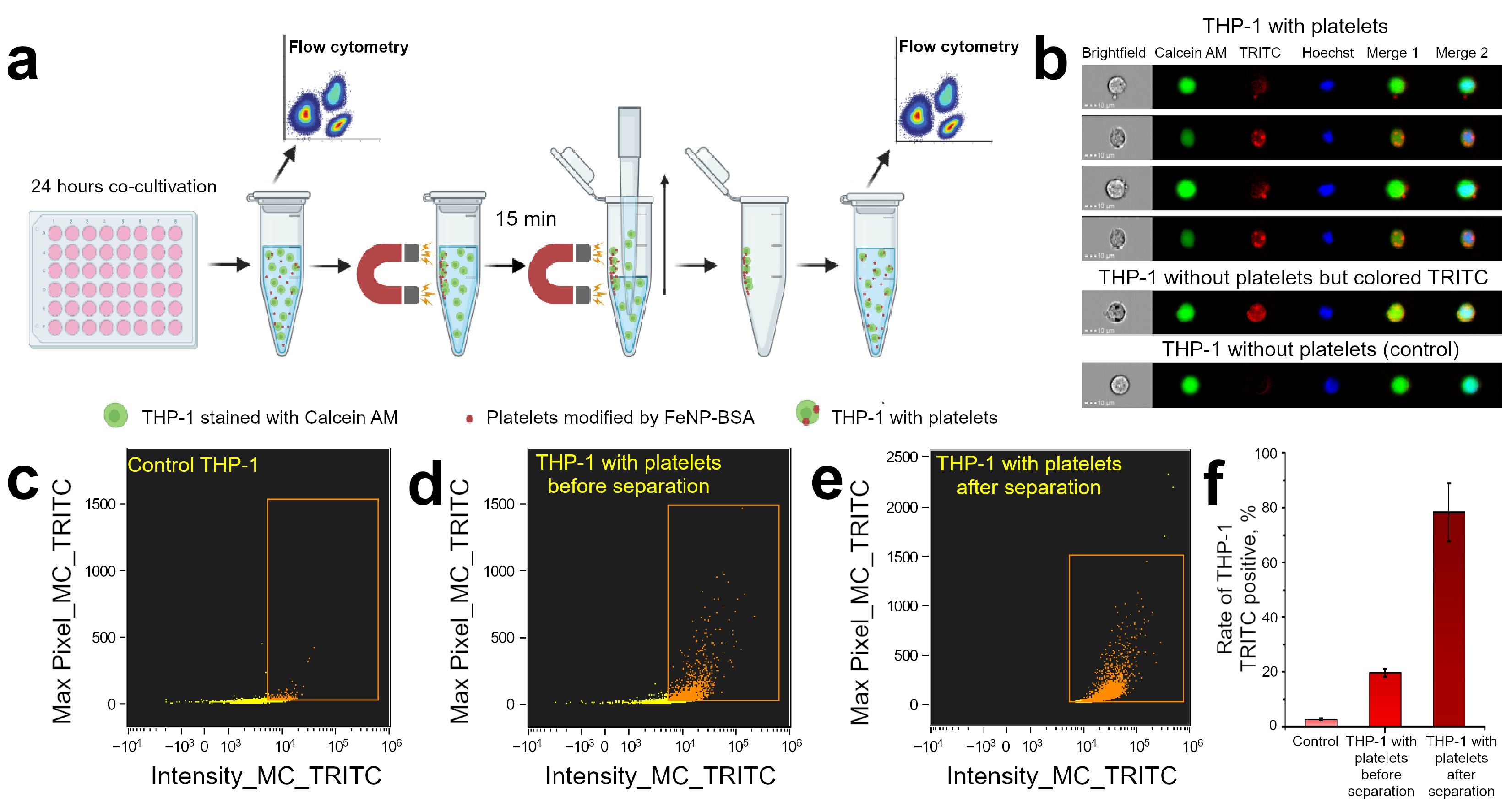
2.10. Magnetic Capturing THP-1 Cells after Platelets Adhesion
Estimation of Magnetic Capturing Efficiency by Flow Cytometry
3. Results and Discussion
3.1. Preparation and Characterization of BSA Conjugated FeNP
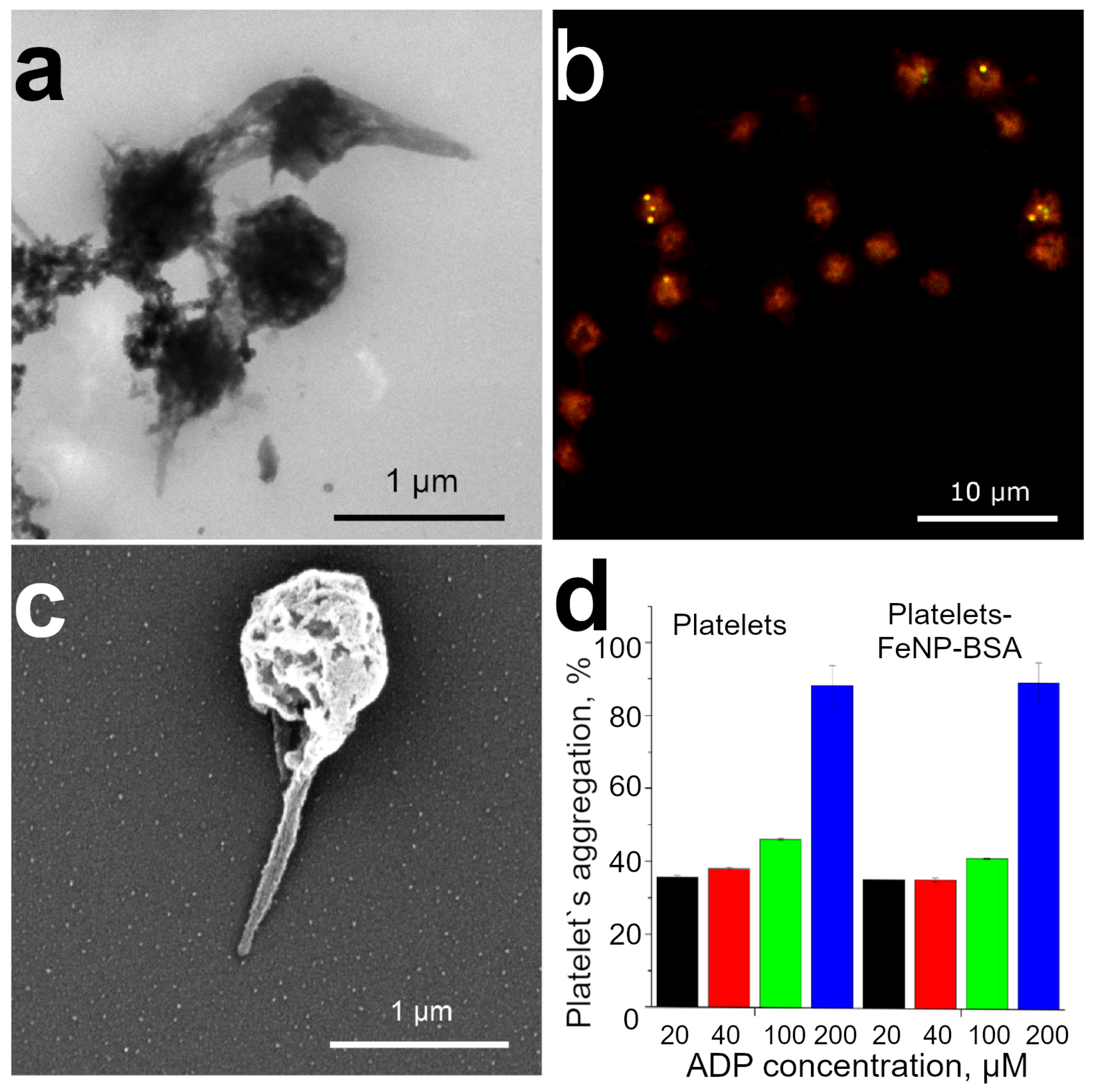
3.2. Functionalization of Platelets by BSA Conjugated FeNP
3.3. Platelet Decoration by BSA Conjugated FeNP
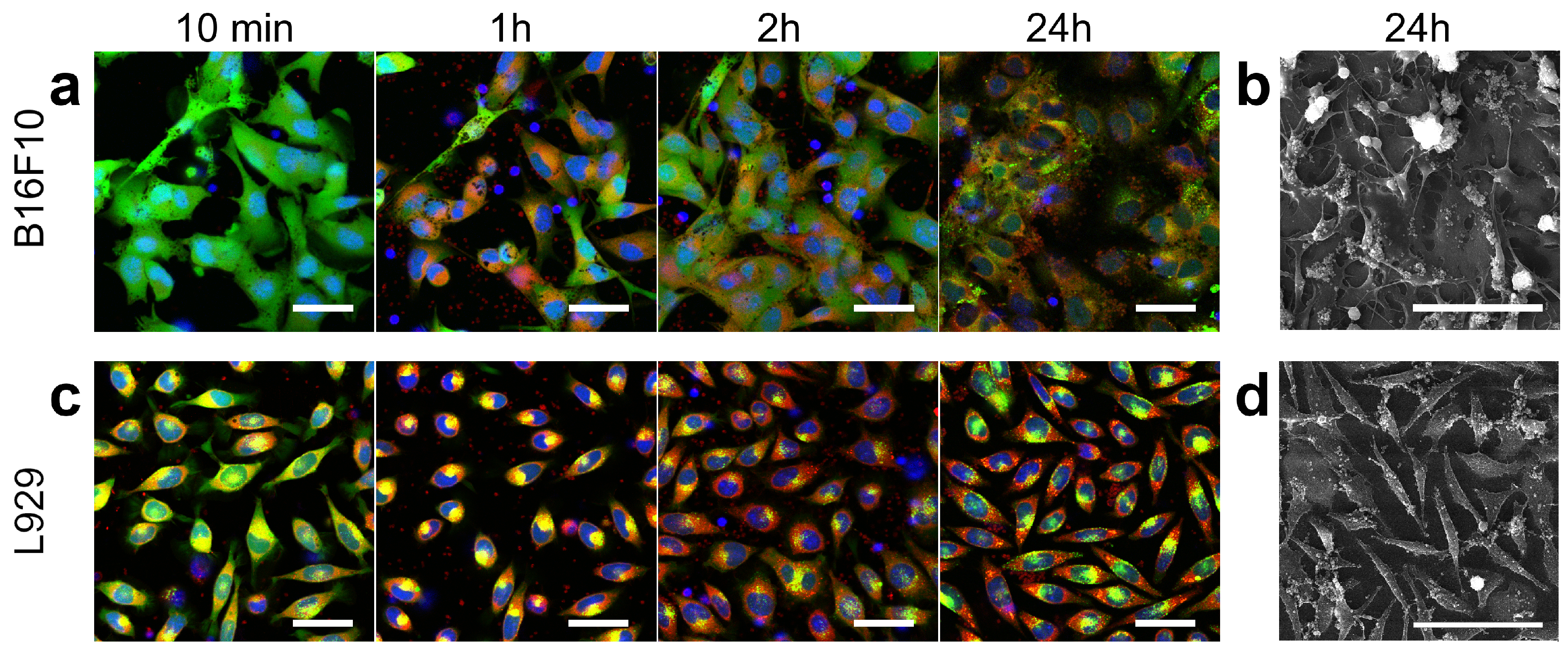
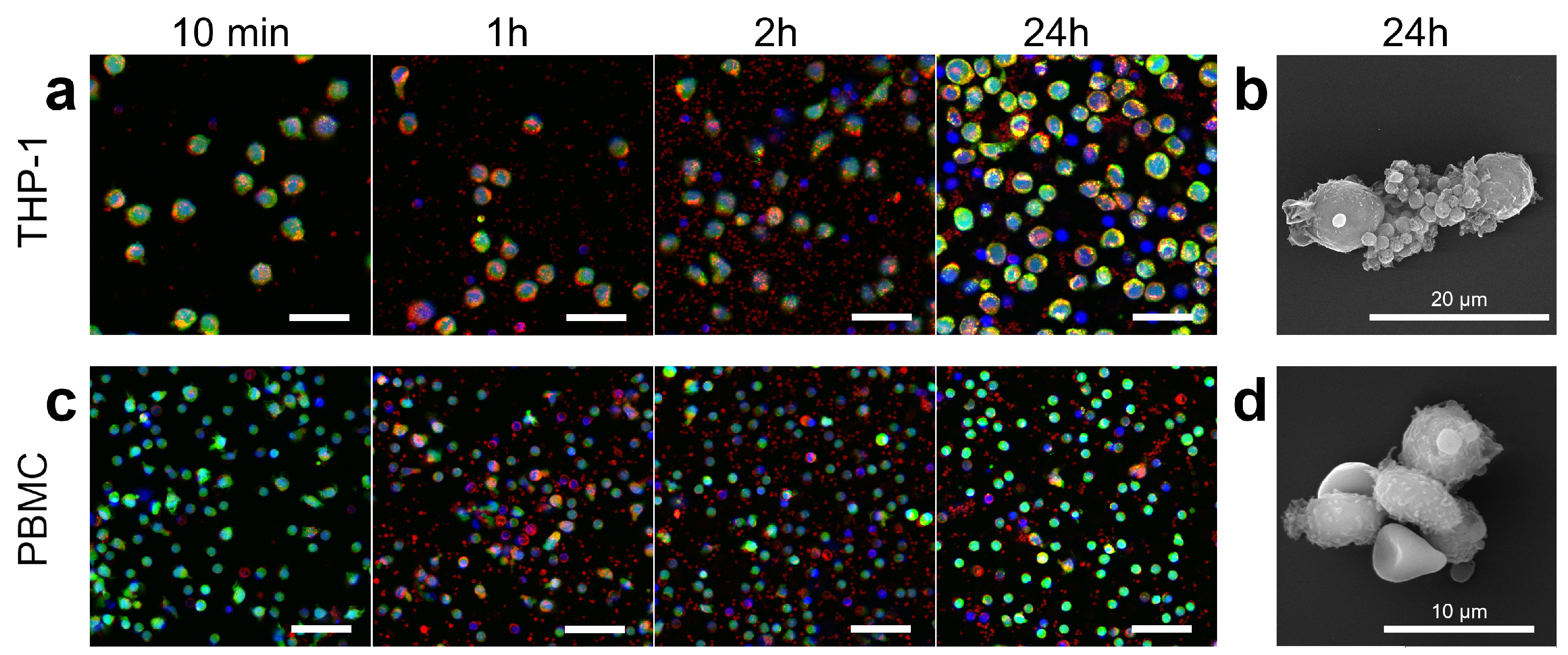
3.4. Platelets Interaction with Cell of Different Origin
3.5. Controlling Cell Movement after Platelet Adherence
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kurmi, B.D.; Kayat, J.; Gajbhiye, V.; Tekade, R.K.; Jain, N.K. Micro- and nanocarrier-mediated lung targeting. Expert Opin. Drug Deliv. 2010, 7, 781–794. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Huang, G. Micro- and Nano-Carrier Mediated Intra-Articular Drug Delivery Systems for the Treatment of Osteoarthritis. J. Nanotechnol. 2012, 2012, 1–11. [Google Scholar] [CrossRef]
- Zhang, L.; Sang, Y.; Feng, J.; Li, Z.; Zhao, A. Polysaccharide-based micro/nanocarriers for oral colon-targeted drug delivery. J. Drug Target. 2016, 24, 579–589. [Google Scholar] [CrossRef] [PubMed]
- Prikhozhdenko, E.S.; Gusliakova, O.I.; Kulikov, O.A.; Mayorova, O.A.; Shushunova, N.A.; Abdurashitov, A.S.; Bratashov, D.N.; Pyataev, N.A.; Tuchin, V.V.; Gorin, D.A.; et al. Target delivery of drug carriers in mice kidney glomeruli via renal artery. Balance between efficiency and safety. J. Control. Release 2021, 329, 175–190. [Google Scholar] [CrossRef]
- Sapach, A.Y.; Sindeeva, O.A.; Nesterchuk, M.V.; Tsitrina, A.A.; Mayorova, O.A.; Prikhozhdenko, E.S.; Verkhovskii, R.A.; Mikaelyan, A.S.; Kotelevtsev, Y.V.; Sukhorukov, G.B. Macrophage In Vitro and In Vivo Tracking via Anchored Microcapsules. ACS Appl. Mater. Interfaces 2022, 14, 51579–51592. [Google Scholar] [CrossRef]
