Cerium-Containing Mesoporous Bioactive Glasses (MBGs)-Derived Scaffolds with Drug Delivery Capability for Potential Tissue Engineering Applications
Abstract
:1. Introduction
2. Materials and Methods
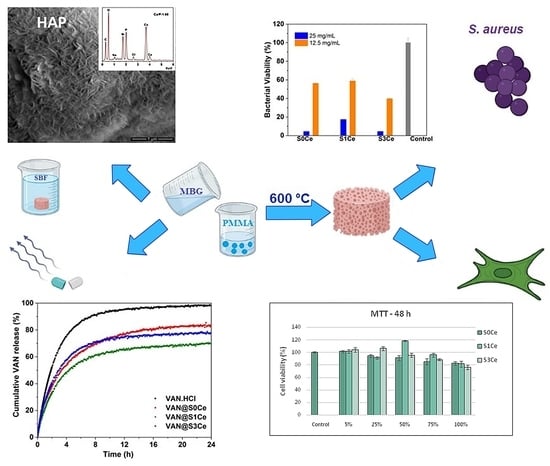
2.1. Preparation of Cerium-Containing MBGsScaffolds
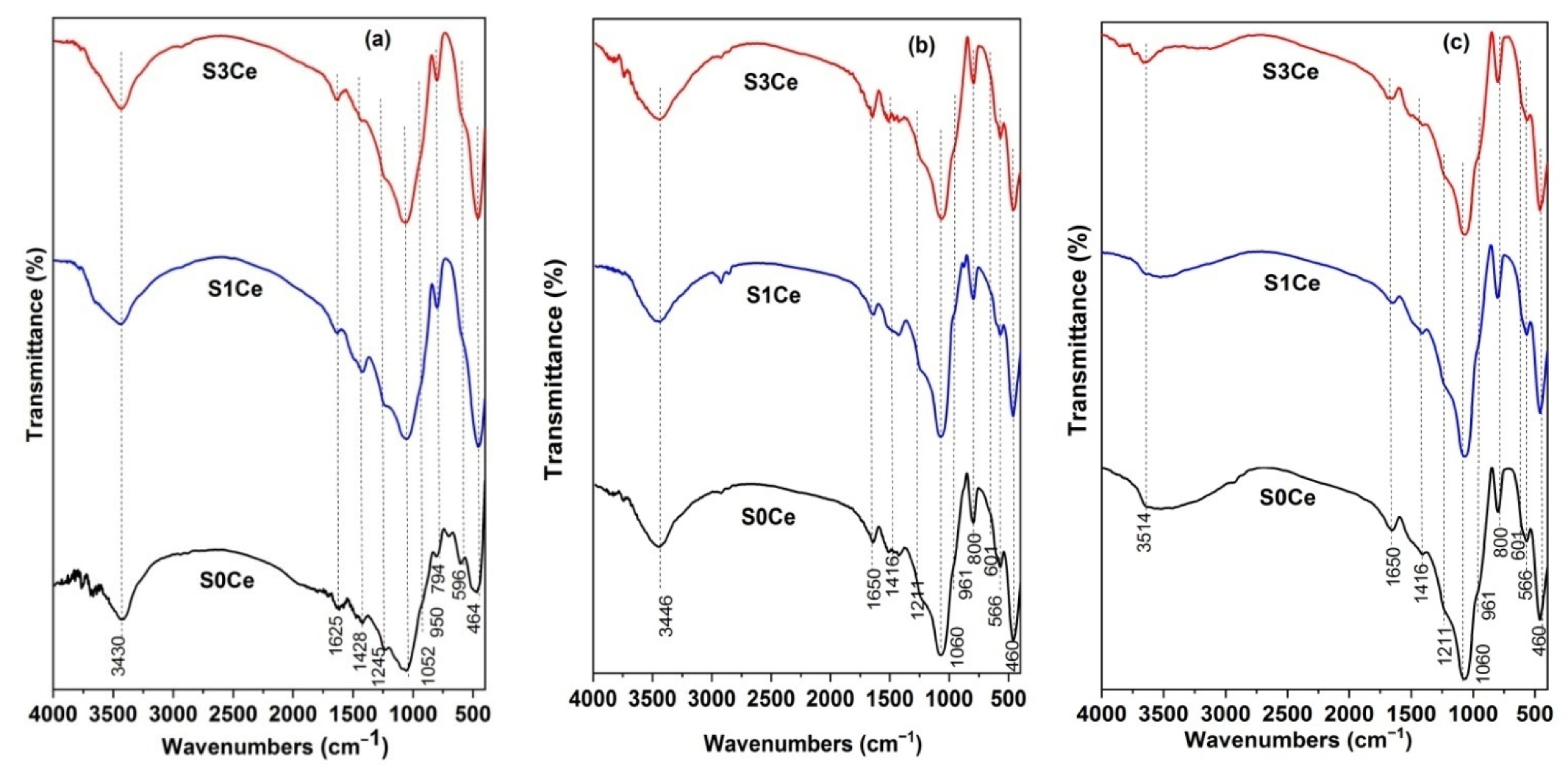
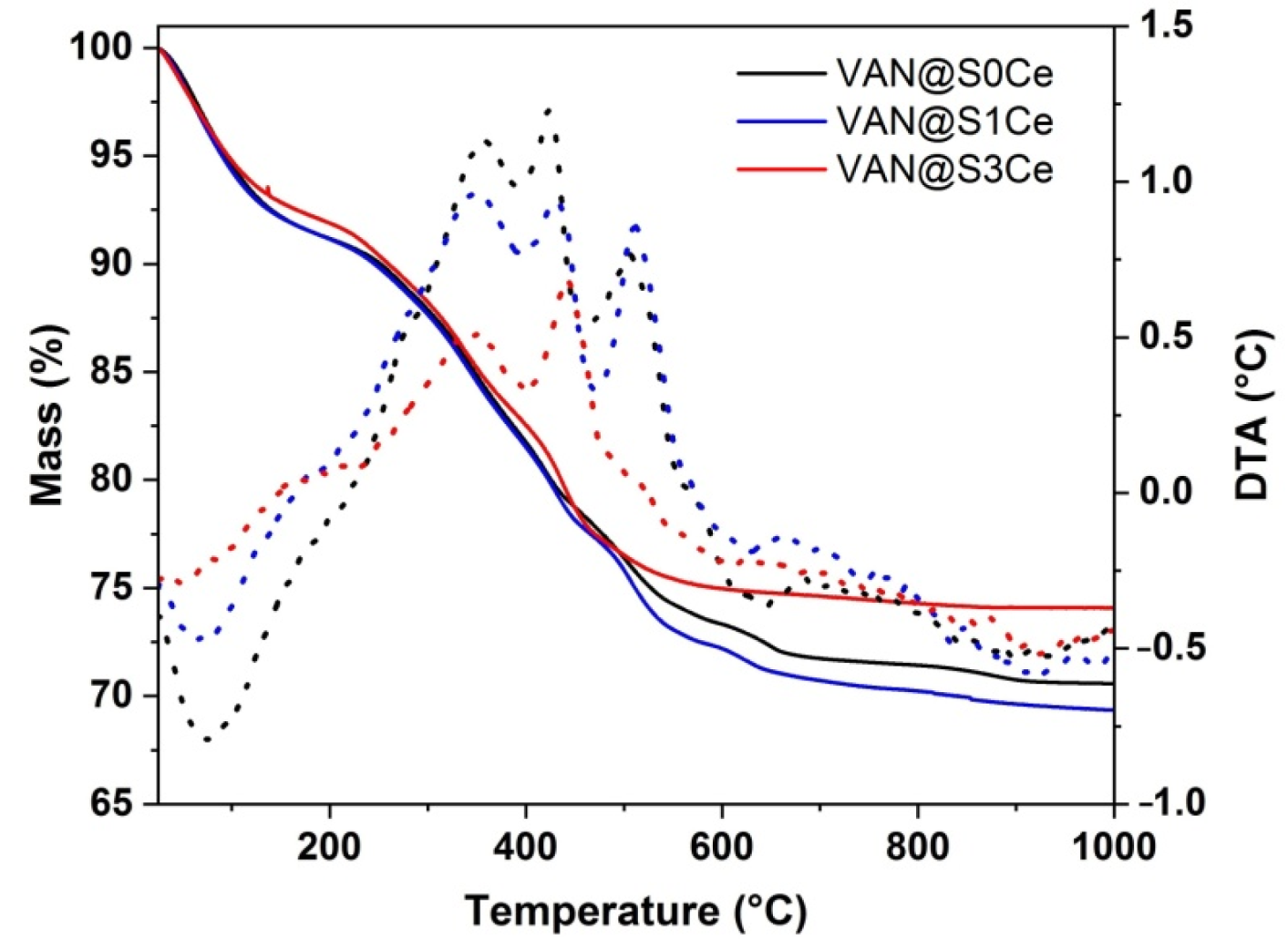
2.2. Characterization of Cerium-Containing MBGs Scaffolds
3. Results and Discussion
3.1. UV-Vis
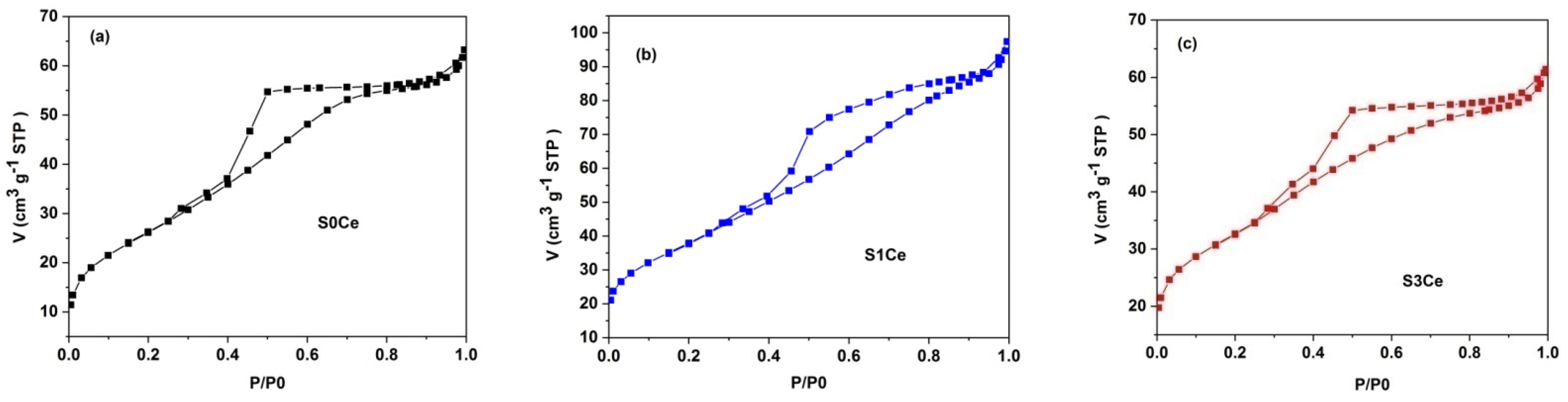
3.2. Textural Characterization
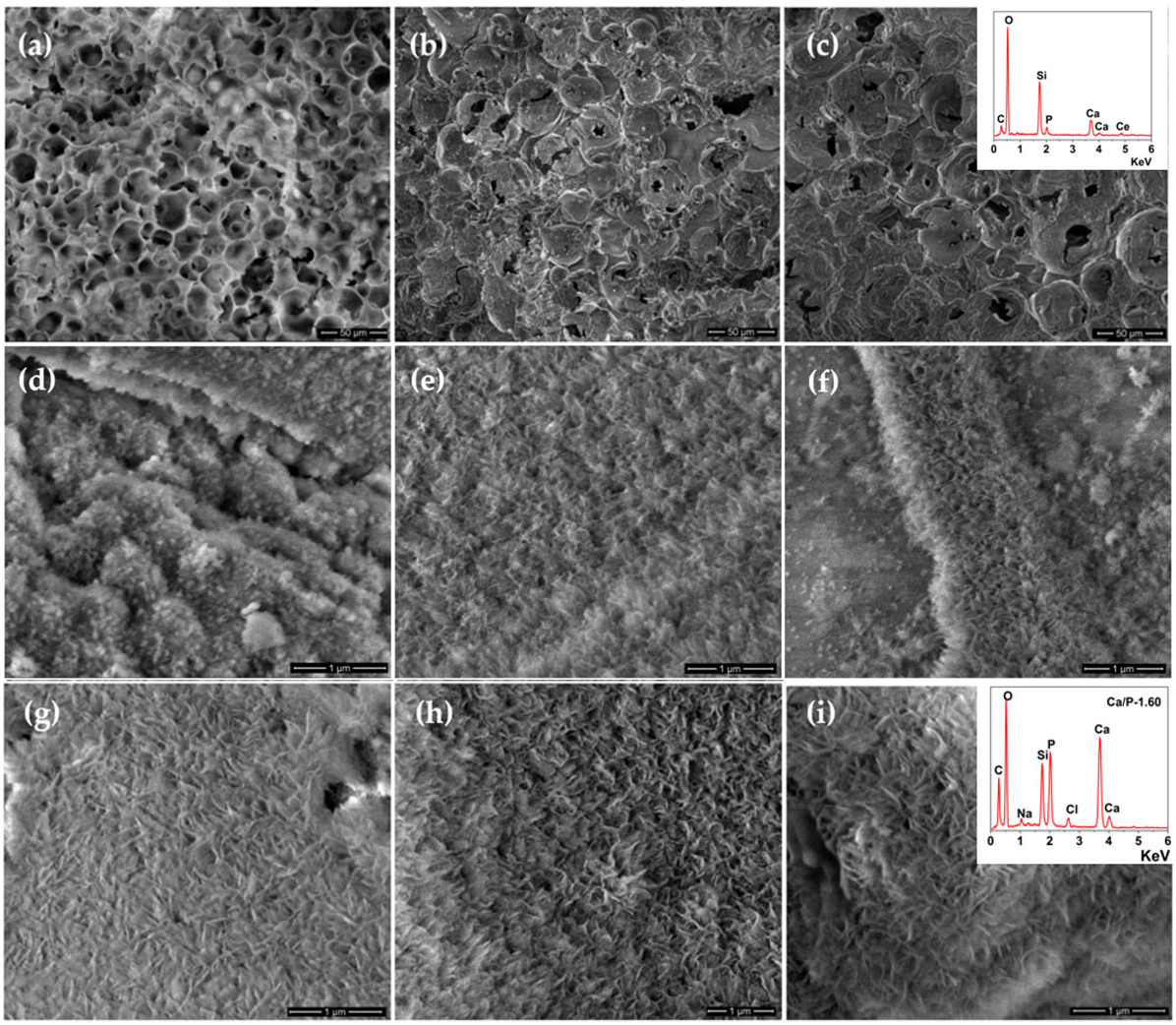
3.3. In Vitro Bioactivity Assessment
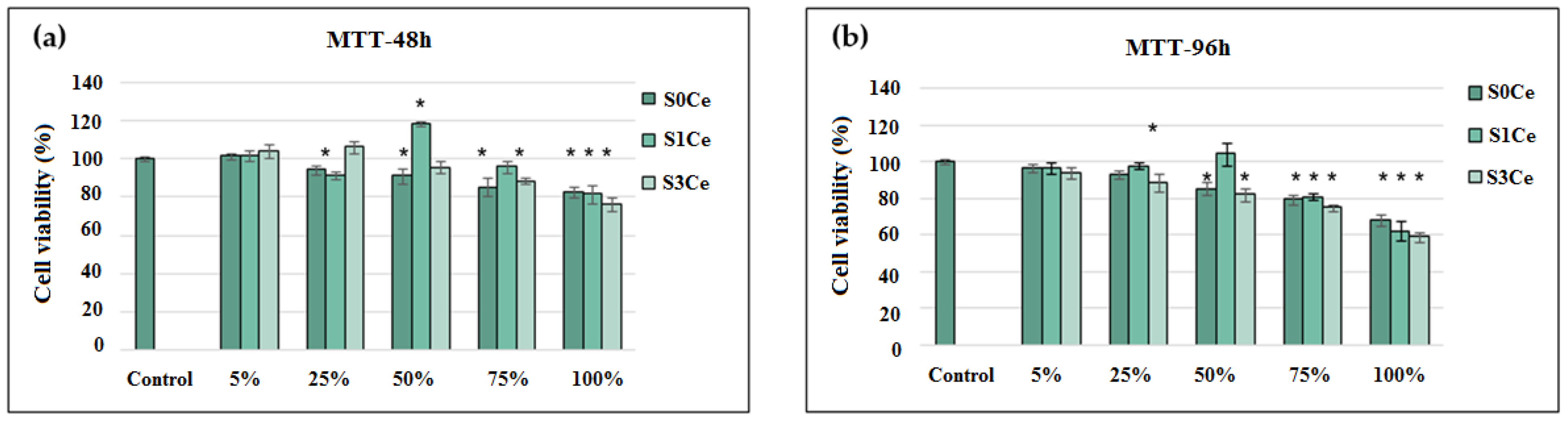
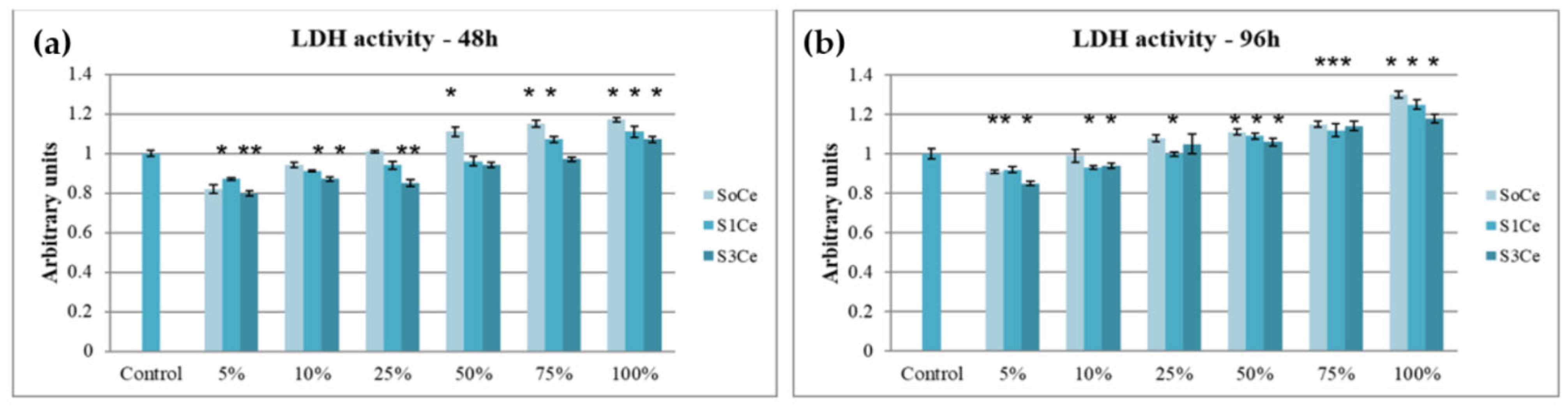
3.4. In Vitro Cytocompatibility Testing
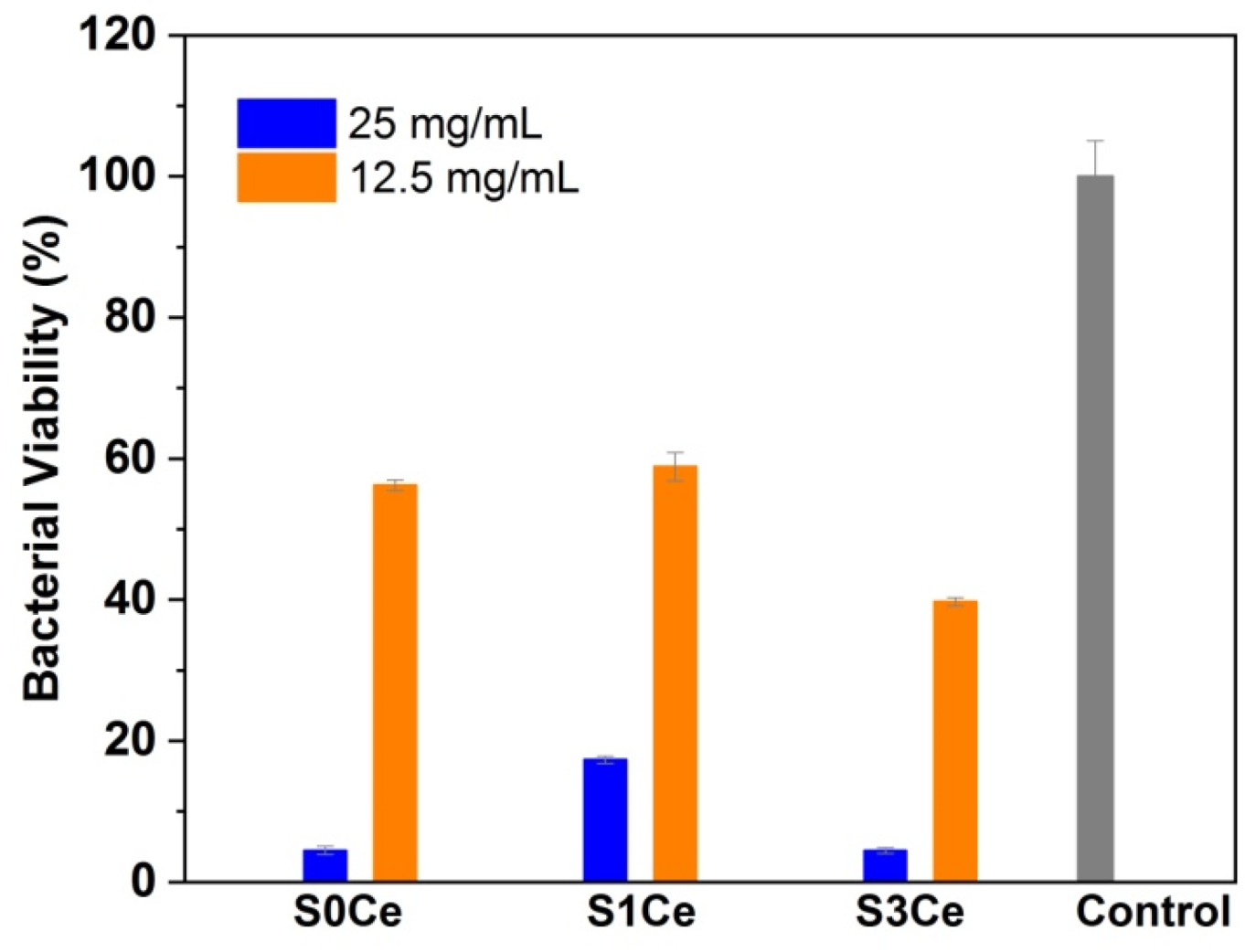
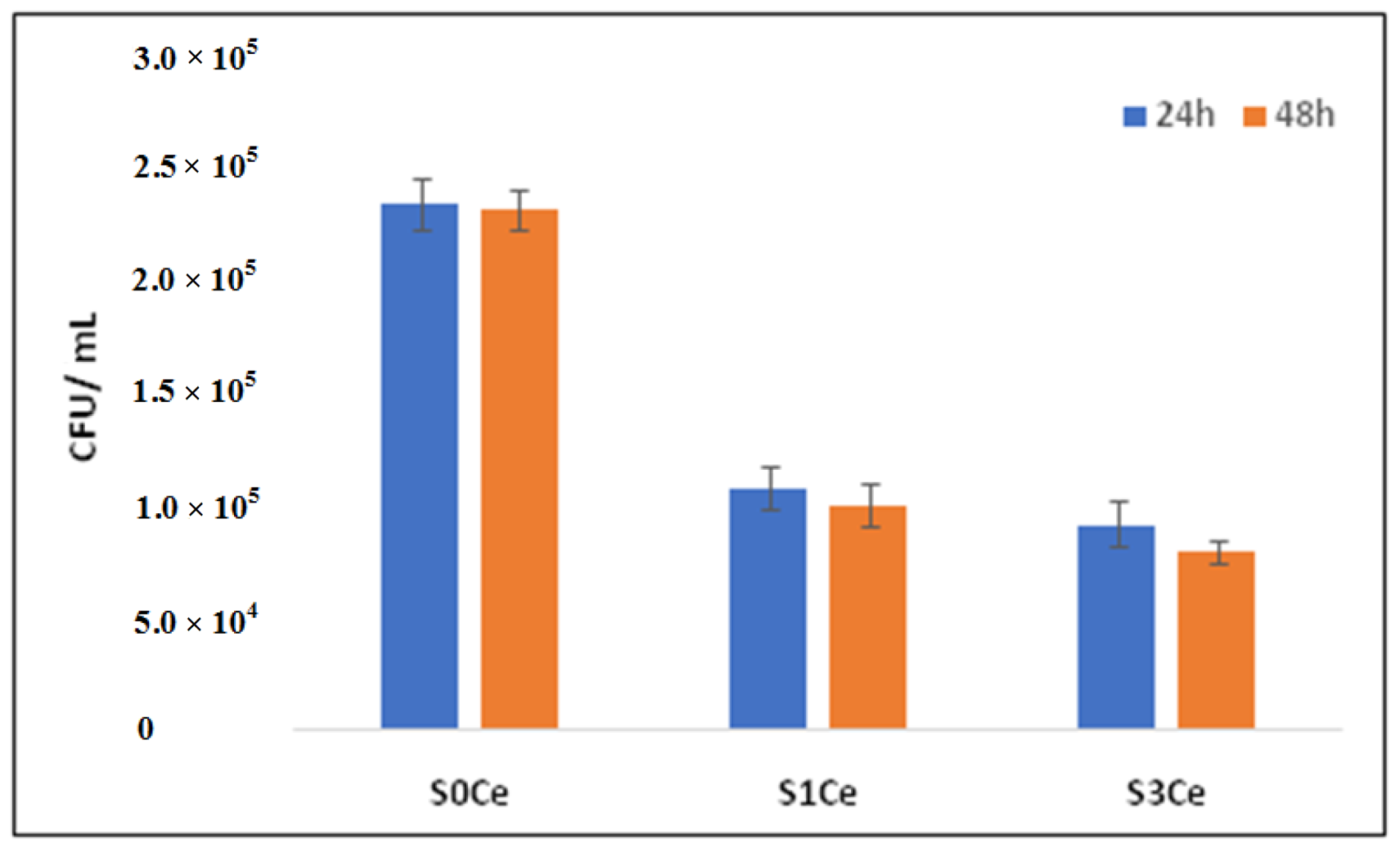
3.5. Antibacterial Activity
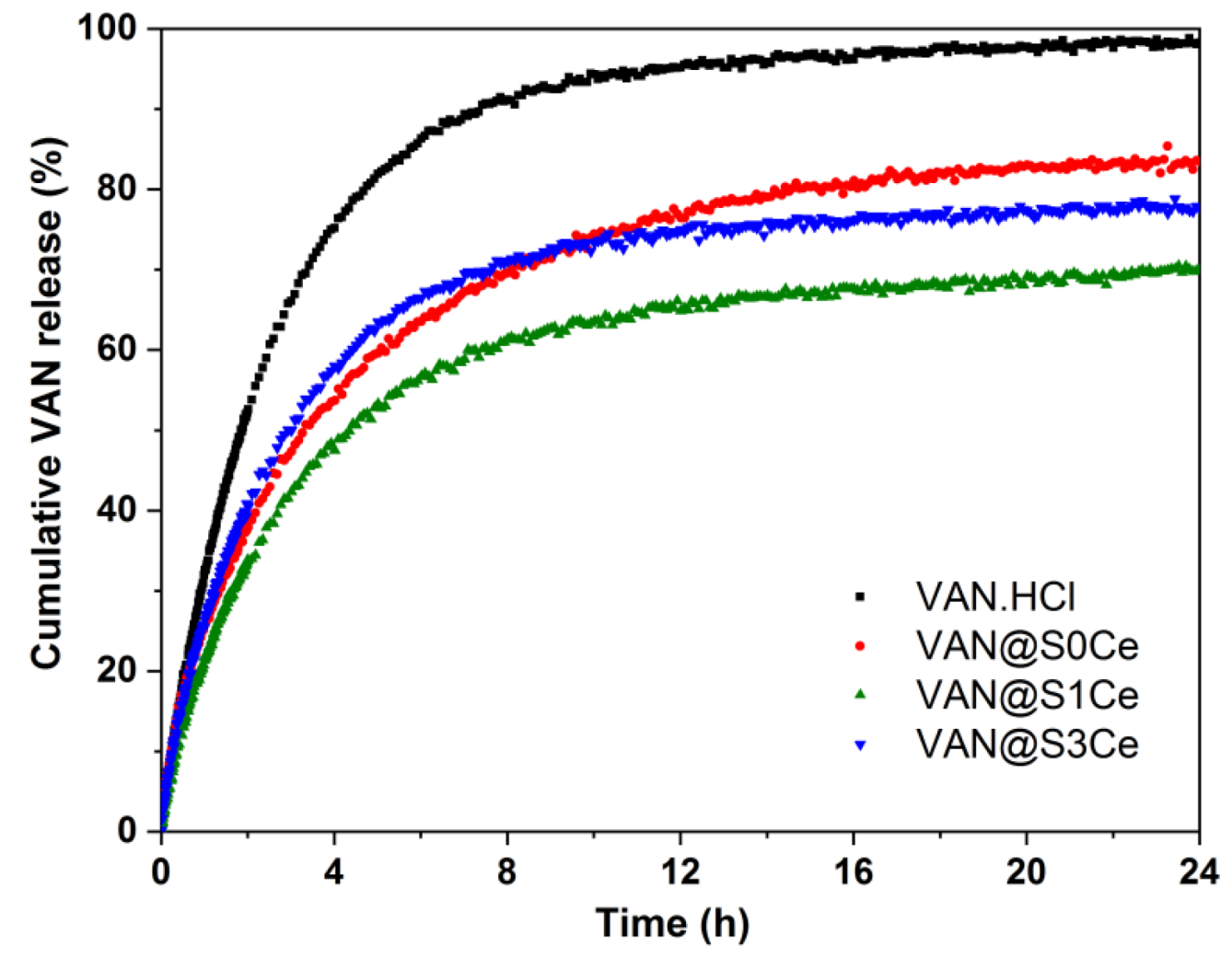
3.6. In Vitro Release Profiles and Kinetic Model
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, W.; Yeung, K.W.K. Bone grafts and biomaterials substitutes for bone defect repair: A review. Bioact. Mater. 2017, 2, 224–247. [Google Scholar] [CrossRef] [PubMed]
- Dhandayuthapani, B.; Yoshida, Y.; Maekawa, T.; Kumar, D.S. Polymeric Scaffolds in Tissue Engineering Application: A Review. Int. J. Polym. Sci. 2011, 2011, 290602. [Google Scholar] [CrossRef]
- Sergi, R.; Bellucci, D.; Cannillo, V. A Review of Bioactive Glass/Natural Polymer Composites: State of the Art. Materials 2020, 13, 5560. [Google Scholar] [CrossRef] [PubMed]
- Chocholata, P.; Kulda, V.; Babuska, V. Fabrication of Scaffolds for Bone-Tissue Regeneration. Materials 2019, 12, 568. [Google Scholar] [CrossRef] [Green Version]
- Baino, F.; Fiume, E. 3D Printing of Hierarchical Scaffolds Based on Mesoporous Bioactive Glasses(MBGs)—Fundamentals and Applications. Materials 2020, 13, 1688. [Google Scholar] [CrossRef] [Green Version]
