Enhanced Ocular Anti-Aspergillus Activity of Tolnaftate Employing Novel Cosolvent-Modified Spanlastics: Formulation, Statistical Optimization, Kill Kinetics, Ex Vivo Trans-Corneal Permeation, In Vivo Histopathological and Susceptibility Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of TOL Basic Spanlastics
2.3. In Vitro TOL Basic Spanlastics Characterization
2.3.1. Entrapment Efficiency (EE%)
2.3.2. Particle Size (PS), Polydispersity Index (PDI), and Zeta Potential (ZP)
2.4. TOL Basic Spanlastics Formulation Optimization
2.5. Modification of the Optimal TOL Basic Spanlastics Using Cosolvents
2.6. Statistical Design for the Preparation of TOL-Cosolvent Spanlastics
2.7. In Vitro TOL-Cosolvent Spanlastics Characterization
2.7.1. Entrapment Efficiency (EE%)
2.7.2. Particle Size (PS), Polydispersity Index (PDI), and Zeta Potential (ZP)
2.8. TOL-Cosolvent Spanlastics Optimization
2.9. Transmission Electron Microscopy (TEM)
2.10. Differential Scanning Calorimetry (DSC)
2.11. Ex Vivo Studies
2.11.1. Corneas Preparation
2.11.2. Corneal Permeation Study
2.12. In Vitro Antifungal Activity
2.12.1. Fungal Strain and Inoculum Preparation
2.12.2. Tested Samples
2.12.3. Minimum Inhibitory Concentration (MIC)
2.12.4. Minimum Fungicidal Concentration (MFC)
2.12.5. Kill Kinetics Assay
2.13. In Vivo Studies
2.13.1. Animals
2.13.2. Draize Test
2.13.3. Histopathology
2.13.4. Susceptibility Test
3. Results and Discussion
3.1. Preparation of TOL Basic Spanlastics
3.2. Factorial Design Analysis of TOL Basic Spanlastics
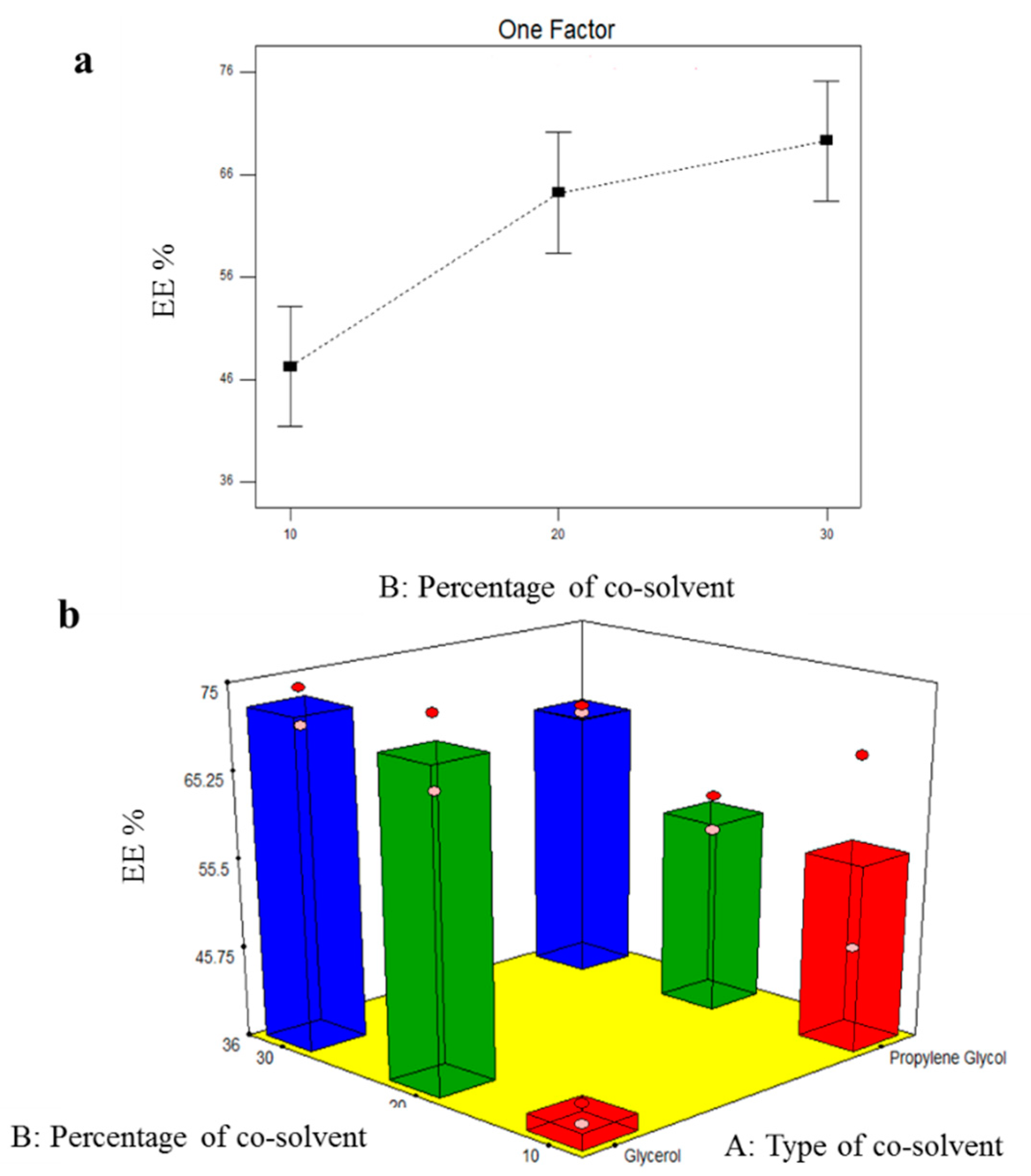
3.2.1. Formulation Variables Effect on EE% of TOL Basic Spanlastics
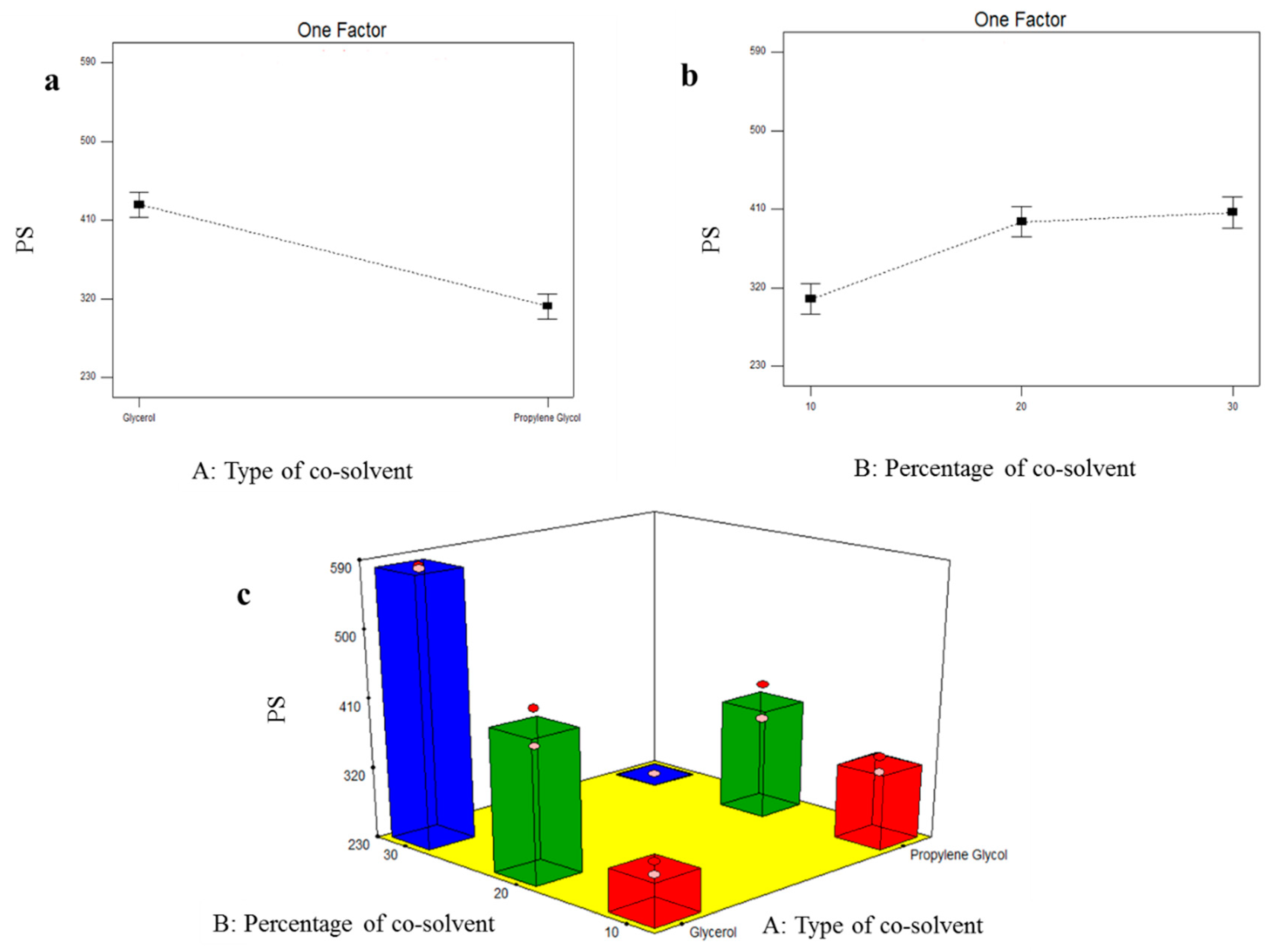
3.2.2. Formulation Variables Effect on PS of TOL Basic Spanlastics
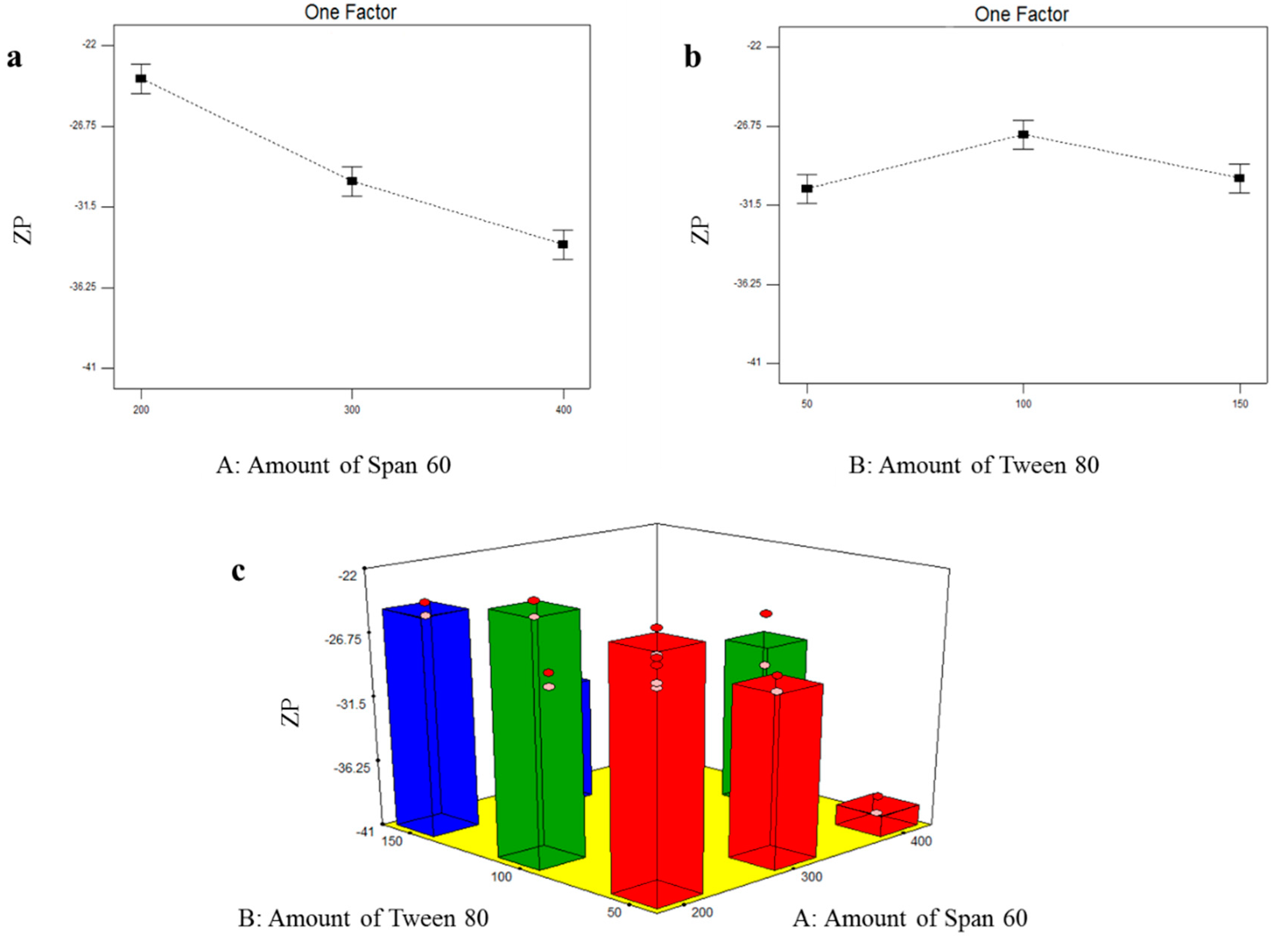
3.2.3. Formulation Variables Effect on ZP of TOL Basic Spanlastics
3.3. Selection of the Optimal TOL Basic Spanlastics
3.4. Factorial Design Analysis of TOL-Cosolvent Spanlastics
3.4.1. Effect of Formulation Variables on EE% of TOL-Cosolvent Spanlastics
3.4.2. Formulation Variables Effect on PS of TOL-Cosolvent Spanlastics
3.4.3. Formulation Variables Effect on ZP of TOL-Cosolvent Spanlastics
3.5. Selection of the Optimal TOL-Cosolvent Spanlastics
3.6. Transmission Electron Microscopy (TEM)
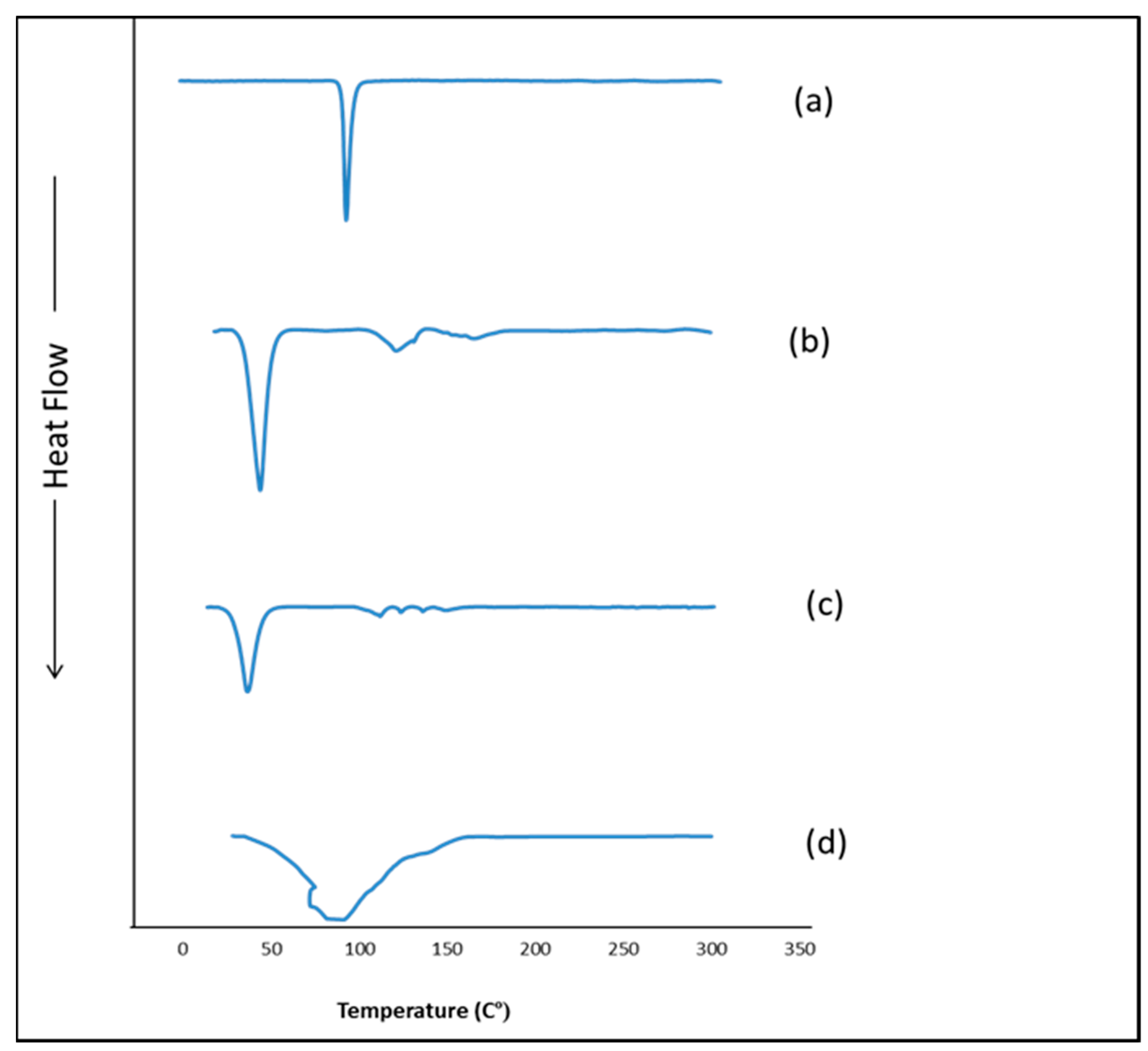
3.7. Differential Scanning Calorimetry (DSC)
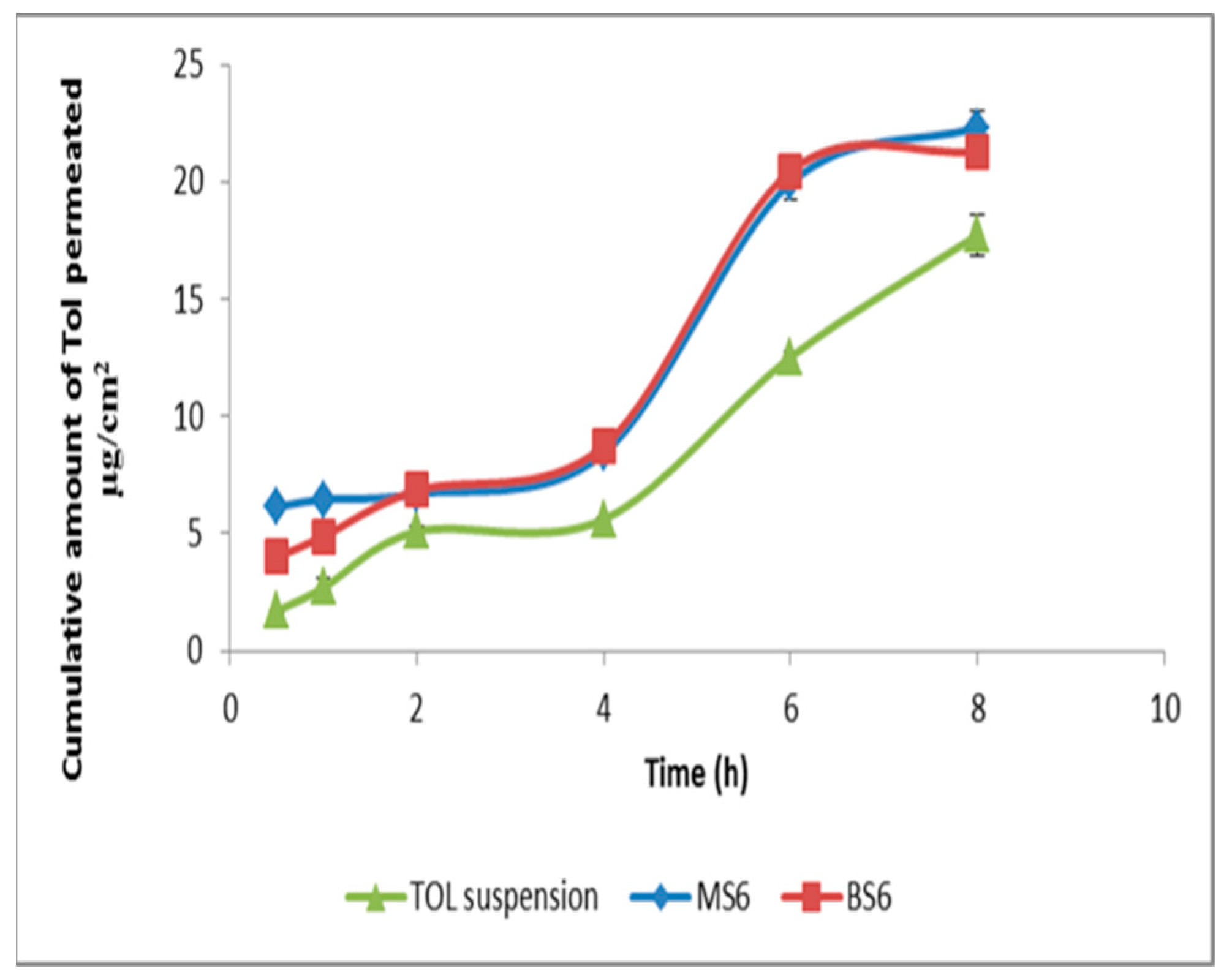
3.8. Ex Vivo Corneal Permeation
3.9. In Vitro Antifungal Activity
3.9.1. Minimum Inhibitory Concentration (MIC)
3.9.2. Minimum Fungicidal Concentration (MFC)
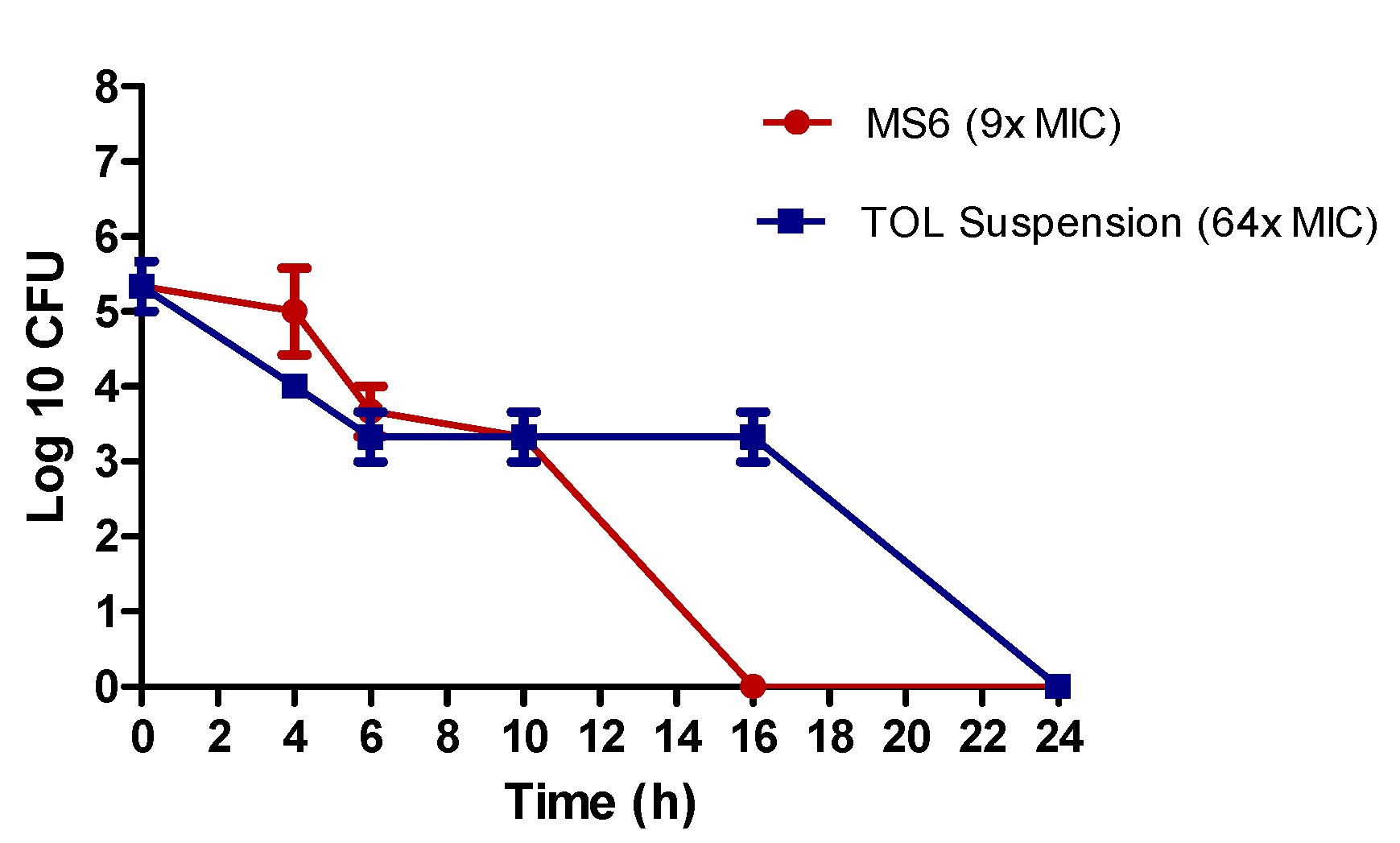
3.9.3. Kill Kinetics Assay
3.10. In Vivo Studies
3.10.1. Draize Test
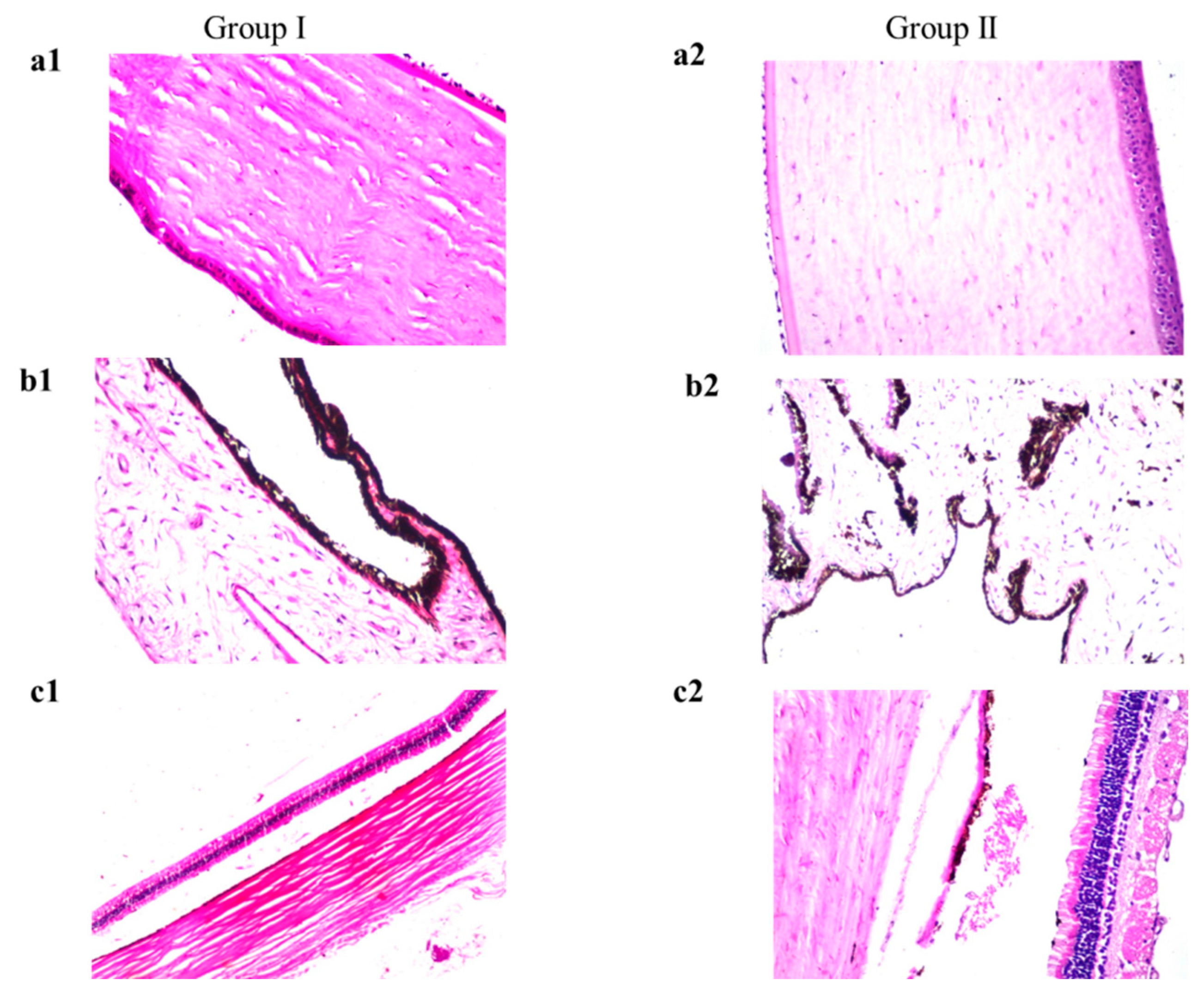
3.10.2. Histopathology
