Abstract
Interest in the use of mesoporous materials as carriers of medicinal substances has been steadily increasing in the last two decades. Mesoporous carriers have application in the preparation of delivery systems for drugs from various therapeutic groups; however, their use as the carriers of anti-inflammatory agents is particularly marked. This review article, with about 170 references, summarizes the achievements in the application of mesoporous materials as the carriers of anti-inflammatory agents in recent years. This article will discuss a variety of mesoporous carriers as well as the characteristics of their porous structure that determine further use of these materials in the field of medical applications. Special attention will be paid to the progress observed in the construction of stimuli-responsive drug carriers and systems providing site-specific drug delivery. Subsequently, a review of the literature devoted to the use of mesoporous matrices as the carriers of anti-inflammatory drugs was carried out.
1. Introduction
According to the IUPAC nomenclature, mesoporous materials are defined as materials with a pore size between 2 and 50 nm [1]. Extraordinary properties of mesoporous materials make them attractive tools in many fields of science including heterogeneous catalysis [2], photocatalysis [3], hydrogen production [4], electronics [5], solar cells [6] or battery components [7]. These materials are of particular interest in the field of medicine and pharmacy. Mesoporous matrices have attracted increasing attention in drug delivery [8], bioanalysis [9], cell and tissue imaging [10], biomolecule separation [11] and tissue engineering [12].
In the last two decades, the use of mesoporous materials as the carriers in drug delivery systems has given scientists almost unlimited possibilities to modify the release of active substances from various therapeutic groups [13]. Mesoporous matrices allow incorporation of numerous classes of drug molecules. They have been shown to store and gradually release therapeutically relevant agents like antibiotics [14], anti-virus drugs [15], anti-cancer drugs [16], vitamins [17], anti-hypertensive drugs [18], lipid-lowering agents [19], anti-fungal drugs [20], antibacterial agents [21], anti-ulcer drugs [22], anti-osteoporosis drugs [23], anti-platelet drugs [24], cardiac drugs [25] and choleretic drugs [26] among others. Mesoporous silica carriers are particularly useful for drug molecules characterized by low solubility and a lack of specificity. Scientists were greatly interested in the use of mesoporous carriers for drugs with anti-inflammatory activity. Numerous anti-inflammatory drugs are poorly water-soluble. Using these medications may lead patients to take high doses of the drug to achieve sufficient therapeutic effect; this is the main cause of adverse drug effects, particularly for drugs with a narrow therapeutic window [27]. Drug delivery systems enable to deliver the specific dose of the drug to the target site in the body while ensuring controlled rate and drug release time to maintain its therapeutic concentration for a long time. Modern stimuli-responsive drug delivery systems ensure controlled release of active substance that depends on the pH [28], temperature [29], light [30], ultrasounds [31], osmotic conditions [32], oxidation state [33] or the presence of enzymes [34] among others.
Mesoporous materials are extremely attractive drug reservoirs due to their physicochemical properties such as high specific surface area, pore ordering, tunable pore size, large pore volume and pore diameter as well as the possibility of surface modification using various chemical functions [35]. Moreover, depending on their chemical structure, mesoporous carriers may exhibit magnetic [36], conducting [37] or fluorescent [38] properties. This group of materials includes substances of different chemical classes, namely carbon, silica, metals, metal salts, metal hydroxides, alkaline and amphoteric metal oxides, transition metal oxides, lanthanide oxides, perovskite-type/spinel-type oxides, organic structures, nitrides/carbides and other hybrid materials that were widely studied in recent years [35].
With the development of the highly ordered mesoporous silica MCM-41 (Mobile Composition of Matter) in 1992, mesoporous materials have become an intense research focus [39]. MCM-41 mesoporous silica possessing organized mesoporous structure, a high degree of porosity, narrow pore size distribution, large surface area, hexagonally formed pores, controllable pore volume and biocompatibility has attracted great research interest as a carrier in drug delivery systems (DDSs) [40]. The exceptional structure of mesoporous materials enables the efficient loading of drugs and their subsequent controlled release.
Mesoporous silicas have been demonstrated to be able to incorporate high dosages of drugs in the internal pore system. The properties that influence the process of drug loading and drug release are specific surface areas, pore volumes, pore diameters, pore arrangement, pore channel length as well as particle size and morphology [41,42,43]. The properties of siliceous material pores (mainly pore size and pore volume) as well as the connectivity and geometry that determine the degree of drug loading and drug release are summarized in Table 1.

Table 1.
The properties of mesoporous materials influencing the drug loading and release.
It has been demonstrated that as the pore size decreases, the amount of drug loaded is reduced. The drug release rate also decreases as a result of increasingly tightly packed molecules in the mesopores [50]. Some data supports the existence of a critical pore width which exerts steric hindrance to drug diffusion through the pores [56]. It has been shown that the release rate of drugs characterized by higher molecular weight is decreased as compared to ones with lower molecular weight from the pores of the same diameter [57]. Some authors claim that to allow for easy access of drug for loading and release, the pore size should be at least three times greater than the drug molecule diameter [58]. It has been reported that there is a pore size threshold limit for individual molecules, after which further increases in pore diameter do not enhance the release rate. It has been shown that long-term stability of amorphous drugs in mesoporous channels is provided [59,60]. However, regarding in silica samples with larger pore sizes, increased recrystallization has been observed due to the loss of nano-confinement properties [61,62]. Some authors claim that the drug molecule cannot recrystallize if the confinement space diameter is less than or equal to 15 times the drug molecule diameter [63,64]. To influence drug loading and release, silica particle properties such as particle size and morphology have been examined to a lesser extent due to the difficulties in examining the effect of particle morphology in isolation. Obviously, it is desired to synthesize particles with monodisperse sizes [65,66]. The large specific surface area of mesoporous carriers is especially important to increase the dissolution rate of poorly water-soluble drugs [67]. Increased surface area improves drug loading and its dissolution rates. However, recent studies demonstrated that there is a threshold level after which increasing surface area does not result in a linear increase in drug release rate [68]. This may be explained due to the effective surface area, which is the real parameter that influences the dissolution rate. Further increase in carrier surface area may not lead to further subdivision of the drug because of the presence of increased cohesive forces/surface tension among the drug particles [56].
Structural properties such as pore size and pore volume as well as surface properties of mesoporous matrices can be altered depending on synthesis conditions (time, temperature, precursor type and its concentration) [69]. Furthermore, the silanol groups present at the external surface can be combined with numerous organic moieties containing a variety of functional groups (-NH2, -Cl, -CN, -CH3, -COOH) to alter their surface characteristics and provide specific drug-carrier interactions [35]. The surface of mesoporous carrier can be functionalized using co-condensation strategy (one-pot synthesis) and grafting method (post-synthesis modification) [35]. The appropriate modification approach can provide desired rate of drug release from the mesoporous carrier. Moreover, surface modification with target-specific ligand facilitates drug transport to the desired site of action, so-called site-specific targeting. Many types of mesoporous silicas like MCM-41 [13], MCM-48 [70], SBA-12 [71], SBA-15 [72], SBA-16 [73], MCF [74] or PHTS [24] were successfully used as carriers in DDSs. Each material is characterized by different structure, morphology and size [27]. In 2001, mesoporous MCM-41 material was used for the first time by Vallet-Regi and co-workers as a carrier for anti-inflammatory drug ibuprofen [13]. This pioneer study initiated the ongoing scientific campaign to search for and use of mesoporous matrices as carriers in drug delivery systems. Over the last twenty years many review papers have been published describing current developments in the usage of mesoporous materials as carriers for various therapeutic agents [8,16,75,76,77,78,79,80,81,82,83,84,85]. Initially, these were simple systems consisting of the mesoporous carrier and the drug molecules. The release of the drugs from as-prepared systems depends mostly on the structure of the mesoporous carrier. Subsequently, the mesoporous surface was modified to create a desired drug-carrier interactions or provide specific target-site delivery of active agent [35]. Nowadays, modern hybrid systems in which the mesoporous matrix is only the element of the complex whole are of interest to many research groups.
Following the first use of mesoporous silica as a carrier for ibuprofen, this paper will review the latest achievements in the application of various mesoporous materials as the carriers for anti-inflammatory agents. Inflammation is the first response of the human immune system to tissue injury, irritation or infection [86]. Long-term inflammation can result in the damage of tissues or even lead to chronic diseases such as rheumatoid arthritis, asthma and other inflammatory vascular diseases [87,88]. It is also considered a serious risk factor for cancer [89]. Anti-inflammatory agents are often prescribed by doctors or even more often used without the prescription as over the counter (OTC) drugs. However, taking many of them is associated with mild but numerous side effects. This review paper summarizes the achievements in terms of developing mesoporous material-based drug delivery systems for synthetic and natural substances with anti-inflammatory activity.
2. Anti-Inflammatory Agents
Pain is a spontaneous sensory sensation and one of the first defence mechanisms [90]. Chronic pain becomes discomfort which subsequently leads to negative changes manifesting, among others, impaired concentration, disordered memorization and even changes in the spinal cord [91]. The consequence is the development of excessive excitability and strengthening pain reactions [92]. Chronic pain can cause anxiety and depression limiting the patient’s functioning in family and society [93]. Hence, it is important to properly treat the pain. Depending on the place of action, two groups of drugs with analgesic effect are distinguished: analgesics of central action and drugs of peripheral action (so-called local anesthetics) [94]. Among analgesics of central action, opioid and non-opioid analgesics are distinguished [95]. The former exhibits numerous side effects primarily on the part of the central nervous system including the depression of the respiratory center [96]. Moreover, opioid use can lead to physical and psychotic addiction [97]. In the treatment of acute and chronic pain, non-opioid analgesics have also been used. Pain therapy always begins with the use of non-opioid drugs. Classic non-opioid anti-pain drugs are aniline derivatives (paracetamol), salicylic acid derivatives (aspirin), derivatives of methylacrylacetic acid (ibuprofen), pyrazolon derivatives (metamizole) and others (e.g., ketorolac) [98]. Drugs from the group of non-steroidal anti-inflammatory drugs (NSAIDS) (salicylic acid derivatives and derivatives of methylacrylacetic acid) exhibit antipyretic and anti-inflammatory modes of action in addition to analgesic effects [99,100]. Among many NSAIDs, a great majority possess a carboxylic function (aspirin, mefenamic acid, indomethacin, diclofenac, ibuprofen). The few exceptions that do not contain this chemical function are phenylbutazone, celecoxib and oxicams (isoxicam, meloxicam, piroxicam) [101]. NSAIDs are commonly used in the treatment of pain, fever and anti-inflammatory diseases [87].
