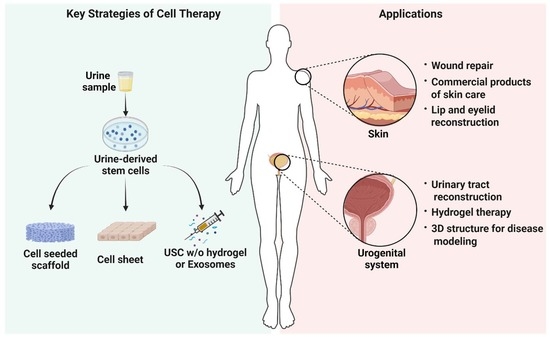
Urine-Derived Stem Cells for Epithelial Tissues Reconstruction and Wound Healing
Abstract
:1. Introduction
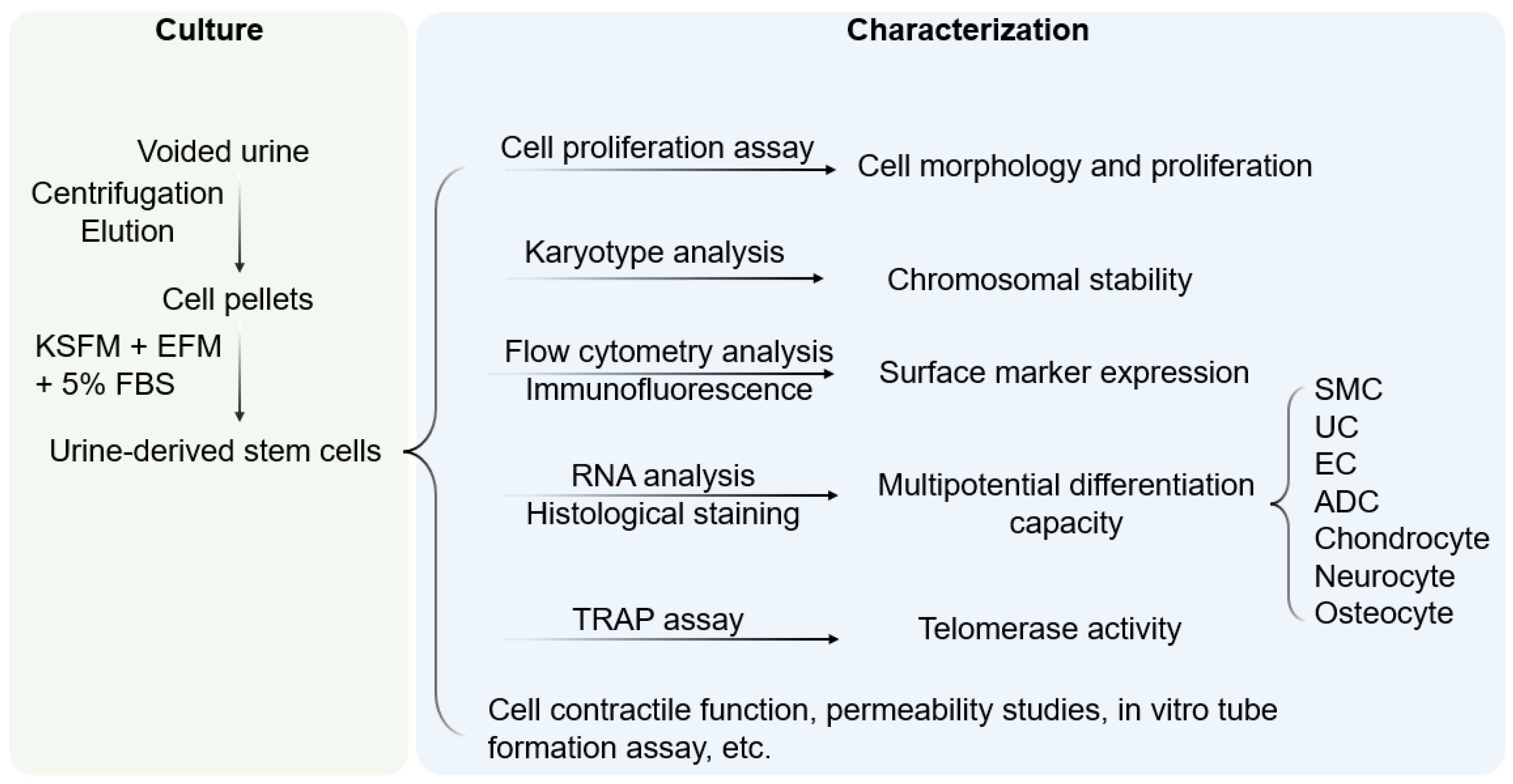
2. Characterization of Urine-Derived Stem Cells
3. Reconstruction of 3D Epithelial Tissue
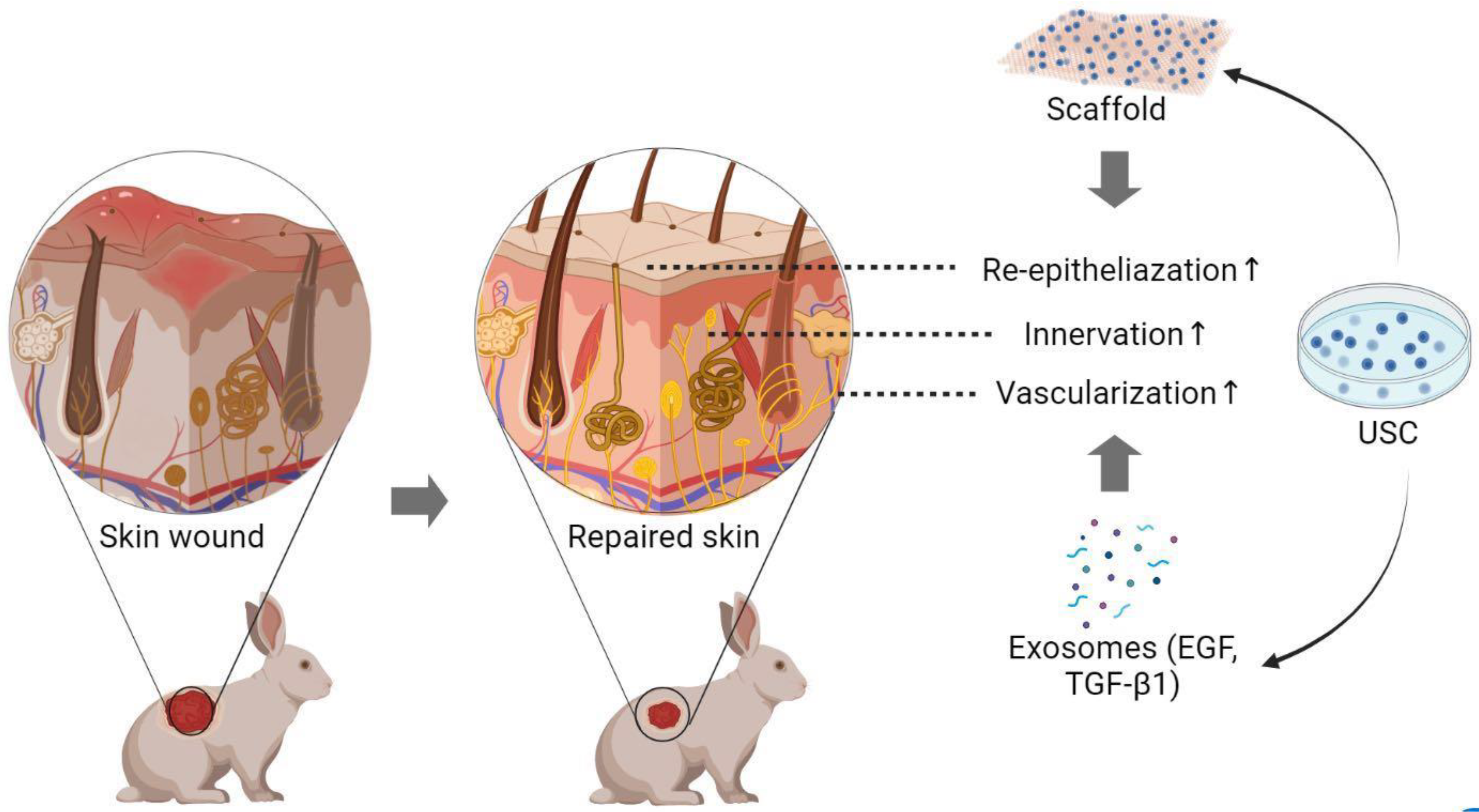
4. Methods to Enhance Skin Regeneration Using USC
4.1. Skin Regeneration Requirements
4.2. Skin Cell-Sheet
4.3. Cell-Seeded Biomatrix for Epidermal Tissue Reconstruction
| Cell Type | Wound Treatment | Outcomes |
|---|---|---|
| BMSC | ||
| Animal expt. | Diabetic/excisional wounds *, **, *** Skin burns ** Radiation burns/dermatitis *, ** | M1 macrophages ↓, M2 macrophages ↑ [75]; Re-epithelialization, granulation tissue, angiogenesis, collagen deposition ↑; inflammation ↓ [76,77,78]; MMP1 expression, pro-collagen ↑ [79] |
| Clin trials | Diabetic foot ulcers *, fibrin spray, *** Bullosis diabeticorum * | Ulcer size, healing time, complications ↓, vascularity ↑ [80,81,82]; clinical outcomes ↑, prevent lower limb amputation [83] |
| AT-MSC | ||
| Animal expt. | Diabetic wounds **, *** Excisional wounds *** Radiation burns * | Innervation, M2 macrophages, granulation tissue, angiogenesis ↑; Inflammation ↓ [8,84]; Wound closure, epidermal/dermal structure ↑ [85,86] |
| Clin trials | Ablative laser: niacinamide cream Low extremity ulcers *** | MMP-1 and MMP-2 expression ↓, type 1 collagen expression ↑ [87]; dermal angiogenesis, wound closure ↑; complete closure time ↓ [88,89] |
| UC-MSC | ||
| Animal expt. | Diabetic wounds *, *** Burn wounds * Excisional wounds ** | Wound closure, re-epithelialization, angiogenesis ↑ [90,91]; inflammation ↓ [92]; fibroblasts-myofibroblasts transition ↓ [89,90,91,92] |
