Therapeutic Drug Monitoring of Sputum Voriconazole in Pulmonary Aspergillosis
Abstract
1. Introduction
2. Materials and Methods
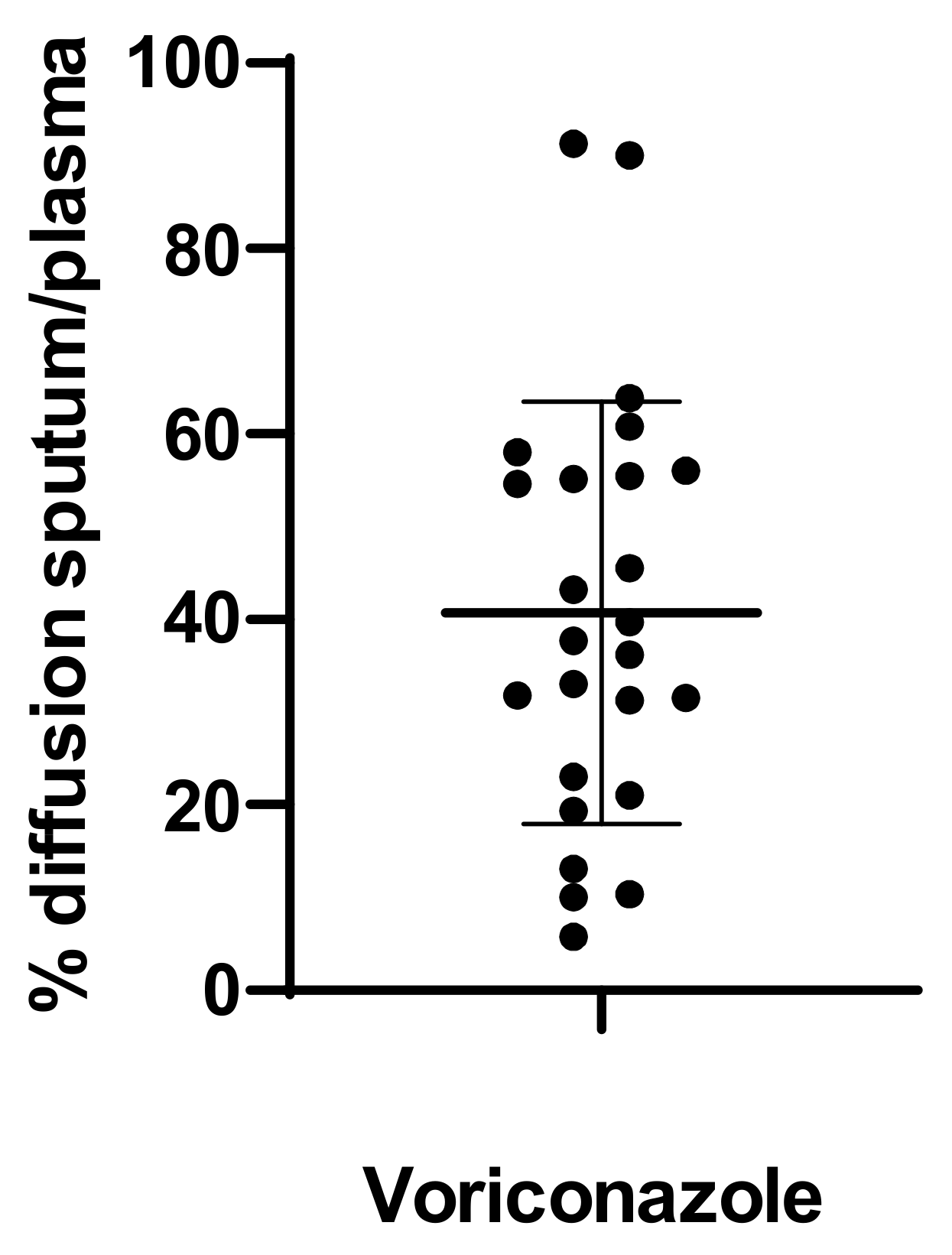
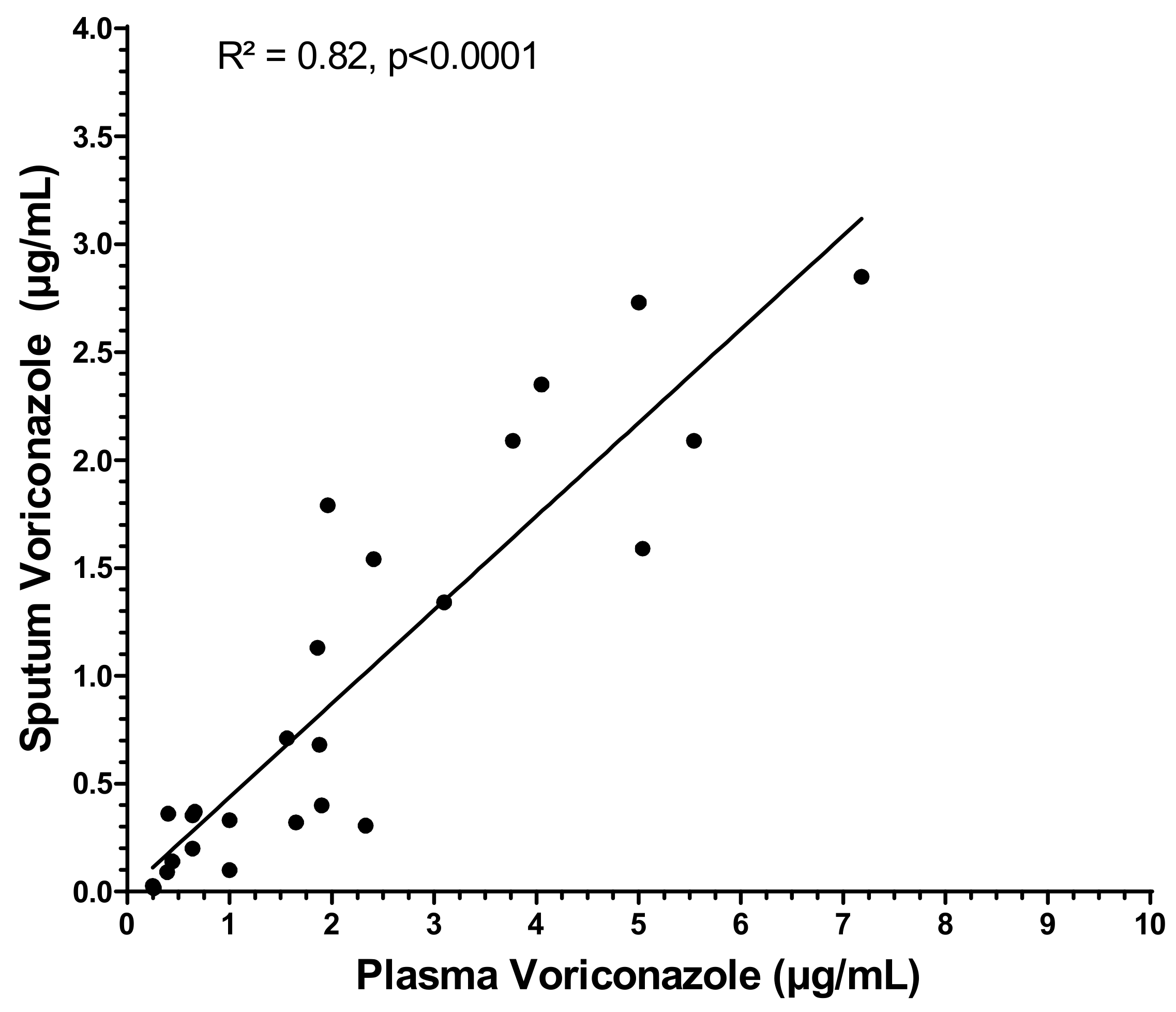
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ullmann, A.J.; Aguado, J.M.; Arikan-Akdagli, S.; Denning, D.W.; Groll, A.H.; Lagrou, K.; Lass-Flörl, C.; Lewis, R.E.; Munoz, P.; Verweij, P.E.; et al. Diagnosis and management of Aspergillus diseases: Executive summary of the 2017 ESCMID-ECMM-ERS guideline. Clin. Microbiol. Infect. 2018, 24 (Suppl. 1), e1–e38. [Google Scholar] [CrossRef] [PubMed]
- Denning, D.W.; Cadranel, J.; Beigelman-Aubry, C.; Ader, F.; Chakrabarti, A.; Blot, S.; Ullmann, A.J.; Dimopoulos, G.; Lange, C. European Society for Clinical Microbiology and Infectious Diseases and European Respiratory Society. Chronic pulmonary aspergillosis: Rationale and clinical guidelines for diagnosis and management. Eur. Respir. J. 2016, 47, 45–68. [Google Scholar] [CrossRef] [PubMed]
- Pascual, A.; Calandra, T.; Bolay, S.; Buclin, T.; Bille, J.; Marchetti, O. Voriconazole therapeutic drug monitoring in patients with invasive mycoses improves efficacy and safety outcomes. Clin. Infect. Dis. 2008, 46, 201–211. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Yim, D.S.; Choi, S.M.; Kwon, J.C.; Han, S.; Lee, D.G.; Park, C.; Kwon, E.Y.; Park, S.H.; Choi, J.H.; et al. Voriconazole-related severe adverse events: Clinical application of therapeutic drug monitoring in Korean patients. Int. J. Infect. Dis. 2011, 15, e753–e758. [Google Scholar] [CrossRef] [PubMed]
