Green Manufacturing for Herbal Remedies with Advanced Pharmaceutical Technology
Abstract
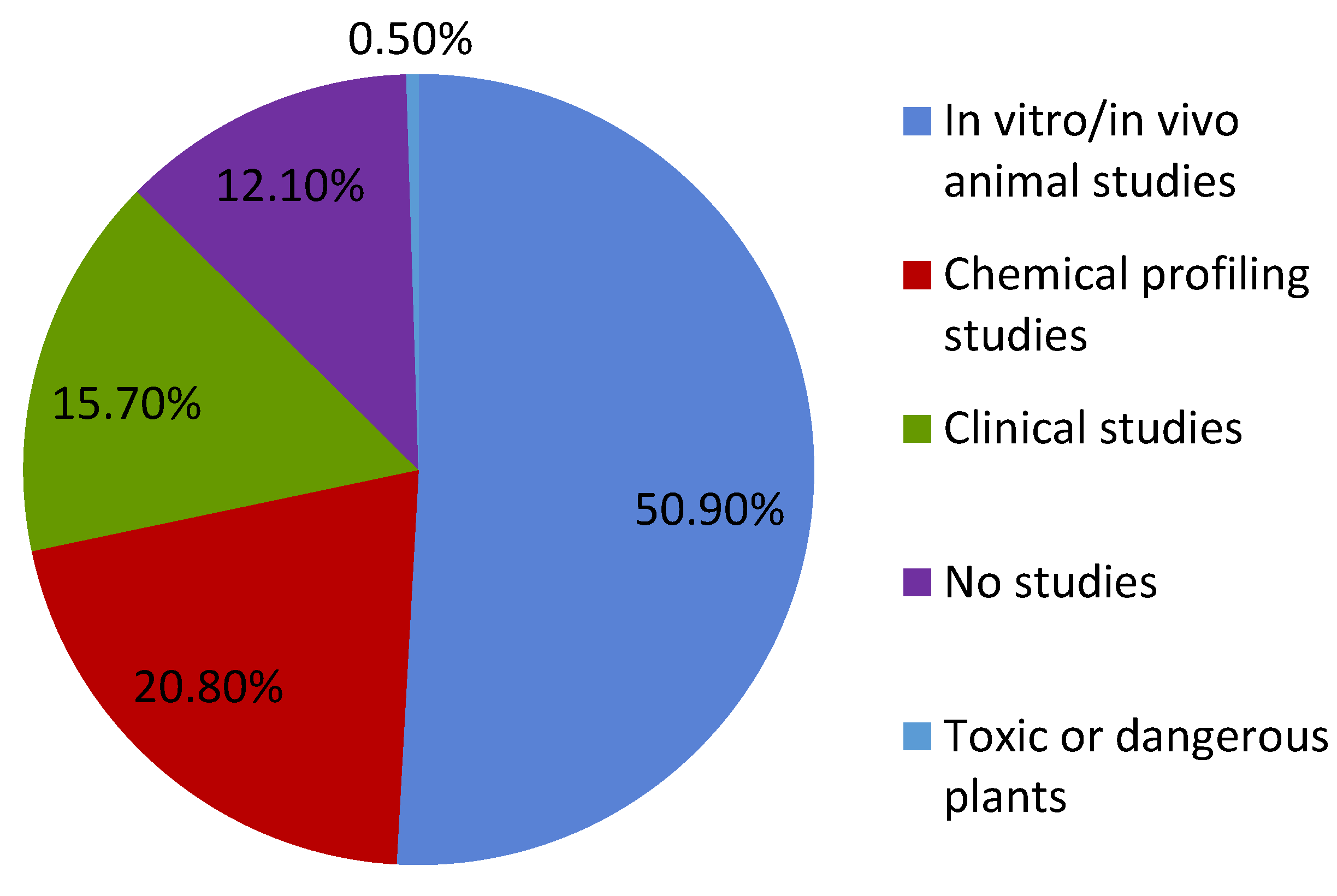
1. Introduction
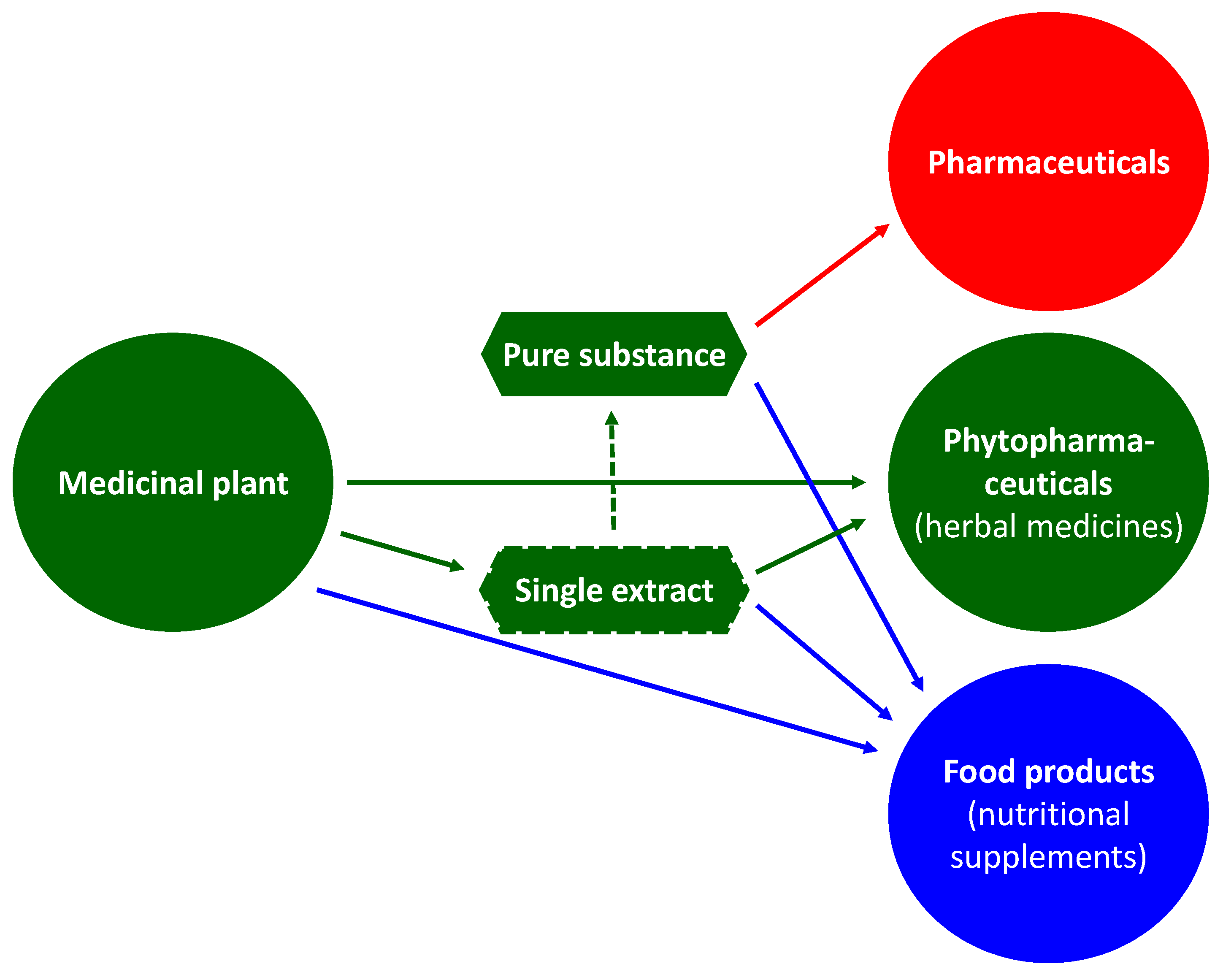
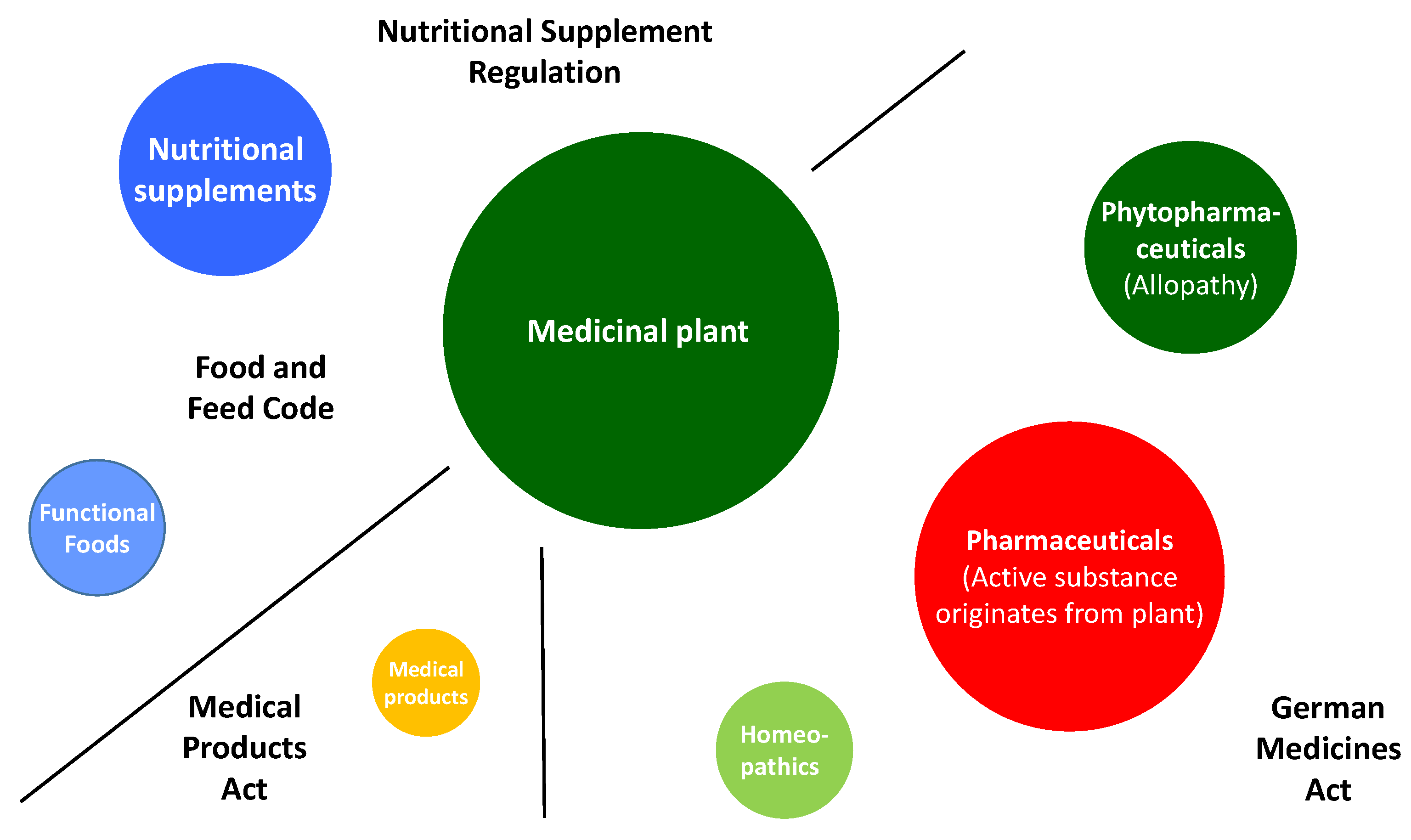
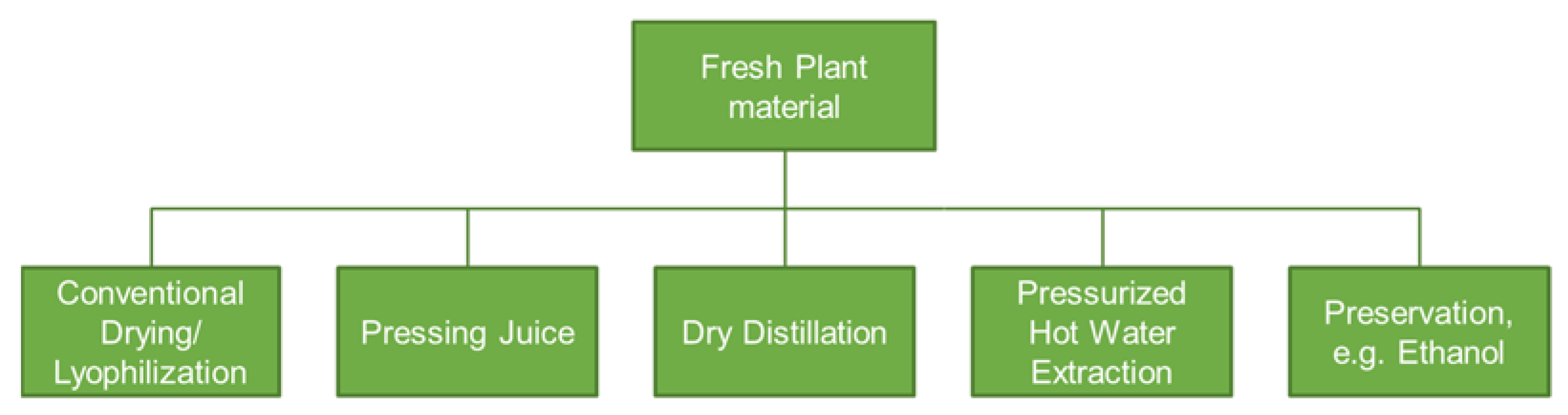
2. Green Manufacturing for Herbal Remedies
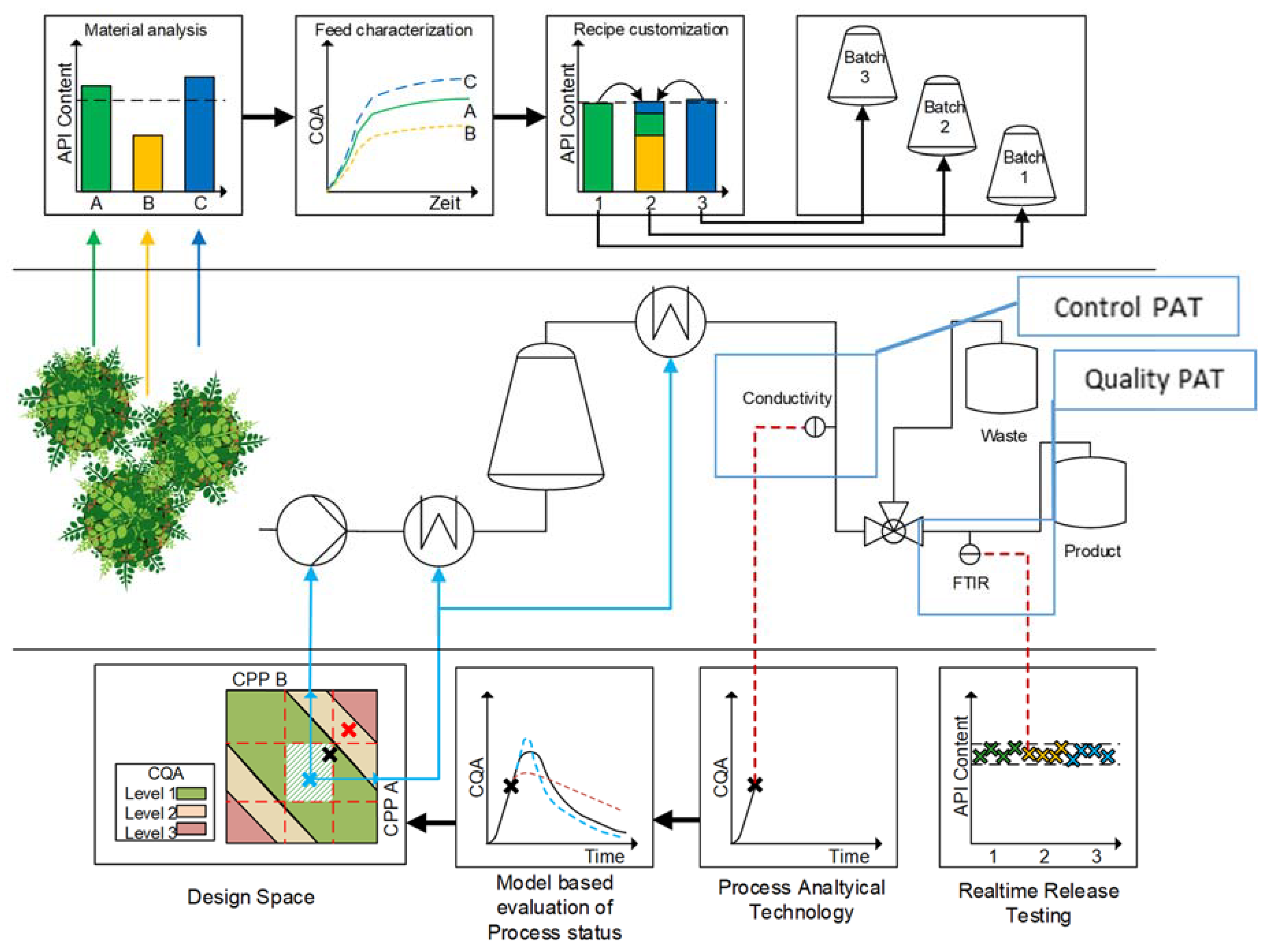
3. Advanced Pharmaceutical Technology
4. Advantages of Processes for the Provision of Pure Substances Taking into Account the Presented Optimizations Using the Example of Artemisinin and Taxol
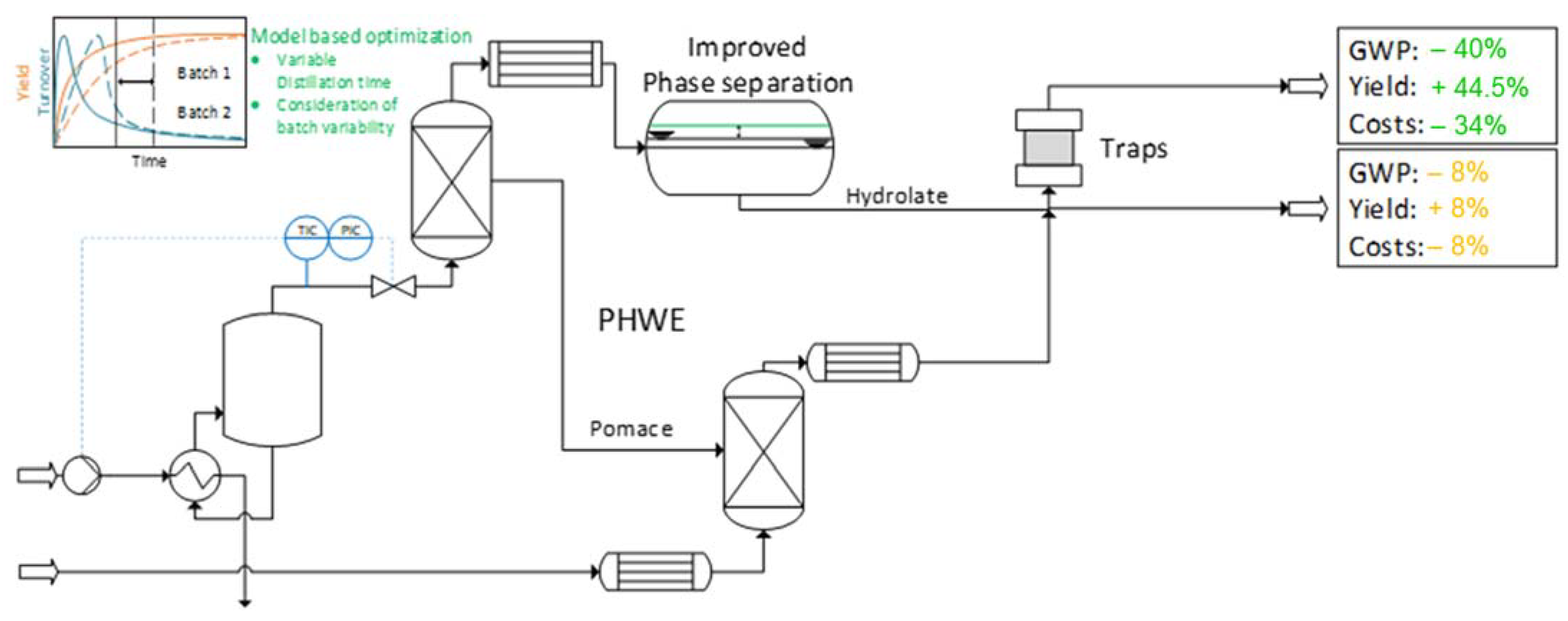
5. The Use of Hot Water Extraction for the Preparation of Extracts of Phytopharmaceuticals Using the Example of Bearberry (Arctostapylos uva-ursi (L.) SPRENG.) with Arbutin as a Reference Substance [55]
6. Optimization of Hawthorn (Craetaegus monogyna JACQ.) Extraction [71]
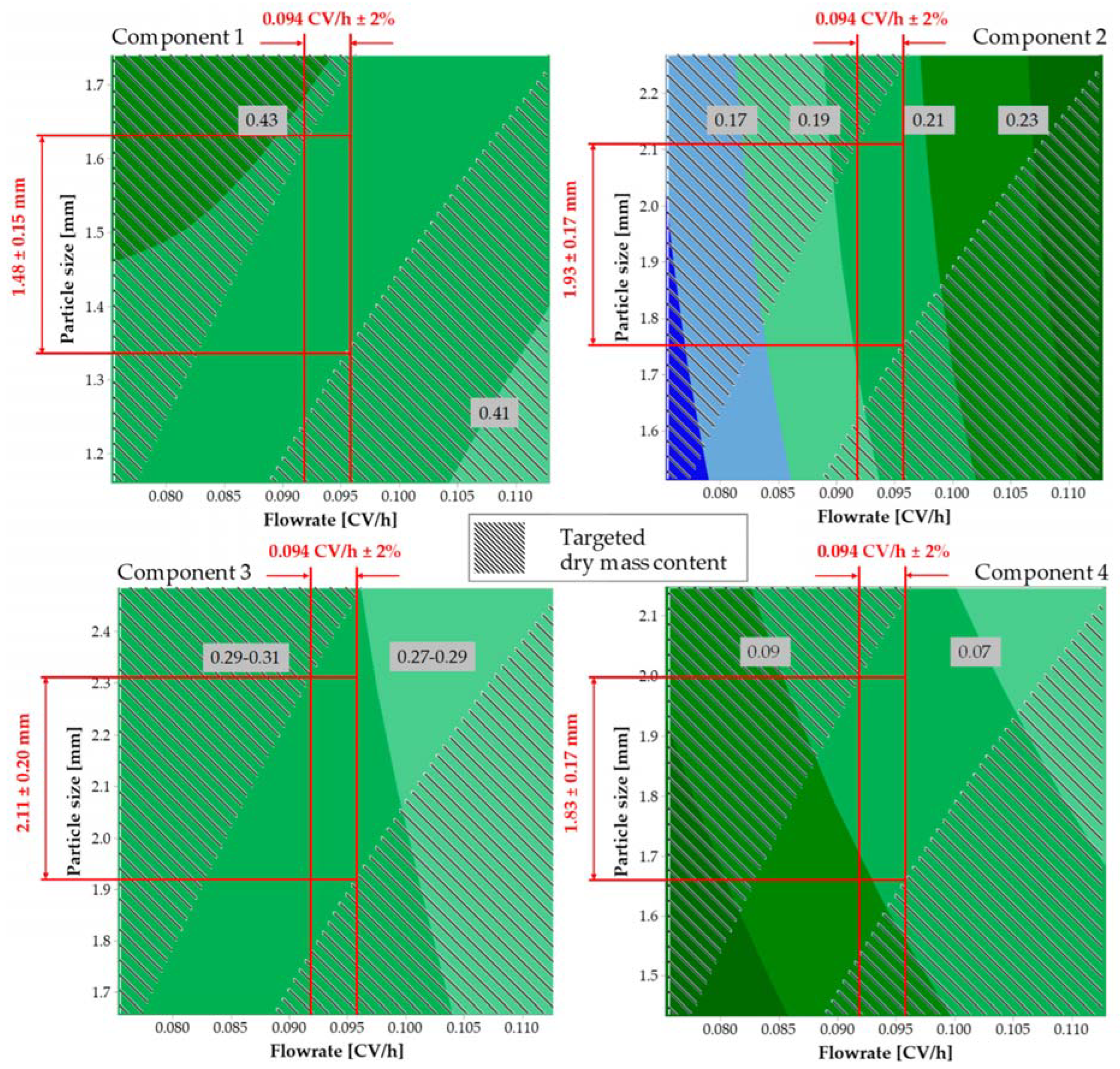
7. Case Study Standard Extract of Four Plant Sources
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 10-DAB | 10-deacetalbaccatin |
| AMG | Medicinal Products Act |
| API | Active pharmaceutical ingredients |
| COG | Cost of Goods |
| DER | Drug Extract Ratio |
| EFSA | European Food Safety Authority |
| EMA | European Medicines Agency |
| FDA | Food and Drug Administration |
| GACP | Good Agricultural and Collection Practice |
| GMP | Good Manufacturing Practice |
| GWP | Global Warming Potential |
| HMPC | Herbal Medicinal Products |
| LMFGB | Food and Feed Code |
| MIT | Massachusetts Institute of Technology |
| MPG | Medical Devices Act |
| NEM | Food Supplements |
| NemV | Food Supplements Ordinance |
| NF | Nanofiltration |
| PAT | Process Analytical Technologies |
| PHWE | Pressurized Hot Water Extraction |
| QbD | Quality by Design |
| SME | Small and Medium Enterprise |
| WEU | Well-established use |
References
- Monslow, M.A.; Elbashir, S.; Sullivan, N.L.; Thiriot, D.S.; Ahl, P.; Smith, J.; Miller, E.; Cook, J.; Cosmi, S.; Thoryk, E.; et al. Immunogenicity generated by mRNA vaccine encoding VZV gE antigen is comparable to adjuvanted subunit vaccine and better than live attenuated vaccine in nonhuman primates. Vaccine 2020, 38, 5793–5802. [Google Scholar] [CrossRef]
- Kensil, C.R.; Patel, U.; Lennick, M.; Marciani, D. Separation and characterization of saponins with adjuvant activity from Quillaja saponaria Molina cortex. J. Immunol. 1991, 146, 431–437. [Google Scholar] [CrossRef] [PubMed]
