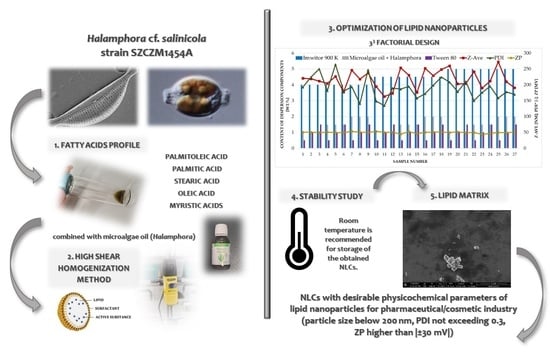
Lipid Constituents of Diatoms (Halamphora) as Components for Production of Lipid Nanoparticles
Abstract
1. Introduction
2. Materials and Methods
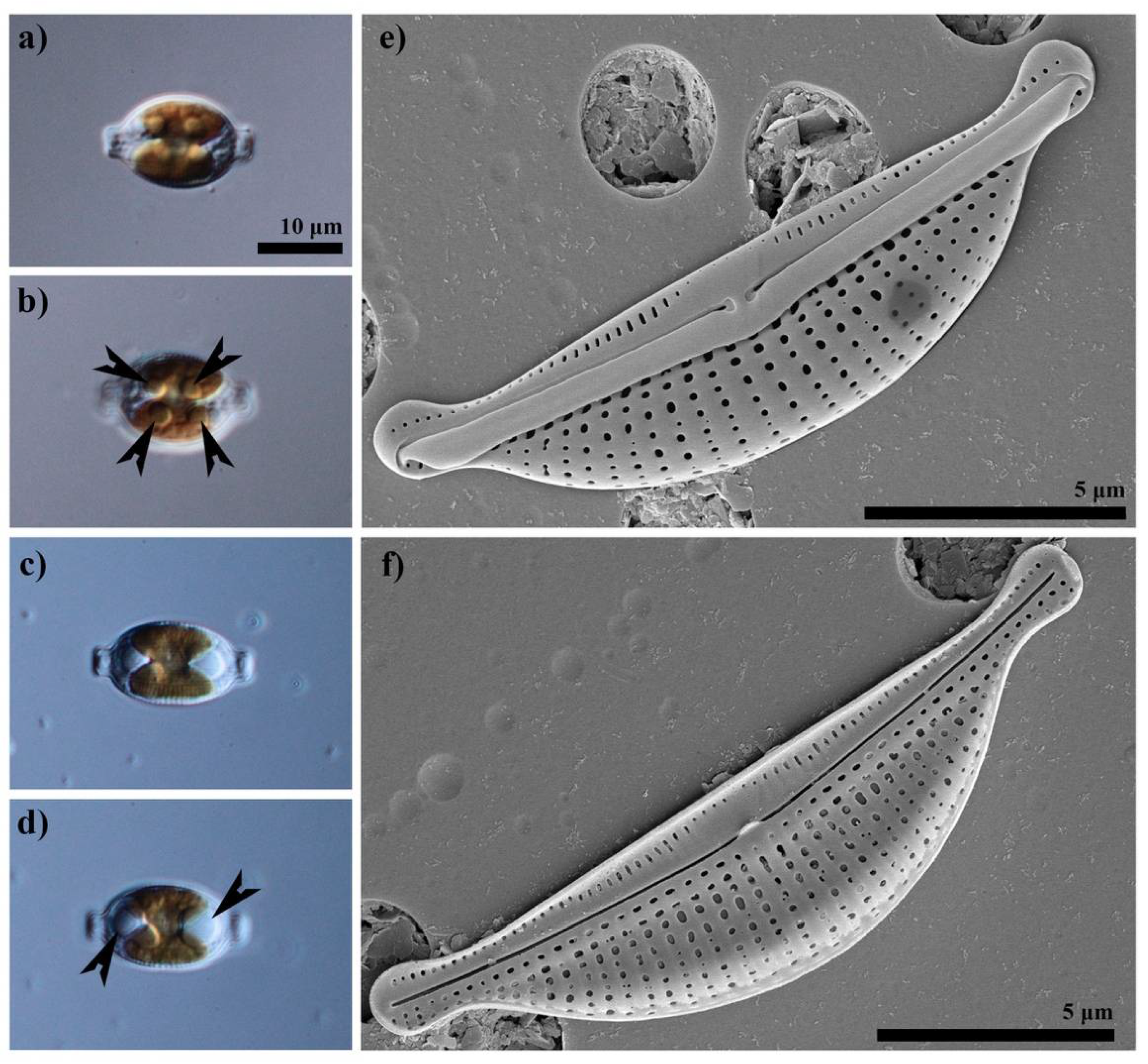
2.1. Diatom Isolation and Culture
2.2. Crude Oil Extraction
2.3. Sample Preparation, Chemicals and Equipment Used in Fatty Acids Determination
2.4. GC-MS Analysis
2.5. Lipid Screening
2.6. Production of Lipid Nanoparticles
