Abstract
Chemotherapy-induced peripheral neuropathy (CIPN) often develops in patients with cancer treated with commonly used anti-cancer drugs. The symptoms of CIPN can occur acutely during chemotherapy or emerge after cessation, and often accompany long-lasting intractable pain. This adverse side effect not only affects the quality of life but also limits the use of chemotherapy, leading to a reduction in the survival rate of patients with cancer. Currently, effective treatments for CIPN are limited, and various interventions are being applied by clinicians and patients because of the unmet clinical need. Potential approaches to ameliorate CIPN include traditional Eastern medicine-based methods. Medicinal substances from traditional Eastern medicine have well-established analgesic effects and are generally safe. Furthermore, many substances can also improve other comorbid symptoms in patients. This article aims to provide information regarding traditional Eastern medicine-based plant extracts and natural compounds for CIPN. In this regard, we briefly summarized the development, mechanisms, and changes in the nervous system related to CIPN, and reviewed the substances of traditional Eastern medicine that have been exploited to treat CIPN in preclinical and clinical settings.
1. Introduction
Chemotherapy-induced peripheral neuropathy (CIPN) is a major adverse effect that may limit the use of cancer chemotherapy agents [1,2]. CIPN usually manifests as sensory neuropathy symptoms in patients. This involves the alteration of sensation induced by mechanical and/or thermal stimuli. Various molecular mechanisms and cellular alterations related to CIPN have been revealed in recent decades. Chemotherapeutic agents act at multiple levels of the somatosensory nervous system, thus affecting the expression of ion channels, myelination of the nerve fibers, the excitability of the sensory neurons located in the dorsal root ganglion and spinal cord, and the connectivity of the pain-related brain network. These changes eventually distort the transmission of sensory signals, causing the normal peripheral sensation to become painful. Despite numerous efforts, there is no effective clinical method for the treatment of CIPN [3,4,5]. Several patients with cancer stop chemotherapy due to this adverse effect. Pain from CIPN not only impairs the quality of life but also becomes a life-threatening factor in these patients by limiting the available cancer treatments [2,3].
As indicated by its name (“peripheral” neuropathy), it is evident that peripheral mechanisms are the initial causes of the development of these symptoms. Recent review papers have described the multiple mechanisms that induce sensory symptoms in CIPN [2,6]. However, the mechanisms of the maintenance of CIPN symptoms are not limited to peripheral changes. Although the initial cause of CIPN is in the peripheral nerve, prolonged injury signals cause abnormalities in the higher levels [7]. This leads to chronic pain, such as neuropathic pain caused by other reasons [8,9]. Previous studies in the research field of general neuropathic pain revealed that central changes, as well as peripheral changes, are critically involved in chronic pain states. Damage to the peripheral nerve that causes pain could induce alterations at multiple levels of the somatosensory nervous system. This includes the spinal cord and brain. These changes could amplify pain transmission even after the initial damage is recovered [10]. Similar to the chronic neuropathic pain caused by other reasons, CIPN symptoms are often prolonged after cessation of the chemotherapeutic agent, and in some cases, the symptoms persist [1,11,12].
Various therapeutic methods have been used to attenuate the symptoms of CIPN in patients. The treatment options include medications from traditional Eastern medicine, due to their beneficial effects in relieving numerous symptoms of CIPN [13,14,15]. Generally, in traditional Eastern medicine, it is recommended to choose multiple substances from many options and combine them for a therapeutic effect against the target symptoms. Treatment strategies should be selected according to individual differences in patients’ general conditions and patterns of comorbid symptoms when applying the traditional Eastern medicine approach. The current understanding of the mechanisms shows that the analgesic effects of traditional Eastern medicinal substances against CIPN symptoms can be induced at different levels of the nervous system. An adequate combination of treatment methods that act on multiple targets or work synergistically would help develop new treatment options for CIPN. In addition, the effects of many substances on cancer have been studied extensively in recent decades [16,17,18,19,20]. Considering that the treatment for CIPN should not deteriorate cancer, these pre-established results are advantageous for selecting the traditional Eastern medicine approach as a treatment option.
In this article, we first review the causes of CIPN and its symptoms for a general understanding of CIPN (Figure 1). The peripheral and spinal mechanisms studied in patients or animal models, and brain changes associated with prolonged pain are also summarized. We further discuss the recent studies seeking novel interventions for CIPN. Particularly, we focus on the methods using plant extracts and natural compounds from traditional Eastern medicine. Considering the clinical difficulties in the investigation of new drugs, utilizing traditional medical knowledge is advantageous for screening drug candidates with long-term safety and effectiveness. In this respect, recent studies on phytochemicals, extracts from medicinal plants, herbal decoctions, and animal venom substances used in traditional Eastern medicine have been summarized and discussed.
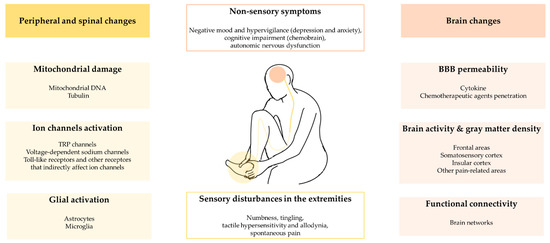
Figure 1.
The summary of peripheral, spinal and brain changes in CIPN.
2. Development of CIPN Symptoms by Chemotherapeutic Agents
Chemotherapeutic agents cause damage to the nervous system, leading to the induction of CIPN. In particular, patients treated with the platinum-based chemotherapeutic agent oxaliplatin or the taxane-based agent paclitaxel are predisposed to develop immediate peripheral neuropathy. Other chemotherapeutic agents, including vinca alkaloids, proteasome inhibitors, and immunomodulatory drugs, also induce CIPN symptoms [6,21]. Sensory symptoms are the most prominent side effects of chemotherapeutic agents. However, chemotherapeutic agents also induce side effects such as dizziness, cognitive dysfunction, and motor dysfunction. Approximately 80% of the patients treated with these agents develop CIPN within 6 months. Pain symptoms are the major cause that limits their use. Most patients reported pain within 6.5 months of the use of the chemotherapeutic agent [11]. Even if the patient does not show pain symptoms in the early period of chemotherapy treatment, the stochastic rate of pain development increases with the duration of chemotherapeutic drug use. Approximately one-third of patients have chronic CIPN six months or more after the end of chemotherapy [22]. In a subset of the patients, the symptoms of CIPN deteriorate after the cessation of chemotherapeutic agents [2,23]. This “coasting” phenomenon includes worsening of the preexisting mild CIPN or development of new CIPN. In this regard, the management of CIPN has to be continued after the cessation of chemotherapy. More so, the sensory symptoms of the patients have to be observed throughout the period.
Although the types and severity of CIPN symptoms can vary depending on the chemotherapeutic agent used, many patients report sensory disturbances in the extremities. Numbness, tingling, mechanical/thermal hypersensitivity, and spontaneous pain are common symptoms. The representative pain symptoms include cold allodynia, which was observed in most patients treated with oxaliplatin or paclitaxel. Heat hyperalgesia is relatively rare, and several patients have reported a loss of heat sensitivity with these agents [24]. Pain-related distress is accompanied by depression, anxiety, and other symptoms related to hypervigilance and negative mood. Mental distress and physical pain influence patients to cease chemotherapy.
Similar to other neuropathic pain caused by nerve damage, the discomfort of pain symptoms caused by chemotherapeutic agents is not controlled by conventional analgesic drugs. Patients rarely adapt to the pain symptoms. Rather, the intensity of pain and discomfort in patients with CIPN often increase with the continued use of chemotherapeutic agents. There have been attempts to suppress CIPN symptoms with treatments such as pregabalin, venlafaxine, or duloxetine. However, only a subset of patients reported having relief from symptoms, which was restricted to partial relief [25].
3. Peripheral and Spinal Mechanisms of the CIPN
Platinum-based chemotherapeutic agents, such as oxaliplatin and cisplatin, induce oxidative stress in the mitochondria. These agent molecules act on mitochondrial DNA in cells and impair the respiratory chain in mitochondria, promoting the production of reactive oxygen species [26,27]. Thus, the apoptotic pathway is activated in cancer cells. Hence, these chemotherapeutic agents easily exert an anti-cancer effect. However, the target cells of these drugs are not restricted to cancer cells. These mechanisms may also affect non-cancer cells, resulting in adverse effects, such as CIPN. Damage to the primary afferent nerve fibers is claimed to be the primary cause of the abnormal pain symptoms in CIPN. The primary afferent fibers of the sensory nerve relay information from the cutaneous skin to the spinal cord, and have very long axons. Chemotherapeutic agents easily affect the mitochondria within sensory afferents due to this characteristic. In the case of paclitaxel, a taxane-based chemotherapeutic agent, alteration of mitochondrial function was claimed to be responsible for the manifestation of CIPN [28]. Many other chemotherapeutic agents also induce mitochondrial dysfunction by altering microtubule dynamics [29,30,31,32]. This effect is exerted by the interaction between the drug and tubulin. In addition to its effect on microtubule dynamics, tubulin can also alter mitochondrial function via its direct interaction with voltage-dependent anion channels located in the mitochondrial membrane [30]. Changes in tubulin have been reported to be common downstream in taxane-based drugs, vinca alkaloids, and proteasome inhibitors [31].
Chemotherapeutic agents also induce abnormal sensory signals and amplify pain through their action on ion channels in the cellular membrane [21,33,34]. These phenomena are mediated by activation of TRP (transient receptor potential) channels [33,35], voltage-dependent sodium channels [34], toll-like receptors [36], and/or other various receptors that indirectly affect ion channels [35,37,38]. The activation of ion channels in the primary afferent fibers also evokes nerve signals similar to the activation of nociceptors in the periphery endings. Accordingly, sensory neurons in the dorsal root ganglion (DRG) fire action potentials that are then transmitted to various brain regions via the spinal cord; thus, the individual perceives the sensory signals as painful distress.
Another mechanism involves glial activation [21,39,40]. For example, the myelin sheath can be damaged by chemotherapeutic agents. The peripheral nerve fibers are myelinated by Schwann cells, and damage or inflammatory changes in this glial cell can amplify the signal transmission of sensory nerve fibers. Glial mechanisms also include changes in the microglia and astrocytes in the central nervous system [41]. Interestingly, the recruitment of microglia and astrocyte in the development of CIPN appears to be related to the chemotherapeutic agent used. Generally, the activation of astrocytes plays a key role in the pathogenesis of CIPN. For example, oxaliplatin affects astrocytes in the spinal cord by activating adenosine kinase and subsequent reduction in adenosine signaling. Activation of astrocytes alters the signaling of other glial cells and neighboring neurons, facilitating signal transmission in the spinal cord [42]. Astrocyte activation was also observed in paclitaxel- and bortezomib-induced CIPN [43,44]. In contrast to the general recruitment of astrocytes across multiple CIPN models, the role of microglial activation in CIPN is somewhat vague and seems to be specific to the chemotherapeutic agent used. In the CIPN model induced by oxaliplatin, paclitaxel, or bortezomib, microglia were not activated in the spinal cord and did not appear to play a significant role in CIPN [43,44]. Conversely, activation of microglia, but not astrocytes, was persistently observed in the spinal cord in the CIPN model induced by cisplatin [45].
All the peripheral and spinal mechanisms are important for the development and maintenance of CIPN. Attempts have been made to develop novel intervention methods based on these mechanisms. Some have shown significant results in clinical trials. However, no treatment has sufficiently suppressed pain symptoms in human patients. Little preclinical understanding of the CIPN is being translated into clinical trials. As such, substances that were found to be promising in the preclinical setting have failed in clinical trials [46].
4. Brain Changes Observed in Subjects with CIPN
The direct effects of chemotherapeutic agents on the brain are unclear in many aspects. Drugs that cause CIPN, such as cisplatin, oxaliplatin, or paclitaxel, are thought to affect the peripheral nervous system rather than the brain. This is because these drugs do not pass through the blood–brain barrier (BBB) [47]. However, studies have shown that small amounts of these substances are found in the brain when they are injected systemically. For example, early studies reported that chemotherapeutic agents, such as cisplatin or doxorubicin, could exert anti-cancer effects against brain tumors, despite limited passage across the BBB [48,49]. Meanwhile, a study reported that a radiotracer form of paclitaxel was found in the brain of animal models when the tracer was intravenously injected, although the amount of the tracer measured in the brain was very small [50]. This shows that the BBB does not completely prevent the penetration of these drugs into the brain. A recent review discussed that cisplatin could migrate into the brain, despite its limited passage across the BBB through the copper transporter 1-mediated mechanisms [51]. Other studies have shown that oxaliplatin affects epithelial cells that constitute the BBB, thereby increasing the chances of penetration of oxaliplatin and cytokines into the brain [52]. This invokes the local release of cytokines in the brain and enables the entrance of more chemotherapeutic agents and cytokines [53,54]. Chemobrain, another major complaint of patients treated with chemotherapy, might be related to this mechanism. Several patients treated with chemotherapy experience cognitive impairment, autonomic nervous dysfunction, decreased motor skills, and other general neurological symptoms. These symptoms may also occur due to the direct effect of chemotherapeutic agents on the brain and/or indirect effects on the BBB [51,53,54].