The anti-inflammatory drugs can be administered to the body via different pathways depending on the disease entity and the patient’s condition among others. The administration routes of most common anti-inflammatory agents available on the market are summarized in Table 2.

Table 2.
The routes of administration of anti-inflammatory drugs.
The use of NSAIDs in the treatment of pain results from the fact that its mediators, mainly prostaglandin PGE2, are responsible for pain and swelling in the inflammatory process [102]. The inflammation leads to the release of pro-inflammatory enzymes and cytokines (interleukins 1 and 6; IL-1, IL-6, tumor necrosis factor α; TNF-α) that activate cyclooxygenase (COX) by the cells of the immune system (monocytes) and thus an increase the synthesis of prostanoids [103]. Prostanoids together with histamine and bradykinin released under the influence of PGE2 cause an increase in the permeability of blood vessel walls and expand them [104]. In this way, they affect not only the movement of blood cells to the site of inflammation but they are also the mediators of pain to the endings of the sensory nerves. Therefore, inhibition of PGE2 synthesis causes a decrease in its concentration, results in anti-inflammatory and analgesic effect. All NSAIDs are inhibitors of COX, an enzyme responsible for the synthesis of prostaglandins [105]. The main side effects resulting from the therapy using NSAIDS are gastric irritation leading to the occurrence of peptic ulcers, irritation of gastrointestinal mucosa, inhibition of platelet aggregation and adverse reactions to the kidneys [86,87,106].
In the therapy of inflammatory bowel disease (e.g., Crohn’s disease, ulcerative colitis), other anti-inflammatory drugs such as mesalazine are necessary [107]. Mesalazine (syn. mesalamine) is a 5-aminosalicylic acid with amine, carboxylic acid and phenolic functions. The substance is rapidly absorbed by the small intestine. It also belongs to the group of COX inhibitors [108].
Inflammation is one of the key factors in the development of corneal angiogenesis [109]. The infiltration of inflammatory cells into the injured cornea upregulates multiple mediators, e.g., IL-1, vascular endothelial growth factor (VEGF), TNF-α and prostaglandins which promote the angiogenic process and stimulate the formation of new corneal vessels, opacity and scars [110,111]. To inhibit the inflammation process in the cornea and avoid the systemic side effects, immunosuppressing drugs are locally applied. An example of such drug is cyclosporin A which decreases the transcription activity of activated T cells and thus reduces the inflammation reactions [110]. Probucol is a diphenolic compound capable of reducing the increased activity of COX through the nuclear factor kappa-light-chain-enhancer of activated C cells of the NF-κB pathway. As a result, the reduced expression of pro-inflammatory markers and the decreased disruption of the blood-brain-barrier integrity was observed in vivo. Furthermore, it has been shown that probucol is a potent free radical scavenger protecting the cellular membranes against oxidative damage [110].
Nimesulide is a chemical compound from the group of sulfonamides [112]. It belongs to the semiselective COX-2 inhibitors. Nimesulide is commonly used for pain relieving in painful osteoarthritis disorders and other acute pain states [113,114]. Another drug often used to relief pain and inflammation symptoms in osteoarthritis and rheumatoid arthritis is flurbiprofen [115,116]. The mechanism of action of this phenylalcanoic acid derivative belonging to the group of NSAIDS also involves blockage of the COX-2 enzyme. Flurbiprofen is a non-selective inhibitor of prostaglandin biosynthesis [117].
Other group of anti-inflammatory drugs are steroids. Natural glucocorticosteroids are cortisone and hydrocortisone. Glucocorticosteroids obtained synthetically were used to increase the desired activity and reduce negative hormonal effects, primarily from mineralogenic activity [118,119]. Synthetic glucocorticosteroids with representatives such as beclomethasone, betamethasone, budesonide, dexamethasone, flumetasone, methylprednisolone, mometasone, prednisolone, prednisone or triamcinolone are widely used in the treatment of numerous disease entities. Glucocorticosteroids affect basal metabolism [120], immune mechanisms [121] and participate in the body’s reactions to stress [122]. The therapeutic effect of glucocorticosteroids is considered to be mediated by four different mechanisms: classical genomic and secondary non-genomic effects caused by cytosolic glucocorticoid receptors, membrane-bound glucocorticoid receptor-mediated non-genomic effects and non-genomic effects caused by interactions with cellular membranes [123]. Glucocorticosteroids are used in substitution therapy in primary adrenal insufficiency (Addison’s disease) [124], in second adrenal insufficiency [125,126], in congenital adrenal hyperplasia [127] and in the diagnosis of Cushing’s syndrome [128]. Moreover, this group of drugs is used to treat inflammations (rheumatoid arthritis, asthma, liver failure, chronic obstructive pulmonary disease, ulcerative colitis, acute myocarditis, neuroimmunological disorders and inflammatory disease of the skin) [129,130,131,132,133,134,135,136,137]. Anti-allergic properties determine the use of glucocorticosteroids in allergic diseases such as bronchial asthma, allergic dermatoses and allergy to drugs [138]. In turn, the antimitotic effect of this group of drugs is used in the treatment of acute lymphoblastic leukemia [139] and some lymphomas [140]. Immunosuppressive activity is the basis for the use of glucocorticosteroids to abolish immune reactions after tissue and organ transplants [141]. An absolute indication for immediate glucocorticosteroid therapy is life-threatening disease, e.g., anaphylactic shock [142] and edema of the brain [143].
Dexamethasone is a glucocorticoid well known to decrease the number of inflammatory cells in airways [144]. It is extensively used for acute lung injury treatment. This inflammatory syndrome is characterized by increased inflammation and severe lung damage. Targeted drug delivery to inflamed lungs has become an attractive research field [145]. Glucocorticosteroids, which are strong anti-inflammatory agents, are commonly used to ameliorate clinical symptoms of acute lung injury. They were shown to limit tissue injury and acute inflammatory response. The dexamethasone therapy decreases the level of proinflammatory cytokines, reduces lung tissue injury and prevents the pulmonary edema [144]. Betamethasone sodium phosphate is the other anti-inflammatory synthetic corticosteroid. This glucocorticosteroid with a strong anti-inflammatory effect was shown to reduce swelling. Thus, it is widely used in various skin dysfunctions like itching, dryness, crusting, redness, scaling or inflammation [146].
The most common side effects of long-term non-substitutive therapy with glucocorticosteroids are osteoporosis, stunted growth in children and youth, steroid diabetes, hypertension, hypokalemia, thromboembolism, increased susceptibility to infections, depression/mood changes, poor wound healing, increased risk for infections, moon face, myopathy or skin atrophy [125,147,148].
To overcome the side effects of synthetic anti-inflammatory drugs the natural compounds with anti-inflammatory activity are proposed in the therapy of many inflammations and infectious diseases. Natural medicines revealing anti-oxidative, anti-inflammatory, antifungal, antiviral, antibiotic and even anticancer properties are used for thousands of years in various corners of the world as a remedy for many diseases manifested by pain. Intensive studies have been carried out on natural anti-inflammatory agents, like curcumin, with fewer adverse effect. It is a natural polyphenol exhibiting anti-inflammatory, antioxidant and anticancerogenic activity. Curcumin occurs in the rhizomes of Curcuma longa [40,108]. Curcumin’s effectiveness in reducing inflammation has been shown to be due to its antiplatelet, antiviral and cytoprotective activity. The main concern limiting medicinal usefulness of curcumin is its low water solubility and bioavailability [86]. Another compound of natural origin demonstrating anti-inflammatory activity is ginsenoside [149]. Ginsenosides (panaxosides) are triterpene saponins. They occur in the plant genus Panax and are usually extracted from the root and the stem of the plant. Panax ginseng is very popular in Chinese medicine. The genus name Panax derives from the Greek and means ‘all-healing’ [149]. Ginsenosides exhibit many biological functions. They possess antiproliferative, neuroprotective, antioxidative, angiogenic and anti-inflammatory properties. Furthermore, it has been demonstrated that ginsenosides increase the activity of osteoblasts, thus leading to enhanced bone tissue healing [150]. Andrographolide is a natural plant-derived diterpene lactone isolated from Andrographis paniculata [151]. This plant is widely recognized for its numerous therapeutic properties in India, China, Korea, Japan and Sri Lanka among others [152]. Andrographolide is the main active ingredient of A. paniculata giving the plant a bitter taste. It possesses anti-inflammatory, antioxidative, antipyretic, hepatoprotective, antiviral, antithrombotic, anticancer and immunostimulant properties [152]. Andrographolide is commonly used in the treatment of inflammation-related diseases, fever, laryngitis and upper respiratory tract infections in South Asian countries. This diterpene lactone has also found application in cartilage inflammations, osteoarthritis and rheumatoid arthritis therapy. Nevertheless, due to its low aqueous solubility, low bioavailability and high lipophilicity resulting in easy clearance from the place of administration after intra-articular injection, its therapeutic efficiency is negligible [151].