- Collins, M.N.; Ren, G.; Young, K.; Pina, S.; Reis, R.L.; Oliveira, J.M. Scaffold Fabrication Technologies and Structure/Function Properties in Bone Tissue Engineering. Adv. Funct. Mater. 2021, 31, 2010609. [Google Scholar] [CrossRef]
- Turnbull, G.; Clarke, J.; Picard, F.; Riches, P.; Jia, L.; Han, F.; Li, B.; Shu, W. 3D bioactive composite scaffolds for bone tissue engineering. Bioact. Mater. 2018, 3, 278–314. [Google Scholar] [CrossRef] [Green Version]
- Bagde, A.D.; Kuthe, A.M.; Quazi, S.; Gupta, V.; Jaiswal, S.; Jyothilal, S.; Lande, N.; Nagdeve, S. State of the Art Technology for Bone Tissue Engineering and Drug Delivery. IRBM 2019, 40, 133–144. [Google Scholar] [CrossRef]
- Schumacher, M.; Habibovic, P.; van Rijt, S. Mesoporous bioactive glass composition effects on degradation and bioactivity. Bioact. Mater. 2021, 6, 1921–1931. [Google Scholar] [CrossRef]
- Lozano, D.; Gil-Albarova, J.; Heras, C.; Sánchez-Salcedo, S.; Gómez-Palacio, V.E.; Gómez-Blasco, A.; Doadrio, J.C.; Vallet-Regí, M.; Salinas, A.J. ZnO-mesoporous glass scaffolds loaded with osteostatin and mesenchymal cells improve bone healing in a rabbit bone defect. J. Mater. Sci. Mater. Med. 2020, 31, 100. [Google Scholar] [CrossRef]
- Zambon, A.; Malavasi, G.; Pallini, A.; Fraulini, F.; Lusvardi, G. Cerium Containing Bioactive Glasses: A Review. ACS Biomater. Sci. Eng. 2021, 7, 4388–4401. [Google Scholar] [CrossRef]
- Mehrabi, T.; Mesgar, A.S.; Mohammadi, Z. Bioactive Glasses: A Promising Therapeutic Ion Release Strategy for Enhancing Wound Healing. ACS Biomater. Sci. Eng. 2020, 6, 5399–5430. [Google Scholar] [CrossRef]
- Xu, C.; Qu, X. Cerium oxide nanoparticle: A remarkably versatile rare earth nanomaterial for biological applications. NPG Asia Mater. 2014, 6, e90. [Google Scholar] [CrossRef]
- Kargozar, S.; Baino, F.; Hoseini, S.J.; Hamzehlou, S.; Darroudi, M.; Verdi, J.; Hasanzadeh, L.; Kim, H.-W.; Mozafari, M. Biomedical applications of nanoceria: New roles for an old player. Nanomedicine 2018, 13, 3051–3069. [Google Scholar] [CrossRef]