- Hagels, H.; Strube, M.; Tegtmeier, M. Pflanzen als Rohstoffbasis. CITplus 2014, 17, 6–8. [Google Scholar]
- Ditz, R.; Gerard, D.; Hagels, H.; Igl, N.; Schäffler, M.; Schulz, H.; Stürtz, M.; Tegtmeier, M.; Treutwein, J.; Strube, J. Phytoextracts: Proposal Towards a New Comprehensive Research Focus: Position Paper of the ProcessNet-Subject Division Plant Based Extracts—Products and Processes and the European Working Group on Phytoextracts; ProcessNet-Subject Division Plant Based Extracts—Products and Processes: Frankfurt, Germany, 2017. [Google Scholar]
- Peschel, W.; Alvarez, B.M. Harmonised European Standards as a Basis for the Safe Use of Herbal Medicinal Products and Their Marketing Authorisation in European Union Member States. Pharm. Med. 2018, 32, 275–293. [Google Scholar] [CrossRef]
- Rote Liste Service GmbH. Rote Liste 2022: Arzneimittelverzeichnis für Deutschland (Einschließlich EU-Zulassungen und bestimmter Medizinprodukte); Rote Liste Service GmbH: Frankfurt, Germany, 2022; ISBN 9783946057741. [Google Scholar]
- Anzivino, N.; Moliterno, C. Vitamins & Dietary Supplements Market trends: Overview. 2020. Available online: https://www.pwc.com/it/it/publications/assets/docs/Vitamins-Dietary-Supplements-Market-Overview.pdf (accessed on 22 November 2022).
- Grand View Research, Inc. Dietary Supplements Market Size, Share & Trends Analysis Report by Ingredient (Vitamins, Minerals), by Form, by Application, by End User, by Distribution, by Region, and Segment Forecasts, 2022–2030; Grand View Research, Inc.: San Francisco, CA, USA, 2022. [Google Scholar]
- European Food Safety Authority—EFSA. Scientific Opinion on Pyrrolizidine Alkaloids in Food and Feed. EFSA J. 2011, 9, 2406. [Google Scholar] [CrossRef]
- Committee on Herbal Medicinal Products—HMPC/EMA. Public Statement on the Use of Herbal Medicinal Products Containing Toxic, Unsaturated Pyrrolizidine Alkaloids (PAs); EMA/HMPC/893108/2011; European Medicines Agency: Amsterdam, The Netherlands, 2014. [Google Scholar]
- Grohs, B.; Steinhoff, B.; Tegtmeier, M. Pyrrolizidinalkaloide in pflanzlichen Arzneimitteln: Herausforderung für Anbau und verarbeitende Industrie. Pharm. Ind. 2016, 78, 1319–1322. [Google Scholar]
- Merz, K.-H.; Schrenk, D. Interim relative potency factors for the toxicological risk assessment of pyrrolizidine alkaloids in food and herbal medicines. Toxicol. Lett. 2016, 263, 44–57. [Google Scholar] [CrossRef]