2.7. Experimental Factorial Design
2.8. Physicochemical Characterization of Lipid Nanoparticles
2.9. Characterization of Lipid Matrices
2.10. Stability Study
3. Results and Discussion
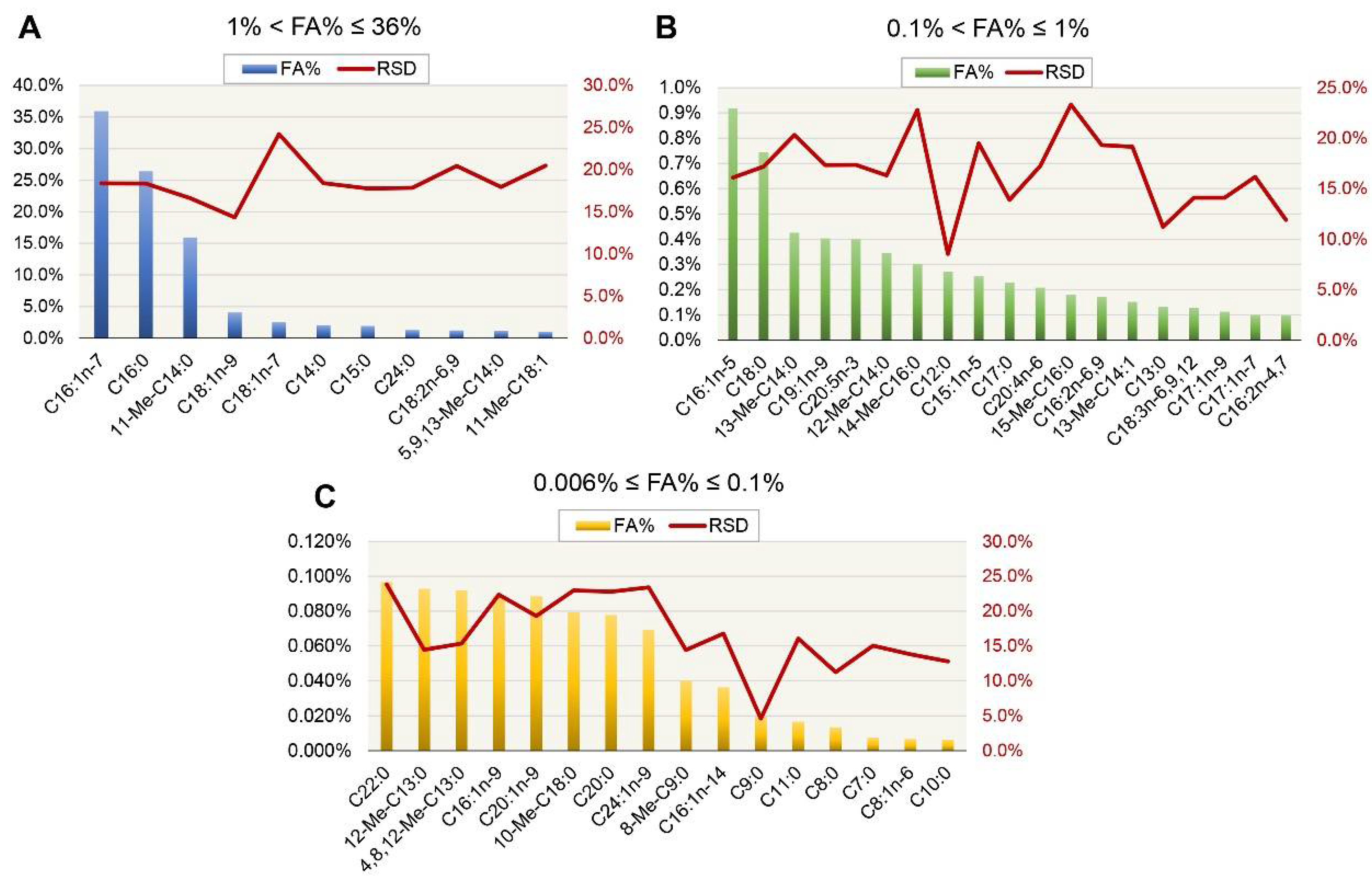
3.1. Fatty Acid Profile of Halamphora cf. salinicola (SZCZM1454A) Crude Oil
3.2. Selection of Solid Lipids Compatible with the Liquid Lipids Tested
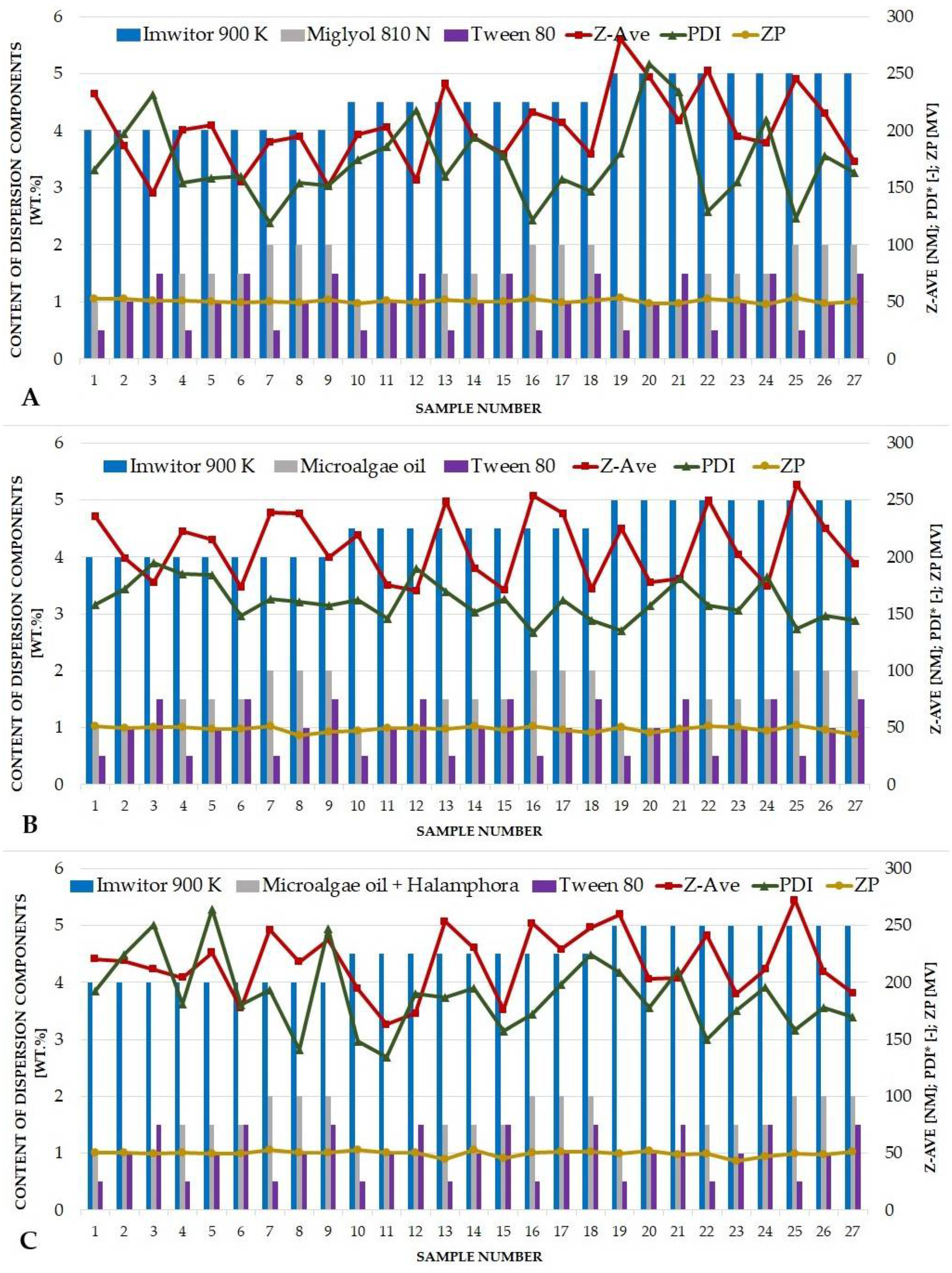
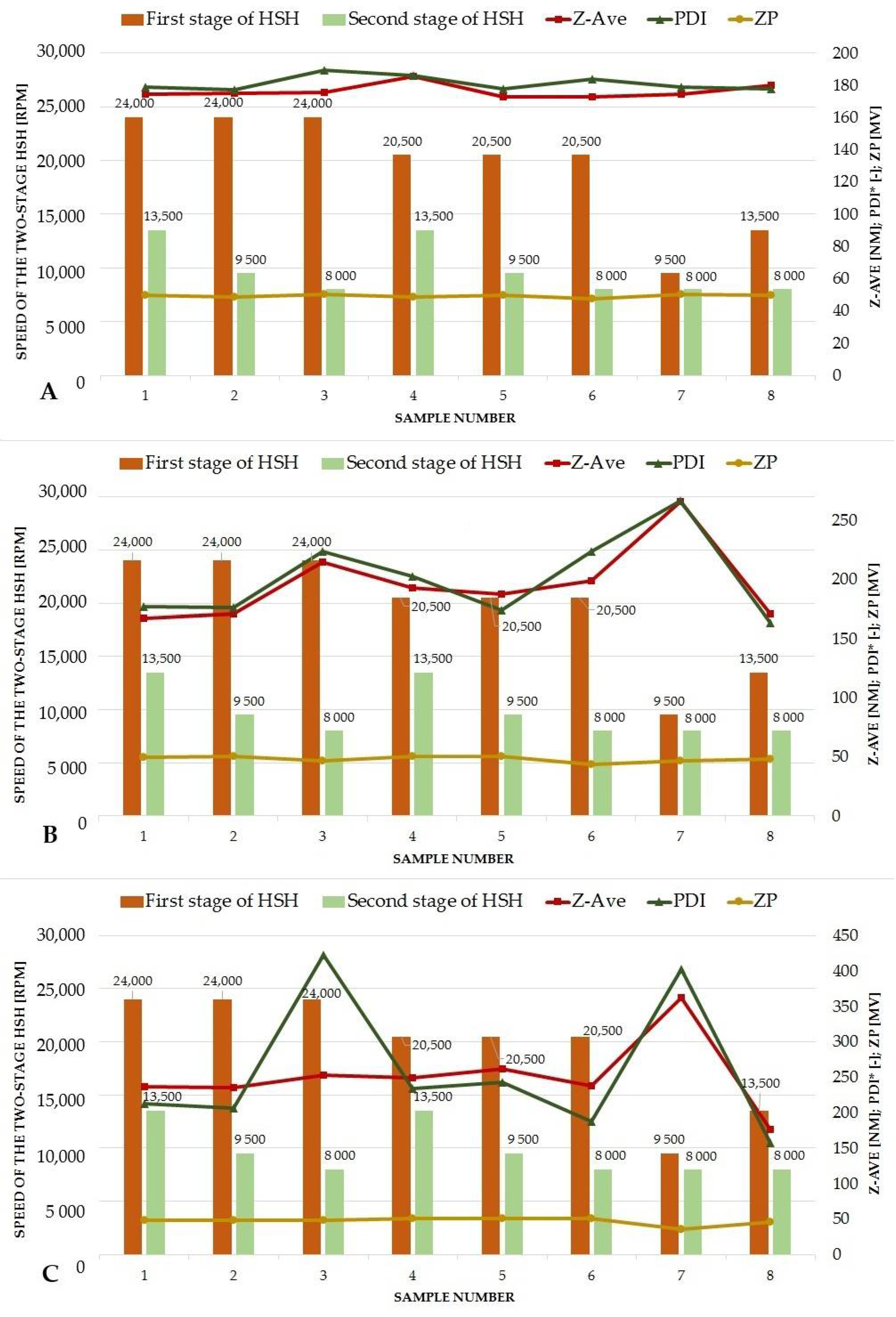