- Nelson, B.C.; Johnson, M.J.; Walker, M.L.; Riley, K.R.; Sims, C.M. Antioxidant Cerium Oxide Nanoparticles in Biology and Medicine. Antioxidants 2016, 5, 15. [Google Scholar] [CrossRef] [Green Version]
- Masadeh, M.M.; Karasneh, G.A.; Al-Akhras, M.-A.; Albiss, B.; Aljarah, K.M.; Al-Azzam, S.I.; Alzoubi, K. Cerium oxide and iron oxide nanoparticles abolish the antibacterial activity of ciprofloxacin against gram positive and gram negative biofilm bacteria. Cytotechnology 2015, 67, 427–435. [Google Scholar] [CrossRef] [Green Version]
- Qi, M.; Li, W.; Zheng, X.; Li, X.; Sun, Y.; Wang, Y.; Li, C.; Wang, L. Cerium and Its Oxidant-Based Nanomaterials for Antibacterial Applications: A State-of-the-Art Review. Front. Mater. 2020, 7, 213. [Google Scholar] [CrossRef]
- Goh, Y.-F.; Alshemary, A.Z.; Akram, M.; Abdul Kadir, M.R.; Hussain, R. In-Vitro Characterization of Antibacterial Bioactive Glass Containing Ceria. Ceram. Int. 2014, 40, 729–737. [Google Scholar] [CrossRef]
- Morais, D.S.; Fernandes, S.; Gomes, P.S.; Fernandes, M.H.; Sampaio, P.; Ferraz, M.P.; Santos, J.D.; Lopes, M.A.; Hussain, N.S. Novel cerium doped glass-reinforced hydroxyapatite with antibacterial and osteoconductive properties for bone tissue regeneration. Biomed. Mater. 2015, 10, 055008. [Google Scholar] [CrossRef]
- Cho, Y.S.; Gwak, S.J.; Cho, Y.S. Fabrication of Polycaprolactone/Nano Hydroxyapatite (PCL/nHA) 3D Scaffold with Enhanced In Vitro Cell Response via Design for Additive Manufacturing (DfAM). Polymers 2021, 13, 1394. [Google Scholar] [CrossRef]
- Perez-Puyana, V.; Jiménez-Rosado, M.; Romero, A.; Guerrero, A. Polymer-Based Scaffolds for Soft-Tissue Engineering. Polymers 2020, 12, 1566. [Google Scholar] [CrossRef]
- Charnley, J. Anchorage of the femoral head prosthesis to the shaft of the femur. J. Bone Joint Surg. Br. 1960, 42, 28–30. [Google Scholar] [CrossRef] [Green Version]