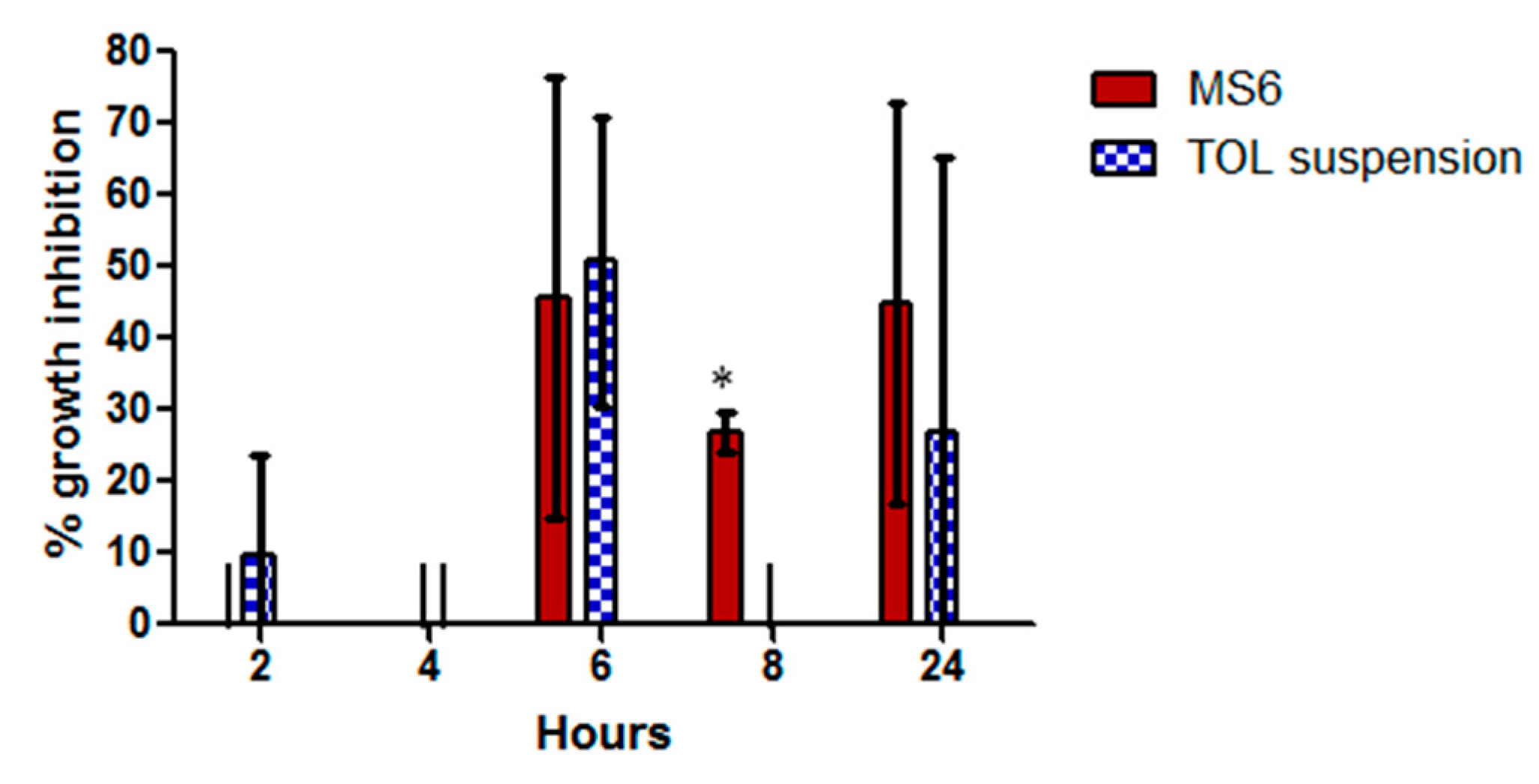
3.10.3. Susceptibility Test
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Acharya, Y.; Acharya, B.; Karki, P. Fungal keratitis: Study of increasing trend and common determinants. Nepal J. Epidemiol. 2017, 7, 685–693. [Google Scholar] [CrossRef] [PubMed]
- Younes, N.F.; Abdel-Halim, S.A.; Elassasy, A.I. Corneal targeted Sertaconazole nitrate loaded cubosomes: Preparation, statistical optimization, in vitro characterization, ex vivo permeation and in vivo studies. Int. J. Pharm. 2018, 553, 386–397. [Google Scholar] [CrossRef] [PubMed]
- Spadea, L.; Giannico, M.I. Diagnostic and management strategies of Aspergillus endophthalmitis: Current insights. Clin. Ophthalmol. 2019, 13, 2573. [Google Scholar] [CrossRef]
- Öz, Y.; Özdemir, H.G.; Gökbolat, E.; Kiraz, N.; Ilkit, M.; Seyedmousavi, S. Time-kill kinetics and in vitro antifungal susceptibility of non-fumigatus Aspergillus species isolated from patients with ocular mycoses. Mycopathologia 2016, 181, 225–233. [Google Scholar] [CrossRef] [PubMed]
- Sherwal, B.; Verma, A. Epidemiology of ocular infection due to bacteria and fungus-a prospective study. JK Sci. 2008, 10, 127–131. [Google Scholar]
- Abdelbary, A.A.; Abd-Elsalam, W.H.; Al-Mahallawi, A.M. Fabrication of novel ultradeformable bilosomes for enhanced ocular delivery of terconazole: In vitro characterization, ex vivo permeation and in vivo safety assessment. Int. J. Pharm. 2016, 513, 688–696. [Google Scholar] [CrossRef]
- Gunda, S.R.C.; Ganesh, G. Formulation and evaluation of tolnaftate loaded topical liposomal gel for effective skin drug delivery to treat fungal diseases. J. Chem. Pharm. Res. 2014, 6, 856–866. [Google Scholar]
- Abousamra, M.M.; Mohsen, A.M. Solid lipid nanoparticles and nanostructured lipid carriers of tolnaftate: Design, optimization and in-vitro evaluation. Int. J. Pharm. Pharm. Sci. 2016, 8, 380–385. [Google Scholar]
- Viswanath, V.; Reddy, R.; Surekha, M.; Bhuvaneswari, S. Effect of edge actuators on the formulation and characterization of tolnaftate proniosomes. Int. J. Res. Pharm. Sci. 2015, 6, 344–357. [Google Scholar]
- AbouSamra, M.M.; Salama, A.H. Enhancement of the topical tolnaftate delivery for the treatment of tinea pedis via provesicular gel systems. J. Liposome Res. 2017, 27, 324–334. [Google Scholar] [CrossRef]
- Ramya, M.G.; Akki, R.; Kumar, P.D. Formulation and evalution of tolnaftate loaded topical niosomal gel. Pharma Innov. J. 2017, 6, 29–34. [Google Scholar]
- Kaur, I.P.; Rana, C.; Singh, H. Development of effective ocular preparations of antifungal agents. J. Ocul. Pharmacol. Ther. 2008, 24, 481–493. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, N.; Sahu, S.; Pathak, K. Antifungal potential of tolnaftate against Candida albicans in the treatment of onychomycosis: Development of nail lacquer and ex vivo characterization. Pharm. Biomed. Res. 2016, 2, 1–12. [Google Scholar] [CrossRef][Green Version]