A few studies investigated the brain-level alterations accompanied by chronic pain symptoms in patients with CIPN [7]. Elaine et al. analyzed the brains of patients with CIPN using fMRI and reported that nociception-related brain regions undergo plastic changes due to pain symptoms [55]. Nudelman et al. analyzed cerebral perfusion and gray matter density of patients with CIPN using MRI [56]. They found that the patients showed increased cerebral perfusion in the frontal areas. They also reported that the degree of CIPN symptoms and related perfusion changes were correlated with the change in gray matter density. Nagasaka et al. studied altered brain activity using fMRI in a macaque model of CIPN [57]. The model animals injected with oxaliplatin showed increased activation in the somatosensory and insular cortices in response to cold stimulation. This was attenuated by duloxetine, a selective serotonin and norepinephrine inhibitor. In addition, inactivation of the brain regions with focal microinjection of GABA agonist muscimol ameliorated cold hypersensitivity symptoms. This indicates that the brain change was in part responsible for the expression of the CIPN. Another recent fMRI study performed by Yeh et al. reported a change in brain connectivity in patients with CIPN [58]. They analyzed brain networks as 11 different subtypes and found that patients treated with analgesic treatment (auricular point acupressure) reported reduced pain and their brain connectivity pattern shifted to a different subtype of the brain network. The difference between the brains of patients with CIPN and the control group did not tell the causal relationship in many reports; however, studies have shown that CIPN symptoms could be ameliorated by various methods related to the manipulation of brain activity [59,60,61].
5. Candidates for New Therapeutics: Approach with Knowledge from Traditional Eastern Medicine
Despite efforts to seek therapeutics for CIPN, heterogeneity in the prevalence, time to symptom incidence, and preexisting cancer condition of the patients make drug development difficult. The ideal intervention for CIPN should not interfere with the general condition of the patients with cancer, or the efficacy of the anti-cancer effect of the chemotherapeutic agents [62,63]. Substances that deteriorate the progression of cancer should be avoided even if they can suppress the pain of patients. One promising approach is to use knowledge from traditional Eastern medicine. Many substances used in traditional Eastern medicine have been studied for decades, and multiple studies have investigated their effects on cancer [16,17,18,19,20]. Furthermore, traditional Eastern medicine has a distinctive classification method for the effects of therapeutic substances. For example, some medicinal plants and natural chemicals are known to relieve “cold” symptoms of the body, including cold stimuli-related pain [64,65]. Considering that the representative symptoms of CIPN in patients treated with oxaliplatin or paclitaxel are cold-evoked allodynia, these “cold-relieving” medicinal substances from Eastern medicine could be promising candidates for new therapeutics for the symptoms [66,67,68]. In the following subsections, the effects of traditional medicinal substances on CIPN symptoms are reviewed. The preclinical and clinical studies of each substance are also summarized in Table 1. The molecular actions of the substances are briefly summarized in Figure 2.

Table 1.
The substances of traditional Eastern medicine that have been exploited to treat CIPN. Per os (p.o.); subcutaneous (s.c.); intraperitoneal (i.p.); intrathecal (i.t.); visual analogue scale (VAS); numerical rating scale (NRS); Common Terminology Criteria for Adverse Events (CTCAE); National Cancer Institute-Common Toxicity Criteria for Adverse Events (NCI-CTCAE); Neurotoxicity Criteria of Debiopharm (DEB-NTC); chemotherapy-induced peripheral neuropathy assessment tool (CIPNAT).
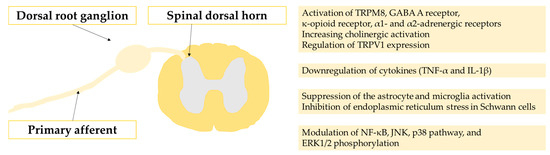
Figure 2.
The molecular actions of the substances presented in the paper.
5.1. Aconitum
In traditional Eastern medicine, substances derived from Aconitum have been used to warm meridian channels, thus ameliorating pain and numbness. Consistent with the traditional knowledge, Suzuki et al. found that Aconiti radix, the root of Aconitum carmichaeli, could alleviate oxaliplatin-induced mechanical and cold pain symptoms in mice with CIPN. This analgesic effect was mediated by its constituent neoline [69]. Jung et al. showed that Aconiti tuber could alleviate neuropathic pain symptoms in rats treated with oxaliplatin by suppressing the activation of astrocytes in the spinal dorsal horn and downregulating the production of pro-inflammatory cytokines, including TNF-α and IL-1β [65]. Previous studies found that bulleyaconitine A, an alkaloid isolated from Aconitum plants, could alleviate paclitaxel-induced CIPN symptoms by inhibiting synaptic transmission in C-fibers and the spinal cord [70,130]. In a clinical retrospective case series study, Aconiti radix-containing herbal formulas were shown to be beneficial for human patients with CIPN symptoms [131]. Although the effects were proven in the clinical and preclinical settings as shown above, Aconitum should be carefully administered to patients because it is toxic and can be lethal if not processed properly [132,133].
5.2. Astragalus
Astragalus belongs to the Fabaceae family and is believed to increase overall vitality. Astragali radix, the dried root of Astragalus membranaceus, has long been used as a remedy for various chronic diseases associated with energy shortages caused by an overactive immune system. Previous studies have shown its pharmacological action in modulating the immune system and its beneficial effects as an adjunct cancer therapy. Studies showed that Astragali radix extracts reduced oxaliplatin-induced pain symptoms via suppression of microglia and astrocyte activation [72,73]. Notably, the treatment showed a neuroprotective effect not only in the peripheral nerves and spinal cord, but also at the level of the brain [73]. Human studies also showed that the administration of this herb is beneficial for the prevention and treatment of neurotoxicity induced by oxaliplatin [134,135]. Deng et al. analyzed the effect of an Astragali radix-based herbal prescription (Wen-Luo-Tong) on CIPN symptoms in human patients and concluded that it is clinically effective for relieving symptoms [136,137]. Lee et al. showed the other Astragalus-based herbal prescription (SH003) could alleviate docetaxel-induced neuropathic pain [138]. Studies also showed that the components included in Astragali radix have an anti-cancer effect [139,140] and help to prevent nephrotoxicity and hepatotoxicity induced by the anti-cancer drugs [73]. These further suggest the usefulness of this substance in anti-cancer therapy.
5.3. Coptis
Coptis chinensis and Coptis teeta have been medicinally used in Eastern Asian regions for treating symptoms related to “damp-heat” of the body, such as fever, swelling, or dyspepsia. Berberine, a monomeric alkaloid compound included in Coptis, has been studied in previous studies and proven to exert anti-cancer effects by multiple mechanisms [141,142,143,144,145]. A recent study showed that berberine could alleviate CIPN symptoms induced by cisplatin treatment [74] as well. The authors of the study found that the alleviating effect of berberine on CIPN was mediated by the regulation of TRPV1 expression in DRG neurons. NF-κB expression and JNK/p38 MAPK/ERK pathways were involved in this modulatory effect of berberine.
5.4. Cinnamommum
Cinnamomum cassia Presl, a cinnamon tree (broiler tree) of the camphor family, has been widely used in East Asia to treat the symptoms of various diseases. Cinnamomi cortex is a representative “warm” medicinal herb and is believed to improve blood circulation, enhance the immune system, alleviate inflammation, and exert an analgesic effect. Studies have found that Cinnamomi cortex extract could attenuate oxaliplatin-induced mechanical and cold hypersensitivity in a rodent model of CIPN [66,67]. Kim et al. found that treatment with Cinnamomi cortex reduced CIPN symptoms by the suppression of glial activation and inhibition of IL-1β and TNF in the spinal cord [66]. The authors also revealed that coumarin and cinnamic acid in the Cinnamomi cortex are the major compounds responsible for the analgesic effect. Both these compounds also have anti-cancer effects [146,147,148,149].
5.5. Curcuma
Curcumin, a chemical from Curcuma longa plants, is known to promote blood circulation, eliminate blood stagnation and ameliorate pain. The analgesic effect of curcumin on CIPN symptoms can be attributed to multiple actions [75,76,77,150]. Zhang et al. suggested that curcumin alleviates oxaliplatin-induced pain symptoms by inhibiting the oxidative stress-mediated activation of NF-κB and mitigating inflammation [77]. Babu et al. suggested that the analgesic effect of curcumin on vincristine-induced pain is mediated by the suppression of pro-inflammatory cytokines [75]. Curcumin also has potent anti-cancer effects [151]. In addition, the co-administration of curcumin with chemotherapeutic agents induces synergistic action to increase anti-cancer activity [152,153,154,155]. Studies have shown that any drug administered in combination with curcumin could improve the anti-cancer effect [155,156,157,158,159].
5.6. Dryobalanops
Borneol, an organic terpene derivative, has been isolated from several plant species. In traditional medicine, natural borneol derived from Dryobalanops aromatica has been used to restore consciousness, remove heat, and relieve pain. It has been shown to be safe for human and is currently approved by the US FDA for use as a flavoring substance or adjuvant for food. Zhou et al. confirmed that borneol administration attenuated oxaliplatin-induced neuropathy in mice. The authors found that borneol exerted an analgesic effect against mechanical and cold allodynia via the blockade of transient receptor potential ankyrin 1 (TRPA1) [78]. Other studies found that the analgesic effect of borneol on general neuropathic pain also involves the activation of TRPM8 and GABA A receptors [160,161]. Notably, borneol transiently alters the permeability of the BBB, helping in drug delivery to the brain [162,163,164,165,166]. Multiple studies have shown that borneol treatment with chemotherapeutic agents could enhance the accumulation of the chemotherapeutic drug in the brain tissue and intracellular uptake, thereby improving the survival of glioma-bearing animals [167,168,169,170]. Borneol co-administered with temozolomide also enhanced anti-cancer efficacy of the drug against glioma by triggering mitochondrial dysfunction and ROS-induced DNA damage [171].
5.7. Lithospermum
Lithospermum belongs to the Boraginaceae family, and Lithospermi radix (the dried root of Lithospermum erythrorhizon) has been used to facilitate wound-healing and treatment of various inflammatory symptoms. Cho et al. showed that an aqueous extract of Lithospermi radix could ameliorate oxaliplatin-induced neurotoxicity, and suggested anti-inflammatory activities in the neuronal immune cells as the mechanisms for this effect [79]. The authors showed that treatment with Lithospermum suppressed the spinal activation of astrocytes and microglia, leading to the alleviation of mechanical hypersensitivity in oxaliplatin-induced neuropathy. The treatment also affected intraepidermal nerve fibers in the skin and DRG neurons. Yu et al. showed that herbal extract decoction containing Lithospermum could attenuate capecitabine-associated hand-foot syndrome in human patients. Shikonin, one of the main active ingredients of Lithospermum, has anti-nociceptive and anti-cancer effects [172,173,174], making it an attractive target for further research [175,176].
5.8. Paeonia
Paeonia lactiflora has been used to treat cardiovascular symptoms, muscle pain, and inflammatory and autoimmune diseases in traditional Eastern medicine. Paeoniae radix is included in various herbal formulas used to treat CIPN [68,177]. Paeoniflorin is a compound isolated from Paeonia lactiflora and has anti-oxidative, anti-inflammatory, and anti-cancer effects [178,179,180,181]. Studies have shown that paeoniflorin could alleviate nerve injury-induced neuropathic pain symptoms and suggested the suppression of the p38 MAPK pathway and NF-κB as the underlying mechanisms [182,183]. Andoh et al. showed that topical application of paeoniflorin prevented paclitaxel-induced mechanical allodynia by protecting sensory nerves from demyelination. This effect was mediated by the activation of the adenosine A1 receptor [80].
5.9. Plantago
Plantaginis semen, the dried seeds of Plantago asiatica, Plantago depressa, or Plantago major, have been used in traditional medicine to relieve heat symptoms. In addition, Plantaginis semen is one of the constituents of the Ucha Shinki Hwan (Gosha-jinki-Gan), an extensively studied herbal formula as a treatment for CIPN symptoms [81]. Andoh et al. suggested that the aqueous extract of Plantaginis semen ameliorated CIPN symptoms in paclitaxel-treated mice via its antioxidant activity [82]. The authors also found that aucubin and pedicularis-lactone, the major constituents of Plantaginis semen, played an important role in the anti-allodynic action [81,82,83,84]. The analgesic effect of aucubin on paclitaxel-induced mechanical allodynia is mediated by the inhibition of endoplasmic reticulum stress in peripheral Schwann cells [83]. The authors further studied pedicularis-lactone isolated from Viticis fructus, the dried fruit of Vitex rotundifolia Linné filius, instead of Plantaginis semen, and confirmed that this substance also showed a potent anti-allodynic effect. Unlike aucubin, the effect of pedicularis-lactone was not mediated by the inhibition of endoplasmic reticulum stress [84], and the underlying mechanisms are still unclear.