- Han, X.; Lin, H.; Chen, X.; Li, X.; Guo, G.; Qu, F. One-step method for the preparation of poly(methyl methacrylate) modified titanium-bioactive glass three-dimensional scaffolds for bone tissue engineering. IET Nanobiotech. 2016, 10, 45–53. [Google Scholar] [CrossRef]
- Atkinson, I.; Anghel, E.M.; Petrescu, S.; Seciu, A.M.; Stefan, L.M.; Mocioiu, O.C.; Predoana, L.; Voicescu, M.; Somacescu, S.; Culita, D.; et al. Cerium-containing mesoporous bioactive glasses: Material characterization, in vitro bioactivity, biocompatibility and cytotoxicity evaluation. Microporous Mesoporous Mater. 2019, 276, 76–88. [Google Scholar] [CrossRef]
- Atkinson, I.; Seciu-Grama, A.M.; Mocioiu, O.C.; Mocioiu, A.M.; Predoana, L.; Voicescu, M.; Cusu, J.P.; Grigorescu, R.M.; Ion, R.M.; Craciunescu, O. Preparation and biocompatibility of poly methyl methacrylate (PMMA)-mesoporous bioactive glass (MBG) composite scaffolds. Gels 2021, 7, 180. [Google Scholar] [CrossRef]
- Khoury, Y.E.; Hielscher, R.; Voicescu, M.; Gross, J.; Hellwig, P. On the specificity of the amide VI band for the secondary structure of proteins. Vib. Spectrosc. 2011, 55, 258–266. [Google Scholar] [CrossRef]
- Kokubo, T.; Takadama, H. How useful is SBF in predicting in vivo bone bioactivity? Biomaterials 2006, 277, 2907–2915. [Google Scholar] [CrossRef]
- Craciunescu, O.; Gaspar, A.; Trif, M.; Moisei, M.; Oancea, A.; Moldovan, L.; Zarnescu, O. Preparation and characterization of a collagen-liposome-chondroitin sulfate matrix with potential application for inflammatory disorders treatment. J. Nanomater. 2014, 2014, 903691. [Google Scholar] [CrossRef]
- Craciunescu, O.; Tardei, C.; Moldovan, L.; Zarnescu, O. Magnesium substitution effect on porous scaffolds for bone repairing. Cent. Eur. J. Biol. 2011, 6, 301–311. [Google Scholar] [CrossRef]
- Deaconu, M.; Nicu, I.; Tincu, R.; Brezoiu, A.-M.; Mitran, R.-A.; Vasile, E.; Matei, C.; Berger, D. Tailored doxycycline delivery from MCM-41-type silica carriers. Chem. Pap. 2018, 72, 1869–1880. [Google Scholar] [CrossRef]