- Aziz, D.; Mohamed, S.; Tayel, S.; Makhlouf, A. Implementing polymeric pseudorotaxanes for boosting corneal permeability and antiaspergillus activity of tolnaftate: Formulation development, statistical optimization, ex vivo permeation and in vivo assessment. Drug Deliv. 2022, 29, 2162–2176. [Google Scholar] [CrossRef]
- Fahmy, A.M.; Hassan, M.; El-Setouhy, D.A.; Tayel, S.A.; Al-Mahallawi, A.M. Statistical optimization of hyaluronic acid enriched ultradeformable elastosomes for ocular delivery of voriconazole via Box-Behnken design: In vitro characterization and in vivo evaluation. Drug Deliv. 2021, 28, 77–86. [Google Scholar] [CrossRef]
- Li, J.; Li, Z.; Liang, Z.; Han, L.; Feng, H.; He, S.; Zhang, J. Fabrication of a drug delivery system that enhances antifungal drug corneal penetration. Drug Deliv. 2018, 25, 938–949. [Google Scholar] [CrossRef]
- Fahmy, A.M.; El-Setouhy, D.A.; Habib, B.A.; Tayel, S.A. Enhancement of Transdermal Delivery of Haloperidol via Spanlastic Dispersions: Entrapment Efficiency vs. Particle Size. AAPS PharmSciTech 2019, 20, 95. [Google Scholar] [CrossRef]
- Duangjit, S.; Opanasopit, P.; Rojanarata, T.; Ngawhirunpat, T. Evaluation of meloxicam-loaded cationic transfersomes as transdermal drug delivery carriers. AAPS PharmSciTech 2013, 14, 133–140. [Google Scholar] [CrossRef]
- ElMeshad, A.N.; Mohsen, A.M. Enhanced corneal permeation and antimycotic activity of itraconazole against Candida albicans via a novel nanosystem vesicle. Drug Deliv. 2016, 23, 2115–2123. [Google Scholar] [CrossRef]
- Basha, M.; Abd El-Alim, S.H.; Shamma, R.N.; Awad, G.E. Design and optimization of surfactant-based nanovesicles for ocular delivery of Clotrimazole. J. Liposome Res. 2013, 23, 203–210. [Google Scholar] [CrossRef]
- Tayel, S.A.; El-Nabarawi, M.A.; Tadros, M.I.; Abd-Elsalam, W.H. Duodenum-triggered delivery of pravastatin sodium via enteric surface-coated nanovesicular spanlastic dispersions: Development, characterization and pharmacokinetic assessments. Int. J. Pharm. 2015, 483, 77–88. [Google Scholar] [CrossRef] [PubMed]
- Manconi, M.; Caddeo, C.; Nacher, A.; Diez-Sales, O.; Peris, J.E.; Ferrer, E.E.; Fadda, A.M.; Manca, M.L. Eco-scalable baicalin loaded vesicles developed by combining phospholipid with ethanol, glycerol, and propylene glycol to enhance skin permeation and protection. Colloids Surf. B Biointerfaces 2019, 184, 110504. [Google Scholar] [CrossRef]