- Gusliakova, O.I.; Prikhozhdenko, E.S.; Plastun, V.O.; Mayorova, O.A.; Shushunova, N.A.; Abdurashitov, A.S.; Kulikov, O.A.; Abakumov, M.A.; Gorin, D.A.; Sukhorukov, G.B.; et al. Renal Artery Catheterization for Microcapsules’ Targeted Delivery to the Mouse Kidney. Pharmaceutics 2022, 14, 1056. [Google Scholar] [CrossRef]
- Ahamed, M.; AlSalhi, M.S.; Siddiqui, M. Silver nanoparticle applications and human health. Clin. Chim. Acta 2010, 411, 1841–1848. [Google Scholar] [CrossRef]
- Díez-Pascual, A.; Rahdar, A. LbL Nano-Assemblies: A Versatile Tool for Biomedical and Healthcare Applications. Nanomaterials 2022, 12, 949. [Google Scholar] [CrossRef]
- Izci, M.; Maksoudian, C.; Manshian, B.B.; Soenen, S.J. The Use of Alternative Strategies for Enhanced Nanoparticle Delivery to Solid Tumors. Chem. Rev. 2021, 121, 1746–1803. [Google Scholar] [CrossRef]
- Sindeeva, O.A.; Verkhovskii, R.A.; Abdurashitov, A.S.; Voronin, D.V.; Gusliakova, O.I.; Kozlova, A.A.; Mayorova, O.A.; Ermakov, A.V.; Lengert, E.V.; Navolokin, N.A.; et al. Effect of Systemic Polyelectrolyte Microcapsule Administration on the Blood Flow Dynamics of Vital Organs. ACS Biomater. Sci. Eng. 2020, 6, 389–397. [Google Scholar] [CrossRef]
- Mayorova, O.A.; Sindeeva, O.A.; Lomova, M.V.; Gusliakova, O.I.; Tarakanchikova, Y.V.; Tyutyaev, E.V.; Pinyaev, S.I.; Kulikov, O.A.; German, S.V.; Pyataev, N.A.; et al. Endovascular addressing improves the effectiveness of magnetic targeting of drug carrier. Comparison with the conventional administration method. Nanomed. Nanotechnol. Biol. Med. 2020, 28, 102184. [Google Scholar] [CrossRef] [PubMed]
- Shanina, B.D.; Konchits, A.A.; Krasnovyd, S.V.; Shevchenko, Y.B.; Petranovs’ka, A.L.; Rieznichenko, L.S. Magnetic nanoparticle ensembles with promising biophysical applications: An EPR study. J. Appl. Phys. 2022, 132, 163905. [Google Scholar] [CrossRef]
- Su, Y.; Xie, Z.; Kim, G.B.; Dong, C.; Yang, J. Design Strategies and Applications of Circulating Cell-Mediated Drug Delivery Systems. ACS Biomater. Sci. Eng. 2015, 1, 201–217. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Han, K.; Ma, S.; Qi, X.; Guo, L.; Li, X. Blood cells as supercarrier systems for advanced drug delivery. Med. Drug Discov. 2022, 13, 100119. [Google Scholar] [CrossRef]
