Flavonoids Enhance Lipofection Efficiency and Ameliorate Cytotoxicity in Colon26 and HepG2 Cells via Oxidative Stress Regulation
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Plasmid DNA
2.3. Cell Culture
2.4. Preparation of Various Concentrations of Flavonoids
2.5. Measurement of Gene Expression and Cell Viability
2.6. Flow Cytometry
2.7. Confocal Microscopy
2.8. Statistical Analysis
3. Results and Discussion
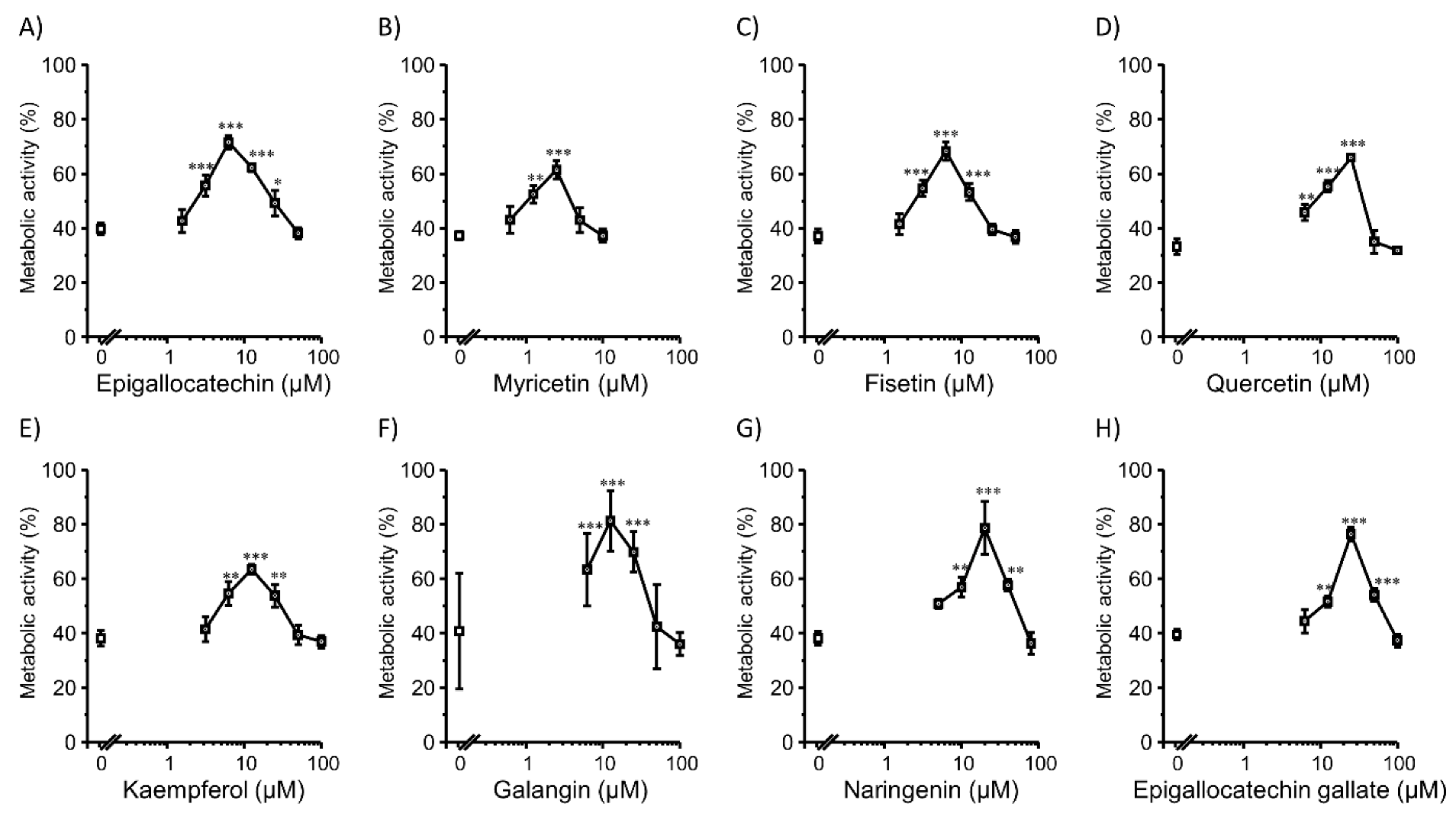
3.1. Transfection Efficiency and Cell Viability with Flavonoids in 2D Colon26 Cells
3.2. Transfection Efficiency and Cell Viability with Flavonoids in 2D HepG2 Cells
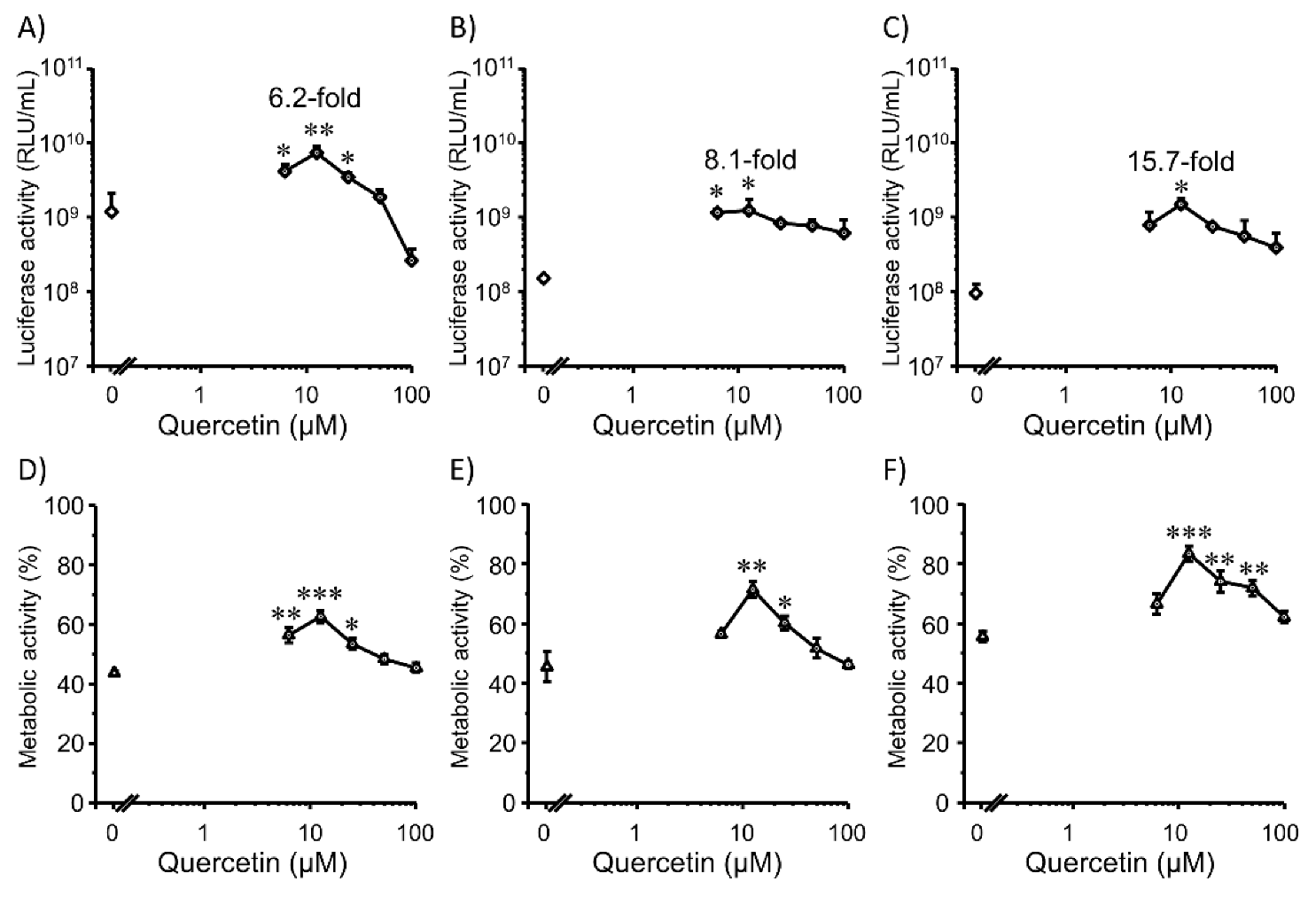
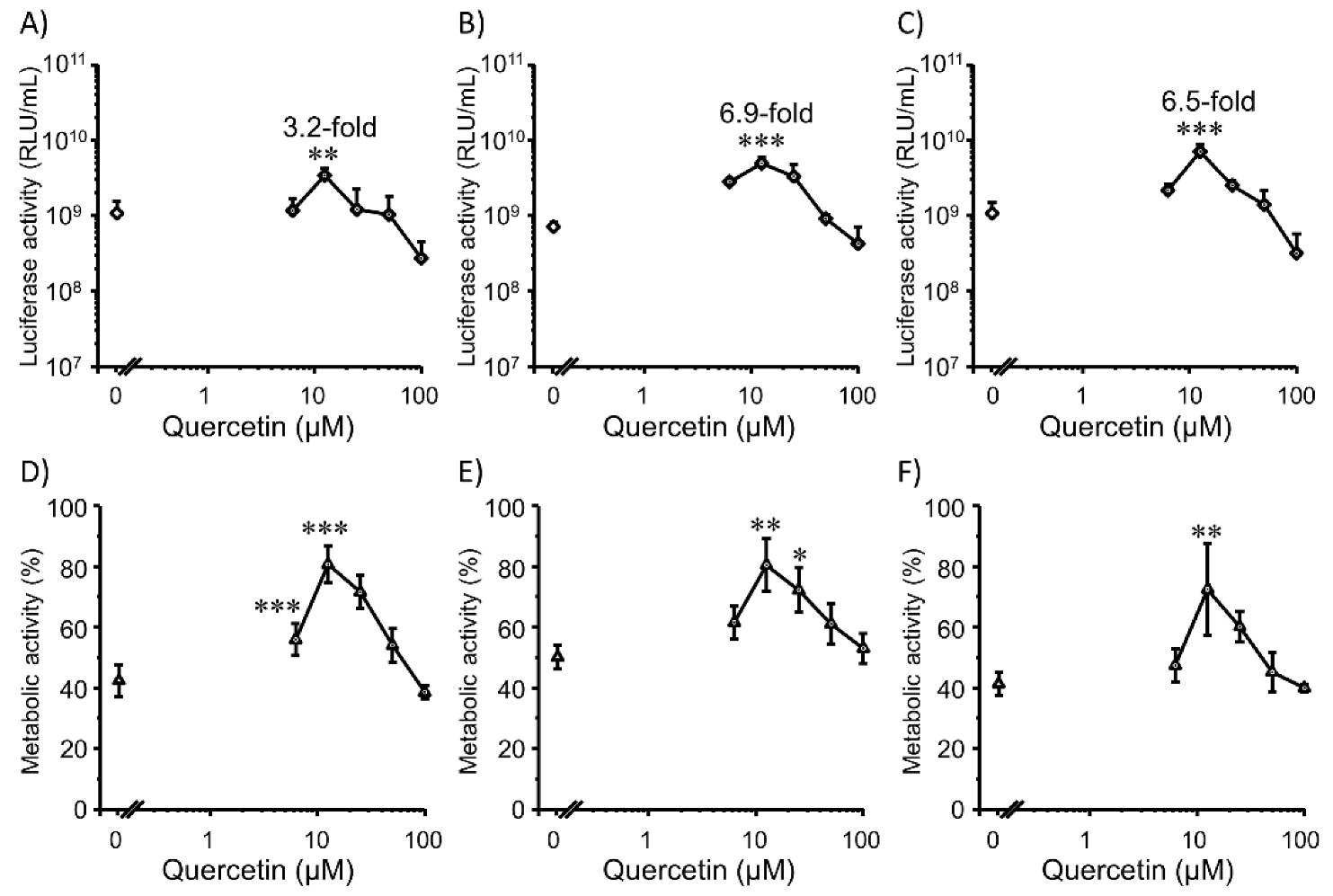
3.3. Transfection Efficiency and Cell Viability with Quercetin in 3D Colon26 Cells
3.4. Transfection Efficiency and Cell Viability with Quercetin in 3D HepG2 Cells
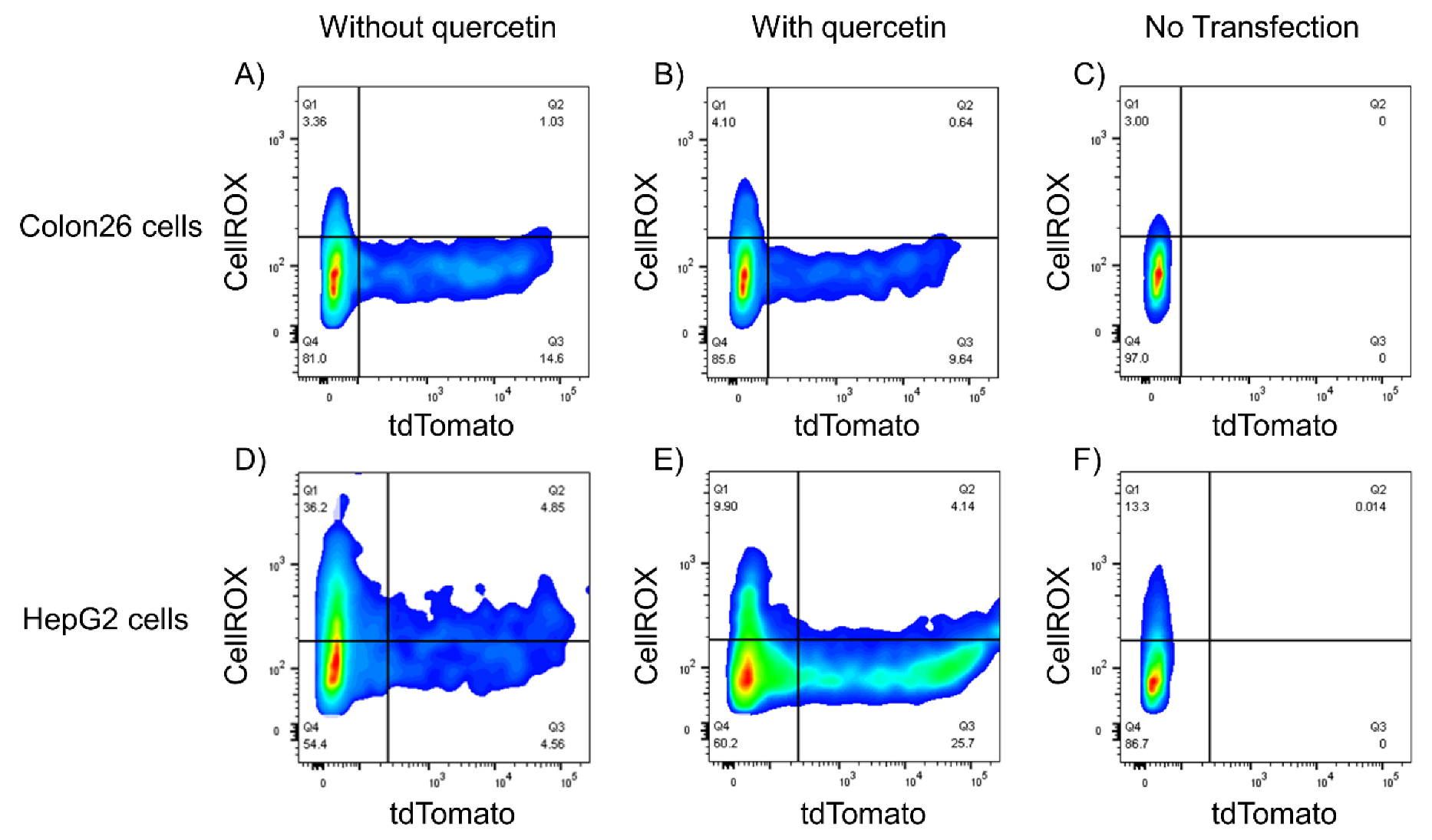
3.5. Relationship between ROS Levels and Gene Expression of Lipoplexes
3.6. Relationships among ROS, Autophagy, Cellular Uptake, and Transfection Efficiency
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zu, H.; Gao, D. Non-viral vectors in gene therapy: Recent development, challenges, and prospects. AAPS J. 2021, 23, 78. [Google Scholar] [CrossRef]
- Wan, N.; Jia, Y.Y.; Hou, Y.L.; Ma, X.X.; He, Y.S.; Li, C.; Zhou, S.Y.; Zhang, B.L. Preparation, physicochemical properties, and transfection activities of tartaric acid-based cationic lipids as effective nonviral gene delivery vectors. Biol. Pharm. Bull. 2016, 39, 1112–1120. [Google Scholar] [CrossRef]
- Yun, C.H.; Bae, C.S.; Ahn, T. Cargo-free nanoparticles containing cationic lipids induce reactive oxygen species and cell death in HepG2 cells. Biol. Pharm. Bull. 2016, 39, 1338–1346. [Google Scholar] [CrossRef]
- Wang, S.; Fumoto, S.; Miyamoto, H.; Tanaka, M.; Nishida, K. Edaravone, a cytoprotective drug, enhances transgene expression mediated by lipoplexes in HepG2 cells and mice. Int. J. Pharm. 2018, 548, 173–181. [Google Scholar] [CrossRef] [PubMed]