- Chessa, M.; Caddeo, C.; Valenti, D.; Manconi, M.; Sinico, C.; Fadda, A.M. Effect of Penetration Enhancer Containing Vesicles on the Percutaneous Delivery of Quercetin through New Born Pig Skin. Pharmaceutics 2011, 3, 497–509. [Google Scholar] [CrossRef] [PubMed]
- Manca, M.L.; Zaru, M.; Manconi, M.; Lai, F.; Valenti, D.; Sinico, C.; Fadda, A.M. Glycerosomes: A new tool for effective dermal and transdermal drug delivery. Int. J. Pharm. 2013, 455, 66–74. [Google Scholar] [CrossRef] [PubMed]
- Kakkar, S.; Kaur, I.P. Spanlastics—A novel nanovesicular carrier system for ocular delivery. Int. J. Pharm. 2011, 413, 202–210. [Google Scholar] [CrossRef] [PubMed]
- Fahmy, A.M.; El-Setouhy, D.A.; Ibrahim, A.B.; Habib, B.A.; Tayel, S.A.; Bayoumi, N.A. Penetration enhancer-containing spanlastics (PECSs) for transdermal delivery of haloperidol: In vitro characterization, ex vivo permeation and in vivo biodistribution studies. Drug Deliv. 2018, 25, 12–22. [Google Scholar] [CrossRef]
- Aburahma, M.H.; Abdelbary, G.A. Novel diphenyl dimethyl bicarboxylate provesicular powders with enhanced hepatocurative activity: Preparation, optimization, in vitro/in vivo evaluation. Int. J. Pharm. 2012, 422, 139–150. [Google Scholar] [CrossRef]
- Fares, A.R.; ElMeshad, A.N.; Kassem, M.A.A. Enhancement of dissolution and oral bioavailability of lacidipine via pluronic P123/F127 mixed polymeric micelles: Formulation, optimization using central composite design and in vivo bioavailability study. Drug Deliv. 2018, 25, 132–142. [Google Scholar] [CrossRef]
- Eldeeb, A.E.; Salah, S.; Ghorab, M. Formulation and evaluation of cubosomes drug delivery system for treatment of glaucoma: Ex-vivo permeation and in-vivo pharmacodynamic study. J. Drug Deliv Sci. Technol. 2019, 52, 236–247. [Google Scholar] [CrossRef]
- Sayed, S.; Elsayed, I.; Ismail, M.M. Optimization of β-cyclodextrin consolidated micellar dispersion for promoting the transcorneal permeation of a practically insoluble drug. Int. J. Pharm. 2018, 549, 249–260. [Google Scholar] [CrossRef]
- Kezutyte, T.; Kornysova, O.; Maruska, A.; Briedis, V. Assay of tolnaftate in human skin samples after in vitro penetration studies using high performance liquid chromatography. Acta Pol. Pharm. 2010, 67, 327–334. [Google Scholar] [PubMed]
- El Zaafarany, G.M.; Awad, G.A.; Holayel, S.M.; Mortada, N.D. Role of edge activators and surface charge in developing ultradeformable vesicles with enhanced skin delivery. Int. J. Pharm. 2010, 397, 164–172. [Google Scholar] [CrossRef]
- Humphries, R.M.; Ambler, J.; Mitchell, S.L.; Castanheira, M.; Dingle, T.; Hindler, J.A.; Koeth, L.; Sei, K. CLSI methods development and standardization working group best practices for evaluation of antimicrobial susceptibility tests. J. Clin. Microbiol. 2018, 56, e01934-17. [Google Scholar] [CrossRef] [PubMed]