5.10. Sophora
Matrine is an alkaloid isolated from the herb Sophora flavescens, Sophora angustifolia, and Echinosophora koreensis. In traditional Eastern medicine, these herbs have been used as anti-inflammatory and analgesic drugs. The pain-relieving effect of matrine is mediated by multiple mechanisms. Studies showed that matrine exerted analgesic effects on vincristine-induced pain symptoms via its anti-oxidative, anti-inflammatory, and calcium antagonistic actions [85,86]. Other antinociceptive mechanisms of matrine include κ-opioid receptor agonism [184] and cholinergic activation in the nervous system [185]. Recent studies have shown that this substance can be used to reduce the side effects of opioid drugs, such as tolerance and withdrawal symptoms [186,187,188]. Matrine also has an anti-cancer effect, and could strengthen the anti-cancer capacity of other chemotherapeutic drugs [189,190]. Other beneficial effects of matrine include reversing anti-cancer drug resistance and reducing the toxicity of anti-cancer drugs [191].
5.11. Bee Venom Therapy
Bee venom has been traditionally used as a part of acupuncture therapy to treat diseases such as neuralgia, arthritis, facial paralysis, numbness, and various pain symptoms. Bee venom acupuncture, also referred to as apipuncture, involves the stimulation of acupoints with diluted bee venom. The main ingredients include melittin, apamin, and phospholipase A2. It has been used to exert healing effects in a variety of painful conditions in traditional Eastern medicine. CIPN can be mitigated or prevented by treatment with bee venom [87,88,90,91,92,93,95,97,98,99,192] or its constituents [89,94,193,194] in preclinical and clinical settings. Recent preclinical studies showed that the therapeutic effect is mediated by the activation of spinal α1- and α2-adrenergic receptors [92,94,193], although other mechanisms also exist [90,99,194]. Li et al. further suggested that the activation of spinal adrenergic receptors is mediated by the bee venom-induced release of norepinephrine from the locus coeruleus in the brain [96]. Bee venom treatment showed long-lasting and additive analgesic effects when combined with venlafaxine, a selective serotonin and noradrenaline reuptake inhibitor [90].
5.12. Pharmacopuncture Therapies
In clinical settings, pharmacopuncture therapy (acupoint injection of medicinal substances) is used to ameliorate the side effects of chemotherapy agents. A case report suggested that pharmacopuncture using the dried resin of Toxicodendron vernicifluum (Rhus verniciflua stokes) mixed with Cinnamomi cortex extracts ameliorated CIPN symptoms [102]. Another case study reported that snake venom pharmacopuncture could alleviate CIPN symptoms [100]. Yoon et al. showed that pharmacopuncture with Scolopendra subspinipes could suppress oxaliplatin-induced mechanical allodynia in mice, and the analgesic effect is mediated by the activation of α2-adrenergic receptors in the spinal cord [101].
5.13. Gyeji Ga Chul Bu Tang
Gyeji ga Chul Bu Tang (in Korean), also called Gui Zhi Jia Shu Fu Tang (in Chinese) and Keishi-ka-jutsu-bu-To (in Japanese), is a herbal formula including Cinnamomi cortex, Aconiti tuber, Atractylodis lanceae rhizome, Glycyrrhizae radix, Paeoniae radix, Zingiberis rhizoma, and Zizyphi fructus. This formula has been widely used for treating cold-related symptoms and pain symptoms in traditional medicine. Preclinical and clinical studies have shown that treatment with this formula has beneficial effects on CIPN symptoms. Cinnamomi cortex and Aconiti tuber are mainly responsible for relieving sensory symptoms [64,103,195].
5.14. Siwei Jianbu Tang
Siwei Jianbu Tang, a medicine created recently in China based on traditional medicinal knowledge, is made up of the following four herbs: Paeonia veitchii Lynch, Salvia miltiorrhiza Bge, Achyranthes bidentata Blume, and Dendrobium nobile Lindl. The main effect of this formula is to improve blood circulation, restore lower limb function, and relieve pain. Zhang et al. and Suo et al. showed that Siwei Jianbu could prevent CIPN symptoms by inhibiting the expression of NF-κB via the downregulation of phosphorylated ERK1/2 and p38 [104,105].
5.15. Ucha Shinki Hwan, Also Referred to as Jeseng Singi Hwan
Ucha Shinki Hwan (in Korean), also called Niu Che Shen Qi Wan (in Chinese), Gosha-jinki-Gan (in Japanese), Jeseng Singi Hwan (in Korean), and Ji Sheng Shen Qi Wan (in Chinese), is formulated from the following herbal ingredients: Cinnamomi cortex, Aconiti tuber, Rehmanniae radix, Achyranthis radix, Corni fructus, Moutan cortex, Alismatis rhizome, Dioscoreae rhizome, Plantaginis semen, and Poria sclerotium. In traditional medicine, this prescription has been used to treat numbness or cold hypersensitivity in the extremities, fatigue, and pain. Studies have found that treatment with this formula could ameliorate CIPN in preclinical [71,106,107,108,109,110,111,112,118] and clinical settings [113,114,115,196,197,198], with controversial prevention effects [116,117,196,197,199].
5.16. Yukgunja Tang
Yukgunja Tang, also referred to as Liu Jun Zi Tang (in Chinese), and Rikkunshi-To (in Japanese), consists of ginseng, Atractylodes, Poria cocos, Glycyrrhizae, Pericarpium Citri Reticulatae, and Pinellia ternata. Its main chemical constituents include succinic acid, hesperidin, ginsenoside Rb1, glycyrrhizic acid I, 2-atractylenolide, and pachymic acid. This decoction has been used to treat functional dyspepsia in traditional Eastern medicine. With its modulatory effects on gastrointestinal disturbance, this medicine is currently used clinically as a complementary therapy to attenuate cisplatin-induced side effects. Chiou et al. showed that this formula could attenuate CIPN symptoms in mice treated with platinum compounds through its anti-oxidative effect and regulatory action on mitochondria [119].
5.17. Other Herbal Formulas
Park et al. studied the nerve regeneration effect of Bogi Jetong Tang, a traditional decoction that consists of 18 medicinal ingredients, on a CIPN rat model [120]. Jeong et al. further showed the effects of Yideung Jetong Tang, which is a compressed version of Bogi Jetong Tang and consists of 12 ingredients, in a similar manner [121]. An et al. reported a case of a patient who experienced CIPN symptoms for over 2 years and was relieved by Ohjeok San treatment [122]. Hwanggi Gyeji Omul Tang (in Korean), also called Huang Qi Gui Zhi Wu Wu Tang (in Chinese) [123,124,125,200] and Ogi-keishi-gomotsu-To (in Japanese) [126], ameliorated symptoms of CIPN. Jakyak Gamcho Tang (in Korean), also called Shao Yao Gan Cao Tang (in Chinese) and Shakuyaku-kanzo-To (in Japanese), could help reduce CIPN symptoms [127,128,129].
6. Barriers in the Spread of Therapeutics for CIPN Based on Traditional Eastern Medicine
As introduced above, traditional medicinal approaches are frequently utilized to treat symptoms of CIPN in Asian countries and have shown significant results in clinical or preclinical settings. Nevertheless, therapeutic methods are not frequently introduced to the international community. The clinicians and patients in non-Asian countries have fewer opportunities to use those natural compounds that are under quality control by national healthcare systems, and the beneficial effects of the substances are sometimes controversial. As a result, these natural compounds ameliorating the adverse effects of chemotherapeutic agents tend to be used only in a subset of Asian countries. There exists several barriers that impede the spread of the traditional medicine against CIPN. Firstly, in the aspect of basic research, clinicians and researchers in non-Asian countries have less access to studies of traditional Eastern medicine. Studies on the traditional medicinal approach are fragmented according to the mixture of medicinal products (i.e., herbal formulas), natural materials that consist of the mixture, and individual compounds contained in the materials. In these circumstances, it is difficult for researchers and clinicians to know which substances should be of interest. Moreover, different Asian countries may have different names for each component. For example, research papers on herbal formulas use different notations depending on the country where the research was conducted (see alias of herbal formulas in Table 1). This makes it more difficult for Western researchers to search for and identify relevant studies. Next, from a clinical point of view, the issue of priority in the treatment arises. All CIPN patients have suffered from cancer, and the anti-cancer treatment inevitably becomes a priority for them. The patients and clinicians are often concerned about whether the interventions for CIPN may interfere with chemotherapy and/or facilitate cancer development. This makes them hesitant to try these seemingly speculative methods. They often decide to ignore CIPN symptoms or stick to the ineffective conventional treatments, rather than exploiting interventions in which the molecular pathways of action are not fully explained. To address this concern, future studies should provide detailed analgesic mechanisms along with more evidence that can prove the interventions do not exacerbate cancer. Clinical studies that can show strong statistical evidence are also needed.
7. Concluding Remarks
Knowledge of traditional Eastern medicine is useful not only for clinicians caring for patients with CIPN but also for researchers seeking new treatments. The substances derived from Eastern medicine can act on multiple targets with various mechanisms to exert analgesic effects against CIPN. In addition, some have anti-cancer effects on their own, and others can increase the efficiency of other co-treated anti-cancer drugs, with enhanced drug delivery and/or other molecular mechanisms. Generally, many substances act on multiple biological targets and exert beneficial effects, such as anti-inflammatory, anti-oxidative, anti-microbial, anti-obesity, neuroprotective, cardioprotective, and wound-healing effects. Utilizing this knowledge would help ameliorate the symptoms of patients and develop new treatments. However, we are not claiming that the treatments with natural compounds from traditional Eastern medicine substitute the chemotherapy. The current limitation is that the mechanisms involved in these actions have not been fully elucidated. More research is needed for a detailed understanding of the mechanisms underlying the molecular actions of the substances and for developing novel drugs based on this knowledge. The appropriate use of traditional knowledge would help in the discovery of efficient molecules in preclinical settings and lower the barrier to the development of new clinical pharmaceutics.