- Ghose, A.K.; Viswanadhan, V.N.; Wendoloski, J.J. A knowledge-based approach in designing combinatorial or medicinal chemistry libraries for drug discovery. 1. A qualitative and quantitative characterization of known drug databases. J. Comb. Chem. 1999, 1, 55–68. [Google Scholar] [CrossRef]
- Kan, X.; Liu, J.; Chen, Y.; Guo, W.; Xu, D.; Cheng, J.; Cao, Y.; Yang, Z.; Fu, S. Myricetin protects against H2O2-induced oxidative damage and apoptosis in bovine mammary epithelial cells. J. Cell Physiol. 2021, 236, 2684–2695. [Google Scholar] [CrossRef] [PubMed]
- Rodius, S.; de Klein, N.; Jeanty, C.; Sánchez-Iranzo, H.; Crespo, I.; Ibberson, M.; Xenarios, I.; Dittmar, G.; Mercader, N.; Nicolau, S.P.; et al. Fisetin protects against cardiac cell death through reduction of ROS production and caspases activity. Sci. Rep. 2020, 10, 2896. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Xu, L.; Yang, L.; Wang, X. Epigallocatechin gallate is the most effective catechin against antioxidant stress via hydrogen peroxide and radical scavenging activity. Med. Sci. Monit. 2018, 24, 8198–8206. [Google Scholar] [CrossRef]
- Wu, P.; Meng, X.; Zheng, H.; Zeng, Q.; Chen, T.; Wang, W.; Zhang, X.; Su, J. Kaempferol attenuates ROS-induced hemolysis and the molecular mechanism of its induction of apoptosis on bladder cancer. Molecules 2018, 23, 2592. [Google Scholar] [CrossRef]
- Wang, K.; Chen, Z.; Huang, L.; Meng, B.; Zhou, X.; Wen, X.; Ren, D. Naringenin reduces oxidative stress and improves mitochondrial dysfunction via activation of the Nrf2/ARE signaling pathway in neurons. Int, J. Mol. Med. 2017, 40, 1582–1590. [Google Scholar] [CrossRef]
- Engstrand, J.; Nilsson, H.; Strömberg, C.; Jonas, E.; Freedman, J. Colorectal cancer liver metastases—A population-based study on incidence, management and survival. BMC Cancer 2018, 18, 78. [Google Scholar] [CrossRef]
- Arellano, R.S. What’s New in Percutaneous Ablative Strategies for Hepatocellular Carcinoma and Colorectal Hepatic Metastases? 2020 Update. Curr. Oncol. Rep. 2020, 22, 105. [Google Scholar] [CrossRef]
- Sendi, H.; Yazdimamaghani, M.; Hu, M.; Sultanpuram, N.; Wang, J.; Moody, A.S.; McCabe, E.; Zhang, J.; Graboski, A.; Li, L.; et al. Nanoparticle Delivery of miR-122 Inhibits Colorectal Cancer Liver Metastasis. Cancer Res. 2022, 82, 105–113. [Google Scholar] [CrossRef]
