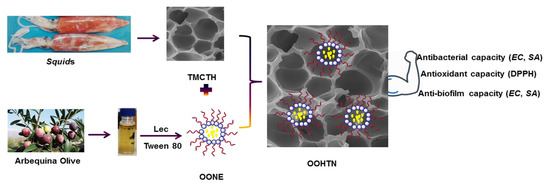
Quaternized Chitosan Thiol Hydrogel-Thickened Nanoemulsion: A Multifunctional Platform for Upgrading the Topical Applications of Virgin Olive Oil
Abstract
:1. Introduction
2. Materials and Methods
2.1. VOO Extraction
2.1.1. Sampling
2.1.2. Oil Extraction
2.1.3. GC-MS Analysis
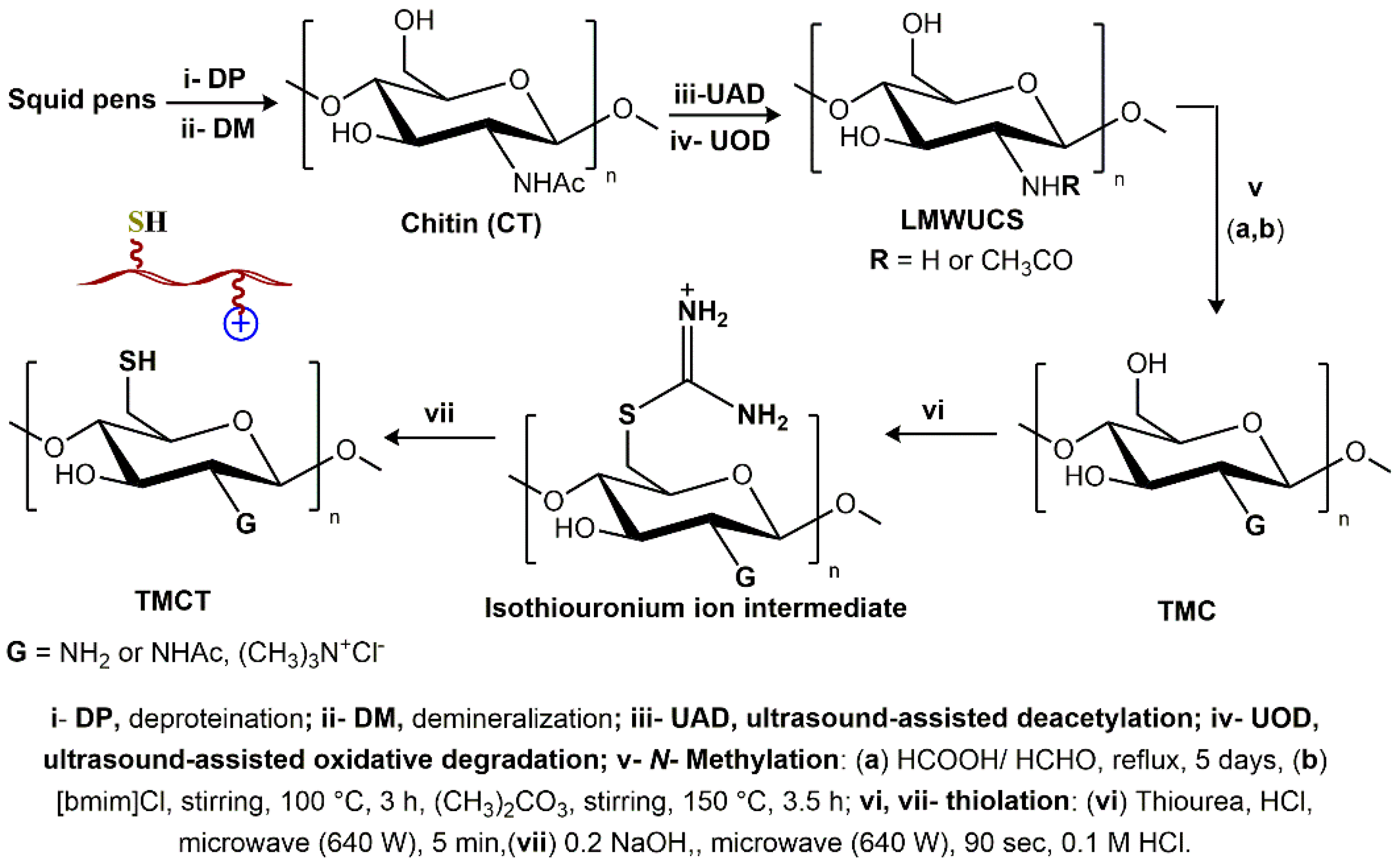
2.2. Preparation of Trimethyl Chitosan Thiol (TMC-Thiol) Hydrogel (TMCTH)
2.3. Preparation of VOO Primary Nanoemulsion (OONE)
2.4. Preparation of Olive Oil Hydrogel-Thickened Nanoemulsion (OOHTN)
2.5. In Vitro VOO Release
2.6. Ex Vivo Skin Permeability Study
2.7. Cytotoxicity Study
2.8. Antimicrobial Study
2.9. Anti-Biofilm Activity
2.10. Free Radical Scavenging Activity (DPPH Assay)
2.11. Statistical Analysis
3. Results and Discussion
3.1. Chemical Characterization of Oil
3.2. Formation Chemistry
3.3. Physicochemical Characterization
3.3.1. Microanalytical Analyses
3.3.2. Infrared Spectroscopy
3.3.3. Droplet Size, Polydispersity Index, Zeta Potential, and Storage Stability
3.4. Morphological Characterization
SEM Analysis
3.5. Pharmacological Performances
3.5.1. Entrapment Efficiency (EE) and Oil Loading (OL)
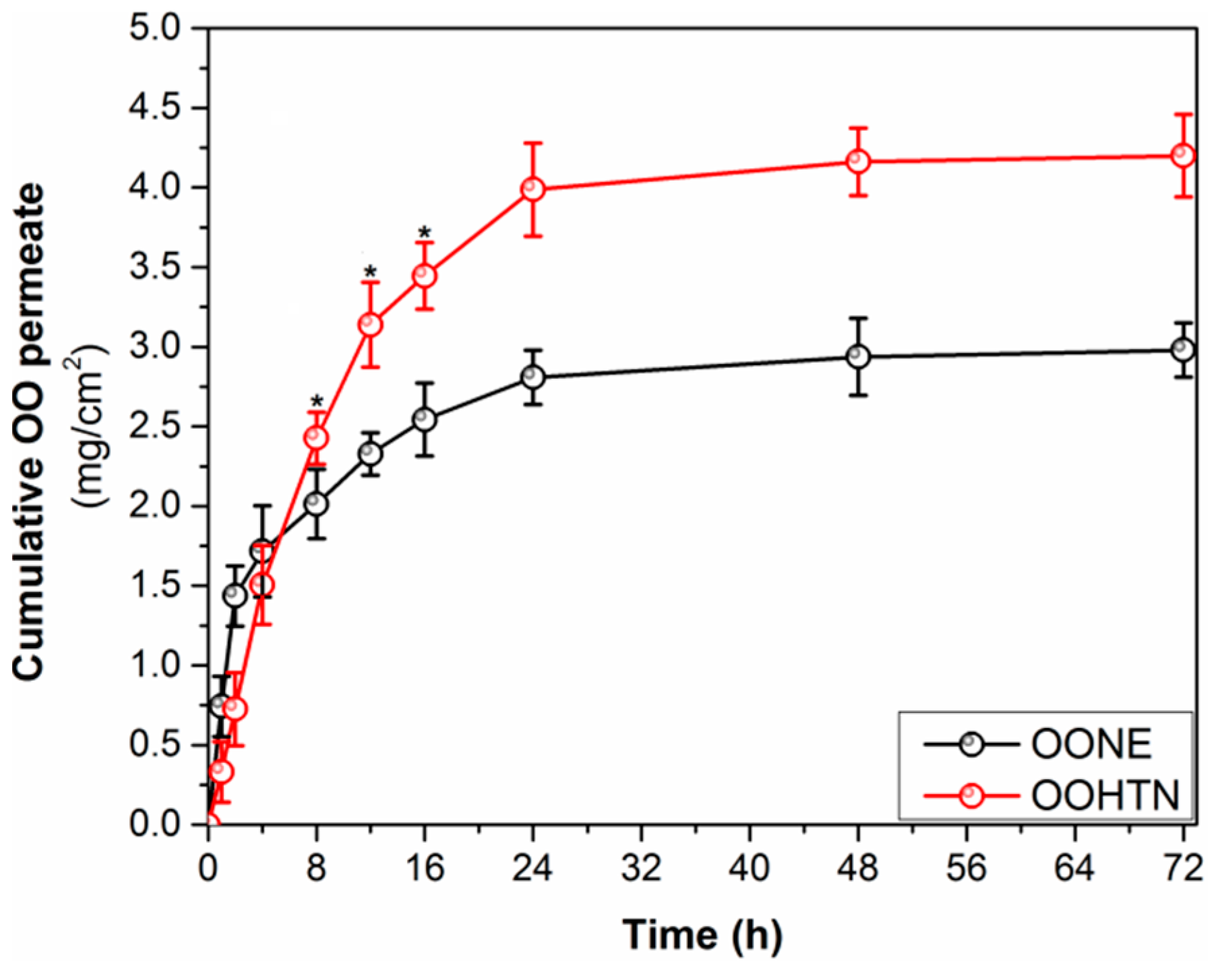
3.5.2. Ex Vivo Skin Permeability Study and Release Kinetics
3.5.3. Cytotoxicity
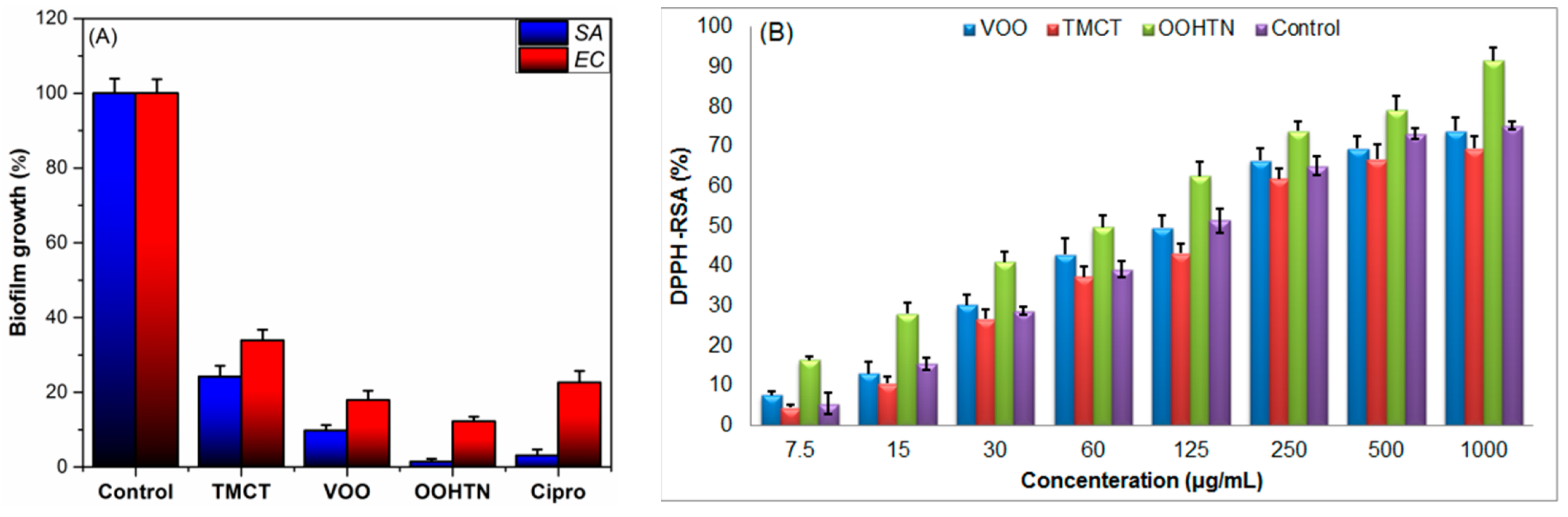
3.5.4. In Vitro Antimicrobial Study
3.5.5. Anti-Biofilm Assessment
3.5.6. Antioxidant Study
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Franklyne, J.S.; Gopinath, P.M.; Mukherjee, A.; Chandrasekaran, N. Nanoemulsions: The rising star of antiviral therapeutics and nanodelivery system—Current status and prospects. Curr. Opin. Colloid Interface Sci. 2021, 54, 101458. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, C.; Chow, A.H.L.; Ren, K.; Gong, T.; Zhang, Z.; Zheng, Y. Self-nanoemulsifying drug delivery system (SNEDDS) for oral delivery of Zedoary essential oil: Formulation and bioavailability studies. Int. J. Pharm. 2010, 383, 170–177. [Google Scholar] [CrossRef]