3. Physicochemical and Biological Characteristics of Mesoporous Material-Based Drug Delivery Systems (DDSs)
The most common mesoporous material used as a carrier in drug delivery systems (DDSs) is mesoporous silica and its more advanced forms such as mesoporous silica nanoparticles, mesoporous silica nanorods or bioactive glasses [153,154,155,156]. Other materials often used for this purpose are mesoporous carbon [157], mesoporous calcium silicate and mesoporous calcium sulfate [150]. The insightful characterization of the physicochemical and biological properties of mesoporous materials is very helpful to confirm the success of the material’s synthesis and in its future bio application. Mesoporous materials are characterized by large pore volume and pore diameter, good mechanical and chemical stability, possibility of surface modification and tunable particle size [35,88]. Moreover, some mesoporous matrices are characterized by biodegradability and biocompatibility [35,158]. In modern DDSs the mesoporous structure modified with wide range of functional groups (organic functions providing drug-carrier interactions, ligands for receptors enabling targeted delivery, ligands providing stimuli-responsiveness) usually act as a reservoir for the drug [154]. DDSs can be characterized using numerous physicochemical methods. Table 3 lists the most common techniques used for physicochemical and biological characterization of mesoporous material-based DDSs.

Table 3.
Physicochemical and biological characterization methods of mesoporous material-based drug delivery systems.
Mesoporous materials exhibit a large specific surface area of several hundred meters. These matrices include a wide range of materials characterized by different structure, ordering of mesoporous channels and pore geometry [35,69]. In order to obtain the structural information at a nanometer scale resolution for porous materials, transmission electron microscopy (TEM) technique can be used [69]. In Figure 1 the TEM micrographs of well-known mesoporous materials are depicted.
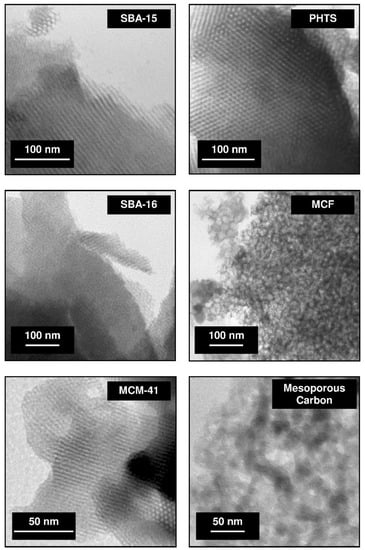
Figure 1.
TEM micrographs of various mesoporous materials (abbreviations: SBA-15, 16–Santa Barbara acid; PHTS–plugged hexagonal templated silica; MCF–mesocellular foam; MCM-41–Mobil Composition of Matter). The micrographs were collected using Jeol JEM 1200 EX electron microscope and come from the authors’ own collection.
The hexagonal channel arrangement in the structure of SBA-15, PHTS and MCM-41 silicas can be distinguished. SBA-16 silica exhibits the regular structure, MCF matrix reveals the foam-like structure meanwhile mesoporous carbon possesses disordered arrangement. The topography and morphology of mesoporous materials can be studied using scanning electron microscopy [69]. The morphology of SBA-15, PHTS, SBA-16, MCF, MCM-41 silicas and mesoporous carbon are presented in Figure 2.

Figure 2.
SEM images of various mesoporous materials. The micrographs were collected using Zeiss ELO-40 electron microscope come from the authors’ own collection.
SBA-15 material reveals chain-like morphology, PHTS–rough fibers, SBA-16–spherical morphology having diameter of several micrometers, MCF–almost spherical morphology resembling the coral reef, MCM-41 and mesoporous carbon–irregular morphology. The textural characterization of mesoporous solids is usually confirmed by nitrogen adsorption/desorption studies. This technique provides information about micro- and mesoporosity of mesoporous structures. It allows determination of the specific surface area of the carrier, its pore volume and pore diameter as well as the pore geometries of mesoporous channels [69]. By using the powder small angle X-ray diffraction (XRD) method, the ordered structure of mesoporous matrices can be committed. X-ray diffraction is a commonly used technique to evaluate the structure (shape) of the mesoporous molecular sieve. The diffractograms of all ordered nanoporous phases exhibit reflexes in small angle range. Figure 3 demonstrates the typical diffractograms of SBA-15, PHTS, SBA-16, MCF, MCM-41 and MCM-48 silicas.
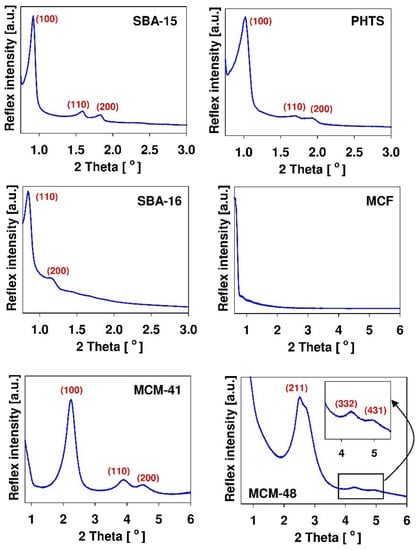
Figure 3.
Typical XRD patterns of chosen mesoporous silicas with indices of the diffraction planes. The diffractograms were collected using Bruker D2 PHASER apparatus and come from the authors’ own collection.
The presence of reflexes enables the confirmation of mesoporous structure of synthesized materials. Among examined materials, only MCF silica do not exhibit the reflexes. It results from the fact that the large dimensions of the structural motif of MCF material precluded the observation of any X-ray reflexes in the low angle region [165]. Since all mesoporous matrices consist of amorphous silica which exhibit no crystallinity at the atomic level, no reflections can be observed higher than 2θ degrees [69]. Nevertheless, the X-ray diffractogram patterns obtained in the wider range of angles can be useful for confirmation of successful adsorption of the drug within the mesoporous channels [35]. In this case, the amorphous nature of the drug confirms its molecular dispersion on the large surface area of mesoporous carrier. The Fourier transform-infrared (FT-IR) spectroscopy is useful in confirmation of the presence of organic moieties [35]. This technique is based on the fact that each molecule exhibits a specific frequency of internal vibrations that occur in the infrared region of the electromagnetic spectrum [69]. This analytical tool gives information concerning the presence of organic functions at the siliceous carrier surface, thus confirming its successful functionalization. There exist also several other analytical methods used to reveal certain characteristics of mesoporous materials. The size and stability of material can be estimated using the dynamic light scattering (DLS) method. The elemental composition of the surface can be analyzed by using the X-ray photoelectron spectroscopy (XPS) method. The thermal properties can be determined from thermogravimetry analysis (TGA) or differential scanning colorimetry (DSC) analysis whereas the information obtained by solid-state nuclear magnetic resonance (NMR) spectroscopy allows for the study of the local structure of mesoporous matrices. Thus, this technique is complementary to the X-ray diffraction method. The principle of this method is that the number of atoms in the structure of solid samples possess isotopes with nuclear spin, thus, it is possible to observe these isotopes by NMR [69].
The most common techniques used for the characterization of active pharmaceutical ingredients (APIs) loaded in the siliceous matrices are summarized in Table 4.

Table 4.
Methods used for analysis of drugs loaded in mesoporous siliceous matrices.
Advanced DDSs that revolutionized drug delivery studies are sophisticated systems in which the release of the drug depends on many parameters such as the solubility of the drug, its diffusion through the pores and the strength of drug-carrier interactions [35]. The systems are designed to prevent premature drug release (resulting from the usage of so-called gate keepers [16]) and to provide stimuli-responsive drug release using various chemical and physical factors including light, redox state, pH or magnetism [78]. To be used in medicine, these sophisticated materials have to be thoroughly examined both in vitro and in vivo [166]. The toxicity of the systems is usually examined on various cell lines via MTT assay or fluorescence microscopy. Other in vitro experiments include drug loading and release studies, biocompatibility tests, hemolysis assays, biodegradability studies as well as the estimation of anti-inflammatory properties and antibacterial activity (see Table 3). In turn, the in vivo experiments being the final stage of research conducted on living organisms, consist of defining pharmacokinetic and pharmacodynamic properties. The results of these studies allow to determine the therapeutic efficacy (anti-inflammatory response) and biosafety of the systems.
4. Mesoporous Material-Based Drug Delivery Systems for Anti-Inflammatory Agents
Among numerous drug delivery platforms gaining attention of the scientific world in the last two decades, mesoporous material-based DDSs exhibit extraordinary properties such as high drug loading capacity, well-defined pore structure, tunable surface chemistry, easily controllable morphology, physical stability and satisfying biocompatibility [30]. Large specific surface area is the main advantageous feature which make mesoporous materials ideal carriers to design multifunctional systems. Mesoporous materials attracted huge scientific attention and several interesting topical reviews are published annually by the leaders in the field, discussing the trends in research and application of these materials as the elements of unique DDSs for use in modern medicine.
Among various porous materials, mesoporous silica and mesoporous carbon have especially gained great attention in recent years due to their superlative properties and structural features. The latter may occur in the form of carbon aerogels which is a special class of lightweight nanoporous materials with tunable porosity and chemical inertness [157]. Mesoporous silica is also the main component of so-called bioactive glass which is an extraordinary class of biomaterials suitable for numerous biomedical applications including wound healing, bone regeneration and cancer therapy [156]. Bioactive glass is composed of silica networks incorporating calcium and is characterized by its mesoporous structure.
Mesoporous silica-based DDSs are especially useful for the incorporation of poorly water-soluble drug molecules. In order to solve the problem of low solubility, many different approaches have been put forward and investigated in depth to improve the dissolution kinetics and bioavailability of poorly water-soluble drugs such as solid dispersions, nanosuspensions, spray drying, cryogenic technologies and prodrug strategies [87,115,155]. It was shown that the problem of low drug solubility can also be overcome by the usage of a carrier substance like mesoporous silica during the preparation of sophisticated drug delivery platforms.