- Ismail, M.M.; Samir, R.; Saber, F.R.; Ahmed, S.R.; Farag, M.A. Pimenta oil as a potential treatment for Acinetobacter baumannii wound infection: In vitro and in vivo bioassays in relation to its chemical composition. Antibiotics 2020, 9, 679. [Google Scholar] [CrossRef] [PubMed]
- Yousry, C.; Zikry, P.M.; Salem, H.M.; Basalious, E.B.; El-Gazayerly, O.N. Integrated nanovesicular/self-nanoemulsifying system (INV/SNES) for enhanced dual ocular drug delivery: Statistical optimization, in vitro and in vivo evaluation. Drug Deliv. Transl. Res. 2020, 10, 801–814. [Google Scholar] [CrossRef]
- Bancroft, J.; Stevens, A.; Turner, D. Theory and Practice of Histopathological Techniques, 4th ed.; Churchill Livingstone: New York, NY, USA; London, UK; Madrid, Spain, 1996. [Google Scholar]
- Albash, R.; Al-Mahallawi, A.M.; Hassan, M.; Alaa-Eldin, A.A. Development and optimization of terpene-enriched vesicles (Terpesomes) for effective ocular delivery of fenticonazole nitrate: In vitro characterization and in vivo assessment. Int. J. Nanomed. 2021, 16, 609. [Google Scholar] [CrossRef]
- Kaur, I.P.; Rana, C.; Singh, M.; Bhushan, S.; Singh, H.; Kakkar, S. Development and evaluation of novel surfactant-based elastic vesicular system for ocular delivery of fluconazole. J. Ocul. Pharmacol. Ther. Off. J. Assoc. Ocul. Pharmacol. Ther. 2012, 28, 484–496. [Google Scholar] [CrossRef]
- Rao, Y.; Zheng, F.; Zhang, X.; Gao, J.; Liang, W. In vitro percutaneous permeation and skin accumulation of finasteride using vesicular ethosomal carriers. AAPS PharmSciTech 2008, 9, 860–865. [Google Scholar] [CrossRef]
- Lieberman, H. Pharmaceutical Dosage Forms: Disperse Systems; CRC Press: Boca Raton, FL, USA, 1998. [Google Scholar]
- Abd-Elsalam, W.H.; El-Zahaby, S.A.; Al-Mahallawi, A.M. Formulation and in vivo assessment of terconazole-loaded polymeric mixed micelles enriched with Cremophor EL as dual functioning mediator for augmenting physical stability and skin delivery. Drug Deliv. 2018, 25, 484–492. [Google Scholar] [CrossRef]
- De Lima, L.S.; Araujo, M.D.M.; Quináia, S.P.; Migliorine, D.W.; Garcia, J.R. Adsorption modeling of Cr, Cd and Cu on activated carbon of different origins by using fractional factorial design. Chem. Eng. J. 2011, 166, 881–889. [Google Scholar] [CrossRef]
- Aziz, D.E.; Abdelbary, A.A.; Elassasy, A.I. Implementing Central Composite Design for Developing Transdermal Diacerein-Loaded Niosomes: Ex vivo Permeation and In vivo Deposition. Curr. Drug Deliv. 2018, 15, 1330–1342. [Google Scholar] [CrossRef] [PubMed]
- El Menshawe, S.F.; Nafady, M.M.; Aboud, H.M.; Kharshoum, R.M.; Elkelawy, A.; Hamad, D.S. Transdermal delivery of fluvastatin sodium via tailored spanlastic nanovesicles: Mitigated Freund’s adjuvant-induced rheumatoid arthritis in rats through suppressing p38 MAPK signaling pathway. Drug Deliv. 2019, 26, 1140–1154. [Google Scholar] [CrossRef] [PubMed]