- Gautier-Veyret, E.; Truffot, A.; Bailly, S.; Fonrose, X.; Thiebaut-Bertrand, A.; Tonini, J.; Cahn, J.Y.; Stanke-Labesque, F. Inflammation is a potential risk factor of voriconazole overdose in hematological patients. Fundam. Clin. Pharmacol. 2019, 33, 232–238. [Google Scholar] [CrossRef] [PubMed]
- Bolcato, L.; Khouri, C.; Veringa, A.; Alffenaar, J.W.C.; Yamada, T.; Naito, T.; Lamoureux, F.; Fonrose, X.; Stanke-Labesque, F.; Gautier-Veyret, E. Combined Impact of Inflammation and Pharmacogenomic Variants on Voriconazole Trough Concentrations: A Meta-Analysis of Individual Data. J. Clin. Med. 2021, 10, 2089. [Google Scholar] [CrossRef] [PubMed]
- Lamoureux, F.; Duflot, T.; Woillard, J.B.; Metsu, D.; Pereira, T.; Compagnon, P.; Morisse-Pradier, H.; El Kholy, M.; Thiberville, L.; Stojanova, J.; et al. Impact of CYP2C19 genetic polymorphisms on voriconazole dosing and exposure in adult patients with invasive fungal infections. Int. J. Antimicrob. Agents 2016, 47, 124–131. [Google Scholar] [CrossRef] [PubMed]
- Duflot, T.; Schrapp, A.; Bellien, J.; Lamoureux, F. Impact of CYP3A4 Genotype on Voriconazole Exposure. Clin. Pharmacol. Ther. 2018, 103, 185–186. [Google Scholar] [CrossRef] [PubMed]
- Koehler, P.; Bassetti, M.; Chakrabarti, A.; Chen, S.C.A.; Colombo, A.L.; Hoenigl, M.; Klimko, N.; Lass-Flörl, C.; Oladele, R.O.; Vinh, D.C.; et al. Defining and managing COVID-19-associated pulmonary aspergillosis: The 2020 ECMM/ISHAM consensus criteria for research and clinical guidance. Lancet Infect. Dis. 2021, 21, e149–e162. [Google Scholar] [CrossRef]
- Bellmann, R.; Smuszkiewicz, P. Pharmacokinetics of antifungal drugs: Practical implications for optimized treatment of patients. Infection 2017, 45, 737–779. [Google Scholar] [CrossRef] [PubMed]