As mentioned before in the first mesoporous silica-based DDS, the siliceous matrix MCM-41 was employed as the carrier for ibuprofen [13]. The sustained drug release from the system was the result of drug molecular dispersion within the pores of the mesoporous carrier. It was shown that mesoporous silica possesses the ability to prevent crystallization of the loaded drug which enhances its dissolution kinetics [13]. Currently, mesoporous silicas used as the elements of modern DDSs provide controlled and intelligent stimuli-triggered drug release. This can be achieved by proper carrier design, including its desired structure and morphology, as well as by sophisticated surface functionalization. Well-defined surface of mesoporous matrices allows conjunction with pore-blocking materials to prevent premature cargo release, with stimuli-responsive ligands (nanovalves or gate keepers [145]) for controlled release or with specific ligands for targeting [151]. Stimuli-responsive drug release refers to the release of the drug trigged by a variety of external factors including temperature, light, pH, heat, tumors, enzyme presence, chiral environment or redox potential [115,161,162]. These approaches were shown to improve the therapeutic efficacy and reduce side effects of the drugs which is of great importance in pharmaceutical practice and can lift DDSs research to a new level.
One outstanding feature resulting from the usage of mesoporous silica as the carrier in modern drug delivery platforms is the enhancement of dissolution kinetics and bioavailability of poorly water-soluble drugs. It is claimed that over 70% of active pharmaceutical ingredients (APIs) belong to the Biopharmaceutical Classification System (BCS) class II and IV [87,115]. The substances from class II are characterized by low solubility and high permeability through biological membranes (intestinal epithelium) meanwhile the components of class IV exhibit both low solubility and low permeability [161]. Thus, the drugs from the BCS Classes II and IV cannot exert satisfactory therapeutic effects due to the insufficient drug concentration in the site of absorption which seriously limits their therapeutic potential. Poor drug solubility in water is associated with the physically stable crystalline nature of most drugs. Thus, it is of great importance to overcome the forces attaching drug molecules within the crystalline lattice [113]. The development of effective DDSs for poorly water-soluble drugs is still a pressing issue for the pharmaceutical industry [157]. By using mesoporous carrier matrices, higher doses of poorly water-soluble drugs can be administered locally, reducing their adverse effects and improving their biodistribution [144].
Numerous studies have been conducted concerning the design of mesoporous material-based DDSs to be used by various administration routes. Mesoporous material-based drug delivery platforms are gaining attention for use through the pulmonary route. The possibility of using mesoporous carrier for dexamethasone in the treatment of airway inflammation was investigated by Gulin-Sarfraz et al. [144]. It was shown that the dissolution kinetics of practically water-insoluble dexamethasone was enhanced when loaded in mesoporous silica particles. Furthermore, drug-loaded particles revealed an increased ability to reach the lower parts of the lungs as compared to free drugs. It was demonstrated that designed delivery platform improved airway distribution and allowed for local treatment. Moreover, the systemic side effects could be reduced, and the possibility of using higher drug doses locally in the lungs is possible. Thus, dexamethasone-loaded mesoporous silica particles revealed its potential as an ideal therapeutic agent for acute airway inflammation [144]. Similar observations were reported by Garcia and colleagues [145]. In this case, the preferential accumulation of mesoporous silica nanoparticles loaded with dexamethasone in inflamed tissues of lungs was ascribed to the vascular nature, high permeability and retention capacity of the lungs. Similar studies were performed by the group of Rosenholm [144] that investigated the feasibility of mesoporous silica particles as carriers for dexamethasone in the treatment of airway inflammation. The particles were administered as aerosol through inhalation to mice models of neutrophil-induced airway inflammation. Down-modulation of the inflammatory response was observed. It has been demonstrated that prepared particles can be employed as corticosteroid carriers in the anti-inflammatory treatment of lung injury [144].
Owing to its convenience, painlessness, easy administration and high patient compliance, oral administration is one from the most accepted and patient preferred drug delivery pathway [155,161]. However, the challenge remains to improve the oral bioavailability of BCS II molecules. Recently, it has been demonstrated that mesoporous material-based DDSs offer advantages for orally administered drugs owing to their ability to deliver the drug to a specific site, avoid digestion in the gastrointestinal tract and control the release and cellular uptake of incorporated drugs [155]. Zhou et al. examined the usage of mesoporous silica nanoparticles as carriers for orally delivered indomethacin [161]. This non-steroidal anti-inflammatory drug exhibits poor water solubility and causes irritation to gastrointestinal mucosa. The carrier was modified with L- and D-tartaric acid. It was shown that as-prepared drug-loaded MSNs revealed higher drug dissolution, oral bioavailability and anti-inflammatory effects compared with naked particles. The superiorities in delivering indomethacin were ascribed to the binding affinity between surface modifying agents and sodium-glucose linked transporter (SLGT). It has been demonstrated that prepared materials triggered chirality of biological environment based on their molecular level chiral function [161]. The group of Aulia examined the anti-inflammatory activity of curcumin-loaded MSNs after oral administration to white male Wistar rats [86]. After oral administration of curcumin-loaded MSNs the anti-inflammatory activity like the one revealed by diclofenac sodium was observed. Furthermore¸ no significant macroscopic and microscopic changes to gastric organs were observed as opposed to the effects caused by diclofenac. The system revealed high potency for medicinal use in inflammation-related diseases.
Mesoporous silica nanoparticles show great potential as delivery platforms in eye therapy due to the possible silicon element supplement to eyes [110]. Sun and colleagues encapsulated the bevacizumab into the pores of MSNs [110]. Separately, cyclosporine A was dissolved in the thermogel. Finally, bevacizumab-loaded MSNs were dispersed in the cyclosporine A-containing thermogel. As-prepared nanohybrid thermogel was administered to the subconjunctiva. It has been demonstrated that the system significantly improved curative treatment in the rabbit model.
Mesoporous materials are an ideal platform for delivery of active agents to be used in the therapy of numerous diseases. Li et al. studied the potential of the application of folic acid-decorated semiconducting polymer dots hybrid mesoporous silica nanoparticles in the therapy of rheumatoid arthritis [154]. The therapeutic effect using the as-prepared delivery nanoplatform was based on the triple photothermal, photodynamic and chemotherapy synergistic treatment. In these studies, mesoporous material served as the reservoir for the hypoxia-activated tirapazamine prodrug. Upon NIR irradiation, the system generated intracellular hyperthermia and excessive singlet oxygen. Local hypoxia caused by molecular oxygen consumption activated the cytotoxicity of tirapazamine. Finally, activated drug killed macrophages inhibited the progression of arthritis. Zheng et al. designed the nanosized pH-responsive DDS for osteoarthritis treatment by using modified mesoporous silica nanoparticles with pH-responsive polyacrylic acid [151]. Prepared nanoplatform was loaded with andrographolide. Compared with pure diterpenoid, andrographolide-loaded nanoparticles revealed enhanced antiarthritic efficacy and chondro-protective capacity as evidenced by lower expression of inflammatory factors and better prevention of proteoglycan loss.
Mahmoudi et al. used smart polymeric nanocomposite based on protonated aluminosilicate-modified mesoporous silica nanoparticles as the carrier for mesalamine in inflammatory bowel disease [108]. The system characterized by high drug loading efficiency was designed to decrease the side effects of this anti-inflammatory drug and to enhance its permeability in intestinal tissues. Drug release studies from as-prepared systems were performed in media simulating conditions occurring in various parts of the gastrointestinal tract. The release experiments indicated increased drug release rate at higher pH simulating the environment of the colon (pH = 8) as compared to the experiments performed in media characterized by lower pH values.
Targeted-lung delivery of dexamethasone using gated mesoporous silica nanoparticles was studied by García-Fernández et al. [145]. Acute lung injury is a critical inflammatory syndrome characterized by high morbidity and mortality. Targeted drug delivery to inflamed lungs is of crucial importance for patients suffering from severe lung damage. The nanodevice based on mesoporous silica nanoparticles loaded with dexamethasone and capped with a peptide targeting the TNFR1 receptor expressed in pro-inflammatory macrophages was designed. It has been demonstrated that after intravenous injection into mice, the nanodevice accumulated in injured lungs. The controlled dexamethasone release resulted in reduction of the levels of TNF-α, IL-6 and IL-1β cytokines [145].
Mesoporous material-based drug delivery systems have a chance to become a valuable tool in the treatment of neuroinflammation. Brain endothelial cells that provide the proper function and integrity of the blood brain barrier are highly susceptible to cellular damage caused by oxidative stress and inflammation. The group of Garcia-Bennet [164] observed the enhanced antioxidant effect of the anti-inflammatory compound probucol when released from mesoporous silica particles. The increased reduction of ROS, COX enzyme activity and PGE2 production was measured in human brain endothelial cells treated with probucol-loaded mesoporous silica particles. Prepared system opens new possibilities for rapid suppression of neuroinflammation underlying numerous neurodegenerative diseases.
Mesoporous material-based DDSs offer great promise as co-delivery platforms in the treatment of cancer. The disease has become a major threat to humans in the 21st century [167]. Great progress has been made in cancer therapy and many strategies have been developed to treat the disease. Unfortunately, many traditional therapeutic strategies result in serious adverse effects on normal tissues, making patients suffer from more pain [167]. Other important problem of effective anticancer therapy is the resistance of surviving cells against the employed antineoplastic drugs called multidrug resistance (MDR) [160]. It can be solved by combination therapy consisting in usage of mesoporous material as the carrier for two or even more therapeutic agents. It has been demonstrated that such a system may exert simultaneous and synergistic action on critical metabolic pathways which result in increasing of apoptotic effect via the co-delivery of several drugs [160]. The conjugation of anti-cancer drugs and anti-inflammatory agents has shown promising results [168]. Benova et al. studied the co-delivery approach of 5-fluorouracil anticancer drug in combination with naproxen anti-inflammatory agent using β-cyclodextrin-modified SBA-15 mesoporous silica as a carrier [160]. The system exhibited pH-driven drug release. At physiological pH=7.4 only 16% and 20% of 5-fluorouracil and naproxen were released, respectively. It has been ascribed to the fact that not all pores were capped with β-cyclodextrin molecules. Meanwhile, at acidic pH=5.0 (pH of tumor tissues) nearly 90% of 5-fluorouracil and 99% of naproxen was released due to the unlocking of the pores by β-cyclodextrin [160]. The group of Hu used polypyrrole-coated mesoporous TiO2 as a carrier for other pairs of anticancer and anti-inflammatory agents, namely doxorubicin and aspirin prodrugs [167]. It has been demonstrated that under external stimulation of near infra-red and ultrasounds, the system revealed excellent photothermal conversion efficiency and a satisfactory sonodynamic effect. Moreover, simultaneous prodrugs activation and rapid drug release were observed. As a result, a significant tumor inhibition effect obtained through synergistic therapy was achieved. The system was shown to reduce the inflammatory factors such as IL-1β and TNF-α in the blood of mice [167].