- Hamidi, M.; Zarrin, A.; Foroozesh, M.; Mohammadi-Samani, S. Applications of carrier erythrocytes in delivery of biopharmaceuticals. J. Control. Release 2007, 118, 145–160. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Sun, X.; Cheng, L.; Yin, S.; Yang, G.; Li, Y.; Liu, Z. Multifunctional Theranostic Red Blood Cells For Magnetic-Field-Enhanced in vivo Combination Therapy of Cancer. Adv. Mater. 2014, 26, 4794–4802. [Google Scholar] [CrossRef]
- Pierigè, F.; Bigini, N.; Rossi, L.; Magnani, M. Reengineering red blood cells for cellular therapeutics and diagnostics. WIREs Nanomed. Nanobiotechnol. 2017, 9, e1454. [Google Scholar] [CrossRef]
- Biagiotti, S.; Rossi, L.; Bianchi, M.; Giacomini, E.; Pierigè, F.; Serafini, G.; Conaldi, P.G.; Magnani, M. Immunophilin-loaded erythrocytes as a new delivery strategy for immunosuppressive drugs. J. Control. Release 2011, 154, 306–313. [Google Scholar] [CrossRef]
- Bourgeaux, V.; Hequet, O.; Campion, Y.; Delcambre, G.; Chevrier, A.M.; Rigal, D.; Godfrin, Y. Inositol hexaphosphate-loaded red blood cells prevent in vitro sickling. Transfusion 2010, 50, 2176–2184. [Google Scholar] [CrossRef]
- Bhateria, M.; Rachumallu, R.; Singh, R.; Bhatta, R.S. Erythrocytes-based synthetic delivery systems: Transition from conventional to novel engineering strategies. Expert Opin. Drug Deliv. 2014, 11, 1219–1236. [Google Scholar] [CrossRef]
- Elsharkasy, O.M.; Nordin, J.Z.; Hagey, D.W.; de Jong, O.G.; Schiffelers, R.M.; Andaloussi, S.E.; Vader, P. Extracellular vesicles as drug delivery systems: Why and how? Adv. Drug Deliv. Rev. 2020, 159, 332–343. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Oller, L.; Seras-Franzoso, J.; Andrade, F.; Rafael, D.; Abasolo, I.; Gener, P.; Schwartz, S., Jr. Extracellular Vesicles as Drug Delivery Systems in Cancer. Pharmaceutics 2020, 12, 1146. [Google Scholar] [CrossRef] [PubMed]
- Luan, X.; Sansanaphongpricha, K.; Myers, I.; Chen, H.; Yuan, H.; Sun, D. Engineering exosomes as refined biological nanoplatforms for drug delivery. Acta Pharmacol. Sin. 2017, 38, 754–763. [Google Scholar] [CrossRef] [PubMed]