- Weiler, S.; Fiegl, D.; MacFarland, R.; Stienecke, E.; Bellmann-Weiler, R.; Dunzendorfer, S.; Joannidis, M.; Bellmann, R. Human tissue distribution of voriconazole. Antimicrob. Agents Chemother. 2011, 55, 925–928. [Google Scholar] [CrossRef] [PubMed]
- Felton, T.; Troke, P.F.; Hope, W.W. Tissue penetration of antifungal agents. Clin. Microbiol. Rev. 2014, 27, 68–88. [Google Scholar] [CrossRef] [PubMed]
- Crandon, J.L.; Banevicius, M.A.; Fang, A.F.; Crownover, P.H.; Knauft, R.F.; Pope, J.S.; Russomanno, J.H.; Shore, E.; Nicolau, D.P.; Kuti, J.L. Bronchopulmonary disposition of intravenous voriconazole and anidulafungin given in combination to healthy adults. Antimicrob. Agents Chemother. 2009, 53, 5102–5107. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Heng, S.C.; Snell, G.I.; Levvey, B.; Keating, D.; Westall, G.P.; Williams, T.J.; Whitford, H.; Nation, R.L.; Slavin, M.A.; Morrissey, O.; et al. Relationship between trough plasma and epithelial lining fluid concentrations of voriconazole in lung transplant recipients. Antimicrob. Agents Chemother. 2013, 57, 4581–4583. [Google Scholar] [CrossRef] [PubMed]
- Capitano, B.; Potoski, B.A.; Husain, S.; Zhang, S.; Paterson, D.L.; Studer, S.M.; McCurry, K.R.; Venkataramanan, R. Intrapulmonary penetration of voriconazole in patients receiving an oral prophylactic regimen. Antimicrob. Agents Chemother. 2006, 50, 1878–1880. [Google Scholar] [CrossRef] [PubMed]
- Bartlett, R.C. Medical Microbiology: Quality, Cost and Clinical Relevance; John Wiley & Sons: New York, NY, USA, 1974; pp. 24–31. [Google Scholar]
- FDA. Guidance for Industry Bioanalytical Method Validation. 2018. Available online: https://www.fda.gov/media/70858/download (accessed on 18 May 2022).