| Clin trials | Diabetic foot ulcers * Cesarean section skin scars: patch Ablative laser: serum and cream Epidermolysis bullosa * | Ulcer healing ↑ [93]; no evident enhancement in skin repair [94]; post-treatment erythema ↓, recovery time ↓ [95]; M2 macrophage polarization ↑; mast cell infiltration, pain ↓ [96] |
| WJ-MSC | ||
| Animal expt. | Excisional wounds * Atopic dermatitis * | No evident enhancement [97,98] Epidermal thickness, inflammation ↓ [99] |
| Clin trials | Diabetic foot ulcers *** | Wound size, healing time ↓ [100] |
| ASC | ||
| Animal expt. | Pressure sores * Excisional wounds ** | Wound healing ↑ [101]; dermal fibroblasts migration and proliferation, wound closure ↑ [102] |
| UCB-MSC | ||
| Animal expt. | Diabetic wounds *, *** Excisional wounds * | Wound healing, angiogenesis ↑ [103,104,105] |
| USC | ||
| Animal expt. | Excisional wounds *, *** Diabetic wounds ** | Wound closure, re-epithelialization, angiogenesis, collagen deposition ↑ [37,49,50,52] |
5. The Role of USC in Urothelial Mucosa Repair
5.1. Urothelial Regeneration Requirements
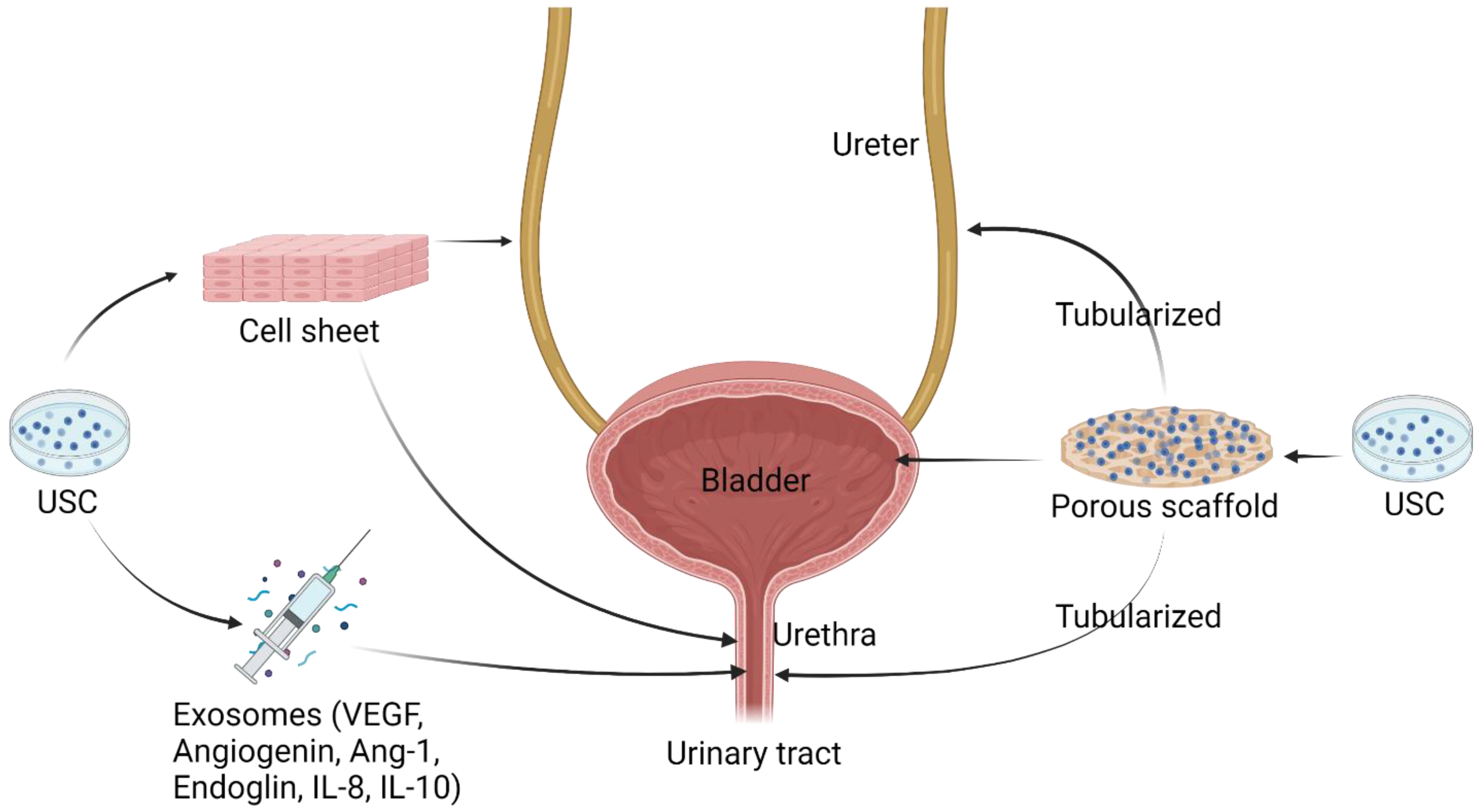
5.2. Implantation of USC with Scaffolds for Urogenital Repair
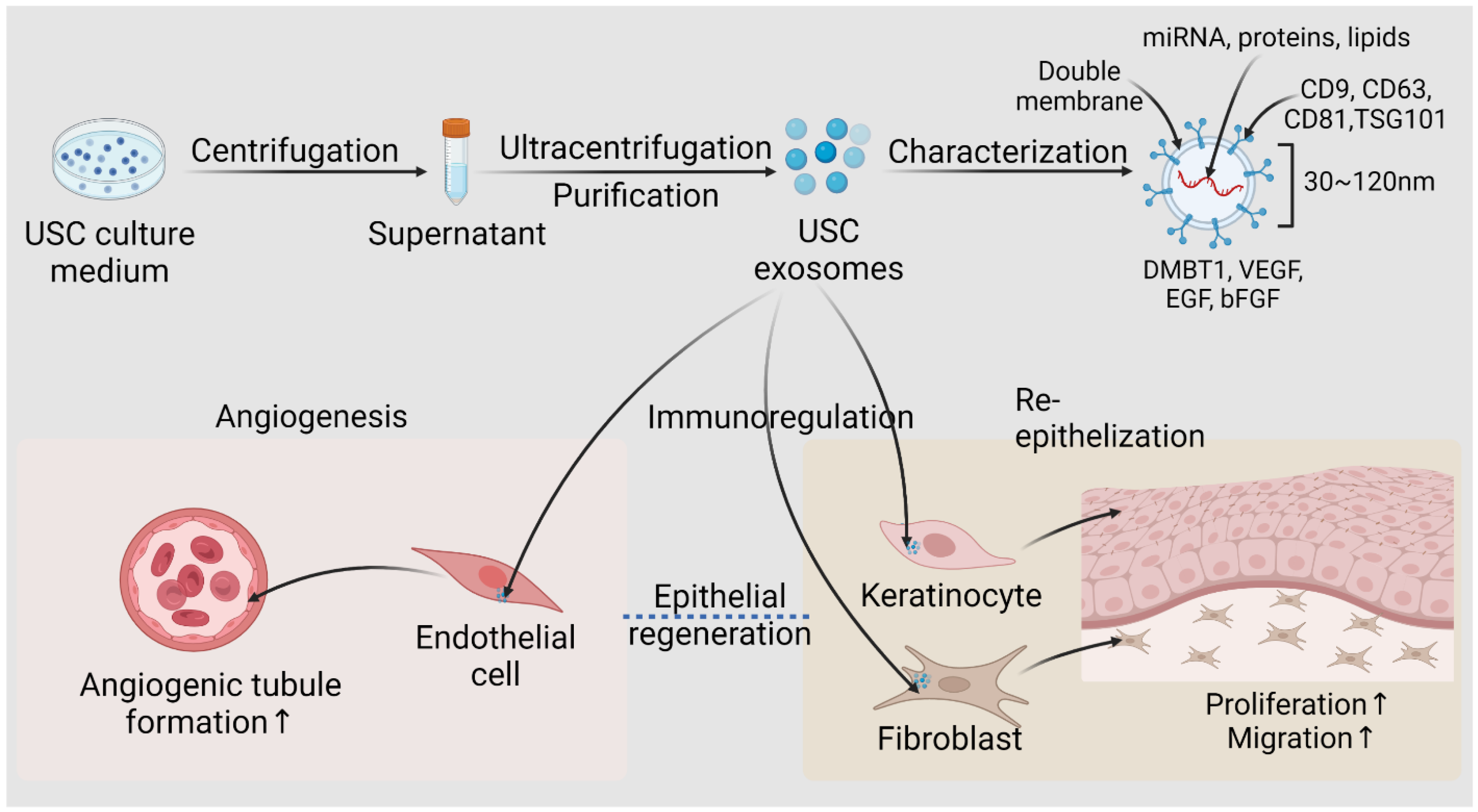
6. Applications of USC-Derived Exosomes
7. Conclusions and Future Directions
- -
- Transplantation of USC cell sheets or USC-seeded scaffolds has potential in the treatment of genitourinary defects or wound repair for patients with a diabetic ulcer or burn injury. Their exosomes can be extracted and manufactured as commercial products for skin wound healing. More studies on optimization of USC therapy are needed before clinical trials start using patients’ USC for skin repair and wound healing are needed in the near future.