- de Jong, O.G.; Kooijmans, S.A.A.; Murphy, D.E.; Jiang, L.; Evers, M.J.W.; Sluijter, J.P.G.; Vader, P.; Schiffelers, R.M. Drug Delivery with Extracellular Vesicles: From Imagination to Innovation. Acc. Chem. Res. 2019, 52, 1761–1770. [Google Scholar] [CrossRef]
- Burger, A.M.; Hartung, G.; Stehle, G.; Sinn, H.; Fiebig, H.H. Pre-clinical evaluation of a methotrexate-albumin conjugate (MTX-HSA) in human tumor xenograftsin vivo. Int. J. Cancer 2001, 92, 718–724. [Google Scholar] [CrossRef]
- Peng, Q.; Mu, H. The potential of protein–nanomaterial interaction for advanced drug delivery. J. Control. Release 2016, 225, 121–132. [Google Scholar] [CrossRef]
- Shi, Q.; Montgomery, R.R. Platelets as delivery systems for disease treatments. Adv. Drug Deliv. Rev. 2010, 62, 1196–1203. [Google Scholar] [CrossRef]
- Bambace, N.M.; Holmes, C.E. The platelet contribution to cancer progression. J. Thromb. Haemost. 2011, 9, 237–249. [Google Scholar] [CrossRef]
- Xu, P.; Zuo, H.; Chen, B.; Wang, R.; Ahmed, A.; Hu, Y.; Ouyang, J. Doxorubicin-loaded platelets as a smart drug delivery system: An improved therapy for lymphoma. Sci. Rep. 2017, 7, 42632. [Google Scholar] [CrossRef]
- Sarkar, S.; Alam, M.A.; Shaw, J.; Dasgupta, A.K. Drug Delivery Using Platelet Cancer Cell Interaction. Pharm. Res. 2013, 30, 2785–2794. [Google Scholar] [CrossRef]
- German, S.V.; Inozemtseva, O.A.; Navolokin, N.A.; Pudovkina, E.E.; Zuev, V.V.; Volkova, E.K.; Bucharskaya, A.B.; Pleskova, S.N.; Maslyakova, G.N.; Gorin, D.A. Synthesis of magnetite hydrosols and assessment of their impact on living systems at the cellular and tissue levels using MRI and morphological investigation. Nanotechnol. Russ. 2013, 8, 573–580. [Google Scholar] [CrossRef]
- Salas, A. Separation of Platelets from Whole Blood. 2000. Available online: http://springerlab.tch.harvard.edu/springer/uploads/Protocols/SeparationofPlateletsfromWholeBlood.pdf (accessed on 15 November 2021).