- EMA. Guideline on Bioanalytical Method Validation. 2011. Available online: https://www.ema.europa.eu/documents/scientific-guideline/guideline-bioanalytical-method-validation_en.pdf (accessed on 18 May 2022).
- Arendrup, M.C.; Friberg, N.; Mares, M.; Kahlmeter, G.; Meletiadis, J.; Guinea, J.; Subcommittee on Antifungal Susceptibility Testing (AFST) of the ESCMID European Committee for Antimicrobial Susceptibility Testing (EUCAST). How to interpret MICs of antifungal compounds according to the revised clinical breakpoints v. 10.0 European committee on antimicrobial susceptibility testing (EUCAST). Clin. Microbiol. Infect. 2020, 26, 1464–1472. [Google Scholar] [CrossRef] [PubMed]


| Characteristic | Value |
|---|---|
| Age (mean ± SD) | 54 ± 14 years |
| Sex, male (n (%)) | 13 (54) |
| Weight (mean ± SD) | 73 ± 13 kg |
| Height (mean ± SD) | 170 ± 8 cm |
| BMI (mean ± SD) | 21 ± 3.7 kg/m2 |
| Positive Aspergillus spp. culture (n (%)) | 16 (66.6%) |
| Species (n (%)) | |
| Aspergillus fumigatus | 14 (87.5%) |
| Aspergillus neoellipticus | 1 (6.25%) |
| Aspergillus wellvitchiae | 1 (6.25%) |
| Voriconazole daily dose | 436 ± 98 mg |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sarfati, S.; Wils, J.; Lambert, T.; Mory, C.; Imbert, L.; Gargala, G.; Morisse-Pradier, H.; Lamoureux, F. Therapeutic Drug Monitoring of Sputum Voriconazole in Pulmonary Aspergillosis. Pharmaceutics 2022, 14, 1598. https://doi.org/10.3390/pharmaceutics14081598
Sarfati S, Wils J, Lambert T, Mory C, Imbert L, Gargala G, Morisse-Pradier H, Lamoureux F. Therapeutic Drug Monitoring of Sputum Voriconazole in Pulmonary Aspergillosis. Pharmaceutics. 2022; 14(8):1598. https://doi.org/10.3390/pharmaceutics14081598
Chicago/Turabian StyleSarfati, Sacha, Julien Wils, Timothée Lambert, Céline Mory, Laurent Imbert, Gilles Gargala, Hélène Morisse-Pradier, and Fabien Lamoureux. 2022. "Therapeutic Drug Monitoring of Sputum Voriconazole in Pulmonary Aspergillosis" Pharmaceutics 14, no. 8: 1598. https://doi.org/10.3390/pharmaceutics14081598
APA StyleSarfati, S., Wils, J., Lambert, T., Mory, C., Imbert, L., Gargala, G., Morisse-Pradier, H., & Lamoureux, F. (2022). Therapeutic Drug Monitoring of Sputum Voriconazole in Pulmonary Aspergillosis. Pharmaceutics, 14(8), 1598. https://doi.org/10.3390/pharmaceutics14081598