- Gasser, U.; Klier, B.; Kühn, A.V.; Steinhoff, B. Current findings on the heavy metal content in herbal drugs. Pharmeuropa 2009, 1, 37–49. [Google Scholar]
- Arcella, D.; Altieri, A.; Horváth, Z. Human acute exposure assessment to tropane alkaloids. EFSA J. 2018, 16, e05160. [Google Scholar] [CrossRef]
- Newman, D.J.; Cragg, G.M. Natural Products as Sources of New Drugs from 1981 to 2014. J. Nat. Prod. 2016, 79, 629–661. [Google Scholar] [CrossRef]
- Uhlenbrock, L.; Sixt, M.; Tegtmeier, M.; Schulz, H.; Hagels, H.; Ditz, R.; Strube, J. Natural Products Extraction of the Future—Sustainable Manufacturing Solutions for Societal Needs. Processes 2018, 6, 177. [Google Scholar] [CrossRef]
- Jensch, C.; Strube, J. Proposal of a New Green Process for Waste Valorization and Cascade Utilization of Essential Oil Plants. Sustainability 2022, 14, 3227. [Google Scholar] [CrossRef]
- Cravotto, G.; Boffa, L.; Genzini, L.; Garella, D. Phytotherapeutics: An evaluation of the potential of 1000 plants. J. Clin. Pharm. Ther. 2010, 35, 11–48. [Google Scholar] [CrossRef] [PubMed]
- European Medicines Agency. Evaluation of Medicines for Human Use, Committee on Herbal Medicinal Products. Guideline on Good Agricultural and Collection Practice (GACP) for Starting Materials of Herbal Origin; EMEA/HMPC/246816/2005; European Medicines Agency: Amsterdam, The Netherlands, 2006. [Google Scholar]
- vom Graf Hagen-Plettenberg, M.; Klier, B.; Tegtmeier, M.; Waimer, F.; Steinhoff, B. Good Agricultural and Collection Practice (GACP)—A Pragmatic and Efficient State-of-the-Art Standard; Europharm: Brussels, Belgium, 2012; pp. 1078–1084. [Google Scholar]
- Hagels, H.; Tegtmeier, M.; Strube, J.; Both, S. GACP regulation for herbal raw materials. Planta Med. 2013, 79, WS22. [Google Scholar] [CrossRef]
- Wagner, B.; Waimer, F.; Klier, B.; Tegtmeier, M.; Steinhoff, B. Implementation of GMP for the Manufacture of Herbal Preparations: An Efficient and Successful Approach for Initial Process Steps. Pharm. Ind. 2014, 76, 222–230. [Google Scholar]
- Kircher. Bioeconomy: Markets, Implications, and Investment Opportunities. Economies 2019, 7, 73. [Google Scholar] [CrossRef]
- Kaufmann, B.; Christen, P. Recent extraction techniques for natural products: Microwave-assisted extraction and pressurised solvent extraction. Phytochem. Anal. 2002, 13, 105–113. [Google Scholar] [CrossRef]