- Chinnaiyan, S.K.; Pandiyan, R.; Natesan, S.; Chindam, S.; Gouti, A.K.; Sugumaran, A. Fabrication of basil oil Nanoemulsion loaded gellan gum hydrogel—Evaluation of its antibacterial and anti-biofilm potential. J. Drug Deliv. Sci. Technol. 2022, 68, 103129. [Google Scholar] [CrossRef]
- Barradas, T.N.; de Holanda e Silva, K.G. Nanoemulsions of essential oils to improve solubility, stability and permeability: A review. Environ. Chem. Lett. 2021, 19, 1153–1171. [Google Scholar] [CrossRef]
- Barradas, T.N.; Senna, J.P.; Cardoso, S.A.; de Holanda e Silva, K.G.; Elias Mansur, C.R. Formulation characterization and in vitro drug release of hydrogel-thickened nanoemulsions for topical delivery of 8-methoxypsoralen. Mater. Sci. Eng. C 2018, 92, 245–253. [Google Scholar] [CrossRef]
- dos Santos, M.K.; Kreutz, T.; Danielli, L.J.; De Marchi, J.G.B.; Pippi, B.; Koester, L.S.; Fuentefria, A.M.; Limberger, R.P. A chitosan hydrogel-thickened nanoemulsion containing Pelargonium graveolens essential oil for treatment of vaginal candidiasis. J. Drug Deliv. Sci. Technol. 2020, 56, 101527. [Google Scholar] [CrossRef]
- Elshaarawy, R.F.M.; El-Azim, H.A.; Hegazy, W.H.; Mustafa, F.H.A.; Talkhan, T.A. Poly(ammonium/ pyridinium)-chitosan Schiff base as a smart biosorbent for scavenging of Cu2+ ions from aqueous effluents. Polym. Test. 2020, 83, 106244. [Google Scholar] [CrossRef]
- Alfaifi, M.Y.; Alkabli, J.; Elshaarawy, R.F.M. Suppressing of milk-borne pathogenic using new water-soluble chitosan-azidopropanoic acid conjugate: Targeting milk-preservation quality improvement. Int. J. Biol. Macromol. 2020, 164, 1519–1526. [Google Scholar] [CrossRef]
- Maurya, A.; Singh, V.K.; Das, S.; Prasad, J.; Kedia, A.; Upadhyay, N.; Dubey, N.K.; Dwivedy, A.K. Essential Oil Nanoemulsion as Eco-Friendly and Safe Preservative: Bioefficacy Against Microbial Food Deterioration and Toxin Secretion, Mode of Action, and Future Opportunities. Front. Microbiol. 2021, 12, 1–17. [Google Scholar] [CrossRef]