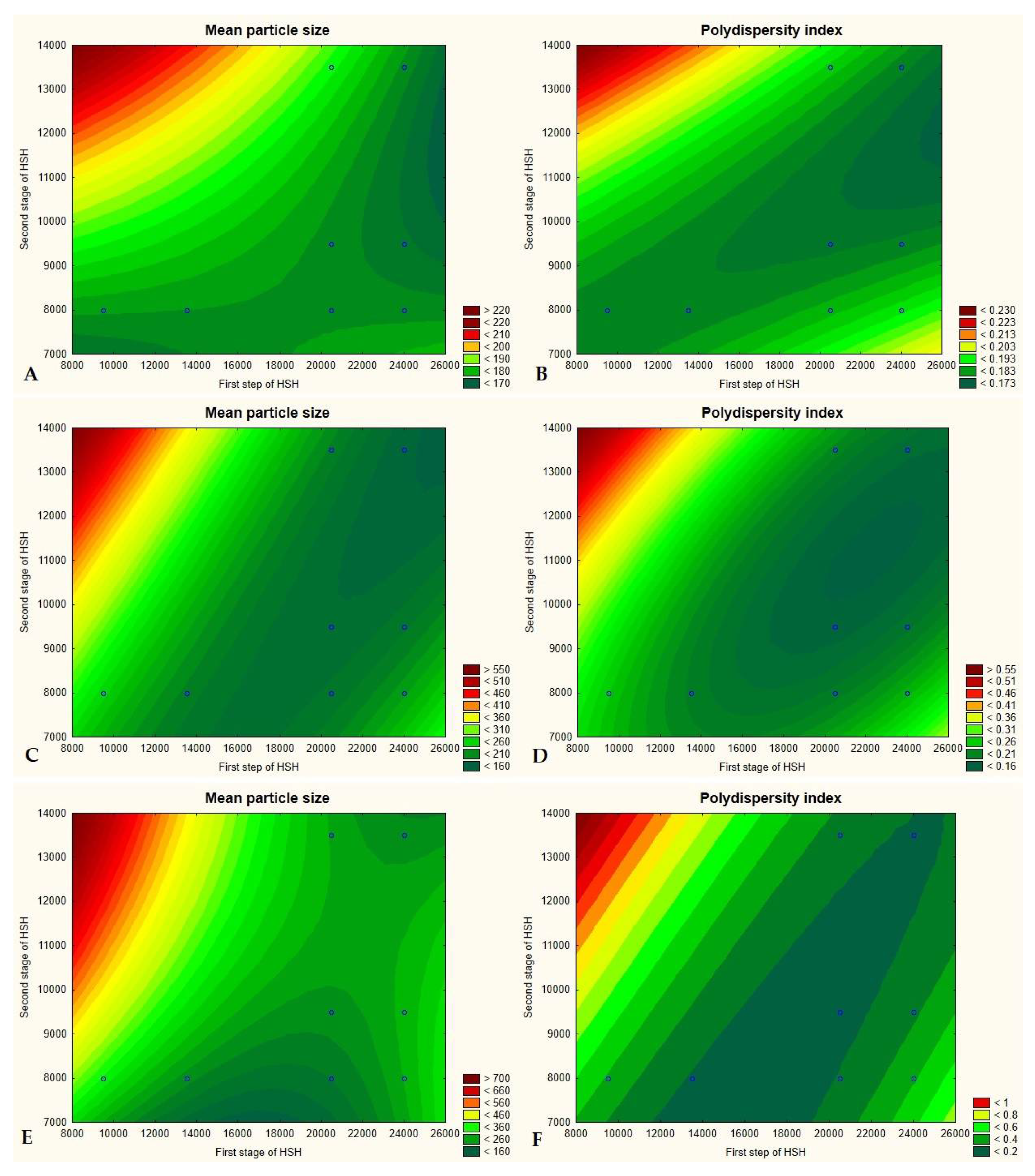
3.3. Optimization of the Quantitative Composition of Lipid Nanoparticles and the Conditions of Their Production
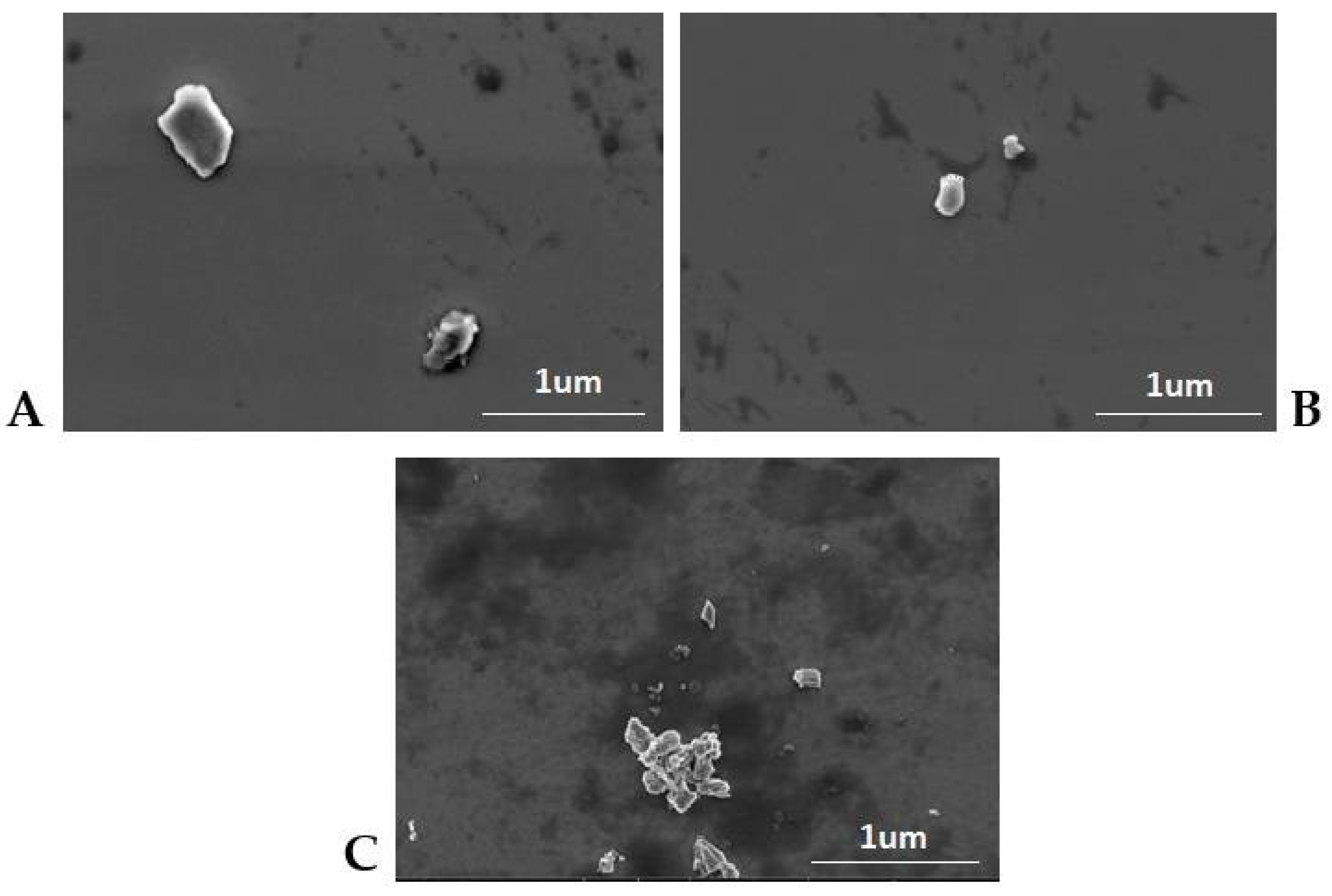
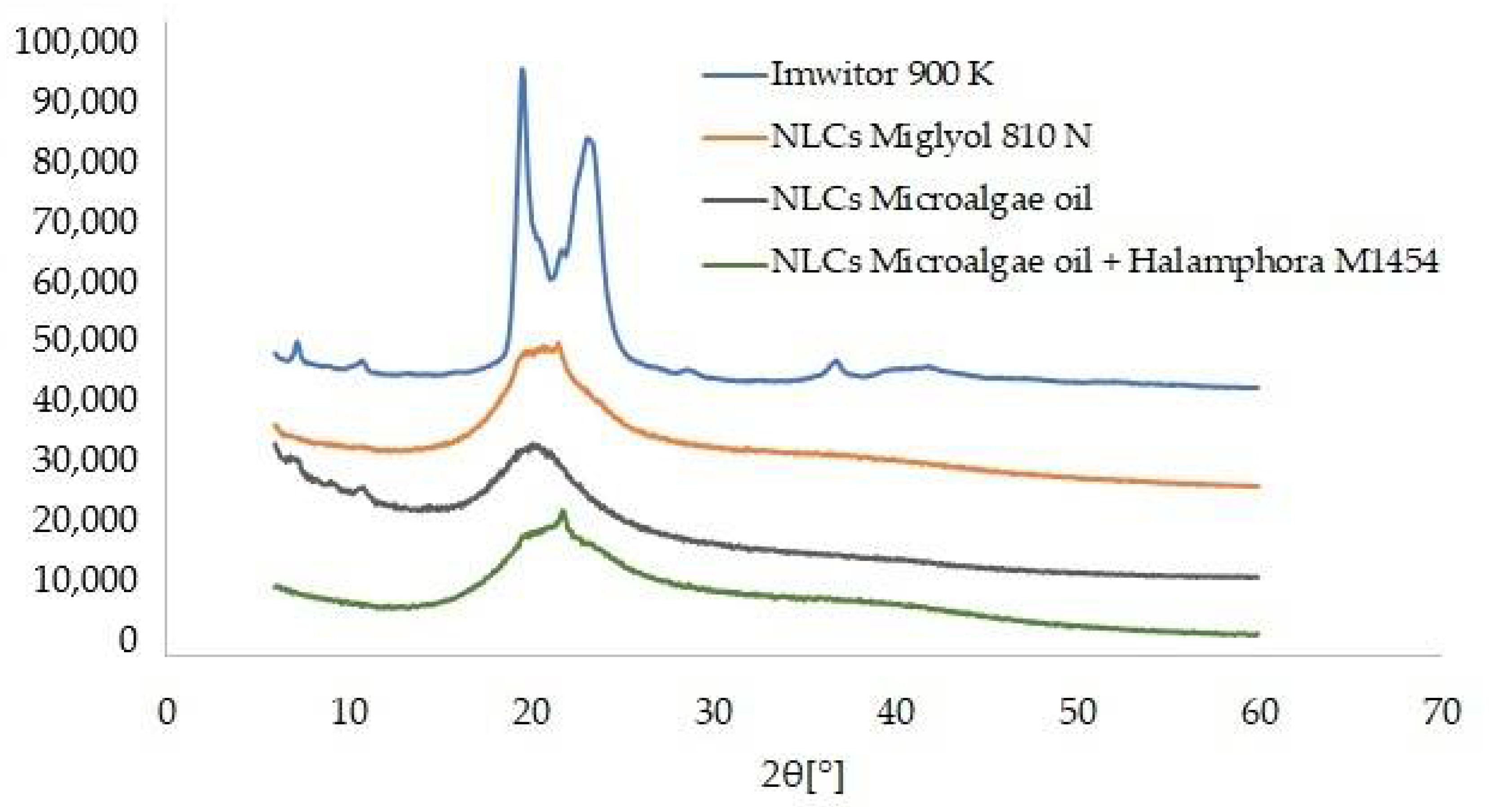
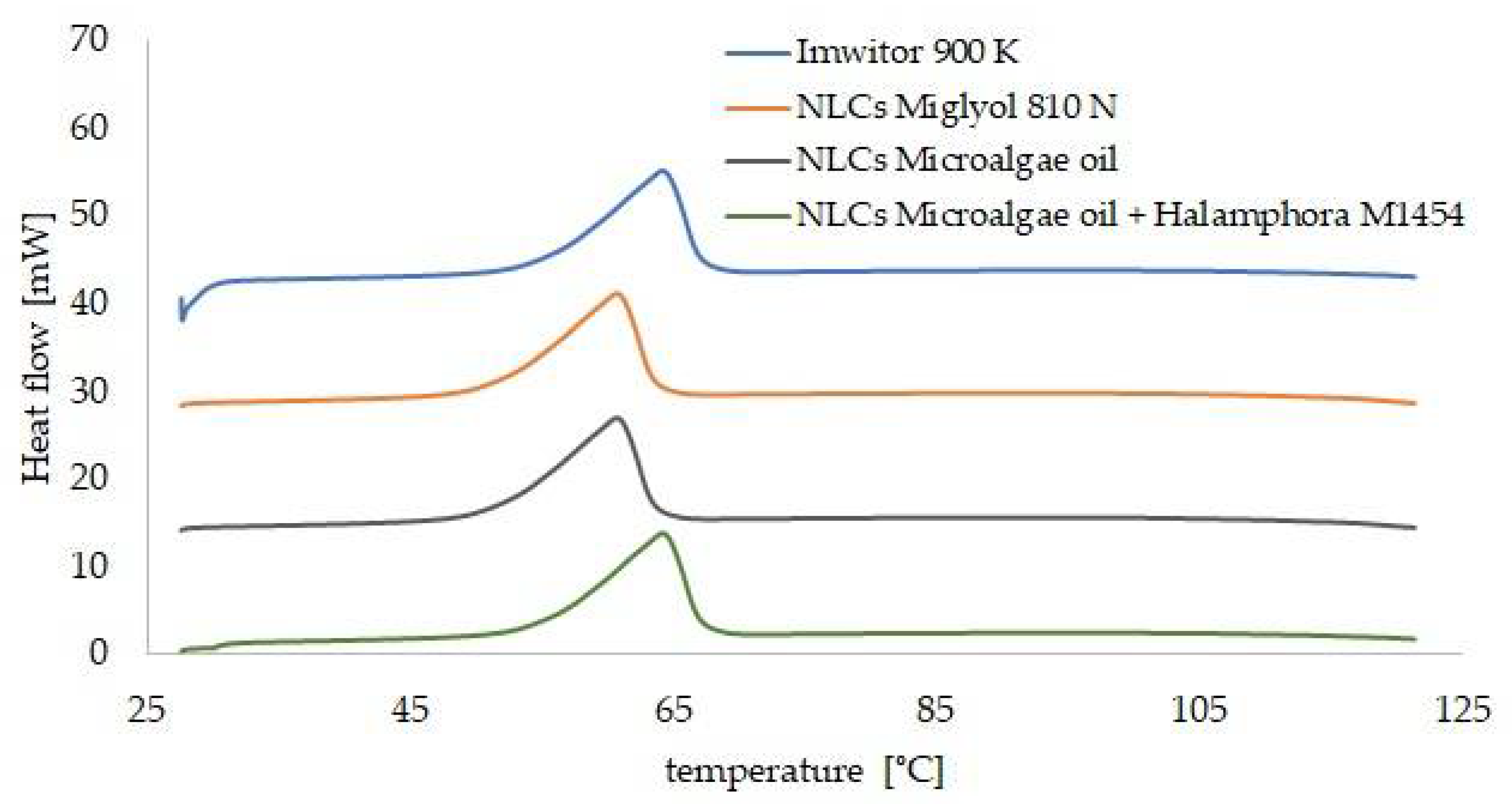
3.4. Analysis of the Results Characterizing the Lipid Matrices (SEM, DSC, XRD)
3.5. Evaluation of Stability of Investigated Lipid Nanoparticles
4. Conclusions
5. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Round, F.E.; Crawford, R.M.; Mann, D.G. The Diatoms: Biology and Morphology of the Genera; Cambridge University Press: Cambridge, UK, 1990; p. 747. [Google Scholar]
- Kociolek, J.P.; Blanco, S.; Coste, M.; Ector, L.; Liu, Y.; Karthick, B.; Kulikovskiy, M.; Lundholm, N.; Ludwig, T.; Potapova, M.; et al. DiatomBase. Available online: http://www.diatombase.org (accessed on 20 December 2021).