- Chemat, F.; Abert-Vian, M.; Fabiano-Tixier, A.S.; Strube, J.; Uhlenbrock, L.; Gunjevic, V.; Cravotto, G. Green extraction of natural products. Origins, current status, and future challenges. TrAC Trends Anal. Chem. 2019, 118, 248–263. [Google Scholar] [CrossRef]
- Jensch, C.; Schmidt, A.; Strube, J. Versatile Green Processing for Recovery of Phenolic Compounds from Natural Product Extracts towards Bioeconomy and Cascade Utilization for Waste Valorization on the Example of Cocoa Bean Shell (CBS). Sustainability 2022, 14, 3126. [Google Scholar] [CrossRef]
- Arzneibuch, E. Amtliche Deutsche Ausgabe; Deutscher Apotheker: Stuttgart, Germany, 2020; ISBN 9783769275155. [Google Scholar]
- Voigt, R.; Bunjes, H.; Daniels, R.; Kuntsche, J.; May, S.; Scheler, S.; Tegtmeier, M. Voigt Pharmazeutische Technologie: Für Studium und Beruf, 13th ed.; Völlig neu Bearbeitete Auflage; Deutscher Apotheker: Stuttgart, Germany, 2021; ISBN 9783769277487. [Google Scholar]
- Chestnut, L.G.; Mills, D.M. A fresh look at the benefits and costs of the US acid rain program. J. Environ. Manag. 2005, 77, 252–266. [Google Scholar] [CrossRef] [PubMed]
- Ratti, C. Hot air and freeze-drying of high-value foods: A review. J. Food Eng. 2001, 49, 311–319. [Google Scholar] [CrossRef]
- Woodstock, J. Modernizing Pharmaceutical Manufacturing—Continuous Manufacturing as a Key Enabler. In Proceedings of the MIT-CMAC International Symposium on Continuous Manufacturing of Pharmaceuticals, Cambridge, MA, USA, 20–21 May 2014. [Google Scholar]
- Novartis-MIT. Center for Continuous Manufacturing. Available online: http://novartis-mit.mit.edu/ (accessed on 12 December 2015).
- Pollard, D. Advances Towards Automated Continuous mAb Processing. Available online: www.merck.com (accessed on 7 December 2015).
- Schmidt, A.; Helgers, H.; Vetter, F.L.; Zobel-Roos, S.; Hengelbrock, A.; Strube, J. Process Automation and Control Strategy by Quality-by-Design in Total Continuous mRNA Manufacturing Platforms. Processes 2022, 10, 1783. [Google Scholar] [CrossRef]
- Hengelbrock, A.; Helgers, H.; Schmidt, A.; Vetter, F.L.; Juckers, A.; Rosengarten, J.F.; Stitz, J.; Strube, J. Digital Twin for HIV-Gag VLP Production in HEK293 Cells. Processes 2022, 10, 866. [Google Scholar] [CrossRef]
- Helgers, H.; Hengelbrock, A.; Schmidt, A.; Vetter, F.L.; Juckers, A.; Strube, J. Digital Twins for scFv Production in Escherichia coli. Processes 2022, 10, 809. [Google Scholar] [CrossRef]