- Barradas, T.N.; Senna, J.P.; Cardoso, S.A.; Nicoli, S.; Padula, C.; Santi, P.; Rossi, F.; de Holanda e Silva, K.G.; Mansur, C.R.E. Hydrogel-thickened nanoemulsions based on essential oils for topical delivery of psoralen: Permeation and stability studies. Eur. J. Pharm. Biopharm. 2017, 116, 38–50. [Google Scholar] [CrossRef]
- Soni, M.; Burdock, G.; Christian, M.; Bitler, C.; Crea, R. Safety assessment of aqueous olive pulp extract as an antioxidant or antimicrobial agent in foods. Food Chem. Toxicol. 2006, 44, 903–915. [Google Scholar] [CrossRef] [PubMed]
- Binkoski, A.E.; Kris-Etherton, P.M.; Wilson, T.A.; Mountain, M.L.; Nicolosi, R.J. Balance of unsaturated fatty acids is important to a cholesterol-lowering diet: Comparison of mid-oleic sunflower oil and olive oil on cardiovascular disease risk factors. J. Am. Diet. Assoc. 2005, 105, 1080–1086. [Google Scholar] [CrossRef] [PubMed]
- Cicerale, S.; Lucas, L.; Keast, R. Antimicrobial, antioxidant and anti-inflammatory phenolic activities in extra virgin olive oil. Curr. Opin. Biotechnol. 2012, 23, 129–135. [Google Scholar] [CrossRef] [PubMed]
- Tavolaro, P.; Catalano, S.; Tavolaro, A. Anticancer activity modulation of an innovative solid formulation of extra virgin olive oil by cultured zeolite scaffolds. Food Chem. Toxicol. 2019, 124, 139–150. [Google Scholar] [CrossRef] [PubMed]
- Boggia, R.; Evangelisti, F.; Rossi, N.; Salvadeo, P.; Zunin, P. Chemical composition of olive oils of the cultivar Colombaia. Grasas Aceites 2005, 56, 276–283. [Google Scholar] [CrossRef]
- Khalifa, I.; Barakat, H.; El-Mansy, H.A.; Soliman, S.A. Effect of Chitosan–Olive Oil Processing Residues Coatings on Keeping Quality of Cold-Storage Strawberry (Fragaria ananassa. Var. Festival). J. Food Qual. 2016, 39, 504–515. [Google Scholar] [CrossRef]