- Fang, J.K.; Chen, L.; Lu, X.G.; Cao, D.; Guo, L.L.; Zhang, Y.S.; Li, L.B.; Zhang, L.F.; Kuang, Y.T.; Wang, S.L. Optimization of Transforming Growth Factor-β1 siRNA Loaded Chitosan-Tripolyphosphate Nanoparticles for the Treatment of Colorectal Cancer Hepatic Metastasis in a Mouse Model. J. Biomed. Nanotechnol. 2016, 12, 1489–1500. [Google Scholar] [CrossRef]
- Nie, G.; Jin, C.; Cao, Y.; Shen, S.; Zhao, B. Distinct effects of tea catechins on 6-hydroxydopamine-induced apoptosis in PC12 cells. Arch. Biochem. Biophys. 2002, 397, 84–90. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Abe, N.; Qi, H.; Zhu, B.; Murata, Y.; Nakamura, Y. Tea catechins inhibit cell proliferation through hydrogen peroxide-dependent and-independent pathways in human T lymphocytic leukemia Jurkat cells. Food Sci. Technol. Res. 2014, 20, 1245–1249. [Google Scholar] [CrossRef][Green Version]
- Jo, S.; Ha, T.K.; Han, S.H.; Kim, M.E.; Jung, I.; Lee, H.W.; Bae, S.K.; Lee, J.S. Myricetin Induces Apoptosis of Human Anaplastic Thyroid Cancer Cells via Mitochondria Dysfunction. Anticancer Res. 2017, 37, 1705–1710. [Google Scholar] [CrossRef]
- Kang, K.A.; Wang, Z.H.; Zhang, R.; Piao, M.J.; Kim, K.C.; Kang, S.S.; Kim, Y.W.; Lee, J.; Park, D.; Hyun, J.W. Myricetin protects cells against oxidative stress-induced apoptosis via regulation of PI3K/Akt and MAPK signaling pathways. Int. J. Mol. Sci. 2010, 11, 4348–4360. [Google Scholar] [CrossRef]
- Chen, P.Y.; Ho, Y.R.; Wu, M.J.; Huang, S.P.; Chen, P.K.; Tai, M.H.; Ho, C.T.; Yen, J.H. Cytoprotective effects of fisetin against hypoxia-induced cell death in PC12 cells. Food Funct. 2015, 6, 287–296. [Google Scholar] [CrossRef]
- Sundarraj, K.; Raghunath, A.; Panneerselvam, L.; Perumal, E. Fisetin Inhibits Autophagy in HepG2 Cells via PI3K/Akt/mTOR and AMPK Pathway. Nutr. Cancer 2021, 73, 2502–2514. [Google Scholar] [CrossRef]
- Kook, D.; Wolf, A.H.; Yu, A.L.; Neubauer, A.S.; Priglinger, S.G.; Kampik, A.; Welge-Lüssen, U.C. The protective effect of quercetin against oxidative stress in the human RPE in vitro. Investig. Ophthalmol. Vis. Sci. 2008, 49, 1712–1720. [Google Scholar] [CrossRef]