Author Contributions
Conceptualization, G.C. and S.K.K.; methodology, G.C.; software, G.C.; validation, G.C. and S.K.K.; formal analysis, G.C.; investigation, G.C.; resources, G.C. and S.K.K.; data curation, G.C.; writing—original draft preparation, G.C.; writing—review and editing, G.C. and S.K.K.; visualization, G.C.; supervision, G.C. and S.K.K.; project administration, G.C. and S.K.K.; funding acquisition, G.C. and S.K.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Research Foundation of Korea (NRF), grant number NRF-2020R1C1C1009162 to G.C., and NRF-2017M3A9E4057926 to S.K.K.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Kerckhove, N.; Collin, A.; Condé, S.; Chaleteix, C.; Pezet, D.; Balayssac, D. Long-term effects, pathophysiological mechanisms, and risk factors of chemotherapy-induced peripheral neuropathies: A comprehensive literature review. Front. Pharmacol. 2017, 8, 86. [Google Scholar] [CrossRef] [PubMed]
- Flatters, S.J.L.; Dougherty, P.M.; Colvin, L.A. Clinical and preclinical perspectives on Chemotherapy-Induced Peripheral Neuropathy (CIPN): A narrative review. Br. J. Anaesth. 2017, 119, 737–749. [Google Scholar] [CrossRef] [PubMed]
- Jones, D.; Zhao, F.; Brell, J.; Lewis, M.A.; Loprinzi, C.L.; Weiss, M.; Fisch, M.J. Neuropathic symptoms, quality of life, and clinician perception of patient care in medical oncology outpatients with colorectal, breast, lung, and prostate cancer. J. Cancer Surviv. 2015, 9, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Staff, N.P.; Grisold, A.; Grisold, W.; Windebank, A.J. Chemotherapy-induced peripheral neuropathy: A current review. Ann. Neurol. 2017, 81, 772–781. [Google Scholar] [CrossRef] [PubMed]
- Loprinzi, C.L.; Lacchetti, C.; Bleeker, J.; Cavaletti, G.; Chauhan, C.; Hertz, D.L.; Kelley, M.R.; Lavino, A.; Lustberg, M.B.; Paice, J.A. Prevention and management of chemotherapy-induced peripheral neuropathy in survivors of adult cancers: ASCO guideline update. J. Clin. Oncol. 2020, 38, 3325–3348. [Google Scholar] [CrossRef] [PubMed]
- Zajaczkowską, R.; Kocot-Kępska, M.; Leppert, W.; Wrzosek, A.; Mika, J.; Wordliczek, J. Mechanisms of chemotherapy-induced peripheral neuropathy. Int. J. Mol. Sci. 2019, 20, 1451. [Google Scholar] [CrossRef] [PubMed]
- Omran, M.; Belcher, E.K.; Mohile, N.A.; Kesler, S.R.; Janelsins, M.C.; Hohmann, A.G.; Kleckner, I.R. Review of the Role of the Brain in Chemotherapy-Induced Peripheral Neuropathy. Front. Mol. Biosci. 2021, 8, 693133. [Google Scholar] [CrossRef]
- Kim, S.K.; Hayashi, H.; Ishikawa, T.; Shibata, K.; Shigetomi, E.; Shinozaki, Y.; Inada, H.; Roh, S.E.; Kim, S.J.; Lee, G.; et al. Cortical astrocytes rewire somatosensory cortical circuits for peripheral neuropathic pain. J. Clin. Investig. 2016, 126, 1983–1997. [Google Scholar] [CrossRef]
- Chung, G.; Shim, H.G.; Kim, C.Y.; Ryu, H.H.; Jang, D.C.; Kim, S.H.; Lee, J.; Kim, C.E.; Kim, Y.K.; Lee, Y.S.; et al. Persistent Activity of Metabotropic Glutamate Receptor 5 in the Periaqueductal Gray Constrains Emergence of Chronic Neuropathic Pain. Curr. Biol. 2020, 30, 4631–4642.e6. [Google Scholar] [CrossRef]
- Meacham, K.; Shepherd, A.; Mohapatra, D.P.; Haroutounian, S. Neuropathic Pain: Central vs. Peripheral Mechanisms. Curr. Pain Headache Rep. 2017, 21, 28. [Google Scholar] [CrossRef]
- Beijers, A.; Mols, F.; Dercksen, W.; Driessen, C.; Vreugdenhil, G. Chemotherapy-induced peripheral neuropathy and impact on quality of life 6 months after treatment with chemotherapy. J. Community Support. Oncol. 2014, 12, 401–406. [Google Scholar] [CrossRef]
- Selvy, M.; Pereira, B.; Kerckhove, N.; Gonneau, C.; Feydel, G.; Pétorin, C.; Vimal-Baguet, A.; Melnikov, S.; Kullab, S.; Hebbar, M.; et al. Long-Term Prevalence of Sensory Chemotherapy-Induced Peripheral Neuropathy for 5 Years after Adjuvant FOLFOX Chemotherapy to Treat Colorectal Cancer: A Multicenter Cross-Sectional Study. J. Clin. Med. 2020, 9, 2400. [Google Scholar] [CrossRef]
- Noh, H.; Yoon, S.W.; Park, B. A Systematic Review of Herbal Medicine for Chemotherapy Induced Peripheral Neuropathy. Evid.-Based Complement. Altern. Med. 2018, 2018, 6194184. [Google Scholar] [CrossRef]
- Li, Z.; Jin, H.; Yan, Q.; Sun, L.; Wasan, H.S.; Shen, M.; Ruan, S. The Method of Activating Blood and Dredging Collaterals for Reducing Chemotherapy-Induced Peripheral Neuropathy: A Systematic Review and Meta-Analysis. Evid.-Based Complement. Altern. Med. 2019, 2019, 1029626. [Google Scholar] [CrossRef]
- Hao, J.; Zhu, X.; Bensoussan, A. Effects of Nonpharmacological Interventions in Chemotherapy-Induced Peripheral Neuropathy: An Overview of Systematic Reviews and Meta-Analyses. Integr. Cancer Ther. 2020, 19, 1534735420945027. [Google Scholar] [CrossRef]
- Wang, S.-F.; Wu, M.-Y.; Cai, C.-Z.; Li, M.; Lu, J.-H. Autophagy modulators from traditional Chinese medicine: Mechanisms and therapeutic potentials for cancer and neurodegenerative diseases. J. Ethnopharmacol. 2016, 194, 861–876. [Google Scholar] [CrossRef]
- Xiang, Y.; Guo, Z.; Zhu, P.; Chen, J.; Huang, Y. Traditional Chinese medicine as a cancer treatment: Modern perspectives of ancient but advanced science. Cancer Med. 2019, 8, 1958–1975. [Google Scholar] [CrossRef]
- Zhang, Y.; Lou, Y.; Wang, J.; Yu, C.; Shen, W. Research Status and Molecular Mechanism of the Traditional Chinese Medicine and Antitumor Therapy Combined Strategy Based on Tumor Microenvironment. Front. Immunol. 2021, 11, 609705. [Google Scholar] [CrossRef]
- Park, J.; Jeong, D.; Song, M.; Kim, B. Recent Advances in Anti-Metastatic Approaches of Herbal Medicines in 5 Major Cancers: From Traditional Medicine to Modern Drug Discovery. Antioxidants 2021, 10, 527. [Google Scholar] [CrossRef]
- Kim, A.; Ha, J.; Kim, J.; Cho, Y.; Ahn, J.; Cheon, C.; Kim, S.H.; Ko, S.G.; Kim, B. Natural Products for Pancreatic Cancer Treatment: From Traditional Medicine to Modern Drug Discovery. Nutrients 2021, 13, 3801. [Google Scholar] [CrossRef]
- Colvin, L.A. Chemotherapy-induced peripheral neuropathy: Where are we now? Pain 2019, 160, S1–S10. [Google Scholar] [CrossRef]
- Seretny, M.; Currie, G.L.; Sena, E.S.; Ramnarine, S.; Grant, R.; Macleod, M.R.; Colvin, L.A.; Fallon, M. Incidence, prevalence, and predictors of chemotherapy-induced peripheral neuropathy: A systematic review and meta-analysis. Pain 2014, 155, 2461–2470. [Google Scholar] [CrossRef]
- Siegal, T.; Haim, N. Cisplatin-induced peripheral neuropathy. Frequent off-therapy deterioration, demyelinating syndromes, and muscle cramps. Cancer 1990, 66, 1117–1123. [Google Scholar] [CrossRef]
- Han, Y.; Smith, M.T. Pathobiology of cancer chemotherapy-induced peripheral neuropathy (CIPN). Front. Pharmacol. 2013, 4, 156. [Google Scholar] [CrossRef]
- Starobova, H.; Vetter, I. Pathophysiology of chemotherapy-induced peripheral neuropathy. Front. Mol. Neurosci. 2017, 10, 174. [Google Scholar] [CrossRef]
- Krauss, R.; Bosanac, T.; Devraj, R.; Engber, T.; Hughes, R.O. Axons Matter: The Promise of Treating Neurodegenerative Disorders by Targeting SARM1-Mediated Axonal Degeneration. Trends Pharmacol. Sci. 2020, 41, 281–293. [Google Scholar] [CrossRef]
- Canta, A.; Pozzi, E.; Carozzi, V.A. Mitochondrial dysfunction in chemotherapy-induced peripheral neuropathy (CIPN). Toxics 2015, 3, 198–223. [Google Scholar] [CrossRef]
- Yilmaz, E.; Watkins, S.C.; Gold, M.S. Paclitaxel-induced increase in mitochondrial volume mediates dysregulation of intracellular Ca2+ in putative nociceptive glabrous skin neurons from the rat. Cell Calcium 2017, 62, 16–28. [Google Scholar] [CrossRef]
- Rovini, A. Tubulin-VDAC interaction: Molecular basis for mitochondrial dysfunction in chemotherapy-induced peripheral neuropathy. Front. Physiol. 2019, 10, 671. [Google Scholar] [CrossRef]
- Genualdi, C.; Feinstein, S.C.; Wilson, L.; Jordan, M.A.; Stagg, N.J. Assessing the utility of in vitro microtubule assays for studying mechanisms of peripheral neuropathy with the microtubule inhibitor class of cancer chemotherapy. Chem. Biol. Interact. 2020, 315, 108906. [Google Scholar] [CrossRef]
- Pero, M.E.; Meregalli, C.; Qu, X.; Shin, G.J.E.; Kumar, A.; Shorey, M.; Rolls, M.M.; Tanji, K.; Brannagan, T.H.; Alberti, P.; et al. Pathogenic role of delta 2 tubulin in bortezomib-induced peripheral neuropathy. Proc. Natl. Acad. Sci. USA 2021, 118, e2012685118. [Google Scholar] [CrossRef] [PubMed]
- Chua, K.C.; El-Haj, N.; Priotti, J.; Kroetz, D.L. Mechanistic insights into the pathogenesis of microtubule-targeting agent-induced peripheral neuropathy from pharmacogenetic and functional studies. Basic Clin. Pharmacol. Toxicol. 2022, 130 (Suppl. 1), 60–74. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, J.C.; Muñoz, L.V.; Ehrlich, B.E. Modulating TRPV4 channels with paclitaxel and lithium. Cell Calcium 2020, 91, 102266. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Huang, J.; Benson, C.; Lankford, K.L.; Zhao, P.; Carrara, J.; Tan, A.M.; Kocsis, J.D.; Waxman, S.G.; Dib-Hajj, S.D. Sodium channel Nav1.6 in sensory neurons contributes to vincristine-induced allodynia. Brain 2020, 143, 2421–2436. [Google Scholar] [CrossRef]
- Brandolini, L.; d’Angelo, M.; Novelli, R.; Castelli, V.; Giorgio, C.; Sirico, A.; Cocchiaro, P.; D’Egidio, F.; Benedetti, E.; Cristiano, C.; et al. Paclitaxel binds and activates C5aR1: A new potential therapeutic target for the prevention of chemotherapy-induced peripheral neuropathy and hypersensitivity reactions. Cell Death Dis. 2022, 13, 500. [Google Scholar] [CrossRef]
- Illias, A.M.; Yu, K.-J.; Hwang, S.-H.; Solis, J.; Zhang, H.; Velasquez, J.F.; Cata, J.P.; Dougherty, P.M. Dorsal root ganglion toll-like receptor 4 signaling contributes to oxaliplatin-induced peripheral neuropathy. Pain 2022, 163, 923–935. [Google Scholar] [CrossRef]
- Domoto, R.; Sekiguchi, F.; Kamaguchi, R.; Iemura, M.; Yamanishi, H.; Tsubota, M.; Wang, D.; Nishibori, M.; Kawabata, A. Role of neuron-derived ATP in paclitaxel-induced HMGB1 release from macrophages and peripheral neuropathy. J. Pharmacol. Sci. 2022, 148, 156–161. [Google Scholar] [CrossRef]
- Sun, W.; Yang, S.; Wu, S.; Ba, X.; Xiong, D.; Xiao, L.; Hao, Y. Transcriptome analysis reveals dysregulation of inflammatory and neuronal function in dorsal root ganglion of paclitaxel-induced peripheral neuropathy rats. Mol. Pain 2022, 174480692211061. [Google Scholar] [CrossRef]