- -
- Autologous USC is an optimal stem cell source for the treatment of other urological disorders, such as intrinsic urethral sphincter deficiency in elder women with stress urinary inconstancy, erectile dysfunction, and renal insufficiency. USC-hydrogel will be an easily injectable, variable, degradable material with high biocompatibility and biosafety for cell therapy.
- -
- -
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Torras, N.; Garcia-Diaz, M.; Fernandez-Majada, V.; Martinez, E. Mimicking Epithelial Tissues in Three-Dimensional Cell Culture Models. Front. Bioeng. Biotechnol. 2018, 6, 197. [Google Scholar] [CrossRef]
- Mescher, A. Junqueira’s Basic Histology Text and Atlas, 15th ed.; McGraw-Hill Education: New York, NY, USA, 2018. [Google Scholar]
- Han, G.; Ceilley, R. Chronic wound healing: A review of current management and treatments. Adv. Ther. 2017, 34, 599–610. [Google Scholar] [CrossRef]
- Chua, A.W.C.; Khoo, Y.C.; Tan, B.K.; Tan, K.C.; Foo, C.L.; Chong, S.J. Skin tissue engineering advances in severe burns: Review and therapeutic applications. Burn. Trauma 2016, 4, 3. [Google Scholar] [CrossRef]
- Vaegler, M.; Maurer, S.; Toomey, P.; Amend, B.; Sievert, K.D. Tissue engineering in urothelium regeneration. Adv. Drug Deliv. Rev. 2015, 82–83, 64–68. [Google Scholar] [CrossRef] [PubMed]
- Pederzoli, F.; Joice, G.; Salonia, A.; Bivalacqua, T.J.; Sopko, N.A. Regenerative and engineered options for urethroplasty. Nat. Rev. Urol. 2019, 16, 453–464. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Lin, H.K.; Frimberger, D.; Epstein, R.B.; Kropp, B.P. Growth of bone marrow stromal cells on small intestinal submucosa: An alternative cell source for tissue engineered bladder. BJU Int. 2005, 96, 1120–1125. [Google Scholar] [CrossRef] [PubMed]
- Kato, Y.; Iwata, T.; Morikawa, S.; Yamato, M.; Okano, T.; Uchigata, Y. Allogeneic Transplantation of an Adipose-Derived Stem Cell Sheet Combined With Artificial Skin Accelerates Wound Healing in a Rat Wound Model of Type 2 Diabetes and Obesity. Diabetes 2015, 64, 2723–2734. [Google Scholar] [CrossRef]
- Zhang, Y.; McNeill, E.; Tian, H.; Soker, S.; Andersson, K.E.; Yoo, J.J.; Atala, A. Urine derived cells are a potential source for urological tissue reconstruction. J. Urol. 2008, 180, 2226–2233. [Google Scholar] [CrossRef]
- Wu, S.; Liu, Y.; Bharadwaj, S.; Atala, A.; Zhang, Y. Human urine-derived stem cells seeded in a modified 3D porous small intestinal submucosa scaffold for urethral tissue engineering. Biomaterials 2011, 32, 1317–1326. [Google Scholar] [CrossRef]
- Yang, H.; Chen, B.; Deng, J.; Zhuang, G.; Wu, S.; Liu, G.; Deng, C.; Yang, G.; Qiu, X.; Wei, P.; et al. Characterization of rabbit urine-derived stem cells for potential application in lower urinary tract tissue regeneration. Cell Tissue Res. 2018, 374, 303–315. [Google Scholar] [CrossRef]
- Liu, Y.; Ma, W.; Liu, B.; Wang, Y.; Chu, J.; Xiong, G.; Shen, L.; Long, C.; Lin, T.; He, D.; et al. Urethral reconstruction with autologous urine-derived stem cells seeded in three-dimensional porous small intestinal submucosa in a rabbit model. Stem Cell Res. Ther. 2017, 8, 63. [Google Scholar] [CrossRef]
- Bharadwaj, S.; Liu, G.; Shi, Y.; Wu, R.; Yang, B.; He, T.; Fan, Y.; Lu, X.; Zhou, X.; Liu, H.; et al. Multipotential differentiation of human urine-derived stem cells: Potential for therapeutic applications in urology. Stem Cells 2013, 31, 1840–1856. [Google Scholar] [CrossRef]
- Bharadwaj, S.; Liu, G.; Shi, Y.; Markert, C.; Andersson, K.E.; Atala, A.; Zhang, Y. Characterization of urine-derived stem cells obtained from upper urinary tract for use in cell-based urological tissue engineering. Tissue Eng. Part A 2011, 17, 2123–2132. [Google Scholar] [CrossRef]
- Lang, R.; Liu, G.; Shi, Y.; Bharadwaj, S.; Leng, X.; Zhou, X.; Liu, H.; Atala, A.; Zhang, Y. Self-renewal and differentiation capacity of urine-derived stem cells after urine preservation for 24 hours. PLoS ONE 2013, 8, e53980. [Google Scholar] [CrossRef]
- Bodin, A.; Bharadwaj, S.; Wu, S.; Gatenholm, P.; Atala, A.; Zhang, Y. Tissue-engineered conduit using urine-derived stem cells seeded bacterial cellulose polymer in urinary reconstruction and diversion. Biomaterials 2010, 31, 8889–8901. [Google Scholar] [CrossRef]
- Chun, S.Y.; Kim, H.T.; Lee, J.S.; Kim, M.J.; Kim, B.S.; Kim, B.W.; Kwon, T.G. Characterization of urine-derived cells from upper urinary tract in patients with bladder cancer. Urology 2012, 79, 1186.e1–1186.e7. [Google Scholar] [CrossRef]
- Benda, C.; Zhou, T.; Wang, X.; Tian, W.; Grillari, J.; Tse, H.F.; Grillari-Voglauer, R.; Pei, D.; Esteban, M.A. Urine as a source of stem cells. Adv. Biochem. Eng./Biotechnol. 2013, 129, 19–32. [Google Scholar] [CrossRef]
- Guan, J.J.; Niu, X.; Gong, F.X.; Hu, B.; Guo, S.C.; Lou, Y.L.; Zhang, C.Q.; Deng, Z.F.; Wang, Y. Biological characteristics of human-urine-derived stem cells: Potential for cell-based therapy in neurology. Tissue Eng. Part A 2014, 20, 1794–1806. [Google Scholar] [CrossRef]
- He, W.; Zhu, W.; Cao, Q.; Shen, Y.; Zhou, Q.; Yu, P.; Liu, X.; Ma, J.; Li, Y.; Hong, K. Generation of Mesenchymal-Like Stem Cells From Urine in Pediatric Patients. Transpl. Proc. 2016, 48, 2181–2185. [Google Scholar] [CrossRef]
- Self, M.; Lagutin, O.V.; Bowling, B.; Hendrix, J.; Cai, Y.; Dressler, G.R.; Oliver, G. Six2 is required for suppression of nephrogenesis and progenitor renewal in the developing kidney. Embo J. 2006, 25, 5214–5228. [Google Scholar] [CrossRef]
- Wu, R.; Liu, G.; Fan, Y.; Rohozinski, J.; Lu, X.; Rodriguez, G.; Farney, A.; Atala, A.; Zhang, Y. 249 Human urine-derived stem cells originate from parietal stem cells. J. Urol. 2013, 189, e103. [Google Scholar] [CrossRef]
- Qin, D.; Long, T.; Deng, J.; Zhang, Y. Urine-derived stem cells for potential use in bladder repair. Stem Cell Res. Ther. 2014, 5, 69. [Google Scholar] [CrossRef]