- Wang, D.; He, X.; Wang, D.; Peng, P.; Xu, X.; Gao, B.; Zheng, C.; Wang, H.; Jia, H.; Shang, Q.; et al. Quercetin Suppresses Apoptosis and Attenuates Intervertebral Disc Degeneration via the SIRT1-Autophagy Pathway. Front. Cell Dev. Biol. 2020, 8, 613006. [Google Scholar] [CrossRef] [PubMed]
- Varshney, R.; Gupta, S.; Roy, P. Cytoprotective effect of kaempferol against palmitic acid-induced pancreatic β-cell death through modulation of autophagy via AMPK/mTOR signaling pathway. Mol. Cell Endocrinol. 2017, 448, 1–20. [Google Scholar] [CrossRef]
- Kim, C.J.; Shin, S.H.; Kim, B.J.; Kim, C.H.; Kim, J.H.; Kang, H.M.; Park, B.S.; Kim, I.R. The Effects of Kaempferol-Inhibited Autophagy on Osteoclast Formation. Int. J. Mol. Sci. 2018, 19, 125. [Google Scholar] [CrossRef]
- Zhang, T.; Mei, X.; Ouyang, H.; Lu, B.; Yu, Z.; Wang, Z.; Ji, L. Natural flavonoid galangin alleviates microglia-trigged blood-retinal barrier dysfunction during the development of diabetic retinopathy. J. Nutr. Biochem. 2019, 65, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Wen, M.; Wu, J.; Luo, H.; Zhang, H. Galangin induces autophagy through upregulation of p53 in HepG2 cells. Pharmacology 2012, 89, 247–255. [Google Scholar] [CrossRef] [PubMed]
- Podder, B.; Song, H.Y.; Kim, Y.S. Naringenin exerts cytoprotective effect against paraquat-induced toxicity in human bronchial epithelial BEAS-2B cells through NRF2 activation. J. Microbiol. Biotechnol. 2014, 24, 605–613. [Google Scholar] [CrossRef] [PubMed]
- Ahamad, M.S.; Siddiqui, S.; Jafri, A.; Ahmad, S.; Afzal, M.; Arshad, M. Induction of apoptosis and antiproliferative activity of naringenin in human epidermoid carcinoma cell through ROS generation and cell cycle arrest. PLoS ONE 2014, 9, e110003. [Google Scholar] [CrossRef] [PubMed]
- Krupkova, O.; Handa, J.; Hlavna, M.; Klasen, J.; Ospelt, C.; Ferguson, S.J.; Wuertz-Kozak, K. The Natural Polyphenol Epigallocatechin Gallate Protects Intervertebral Disc Cells from Oxidative Stress. Oxid. Med. Cell Longev. 2016, 2016, 7031397. [Google Scholar] [CrossRef] [PubMed]