- Aboud, H.M.; Hassan, A.H.; Ali, A.A.; Abdel-Razik, A.H. Novel in situ gelling vaginal sponges of sildenafil citrate-based cubosomes for uterine targeting. Drug Deliv. 2018, 25, 1328–1339. [Google Scholar] [CrossRef] [PubMed]
- Abdelrahman, F.E.; Elsayed, I.; Gad, M.K.; Elshafeey, A.H.; Mohamed, M.I. Response surface optimization, Ex vivo and In vivo investigation of nasal spanlastics for bioavailability enhancement and brain targeting of risperidone. Int. J. Pharm. 2017, 530, 1–11. [Google Scholar] [CrossRef]
- Younes, N.F.; Abdel-Halim, S.A.; Elassasy, A.I. Solutol HS15 based binary mixed micelles with penetration enhancers for augmented corneal delivery of sertaconazole nitrate: Optimization, in vitro, ex vivo and in vivo characterization. Drug Deliv. 2018, 25, 1706–1717. [Google Scholar] [CrossRef]
- Wilson, B.; Samanta, M.K.; Santhi, K.; Kumar, K.P.S.; Paramakrishnan, N.; Suresh, B. Poly (n-butylcyanoacrylate) nanoparticles coated with polysorbate 80 for the targeted delivery of rivastigmine into the brain to treat Alzheimer’s disease. Brain Res. 2008, 1200, 159–168. [Google Scholar] [CrossRef]
- Alexander, D.L.; Tropsha, A.; Winkler, D.A. Beware of R(2): Simple, Unambiguous Assessment of the Prediction Accuracy of QSAR and QSPR Models. J. Chem. Inf. Modeling 2015, 55, 1316–1322. [Google Scholar] [CrossRef]
- Elmoslemany, R.M.; Abdallah, O.Y.; El-Khordagui, L.K.; Khalafallah, N.M. Propylene glycol liposomes as a topical delivery system for miconazole nitrate: Comparison with conventional liposomes. AAPS PharmSciTech 2012, 13, 723–731. [Google Scholar] [CrossRef]
- Manconi, M.; Mura, S.; Sinico, C.; Fadda, A.; Vila, A.; Molina, F. Development and characterization of liposomes containing glycols as carriers for diclofenac. Colloids Surf. A Physicochem. Eng. Asp. 2009, 342, 53–58. [Google Scholar] [CrossRef]
- Salem, H.F.; Kharshoum, R.M.; Sayed, O.M.; Abdel Hakim, L.F. Formulation design and optimization of novel soft glycerosomes for enhanced topical delivery of celecoxib and cupferron by Box-Behnken statistical design. Drug Dev. Ind. Pharm. 2018, 44, 1871–1884. [Google Scholar] [CrossRef]
- Kumari, P.; Misra, S.; Pandey, S. Formulation and Evaluation of Tolnaftate Microsponges Loaded Gels for Treatment of Dermatophytosis. Eur. J. Pharm. Med. Res. 2017, 4, 326–335. [Google Scholar]
- Al-Mahallawi, A.M.; Abdelbary, A.A.; Aburahma, M.H. Investigating the potential of employing bilosomes as a novel vesicular carrier for transdermal delivery of tenoxicam. Int. J. Pharm. 2015, 485, 329–340. [Google Scholar] [CrossRef] [PubMed]