The potential of using mesoporous material-based devices as suitable materials for medical and hygienic applications was also reported. Hashemikia et al. grafted betamethasone-loaded SBA-15 mesoporous silica particles on the surface of cotton fabric in order to obtain an antibacterial nanosurface with drug delivery properties [146]. Prepared fabrics exhibited excellent antibacterial activity against Escherichia coli and Staphylococcus aureus even after several washing cycles. Obtained textile surface revealed the potential to be used in wound dressing acting as a gradually releasing corticosteroid reservoir at the affected site for a certain period.
It is also possible to use mesoporous material-based DDSs in bone regeneration [150]. Bone defects are very common in older generations which can be ascribed to osteoporosis among other diseases. Bone scaffolds enhance bone regeneration acting as biological extracellular support for cells. Chen et al. designed a 3D-printed mesoporous calcium silicate/calcium sulfate scaffold loaded with ginsenoside Rb1 that provided mechanical stability and promoted cell viability [150]. Experiments revealed that human dental pulp stem cells (hDPSCs) cultivated in ginsenoside-containing scaffold exhibited proliferative ability and higher expression of osteogenic-related proteins and could effectively inhibit inflammation. Very recently, mesoporous bioactive glass nanoparticles containing cerium element were synthesized by Boccaccini et al.as anti-inflammatory and anti-bacterial agents with potential to be used in inflammatory bone diseases and bone infections [156]. Mesoporous bioactive glasses focused the attention of many scientific groups as they found applications in many fields of biomedicine, e.g., as delivery carriers, bioactive fillers and injectable biomaterials. Mesoporous glasses are osteoinductive and they exhibit superior mineralization capability. They were shown to form strong interfacial bonding with both soft and hard tissues. Moreover, similar to other mesoporous matrices, they possess large specific surface area, high pore volume and pore diameter. Depending on their composition and morphology these materials can enhance vascularization and wound healing. It was demonstrated that cerium-incorporated mesoporous glass nanoparticles revealed strong anti-inflammatory activity on lipopolysaccharide-induced RAW 264.7 macrophage cells. Moreover, the antibacterial properties of the material against Staphylococcus aureus and Escherichia coli were demonstrated [156].
Other therapeutic achievements resulting from the usage of mesoporous material-based DDS are briefly described in Table 5.

Table 5.
Therapeutic achievements resulting from the usage of mesoporous materials-based drug delivery systems (DDSs).
5. Conclusions and Perspectives
This review describes the most recent achievements in using mesoporous materials as the elements of sophisticated drug delivery systems (DDSs). During the past two decades, increasing progress has been made in the synthesis and functionalization of mesoporous matrices to be used as carriers for anti-inflammatory therapeutic agents. The practical utilization of knowledge from the field of material science to biomedical applications offer the promising possibilities for more effective treatment of pain and inflammation-related diseases. Unique properties of mesoporous materials including their large specific surface area, large pore volumes and pore diameters, tunability of the pore sizes, ease of chemical synthesis, possibility of surface functionalization and biocompatibility make these structures very interesting candidates as drug carriers. It is worth to be mentioned that the main advantage of mesoporous matrices is the possibility of their usage to merge different materials and thus to provide various functionalities to the designed system. Mesoporous matrices can act as a basis for the preparation of multifunctional tools for biomedical applications such as promising drug carriers. The outstanding pharmaceutical features resulting from the usage of mesoporous matrices as the carriers of anti-inflammatory agents is the possibility of increasing the dissolution kinetics of drugs that are usually characterized by low water solubility. Moreover, the proper surface functionalization makes mesoporous matrices promising carriers to effectively transport and site-specifically deliver drugs while also minimizing undesired side effects. Mesoporous matrices are superior candidates, especially for the construction of stimuli-responsive drug carriers. In these systems, there is a possibility to control the kinetics of drug release through external stimuli. In this area, huge effort has been made by scientists to develop various gatekeepers that could enable controlled exposure of entrapped drugs upon diverse stimuli. Overall, the research advancements in the use of mesoporous materials as elements of DDSs are exciting and hold great potential for future biomedical applications.
Although mesoporous material-based drug delivery systems are promising tools to be used in the therapy of inflammation, several critical issues regarding their safe use need to be addressed. First, the synthesis and functionalization steps during DDS manufacture have to be reproducible in order to provide valuable material for further in vitro and in vivo testing. Careful analyses of in vitro and in vivo toxicity regarding the chemical composition, size, shape, surface area, porosity, functionalization and charge of the adequate system are needed to facilitate understanding of its behavior in a biological environment. A comprehensive study of biocompatibility, biodegradability and biodistribution of the systems is needed. Although many in vitro and in vivo studies of DDS toxicities are performed, the toxicity studies in the human body are very limited. To summarize, before the introducing the final product to the market, its safety for the human body must be confirmed in detail which is time consuming, tedious and expensive. It seems that the toxicity aspect is the major barrier in the process of DDS commercialization. It should be kept in mind that all components used to fabricate the system have to be biocompatible. The main challenges related to the application of mesoporous material-based DDSs in biomedicine are summarized in Table 6.

Table 6.
Challenges related to the application of mesoporous material-based DDSs in biomedicine.
Author Contributions
Conceptualization, M.M. and M.G.-M.; methodology, M.M. and M.G.-M.; software, M.M. and M.G.-M.; formal analysis, M.M. and M.G.-M.; investigation, M.M. and M.G.-M.; resources, M.M. and M.G.-M.; data curation, M.M. and M.G.-M.; writing—original draft preparation, M.M. and M.G.-M.; writing—review and editing, M.M. and M.G.-M.; visualization, M.M. and M.G.-M.; supervision, M.M. and M.G.-M.; project administration, M.M. and M.G.-M.; funding acquisition, M.M. and M.G.-M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by POMERANIAN MEDICAL UNIVERSITY (WFB-405-01/S/21/2022 and WFB-406-01/S/21/2022).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Sing, K.S.W.; Everett, D.H.; Haul, R.A.W.; Moscou, L.; Pierotti, R.A.; Rouquérol, J.; Siemieniewska, T. Reporting physisorption data for gas/solid systems with special references to the determination of surface area and porosity. Pure Appl. Chem. 1985, 57, 603–619. [Google Scholar] [CrossRef]
- Perego, A.; Millini, R. Porous materials in catalysis: Challenges for mesoporous materials. Chem. Soc. Rev. 2013, 42, 3956–3976. [Google Scholar] [CrossRef] [PubMed]
- Niu, B.; Wang, X.; Wu, K.; He, X.; Zhang, R. Mesoporous titanium dioxide: Synthesis and applications in photocatalysis, energy and biology. Materials 2018, 11, 1910. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, X.; Yu, T.; Fan, X.; Zhang, H.; Li, Z.; Ye, J.; Zou, Z. Enhanced activity of mesoporous Nb2O5 for photocatalytic hydrogen production. Appl. Surf. Sci. 2007, 253, 5800–8506. [Google Scholar] [CrossRef]
- Laskowski, Ł.; Laskowska, M.; Villa, N.; Schabikowski, M.; Walcarius, A. Mesoporous silica-based materials for electronics-oriented applications. Molecules 2019, 24, 2395. [Google Scholar] [CrossRef] [Green Version]