- Woller, S.A.; Choi, S.H.; An, E.J.; Low, H.; Schneider, D.A.; Ramachandran, R.; Kim, J.; Bae, Y.S.; Sviridov, D.; Corr, M.; et al. Inhibition of Neuroinflammation by AIBP: Spinal Effects upon Facilitated Pain States. Cell Rep. 2018, 23, 2667–2677. [Google Scholar] [CrossRef]
- Lee, J.H.; Kim, N.; Park, S.; Kim, S.K. Analgesic effects of medicinal plants and phytochemicals on chemotherapy-induced neuropathic pain through glial modulation. Pharmacol. Res. Perspect. 2021, 9, e00819. [Google Scholar] [CrossRef]
- Fumagalli, G.; Monza, L.; Cavaletti, G.; Rigolio, R.; Meregalli, C. Neuroinflammatory Process Involved in Different Preclinical Models of Chemotherapy-Induced Peripheral Neuropathy. Front. Immunol. 2021, 11, 626687. [Google Scholar] [CrossRef]
- Hald, A. Spinal astrogliosis in pain models: Cause and effects. Cell. Mol. Neurobiol. 2009, 29, 609–619. [Google Scholar] [CrossRef]
- Robinson, C.R.; Zhang, H.; Dougherty, P.M. Astrocytes, but not microglia, are activated in oxaliplatin and bortezomib-induced peripheral neuropathy in the rat. Neuroscience 2014, 274, 308–317. [Google Scholar] [CrossRef]
- Zhang, H.; Yoon, S.Y.; Zhang, H.; Dougherty, P.M. Evidence that spinal astrocytes but not microglia contribute to the pathogenesis of paclitaxel-induced painful neuropathy. J. Pain 2012, 13, 293–303. [Google Scholar] [CrossRef]
- Hu, L.Y.; Zhou, Y.; Cui, W.Q.; Hu, X.M.; Du, L.X.; Mi, W.L.; Chu, Y.X.; Wu, G.C.; Wang, Y.Q.; Mao-Ying, Q.L. Triggering receptor expressed on myeloid cells 2 (TREM2) dependent microglial activation promotes cisplatin-induced peripheral neuropathy in mice. Brain. Behav. Immun. 2018, 68, 132–145. [Google Scholar] [CrossRef]
- St. Germain, D.C.; O’Mara, A.M.; Robinson, J.L.; Torres, A.D.; Minasian, L.M. Chemotherapy-induced peripheral neuropathy: Identifying the research gaps and associated changes to clinical trial design. Cancer 2020, 126, 4602–4613. [Google Scholar] [CrossRef]
- Jacobs, S.S.; Fox, E.; Dennie, C.; Morgan, L.B.; McCully, C.L.; Balis, F.M. Plasma and cerebrospinal fluid pharmacokinetics of intravenous oxaliplatin, cisplatin, and carboplatin in nonhuman primates. Clin. Cancer Res. 2005, 11, 1669–1674. [Google Scholar] [CrossRef]
- Dorigo, O.; Turla, S.T.; Lebedeva, S.; Gjerset, R.A. Sensitization of rat glioblastoma multiforme to cisplatin in vivo following restoration of wild-type p53 function. J. Neurosurg. 1998, 88, 535–540. [Google Scholar] [CrossRef]
- Steiniger, S.C.J.; Kreuter, J.; Khalansky, A.S.; Skidan, I.N.; Bobruskin, A.I.; Smirnova, Z.S.; Severin, S.E.; Uhl, R.; Kock, M.; Geiger, K.D. Chemotherapy of glioblastoma in rats using doxorubicin-loaded nanoparticles. Int. J. Cancer 2004, 109, 759–767. [Google Scholar] [CrossRef]
- Gangloff, A.; Hsueh, W.-A.; Kesner, A.L.; Kiesewetter, D.O.; Pio, B.S.; Pegram, M.D.; Beryt, M.; Townsend, A.; Czernin, J.; Phelps, M.E. Estimation of paclitaxel biodistribution and uptake in human-derived xenografts in vivo with 18F-fluoropaclitaxel. J. Nucl. Med. 2005, 46, 1866–1871. [Google Scholar]
- Ongnok, B.; Chattipakorn, N.; Chattipakorn, S.C. Doxorubicin and cisplatin induced cognitive impairment: The possible mechanisms and interventions. Exp. Neurol. 2020, 324, 113118. [Google Scholar] [CrossRef]
- Branca, J.J.V.; Maresca, M.; Morucci, G.; Becatti, M.; Paternostro, F.; Gulisano, M.; Ghelardini, C.; Salvemini, D.; Mannelli, L.D.C.; Pacini, A. Oxaliplatin-induced blood brain barrier loosening: A new point of view on chemotherapy-induced neurotoxicity. Oncotarget 2018, 9, 23426–23438. [Google Scholar] [CrossRef]
- Ren, X.; Clair, D.K.S.; Butterfield, D.A. Dysregulation of cytokine mediated chemotherapy induced cognitive impairment. Pharmacol. Res. 2017, 117, 267–273. [Google Scholar] [CrossRef]
- Nguyen, L.D.; Ehrlich, B.E. Cellular mechanisms and treatments for chemobrain: Insight from aging and neurodegenerative diseases. EMBO Mol. Med. 2020, 12, e12075. [Google Scholar] [CrossRef]
- Boland, E.G.; Selvarajah, D.; Hunter, M.; Ezaydi, Y.; Tesfaye, S.; Ahmedzai, S.H.; Snowden, J.A.; Wilkinson, I.D. Central pain processing in chronic chemotherapy- induced peripheral neuropathy: A functional magnetic resonance imaging study. PLoS ONE 2014, 9, e96474. [Google Scholar] [CrossRef]
- Nudelman, K.N.H.; McDonald, B.C.; Wang, Y.; Smith, D.J.; West, J.D.; O’Neill, D.P.; Zanville, N.R.; Champion, V.L.; Schneider, B.P.; Saykin, A.J. Cerebral perfusion and gray matter changes associated with chemotherapy-induced peripheral neuropathy. J. Clin. Oncol. 2016, 34, 677–683. [Google Scholar] [CrossRef]
- Nagasaka, K.; Yamanaka, K.; Ogawa, S.; Takamatsu, H.; Higo, N. Brain activity changes in a macaque model of oxaliplatin-induced neuropathic cold hypersensitivity. Sci. Rep. 2017, 7, 4305. [Google Scholar] [CrossRef]
- Yeh, C.H.; Caswell, K.; Pandiri, S.; Sair, H.; Lukkahatai, N.; Campbell, C.M.; Stearns, V.; Van de Castle, B.; Perrin, N.; Smith, T.J.; et al. Dynamic Brain Activity Following Auricular Point Acupressure in Chemotherapy-Induced Neuropathy: A Pilot Longitudinal Functional Magnetic Resonance Imaging Study. Glob. Adv. Health Med. 2020, 9, 216495612090609. [Google Scholar] [CrossRef]
- Prinsloo, S.; Novy, D.; Driver, L.; Lyle, R.; Ramondetta, L.; Eng, C.; Lopez, G.; Li, Y.; Cohen, L. The Long-Term Impact of Neurofeedback on Symptom Burden and Interference in Patients With Chronic Chemotherapy-Induced Neuropathy: Analysis of a Randomized Controlled Trial. J. Pain Symptom Manag. 2018, 55, 1276–1285. [Google Scholar] [CrossRef]
- Vollmers, P.L.; Mundhenke, C.; Maass, N.; Bauerschlag, D.; Kratzenstein, S.; Röcken, C.; Schmidt, T. Evaluation of the effects of sensorimotor exercise on physical and psychological parameters in breast cancer patients undergoing neurotoxic chemotherapy. J. Cancer Res. Clin. Oncol. 2018, 144, 1785–1792. [Google Scholar] [CrossRef]
- Goto, Y.; Hosomi, K.; Shimokawa, T.; Shimizu, T.; Yoshino, K.; Kim, S.J.; Mano, T.; Kishima, H.; Saitoh, Y. Pilot study of repetitive transcranial magnetic stimulation in patients with chemotherapy-induced peripheral neuropathy. J. Clin. Neurosci. 2020, 73, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Gewandter, J.S.; Brell, J.; Cavaletti, G.; Dougherty, P.M.; Evans, S.; Howie, L.; McDermott, M.P.; O’Mara, A.; Smith, A.G.; Dastros-Pitei, D.; et al. Trial designs for chemotherapy-induced peripheral neuropathy prevention. Neurology 2018, 91, 403–413. [Google Scholar] [CrossRef] [PubMed]
- Sałat, K. Chemotherapy-induced peripheral neuropathy—Part 2: Focus on the prevention of oxaliplatin-induced neurotoxicity. Pharmacol. Rep. 2020, 72, 508–527. [Google Scholar]
- Ahn, B.-S.; Kim, S.-K.; Kim, H.N.; Lee, J.-H.; Lee, J.-H.; Hwang, D.S.; Bae, H.; Min, B.-I.; Kim, S.K. Gyejigachulbu-tang relieves oxaliplatin-induced neuropathic cold and mechanical hypersensitivity in rats via the suppression of spinal glial activation. Evid.-Based Complement. Altern. Med. 2014, 2014, 436482. [Google Scholar] [CrossRef] [PubMed]
- Jung, Y.; Lee, J.H.; Kim, W.; Yoon, S.H.; Kim, S.K. Anti-allodynic effect of Buja in a rat model of oxaliplatin-induced peripheral neuropathy via spinal astrocytes and pro-inflammatory cytokines suppression. BMC Complement. Altern. Med. 2017, 17, 48. [Google Scholar] [CrossRef]
- Kim, C.; Lee, J.H.; Kim, W.; Li, D.; Kim, Y.; Lee, K.; Kim, S.K. The suppressive effects of Cinnamomi Cortex and its phytocompound coumarin on oxaliplatin-induced neuropathic cold allodynia in rats. Molecules 2016, 21, 1253. [Google Scholar] [CrossRef]
- Chae, H.K.; Kim, W.; Kim, S.K. Phytochemicals of cinnamomi cortex: Cinnamic acid, but not cinnamaldehyde, attenuates oxaliplatin-induced cold and mechanical hypersensitivity in rats. Nutrients 2019, 11, 432. [Google Scholar] [CrossRef]
- Lee, G.; Kim, S.K. Therapeutic effects of phytochemicals and medicinal herbs on chemotherapy-induced peripheral neuropathy. Molecules 2016, 21, 1252. [Google Scholar] [CrossRef]
- Suzuki, T.; Miyamoto, K.; Yokoyama, N.; Sugi, M.; Kagioka, A.; Kitao, Y.; Adachi, T.; Ohsawa, M.; Mizukami, H.; Makino, T. Processed aconite root and its active ingredient neoline may alleviate oxaliplatin-induced peripheral neuropathic pain. J. Ethnopharmacol. 2016, 186, 44–52. [Google Scholar] [CrossRef]
- Zhu, H.Q.; Xu, J.; Shen, K.F.; Pang, R.P.; Wei, X.H.; Liu, X.G. Bulleyaconitine A depresses neuropathic pain and potentiation at C-fiber synapses in spinal dorsal horn induced by paclitaxel in rats. Exp. Neurol. 2015, 273, 263–272. [Google Scholar] [CrossRef]
- Higuchi, H.; Yamamoto, S.; Ushio, S.; Kawashiri, T.; Egashira, N. Goshajinkigan reduces bortezomib-induced mechanical allodynia in rats: Possible involvement of kappa opioid receptor. J. Pharmacol. Sci. 2015, 129, 196–199. [Google Scholar] [CrossRef]
- Mannelli, L.D.C.; Zanardelli, M.; Bartolucci, G.; Karioti, A.; Bilia, A.R.; Vannacci, A.; Mugelli, A.; Ghelardini, C. In vitro evidence for the use of astragali radix extracts as adjuvant against oxaliplatin-induced neurotoxicity. Planta Med. 2015, 81, 1045–1055. [Google Scholar]
- Mannelli, L.D.C.; Pacini, A.; Micheli, L.; Femia, A.P.; Maresca, M.; Zanardelli, M.; Vannacci, A.; Gallo, E.; Bilia, A.R.; Caderni, G. Astragali radix: Could it be an adjuvant for oxaliplatin-induced neuropathy? Sci. Rep. 2017, 7, 42021. [Google Scholar] [CrossRef]
- Meng, J.; Qiu, S.; Zhang, L.; You, M.; Xing, H.; Zhu, J. Berberine Alleviate Cisplatin-Induced Peripheral Neuropathy by Modulating Inflammation Signal via TRPV1. Front. Pharmacol. 2022, 12, 774795. [Google Scholar] [CrossRef]
- Babu, A.; Prasanth, K.G.; Balaji, B. Effect of curcumin in mice model of vincristine-induced neuropathy. Pharm. Biol. 2015, 53, 838–848. [Google Scholar] [CrossRef]
- Agthong, S.; Kaewsema, A.; Charoensub, T. Curcumin ameliorates functional and structural abnormalities in cisplatin-induced neuropathy. Exp. Neurobiol. 2015, 24, 139–145. [Google Scholar] [CrossRef]
- Zhang, X.; Guan, Z.; Wang, X.; Sun, D.; Wang, D.; Li, Y.; Pei, B.; Ye, M.; Xu, J.; Yue, X. Curcumin alleviates oxaliplatin-induced peripheral neuropathic pain through inhibiting oxidative stress-mediated activation of NF-κB and mitigating inflammation. Biol. Pharm. Bull. 2020, 43, 348–355. [Google Scholar] [CrossRef]
- Zhou, H.H.; Zhang, L.; Zhou, Q.G.; Fang, Y.; Ge, W.H. (+)-Borneol attenuates oxaliplatin-induced neuropathic hyperalgesia in mice. Neuroreport 2016, 27, 160–165. [Google Scholar] [CrossRef]