- Nicolini, V.; Gambuzzi, E.; Malavasi, G.; Menabue, L.; Menziani, M.C.; Lusvardi, G.; Pedone, A.; Benedetti, F.; Luches, P.; D’Addato, S.; et al. Evidence of Catalase Mimetic Activity in Ce3+/Ce4+ Doped Bioactive Glasses. J. Phys. Chem. B 2015, 119, 4009–4019. [Google Scholar] [CrossRef] [PubMed]
- Sing, K.S.W.; Everett, D.H.; Haul, R.A.W.; Moscou, L.; Pierotti, R.A.; Rouquerol, J.; Siemieniewska, T. Reporting physisorption data for gas/solid systems with special reference to the determination of surface area and porosity (IUPAC Recommendations 1984). Pure Appl. Chem. 1985, 57, 603–619. [Google Scholar] [CrossRef]
- Grosman, A.; Ortega, C. Capillary Condensation in Porous Materials. Hysteresis and Interaction Mechanism without Pore Blocking/Percolation Process. Langmuir 2008, 24, 3977–3986. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, C.; Chang, J. Mesoporous bioactive glasses: Structure characteristics, drug/growth factor delivery and bone regeneration application. Interface Focus 2012, 2, 229–306. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Varini, E.; Sánchez-Salcedo, S.; Malavasi, G.; Lusvardi, G.; Vallet-Regí, M.; Salinas, A.J. Cerium (III) and (IV) containing mesoporous glasses/alginate beads for bone regeneration: Bioactivity, biocompatibility and reactive oxygen species activity. Mater. Sci. Eng. C 2019, 105, 109971. [Google Scholar] [CrossRef] [PubMed]
- García, A.; Cabañas, M.V.; Peña, J.; Sánchez-Salcedo, S. Design of 3D Scaffolds for Hard Tissue Engineering: From Apatites to Silicon Mesoporous Materials. Pharmaceutics 2021, 13, 1981. [Google Scholar] [CrossRef] [PubMed]
- Shruti, S.; Salinas, A.J.; Malavasi, G.; Lusvardi, G.; Menabue, L.; Ferrara, C.; Mustarelli, P.; Vallet-Regi, M. Structural and in vitro study of cerium, gallium and zinc containing sol–gel bioactive glasses. J. Mater. Chem. 2012, 22, 13698–13706. [Google Scholar] [CrossRef]
- Zhang, E.; Zou, C.; Yu, G. Surface microstructure and cell biocompatibility of silicon-substituted hydroxyapatite coating on titanium substrate prepared by a biomimetic process. Mater. Sci. Eng. C 2009, 29, 298–305. [Google Scholar] [CrossRef]