- Dovale-Rosabal, G.; Casariego, A.; Forbes-Hernandez, T.Y.; García, M.A. Effect of chitosan-olive oil emulsion coating on quality of tomatoes during storage at ambient conditions. J. Berry Res. 2015, 5, 207–218. [Google Scholar] [CrossRef] [Green Version]
- Vieira, T.M.; Moldão-Martins, M.; Alves, V.D. Composite Coatings of Chitosan and Alginate Emulsions with Olive Oil to Enhance Postharvest Quality and Shelf Life of Fresh Figs (Ficus carica L. cv. ‘Pingo De Mel’). Foods 2021, 10, 718. [Google Scholar] [CrossRef]
- Taghizadeh, E.; Khamesipour, A.; Khoee, S.; Jaafari, M.R.; Hosseini, S.A. Improvement of the Solubility Amphotericin B Using Olive Oil Nanoemulsion Coated with Chitosan for More Effective Treatment of Zoonotic Cutaneous Leishmaniasis. Iran. J. Pharm. Res. 2021, 20, 289–299. [Google Scholar]
- Federer, C.; Kurpiers, M.; Bernkop-Schnürch, A.J.B. Thiolated chitosans: A multi-talented class of polymers for various applications. Biomacromol. 2020, 22, 24–56. [Google Scholar] [CrossRef]
- Rai, M.K.; Deshmukh, S.; Ingle, A.; Gade, A. Silver nanoparticles: The powerful nanoweapon against multidrug-resistant bacteria. J. Appl. Microbiol. 2012, 112, 841–852. [Google Scholar] [CrossRef] [PubMed]
- Elshaarawy, R.F.; Ismail, L.A.; Alfaifi, M.Y.; Rizk, M.A.; Eltamany, E.E.; Janiak, C. Inhibitory activity of biofunctionalized silver-capped N-methylated water-soluble chitosan thiomer for microbial and biofilm infections. Int. J. Biol. Macromol. 2020, 152, 709–717. [Google Scholar] [CrossRef] [PubMed]
- El-Sayed, W.N.; Alkabli, J.; Aloqbi, A.; Elshaarawy, R.F.M. Optimization enzymatic degradation of chitosan into amphiphilic chitooligosaccharides for application in mitigating liver steatosis and cholesterol regulation. Eur. Polym. J. 2021, 153, 110507. [Google Scholar] [CrossRef]
- Ibrahim, H.K.; El-Tamany, S.H.; El-Shaarawy, R.F.; El-Deen, I.M. Synthesis and investigation of mass spectra of some novel benzimidazole derivatives. Maced. J. Chem. Chem. Eng. 2008, 27, 65–79. [Google Scholar] [CrossRef] [Green Version]