- Falkowski, P.; Scholes, R.J.; Boyle, E.; Canadell, J.; Canfield, D.; Elser, J.; Gruber, N.; Hibbard, K.; Högberg, P.; Linder, S.; et al. The global carbon cycle: A test of our knowledge of earth as a system. Science 2000, 290, 291–296. [Google Scholar] [CrossRef] [PubMed]
- Armbrust, E.V. The life of diatoms in the world’s oceans. Nature 2009, 459, 185–192. [Google Scholar] [CrossRef] [PubMed]
- Cox, T.E.; Cebrian, J.; Tabor, M.; West, L.; Krause, J.W. Do diatoms dominate benthic production in shallow systems? A case study from a mixed seagrass bed. Limnol. Oceanogr. Lett. 2020, 5, 425–434. [Google Scholar] [CrossRef]
- Hildebrand, M.; Manandhar-Shrestha, K.; Abbriano, R. Effects of chrysolaminarin synthase knockdown in the diatom Thalassiosira pseudonana: Implications of reduced carbohydrate storage relative to green algae. Algal Res. 2017, 23, 66–77. [Google Scholar] [CrossRef]
- Caballero, M.A.; Jallet, D.; Shi, S.; Rithner, C.; Zhang, Y.; Peers, G. Quantification of chrysolaminarin from the model diatom Phaeodactylum tricornutum. Algal Res. 2016, 20, 180–188. [Google Scholar] [CrossRef]
- Chisti, Y. Biodiesel from microalgae. Biotechnol. Adv. 2007, 25, 294–306. [Google Scholar] [CrossRef] [PubMed]
- Mishra, M.; Arukha, A.P.; Bashir, T.; Yadav, D.; Prasad, G. All New Faces of Diatoms: Potential Source of Nanomaterials and Beyond. Front. Microbiol. 2017, 8, 1239. [Google Scholar] [CrossRef]
- Guéguen, N.; Le Moigne, D.; Amato, A.; Salvaing, J.; Maréchal, E. Lipid Droplets in Unicellular Photosynthetic Stramenopiles. Front. Plant Sci. 2021, 12, 639276. [Google Scholar] [CrossRef]
- Matsumoto, M.; Mayama, S.; Nemoto, M.; Fukuda, Y.; Muto, M.; Yoshino, T.; Matsunaga, T.; Tanaka, T. Morphological and molecular phylogenetic analysis of the high triglyceride-producing marine diatom, Fistulifera solaris sp. nov. (Bacillariophyceae). Phycol. Res. 2014, 62, 257–268. [Google Scholar] [CrossRef]
- Yi, Z.; Xu, M.; Di, X.; Brynjolfsson, S.; Fu, W. Exploring Valuable Lipids in Diatoms. Front. Mar. Sci. 2017, 4, 17. [Google Scholar] [CrossRef]
- Stepanek, J.G.; Fields, F.J.; Kociolek, P.J. A comparison of lipid content metrics using six species from the genus Halamphora (Bacillariophyta). Biofuels 2016, 7, 521–528. [Google Scholar] [CrossRef]
- Oliver, A.; Podell, S.; Pinowska, A.; Traller, J.; Smith, S.; McClure, R.; Beliaev, A.; Bohutskyi, P.; Hill, E.; Rabines, A.; et al. Diploid genomic architecture of Nitzschia inconspicua, an elite biomass production diatom. Sci. Rep. 2021, 11, 15592. [Google Scholar] [CrossRef] [PubMed]
- Govindan, N.; Maniam, G.P.; Ab Rahim, M.H.; Sulaiman, A.Z.; Ajit, A.; Chatsungnoen, T.; Chisti, Y. Production of Renewable Lipids by the Diatom Amphora copulata. Fermentation 2021, 7, 37. [Google Scholar] [CrossRef]
- Yu, E.T.; Zendejas, F.J.; Lane, P.D.; Gaucher, S.; Simmons, B.A.; Lane, T.W. Triacylglycerol accumulation and profiling in the model diatoms Thalassiosira pseudonana and Phaeodactylum tricornutum (Bacillariophyceae) during starvation. J. Appl. Phycol. 2009, 21, 669–681. [Google Scholar] [CrossRef]
- Hu, Q.; Sommerfeld, M.; Jarvis, E.; Ghirardi, M.; Posewitz, M.; Seibert, M.; Darzins, A. Microalgal triacylglycerols as feedstock for biofuels production: Perspectives and advances. Plant J. 2008, 54, 621–639. [Google Scholar] [CrossRef]
- Manaia, E.B.; Abuçafy, M.P.; Chiari-Andréo, B.G.; Silva, B.L.; Oshiro, J.A., Jr.; Chiavacci, L.A. Physicochemical characterization of drug nanocarriers. Int. J. Nanomed. 2017, 12, 4991–5011. [Google Scholar] [CrossRef]
- Müller, R.H.; Radtke, M.; Wissing, S.A. Solid Lipid Nanoparticles (SLN) and Nanostructured Lipid Carriers (NLC) in Cosmetic and Dermatological Preparations. Adv. Drug Deliv. Rev. 2002, 54, 131–155. [Google Scholar] [CrossRef]
- Souto, E.B.; Baldim, I.; Oliveira, W.P.; Rao, R.; Yadav, N.; Gama, F.M.; Mahant, S. SLN and NLC for topical, dermal and transdermal drug delivery. Expert Opin. Drug Deliv. 2020, 17, 357–377. [Google Scholar] [CrossRef]
- Souto, E.B.; Müller, R.H. Lipid nanoparticles (solid lipid nanoparticles and nanostructured lipid carriers) for cosmetic, dermal, and transdermal applications. In Nanoparticulate Drug Delivery Systems; Thassu, D., Ed.; CRC Press: Boca Raton, FL, USA, 2007; pp. 213–233. [Google Scholar]
- Badea, G.; Lăcătuşu, I.; Badea, N.; Ott, C.; Meghea, A. Use of various vegetable oils in designing photoprotective nanostructured formulations for UV protection and antioxidant activity. Ind. Crops Prod. 2015, 67, 18–24. [Google Scholar] [CrossRef]
- Bojarowicz, H.; Woźniak, B. Polyunsaturated fatty acids and their influence on skin condition. Probl. Hig. Epidemiol. 2008, 89, 471–475. [Google Scholar]
- Lin, T.K.; Zhong, L.; Santiago, J.L. Anti-Inflammatory and Skin Barrier Repair Effects of Topical Application of Some Plant Oils. Int. J. Mol. Sci. 2018, 19, 70. [Google Scholar] [CrossRef] [PubMed]