- Shi, Y.; Liu, G.; Shantaram, B.; Atala, A.; Zhang, Y. 736 Urine Derived Stem Cells with High TelomeRase Activity for Cell Based Therapy in Urology. J. Urol. 2012, 187, e302. [Google Scholar] [CrossRef]
- Wu, S.; Wang, Z.; Bharadwaj, S.; Hodges, S.J.; Atala, A.; Zhang, Y. Implantation of autologous urine derived stem cells expressing vascular endothelial growth factor for potential use in genitourinary reconstruction. J. Urol. 2011, 186, 640–647. [Google Scholar] [CrossRef]
- Lee, J.N.; Chun, S.Y.; Lee, H.J.; Jang, Y.J.; Choi, S.H.; Kim, D.H.; Oh, S.H.; Song, P.H.; Lee, J.H.; Kim, J.K.; et al. Human Urine-derived Stem Cells Seeded Surface Modified Composite Scaffold Grafts for Bladder Reconstruction in a Rat Model. J. Korean Med. Sci. 2015, 30, 1754–1763. [Google Scholar] [CrossRef] [PubMed]
- Lazzeri, E.; Ronconi, E.; Angelotti, M.L.; Peired, A.; Mazzinghi, B.; Becherucci, F.; Conti, S.; Sansavini, G.; Sisti, A.; Ravaglia, F.; et al. Human Urine-Derived Renal Progenitors for Personalized Modeling of Genetic Kidney Disorders. J. Am. Soc. Nephrol. JASN 2015, 26, 1961–1974. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.Z.; Liu, Y.M.; Niu, X.; Yin, J.Y.; Hu, B.; Guo, S.C.; Fan, Y.; Wang, Y.; Wang, N.S. Exosomes secreted by human urine-derived stem cells could prevent kidney complications from type I diabetes in rats. Stem Cell Res. Ther. 2016, 7, 24. [Google Scholar] [CrossRef] [PubMed]
- Oliveira Arcolino, F.; Tort Piella, A.; Papadimitriou, E.; Bussolati, B.; Antonie, D.J.; Murray, P.; van den Heuvel, L.; Levtchenko, E. Human Urine as a Noninvasive Source of Kidney Cells. Stem Cells Int. 2015, 2015, 362562. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, B.; Sun, X.; Han, D.; Chen, S.; Yao, B.; Gao, Y.; Bian, J.; Huang, Y.; Zhang, Y.; Wan, Z.; et al. Human urine-derived stem cells alone or genetically-modified with FGF2 Improve type 2 diabetic erectile dysfunction in a rat model. PLoS ONE 2014, 9, e92825. [Google Scholar] [CrossRef]
- Liu, G.; Pareta, R.A.; Wu, R.; Shi, Y.; Zhou, X.; Liu, H.; Deng, C.; Sun, X.; Atala, A.; Opara, E.C.; et al. Skeletal myogenic differentiation of urine-derived stem cells and angiogenesis using microbeads loaded with growth factors. Biomaterials 2013, 34, 1311–1326. [Google Scholar] [CrossRef]
- Guan, J.; Zhang, J.; Guo, S.; Zhu, H.; Zhu, Z.; Li, H.; Wang, Y.; Zhang, C.; Chang, J. Human urine-derived stem cells can be induced into osteogenic lineage by silicate bioceramics via activation of the Wnt/β-catenin signaling pathway. Biomaterials 2015, 55, 1–11. [Google Scholar] [CrossRef]
- Guan, J.; Zhang, J.; Li, H.; Zhu, Z.; Guo, S.; Niu, X.; Wang, Y.; Zhang, C. Human Urine Derived Stem Cells in Combination with β-TCP Can Be Applied for Bone Regeneration. PLoS ONE 2015, 10, e0125253. [Google Scholar] [CrossRef]
- Guan, J.; Zhang, J.; Zhu, Z.; Niu, X.; Guo, S.; Wang, Y.; Zhang, C. Bone morphogenetic protein 2 gene transduction enhances the osteogenic potential of human urine-derived stem cells. Stem Cell Res. Ther. 2015, 6, 5. [Google Scholar] [CrossRef]
- Qin, H.; Zhu, C.; An, Z.; Jiang, Y.; Zhao, Y.; Wang, J.; Liu, X.; Hui, B.; Zhang, X.; Wang, Y. Silver nanoparticles promote osteogenic differentiation of human urine-derived stem cells at noncytotoxic concentrations. Int. J. Nanomed. 2014, 9, 2469–2478. [Google Scholar] [CrossRef]
- Wang, C.; Hei, F.; Ju, Z.; Yu, J.; Yang, S.; Chen, M. Differentiation of Urine-Derived Human Induced Pluripotent Stem Cells to Alveolar Type II Epithelial Cells. Cell. Reprogram. 2016, 18, 30–36. [Google Scholar] [CrossRef]
- Fu, Y.; Guan, J.; Guo, S.; Guo, F.; Niu, X.; Liu, Q.; Zhang, C.; Nie, H.; Wang, Y. Human urine-derived stem cells in combination with polycaprolactone/gelatin nanofibrous membranes enhance wound healing by promoting angiogenesis. J. Transl. Med. 2014, 12, 274. [Google Scholar] [CrossRef]
- Pei, M.; Li, J.; Zhang, Y.; Liu, G.; Wei, L.; Zhang, Y. Expansion on a matrix deposited by nonchondrogenic urine stem cells strengthens the chondrogenic capacity of repeated-passage bone marrow stromal cells. Cell Tissue Res. 2014, 356, 391–403. [Google Scholar] [CrossRef]
- Zhou, T.; Benda, C.; Dunzinger, S.; Huang, Y.; Ho, J.C.; Yang, J.; Wang, Y.; Zhang, Y.; Zhuang, Q.; Li, Y.; et al. Generation of human induced pluripotent stem cells from urine samples. Nat. Protoc. 2012, 7, 2080–2089. [Google Scholar] [CrossRef]
- Xue, Y.; Cai, X.; Wang, L.; Liao, B.; Zhang, H.; Shan, Y.; Chen, Q.; Zhou, T.; Li, X.; Hou, J.; et al. Generating a non-integrating human induced pluripotent stem cell bank from urine-derived cells. PLoS ONE 2013, 8, e70573. [Google Scholar] [CrossRef]
- Jia, B.; Chen, S.; Zhao, Z.; Liu, P.; Cai, J.; Qin, D.; Du, J.; Wu, C.; Chen, Q.; Cai, X.; et al. Modeling of hemophilia A using patient-specific induced pluripotent stem cells derived from urine cells. Life Sci. 2014, 108, 22–29. [Google Scholar] [CrossRef]
- Chen, Y.; Luo, R.; Xu, Y.; Cai, X.; Li, W.; Tan, K.; Huang, J.; Dai, Y. Generation of systemic lupus erythematosus-specific induced pluripotent stem cells from urine. Rheumatol. Int. 2013, 33, 2127–2134. [Google Scholar] [CrossRef]
- Zhou, T.; Benda, C.; Duzinger, S.; Huang, Y.; Li, X.; Li, Y.; Guo, X.; Cao, G.; Chen, S.; Hao, L. Generation of induced pluripotent stem cells from urine. J. Am. Soc. Nephrol. 2011, 22, 1221–1228. [Google Scholar] [CrossRef]
- Zhou, J.; Wang, X.; Zhang, S.; Gu, Y.; Yu, L.; Wu, J.; Gao, T.; Chen, F. Generation and characterization of human cryptorchid-specific induced pluripotent stem cells from urine. Stem Cells Dev. 2013, 22, 717–725. [Google Scholar] [CrossRef]
- Afzal, M.Z.; Strande, J.L. Generation of induced pluripotent stem cells from muscular dystrophy patients: Efficient integration-free reprogramming of urine derived cells. J. Vis. Exp. JoVE 2015, 95, 52032. [Google Scholar] [CrossRef]