- Cho, E.-S.; Yi, J.-M.; Park, J.-S.; Lee, Y.J.; Lim, C.J.; Bang, O.-S.; Kim, N.S. Aqueous extract of Lithospermi radix attenuates oxaliplatin-induced neurotoxicity in both in vitro and in vivo models. BMC Complement. Altern. Med. 2016, 16, 419. [Google Scholar] [CrossRef]
- Andoh, T.; Kobayashi, N.; Uta, D.; Kuraishi, Y. Prophylactic topical paeoniflorin prevents mechanical allodynia caused by paclitaxel in mice through adenosine A1 receptors. Phytomedicine 2017, 25, 1–7. [Google Scholar] [CrossRef]
- Toume, K.; Hou, Z.; Yu, H.; Kato, M.; Maesaka, M.; Bai, Y.; Hanazawa, S.; Ge, Y.; Andoh, T.; Komatsu, K. Search of anti-allodynic compounds from Plantaginis Semen, a crude drug ingredient of Kampo formula “Goshajinkigan”. J. Nat. Med. 2019, 73, 761–768. [Google Scholar] [CrossRef]
- Andoh, T.; Kato, M.; Kitamura, R.; Mizoguchi, S.; Uta, D.; Toume, K.; Komatsu, K.; Kuraishi, Y. Prophylactic administration of an extract from Plantaginis Semen and its major component aucubin inhibits mechanical allodynia caused by paclitaxel in mice. J. Tradit. Complement. Med. 2016, 6, 305–308. [Google Scholar] [CrossRef]
- Andoh, T.; Uta, D.; Kato, M.; Toume, K.; Komatsu, K.; Kuraishi, Y. Prophylactic administration of aucubin inhibits paclitaxel-induced mechanical allodynia via the inhibition of endoplasmic reticulum stress in peripheral Schwann cells. Biol. Pharm. Bull. 2017, 40, 473–478. [Google Scholar] [CrossRef]
- Yu, H.; Toume, K.; Kurokawa, Y.; Andoh, T.; Komatsu, K. Iridoids isolated from Viticis Fructus inhibit paclitaxel-induced mechanical allodynia in mice. J. Nat. Med. 2020, 75, 48–55. [Google Scholar] [CrossRef]
- Gong, S.-S.; Li, Y.-X.; Zhang, M.-T.; Du, J.; Ma, P.-S.; Yao, W.-X.; Zhou, R.; Niu, Y.; Sun, T.; Yu, J.-Q. Neuroprotective effect of matrine in mouse model of vincristine-induced neuropathic pain. Neurochem. Res. 2016, 41, 3147–3159. [Google Scholar] [CrossRef]
- Dun, L.; Li, Y.; Xu, Y.; Zhou, R.; Ma, L.; Jin, S.; Du, J.; Sun, T.; Yu, J. Antinociceptive effect of matrine on vincristine-induced neuropathic pain model in mice. Neurol. Sci. 2014, 35, 815–821. [Google Scholar] [CrossRef]
- Yoon, S.-Y.Y.; Yeo, J.-H.H.; Han, S.-D.D.; Bong, D.-J.J.; Oh, B.; Roh, D.-H.H. Diluted bee venom injection reduces ipsilateral mechanical allodynia in oxaliplatin-induced neuropathic mice. Biol. Pharm. Bull. 2013, 36, 1787–1793. [Google Scholar] [CrossRef]
- Kim, W.; Kim, M.J.; Go, D.; Min, B.I.; Na, H.S.; Kim, S.K. Combined effects of bee venom acupuncture and morphine on Oxaliplatin-induced neuropathic pain in mice. Toxins 2016, 8, 33. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Lee, Y.; Kim, W.; Lee, K.; Bae, H.; Kim, S.K. Analgesic effects of bee venom derived phospholipase A2 in a mouse model of oxaliplatin-induced neuropathic pain. Toxins 2015, 7, 2422–2434. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Yoo, J.H.; Kim, S.K. Long-Lasting and Additive Analgesic Effects of Combined Treatment of Bee Venom Acupuncture and Venlafaxine on Paclitaxel-Induced Allodynia in Mice. Toxins 2020, 12, 620. [Google Scholar] [CrossRef] [PubMed]
- Lim, B.S.; Moon, H.J.; Li, D.X.; Gil, M.; Min, J.K.; Lee, G.; Bae, H.; Kim, S.K.; Min, B.I. Effect of bee venom acupuncture on oxaliplatin-induced cold allodynia in rats. Evid.-Based Complement. Altern. Med. 2013, 2013, 369324. [Google Scholar] [CrossRef]
- Lee, J.H.; Li, D.X.; Yoon, H.; Go, D.; Quan, F.S.; Min, B.I.; Kim, S.K. Serotonergic mechanism of the relieving effect of bee venom acupuncture on oxaliplatin-induced neuropathic cold allodynia in rats. BMC Complement. Altern. Med. 2014, 14, 471. [Google Scholar] [CrossRef]
- Yeo, J.H.; Yoon, S.Y.; Kwon, S.K.; Kim, S.J.; Lee, J.H.; Beitz, A.J.; Roh, D.H. Repetitive acupuncture point treatment with diluted bee venom relieves mechanical allodynia and restores intraepidermal nerve fiber loss in oxaliplatin-induced neuropathic mice. J. Pain 2016, 17, 298–309. [Google Scholar] [CrossRef]
- Choi, S.; Chae, H.K.; Heo, H.; Hahm, D.-H.; Kim, W.; Kim, S.K. Analgesic effect of melittin on oxaliplatin-induced peripheral neuropathy in rats. Toxins 2019, 11, 396. [Google Scholar] [CrossRef]
- Choi, J.; Jeon, C.; Lee, J.H.; Jang, J.U.; Quan, F.S.; Lee, K.; Kim, W.; Kim, S.K. Suppressive Effects of Bee Venom Acupuncture on Paclitaxel-Induced Neuropathic Pain in Rats: Mediation by Spinal α2-Adrenergic Receptor. Toxins 2017, 9, 351. [Google Scholar] [CrossRef]
- Li, D.; Chung, G.; Kim, S.K. The Involvement of Central Noradrenergic Pathway in the Analgesic Effect of Bee Venom Acupuncture on Vincristine-Induced Peripheral Neuropathy in Rats. Toxins 2020, 12, 775. [Google Scholar] [CrossRef]
- Park, B.-R.; Kim, J.-M.; Cho, C.-K.; Shin, S.-H.; Yoo, H.-S. Effect of Bee Venom Ointment Treatment for Chemotherapy-induced Peripheral Neuropathy: A Case Series. J. Haehwa Med. 2014, 22, 111–117. [Google Scholar]
- Yoon, J.; Jeon, J.H.; Lee, Y.W.; Cho, C.K.; Kwon, K.R.; Shin, J.E.; Sagar, S.; Wong, R.; Yoo, H.S. Sweet Bee Venom Pharmacopuncture for Chemotherapy-Induced Peripheral Neuropathy. JAMS J. Acupunct. Meridian Stud. 2012, 5, 156–165. [Google Scholar] [CrossRef]
- Park, J.W.; Jeon, J.H.; Yoon, J.; Jung, T.Y.; Kwon, K.R.; Cho, C.K.; Lee, Y.W.; Sagar, S.; Wong, R.; Yoo, H.S. Effects of sweet bee venom pharmacopuncture treatment for chemotherapy-induced peripheral neuropathy: A case series. Integr. Cancer Ther. 2012, 11, 166–171. [Google Scholar] [CrossRef]
- Song, S.Y.; Bae, K.; Shin, K.H.; Yoo, H.-S. A Case Series of Snake Venom Pharmacopuncture for Chemotherapy-Induced Peripheral Neuropathy: A Retrospective Observational Study. J. Pharmacopunct. 2017, 20, 280. [Google Scholar] [CrossRef]
- Yoon, S.Y.; Lee, J.Y.; Roh, D.H.; Oh, S.B. Pharmacopuncture With Scolopendra subspinipes Suppresses Mechanical Allodynia in Oxaliplatin-Induced Neuropathic Mice and Potentiates Clonidine-induced Anti-allodynia Without Hypotension or Motor Impairment. J. Pain 2018, 19, 1157–1168. [Google Scholar] [CrossRef]
- Hong, S.H.; Jung, Y. Effect of Korean Medicine Including Pharmacopuncture on Chemotherapy Induced Peripheral Neuropathy. J. Korean Tradit. Oncol. 2019, 24, 23–31. [Google Scholar]
- Yamada, T.; Kan, H.; Matsumoto, S.; Koizumi, M.; Sasaki, J.; Tani, A.; Yokoi, K.; Uchida, E. Reduction in oxaliplatin-related neurotoxicity by the administration of Keishikajutsubuto (TJ-18) and powdered processed aconite root. Gan To Kagaku Ryoho. 2012, 39, 1687. [Google Scholar]
- Zhang, P.; Lu, Y.; Yang, C.; Zhang, Q.; Qian, Y.; Suo, J.; Cheng, P.; Zhu, J. Based on Systematic Pharmacology: Molecular Mechanism of Siwei Jianbu Decoction in Preventing Oxaliplatin-Induced Peripheral Neuropathy. Neural Plast. 2020, 2020, 8880543. [Google Scholar] [CrossRef]
- Suo, J.; Wang, M.; Zhang, P.; Lu, Y.; Xu, R.; Zhang, L.; Qiu, S.; Zhang, Q.; Qian, Y.; Meng, J.; et al. Siwei Jianbu decoction improves painful paclitaxel-induced peripheral neuropathy in mouse model by modulating the NF-κB and MAPK signaling pathways. Regen. Med. Res. 2020, 8, 2. [Google Scholar] [CrossRef]
- Kitamura, R.; Andoh, T.; Fushimi, H.; Komatsu, K.; Shibahara, N.; Kuraishi, Y. Involvement of descending monoaminergic systems in antiallodynic effect of goshajinkigan in oxaliplatin-treated mice. J. Tradit. Med. 2013, 30, 183–189. [Google Scholar]
- Andoh, T.; Kitamura, R.; Fushimi, H.; Komatsu, K.; Shibahara, N.; Kuraishi, Y. Effects of goshajinkigan, hachimijiogan, and rokumigan on mechanical allodynia induced by Paclitaxel in mice. J. Tradit. Complement. Med. 2014, 4, 293–297. [Google Scholar] [CrossRef]
- Mizuno, K.; Kono, T.; Suzuki, Y.; Miyagi, C.; Omiya, Y.; Miyano, K.; Kase, Y.; Uezono, Y. Goshajinkigan, a traditional Japanese medicine, prevents oxaliplatin-induced acute peripheral neuropathy by suppressing functional alteration of TRP channels in rat. J. Pharmacol. Sci. 2014, 125, 91–98. [Google Scholar] [CrossRef]
- Kono, T.; Suzuki, Y.; Mizuno, K.; Miyagi, C.; Omiya, Y.; Sekine, H.; Mizuhara, Y.; Miyano, K.; Kase, Y.; Uezono, Y. Preventive effect of oral goshajinkigan on chronic oxaliplatin-induced hypoesthesia in rats. Sci. Rep. 2015, 5, 16078. [Google Scholar] [CrossRef]
- Mizuno, K.; Shibata, K.; Komatsu, R.; Omiya, Y.; Kase, Y.; Koizumi, S. An effective therapeutic approach for oxaliplatin-induced peripheral neuropathy using a combination therapy with goshajinkigan and bushi. Cancer Biol. Ther. 2016, 17, 1206–1212. [Google Scholar] [CrossRef]
- Hashimoto, K.; Sakuma, Y.; Kotani, J. Goshajinkigan improves paclitaxel-induced peripheral neuropathy in rats. J. Osaka Dent. Univ. 2006, 40, 47–52. [Google Scholar]
- Matsumura, Y.; Yokoyama, Y.; Hirakawa, H.; Shigeto, T.; Futagami, M.; Mizunuma, H. The prophylactic effects of a traditional Japanese medicine, goshajinkigan, on paclitaxel-induced peripheral neuropathy and its mechanism of action. Mol. Pain 2014, 10, 1744–8069. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, N.; Hosokawa, T.; Ishikawa, T.; Yagi, N.; Kokura, S.; Naito, Y.; Nakanishi, M.; Kokuba, Y.; Otsuji, E.; Kuroboshi, H. Efficacy of goshajinkigan for oxaliplatin-induced peripheral neuropathy in colorectal cancer patients. J. Oncol. 2013, 2013, 139740. [Google Scholar] [CrossRef] [PubMed]
- Fukazawa, K.; Tsukagoshi, M.; Ozawa, D.; Ogata, K.; Kamiyama, Y.; Aihara, R. Preventive and inhibitory effects of goshajinkigan with respect to neurotoxicity induced by mFOLFOX6 in colorectal cancer therapy. Jpn. J. Pharm. Health Care Sci. 2011, 37, 625–630. [Google Scholar] [CrossRef][Green Version]
- Lee, J.H.; Park, H.L.; Lee, H.Y.; Cho, M.K.; Hong, M.N.; Han, C.W.; Choi, J.Y.; Park, S.H.; Kwon, J.N.; Lee, I. Case Report of Chemotherapy Induced Peripheral Neuropathy Treated with Korean Medicine. J. Physiol. Pathol. Korean Med. 2014, 28, 565–570. [Google Scholar] [CrossRef]
- Shindo, Y.; Tenma, K.; Imano, H.; Hibino, M.; Yoshino, K.; Nakamura, M. Reduction of oxaliplatin-related neurotoxicity by Gosha-jinki-gan. Gan To Kagaku Ryoho. 2008, 35, 863. [Google Scholar]
- Nishioka, M.; Shimada, M.; Kurita, N.; Iwata, T.; Morimoto, S.; Yoshikawa, K.; Higashijima, J.; Miyatani, T.; Kono, T. The Kampo medicine, Goshajinkigan, prevents neuropathy in patients treated by FOLFOX regimen. Int. J. Clin. Oncol. 2011, 16, 322–327. [Google Scholar] [CrossRef]