- Newhouse, P.; Clark, C. The variability of platelet aggregation. In Platelet Function: Laboratory Evaluation and Clinical Application; Triplett, D.A., Ed.; Educational Products Division, American Society of Clinical Pathologists, ASCP: Chicago, IL, USA, 1978; pp. 63–107. [Google Scholar]
- Nguyen, T.H.; Schuster, N.; Greinacher, A.; Aurich, K. Uptake Pathways of Protein-Coated Magnetic Nanoparticles in Platelets. ACS Appl. Mater. Interfaces 2018, 10, 28314–28321. [Google Scholar] [CrossRef] [PubMed]
- Easo, S.L.; Mohanan, P. In vitro hematological and in vivo immunotoxicity assessment of dextran stabilized iron oxide nanoparticles. Colloids Surf. B Biointerfaces 2015, 134, 122–130. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Gao, H.; Bao, G. Physical Principles of Nanoparticle Cellular Endocytosis. ACS Nano 2015, 9, 8655–8671. [Google Scholar] [CrossRef]
- Nosrati, H.; Sefidi, N.; Sharafi, A.; Danafar, H.; Kheiri Manjili, H. Bovine Serum Albumin (BSA) coated iron oxide magnetic nanoparticles as biocompatible carriers for curcumin-anticancer drug. Bioorg. Chem. 2018, 76, 501–509. [Google Scholar] [CrossRef]
- Aurich, K.; Wesche, J.; Palankar, R.; Schlüter, R.; Bakchoul, T.; Greinacher, A. Magnetic Nanoparticle Labeling of Human Platelets from Platelet Concentrates for Recovery and Survival Studies. ACS Appl. Mater. Interfaces 2017, 9, 34666–34673. [Google Scholar] [CrossRef]
- Albanese, A.; Tang, P.S.; Chan, W.C. The Effect of Nanoparticle Size, Shape, and Surface Chemistry on Biological Systems. Annu. Rev. Biomed. Eng. 2012, 14, 1–16. [Google Scholar] [CrossRef]
- Matuszak, J.; Dörfler, P.; Zaloga, J.; Unterweger, H.; Lyer, S.; Dietel, B.; Alexiou, C.; Cicha, I. Shell matters: Magnetic targeting of SPIONs and in vitro effects on endothelial and monocytic cell function. Clin. Hemorheol. Microcirc. 2015, 61, 259–277. [Google Scholar] [CrossRef]
- Chan, M.V.; Armstrong, P.C.J.; Papalia, F.; Kirkby, N.S.; Warner, T.D. Optical multichannel (optimul) platelet aggregometry in 96-well plates as an additional method of platelet reactivity testing. Platelets 2011, 22, 485–494. [Google Scholar] [CrossRef]