- Kang, H.S.; Choi, S.H.; Kim, B.S.; Choi, J.Y.; Park, G.B.; Kwon, T.G.; Chun, S.Y. Advanced Properties of Urine Derived Stem Cells Compared to Adipose Tissue Derived Stem Cells in Terms of Cell Proliferation, Immune Modulation and Multi Differentiation. J. Korean Med. Sci. 2015, 30, 1764–1776. [Google Scholar] [CrossRef]
- Nersesyan, A.; Kundi, M.; Fenech, M.; Bolognesi, C.; Misik, M.; Wultsch, G.; Hartmann, M.; Knasmueller, S. Micronucleus assay with urine derived cells (UDC): A review of its application in human studies investigating genotoxin exposure and bladder cancer risk. Mutat. Res. Rev. Mutat. Res. 2014, 762, 37–51. [Google Scholar] [CrossRef]
- Zhang, S.Z.; Li, H.F.; Ma, L.X.; Qian, W.J.; Wang, Z.F.; Wu, Z.Y. Urine-derived induced pluripotent stem cells as a modeling tool for paroxysmal kinesigenic dyskinesia. Biol. Open 2015, 4, 1744–1752. [Google Scholar] [CrossRef]
- Chen, C.Y.; Rao, S.S.; Ren, L.; Hu, X.K.; Tan, Y.J.; Hu, Y.; Luo, J.; Liu, Y.W.; Yin, H.; Huang, J.; et al. Exosomal DMBT1 from human urine-derived stem cells facilitates diabetic wound repair by promoting angiogenesis. Theranostics 2018, 8, 1607–1623. [Google Scholar] [CrossRef]
- Cao, Y.M.; Liu, M.Y.; Xue, Z.W.; Qiu, Y.; Li, J.; Wang, Y.; Wu, Q.K. Surface-structured bacterial cellulose loaded with hUSCs accelerate skin wound healing by promoting angiogenesis in rats. Biochem. Biophys. Res. Commun. 2019, 516, 1167–1174. [Google Scholar] [CrossRef]
- Wu, R.; Soland, M.; Liu, G.; Shi, Y.; Bharadwaj, S.; Atala, A.; Almeida-Porada, G.; Zhang, Y. Immunomodulatory properties of urine derived stem cells. In Proceedings of the 3rd Annual Regenerative Medicine Foundation Conference, Charlotte, NC, USA, 18–19 October 2012; pp. 18–19. [Google Scholar]
- Zhang, X.R.; Huang, Y.Z.; Gao, H.W.; Jiang, Y.L.; Hu, J.G.; Pi, J.K.; Chen, A.J.; Zhang, Y.; Zhou, L.; Xie, H.Q. Hypoxic preconditioning of human urine-derived stem cell-laden small intestinal submucosa enhances wound healing potential. Stem Cell Res. Ther. 2020, 11, 150. [Google Scholar] [CrossRef]
- Hu, C.; He, Y.; Fang, S.; Tian, N.; Gong, M.; Xu, X.; Zhao, L.; Wang, Y.; He, T.; Zhang, Y. Urine-derived stem cells accelerate the recovery of injured mouse hepatic tissue. Am. J. Transl. Res. 2020, 12, 5131. [Google Scholar] [PubMed]
- Liu, G.; Wang, X.; Sun, X.; Deng, C.; Atala, A.; Zhang, Y. The effect of urine-derived stem cells expressing VEGF loaded in collagen hydrogels on myogenesis and innervation following after subcutaneous implantation in nude mice. Biomaterials 2013, 34, 8617–8629. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Chen, L.; Huang, Y.Z.; Huang, Y.; Parolini, O.; Zhong, Q.; Tian, X.; Deng, L. Comparison of the Proliferation and Differentiation Potential of Human Urine-, Placenta Decidua Basalis-, and Bone Marrow-Derived Stem Cells. Stem Cells Int. 2018, 2018, 7131532. [Google Scholar] [CrossRef] [PubMed]
- Wan, Q.; Xiong, G.; Liu, G.; Shupe, T.D.; Wei, G.; Zhang, D.; Liang, D.; Lu, X.; Atala, A.; Zhang, Y. Urothelium with barrier function differentiated from human urine-derived stem cells for potential use in urinary tract reconstruction. Stem Cell Res. Ther. 2018, 9, 304. [Google Scholar] [CrossRef]
- Shi, Y.; Liu, G.; Wu, R.; Mack, D.L.; Sun, X.S.; Maxwell, J.; Guan, X.; Atala, A.; Zhang, Y. Differentiation Capacity of Human Urine-Derived Stem Cells to Retain Telomerase Activity. Front. Cell Dev. Biol. 2022, 10, 890574. [Google Scholar] [CrossRef]
- Zhang, D.X.; Xing, G.; Tao, L.; Gong, M.; Ma, W.; Chu, J.; He, D.; Wei, G.; Zhang, Y. Autologous Urine-derived Stem Cells for Kidney Tissue Repair. Ann. Nephrol. 2018, 3, 28–30. [Google Scholar]
- Dalghi, M.G.; Montalbetti, N.; Carattino, M.D.; Apodaca, G. The Urothelium: Life in a Liquid Environment. Physiol. Rev. 2020, 100, 1621–1705. [Google Scholar] [CrossRef]
- Lee, J.; Rabbani, C.C.; Gao, H.; Steinhart, M.R.; Woodruff, B.M.; Pflum, Z.E.; Kim, A.; Heller, S.; Liu, Y.; Shipchandler, T.Z.; et al. Hair-bearing human skin generated entirely from pluripotent stem cells. Nature 2020, 582, 399–404. [Google Scholar] [CrossRef]
- Sen, C.K. Human Wound and Its Burden: Updated 2020 Compendium of Estimates. Adv. Wound Care 2021, 10, 281–292. [Google Scholar] [CrossRef]
- Ronfard, V.; Rives, J.-M.; Neveux, Y.; Carsin, H.; Barrandon, Y. Long-term regeneration of human epidermis on third degree burns transplanted with autologous cultured epithelium grown on a fibrin matrix1, 2. Transplantation 2000, 70, 1588–1598. [Google Scholar] [CrossRef]
- Oualla-Bachiri, W.; Fernández-González, A.; Quiñones-Vico, M.I.; Arias-Santiago, S. From Grafts to Human Bioengineered Vascularized Skin Substitutes. Int. J. Mol. Sci. 2020, 21, 8197. [Google Scholar] [CrossRef]
- Cerqueira, M.T.; Pirraco, R.P.; Martins, A.R.; Santos, T.C.; Reis, R.L.; Marques, A.P. Cell sheet technology-driven re-epithelialization and neovascularization of skin wounds. Acta Biomater. 2014, 10, 3145–3155. [Google Scholar] [CrossRef]
- Zhang, Y.; Atala, A. Urothelial cell culture. Methods Mol. Biol. 2013, 1037, 27–43. [Google Scholar] [CrossRef]
- Zhang, Y.; Atala, A. Urothelial cell culture: Stratified urothelial sheet and three-dimensional growth of urothelial structure. Methods Mol. Biol. 2013, 945, 383–399. [Google Scholar] [CrossRef]
- Chen, Y.; Xu, Y.; Li, M.; Shi, Q.; Chen, C. Application of Autogenous Urine-Derived Stem Cell Sheet Enhances Rotator Cuff Healing in a Canine Model. Am. J. Sports Med. 2020, 48, 3454–3466. [Google Scholar] [CrossRef]
- Yang, X.; Xiong, X.; Zhou, W.; Feng, G.; Zhang, Y.; Dai, H.; Zhou, J. Effects of human urine-derived stem cells on the cementogenic differentiation of indirectly-cocultured periodontal ligament stem cells. Am. J. Transl. Res. 2020, 12, 361–378. [Google Scholar]