- Kamal, I.; Khedr, A.I.M.; Alfaifi, M.Y.; Elbehairi, S.E.I.; Elshaarawy, R.F.M.; Saad, A.S. Chemotherapeutic and chemopreventive potentials of ρ-coumaric acid—Squid chitosan nanogel loaded with Syzygium aromaticum essential oil. Int. J. Biol. Macromol. 2021, 188, 523–533. [Google Scholar] [CrossRef]
- El Sharnouby, G. Physicochemical and phytonutrients Evaluation of Arbequina Extra Virgin Olive oil Cultivated Recently in Egypt. Bull. Nat. Nut. Inst. Arab Repub. Egypt 2017, 50, 66–96. [Google Scholar]
- Cavaliere, B.; De Nino, A.; Hayet, F.; Lazez, A.; Macchione, B.; Moncef, C.; Perri, E.; Sindona, G.; Tagarelli, A. A metabolomic approach to the evaluation of the origin of extra virgin olive oil: A convenient statistical treatment of mass spectrometric analytical data. J. Agric. Food Chem. 2007, 55, 1454–1462. [Google Scholar] [CrossRef]
- Ghiasi, Z.; Esmaeli, F.; Aghajani, M.; Ghazi-Khansari, M.; Faramarzi, M.A.; Amani, A. Enhancing analgesic and anti-inflammatory effects of capsaicin when loaded into olive oil nanoemulsion: An in vivo study. Int. J. Pharm. 2019, 559, 341–347. [Google Scholar] [CrossRef]
- Muzzalupo, I.; Badolati, G.; Chiappetta, A.; Picci, N.; Muzzalupo, R. In vitro Antifungal Activity of Olive (Olea europaea) Leaf Extracts Loaded in Chitosan Nanoparticles. Front. Bioeng. Biotechnol. 2020, 8, 151. [Google Scholar] [CrossRef] [Green Version]
- Lin, H.; Xie, Q.; Huang, X.; Ban, J.; Wang, B.; Wei, X.; Chen, Y.; Lu, Z. Increased skin permeation efficiency of imperatorin via charged ultradeformable lipid vesicles for transdermal delivery. Int. J. Nanomed. 2018, 13, 831. [Google Scholar] [CrossRef] [Green Version]
- Elshaarawy, R.F.; Eldeen, I.M.; Hassan, E.M. Efficient synthesis and evaluation of bis-pyridinium/bis-quinolinium metallosalophens as antibiotic and antitumor candidates. J. Mol. Struct. 2017, 1128, 162–173. [Google Scholar] [CrossRef]