- Cia, D.; Vergnaud-Gauduchon, J.; Jacquemot, N.; Doly, M. Epigallocatechin gallate (EGCG) prevents H2O2-induced oxidative stress in primary rat retinal pigment epithelial cells. Curr. Eye Res. 2014, 39, 944–952. [Google Scholar] [CrossRef]
- Treml, J.; Šmejkal, K. Flavonoids as potent scavengers of hydroxyl radicals. Compr. Rev. Food Sci. Food Saf. 2016, 15, 720–738. [Google Scholar] [CrossRef] [PubMed]
- Rietjens, I.M.; Boersma, M.G.; Haan, L.D.; Spenkelink, B.; Awad, H.M.; Cnubben, N.H.; van Zanden, J.J.; Woude, H.V.; Alink, G.M.; Koeman, J.H. The pro-oxidant chemistry of the natural antioxidants vitamin C, vitamin E, carotenoids and flavonoids. Environ. Toxicol. Pharmacol. 2002, 11, 321–333. [Google Scholar] [CrossRef]
- Yen, G.C.; Duh, P.D.; Tsai, H.L.; Huang, S.L. Pro-oxidative properties of flavonoids in human lymphocytes. Biosci. Biotechnol. Biochem. 2003, 67, 1215–1222. [Google Scholar] [CrossRef] [PubMed]
- Bedard, K.; Krause, K.H. The NOX family of ROS-generating NADPH oxidases: Physiology and pathophysiology. Physiol. Rev. 2007, 87, 245–313. [Google Scholar] [CrossRef] [PubMed]
- Tarafdar, A.; Pula, G. The role of NADPH oxidases and oxidative stress in neurodegenerative disorders. Int. J. Mol. Sci. 2018, 19, 3824. [Google Scholar] [CrossRef]
- Gao, H.M.; Zhou, H.; Hong, J.S. NADPH oxidases: Novel therapeutic targets for neurodegenerative diseases. Trends Pharmacol. Sci. 2012, 33, 295–303. [Google Scholar] [CrossRef]
- Wisastra, R.; Dekker, F.J. Inflammation, cancer, and oxidative lipoxygenase activity are intimately linked. Cancers 2014, 6, 1500–1521. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.Y.; Kim, T.B.; Moon, K.A.; Kim, T.J.; Shin, D.; Cho, Y.S.; Moon, H.B.; Lee, K.Y. Regulation of pro-inflammatory responses by lipoxygenases via intracellular reactive oxygen species in vitro and in vivo. Exp. Mol. Med. 2008, 40, 461–476. [Google Scholar] [CrossRef]