- Konop, M.; Czuwara, J.; Kłodzińska, E.; Laskowska, A.K.; Sulejczak, D.; Damps, T.; Zielenkiewicz, U.; Brzozowska, I.; Sureda, A.; Kowalkowski, T.; et al. Evaluation of keratin biomaterial containing silver nanoparticles as a potential wound dressing in full-thickness skin wound model in diabetic mice. J. Tissue Eng. Regen. Med. 2020, 14, 334–346. [Google Scholar] [CrossRef]
- Konop, M.; Rybka, M.; Drapała, A. Keratin Biomaterials in Skin Wound Healing, an Old Player in Modern Medicine: A Mini Review. Pharmaceutics 2021, 13, 2029. [Google Scholar] [CrossRef]
- Konop, M.; Laskowska, A.K.; Rybka, M.; Kłodzińska, E.; Sulejczak, D.; Schwartz, R.A.; Czuwara, J. Keratin Scaffolds Containing Casomorphin Stimulate Macrophage Infiltration and Accelerate Full-Thickness Cutaneous Wound Healing in Diabetic Mice. Molecules 2021, 26, 2554. [Google Scholar] [CrossRef]
- Blais, M.; Parenteau-Bareil, R.; Cadau, S.; Berthod, F. Concise review: Tissue-engineered skin and nerve regeneration in burn treatment. Stem Cells Transl. Med. 2013, 2, 545–551. [Google Scholar] [CrossRef]
- Liu, Y.; Zheng, Y.; Li, S.; Xue, H.; Schmitt, K.; Hergenroeder, G.W.; Wu, J.; Zhang, Y.; Kim, D.H.; Cao, Q. Human neural progenitors derived from integration-free iPSCs for SCI therapy. Stem Cell Res. 2017, 19, 55–64. [Google Scholar] [CrossRef]
- Shi, T.; Cheung, M. Urine-derived induced pluripotent/neural stem cells for modeling neurological diseases. Cell Biosci. 2021, 11, 85. [Google Scholar] [CrossRef]
- Bai, H.; Kyu-Cheol, N.; Wang, Z.; Cui, Y.; Liu, H.; Liu, H.; Feng, Y.; Zhao, Y.; Lin, Q.; Li, Z. Regulation of inflammatory microenvironment using a self-healing hydrogel loaded with BM-MSCs for advanced wound healing in rat diabetic foot ulcers. J. Tissue Eng. 2020, 11, 2041731420947242. [Google Scholar] [CrossRef]
- Wu, Y.; Chen, L.; Scott, P.G.; Tredget, E.E. Mesenchymal stem cells enhance wound healing through differentiation and angiogenesis. Stem Cells 2007, 25, 2648–2659. [Google Scholar] [CrossRef]
- De Mayo, T.; Conget, P.; Becerra-Bayona, S.; Sossa, C.L.; Galvis, V.; Arango-Rodríguez, M.L. The role of bone marrow mesenchymal stromal cell derivatives in skin wound healing in diabetic mice. PLoS ONE 2017, 12, e0177533. [Google Scholar] [CrossRef]
- Zhang, B.; Wang, M.; Gong, A.; Zhang, X.; Wu, X.; Zhu, Y.; Shi, H.; Wu, L.; Zhu, W.; Qian, H.; et al. HucMSC-Exosome Mediated-Wnt4 Signaling Is Required for Cutaneous Wound Healing. Stem Cells 2015, 33, 2158–2168. [Google Scholar] [CrossRef]
- Kwon, T.R.; Oh, C.T.; Choi, E.J.; Kim, S.R.; Jang, Y.J.; Ko, E.J.; Yoo, K.H.; Kim, B.J. Conditioned medium from human bone marrow-derived mesenchymal stem cells promotes skin moisturization and effacement of wrinkles in UVB-irradiated SKH-1 hairless mice. Photodermatol. Photoimmunol. Photomed. 2016, 32, 120–128. [Google Scholar] [CrossRef]
- Portas, M.; Mansilla, E.; Drago, H.; Dubner, D.; Radl, A.; Coppola, A.; Di Giorgio, M. Use of Human Cadaveric Mesenchymal Stem Cells for Cell Therapy of a Chronic Radiation-Induced Skin Lesion: A Case Report. Radiat. Prot. Dosim. 2016, 171, 99–106. [Google Scholar] [CrossRef]
- Falanga, V.; Iwamoto, S.; Chartier, M.; Yufit, T.; Butmarc, J.; Kouttab, N.; Shrayer, D.; Carson, P. Autologous bone marrow-derived cultured mesenchymal stem cells delivered in a fibrin spray accelerate healing in murine and human cutaneous wounds. Tissue Eng. 2007, 13, 1299–1312. [Google Scholar] [CrossRef]
- Vojtassák, J.; Danisovic, L.; Kubes, M.; Bakos, D.; Jarábek, L.; Ulicná, M.; Blasko, M. Autologous biograft and mesenchymal stem cells in treatment of the diabetic foot. Neuro Endocrinol. Lett. 2006, 27 (Suppl. 2), 134–137. [Google Scholar]
- Chen, Y.; Ma, Y.; Li, N.; Wang, H.; Chen, B.; Liang, Z.; Ren, R.; Lu, D.; Boey, J.; Armstrong, D.G.; et al. Efficacy and long-term longitudinal follow-up of bone marrow mesenchymal cell transplantation therapy in a diabetic patient with recurrent lower limb bullosis diabeticorum. Stem Cell Res. Ther. 2018, 9, 99. [Google Scholar] [CrossRef] [PubMed]
- De Gregorio, C.; Contador, D.; Díaz, D.; Cárcamo, C.; Santapau, D.; Lobos-Gonzalez, L.; Acosta, C.; Campero, M.; Carpio, D.; Gabriele, C.; et al. Human adipose-derived mesenchymal stem cell-conditioned medium ameliorates polyneuropathy and foot ulceration in diabetic BKS db/db mice. Stem Cell Res. Ther. 2020, 11, 168. [Google Scholar] [CrossRef] [PubMed]
- Strong, A.L.; Bowles, A.C.; MacCrimmon, C.P.; Frazier, T.P.; Lee, S.J.; Wu, X.; Katz, A.J.; Gawronska-Kozak, B.; Bunnell, B.A.; Gimble, J.M. Adipose stromal cells repair pressure ulcers in both young and elderly mice: Potential role of adipogenesis in skin repair. Stem Cells Transl. Med. 2015, 4, 632–642. [Google Scholar] [CrossRef] [PubMed]
- Riccobono, D.; Agay, D.; Scherthan, H.; Forcheron, F.; Vivier, M.; Ballester, B.; Meineke, V.; Drouet, M. Application of adipocyte-derived stem cells in treatment of cutaneous radiation syndrome. Health Phys. 2012, 103, 120–126. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.I.; Kim, S.; Kim, J.; Kim, J.; Chung, K.B.; Lee, J.H. Randomized controlled study for the anti-aging effect of human adipocyte-derived mesenchymal stem cell media combined with niacinamide after laser therapy. J. Cosmet Derm. 2021, 20, 1774–1781. [Google Scholar] [CrossRef] [PubMed]
- Moon, K.C.; Suh, H.S.; Kim, K.B.; Han, S.K.; Young, K.W.; Lee, J.W.; Kim, M.H. Potential of Allogeneic Adipose-Derived Stem Cell-Hydrogel Complex for Treating Diabetic Foot Ulcers. Diabetes 2019, 68, 837–846. [Google Scholar] [CrossRef]
- Lafosse, A.; Desmet, C.; Aouassar, N.; André, W.; Hanet, M.S.; Beauloye, C.; Vanwijck, R.; Poirel, H.A.; Gallez, B.; Dufrane, D. Autologous Adipose Stromal Cells Seeded onto a Human Collagen Matrix for Dermal Regeneration in Chronic Wounds: Clinical Proof of Concept. Plast Reconstr. Surg. 2015, 136, 279–295. [Google Scholar] [CrossRef]
- Zhao, Q.S.; Xia, N.; Zhao, N.; Li, M.; Bi, C.L.; Zhu, Q.; Qiao, G.F.; Cheng, Z.F. Localization of human mesenchymal stem cells from umbilical cord blood and their role in repair of diabetic foot ulcers in rats. Int. J. Biol. Sci. 2013, 10, 80–89. [Google Scholar] [CrossRef]