- Kaku, H.; Kumagai, S.; Onoue, H.; Takada, A.; Shoji, T.; Miura, F.; Yoshizaki, A.; Sato, S.; Kigawa, J.; Arai, T. Objective evaluation of the alleviating effects of Goshajinkigan on peripheral neuropathy induced by paclitaxel/carboplatin therapy: A multicenter collaborative study. Exp. Ther. Med. 2012, 3, 60–65. [Google Scholar] [CrossRef]
- Chiou, C.-T.; Wang, K.-C.; Yang, Y.-C.; Huang, C.-L.; Yang, S.-H.; Kuo, Y.-H.; Huang, N.-K. Liu Jun Zi Tang—A Potential, Multi-Herbal Complementary Therapy for Chemotherapy-Induced Neurotoxicity. Int. J. Mol. Sci. 2018, 19, 1258. [Google Scholar] [CrossRef]
- Park, S.; Kim, C.; Cho, C. Effects of Nerve Regeneration by Bogijetong-tang Treatment on Peripheral Nerves Damaged by Taxol and Crush Injury. J. Intern. Korean Med. 2013, 34, 384–404. [Google Scholar]
- Jeong, H.Y.; Kim, C.J.; Cho, C.S. Effects of YideungJetong-Tang on Peripheral Neuropathy Induced by Taxol and Compression Injury in the Rat Sciatic Nerve. J. Korean Med. 2012, 33, 133–146. [Google Scholar]
- An, Y.; Lee, Y.-N.; Baek, K.; Jang, W.-S. A Case Report of Chronic Chemotherapy-Induced Peripheral Neuropathy Treated by Korean Traditional Medicine. J. Intern. Korean Med. 2020, 41, 892–901. [Google Scholar] [CrossRef]
- Cheng, X.; Huo, J.; Wang, D.; Cai, X.; Sun, X.; Lu, W.; Yang, Y.; Hu, C.; Wang, X.; Cao, P. Herbal medicine AC591 prevents oxaliplatin-induced peripheral neuropathy in animal model and cancer patients. Front. Pharmacol. 2017, 8, 344. [Google Scholar] [CrossRef]
- Shen, J.; He, S.; Sun, X.; Hu, N.; Cai, Y. Clinical study on external bath of modified huangqi guizhi wuwu decoction for peripheral neurotoxicity induced by oxaliplatin. Chin. J. Inf. Tradit. Chin. Med. 2015, 22, 13–15. [Google Scholar]
- Li, Y.; Gui, H.; Huang, J.; Wu, X. Clinical study of Jiawei Huangqi Guizhi Wuwu Decoction in preventing and treating peripheral neuro-sensory toxicity caused by oxaliplatin. Chin. J. Integr. Med. 2006, 12, 19–23. [Google Scholar]
- Tatsumi, T.; Kishi, D.; Kogure, T. The efficacy of ogikeishigomotsuto on chronic cumulative sensory neuropathy induced by Oxaliplatin-Case report and Literature view. J. Tradit. Med. 2009, 26, 136–140. [Google Scholar]
- Hidaka, T.; Shima, T.; Nagira, K.; Ieki, M.; Nakamura, T.; Aono, Y.; Kuraishi, Y.; Arai, T.; Saito, S. Herbal medicine Shakuyaku-kanzo-to reduces paclitaxel-induced painful peripheral neuropathy in mice. Eur. J. Pain 2009, 13, 22–27. [Google Scholar] [CrossRef]
- Fujii, K.; Okamoto, S.; Saitoh, K.; Sasaki, N.; Takano, M.; Tanaka, S.; Kudoh, K.; Kita, T.; Tode, T.; Kikuchi, Y. The efficacy of Shakuyaku-Kanzo-to for peripheral nerve dysfunction in paclitaxel combination chemotherapy for epithelial ovarian carcinoma. Gan To Kagaku Ryoho 2004, 31, 1537–1540. [Google Scholar]
- Yamamoto, K. Effects of shakuyaku-kanzo-to on muscle pain from combination chemotherapy with paclitaxel and carboplatin. Gynecol Oncol. 2001, 81, 333–334. [Google Scholar] [CrossRef]
- Xie, M.X.; Zhu, H.Q.; Pang, R.P.; Wen, B.T.; Liu, X.G. Mechanisms for therapeutic effect of bulleyaconitine A on chronic pain. Mol. Pain 2018, 14, 1744806918797243. [Google Scholar] [CrossRef]
- Kimata, Y.; Ogawa, K.; Okamoto, H.; Chino, A.; Namiki, T. Efficacy of Japanese traditional (Kampo) medicine for treating chemotherapy-induced peripheral neuropathy: A retrospective case series study. World J. Clin. Cases 2016, 4, 310. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Li, F.; Li, Y.; Li, W.; Xu, J.; Du, H. A review of traditional and current methods used to potentially reduce toxicity of Aconitum roots in Traditional Chinese Medicine. J. Ethnopharmacol. 2017, 207, 237–250. [Google Scholar] [CrossRef] [PubMed]
- Chan, Y.T.; Wang, N.; Feng, Y. The toxicology and detoxification of Aconitum: Traditional and modern views. Chin. Med. 2021, 16, 61. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.; Li, O.; Tan, H.; Li, Y. Clinical observation of efficacy of Huangqi injection in prevention and treatment of neuroto-xicity induced by oxaliplatin-containing chemotherapy regimen. Advers. Drug React. J. 2009, 11, 249–252. [Google Scholar]
- Zhang, Y.; Lu, X. Clinical study of the protective effect of thioctic acid combined with Huangqi Oral Liquid on oxaliplatin-induced neurotoxicity. China J. Chin. Med. 2013, 28, 1617–1618. [Google Scholar]
- Deng, B.; Jia, L.; Cheng, Z. Radix astragali-based chinese herbal medicine for oxaliplatin-induced peripheral neuropathy: A systematic review and meta-analysis. Evid.-Based Complement. Altern. Med. 2016, 2016, 2421876. [Google Scholar] [CrossRef]
- Deng, B.; Jia, L.; Wan, D.; Wang, B.; Cheng, Z.; Deng, C. Efficacy of Wen-Luo-Tong on Peripheral Neuropathy Induced by Chemotherapy or Target Therapy: A Randomized, Double-Blinded, Placebo-Controlled Trial. Chin. J. Integr. Med. 2022, 28, 579–585. [Google Scholar] [CrossRef]
- Lee, K.; Ku, J.M.; Choi, Y.J.; Hwang, H.H.; Jeong, M.; Kim, Y.G.; Kim, M.J.; Ko, S.G. Herbal Prescription SH003 Alleviates Docetaxel-Induced Neuropathic Pain in C57BL/6 Mice. Evid.-Based. Complement. Altern. Med. 2021, 2021, 4120334. [Google Scholar] [CrossRef]
- Guo, Z.; Lou, Y.; Kong, M.; Luo, Q.; Liu, Z.; Wu, J. A systematic review of phytochemistry, pharmacology and pharmacokinetics on astragali radix: Implications for astragali radix as a personalized medicine. Int. J. Mol. Sci. 2019, 20, 1463. [Google Scholar] [CrossRef]
- Wang, Y.; Dong, B.; Xue, W.; Feng, Y.; Yang, C.; Liu, P.; Cao, J.; Zhu, C. Anticancer effect of radix astragali on cholangiocarcinoma in vitro and its mechanism via network pharmacology. Med. Sci. Monit. 2020, 26, e921162. [Google Scholar] [CrossRef]
- Okuno, K.; Garg, R.; Yuan, Y.-C.; Tokunaga, M.; Kinugasa, Y.; Goel, A. Berberine and Oligomeric Proanthocyanidins Exhibit Synergistic Efficacy Through Regulation of PI3K-Akt Signaling Pathway in Colorectal Cancer. Front. Oncol. 2022, 12, 855860. [Google Scholar] [CrossRef]
- Chen, H.; Ye, C.; Cai, B.; Zhang, F.; Wang, X.; Zhang, J.; Zhang, Z.; Guo, Y.; Yao, Q. Berberine inhibits intestinal carcinogenesis by suppressing intestinal pro-inflammatory genes and oncogenic factors through modulating gut microbiota. BMC Cancer 2022, 22, 566. [Google Scholar] [CrossRef]
- Jiang, X.; Jiang, Z.; Jiang, M.; Sun, Y. Berberine as a Potential Agent for the Treatment of Colorectal Cancer. Front. Med. 2022, 9, 886996. [Google Scholar] [CrossRef]
- Huang, C.; Sun, Y.; Liao, S.; Chen, Z.; Lin, H.; Shen, W. Suppression of Berberine and Probiotics (in vitro and in vivo) on the Growth of Colon Cancer With Modulation of Gut Microbiota and Butyrate Production. Front. Microbiol. 2022, 13, 869931. [Google Scholar] [CrossRef]
- Zhu, Y.; Xie, N.; Chai, Y.; Nie, Y.; Liu, K.; Liu, Y.; Yang, Y.; Su, J.; Zhang, C. Apoptosis Induction, a Sharp Edge of Berberine to Exert Anti-Cancer Effects, Focus on Breast, Lung, and Liver Cancer. Front. Pharmacol. 2022, 13, 803717. [Google Scholar] [CrossRef]
- Wu, Y.; Xu, J.; Liu, Y.; Zeng, Y.; Wu, G. A Review on Anti-Tumor Mechanisms of Coumarins. Front. Oncol. 2020, 10, 592853. [Google Scholar] [CrossRef]
- Al-Warhi, T.; Sabt, A.; Elkaeed, E.B.; Eldehna, W.M. Recent advancements of coumarin-based anticancer agents: An up-to-date review. Bioorg. Chem. 2020, 103, 104163. [Google Scholar] [CrossRef]
- Akkol, E.K.; Genç, Y.; Karpuz, B.; Sobarzo-Sánchez, E.; Capasso, R. Coumarins and coumarin-related compounds in pharmacotherapy of cancer. Cancers 2020, 12, 1959. [Google Scholar] [CrossRef]
- Ruwizhi, N.; Aderibigbe, B.A. Cinnamic acid derivatives and their biological efficacy. Int. J. Mol. Sci. 2020, 21, 5712. [Google Scholar] [CrossRef]
- Al Moundhri, M.S.; Al-Salam, S.; Al Mahrouqee, A.; Beegam, S.; Ali, B.H. The effect of curcumin on oxaliplatin and cisplatin neurotoxicity in rats: Some behavioral, biochemical, and histopathological studies. J. Med. Toxicol. 2013, 9, 25–33. [Google Scholar] [CrossRef]
- Giordano, A.; Tommonaro, G. Curcumin and cancer. Nutrients 2019, 11, 2376. [Google Scholar] [CrossRef]
- Rizeq, B.; Gupta, I.; Ilesanmi, J.; AlSafran, M.; Rahman, M.D.M.; Ouhtit, A. The power of phytochemicals combination in cancer chemoprevention. J. Cancer 2020, 11, 4521–4533. [Google Scholar] [CrossRef]
- Ashrafizadeh, M.; Zarrabi, A.; Hashemi, F.; Zabolian, A.; Saleki, H.; Bagherian, M.; Azami, N.; Bejandi, A.K.; Hushmandi, K.; Ang, H.L.; et al. Polychemotherapy with curcumin and doxorubicin via biological nanoplatforms: Enhancing antitumor activity. Pharmaceutics 2020, 12, 1084. [Google Scholar] [CrossRef]
- Gupta, N.; Verma, K.; Nalla, S.; Kulshreshtha, A.; Lall, R.; Prasad, S. Free Radicals as a Double-Edged Sword: The Cancer Preventive and Therapeutic Roles of Curcumin. Molecules 2020, 25, 5390. [Google Scholar] [CrossRef]
- Tan, B.L.; Norhaizan, M.E. Curcumin combination chemotherapy: The implication and efficacy in cancer. Molecules 2019, 24, 2527. [Google Scholar] [CrossRef]
- Grover, M.; Behl, T.; Sachdeva, M.; Bungao, S.; Aleya, L.; Setia, D. Focus on Multi-targeted Role of Curcumin: A Boon in Therapeutic Paradigm. Environ. Sci. Pollut. Res. 2021, 28, 18893–18907. [Google Scholar] [CrossRef]
- Bordoloi, D.; Roy, N.K.; Monisha, J.; Padmavathi, G.; Kunnumakkara, A.B. Multi-Targeted Agents in Cancer Cell Chemosensitization: What We Learnt from Curcumin Thus Far. Recent Pat. Anticancer. Drug Discov. 2015, 11, 67–97. [Google Scholar] [CrossRef]
- Kasi, P.D.; Tamilselvam, R.; Skalicka-Woźniak, K.; Nabavi, S.F.; Daglia, M.; Bishayee, A.; Pazoki-Toroudi, H.; Nabavi, S.M. Molecular targets of curcumin for cancer therapy: An updated review. Tumor Biol. 2016, 37, 13017–13028. [Google Scholar] [CrossRef]
- Panda, A.K.; Chakraborty, D.; Sarkar, I.; Khan, T.; Sa, G. New insights into therapeutic activity and anticancer properties of curcumin. J. Exp. Pharmacol. 2017, 9, 31–45. [Google Scholar] [CrossRef]