- Fruehauf, J.P.; Meyskens, F.L., Jr. Reactive oxygen species: A breath of life or death? Clin. Cancer Res. 2007, 13, 789–794. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Wang, Z.; Liu, Y.; Liu, Y.; Li, H.; Di, C.; Wu, Z.; Gan, L.; Zhang, H. Carbon ion beams induce hepatoma cell death by NADPH oxidase-mediated mitochondrial damage. J. Cell Physiol. 2014, 229, 100–107. [Google Scholar] [CrossRef]
- Mashima, R.; Okuyama, T. The role of lipoxygenases in pathophysiology: New insights and future perspectives. Redox Biol. 2015, 6, 297–310. [Google Scholar] [CrossRef] [PubMed]
- Sekher Pannala, A.; Chan, T.S.; O’Brien, P.J.; Rice-Evans, C.A. Flavonoid B-ring chemistry and antioxidant activity: Fast reaction kinetics. Biochem. Biophys. Res. Commun. 2001, 282, 1161–1168. [Google Scholar] [CrossRef]
- Vinci, M.; Gowan, S.; Boxall, F.; Patterson, L.; Zimmermann, M.; Court, W.; Lomas, C.; Mendiola, M.; Hardisson, D.; Eccles, S.A. Advances in establishment and analysis of three-dimensional tumor spheroid-based functional assays for target validation and drug evaluation. BMC Biol. 2012, 10, 29. [Google Scholar] [CrossRef] [PubMed]
- Tibbitt, M.W.; Anseth, K.S. Hydrogels as extracellular matrix mimics for 3D cell culture. Biotechnol. Bioeng. 2009, 103, 655–663. [Google Scholar] [CrossRef]
- Taniguchi, M.; Miyamoto, H.; Tokunaga, A.; Fumoto, S.; Tanaka, T.; Nishida, K. Evaluation of mRNA expression of drug-metabolizing enzymes in acetaminophen-induced hepatotoxicity using a three-dimensional hepatocyte culture system. Xenobiotica 2020, 50, 654–662. [Google Scholar] [CrossRef] [PubMed]
- Ikari, R.; Mukaisho, K.I.; Kageyama, S.; Nagasawa, M.; Kubota, S.; Nakayama, T.; Murakami, S.; Taniura, N.; Tanaka, H.; Kushima, R.P.; et al. Differences in the central energy metabolism of cancer cells between conventional 2D and novel 3D culture systems. Int. J. Mol. Sci. 2021, 22, 1805. [Google Scholar] [CrossRef]
- Brunner, S.; Sauer, T.; Carotta, S.; Cotton, M.; Saltik, M.; Wagner, E. Cell cycle dependence of gene transfer by lipoplex, polyplex, and recombinant adenovirus. Gene Ther. 2000, 7, 401–407. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.Y.; Kim, M.K.; Park, K.S.; Choo, H.; Chong, Y. Quercetin-POC conjugates: Differential stability and bioactivity profiles between breast cancer (MCF-7) and colorectal carcinoma (HCT116) cell lines. Bioorg. Med. Chem. 2013, 21, 1671–1679. [Google Scholar] [CrossRef]