- Elshaarawy, R.F.; Lan, Y.; Janiak, C. Oligonuclear homo-and mixed-valence manganese complexes based on thiophene-or aryl-carboxylate ligation: Synthesis, characterization and magnetic studies. Inorg. Chim. Acta 2013, 401, 85–94. [Google Scholar] [CrossRef]
- Refaee, A.A.; El-Naggar, M.E.; Mostafa, T.B.; Elshaarawy, R.F.M.; Nasr, A.M. Nano-bio finishing of cotton fabric with quaternized chitosan Schiff base-TiO2-ZnO nanocomposites for antimicrobial and UV protection applications. Eur. Polym. J. 2022, 166, 111040. [Google Scholar] [CrossRef]
- Batool, R.; Ayub, S.; Akbar, I. Isolation of biosurfactant producing bacteria from petroleum contaminated sites and their characterization. Soil Environ. 2017, 36, 35–44. [Google Scholar] [CrossRef]
- Nzai, J.; Proctor, A. Soy lecithin phospholipid determination by fourier transform infrared spectroscopy and the acid digest/arseno-molybdate method: A comparative study. J. Am. Chem. Soc. 1999, 76, 61–66. [Google Scholar] [CrossRef]
- Ryu, V.; McClements, D.J.; Corradini, M.G.; McLandsborough, L. Effect of ripening inhibitor type on formation, stability, and antimicrobial activity of thyme oil nanoemulsion. Food Chem. 2018, 245, 104–111. [Google Scholar] [CrossRef]
- da Silva Gündel, S.; Velho, M.C.; Diefenthaler, M.K.; Favarin, F.R.; Copetti, P.M.; de Oliveira Fogaça, A.; Klein, B.; Wagner, R.; Gündel, A.; Sagrillo, M.R.; et al. Basil oil-nanoemulsions: Development, cytotoxicity and evaluation of antioxidant and antimicrobial potential. J. Drug Del. Sci. Technol. 2018, 46, 378–383. [Google Scholar] [CrossRef]
- Ahmadi, O.; Jafarizadeh-Malmiri, H. Intensification process in thyme essential oil nanoemulsion preparation based on subcritical water as green solvent and six different emulsifiers. Green Process. Synth. 2021, 10, 430–439. [Google Scholar] [CrossRef]