- Medina, C.; Tomaszewski, K.; Gilmer, J.; Bazou, D.; Santos-Martinez, M.; Radomski, M. Pharmacological characterization of nanoparticle-induced platelet microaggregation using quartz crystal microbalance with dissipation: Comparison with light aggregometry. Int. J. Nanomed. 2015, 10, 5107–5119. [Google Scholar] [CrossRef]
- Tong, J.; Wu, J.; Xu, X.; Wu, S.; Tan, B.; Shi, M.J.; Wang, J.; Zhao, W.; Jiang, H.; Jin, S.; et al. Preclinical evaluation of recombinant human IFNα2b-containing magnetoliposomes for treating hepatocellular carcinoma. Int. J. Nanomed. 2014, 9, 4533–4550. [Google Scholar] [CrossRef]
- Zhou, L.; Schmaier, A.H. Platelet Aggregation Testing in Platelet-Rich Plasma: Description of Procedures With the Aim to Develop Standards in the Field. Am. J. Clin. Pathol. 2005, 123, 172–183. [Google Scholar] [CrossRef] [PubMed]
- Aurich, K.; Spoerl, M.C.; Fürll, B.; Sietmann, R.; Greinacher, A.; Hosten, N.; Weitschies, W. Development of a method for magnetic labeling of platelets. Nanomed. Nanotechnol. Biol. Med. 2012, 8, 537–544. [Google Scholar] [CrossRef] [PubMed]
- Reimer, P.; Balzer, T. Ferucarbotran (Resovist): A new clinically approved RES-specific contrast agent for contrast-enhanced MRI of the liver: Properties, clinical development, and applications. Eur. Radiol. 2003, 13, 1266–1276. [Google Scholar] [CrossRef] [PubMed]
- Santoyo Salazar, J.; Perez, L.; de Abril, O.; Truong Phuoc, L.; Ihiawakrim, D.; Vazquez, M.; Greneche, J.M.; Begin-Colin, S.; Pourroy, G. Magnetic Iron Oxide Nanoparticles in 10–40 nm Range: Composition in Terms of Magnetite/Maghemite Ratio and Effect on the Magnetic Properties. Chem. Mater. 2011, 23, 1379–1386. [Google Scholar] [CrossRef]
- Itoh, H.; Sugimoto, T. Systematic control of size, shape, structure, and magnetic properties of uniform magnetite and maghemite particles. J. Colloid Interface Sci. 2003, 265, 283–295. [Google Scholar] [CrossRef]