- Bach, U.; Lupo, D.; Comte, P.; Moser, J.E.; Weissörtel, F.; Salbeck, J.; Spreitzer, H.; Grätzel, M. Solid-state dye-sensitized mesoporous TiO2 solar cells with high photon-to-electron conversion efficiencies. Nature 1998, 395, 583–585. [Google Scholar] [CrossRef]
- Eftekhari, A. Ordered mesoporous materials for lithium-ion batteries. Mesoporous Microporous Mater. 2017, 243, 355–369. [Google Scholar] [CrossRef]
- Yang, P.; Gai, S.; Lin, J. Functionalized mesoporous silica materials for controlled drug delivery. Chem. Soc. Rev. 2012, 41, 3679–3698. [Google Scholar] [CrossRef]
- Ispas, C.; Sokolov, I.; Andreescu, S. Enzyme-functionalized mesoporous silica for bioanalytical applications. Anal. Bioanal. Chem. 2009, 393, 543–554. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Lu, Y.; Jiang, C.; Zhu, Y.; Yang, X.; Hu, X.; Lin, Z.; Zhang, Y.; Peng, M.; Xia, H.; et al. Actively targeted deep tissue imaging and photothermal-chemo therapy of breast cancer by antibody-functionalized drug-loaded X-ray-responsive bismuth sulfide@mesoporous silica core-shell nanoparticles. Adv. Funct. Mater. 2018, 28, 1704623. [Google Scholar] [CrossRef]
- Liu, Z.; Li, M.; Yang, X.; Yin, M.; Ren, J.; Qu, X. The use of multifunctional magnetic mesoporous core/shell heterostructures in a biomolecule separation system. Biomaterials 2011, 32, 4683–4690. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Zhou, X.; He, C. Mesoporous silica nanoparticles for tissue-engineering applications. WIREs Nanomed. Nanobiotechnol. 2019, 11, e1573. [Google Scholar] [CrossRef] [PubMed]
- Vallet-Regí, M.; Rámila, A.; del Real, R.P.; Pérez-Pariente, J. A new property of MCM-41: Drug delivery system. Chem. Mater. 2001, 13, 308–311. [Google Scholar] [CrossRef]
- Atkinson, I.; Seciu-Grama, A.M.; Petrescu, S.; Culita, D.; Mocioiu, O.C.; Voicescu, M.; Mitran, R.-A.; Lincu, D.; Prelipcean, A.-M.; Craciumescu, O. Cerium-containing mesoporous bioactive glasses (MBGs)-derived scaffolds with drug delivery capability for potential tissue engineering applications. Pharmaceutics 2022, 14, 1169. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.C.; Nguyen, C.T.H.; Strounina, E.; Davis-Poynter, N.; Ross, B.P. Structure-activity relationships of GAG mimetic-functionalized mesoporous silica nanoparticles and evaluation of acyclovir-loaded antiviral nanoparticles with dual mechanisms of action. ACS Omega 2018, 3, 1689–1699. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.-N.; Zhang, C.-Q.; Wang, W.; Wang, P.C.; Zhou, J.-P.; Liang, X.-J. pH-Responsive mesoporous silica nanoparticles employed un controlled drug delivery systems for cancer treatment. Cancer Biol. Med. 2014, 11, 34–43. [Google Scholar]
- Moritz, M. Solvent optimization for niacinamide adsorption on organo-functionalized SBA-15 mesoporous silica. Appl. Surf. Sci. 2013, 283, 537–545. [Google Scholar] [CrossRef]
- Moritz, M.; Łaniecki, M. Modified SBA-15 as the carrier for metoprolol and papaverine: Adsorption and release study. J. Solid State Chem. 2011, 184, 1761–1767. [Google Scholar] [CrossRef]
- Jadhav, N.V.; Vavia, P.R. Dodecylamine template-based hexagonal mesoporous silica (HMS) as a carrier for improved oral delivery of fenofibrate. AAPS PharmSciTech 2017, 18, 2764–2773. [Google Scholar] [CrossRef]
- Lengert, E.; Verkhovskii, R.; Yurasov, N.; Genina, E.; Svenskaya, Y. Mesoporous carriers for transdermal delivery of antifungal drug. Mater. Lett. 2019, 248, 211–213. [Google Scholar] [CrossRef]
- Moritz, M.; Geszke-Moritz, M. Mesoporous silica materials with different structures as the carriers for antimicrobial agent. Modeling of chlorhexidine adsorption and release. Appl. Surf. Sci. 2015, 356, 1327–1340. [Google Scholar] [CrossRef]
- Pajzderska, A.; Drużbicki, K.; Bilski, P.; Jenczyk, J.; Jarek, M.; Mielcarek, J.; Wąskicki, J. Environmental effects of the molecular mobility of ranitidine hydrochloride: Crystalline state versus drug loaded into the silica matrix. J. Phys. Chem. C 2019, 123, 18364–18375. [Google Scholar] [CrossRef]
- López-Noriega, A.; Arcos, D.; Vallet-Regí, M. Functionalizing mesoporous bioglasses for long-term anti-osteoporotic drug delivery. Chem. Eur. J. 2010, 16, 10879–10886. [Google Scholar] [CrossRef] [PubMed]
- Moritz, M.; Geszke-Moritz, M. Sulfonic acid derivative-modified SBA-15, PHTS and MCM-41 mesoporous silicas as carriers for a new antiplatelet drug: Ticagrelor adsorption and release studies. Materials 2020, 13, 2913. [Google Scholar] [CrossRef]
- Han, C.; Huang, H.; Dong, Y.; Sui, X.; Jian, B.; Zhu, W. A comparative study of the use of mesoporous carbon and mesoporous silica as drug carriers for oral delivery of the water-insoluble drug carvedilol. Molecules 2019, 24, 1770. [Google Scholar] [CrossRef] [Green Version]
- Geszke-Moritz, M.; Moritz, M. Modeling of boldine alkaloid adsorption onto pure and propyl-sulfonic acid-modified mesoporous silicas. A comparative study. Mater. Sci. Eng. C 2016, 69, 815–830. [Google Scholar] [CrossRef]
- Žid, L.; Zeleňák, V.; Almáši, M.; Zeleňáková, A.; Szücsová, J.; Bednarčik, J.; Šuleková, M.; Hudák, A.; Váhovská, L. Mesoporous silica as a drug delivery system for naproxen: Influence of surface functionalization. Molecules 2020, 25, 4722. [Google Scholar] [CrossRef]
- Gisbert-Garzarán, M.; Manzano, M.; Vallet-Regí, M. pH-Responsive mesoporous silica and carbon nanoparticles for drug delivery. Bioengineering 2017, 41, 3. [Google Scholar] [CrossRef] [Green Version]
- Yao, P.; Zou, A.; Tian, Z.; Meng, W.; Fang, X.; Wu, T.; Cheng, J. Construction and characterization of a temperature-responsive nanocarrier for imidacloprid based on mesoporous silica nanoparticles. Colloids Surf. B 2021, 198, 111464. [Google Scholar] [CrossRef]
- Almáši, M.; Matiašová, A.A.; Šuleková, M.; Beňová, E.; Ševc, J.; Váhovská, L.; Lisnichuk, M.; Girman, V.; Zeleňáková, A.; Hudák, A.; et al. In vivo study of light-driven naproxen release from gated mesoporous silica drug delivery system. Sci. Rep. 2021, 11, 20191. [Google Scholar] [CrossRef]
- Paris, J.L.; Cabañas, V.; Manzano, M.; Vallet-Regí, M. Polymer-grafted mesoporous silica nanoparticles as ultrasound-responsive drug carriers. ACS Nano 2015, 9, 11023–11033. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, Z.; Wu, C.; Zhao, Y.; Hao, Y.; Liu, Y.; Zhao, W. Development of an oral push-pull osmotic pump of fenofibrate-loaded mesoporous silica nanoparticles. Int. J. Nanomed. 2015, 10, 1691–1701. [Google Scholar] [CrossRef] [PubMed]
- Wu, E.C.; Park, J.-H.; Park, J.; Segal, E.; Cunin, F.; Sailor, M.J. Oxidation-triggered release of fluorescent molecules or drugs from mesoporous Si microparticles. ACS Nano 2008, 11, 2401–2409. [Google Scholar] [CrossRef]
- Cheng, Y.-J.; Luo, G.-F.; Zhu, J.-Y.; Xu, X.-D.; Zeng, X.; Cheng, D.-B.; Li, Y.-M.; Wu, Y.; Zhang, X.-Z.; Zhuo, R.-X.; et al. Enzyme-induced and tumor-targeted drug delivery system based on multifunctional mesoporous silica nanoparticles. ACS Appl. Mater. Interfaces 2015, 7, 9078–9087. [Google Scholar] [CrossRef] [PubMed]
- Moritz, M.; Geszke-Moritz, M. Mesoporous materials as multifunctional tools in biosciences: Principles and applications. Mater. Sci. Eng. C 2015, 49, 114–151. [Google Scholar] [CrossRef]
- Wu, J.; Jiang, W.; Shen, Y.; Jiang, W.; Tian, R. Synthesis and characterization of mesoporous magnetic nanocomposites wrapped with chitosan gatekeepers for pH-sensitive controlled release of doxorubicin. Mater. Sci. Eng. C 2017, 70, 132–140. [Google Scholar] [CrossRef] [Green Version]
- Cho, Y.; Shi, R.; Ivanisevic, A.; Borgens, R.B. A mesoporous silica nanosphere-based drug delivery system using an electrically conducting polymer. Nanotechnology 2009, 20, 275102. [Google Scholar] [CrossRef] [PubMed]
- Palantavida, S.; Tang, R.; Sudlow, G.P.; Akers, W.J.; Schilefu, S.; Sokolov, I. Ultrabright NIR fluorescent mesoporous silica nanoparticles. J. Mater. Chem. B 2014, 2, 3107–3114. [Google Scholar] [CrossRef]
- Kresge, C.T.; Leonowicz, M.E.; Roth, W.J.; Vartuli, J.C.; Beck, J.S. Ordered mesoporous molecular sieves synthesized by a liquid-crystal template mechanism. Nature 1992, 359, 710–712. [Google Scholar] [CrossRef]