- Hussain, A.; Khan, G.M.; Jan, S.U.; Shah, S.U.; Shah, K.; Akhlaq, M.; Rahim, N.; Nawaz, A.; Wahab, A. Effect of olive oil on transdermal penetration of flurbiprofen from topical gel as enhancer. Pak. J. Pharm. Sci. 2012, 25, 365–369. [Google Scholar]
- Baboota, S.; Shakeel, F.; Ahuja, A.; Ali, J.; Shafiq, S. Design, development and evaluation of novel nanoemulsion formulations for transdermal potential of celecoxib. Acta Pharm. 2007, 57, 315. [Google Scholar] [CrossRef] [Green Version]
- Piroddi, M.; Albini, A.; Fabiani, R.; Giovannelli, L.; Luceri, C.; Natella, F.; Rosignoli, P.; Rossi, T.; Taticchi, A.; Servili, M. Nutrigenomics of extra-virgin olive oil: A review. Biofactors 2017, 43, 17–41. [Google Scholar] [CrossRef]
- Dash, M.; Chiellini, F.; Ottenbrite, R.M.; Chiellini, E. Chitosan—A versatile semi-synthetic polymer in biomedical applications. Prog. Polym. Sci. 2011, 36, 981–1014. [Google Scholar] [CrossRef]
- MI, R.; Gomaa, H.; MI, M.; Zaki, B. Management of aggressive periodontitis using ozonized water. Egypt. Med. J. NRC 2005, 6, 229–245. [Google Scholar]
- Aranaz, I.; Harris, R.; Heras, A.J.C.O.C. Chitosan amphiphilic derivatives. Chem. Appl. 2010, 14, 308–330. [Google Scholar]
- Yubero-Serrano, E.M.; Lopez-Moreno, J.; Gomez-Delgado, F.; Lopez-Miranda, J. Extra virgin olive oil: More than a healthy fat. Eur. J. Clin. Nut. 2019, 72, 8–17. [Google Scholar] [CrossRef] [Green Version]
- Abd El-Hack, M.E.; El-Saadony, M.T.; Shafi, M.E.; Zabermawi, N.M.; Arif, M.; Batiha, G.E.; Khafaga, A.F.; Abd El-Hakim, Y.M.; Al-Sagheer, A.A. Antimicrobial and antioxidant properties of chitosan and its derivatives and their applications: A review. Int. J. Biol. Macromol. 2020, 164, 2726–2744. [Google Scholar] [CrossRef]






| Sample | [η] (mL/g) | Mw (kDa) | EA (%) | DA | DQ | DS | |||
|---|---|---|---|---|---|---|---|---|---|
| C | H | N | S | ||||||
| UCS | 488.67 | 691.6 | 40.14 | 7.21 | 7.65 | – | 6.2 | – | – |
| LMWUCS | 21.18 | 23.8 | 40.11 | 7.28 | 7.49 | – | 7.5 | – | – |
| TMC | 35.51 | 41.7 | 48.87 | 7.97 | 6.07 | – | – | 13.4 | – |