- Schmidt, A.; Zobel-Roos, S.; Helgers, H.; Lohmann, L.; Vetter, F.; Jensch, C.; Juckers, A.; Strube, J. Digital Twins for Continuous Biologics Manufacturing. In Process Control, Intensification, and Digitalisation in Continuous Biomanufacturing; Wiley-VCH: Weinheim, Germany, 2022; Volume 7, pp. 265–350. [Google Scholar] [CrossRef]
- Herwig, C.; Pörtner, R.; Möller, J. Digital Twins: Tools and Concepts For Smart Biomanufacturing; Springer: Berlin/Heidelberg, Germany, 2021; Volume 176. [Google Scholar] [CrossRef]
- Zobel-Roos, S.; Schmidt, A.; Uhlenbrock, L.; Ditz, R.; Köster, D.; Strube, J. Digital Twins in Biomanufacturing. Adv. Biochem. Eng. Biotechnol. 2021, 176, 181–262. [Google Scholar] [CrossRef] [PubMed]
- Uhlenbrock, L.; Jensch, C.; Tegtmeier, M.; Strube, J. Digital Twin for Extraction Process Design and Operation. Processes 2020, 8, 866. [Google Scholar] [CrossRef]
- Ich Harmonised Tripartite Guideline. Development And Manufacture of Drug Substances: (Chemical Entities and Biotechnological/Biological Entities) Q11; European Medicines Agency: London, UK, 2011. [Google Scholar]
- Udugama, I.A.; Lopez, P.C.; Gargalo, C.L.; Li, X.; Bayer, C.; Gernaey, K.V. Digital Twin in biomanufacturing: Challenges and opportunities towards its implementation. Syst. Microbiol. Biomanuf. 2021, 1, 257–274. [Google Scholar] [CrossRef]
- Sixt, M.; Uhlenbrock, L.; Strube, J. Toward a Distinct and Quantitative Validation Method for Predictive Process Modelling—On the Example of Solid-Liquid Extraction Processes of Complex Plant Extracts. Processes 2018, 6, 66. [Google Scholar] [CrossRef]
- Sixt, M.; Gudi, G.; Schulz, H.; Strube, J. In-line Raman spectroscopy and advanced process control for the extraction of anethole and fenchone from fennel (Foeniculum vulgare L. MILL.). C. R. Chim. 2018, 21, 97–103. [Google Scholar] [CrossRef]
- Both, S.; Koudous, I.; Jenelten, U.; Strube, J. Model-based equipment-design for plant-based extraction processes—Considering botanic and thermodynamic aspects. C. R. Chim. 2014, 17, 187–196. [Google Scholar] [CrossRef]
- Kassing, M.; Jenelten, U.; Schenk, J.; Hänsch, R.; Strube, J. Combination of Rigorous and Statistical Modeling for Process Development of Plant-Based Extractions Based on Mass Balances and Biological Aspects. Chem. Eng. Technol. 2012, 35, 109–132. [Google Scholar] [CrossRef]
- Roth, T.; Uhlenbrock, L.; Strube, J. Distinct and Quantitative Validation for Predictive Process Modelling in Steam Distillation of Caraway Fruits and Lavender Flower Following a Quality-By-Design (QbD) Approach. Processes 2020, 8, 594. [Google Scholar] [CrossRef]