- Andersen, R.A.; Kawachi, M. Traditional microalgae isolation techniques. In Algal Culturing Techniques; Andersen, R.A., Ed.; Elsevier: London, UK, 2005; pp. 83–100. [Google Scholar]
- Dabek, P.; Ashworth, M.P.; Górecka, E.; Krzywda, M.; Bornman, T.G.; Sato, S.; Witkowski, A. Towards a multigene phylogeny of the Cymatosiraceae (Bacillariophyta, Mediophyceae): Morphological and molecular insights into the taxonomy of the forgotten species Campylosira africana and of Extubocellulus, with a description of two new taxa. J. Phycol. 2019, 55, 425–441. [Google Scholar] [CrossRef]
- Guillard, R.R.L. Culture of phytoplankton for feeding marine invertebrates. In Culture of Marine Invertebrate Animals; Smith, W.L., Chanley, M.H., Eds.; Plenum Press: New York, NY, USA, 1975; pp. 26–60. [Google Scholar]
- Axelsson, M.; Gentili, F. A single-step method for rapid extraction of total lipids from green microalgae. PLoS ONE 2014, 9, e89643. [Google Scholar] [CrossRef] [PubMed]
- Martínez, B.; Miranda, J.M.; Franco, C.M.; Cepeda, A.; Rodríguez, J.L. Development of a simple method for the quantitative determination of fatty acids in milk with special emphasis on long-chain fatty acids, CyTA. J. Food 2012, 10, 27–35. [Google Scholar] [CrossRef][Green Version]
- Gaumet, M.; Vargas, A.; Gurny, R.; Delie, F. Nanoparticles for drug delivery: The need for precision in reporting particle size parameters. Eur. J. Pharm. Biopharm. 2008, 69, 1–9. [Google Scholar] [CrossRef]
- D’Ippolito, G.; Sardo, A.; Paris, D.; Vella, F.M.; Adelfi, M.G.; Botte, P.; Gallo, C.; Fontana, A. Potential of lipid metabolism in marine diatoms for biofuel production. Biotechnol. Biofuels 2015, 8, 28. [Google Scholar] [CrossRef]
- Cointet, E.; Wielgosz-Collin, G.; Bougaran, G.; Rabesaotra, V.; Gonçalves, O.; Méléder, V. Effects of light and nitrogen availability on photosynthetic efficiency and fatty acid content of three original benthic diatom strains. PLoS ONE 2019, 14, e0224701. [Google Scholar] [CrossRef]
- Soler, C.; Claquin, P.; Goutx, M.; Ragueneau, O.; Moriceau, B. Impact of nutrient starvation on the biochemical composition of the marine diatom Thalassiosira weissflogii: From the whole cell to the frustule fraction. Biogeosci. Discuss. 2010, 7, 5953–5995. [Google Scholar]
- Zulu, N.N.; Zienkiewicz, K.; Vollheyde, K.; Feussner, I. Current trends to comprehend lipid metabolism in diatoms. Prog. Lipid Res. 2018, 70, 1–16. [Google Scholar] [CrossRef]
- Grumezescu, A. Design of Nanostructures for Versatile Therapeutic Applications, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 97–136. [Google Scholar]
- Gonzalez-Rodriguez, M.L.; Rabasco, A.M. Charged liposomes as carriers to enhance the permeation through the skin. Expert Opin. Drug Deliv. 2011, 8, 857–871. [Google Scholar] [CrossRef]
- Dąbrowska, M.; Souto, E.B.; Nowak, I. Lipid Nanoparticles Loaded with Iridoid Glycosides: Development and Optimization Using Experimental Factorial Design. Molecules 2021, 26, 3161. [Google Scholar] [CrossRef] [PubMed]
- Fangueiro, J.F.; Andreani, T.; Egea, M.A.; Garcia, M.L.; Souto, S.B.; Souto, E.B. Experimental factorial design applied to mucoadhesive lipid nanoparticles via multiple emulsion process. Colloids Surf. B Biointerfaces 2012, 100, 84–89. [Google Scholar] [CrossRef] [PubMed]
- Jaiswal, P.; Gidwani, B.; Vyas, A. Nanostructured lipid carriers and their current application in targeted drug delivery. Artif. Cells Nanomed. Biotechnol. 2016, 44, 27–40. [Google Scholar] [CrossRef] [PubMed]
- Souto, E.B.; Müller, R.H. Lipid nanoparticles: Effect on bioavailability and pharmacokinetic changes. Handb. Exp. Pharmacol. 2010, 197, 115–141. [Google Scholar] [CrossRef]
- Guimarães, K.L.; Ré, M.I. Lipid Nanoparticles as Carriers for Cosmetic Ingredients: The First (SLN) and the Second Generation (NLC); Springer: Berlin/Heidelberg, Germany, 2011; pp. 101–122. [Google Scholar]
- Averina, E.S.; Seewald, G.; Müller, R.H.; Radnaeva, L.D.; Popov, D.V. Nanostructured lipid carriers (NLC) on the basis of Siberian pine (Pinus sibirica) seed oil. Pharmazie 2010, 65, 25–31. [Google Scholar] [CrossRef]
- Yang, Y.; Corona, A., III; Schubert, B.; Reeder, R.; Henson, M.A. The effect of oil type on the aggregation stability of nanostructured lipid carriers. J. Colloid Interface Sci. 2014, 15, 261–272. [Google Scholar] [CrossRef] [PubMed]
| Quantity of Ingredient (wt.% ± 0.1) | |||
|---|---|---|---|
| Sample No. | Solid Lipid | Liquid Lipid | Non-Ionic Surfactant |
| 1 | 4.0 | 1.0 | 0.5 |
| 2 | 4.0 | 1.0 | 1.0 |
| 3 | 4.0 | 1.0 | 1.5 |
| 4 | 4.0 | 1.5 | 0.5 |
| 5 | 4.0 | 1.5 | 1.0 |
| 6 | 4.0 | 1.5 | 1.5 |
| 7 | 4.0 | 2.0 | 0.5 |
| 8 | 4.0 | 2.0 | 1.0 |
| 9 | 4.0 | 2.0 | 1.5 |
| 10 | 4.5 | 1.0 | 0.5 |
| 11 | 4.5 | 1.0 | 1.0 |
| 12 | 4.5 | 1.0 | 1.5 |
| 13 | 4.5 | 1.5 | 0.5 |
| 14 | 4.5 | 1.5 | 1.0 |
| 15 | 4.5 | 1.5 | 1.5 |
| 16 | 4.5 | 2.0 | 0.5 |
| 17 | 4.5 | 2.0 | 1.0 |
| 18 | 4.5 | 2.0 | 1.5 |