- Shrestha, C.; Zhao, L.; Chen, K.; He, H.; Mo, Z. Enhanced healing of diabetic wounds by subcutaneous administration of human umbilical cord derived stem cells and their conditioned media. Int. J. Endocrinol. 2013, 2013, 592454. [Google Scholar] [CrossRef]
- Liu, L.; Yu, Y.; Hou, Y.; Chai, J.; Duan, H.; Chu, W.; Zhang, H.; Hu, Q.; Du, J. Human umbilical cord mesenchymal stem cells transplantation promotes cutaneous wound healing of severe burned rats. PLoS ONE 2014, 9, e88348. [Google Scholar] [CrossRef]
- Qin, H.L.; Zhu, X.H.; Zhang, B.; Zhou, L.; Wang, W.Y. Clinical Evaluation of Human Umbilical Cord Mesenchymal Stem Cell Transplantation After Angioplasty for Diabetic Foot. Exp. Clin. Endocrinol. Diabetes 2016, 124, 497–503. [Google Scholar] [CrossRef]
- Fan, D.; Zeng, M.; Xia, Q.; Wu, S.; Ye, S.; Rao, J.; Lin, D.; Zhang, H.; Ma, H.; Han, Z.; et al. Efficacy and safety of umbilical cord mesenchymal stem cells in treatment of cesarean section skin scars: A randomized clinical trial. Stem Cell Res. Ther. 2020, 11, 244. [Google Scholar] [CrossRef]
- Kim, J.; Kim, B.; Kim, S.; Lee, Y.I.; Kim, J.; Lee, J.H. The effect of human umbilical cord blood-derived mesenchymal stem cell media containing serum on recovery after laser treatment: A double-blinded, randomized, split-face controlled study. J. Cosmet Derm. 2020, 19, 651–656. [Google Scholar] [CrossRef]
- Lee, S.E.; Lee, S.J.; Kim, S.E.; Kim, K.; Cho, B.; Roh, K.; Kim, S.C. Intravenous allogeneic umbilical cord blood-derived mesenchymal stem cell therapy in recessive dystrophic epidermolysis bullosa patients. JCI Insight 2021, 6, e143606. [Google Scholar] [CrossRef]
- Arno, A.I.; Amini-Nik, S.; Blit, P.H.; Al-Shehab, M.; Belo, C.; Herer, E.; Tien, C.H.; Jeschke, M.G. Human Wharton’s jelly mesenchymal stem cells promote skin wound healing through paracrine signaling. Stem Cell Res. Ther. 2014, 5, 28. [Google Scholar] [CrossRef]
- Doi, H.; Kitajima, Y.; Luo, L.; Yan, C.; Tateishi, S.; Ono, Y.; Urata, Y.; Goto, S.; Mori, R.; Masuzaki, H.; et al. Potency of umbilical cord blood- and Wharton’s jelly-derived mesenchymal stem cells for scarless wound healing. Sci Rep. 2016, 6, 18844. [Google Scholar] [CrossRef]
- Formigli, L.; Paternostro, F.; Tani, A.; Mirabella, C.; Quattrini Li, A.; Nosi, D.; D’Asta, F.; Saccardi, R.; Mazzanti, B.; Lo Russo, G.; et al. MSCs seeded on bioengineered scaffolds improve skin wound healing in rats. Wound Repair. Regen. 2015, 23, 115–123. [Google Scholar] [CrossRef]
- Hashemi, S.S.; Mohammadi, A.A.; Kabiri, H.; Hashempoor, M.R.; Mahmoodi, M.; Amini, M.; Mehrabani, D. The healing effect of Wharton’s jelly stem cells seeded on biological scaffold in chronic skin ulcers: A randomized clinical trial. J. Cosmet Derm. 2019, 18, 1961–1967. [Google Scholar] [CrossRef]
- Iacono, E.; Merlo, B.; Pirrone, A.; Antonelli, C.; Brunori, L.; Romagnoli, N.; Castagnetti, C. Effects of mesenchymal stem cells isolated from amniotic fluid and platelet-rich plasma gel on severe decubitus ulcers in a septic neonatal foal. Res. Vet. Sci. 2012, 93, 1439–1440. [Google Scholar] [CrossRef]
- Jun, E.K.; Zhang, Q.; Yoon, B.S.; Moon, J.H.; Lee, G.; Park, G.; Kang, P.J.; Lee, J.H.; Kim, A.; You, S. Hypoxic conditioned medium from human amniotic fluid-derived mesenchymal stem cells accelerates skin wound healing through TGF-β/SMAD2 and PI3K/Akt pathways. Int. J. Mol. Sci. 2014, 15, 605–628. [Google Scholar] [CrossRef]
- Myung, H.; Jang, H.; Myung, J.K.; Lee, C.; Lee, J.; Kang, J.; Jang, W.S.; Lee, S.J.; Kim, H.; Kim, H.Y.; et al. Platelet-rich plasma improves the therapeutic efficacy of mesenchymal stem cells by enhancing their secretion of angiogenic factors in a combined radiation and wound injury model. Exp. Derm. 2020, 29, 158–167. [Google Scholar] [CrossRef]
- Lee, C.; Shim, S.; Jang, H.; Myung, H.; Lee, J.; Bae, C.H.; Myung, J.K.; Kim, M.J.; Lee, S.B.; Jang, W.S.; et al. Human umbilical cord blood-derived mesenchymal stromal cells and small intestinal submucosa hydrogel composite promotes combined radiation-wound healing of mice. Cytotherapy 2017, 19, 1048–1059. [Google Scholar] [CrossRef]
- Luo, G.; Cheng, W.; He, W.; Wang, X.; Tan, J.; Fitzgerald, M.; Li, X.; Wu, J. Promotion of cutaneous wound healing by local application of mesenchymal stem cells derived from human umbilical cord blood. Wound Repair. Regen. 2010, 18, 506–513. [Google Scholar] [CrossRef]
- Chapple, C.R. Urethral injury. BJU Int. 2000, 86, 318–326. [Google Scholar] [CrossRef]
- Vasyutin, I.; Butnaru, D.; Lyundup, A.; Timashev, P.; Vinarov, A.; Kuznetsov, S.; Atala, A.; Zhang, Y. Frontiers in urethra regeneration: Current state and future perspective. Biomed. Mater. 2021, 16, 042004. [Google Scholar] [CrossRef]
- Ram-Liebig, G.; Barbagli, G.; Heidenreich, A.; Fahlenkamp, D.; Romano, G.; Rebmann, U.; Standhaft, D.; van Ahlen, H.; Schakaki, S.; Balsmeyer, U. Results of use of tissue-engineered autologous oral mucosa graft for urethral reconstruction: A multicenter, prospective, observational trial. EBioMedicine 2017, 23, 185–192. [Google Scholar] [CrossRef]
- Prabhasawat, P.; Ekpo, P.; Uiprasertkul, M.; Chotikavanich, S.; Tesavibul, N.; Pornpanich, K.; Luemsamran, P. Long-term result of autologous cultivated oral mucosal epithelial transplantation for severe ocular surface disease. Cell Tissue Bank. 2016, 17, 491–503. [Google Scholar] [CrossRef]
- Fu, W.J.; Xu, Y.D.; Wang, Z.X.; Li, G.; Shi, J.G.; Cui, F.Z.; Zhang, Y.; Zhang, X. New ureteral scaffold constructed with composite poly(L-lactic acid)-collagen and urothelial cells by new centrifugal seeding system. J. Biomed. Mater. Res. Part A 2012, 100, 1725–1733. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, G.; Kropp, B.P. Re-epithelialization of demucosalized stomach patch with tissue-engineered urothelial mucosa combined with Botox A in bladder augmentation. BJU Int. 2012, 110, E106–E112. [Google Scholar] [CrossRef]