- Jiang, J.; Shen, Y.Y.; Li, J.; Lin, Y.H.; Luo, C.X.; Zhu, D.Y. (+)-Borneol alleviates mechanical hyperalgesia in models of chronic inflammatory and neuropathic pain in mice. Eur. J. Pharmacol. 2015, 757, 53–58. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, D.; Hu, J.; Jia, Q.; Xu, W.; Su, D.; Song, H.; Xu, Z.; Cui, J.; Zhou, M.; et al. A clinical and mechanistic study of topical borneol-induced analgesia. EMBO Mol. Med. 2017, 9, 802–815. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Wu, D.; Wu, J.; Ou, Y.; Mu, C.; Han, B.; Zhang, Q. Improved blood-brain barrier distribution: Effect of borneol on the brain pharmacokinetics of kaempferol in rats by in vivo microdialysis sampling. J. Ethnopharmacol. 2015, 162, 270–277. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.L.; Fu, B.M.; Zhang, Z.J. Borneol, a novel agent that improves central nervous system drug delivery by enhancing blood–brain barrier permeability. Drug Deliv. 2017, 24, 1037–1044. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Zhang, A.; Lu, H.; Cheng, Q. The Role and Mechanism of Borneol to Open the Blood-Brain Barrier. Integr. Cancer Ther. 2018, 17, 806–812. [Google Scholar] [CrossRef]
- Zheng, Q.; Chen, Z.X.; Xu, M.B.; Zhou, X.L.; Huang, Y.Y.; Zheng, G.Q.; Wang, Y. Borneol, a messenger agent, improves central nervous system drug delivery through enhancing blood–brain barrier permeability: A preclinical systematic review and meta-analysis. Drug Deliv. 2018, 25, 1617–1633. [Google Scholar] [CrossRef]
- Wu, J.Y.; Li, Y.J.; Yang, L.; Hu, Y.Y.; Hu, X.B.; Tang, T.T.; Wang, J.M.; Liu, X.Y.; Xiang, D.X. Borneol and a-asarone as adjuvant agents for improving blood-brain barrier permeability of puerarin and tetramethylpyrazine by activating adenosine receptors. Drug Deliv. 2018, 25, 1858–1864. [Google Scholar] [CrossRef]
- Yin, Y.; Cao, L.; Ge, H.; Duanmu, W.; Tan, L.; Yuan, J.; Tunan, C.; Li, F.; Hu, R.; Gao, F.; et al. L-Borneol induces transient opening of the blood-brain barrier and enhances the therapeutic effect of cisplatin. Neuroreport 2017, 28, 506–513. [Google Scholar] [CrossRef]
- Meng, L.; Chu, X.; Xing, H.; Liu, X.; Xin, X.; Chen, L.; Jin, M.; Guan, Y.; Huang, W.; Gao, Z. Improving glioblastoma therapeutic outcomes via doxorubicin-loaded nanomicelles modified with borneol. Int. J. Pharm. 2019, 567, 118485. [Google Scholar] [CrossRef]
- Cao, W.; Li, Y.; Hou, Y.; Yang, M.; Fu, X.; Zhao, B.; Jiang, H.; Fu, X. Enhanced anticancer efficiency of doxorubicin against human glioma by natural borneol through triggering ROS-mediated signal. Biomed. Pharmacother. 2019, 118, 109261. [Google Scholar] [CrossRef]
- Lv, L.; Li, X.; Qian, W.; Li, S.; Jiang, Y.; Xiong, Y.; Xu, J.; Lv, W.; Liu, X.; Chen, Y.; et al. Enhanced Anti-Glioma Efficacy by Borneol Combined With CGKRK-Modified Paclitaxel Self-Assembled Redox-Sensitive Nanoparticles. Front. Pharmacol. 2020, 11, 558. [Google Scholar] [CrossRef]
- Liu, W.J.; Yin, Y.B.; Sun, J.Y.; Feng, S.; Ma, J.K.; Fu, X.Y.; Hou, Y.J.; Yang, M.F.; Sun, B.L.; Fan, C.D. Natural borneol is a novel chemosensitizer that enhances temozolomide-induced anticancer efficiency against human glioma by triggering mitochondrial dysfunction and reactive oxide species-mediated oxidative damage. OncoTargets Ther. 2018, 11, 5429–5439. [Google Scholar] [CrossRef]
- Boulos, J.C.; Rahama, M.; Hegazy, M.E.F.; Efferth, T. Shikonin derivatives for cancer prevention and therapy. Cancer Lett. 2019, 459, 248–267. [Google Scholar] [CrossRef]
- Zhao, Q.; Kretschmer, N.; Bauer, R.; Efferth, T. Shikonin and its derivatives inhibit the epidermal growth factor receptor signaling and synergistically kill glioblastoma cells in combination with erlotinib. Int. J. Cancer 2015, 137, 1446–1456. [Google Scholar] [CrossRef]
- Gupta, B.; Chakraborty, S.; Saha, S.; Chandel, S.G.; Baranwal, A.K.; Banerjee, M.; Chatterjee, M.; Chaudhury, A. Antinociceptive properties of shikonin: In vitro and in vivo studies. Can. J. Physiol. Pharmacol. 2016, 94, 788–796. [Google Scholar] [CrossRef]
- Guo, C.; He, J.; Song, X.; Tan, L.; Wang, M.; Jiang, P.; Li, Y.; Cao, Z.; Peng, C. Pharmacological properties and derivatives of shikonin—A review in recent years. Pharmacol. Res. 2019, 149, 104463. [Google Scholar] [CrossRef]
- Andújar, I.; Ríos, J.L.; Giner, R.M.; Recio, M.C. Pharmacological properties of shikonin—A review of literature since 2002. Planta Med. 2013, 79, 1685–1697. [Google Scholar]
- Schröder, S.; Beckmann, K.; Franconi, G.; Meyer-Hamme, G.; Friedemann, T.; Greten, H.J.; Rostock, M.; Efferth, T. Can Medical Herbs Stimulate Regeneration or Neuroprotection and Treat Neuropathic Pain in Chemotherapy-Induced Peripheral Neuropathy? Evid.-Based Complement. Altern. Med. 2013, 2013, 423713. [Google Scholar] [CrossRef]
- Feng, L.; Jia, X. Antioxidative and anti-inflammatory activities of paeoniflorin and oxypaeoniflora on AGEs-induced mesangial cell damage. Planta Med. 2013, 79, 1319–1323. [Google Scholar]
- Wang, C.; Yuan, J.; Wu, H.; Chang, Y.; Wang, Q.; Wu, Y.; Liu, L.; Wei, W. Paeoniflorin inhibits inflammatory responses in mice with allergic contact dermatitis by regulating the balance between inflammatory and anti-inflammatory cytokines. Inflamm. Res. 2013, 62, 1035–1044. [Google Scholar] [CrossRef]
- Chang, Y.; Zhang, L.; Wang, C.; Jia, X.-Y.; Wei, W. Paeoniflorin inhibits function of synoviocytes pretreated by rIL-1α and regulates EP4 receptor expression. J. Ethnopharmacol. 2011, 137, 1275–1282. [Google Scholar] [CrossRef]
- Wu, J.-J.; Sun, W.-Y.; Hu, S.-S.; Zhang, S.; Wei, W. A standardized extract from Paeonia lactiflora and Astragalus membranaceus induces apoptosis and inhibits the proliferation, migration and invasion of human hepatoma cell lines. Int. J. Oncol. 2013, 43, 1643–1651. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Cheng, J.; Ma, S.; Zhou, J. Paeoniflorin attenuates chronic constriction injury-induced neuropathic pain by suppressing spinal NLRP3 inflammasome activation. Inflammopharmacology 2020, 28, 1495–1508. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Wang, L.; Wang, J.; Wang, C.; Yang, Z.; Wang, C.; Zhu, Y.; Zhang, J. Paeoniflorin and Albiflorin Attenuate Neuropathic Pain via MAPK Pathway in Chronic Constriction Injury Rats. Evid.-Based Complement. Altern. Med. 2016, 2016, 8082753. [Google Scholar] [CrossRef] [PubMed]
- Teramoto, H.; Yamauchi, T.; Terado, Y.; Odagiri, S.; Sasaki, S.; Higashiyama, K. Design and synthesis of a piperidinone scaffold as an analgesic through kappa-opioid receptor: Structure-activity relationship study of matrine alkaloids. Chem. Pharm. Bull. 2016, 64, 410–419. [Google Scholar] [CrossRef]
- Yin, L.L.; Zhu, X.Z. The involvement of central cholinergic system in (+)-matrine-induced antinociception in mice. Pharmacol. Biochem. Behav. 2005, 80, 419–425. [Google Scholar] [CrossRef]
- Sun, Y.Z.; You, R.L.; Wang, L.; Ren, J.S.; Wang, D.Y.; Su, S.J.; Xu, R.F. Compound matrine injection reduces morphine tolerance of the mice with lung cancer by inhibiting expression of multidrug resistance gene 1 and P-glycoprotein. Zhonghua Zhong Liu Za Zhi 2020, 42, 216–221. [Google Scholar] [CrossRef]
- Kianbakht, S.; Hajiaghaee, R.; Akhondzadeh, S. Efficacy and safety of Sophora alopecuroides var. alopecuroides seed extract for opioid detoxification: A randomized, double-blind, and placebo-controlled clinical trial. Phyther. Res. 2020, 34, 1108–1113. [Google Scholar] [CrossRef]
- Kianbakht, S.; Dabaghian, F.H. Sophora alopecuroides L. var. alopecuroides alleviates morphine withdrawal syndrome in mice: Involvement of alkaloid fraction and matrine. Iran. J. Basic Med. Sci. 2016, 19, 1090–1095. [Google Scholar]
- Ge, L.; Wang, Y.; Tian, J.; Mao, L.; Zhang, J.; Zhang, J.; Shen, X.; Yang, K. Network meta-analysis of Chinese herb injections combined with FOLFOX chemotherapy in the treatment of advanced colorectal cancer. J. Clin. Pharm. Ther. 2016, 41, 383–391. [Google Scholar] [CrossRef]
- Hu, G.; Cao, C.; Deng, Z.; Li, J.; Zhou, X.; Huang, Z.; Cen, C. Effects of matrine in combination with cisplatin on liver cancer. Oncol. Lett. 2021, 21, 1. [Google Scholar] [CrossRef]
- Zhang, H.; Chen, L.; Sun, X.; Yang, Q.; Wan, L.; Guo, C. Matrine: A promising natural product with various pharmacological activities. Front. Pharmacol. 2020, 11, 588. [Google Scholar] [CrossRef]
- Kim, H.; Lee, G.; Park, S.; Chung, H.S.; Lee, H.; Kim, J.Y.; Nam, S.; Kim, S.K.; Bae, H. Bee venom mitigates cisplatin-induced nephrotoxicity by regulating CD4+CD25+Foxp3+ regulatory T cells in mice. Evid.-Based Complement. Altern. Med. 2013, 2013, 879845. [Google Scholar] [CrossRef]
- Woo, S.; Chung, G.; Bae, H.; Kim, S.K. Suppressive effects of bee venom-derived phospholipase a2 on mechanical allodynia in a rat model of neuropathic pain. Toxins 2019, 11, 477. [Google Scholar] [CrossRef]
- Li, D.; Kim, W.; Shin, D.; Jung, Y.; Bae, H.; Kim, S.K. Preventive effects of bee venom derived phospholipase A2 on oxaliplatin-induced neuropathic pain in mice. Toxins 2016, 8, 27. [Google Scholar] [CrossRef]
- Nakanishi, M.; Arimitsu, J.; Kageyama, M.; Otsuka, S.; Inoue, T.; Nishida, S.; Yoshikawa, H.; Kishida, Y. Efficacy of traditional Japanese herbal medicines—Keishikajutsubuto (TJ-18) and Bushi-matsu (TJ-3022)—Against postherpetic neuralgia aggravated by self-reported cold stimulation: A case series. J. Altern. Complement. Med. 2012, 18, 686–692. [Google Scholar] [CrossRef]
- Hoshino, N.; Ganeko, R.; Hida, K.; Sakai, Y. Goshajinkigan for reducing chemotherapy-induced peripheral neuropathy: A systematic review and meta-analysis. Int. J. Clin. Oncol. 2018, 23, 434–442. [Google Scholar] [CrossRef]
- Kuriyama, A.; Endo, K. Goshajinkigan for prevention of chemotherapy-induced peripheral neuropathy: A systematic review and meta-analysis. Support. Care Cancer 2018, 26, 1051–1059. [Google Scholar] [CrossRef]
- Cascella, M.; Muzio, M.R. Potential application of the Kampo medicine goshajinkigan for prevention of chemotherapy-induced peripheral neuropathy. J. Integr. Med. 2017, 15, 77–87. [Google Scholar] [CrossRef]
- Oki, E.; Emi, Y.; Kojima, H.; Higashijima, J.; Kato, T.; Miyake, Y.; Kon, M.; Ogata, Y.; Takahashi, K.; Ishida, H. Preventive effect of Goshajinkigan on peripheral neurotoxicity of FOLFOX therapy (GENIUS trial): A placebo-controlled, double-blind, randomized phase III study. Int. J. Clin. Oncol. 2015, 20, 767–775. [Google Scholar] [CrossRef]
- Gu, J.; Wei, G.; Ma, Y.; Zhang, J.; Ji, Y.; Li, L.; Yu, J.; Hu, C.; Huo, J. Exploring the Possible Mechanism and Drug Targets of Huang-Qi-Gui-Zhi-Wu-Wu Decoction for the Treatment of Chemotherapy-Induced Peripheral Neuropathy on Network Pharmacology. Evid.-Based Complement. Altern. Med. 2020, 2020, 2363262. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).