| 19 | 5.0 | 1.0 | 0.5 |
| 20 | 5.0 | 1.0 | 1.0 |
| 21 | 5.0 | 1.0 | 1.5 |
| 22 | 5.0 | 1.5 | 0.5 |
| 23 | 5.0 | 1.5 | 1.0 |
| 24 | 5.0 | 1.5 | 1.5 |
| 25 | 5.0 | 2.0 | 0.5 |
| 26 | 5.0 | 2.0 | 1.0 |
| 27 | 5.0 | 2.0 | 1.5 |
| Speed of the Two-Stage High Shear Homogenization (rpm) | ||
|---|---|---|
| Sample No. | First Stage | Second Stage |
| 1 | 24,000 | 13,500 |
| 2 | 24,000 | 9500 |
| 3 | 24,000 | 8000 |
| 4 | 20,500 | 13,500 |
| 5 | 20,500 | 9500 |
| 6 | 20,500 | 8000 |
| 7 | 9500 | 8000 |
| 8 | 13,500 | 8000 |
| Assigned FA 1 | Name | RSD 2 (%) | TFA 3 (%) |
|---|---|---|---|
| C7:0 | Heptanoic acid | 15.03 | 0.01 |
| C8:0 | Octanoic acid | 11.22 | 0.01 |
| C8:1n-6 | 2-Octenoic acid | 13.79 | 0.01 |
| C9:0 | Nonanoic acid | 4.61 | 0.02 |
| 8-Me-C9:0 | 8-Methylnonanoic acid | 14.41 | 0.04 |
| C10:0 | Decanoic acid | 12.77 | 0.01 |
| C11:0 | Undecanoic acid | 16.05 | 0.02 |
| C12:0 | Dodecanoic acid | 8.54 | 0.27 |
| C13:0 | Tridecanoic acid | 11.22 | 0.13 |
| 12-Me-C13:0 | 12-Methyltridecanoic acid | 14.44 | 0.09 |
| C14:0 | Tetradecanoic acid | 18.40 | 2.08 |
| 11-Me-C14:0 | 11-Methyltetradecanoic acid | 16.61 | 15.92 |
| 13-Me-C14:1 | 13-Methyltetradec-9-enoic acid | 19.15 | 0.15 |
| 4,8,12-Me-C13:0 | 4,8,12-Trimethyltridecanoic acid | 15.35 | 0.09 |
| 13-Me-C14:0 | 13-Methyltetradecanoic acid | 20.33 | 0.43 |
| 12-Me-C14:0 | 12-Methyltetradecanoic acid | 16.33 | 0.35 |
| C15:1n-5 | 10-Pentadecenoic acid | 19.48 | 0.25 |
| C15:0 | Pentadecanoic acid | 17.75 | 1.95 |
| C16:1n-14 | 2-Hexadecenoic acid | 16.75 | 0.04 |
| C16:1n-7 | 9-Hexadecenoic acid | 18.38 | 35.94 |
| C16:1n-5 | 11-Hexadecenoic acid | 16.11 | 0.92 |
| C16:1n-9 | 7-Hexadecenoic acid | 22.37 | 0.09 |
| C16:0 | Hexadecanoic acid | 18.34 | 26.44 |
| C16:2n-4,7 | 9,12-Hexadecadienoic acid | 11.90 | 0.10 |
| 5,9,13-Me-C14:0 | 5,9,13-Trimethyltetradecanoic acid | 17.94 | 1.18 |
| C16:2n-6,9 | 7,10-Hexadecadienoic acid | 19.32 | 0.17 |
| 15-Me-C16:0 | 15-Methylhexadecanoic acid | 23.32 | 0.18 |
| 14-Me-C16:0 | 14-Methylhexadecanoic acid | 22.80 | 0.30 |
| C17:1n-9 | 8-Heptadecenoic acid | 14.11 | 0.11 |
| C17:1n-7 | 10-Heptadecenoic acid | 16.17 | 0.10 |
| C17:0 | Heptadecanoic acid | 13.90 | 0.23 |
| C18:3n-6,9,12 | 6,9,12-Octadecatrienoic acid | 14.10 | 0.13 |
| C18:2n-6,9 | 9,12-Octadecadienoic acid | 20.43 | 1.21 |
| C18:1n-9 | 9-Octadecenoic acid | 14.34 | 4.06 |
| C18:1n-7 | 11-Octadecenoic acid | 24.21 | 2.52 |
| C18:0 | Octadecanoic acid | 17.20 | 0.75 |
| 11-Me-C18:1 | 11-Methyloctadec-12-enoic acid | 20.48 | 1.01 |
| 10-Me-C18:0 | 10-Methyloctadecanoic acid | 22.98 | 0.08 |
| C19:1n-9 | 10-Nonadecenoic acid | 17.34 | 0.40 |
| C20:4n-6 | 5,8,11,14-Eicosatetraenoic acid | 17.25 | 0.21 |
| C20:5n-3 | 5,8,11,14,17-Eicosapentaenoic acid | 17.35 | 0.40 |
| C20:1n-9 | 11-Eicosenoic acid | 19.31 | 0.09 |
| C20:0 | Eicosanoic acid | 22.78 | 0.08 |
| C22:0 | Docosanoic acid | 23.84 | 0.10 |
| C24:1n-9 | 15-Tetracosenoic acid | 23.42 | 0.07 |
| C24:0 | Tetracosanoic acid | 17.83 | 1.28 |
| Liquid Lipid | Solid Lipid | Solubility | ||||
|---|---|---|---|---|---|---|
| 15 min | 30 min | 1 h | 24 h | 72 h | ||
| Miglyol® 810 N | Compritol® 888 ATO | + | + | + | + | + |
| Precirol® ATO 5 | - | - | - | - | - | |
| Imwitor® 900 K | + | + | + | + | + | |
| Softisan® 601 | + | + | + | + | + | |
| Microalgae oil (Schizochytrium) | Compritol® 888 ATO | + | + | + | + | + |
| Precirol® ATO 5 | - | - | - | - | - | |
| Imwitor® 900 K | + | + | + | + | + | |
| Softisan® 601 | + | + | + | - | - | |
| Halamphora cf. salinicola crude oil with microalgae oil | Compritol® 888 ATO | - | - | - | - | - |
| Precirol® ATO 5 | - | - | - | - | - | |
| Imwitor® 900 K | + | + | + | + | + | |
| Softisan® 601 | + | + | + | - | - | |
| Miglyol® 810 N | Microalgae Oil (Schizochytrium) | Halamphora cf. salinicola Crude Oil with Microalgae Oil | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Sample No. | Z-Ave 1 (nm) | PDI 2 (-) | ZP 3 (mV) | Z-Ave 1 (nm) | PDI 2 (-) | ZP 3 (mV) | Z-Ave 1 (nm) | PDI 2 (-) | ZP 3 (mV) |
| 1 | 232.6 ± 5.9 | 0.166 ± 0.016 | 53.0 ± 1.4 | 236.1 ± 1.0 | 0.158 ± 0.031 | 51.4 ± 1.4 | 220.3 ± 3.6 | 0.193 ± 0.011 | 50.8 ± 1.3 |
| 2 | 186.8 ± 5.3 | 0.198 ± 0.018 | 52.6 ± 0.2 | 198.8 ± 6.8 | 0.172 ± 0.019 | 49.4 ± 2.4 | 218.9 ± 2.8 | 0.224 ± 0.011 | 50.7 ± 1.3 |
| 3 | 145.8 ± 1.6 | 0.232 ± 0.012 | 51.1 ± 1.0 | 178.1 ± 3.0 | 0.195 ± 0.012 | 50.7 ± 1.8 | 211.5 ± 6.5 | 0.250 ± 0.010 | 49.5 ± 0.6 |
| 4 | 201.1 ± 4.7 | 0.155 ± 0.024 | 51.0 ± 1.3 | 223.0 ± 4.9 | 0.185 ± 0.010 | 50.8 ± 0.6 | 204.1 ± 0.7 | 0.181 ± 0.018 | 50.8 ± 1.1 |
| 5 | 205.2 ± 2.6 | 0.159 ± 0.013 | 50.5 ± 0.5 | 215.3 ± 2.8 | 0.184 ± 0.008 | 49.0 ± 1.5 | 226.0 ± 2.2 | 0.264 ± 0.013 | 49.6 ± 1.9 |
| 6 | 155.4 ± 1.8 | 0.160 ± 0.012 | 49.1 ± 2.1 | 173.9 ± 1.3 | 0.149 ± 0.009 | 48.9 ± 0.4 | 177.3 ± 2.9 | 0.180 ± 0.005 | 49.4 ± 0.6 |
| 7 | 190.1 ± 2.2 | 0.119 ± 0.004 | 50.6 ± 1.0 | 239.3 ± 5.3 | 0.163 ± 0.013 | 51.6 ± 0.1 | 246.2 ± 4.2 | 0.194 ± 0.013 | 53.3 ± 1.8 |