- Schmidt, A.; Strube, J. Transport Phenomena in Pervaporation. In Current Trends and Future Developments on (Bio-) Membranes: Transport Phenomena in Membranes; Basile, A., Ed.; Elsevier: San Diego, CA, USA, 2022; pp. 165–192. ISBN 9780128222577. [Google Scholar]
- Huter, M.J.; Jensch, C.; Strube, J. Model Validation and Process Design of Continuous Single Pass Tangential Flow Filtration Focusing on Continuous Bioprocessing for High Protein Concentrations. Processes 2019, 7, 781. [Google Scholar] [CrossRef]
- Schmidt, A.; Strube, J. Distinct and Quantitative Validation Method for Predictive Process Modeling with Examples of Liquid-Liquid Extraction Processes of Complex Feed Mixtures. Processes 2019, 7, 298. [Google Scholar] [CrossRef]
- Schmidt, A.; Sixt, M.; Huter, M.; Mestmäcker, F.; Strube, J. Systematic and Model-Assisted Process Design for the Extraction and Purification of Artemisinin from Artemisia annua L.—Part II: Model-Based Design of Agitated and Packed Columns for Multistage Extraction and Scrubbing. Processes 2018, 6, 179. [Google Scholar] [CrossRef]
- Vetter, F.L.; Strube, J. Enabling Total Process Digital Twin in Sugar Refining through the Integration of Secondary Crystallization Influences. Processes 2022, 10, 373. [Google Scholar] [CrossRef]
- Huter, M.; Schmidt, A.; Mestmäcker, F.; Sixt, M.; Strube, J. Systematic and Model-Assisted Process Design for the Extraction and Purification of Artemisinin from Artemisia annua L.—Part IV: Crystallization. Processes 2018, 6, 181. [Google Scholar] [CrossRef]
- Lohmann, L.J.; Strube, J. Accelerating Biologics Manufacturing by Modeling: Process Integration of Precipitation in mAb Downstream Processing. Processes 2020, 8, 58. [Google Scholar] [CrossRef]
- Jensch, C.; Knierim, L.; Tegtmeier, M.; Strube, J. Development of a General PAT Strategy for Online Monitoring of Complex Mixtures—On the Example of Natural Product Extracts from Bearberry Leaf (Arctostaphylos uva-ursi). Processes 2021, 9, 2129. [Google Scholar] [CrossRef]
- Uhlenbrock, L. Quality-by-Design zur Systematischen Entwicklung von Wertschöpfungsprozessen Pflanzlicher Rohstoffe. Ph.D. Thesis, Technische Universität Clausthal, Clausthal-Zellerfeld, Germany, 2021. [Google Scholar]
- Sixt, M.; Schmidt, A.; Mestmäcker, F.; Huter, M.; Uhlenbrock, L.; Strube, J. Systematic and Model-Assisted Process Design for the Extraction and Purification of Artemisinin from Artemisia annua L.—Part I: Conceptual Process Design and Cost Estimation. Processes 2018, 6, 161. [Google Scholar] [CrossRef]