- Mocioiu, O.C.; Zaharescu, M.; Atkinson, I.; Mocioiu, A.M.; Budrugeac, P. Study of crystallization process of soda lead silicate glasses by thermal and spectroscopic methods. J. Therm. Anal. Calorim. 2014, 117, 131–139. [Google Scholar] [CrossRef] [Green Version]
- Xu, J.; Yang, Y.; Wan, R.; Shen, Y.; Zhang, W. Hydrothermal Preparation and Characterization of Ultralong Strontium-Substituted Hydroxyapatite Whiskers Using Acetamide as Homogeneous Precipitation Reagent. Sci. World J. 2014, 2014, 863137. [Google Scholar] [CrossRef]
- Leonelli, C.; Lusvardi, G.; Malavasi, G.; Menabue, L.; Tonelli, M. Synthesis and characterization of cerium-doped glasses and in vitro evaluation of bioactivity. J. Non Cryst. Solids 2003, 316, 198–216. [Google Scholar] [CrossRef]
- Malavasi, G.; Lusvardi, G. Composition and morphology effects on catalase mimetic activity of potential bioactive glasses. Ceram. Int. 2020, 46, 25854–25864. [Google Scholar] [CrossRef]
- Bellucci, D.; Salvatori, R.; Anesi, A.; Chiarini, L.; Cannillo, V. SBF assays, direct and indirect cell culture tests to evaluate the biological performance of bioglasses and bioglass-based composites: Three paradigmatic cases. Mater. Sci. Eng. C 2019, 96, 757–764. [Google Scholar] [CrossRef]
- Zhenga, K.; Torre, E.; Baric, A.; Taccardi, N.; Cassinelli, C.; Morra, M.; Fiorilli, S.; Vitale-Brovarone, C.; Iviglia, G.; Boccaccini, A.R. Antioxidant mesoporous Ce-doped bioactive glass nanoparticles with anti-inflammatory and pro-osteogenic activities. Mater. Today Bio. 2020, 5, 100041. [Google Scholar] [CrossRef]
- Naganuma, T.; Traversa, E. The effect of cerium valence states at cerium oxide nanoparticle surfaces on cell proliferation. Biomaterials 2014, 35, 4441–4453. [Google Scholar] [CrossRef]
- Michels, R.; Last, K.; Becker, S.L.; Papan, C. Update on Coagulase-Negative Staphylococci—What the Clinician Should Know. Microorganisms 2021, 9, 830. [Google Scholar] [CrossRef]