- Toyoda, T.; Isobe, K.; Tsujino, T.; Koyata, Y.; Ohyagi, F.; Watanabe, T.; Nakamura, M.; Kitamura, Y.; Okudera, H.; Nakata, K.; et al. Direct activation of platelets by addition of CaCl2 leads coagulation of platelet-rich plasma. Int. J. Implant Dent. 2018, 4, 23. [Google Scholar] [CrossRef]
- Tomaiuolo, M.; Litvinov, R.I.; Weisel, J.W.; Stalker, T.J. Use of electron microscopy to study platelets and thrombi. Platelets 2020, 31, 580–588. [Google Scholar] [CrossRef]
- Ito, A.; Azzellini, G.; Silva, S.; Serra, O.; Szabo, A. Optical absorption and fluorescence spectroscopy studies of ground state melanin—Cationic porphyrins complexes. Biophys. Chem. 1992, 45, 79–89. [Google Scholar] [CrossRef]
- Ulyanova, V.; Vershinina, V.; Ilinskaya, O. Barnase and binase: Twins with distinct fates. FEBS J. 2011, 278, 3633–3643. [Google Scholar] [CrossRef]
- Edelweiss, E.; Balandin, T.G.; Ivanova, J.L.; Lutsenko, G.V.; Leonova, O.G.; Popenko, V.I.; Sapozhnikov, A.M.; Deyev, S.M. Barnase as a New Therapeutic Agent Triggering Apoptosis in Human Cancer Cells. PLoS ONE 2008, 3, e2434. [Google Scholar] [CrossRef] [PubMed]
- Haserück, N.; Erl, W.; Pandey, D.; Tigyi, G.; Ohlmann, P.; Ravanat, C.; Gachet, C.; Siess, W. The plaque lipid lysophosphatidic acid stimulates platelet activation and platelet-monocyte aggregate formation in whole blood: Involvement of P2Y1 and P2Y12 receptors. Blood 2004, 103, 2585–2592. [Google Scholar] [CrossRef] [PubMed]
- Pawelski, H.; Lang, D.; Reuter, S. Interactions of monocytes and platelets: Implication for life. Front. Biosci.-Sch. 2014, 6, 75–91. [Google Scholar]
- Freedman, J.E.; Loscalzo, J. Platelet–Monocyte Aggregates. Circulation 2002, 105, 2130–2132. [Google Scholar] [CrossRef] [PubMed]
- Labelle, M.; Begum, S.; Hynes, R.O. Direct Signaling between Platelets and Cancer Cells Induces an Epithelial-Mesenchymal-Like Transition and Promotes Metastasis. Cancer Cell 2011, 20, 576–590. [Google Scholar] [CrossRef]
- Palacios-Acedo, A.L.; Langiu, M.; Crescence, L.; Mège, D.; Dubois, C.; Panicot-Dubois, L. Platelet and Cancer-Cell Interactions Modulate Cancer-Associated Thrombosis Risk in Different Cancer Types. Cancers 2022, 14, 730. [Google Scholar] [CrossRef] [PubMed]