- Mestmäcker, F.; Schmidt, A.; Huter, M.; Sixt, M.; Strube, J. Systematic and Model-Assisted Process Design for the Extraction and Purification of Artemisinin from Artemisia annua L.—Part III: Chromatographic Purification. Processes 2018, 6, 180. [Google Scholar] [CrossRef]
- Sixt, M.; Strube, J. Systematic and Model-Assisted Evaluation of Solvent Based- or Pressurized Hot Water Extraction for the Extraction of Artemisinin from Artemisia annua L. Processes 2017, 5, 86. [Google Scholar] [CrossRef]
- Koudous, I.; Sixt, M.; Strube, J. Model-based systematic interpretation of the extraction and purification of 10-deacetylbaccatin III from Taxus baccata. Ber. Aus Dem Jul. Kühn. Inst. 2016, 183, 26. [Google Scholar]
- Uhlenbrock, L.; Sixt, M.; Strube, J. Quality-by-Design (QbD) process evaluation for phytopharmaceuticals on the example of 10-deacetylbaccatin III from yew. Resour. Effic. Technol. 2017, 3, 137–143. [Google Scholar] [CrossRef]
- Sixt, M.; Strube, J. Pressurized hot water extraction of 10-deacetylbaccatin III from yew for industrial application. Resour. Effic. Technol. 2017, 3, 177–186. [Google Scholar] [CrossRef]
- Glöckl, I.; Blaschke, G.; Veit, M. Validated methods for direct determination of hydroquinone glucuronide and sulfate in human urine after oral intake of bearberry leaf extract by capillary zone electrophoresis. J. Chromatogr. B Biomed. Sci. Appl. 2001, 761, 261–266. [Google Scholar] [CrossRef] [PubMed]
- Committee on Herbal Medicinal Products. European Union Herbal Monograph on Arctostaphylos Uva-Ursi (L.) Spreng., Folium; EMA/HMPC/750269/2016; European Medicines Agency: Amsterdam, The Netherlands, 2018. [Google Scholar]
- Chemat, F.; Strube, J. Green Extraction of Natural Products: Theory And Practice; Wiley VCH: Weinheim, Germany, 2015; ISBN 9783527336531. [Google Scholar]
- Chemat, F.; Vian, M.A. Alternative Solvents for Natural Products Extraction; Springer: Berlin/Heidelberg, Germany, 2014. [Google Scholar]
- Lebovka, N.; Vorobiev, E.; Chémat, F. (Eds.) Enhancing Extraction Processes in the Food Industry, 1st ed.; CRC Press: Boca Raton, FL, USA, 2016; ISBN 9781439845950. [Google Scholar]
- EMA-FDA Pilot Program for Parallel Assessment of Quality by Design Applications; European Medicines Agency, Food and Drug Administration: London, UK, 2011.