- Pelletier, D.A.; Suresh, A.K.; Holton, G.A.; McKeown, C.K.; Wang, W.; Gu, B.; Mortensen, N.P. Effects of engineered cerium oxide nanoprticles on bacterial growth and viability. Appl. Environ. Microb. 2010, 76, 7981–7989. [Google Scholar] [CrossRef] [Green Version]
- Youness, R.A.; Taha, M.A.; El-Kheshen, A.A.; El-Faramawy, N.; Ibrahim, M. In vitro bioactivity evaluation, antimicrobial behavior and mechanical properties of cerium-containing phosphate glasses. Mater. Res. Express 2019, 6, 075212. [Google Scholar] [CrossRef]
- Raimondi, S.; Zambon, A.; Ranieri, R.; Fraulini, F.; Amaretti, A.; Rossi, M.; Lusvardi, G. Investigation on the antimicrobial properties of cerium-doped bioactive glasses. J. Biomed. Mater. Res. 2022, 110, 504–508. [Google Scholar] [CrossRef]
- Goh, Y.-F.; Akram, M.; Alshemary, A.; Hussain, R. Antibacterial polylactic acid/chitosan nanofibers decorated with bioactive glass. Appl. Surf. Sci. 2016, 387, 1–7. [Google Scholar] [CrossRef]
- Zeng, L.; An, L.; Wu, X. Modeling Drug-Carrier Interaction in the Drug Release from Nanocarriers. J. Drug Deliv. 2011, 2011, 370308. [Google Scholar] [CrossRef]
- Mitran, R.-A.; Matei, C.; Berger, D.; Băjenaru, L.; Moisescu, M.G. Controlling drug release from mesoporous silicathrough an amorphous, nanoconfined 1-tetradecanol layer. Eur. J. Pharm. Biopharm. 2018, 127, 318–325. [Google Scholar] [CrossRef]
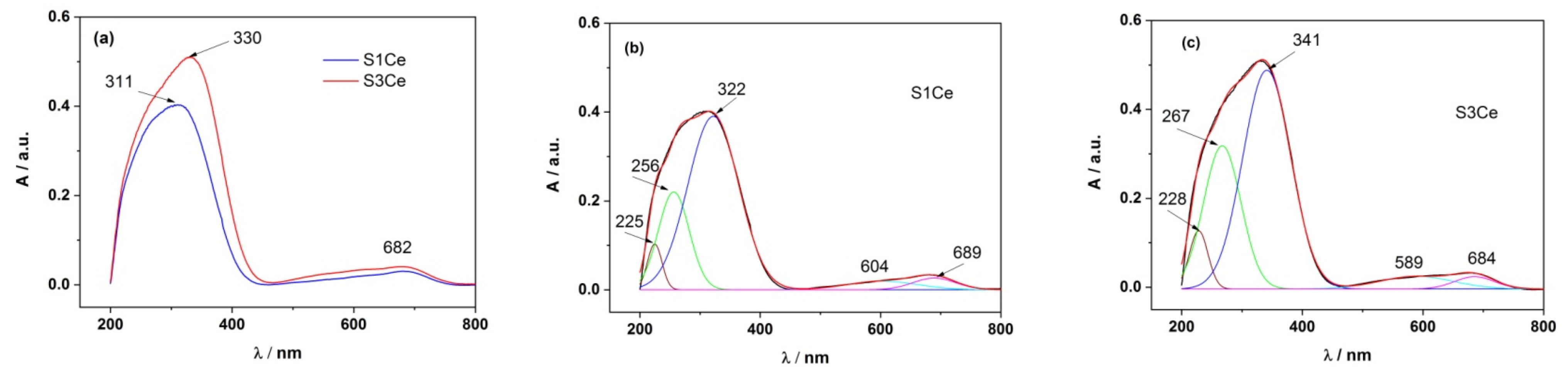

| Sample | PMMA-MBGs | λabs,Ce4+ | λabs,Ce3+ | Impurity/Others |
|---|---|---|---|---|
| S1Ce | 225 nm (4.32%) | 256 nm (19.79%) | 322 nm (64.39%) | 500–750 nm (11.50%) |
| S3Ce | 228 nm (5.48%) | 267 nm (34.85%) | 341 nm (52.72%) | 500–750 nm (6.95%) |
| Sample | SBET (m2/g) | Average Pore Diameter (nm) | Total Pore Volume (cm3/g) |
|---|---|---|---|
| S0Ce | 96.98 ± 0.43 | 4.07 | 0.10 |
| S1Ce | 136.96 ± 0.29 | 4.67 | 0.15 |
| S3Ce | 113.36 ± 0.78 | 3.93 | 0.10 |