- Li, P.; Chang, M. Roles of PRR-mediated signaling pathways in the regulation of oxidative stress and inflammatory diseases. Int. J. Mol. Sci. 2021, 22, 7688. [Google Scholar] [CrossRef]
- Takeshita, F.; Leifer, C.A.; Gursel, I.; Ishii, K.J.; Takeshita, S.; Gursel, M.; Klinman, D.M. Cutting edge: Role of Toll-like receptor 9 in CpG DNA-induced activation of human cells. J. Immunol. 2001, 167, 3555–3558. [Google Scholar] [CrossRef]
- Li, Y.; Deng, S.L.; Lian, Z.X.; Yu, K. Roles of Toll-Like receptors in nitroxidative stress in mammals. Cells 2019, 8, 576. [Google Scholar] [CrossRef]
- Paludan, S.R.; Bowie, A.G. Immune sensing of DNA. Immunity 2013, 38, 870–880. [Google Scholar] [CrossRef] [PubMed]
- Yew, N.S.; Zhao, H.; Przybylska, M.; Wu, I.H.; Tousignant, J.D.; Scheule, R.K.; Cheng, S.H. CpG-depleted plasmid DNA vectors with enhanced safety and long-term gene expression in vivo. Mol. Ther. 2002, 5, 731–738. [Google Scholar] [CrossRef]
- Roberts, R.; Al-Jamal, W.T.; Whelband, M.; Thomas, P.; Jefferson, M.; van den Bossche, J.; Powell, P.P.; Kostarelos, K.; Wileman, T. Autophagy and formation of tubulovesicular autophagosomes provide a barrier against nonviral gene delivery. Autophagy 2013, 9, 667–682. [Google Scholar] [CrossRef] [PubMed]
- Biasizzo, M.; Kopitar-Jerala, N. Interplay between NLRP3 inflammasome and autophagy. Front. Immunol. 2020, 11, 591803. [Google Scholar] [CrossRef] [PubMed]
- Chanjitwiriya, K.; Roytrakul, S.; Kunthalert, D. Quercetin negatively regulates IL-1β production in Pseudomonas aeruginosa-infected human macrophages through the inhibition of MAPK/NLRP3 inflammasome pathways. PLoS ONE 2020, 15, e0237752. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Wang, S.; Gou, H.; Zhang, J.; Li, C. Crosstalk between autophagy and the cGAS-STING signaling pathway in type I interferon production. Front. Cell Dev. Biol. 2021, 9, 748485. [Google Scholar] [CrossRef] [PubMed]