- Guideline for Implimentation of Q9; Food and Drug Administration: Silver Spring, MD, USA, 2006.
- ICH Expert Working Group. Riskmanagement (Q9). ICH Harmonised Tripartite Guideline. Q9; ICH: Geneva, Switzerland, 2005. [Google Scholar]
- Sixt, M.; Strube, J. Systematic Design and Evaluation of an Extraction Process for Traditionally Used Herbal Medicine on the Example of Hawthorn (Crataegus monogyna JACQ.). Processes 2018, 6, 73. [Google Scholar] [CrossRef]
- Committee on Herbal Medicinal Products. European Union Herbal Monograph on Crataegus Spp., Folium Cum Flore; EMA/HMPC/159075/2014; European Medicines Agency: Amsterdam, The Netherlands, 2016. [Google Scholar]
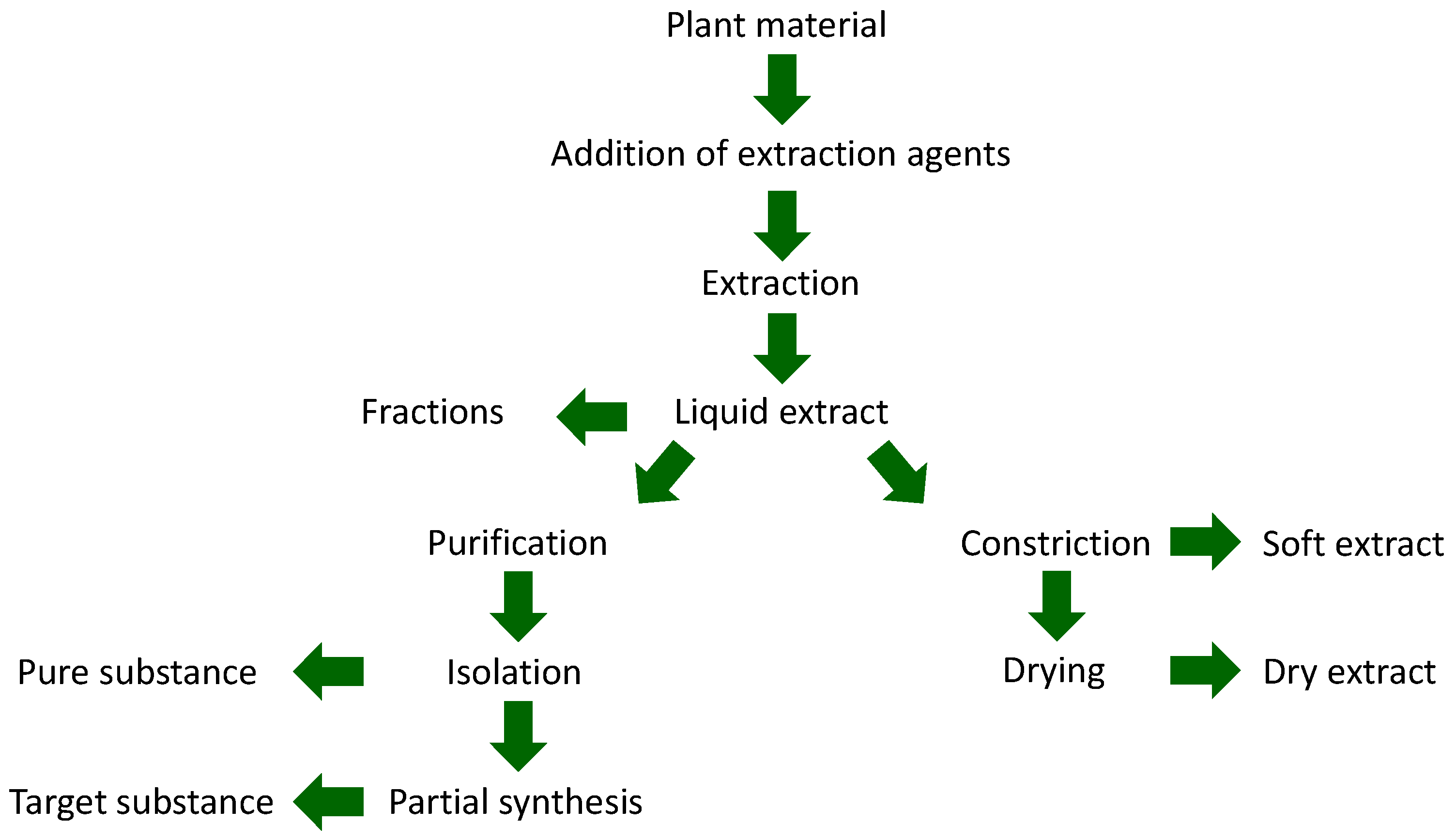

| Type of Extract | Classification of Extract According to the European Pharmacopoeia | |
|---|---|---|
| Designation | Latin Designation | |
| Fluid extracts | Liquid extracts | Praeparationes fluidae ab extractione |
| Extracta fluida | ||
| Tinctures | Tinctura | |
| Concentrates | Soft extracts | Extracta spissa |
| Oleoresins | Oleoresina | |
| Dry extracts | Dry extracts | Extracta sicca |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tegtmeier, M.; Knierim, L.; Schmidt, A.; Strube, J. Green Manufacturing for Herbal Remedies with Advanced Pharmaceutical Technology. Pharmaceutics 2023, 15, 188. https://doi.org/10.3390/pharmaceutics15010188
Tegtmeier M, Knierim L, Schmidt A, Strube J. Green Manufacturing for Herbal Remedies with Advanced Pharmaceutical Technology. Pharmaceutics. 2023; 15(1):188. https://doi.org/10.3390/pharmaceutics15010188
Chicago/Turabian StyleTegtmeier, Martin, Larissa Knierim, Axel Schmidt, and Jochen Strube. 2023. "Green Manufacturing for Herbal Remedies with Advanced Pharmaceutical Technology" Pharmaceutics 15, no. 1: 188. https://doi.org/10.3390/pharmaceutics15010188
APA StyleTegtmeier, M., Knierim, L., Schmidt, A., & Strube, J. (2023). Green Manufacturing for Herbal Remedies with Advanced Pharmaceutical Technology. Pharmaceutics, 15(1), 188. https://doi.org/10.3390/pharmaceutics15010188