| Sample | %VAN (wt.%) | ΔG (10−21 J/Molecule) | ktr (h−1) | kdes (h−1) | kads (h−1) | Adj. R2 (−) |
|---|---|---|---|---|---|---|
| VAN.HCl | 100 | 10.61 | 0.421 | 0.087 | 0.007 | 0.9998 |
| VAN@S0Ce | 20.8 | 2.67 | 0.462 | 0.050 | 0.027 | 0.9957 |
| VAN@S1Ce | 22.0 | 1.99 | 0.404 | 0.015 | 0.009 | 0.9985 |
| VAN@S3Ce | 18.0 | 3.89 | 0.435 | 0.016 | 0.006 | 0.9981 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Atkinson, I.; Seciu-Grama, A.M.; Petrescu, S.; Culita, D.; Mocioiu, O.C.; Voicescu, M.; Mitran, R.-A.; Lincu, D.; Prelipcean, A.-M.; Craciunescu, O. Cerium-Containing Mesoporous Bioactive Glasses (MBGs)-Derived Scaffolds with Drug Delivery Capability for Potential Tissue Engineering Applications. Pharmaceutics 2022, 14, 1169. https://doi.org/10.3390/pharmaceutics14061169
Atkinson I, Seciu-Grama AM, Petrescu S, Culita D, Mocioiu OC, Voicescu M, Mitran R-A, Lincu D, Prelipcean A-M, Craciunescu O. Cerium-Containing Mesoporous Bioactive Glasses (MBGs)-Derived Scaffolds with Drug Delivery Capability for Potential Tissue Engineering Applications. Pharmaceutics. 2022; 14(6):1169. https://doi.org/10.3390/pharmaceutics14061169
Chicago/Turabian StyleAtkinson, Irina, Ana Maria Seciu-Grama, Simona Petrescu, Daniela Culita, Oana Catalina Mocioiu, Mariana Voicescu, Raul-Augustin Mitran, Daniel Lincu, Ana-Maria Prelipcean, and Oana Craciunescu. 2022. "Cerium-Containing Mesoporous Bioactive Glasses (MBGs)-Derived Scaffolds with Drug Delivery Capability for Potential Tissue Engineering Applications" Pharmaceutics 14, no. 6: 1169. https://doi.org/10.3390/pharmaceutics14061169
APA StyleAtkinson, I., Seciu-Grama, A. M., Petrescu, S., Culita, D., Mocioiu, O. C., Voicescu, M., Mitran, R.-A., Lincu, D., Prelipcean, A.-M., & Craciunescu, O. (2022). Cerium-Containing Mesoporous Bioactive Glasses (MBGs)-Derived Scaffolds with Drug Delivery Capability for Potential Tissue Engineering Applications. Pharmaceutics, 14(6), 1169. https://doi.org/10.3390/pharmaceutics14061169