- Hayman, T.J.; Baro, M.; MacNeil, T.; Phoomak, C.; Aung, T.N.; Cui, W.; Leach, K.; Iyer, R.; Challa, S.; Sandoval-Schaefer, T.; et al. STING enhances cell death through regulation of reactive oxygen species and DNA damage. Nat. Commun. 2021, 12, 2327. [Google Scholar] [CrossRef]
- Zheng, W.; Xia, N.; Zhang, J.; Chen, N.; Meurens, F.; Liu, Z.; Zhu, J. How the innate immune DNA sensing cGAS-STING pathway is involved in autophagy. Int. J. Mol. Sci. 2021, 22, 13232. [Google Scholar] [CrossRef] [PubMed]
- Bai, H.; Lester, G.M.S.; Petishnok, L.C.; Dean, D.A. Cytoplasmic transport and nuclear import of plasmid DNA. Biosci. Rep. 2017, 37, BSR20160616. [Google Scholar] [CrossRef]
- Li, Z.; Ji, X.; Wang, D.; Liu, J.; Zhang, X. Autophagic flux is highly active in early mitosis and differentially regulated throughout the cell cycle. Oncotarget 2016, 7, 39705–39718. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Xie, R.; Nguyen, S.; Ye, M.; McKeehan, W.L. Robust autophagy/mitophagy persists during mitosis. Cell Cycle 2009, 8, 1616–1620. [Google Scholar] [CrossRef] [PubMed]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, D.; Fumoto, S.; Miyamoto, H.; Tanaka, M.; Nishida, K. Flavonoids Enhance Lipofection Efficiency and Ameliorate Cytotoxicity in Colon26 and HepG2 Cells via Oxidative Stress Regulation. Pharmaceutics 2022, 14, 1203. https://doi.org/10.3390/pharmaceutics14061203
Hu D, Fumoto S, Miyamoto H, Tanaka M, Nishida K. Flavonoids Enhance Lipofection Efficiency and Ameliorate Cytotoxicity in Colon26 and HepG2 Cells via Oxidative Stress Regulation. Pharmaceutics. 2022; 14(6):1203. https://doi.org/10.3390/pharmaceutics14061203
Chicago/Turabian StyleHu, Die, Shintaro Fumoto, Hirotaka Miyamoto, Masakazu Tanaka, and Koyo Nishida. 2022. "Flavonoids Enhance Lipofection Efficiency and Ameliorate Cytotoxicity in Colon26 and HepG2 Cells via Oxidative Stress Regulation" Pharmaceutics 14, no. 6: 1203. https://doi.org/10.3390/pharmaceutics14061203
APA StyleHu, D., Fumoto, S., Miyamoto, H., Tanaka, M., & Nishida, K. (2022). Flavonoids Enhance Lipofection Efficiency and Ameliorate Cytotoxicity in Colon26 and HepG2 Cells via Oxidative Stress Regulation. Pharmaceutics, 14(6), 1203. https://doi.org/10.3390/pharmaceutics14061203