- Atiyah, N.A.; Albayati, T.M.; Atiya, M.A. Functionalization of mesoporous MCM-41 for the delivery of curcumin as an anti-inflammatory therapy. Adv. Powder Technol. 2022, 33, 103417. [Google Scholar] [CrossRef]
- Strømme, M.; Brohede, U.; Atluri, R.; Garcia-Bennett, A.E. Mesoporous silica-based nanomaterials for drug delivery: Evaluation of structuraal properties associated with release rate. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnology 2009, 1, 140–148. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, C.A.; Ahern, R.J.; Dontireddy, R.; Ryan, K.B.; Crean, A.M. Mesoporous silica formulation strategies for drug dissolution enhancement: A review. Expert Opin. Drug Deliv. 2016, 13, 93–108. [Google Scholar] [CrossRef] [PubMed]
- Bang, A.; Sadekar, A.G.; Buback, C.; Curtin, B.; Acar, S.; Kolasinac, D.; Yin, W.; Rubenstein, D.A.; Lu, H.; Leventis, N.; et al. Evaluation of dysprosia aerogels as drug delivery systems: A comparative study with random and ordered mesoporous silicas. ACS Appl. Mater. Interfaces 2014, 6, 4891–4902. [Google Scholar] [CrossRef]
- Maleki, A.; Hamidi, M. Dissolution enhancement of a model poorly water-soluble drug, atorvastatin, with ordered mesoporous silica: Comparison of MSF with SBA-15 as drug carriers. Expert Opin. Drug Deliv. 2016, 13, 171–181. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Zhi, Z.; Zhao, Q.; Wu, C.; Zhao, P.; Jiang, H.; Jiang, T.; Wang, S. 3D cubic mesoporous silica microsphere as a carrier for poorly soluble drug carvedilol. Microporous Mesoporous Mater. 2012, 147, 94–101. [Google Scholar] [CrossRef]
- Ambrogi, V.; Perioli, L.; Pagano, C.; Latterini, L.; Marmottini, F.; Ricci, M.; Carlo, R. MCM-41 for furosemide dissolution improvement. Microporous Mesoporous Mater. 2012, 147, 343–349. [Google Scholar] [CrossRef]
- Ambrogi, V.; Perioli, L.; Pagano, C.; Marmottini, F.; Ricci, M.; Sagnella, A.; Rossi, C. Use of SBA-15 for furosemide oral delivery enhancement. Eur. J. Pharm. Sci. 2012, 46, 43–48. [Google Scholar] [CrossRef]
- Heikkilä, T.; Salonen, J.; Tuura, J.; Kumar, N.; Salmi, T.; Murzin, D.Y.; Hamdy, M.S.; Mul, G.; Laitinen, L.; Kaukonen, A.M.; et al. Evalauation of mesoporous TCPSi, MCM-41, SBA-15, and TUD-1 materials as API carriers for oral drug delivery. Drug Deliv. 2007, 14, 337–347. [Google Scholar] [CrossRef]
- Manzano, M.; Aina, V.; Arean, C.O.; Balas, F.; Cauda, V.; Colilla, M.; Delgado, M.R.; Vallet-Regí, M. Studies on MCM-41 mesoporous silica for drug delivery: Effect of particle morphology and amine functionalization. Chem. Eng. J. 2008, 137, 30–37. [Google Scholar] [CrossRef]
- Horcajada, P.; Ramilla, A.; Perez-Pariente, J.; Vallet-Regí, M. Influence of pore size of MCM-41 matrices on drug delivery rate. Microporous Mesoporous Mater. 2004, 68, 105–109. [Google Scholar] [CrossRef]
- Izquierdo-Barba, I.; Sousa, E.; Doadrio, J.C.; Doadrio, A.L.; Perez-Pariente, J.; Martínez, A.; Babonneau, F.; Vallet-Regí, M. Influence of mesoporous structure type on the controlled delivery of drugs: Release of ibuprofen from MCM-48, SBA-15 and functionalized SBA-15. J. Sol-Gel Sci. Technol. 2009, 50, 421–429. [Google Scholar] [CrossRef]
- Andersson, J.; Rosenholm, J.; Areva, S.; Linden, M. Influences of material characteristics on ibuprofen drug loading and release profiles from ordered micro- and mesoporous silica matrices. Chem. Mater. 2004, 16, 4160–4167. [Google Scholar] [CrossRef]
- Qu, F.; Zhu, G.; Lin, H.; Zhang, W.; Sun, J.; Li, S.; Qiu, S. A controlled release of ibuprofen by systematically tailoring the morphology of mesoporous silica materials. J. Solid State Chem. 2006, 179, 2027–2035. [Google Scholar] [CrossRef]
- Shen, S.-C.; Ng, W.K.; Chia, L.; Hu, J.; Tan, R.B.H. Physical state and dissolution of ibuprofen formulated by co-spray drying with mesoporous silica: Effect of pore and particle size. Int. J. Pharm. 2011, 410, 188–195. [Google Scholar] [CrossRef] [PubMed]
- Mellaerts, L.; Aerts, C.A.; Van Humbeeck, J.; Augustijns, P.; Van den Mooter, G.; Martens, J.A. Enhanced release of itraconazole from ordered mesoporous SBA-15 silica materials. Chem. Commun. 2007, 13, 1375–1377. [Google Scholar] [CrossRef]
- Tao, Z. Mesoporous silica-based nanodevices for biological applications. RSC Adv. 2014, 4, 18961–18980. [Google Scholar] [CrossRef]
- Izquierdo-Barba, I.; Martínez, A.; Doadrio, A.L.; Perez-Pariente, J.; Vallet-Regí, M. Release evaluation of drugs from ordered three-dimensional silica structures. Eur. J. Pharm. Sci. 2005, 26, 365–373. [Google Scholar] [CrossRef]
- Xu, W.; Riikonen, J.; Lechto, V.-P. Mesoporous systems for poorly soluble drugs. Int. J. Pharm. 2013, 453, 181–197. [Google Scholar] [CrossRef]
- Shen, S.-C.; Ng, W.K.; Chia, L.; Dong, Y.-C.; Tan, R.B.H. Stabilized amorphous state of ibuprofen byco-spray drying with mesoporous SBA-15 to enhance dissolution properties. J. Pharm. Sci. 2010, 99, 1997–2007. [Google Scholar] [CrossRef]
- Mellaerts, R.; Houthoofd, K.; Elen, K.; Chen, H.; Van Speybroeck, M.; Van Humbeeck, J.; Augustijns, P.; Mullens, J.; Van den Mooter, G.; Martens, J.A. Aging behavior of pharmaceutical formulations of itraconazole on SBA-15 ordered mesoporous silica carrier material. Microporous Mesoporous Mater. 2010, 130, 154–161. [Google Scholar] [CrossRef]
- Mellaerts, R.; Jammaer, A.A.G.; Speybroeck, M.V.; Chen, H.; Humbeeck, J.V.; Augustijns, P.; Van den Mooter, G.; Martens, J.A. Physical state of poorly water soluble therapeutic molecules loaded into SBA-15 ordered mesoporous silica carriers: A case study with itraconazole and ibuprofen. Langmuir 2008, 24, 8651–8659. [Google Scholar] [CrossRef] [PubMed]
- Miura, H.; Kanebako, M.; Shirai, H.; Nakao, H.; Inagi, T.; Terada, K. Stability of amorphous drug, 2-benzyl-5-(4-chlorophenyl)-6-[4-methylthio)phenyl]-2H-pyridazin-3-one, in silica mesopores and measurement of its molecular mobility by solid-state 13C NMR spectroscopy. Int. J. Pharm. 2011, 410, 61–67. [Google Scholar] [CrossRef]
- Sliwinska-Bartkowiak, M.; Dudziak, G.; Gras, R.; Sikorski, R.; Radhakrishnan, R.; Gubbins, K.E. Freezing behavior in porous glasses and MCM-41. Colloids Surf. A 2001, 187, 523–529. [Google Scholar] [CrossRef]
- Rengarajan, G.; Enke, D.; Steinhart, M.; Beiner, M. Stabilization of the amorphous state of pharmaceuticals in nanopores. J. Mater. Chem. 2008, 18, 2537–2539. [Google Scholar] [CrossRef]
- Qi, L.; Ma, J.; Cheng, H.; Zhao, Z. Micrometer-sized mesoporous silica spheres grown under static conditions. Chem. Mater. 1998, 10, 1623–1626. [Google Scholar] [CrossRef]
- Slowing, I.I.; Vivero-Escoto, J.L.; Wu, C.-W.; Lin, V.S.-Y. Mesoporous silica nanoparticles as controlled release drug delivery and gene transfection carriers. Adv. Drug Deliv. Rev. 2008, 60, 1278–1288. [Google Scholar] [CrossRef]
- Singh, A.; Worku, Z.A.; Van den Mooter, G. Oral formulation strategies to improve solubility of poorly water-soluble druga. Expert Opin. Drug Deliv. 2011, 8, 1361–1378. [Google Scholar] [CrossRef]
- Kumar, D.; Chirravuri, S.V.S.; Shastri, N.R. Impact of surface area of silica particles on dissolution rate and oral bioavailability of poorly water soluble drugs: A case study with aceclofenac. Int. J. Pharm. 2014, 461, 459–468. [Google Scholar] [CrossRef]
- Meynen, V.; Cool, P.; Vansant, E.F. Verified syntheses of mesoporous materials. Microporous Mesoporous Mater. 2009, 125, 170–223. [Google Scholar] [CrossRef]
- Schumacher, K.; Ravikovitch, P.I.; Chesne, A.D.; Neimark, A.V.; Unger, K.K. Characterization of MCM-48 materials. Langmuir 2000, 16, 4648–4654. [Google Scholar] [CrossRef]
- Zelenak, V.; Halamova, D.; Gaberova, L.; Bloch, E.; Llewellyn, P. Amine-modified SBA-12 mesoporous silica for carbon dioxide capture: Effect of amine basicity on sorption properties. Microporous Mesoporous Mater. 2008, 116, 358–364. [Google Scholar] [CrossRef]
- Moritz, M.; Łaniecki, M. SBA-15 mesoporous material modified with APTES as the carrier for 2-(3-benzoylphenyl)propionic acid. Appl. Surf. Sci. 2012, 258, 7523–7529. [Google Scholar] [CrossRef]
- Geszke-Moritz, M.; Moritz, M. APTES-modified mesoporous silicas as the carriers for poorly water-soluble drug. Modeling of diflunisal adsorption and release. Appl. Surf. Sci. 2016, 368, 348–359. [Google Scholar] [CrossRef]