- Elsayed, I.; Abdelbary, A.A.; Elshafeey, A.H. Nanosizing of a poorly soluble drug: Technique optimization, factorial analysis, and pharmacokinetic study in healthy human volunteers. Int. J. Nanomed. 2014, 9, 2943–2953. [Google Scholar] [CrossRef]
- Kakkar, S.; Kaur, I.P. A novel nanovesicular carrier system to deliver drug topically. Pharm. Dev. Technol. 2013, 18, 673–685. [Google Scholar] [CrossRef]
- Aziz, D.E.; Abdelbary, A.A.; Elassasy, A.I. Fabrication of novel elastosomes for boosting the transdermal delivery of diacerein: Statistical optimization, ex-vivo permeation, in-vivo skin deposition and pharmacokinetic assessment compared to oral formulation. Drug Deliv. 2018, 25, 815–826. [Google Scholar] [CrossRef]
- Zhou, T.; Zhu, L.; Xia, H.; He, J.; Liu, S.; He, S.; Wang, L.; Zhang, J. Micelle carriers based on macrogol 15 hydroxystearate for ocular delivery of terbinafine hydrochloride: In vitro characterization and in vivo permeation. Eur. J. Pharm. Sci. Off. J. Eur. Fed. Pharm. Sci. 2017, 109, 288–296. [Google Scholar] [CrossRef]



| Factors | Levels | ||
| X1: Span 60 amount (mg) | 200 | 300 | 400 |
| X2: Tween 80 amount (mg) | 50 | 100 | 150 |
| Responses (dependent variables) | Desirability Constraints | ||
| Y1: EE% | Maximize | ||
| Y2: PS (nm) | Minimize | ||
| Y3: ZP (mV) | Maximize (as absolute value) | ||
| Factors | Levels | |||
| X1: Type of cosolvent | Glycerol | Propylene glycol | ||
| X2: Percentage of cosolvent | 10 | 20 | 30 | |
| Responses (dependent variables) | Desirability Constraints | |||
| Y1: EE% | Maximize | |||
| Y2: PS (nm) | Minimize | |||
| Y3: ZP (mV) | Maximize (as absolute value) | |||
| RunsRuns | X1 | X2 | Y1 | Y2 | Y3 |
|---|---|---|---|---|---|
| Span 60 Amount (mg) | Tween 80 Amount (mg) | EE % a | PS (nm) a | ZP (mV) a | |
| BS1 | 200 | 50 | 27.85 ± 1.20 | 238 ± 1.98 | −23.75 ± 1.20 |
| BS2 | 300 | 50 | 32.05 ± 3.89 | 271.8 ± 11.03 | −28.35 ± 0.78 |
| BS3 | 400 | 50 | 51.15 ± 0.49 | 436.55 ± 9.97 | −39.45 ± 0.92 |
| BS4 | 200 | 100 | 31.05 ± 1.63 | 349.55 ± 34.44 | −23.25 ± 0.78 |
| BS5 | 300 | 100 | 49.8 ± 12.30 | 300.14 ± 0.14 | −29.75 ± 1.20 |
| BS6 | 400 | 100 | 76.8 ± 7.07 | 282.75 ± 1.34 | −28.75 ± 2.76 |
| BS7 | 200 | 150 | 30.9 ± 2.26 | 231.5 ± 10.18 | −24.85 ± 0.64 |
| BS8 | 300 | 150 | 47.3 ± 2.12 | 330.55 ± 44.48 | −31.85 ± 0.78 |
| BS9 | 400 | 150 | 85.45 ± 1.77 | 400.40 ± 6.51 | −32.95 ± 1.48 |
| Responses | R2 | Adjusted R2 | Predicted R2 | Adequate Precision | Significant Factors |
|---|---|---|---|---|---|
| EE% | 0.9676 | 0.9388 | 0.8703 | 15.990 | X1 (<0.0001), X2 (=0.0004) |
| PS (nm) | 0.9602 | 0.9249 | 0.8409 | 14.628 | X1 (<0.0001) |
| ZP (mV) | 0.9641 | 0.9321 | 0.8562 | 17.310 | X1 (<0.0001), X2(=0.0047) |