- Li, N. Platelets in cancer metastasis: To help the “villain” to do evil. Int. J. Cancer 2016, 138, 2078–2087. [Google Scholar] [CrossRef] [PubMed]
- Ploppa, A.; George, T.C.; Unertl, K.E.; Nohe, B.; Durieux, M.E. ImageStream cytometry extends the analysis of phagocytosis and oxidative burst. Scand. J. Clin. Lab. Investig. 2011, 71, 362–369. [Google Scholar] [CrossRef] [PubMed]
- Stamopoulos, D.; Manios, E.; Gogola, V.; Benaki, D.; Bouziotis, P.; Niarchos, D.; Pissas, M. Bare and protein-conjugated Fe3O4 ferromagnetic nanoparticles for utilization in magnetically assisted hemodialysis: Biocompatibility with human blood cells. Nanotechnology 2008, 19, 505101. [Google Scholar] [CrossRef]
- Phanse, Y.; Ramer-Tait, A.E.; Friend, S.L.; Carrillo-Conde, B.; Lueth, P.; Oster, C.J.; Phillips, G.J.; Narasimhan, B.; Wannemuehler, M.J.; Bellaire, B.H. Analyzing Cellular Internalization of Nanoparticles and Bacteria by Multi-spectral Imaging Flow Cytometry. J. Vis. Exp. 2012, 64, e3884. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mayorova, O.A.; Gusliakova, O.I.; Prikhozhdenko, E.S.; Verkhovskii, R.A.; Bratashov, D.N. Magnetic Platelets as a Platform for Drug Delivery and Cell Trapping. Pharmaceutics 2023, 15, 214. https://doi.org/10.3390/pharmaceutics15010214
Mayorova OA, Gusliakova OI, Prikhozhdenko ES, Verkhovskii RA, Bratashov DN. Magnetic Platelets as a Platform for Drug Delivery and Cell Trapping. Pharmaceutics. 2023; 15(1):214. https://doi.org/10.3390/pharmaceutics15010214
Chicago/Turabian StyleMayorova, Oksana A., Olga I. Gusliakova, Ekaterina S. Prikhozhdenko, Roman A. Verkhovskii, and Daniil N. Bratashov. 2023. "Magnetic Platelets as a Platform for Drug Delivery and Cell Trapping" Pharmaceutics 15, no. 1: 214. https://doi.org/10.3390/pharmaceutics15010214
APA StyleMayorova, O. A., Gusliakova, O. I., Prikhozhdenko, E. S., Verkhovskii, R. A., & Bratashov, D. N. (2023). Magnetic Platelets as a Platform for Drug Delivery and Cell Trapping. Pharmaceutics, 15(1), 214. https://doi.org/10.3390/pharmaceutics15010214