- Liu, Y.; Bharadwaj, S.; Lee, S.J.; Atala, A.; Zhang, Y. Optimization of a natural collagen scaffold to aid cell-matrix penetration for urologic tissue engineering. Biomaterials 2009, 30, 3865–3873. [Google Scholar] [CrossRef]
- Zhang, Y.; Kropp, B.P.; Lin, H.K.; Cowan, R.; Cheng, E.Y. Bladder regeneration with cell-seeded small intestinal submucosa. Tissue Eng. 2004, 10, 181–187. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Xu, Y.-M.; Song, L.-J.; Fu, Q.; Cui, L.; Yin, S. Urethral reconstruction using oral keratinocyte seeded bladder acellular matrix grafts. J. Urol. 2008, 180, 1538–1542. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Yu, H.; Fan, C.; Kong, Q.; Liu, D.; Meng, L. Differentiate into urothelium and smooth muscle cells from adipose tissue-derived stem cells for ureter reconstruction in a rabbit model. Am. J. Transl. Res. 2016, 8, 3757. [Google Scholar] [PubMed]
- Pinho, A.G.; Cibrão, J.R.; Silva, N.A.; Monteiro, S.; Salgado, A.J. Cell Secretome: Basic Insights and Therapeutic Opportunities for CNS Disorders. Pharmaceuticals 2020, 13, 31. [Google Scholar] [CrossRef]
- Wu, R.; Huang, C.; Wu, Q.; Jia, X.; Liu, M.; Xue, Z.; Qiu, Y.; Niu, X.; Wang, Y. Exosomes secreted by urine-derived stem cells improve stress urinary incontinence by promoting repair of pubococcygeus muscle injury in rats. Stem Cell Res. Ther. 2019, 10, 80. [Google Scholar] [CrossRef]
- Duan, Y.R.; Chen, B.P.; Chen, F.; Yang, S.X.; Zhu, C.Y.; Ma, Y.L.; Li, Y.; Shi, J. Exosomal microRNA-16-5p from human urine-derived stem cells ameliorates diabetic nephropathy through protection of podocyte. J. Cell Mol. Med. 2021, 25, 10798–10813. [Google Scholar] [CrossRef]
- Luo, S.; Shao, L.; Geng, R.; Liu, Q.; Jiang, W.; Gong, M.; Zhang, Y.; He, Y. Identification and biological characteristics of clear cell renal cell carcinoma associated urine-derived stem cells. Am. J. Transl. Res. 2021, 13, 2143–2162. [Google Scholar]
- Xiong, G.; Tang, W.; Zhang, D.; He, D.; Wei, G.; Atala, A.; Liang, X.J.; Bleyer, A.J.; Bleyer, M.E.; Yu, J.; et al. Impaired Regeneration Potential in Urinary Stem Cells Diagnosed from the Patients with Diabetic Nephropathy. Theranostics 2019, 9, 4221–4232. [Google Scholar] [CrossRef]
- Yu, P.; Duan, Z.; Liu, S.; Pachon, I.; Ma, J.; Hemstreet, G.P.; Zhang, Y. Drug-Induced Nephrotoxicity Assessment in 3D Cellular Models. Micromachines 2021, 13, 3. [Google Scholar] [CrossRef]
- Ding, H.; Jambunathan, K.; Jiang, G.; Margolis, D.M.; Leng, I.; Ihnat, M.; Ma, J.-X.; Mirsalis, J.; Zhang, Y. 3D Spheroids of Human Primary Urine-Derived Stem Cells in the Assessment of Drug-Induced Mitochondrial Toxicity. Pharmaceutics 2022, 14, 1042. [Google Scholar] [CrossRef]
- Guo, H.; Deng, N.; Dou, L.; Ding, H.; Criswell, T.; Atala, A.; Furdui, C.M.; Zhang, Y. 3-D Human Renal Tubular Organoids Generated from Urine-Derived Stem Cells for Nephrotoxicity Screening. ACS Biomater. Sci. Eng. 2020, 6, 6701–6709. [Google Scholar] [CrossRef]
- Sanabria-de la Torre, R.; Fernández-González, A.; Quiñones-Vico, M.I.; Montero-Vilchez, T.; Arias-Santiago, S. Bioengineered Skin Intended as In Vitro Model for Pharmacosmetics, Skin Disease Study and Environmental Skin Impact Analysis. Biomedicines 2020, 8, 464. [Google Scholar] [CrossRef]
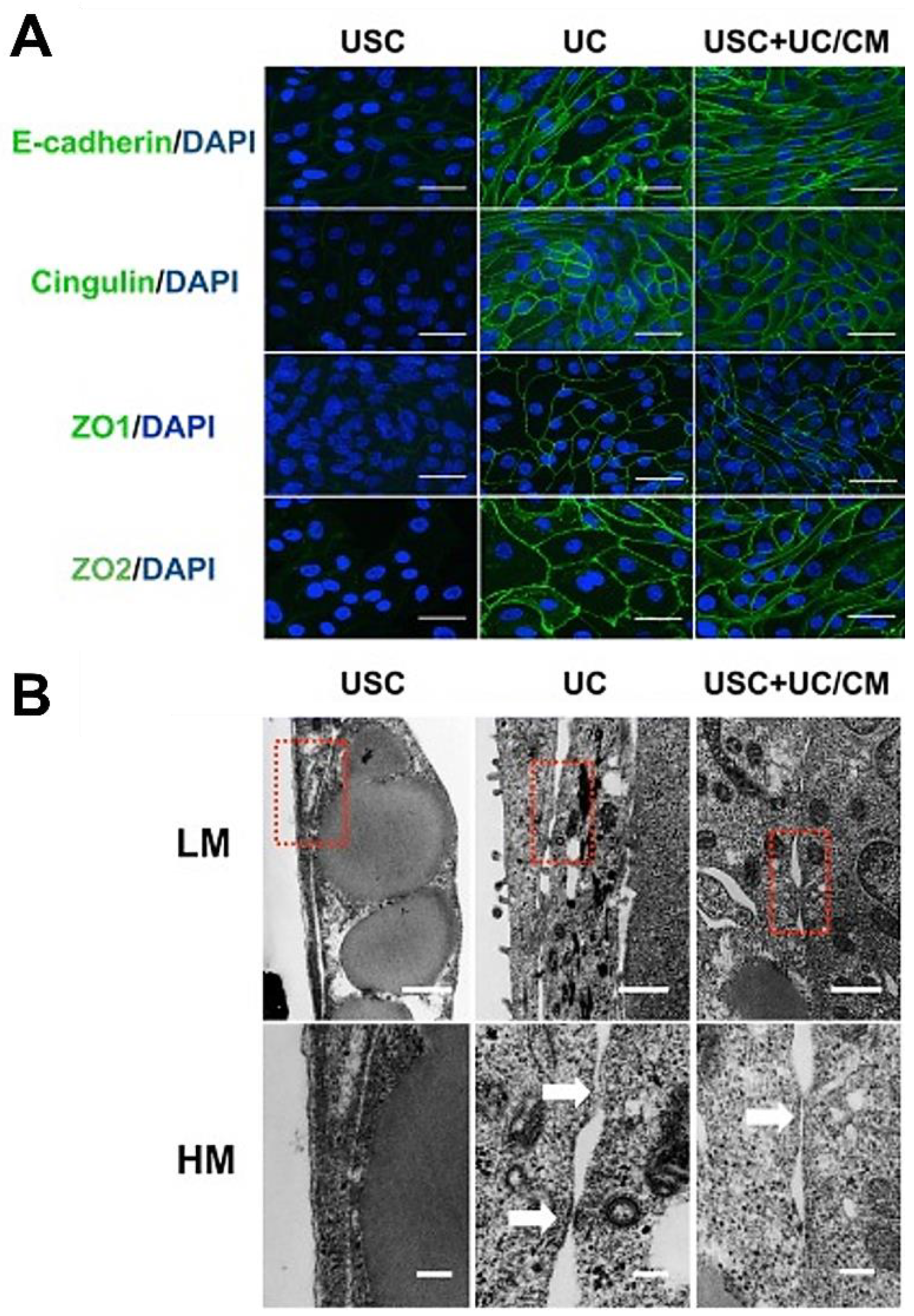
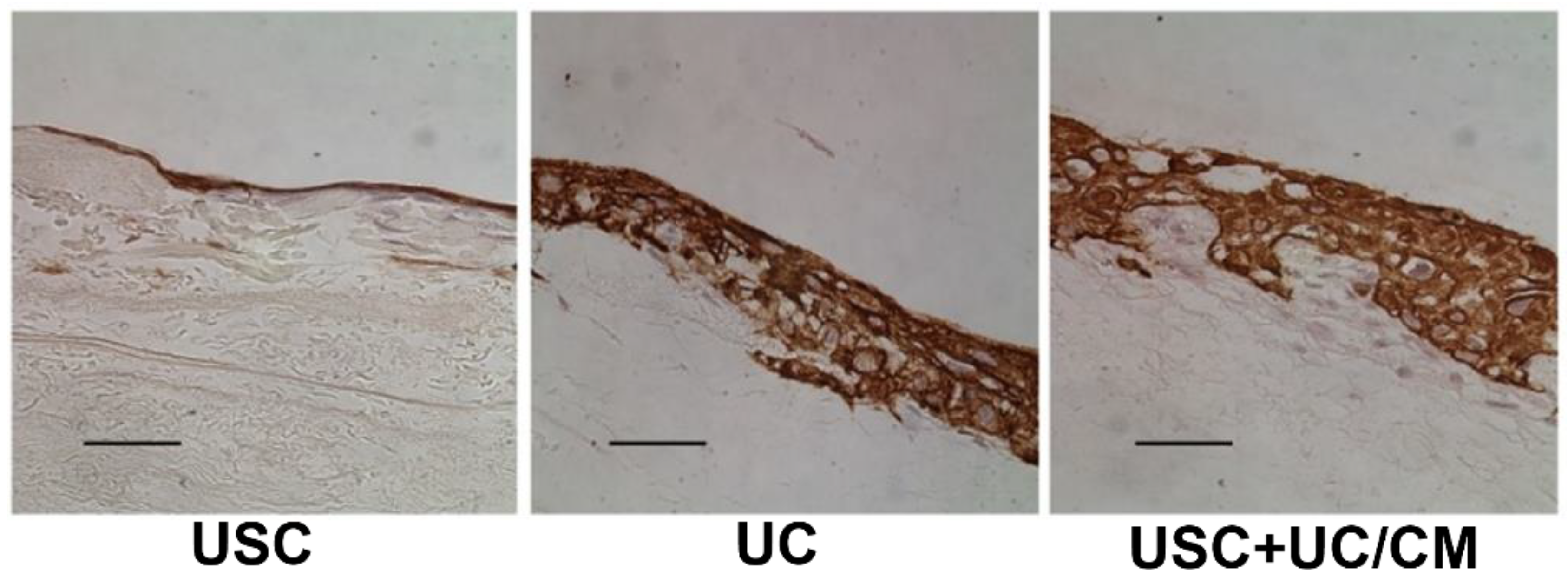
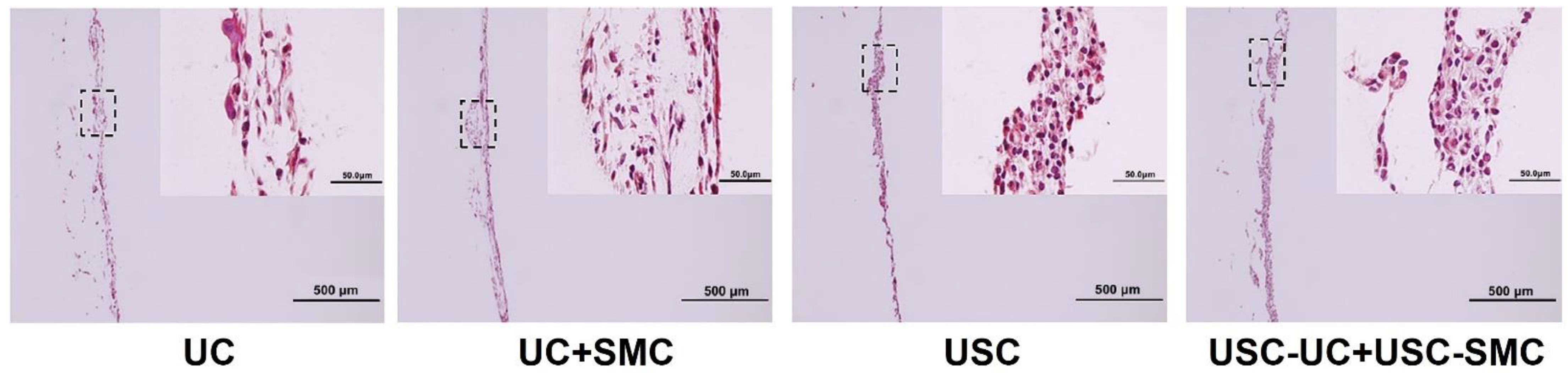
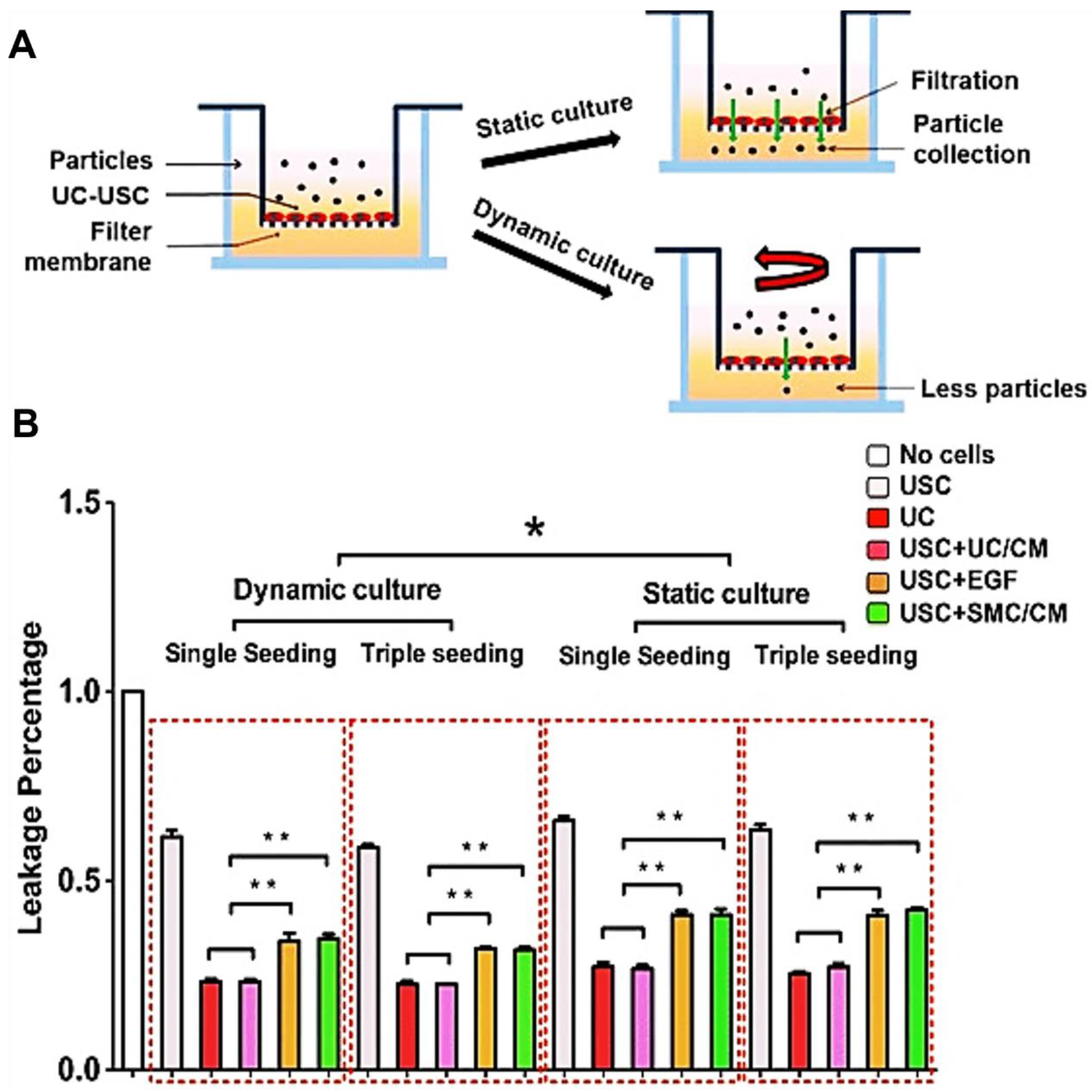
| Epithelial Tissues | Biomaterial | Key Outcomes |
|---|---|---|
| Cell-seeded scaffold for urinary tract reconstruction in vivo | 3D porous SIS Porous bacterial cellulose scaffoldComposite scaffold | Urethral caliber, SM content, vessel density ↑ [12] 3D multilayered urothelium, cell matrix infiltration [10,16] Bladder capacity, compliance, SM content, multi-layered urothelium, submucosa layers ↑ [26] |
| In vitro cell sheet structure | 3D porous SIS | A multilayered urothelium with barrier function [56] |
| Exosome therapy for urinary tract reconstruction in a rodent model | Collagen-I gel | Angiogenesis, in vivo cell survival, myogenic, and nerve regeneration ↑ [25] |
| Cell therapy for SUI in rodent models | Collagen hydrogel Alginate microbeads Collagen-I gel | Cell survival, cell recruitment, myogenesis, innervation, neovascularization ↑ [54] |
| Exosome therapy for ED in diabetic rat model | PBS | Endothelial functional protein eNOS and CD31 expression, ICP ↑ [30] |
| Cell seeded scaffold and exosome therapy for skin defect repair in vivo | Polycaprolactone/gelatin nanofibrous membranes, surface-structured bacterial cellulose, 3D porous SIS | Wound closure rate, re-epithelialization, and angiogenesis ↑ [37,50,52] |
| Exosome therapy for diabetic ulcer in rodent model | PBS | Wound closure rate, re-epithelialization, skin cell proliferation ↑; Scar formation ↓ [49] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yin, X.; Li, Q.; McNutt, P.M.; Zhang, Y. Urine-Derived Stem Cells for Epithelial Tissues Reconstruction and Wound Healing. Pharmaceutics 2022, 14, 1669. https://doi.org/10.3390/pharmaceutics14081669
Yin X, Li Q, McNutt PM, Zhang Y. Urine-Derived Stem Cells for Epithelial Tissues Reconstruction and Wound Healing. Pharmaceutics. 2022; 14(8):1669. https://doi.org/10.3390/pharmaceutics14081669
Chicago/Turabian StyleYin, Xiya, Qingfeng Li, Patrick Michael McNutt, and Yuanyuan Zhang. 2022. "Urine-Derived Stem Cells for Epithelial Tissues Reconstruction and Wound Healing" Pharmaceutics 14, no. 8: 1669. https://doi.org/10.3390/pharmaceutics14081669
APA StyleYin, X., Li, Q., McNutt, P. M., & Zhang, Y. (2022). Urine-Derived Stem Cells for Epithelial Tissues Reconstruction and Wound Healing. Pharmaceutics, 14(8), 1669. https://doi.org/10.3390/pharmaceutics14081669