| Runs | X1 | X2 | Y1 | Y2 | Y3 |
|---|---|---|---|---|---|
| Cosolvent Type | Cosolvent Percentage | EE% a | PS (nm) a | ZP (mV) a | |
| MS1 | Glycerol | 10 | 71 ± 5.66 | 416.5 ± 33.23 | −30.9 ± 1.13 |
| MS2 | Glycerol | 20 | 37.8 ± 1.56 | 285.5 ± 11.17 | −29.75 ± 2.90 |
| MS3 | Glycerol | 30 | 72.5 ± 2.83 | 581.5 ± 2.55 | −31.9 ± 1.13 |
| MS4 | Propylene glycol | 10 | 56.73 ± 15.09 | 328.55 ± 14.21 | −31.8 ± 0.71 |
| MS5 | Propylene glycol | 20 | 57.45 ± 2.76 | 374.85 ± 32.74 | −28.7 ± 2.12 |
| MS6 | Propylene glycol | 30 | 66.1 ± 0.57 | 231.2 ± 0.141 | −32.15 ± 0.07 |
| Responses | R2 | Adjusted R2 | Predicted R2 | Adequate Precision | Significant Factors |
|---|---|---|---|---|---|
| EE% | 0.8557 | 0.7354 | 0.4226 | 7.209 | X2 (=0.0089) |
| PS (nm) | 0.9836 | 0.9699 | 0.9342 | 24.226 | X1 (<0.0001), X2 (=0.0009) |
| ZP (mV) | 0.5441 | 0.1641 | −0.8238 | 2.991 | --------- |
| Formulation | Total Amount of TOL Permeated per Unit Area in 8 h (µg/cm2) a | Jmax (µg/cm2/h) a | ER |
|---|---|---|---|
| MS6 | 22.33 ± 0.67 | 4.36 ± 0.13 | 1.26 |
| BS6 | 21.30 ± 0.07 | 4.16 ± 0.01 | 1.20 |
| Drug suspension | 17.74 ± 0.89 | 3.46 ± 0.17 | 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aziz, D.; Mohamed, S.A.; Tayel, S.; Makhlouf, A. Enhanced Ocular Anti-Aspergillus Activity of Tolnaftate Employing Novel Cosolvent-Modified Spanlastics: Formulation, Statistical Optimization, Kill Kinetics, Ex Vivo Trans-Corneal Permeation, In Vivo Histopathological and Susceptibility Study. Pharmaceutics 2022, 14, 1746. https://doi.org/10.3390/pharmaceutics14081746
Aziz D, Mohamed SA, Tayel S, Makhlouf A. Enhanced Ocular Anti-Aspergillus Activity of Tolnaftate Employing Novel Cosolvent-Modified Spanlastics: Formulation, Statistical Optimization, Kill Kinetics, Ex Vivo Trans-Corneal Permeation, In Vivo Histopathological and Susceptibility Study. Pharmaceutics. 2022; 14(8):1746. https://doi.org/10.3390/pharmaceutics14081746
Chicago/Turabian StyleAziz, Diana, Sally A. Mohamed, Saadia Tayel, and Amal Makhlouf. 2022. "Enhanced Ocular Anti-Aspergillus Activity of Tolnaftate Employing Novel Cosolvent-Modified Spanlastics: Formulation, Statistical Optimization, Kill Kinetics, Ex Vivo Trans-Corneal Permeation, In Vivo Histopathological and Susceptibility Study" Pharmaceutics 14, no. 8: 1746. https://doi.org/10.3390/pharmaceutics14081746
APA StyleAziz, D., Mohamed, S. A., Tayel, S., & Makhlouf, A. (2022). Enhanced Ocular Anti-Aspergillus Activity of Tolnaftate Employing Novel Cosolvent-Modified Spanlastics: Formulation, Statistical Optimization, Kill Kinetics, Ex Vivo Trans-Corneal Permeation, In Vivo Histopathological and Susceptibility Study. Pharmaceutics, 14(8), 1746. https://doi.org/10.3390/pharmaceutics14081746