| 8 | 195.0 ± 2.6 | 0.155 ± 0.007 | 49.4 ± 0.5 | 238.2 ± 5.2 | 0.161 ± 0.018 | 43.4 ± 1.6 | 217.9 ± 2.5 | 0.141 ± 0.019 | 50.5 ± 0.9 |
| 9 | 151.8 ± 1.7 | 0.152 ± 0.018 | 51.5 ± 3.4 | 199.5 ±3.4 | 0.158 ± 0.019 | 46.4 ± 0.8 | 237.4 ± 4.3 | 0.247 ± 0.004 | 50.3 ± 1.4 |
| 10 | 196.7 ± 6.6 | 0.175 ± 0.006 | 48.3 ± 1.2 | 219.6 ±3.5 | 0.162 ± 0.002 | 47.5 ± 1.3 | 194.4 ± 1.3 | 0.149 ±0.010 | 53.2 ± 2.2 |
| 11 | 203.6 ± 4.2 | 0.186 ± 0.010 | 51.4 ± 0.7 | 175.5 ± 2.4 | 0.146 ± 0.010 | 49.7 ± 0.6 | 163.1 ± 2.8 | 0.134 ± 0.016 | 50.4 ± 0.9 |
| 12 | 156.9 ± 3.0 | 0.218 ± 0.006 | 49.7 ± 1.0 | 170.3 ± 3.3 | 0.190 ± 0.015 | 50.0 ± 1.2 | 172.8 ± 1.7 | 0.190 ± 0.005 | 50.5 ± 1.2 |
| 13 | 241.8 ± 5.4 | 0.160 ± 0.020 | 51.5 ± 1.3 | 249.0 ± 3.3 | 0.169 ± 0.014 | 49.0 ± 0.9 | 253.4 ± 1.9 | 0.187 ± 0.004 | 44.5 ± 0.5 |
| 14 | 194.5 ± 3.5 | 0.194 ± 0.014 | 50.1 ± 0.8 | 190.0 ± 2.7 | 0.152 ± 0.012 | 51.3 ± 1.2 | 230.2 ± 4.4 | 0.195 ± 0.008 | 52.9 ± 1.2 |
| 15 | 179.9 ± 3.2 | 0.178 ± 0.018 | 49.9 ± 0.6 | 171.2 ± 1.6 | 0.163 ± 0.019 | 48.3 ± 1.4 | 175.9 ± 0.4 | 0.158 ± 0.006 | 45.9 ± 1.1 |
| 16 | 216.6 ± 3.1 | 0.122 ± 0.021 | 52.7 ± 0.6 | 253.9 ± 3.4 | 0.134 ± 0.025 | 51.1 ± 0.6 | 252.0 ± 0.9 | 0.172 ± 0.033 | 50.2 ± 0.4 |
| 17 | 207.2 ± 5.3 | 0.157 ± 0.010 | 49.4 ± 1.3 | 238.3 ± 5.1 | 0.162 ± 0.031 | 47.7 ± 1.1 | 228.9 ± 3.1 | 0.198 ± 0.010 | 51.4 ± 1.2 |
| 18 | 179.9 ± 3.1 | 0.147 ± 0.005 | 51.2 ± 1.9 | 171.9 ± 3.3 | 0.144 ± 0.012 | 45.7 ± 0.8 | 248.2 ± 3.9 | 0.224 ± 0.018 | 51.3 ± 1.1 |
| 19 | 280.8 ± 6.9 | 0.180 ± 0.018 | 53.9 ± 2.3 | 225.1 ± 5.6 | 0.135 ± 0.005 | 50.2 ± 0.7 | 259.7 ± 5.3 | 0.209 ± 0.013 | 49.7 ± 1.7 |
| 20 | 247.4 ± 7.4 | 0.259 ± 0.006 | 48.4 ± 1.3 | 178.0 ± 3.3 | 0.158 ± 0.015 | 45.1 ± 3.7 | 203.0 ± 3.7 | 0.178 ± 0.009 | 51.7 ± 0.6 |
| 21 | 209.3 ± 4.4 | 0.234 ± 0.006 | 48.7 ± 1.7 | 180.9 ± 4.1 | 0.181 ± 0.020 | 48.8 ± 0.8 | 203.9 ± 3.6 | 0.210 ± 0.025 | 48.9 ± 0.3 |
| 22 | 253.1 ± 9.0 | 0.129 ± 0.018 | 53.1 ± 0.6 | 249.5 ± 2.7 | 0.157 ± 0.012 | 51.3 ± 0.8 | 241.4 ± 4.1 | 0.150 ± 0.017 | 49.9 ± 1.6 |
| 23 | 195.2 ± 3.4 | 0.155 ± 0.015 | 50.8 ± 0.5 | 202.0 ± 1.9 | 0.153 ± 0.016 | 50.3 ± 0.8 | 189.9 ± 1.4 | 0.175 ± 0.010 | 43.4 ± 0.1 |
| 24 | 189.5 ± 4.3 | 0.210 ± 0.009 | 47.9 ± 1.8 | 174.2 ± 5.2 | 0.183 ± 0.018 | 47.3 ± 2.6 | 211.6 ± 1.7 | 0.195 ± 0.023 | 46.8 ± 0.6 |
| 25 | 245.7 ± 3.3 | 0.124 ± 0.021 | 53.1 ± 1.4 | 263.7 ± 5.8 | 0.137 ± 0.041 | 51.7 ± 0.5 | 272.2 ± 4.7 | 0.158 ± 0.016 | 49.6 ± 1.5 |
| 26 | 215.4 ± 4.3 | 0.178 ± 0.005 | 48.5 ± 1.0 | 225.0 ± 2.1 | 0.149 ± 0.024 | 47.8 ± 3.0 | 209.5 ± 3.1 | 0.178 ± 0.014 | 49.0 ± 2.3 |
| 27 | 173.1 ± 3.0 | 0.163 ± 0.011 | 49.9 ± 1.6 | 194.2 ± 3.4 | 0.144 ± 0.010 | 44.1 ± 1.2 | 190.6 ± 2.3 | 0.170 ± 0.031 | 51.4 ± 1.3 |
| Temp. | Day | Miglyol® 810 N | Microalgae Oil (Schizochytrium) | Halamphora cf. salinicola Crude Oil with Microalgae Oil |
|---|---|---|---|---|
| Mean particle Size (nm) | ||||
| 25 °C | 1 | 170.1 ± 0.7 | 184.2 ± 0.8 | 216.4 ± 1.5 |
| 4 °C | 30 | 192.6 ± 11.6 | 465.5 ± 8.7 | 301.3 ± 9.6 |
| 25 °C | 30 | 168.2 ± 1.4 | 168.0 ± 2.1 | 206.9 ± 2.3 |
| 40 °C | 30 | 174.5 ± 1.2 | 486.6 ± 8.4 | 245.3 ± 9.2 |
| Polydispersity Index (-) | ||||
| 25 °C | 1 | 0.196 ± 0.014 | 0.189 ± 0.002 | 0.198 ± 0.012 |
| 4 °C | 30 | 0.293 ± 0.024 | 0.508 ± 0.055 | 0.450 ± 0.020 |
| 25 °C | 30 | 0.168 ± 0.012 | 0.150 ± 0.009 | 0.175 ± 0.003 |
| 40 °C | 30 | 0.192 ± 0.001 | 0.556 ± 0.073 | 0.403 ± 0.044 |
| Zeta Potential (mV) 1 | ||||
| 25 °C | 1 | 48.3 ± 1.6 | 49.1 ± 0.6 | 50.6 ± 1.1 |
| 4 °C | 30 | 49.9 ± 1.0 | 53.6 ± 0.7 | 50.0 ± 1.8 |
| 25 °C | 30 | 52.0 ± 0.7 | 50.1 ± 0.7 | 51.1 ± 0.2 |
| 40 °C | 30 | 51.7 ± 0.4 | 47.2 ± 0.2 | 54.1 ± 2.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marzec, M.; Dąbek, P.; Witkowski, A.; Monedeiro, F.; Pomastowski, P.; Buszewski, B.; Nowak, I. Lipid Constituents of Diatoms (Halamphora) as Components for Production of Lipid Nanoparticles. Pharmaceutics 2022, 14, 1171. https://doi.org/10.3390/pharmaceutics14061171
Marzec M, Dąbek P, Witkowski A, Monedeiro F, Pomastowski P, Buszewski B, Nowak I. Lipid Constituents of Diatoms (Halamphora) as Components for Production of Lipid Nanoparticles. Pharmaceutics. 2022; 14(6):1171. https://doi.org/10.3390/pharmaceutics14061171
Chicago/Turabian StyleMarzec, Marta, Przemysław Dąbek, Andrzej Witkowski, Fernanda Monedeiro, Paweł Pomastowski, Bogusław Buszewski, and Izabela Nowak. 2022. "Lipid Constituents of Diatoms (Halamphora) as Components for Production of Lipid Nanoparticles" Pharmaceutics 14, no. 6: 1171. https://doi.org/10.3390/pharmaceutics14061171
APA StyleMarzec, M., Dąbek, P., Witkowski, A., Monedeiro, F., Pomastowski, P., Buszewski, B., & Nowak, I. (2022). Lipid Constituents of Diatoms (Halamphora) as Components for Production of Lipid Nanoparticles. Pharmaceutics, 14(6), 1171. https://doi.org/10.3390/pharmaceutics14061171