| TMCT | 51.85 | 49.7 | 42.32 | 7.49 | 5.06 | 23.9 | – | – | 1.66 |
| Sample | Permeate in 24 h (mg/cm2 h) | Jss (mg/cm2 h) | Kp × 10−3 (cm/h) | Er | R2 | ||
|---|---|---|---|---|---|---|---|
| Zero Order | 1st Order | Higuchi | |||||
| VOO | – | 0.0511 ± 0.02 | 0.18 ± 0.04 | – | – | – | – |
| OONE | 2.71 ± 0.11 | 0.0824 ± 0.04 | 0.98 ± 0.13 | 1.68 ± 0.26 | 0.85374 | 0.68574 | 0.96313 |
| OOHTN | 4.15 ± 0.16 | 0.2683 ± 0.11 | 4.41 ± 0.85 | 5.55 ± 0.76 | 0.97867 | 0.80386 | 0.99982 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nasr, A.M.; Aboelenin, S.M.; Alfaifi, M.Y.; Shati, A.A.; Elbehairi, S.E.I.; Elshaarawy, R.F.M.; Elwahab, N.H.A. Quaternized Chitosan Thiol Hydrogel-Thickened Nanoemulsion: A Multifunctional Platform for Upgrading the Topical Applications of Virgin Olive Oil. Pharmaceutics 2022, 14, 1319. https://doi.org/10.3390/pharmaceutics14071319
Nasr AM, Aboelenin SM, Alfaifi MY, Shati AA, Elbehairi SEI, Elshaarawy RFM, Elwahab NHA. Quaternized Chitosan Thiol Hydrogel-Thickened Nanoemulsion: A Multifunctional Platform for Upgrading the Topical Applications of Virgin Olive Oil. Pharmaceutics. 2022; 14(7):1319. https://doi.org/10.3390/pharmaceutics14071319
Chicago/Turabian StyleNasr, Ali M., Salama M. Aboelenin, Mohammad Y. Alfaifi, Ali A. Shati, Serag Eldin I. Elbehairi, Reda F. M. Elshaarawy, and Nashwa H. Abd Elwahab. 2022. "Quaternized Chitosan Thiol Hydrogel-Thickened Nanoemulsion: A Multifunctional Platform for Upgrading the Topical Applications of Virgin Olive Oil" Pharmaceutics 14, no. 7: 1319. https://doi.org/10.3390/pharmaceutics14071319
APA StyleNasr, A. M., Aboelenin, S. M., Alfaifi, M. Y., Shati, A. A., Elbehairi, S. E. I., Elshaarawy, R. F. M., & Elwahab, N. H. A. (2022). Quaternized Chitosan Thiol Hydrogel-Thickened Nanoemulsion: A Multifunctional Platform for Upgrading the Topical Applications of Virgin Olive Oil. Pharmaceutics, 14(7), 1319. https://doi.org/10.3390/pharmaceutics14071319