- Moritz, M.; Geszke-Moritz, M. Amine-modified SBA-15 and MCF mesoporous molecular sieves as promising sorbents for natural antioxidant. Modeling of caffeic acid adsorption. Mater. Sci. Eng. C 2016, 61, 411–421. [Google Scholar] [CrossRef]
- Vallet-Regí, M.; Colilla, M.; Izquierdo-Barba, I.; Manzano, M. Mesoporous silica nanoparticles for drug delivery: Current insights. Molecules 2018, 23, 47. [Google Scholar] [CrossRef] [Green Version]
- Stephen, S.; Gorain, B.; Choudhury, H.; Chatterjee, B. Exploring the role of mesoporous silica nanoparticle in the development of novel drug delivery systems. Drug Deliv. Translat. Res. 2021, 12, 105–123. [Google Scholar] [CrossRef]
- Attia, M.S.; Hassaballah, M.Y.; Abdelqawy, M.A.; Emad-Eldin, M.; Farag, A.K.; Negida, A.; Ghaith, H.; Emam, S.E. An uptaded review of mesoporous carbon as a novel drug delivery system. Drug Dev. Ind. Pharm. 2021, 47, 1029–1037. [Google Scholar] [CrossRef]
- Iturrioz-Rodríguez, N.; Correa-Duarte, M.A.; Fanarraga, M.L. Controlled drug delivery systems for cancer based on mesoporous silica nanoparticles. Int. J. Nanomed. 2019, 14, 3389–3401. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Xhao, Q.; Han, N.; Bai, L.; Li, J.; Liu, J.; Che, E.; Hu, L.; Zhang, Q.; Jiang, T.; et al. Mesoporous silica nanoparticles in drug delivery and biomedical applications. Nanomed. Nanotechnol. Biol. Med. 2015, 11, 313–327. [Google Scholar] [CrossRef]
- Wen, J.; Yang, K.; Liu, F.; Li, H.; Xu, Y.; Sun, S. Diverse gatekeepers for mesoporous silica nanoparticle based drug delivery systems. Chem. Soc. Rev. 2017, 46, 6024. [Google Scholar] [CrossRef]
- Rosenholm, J.M.; Sahlgren, C.; Lindén, M. Towards multifunctional, targeted drug delivery systems using mesoporous silica nanoparticles—Opportunities & challenges. Nanoscale 2010, 2, 1870–1883. [Google Scholar] [PubMed]
- Vallet-Regí, M.; Balas, F.; Arcos, D. Mesoporous materials for drug delivery. Angew. Chem. Int. Ed. 2007, 46, 7548–7558. [Google Scholar] [CrossRef] [PubMed]
- Bharti, C.; Nagaich, U.; Pal, A.K.; Gulati, N. Mesoporous silica nanoparticles in target drug delivery system: A review. Int. J. Pharm. Investig. 2015, 5, 124–133. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, Q.; Lin, Y.; Han, N.; Li, X.; Geng, H.; Wang, X.; Cui, Y.; Wang, S. Mesoporous carbon nanomaterials in drug delivery and biomedical applications. Drug Deliv. 2017, 24, 94–107. [Google Scholar] [CrossRef]
- Trzeciak, K.; Chotera-Ouda, A.; Bak-Sypien, I.I.; Potrzebowski, M.J. Mesoporous silica particles as drug delivery systems—The state of the art in loading methods and the recent progress in analytical techniques for monitoring these processes. Pharmaceutics 2021, 13, 950. [Google Scholar] [CrossRef]
- Hadisoewignyo, L.; Hartono, S.B.; Kresnamurti, A.; Soeliono, I.; Nataline, Y.; Prakoso, G.A.; Aulia, D.A.R.E. Evaluation of anti-inflammatory activity and biocompatibility of curcumin loaded mesoporous silica nanoparticles as an oral drug delivery system. Adv. Nat. Sci. Nanosci. Nanotechnol. 2018, 9, 035007. [Google Scholar] [CrossRef]
- Gou, K.; Wang, Y.; Guo, X.; Wang, Y.; Bian, Y.; Zhao, H.; Guo, Y.; Pang, Y.; Xie, L.; Li, S.; et al. Carboxyl-functionalized mesoporous silica nanoparticles for the controlled delivery of poorly water-soluble non-steroidal anti-inflammatory drugs. Acta Biomater. 2021, 134, 576–592. [Google Scholar] [CrossRef]
- Rai, S.K.; Shakambari, G.; Rajan, M.; Praphakar, R.A.; Ashokkumar, B.; Pugazhendhi, A.; Varalakshmi, P. Mesoporous nanoparticles for the delivery of (9S,E)-8-ethyl-9-methylnonadec-6-en-3-one (EME): A study of anti-inflammatory and tumor suppressing potential in RAW 264.7, HeLa and HepG2 cell lines. Process Biochem. 2021, 111, 1–11. [Google Scholar] [CrossRef]
- Crusz, S.M.; Balkwill, F.R. Inflammation and cancer: Advances and new agents. Nat. Rev. Clin. Oncol. 2015, 12, 584–596. [Google Scholar] [CrossRef]
- Świeboda, P.; Filip, R.; Prystupa, A.; Drozd, M. Assessment of pain: Types, mechanisms and treatment. Ann. Agric. Environ. Med. 2013, 1, 2–7. [Google Scholar]
- Honore, P.; Rogers, S.D.; Schwei, M.J.; Salak-Johnson, J.L.; Luger, N.M.; Sabino, M.C.; Clohisy, D.R.; Mantyh, P.W. Murine models of inflammatory, neuropathic and cancer pain each generates a unique set of neurochemical changes in the spinal cord and sensory neurons. Neuroscience 2000, 98, 585–598. [Google Scholar] [CrossRef]
- Caumo, W.; Deitos, A.; Carvahlo, S.; Leite, J.; Carvahlo, F.; Dussán-Sarria, J.A.; Tarrago, M.G.L.; Souza, A.; Torres, I.L.S.; Fregni, F. Motor cortex excitability and BDNF levels in chronic musculoskeletal pain according to structural pathology. Front. Hum. Neurosci. 2016, 10, 357. [Google Scholar] [CrossRef] [PubMed]
- Lerman, S.F.; Rudich, Z.; Brill, S.; Shalev, H.; Shahar, G. Longitudinal associations between depression, anxiety, pain, and pain-related disability in chronic pain patients. Psychosom. Med. 2015, 77, 333–341. [Google Scholar] [CrossRef] [PubMed]
- Minami, K.; Uezono, Y. The recent progress in research on effects of anesthetics and analgesics on G protein-coupled receptors. J. Anesth. 2013, 27, 284–292. [Google Scholar] [CrossRef]
- Schug, S.A.; Garrett, W.R.; Gillespie, G. Opioid and non-opioid analgesics. Best Pract. Res. Clin. Anaesthesiol. 2003, 17, 91–110. [Google Scholar] [CrossRef]
- Berde, A.; Nurko, S. Opioid side effects—Mechanism-based therapy. N. Eng. J. Med. 2008, 358, 2400–2402. [Google Scholar] [CrossRef]
- Hser, Y.-I.; Evans, E.; Grella, C.; Ling, W.; Anglin, D. Long-term course of opioid addiction. Harv. Rev. Psychiatry 2015, 23, 76–89. [Google Scholar] [CrossRef]
- Hebbes, C.; Lambert, D. Non-opioid analgesics. Anaesth. Intensive Care Med. 2011, 12, 69–72. [Google Scholar] [CrossRef]
- Rainsford, K.D. Ibuprofen: Pharmacology, efficacy and safety. Inflammopharmacology 2009, 17, 275–342. [Google Scholar] [CrossRef]
- Fadeyi, O.O.; Obafemi, C.A.; Adewunmi, C.O.; Iwalewa, E.O. Antipyretic, analgesic, anti-inflammatory and cytotoxic effects of four derivatives of salicylic acid and anthranilic acid in mice and rats. Afr. J. Biotechnol. 2004, 3, 426–431. [Google Scholar]
- Xu, S.; Rouzer, C.A.; Marnett, L.J. Oxicams, a class of nonsteroidal anti-inflammatory drugs and beyond. IUBMB Life 2014, 66, 803–811. [Google Scholar] [CrossRef] [PubMed]
- Grösch, S.; Niederberger, E.; Geisslinger, G. Investigational drugs targeting the prostaglandin E2 signaling pathway for the treaatment of inflammatory pain. Expert Opin. Investig. Drugs 2017, 26, 51–61. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.-M.; An, J. Cytokines, inflammation and pain. Int. Anesthesiol. Clin. 2007, 45, 27–37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Williams, T.J.; Morley, J. Prostaglandins as potentiators of increased vascular permeability in inflammation. Nature 1973, 246, 215–217. [Google Scholar] [CrossRef]
- Hawkey, C.J. COX-2 inhibitors. Lancet 1999, 353, 307–314. [Google Scholar] [CrossRef]
- Rainsford, K.D. Profile and mechanisms of gastrointestinal and other side effects of nonsteroidal anti-inflammatory drugs (NSAIDs). Am. J. Med. 1999, 107, 27–35. [Google Scholar] [CrossRef]
- Lochs, H.; Mayer, M.; Fleig, W.E.; Mortensen, P.B.; Bauer, P.; Genser, D.; Petritsch, W.; Raithel, M.; Hoffmann, R.; Gross, V.; et al. Prophylaxis of postoperative relapse in Crohn’s disease with mesalamine: European Cooperative Crohn’s Disease Study VI. Gastroenterology 2000, 118, 264–273. [Google Scholar] [CrossRef]
- Amiry, F.; Sazegar, M.R.; Mahmoudi, A. Smart polymeric nanocomposite based on protonated aluminosilicate curcumin, and chitosan for mesalamine drug delivery as an anti-inflammatory nanocarrier. Microporous Mesoporous Mater. 2022, 330, 111533. [Google Scholar] [CrossRef]
- Chang, J.-H.; Gabison, E.E.; Kato, T.; Azar, D.T. Corneal neovascularization. Curr. Opin. Ophthalmol. 2001, 12, 242–249. [Google Scholar] [CrossRef] [Green Version]
- Lyu, N.; Zhao, Y.; Xiang, J.; Fan, X.; Huang, C.; Sun, X.; Xu, J.; Xu, Z.P.; Sun, J. Inhibiting corneal neovascularization by sustainably releasing anti-VEGF and anti-inflammation drugs from silica-thermogel nanohybrids. Mater. Sci. Eng. C 2021, 128, 112274. [Google Scholar] [CrossRef]
- Siemerink, M.J.; Augustin, A.J.; Schlingemann, R.O. Mechanisms of ocular angiogenesis and its molecular mediators. Anti-VEGF 2010, 46, 4–20. [Google Scholar]
- Suleyman, H.; Cadirci, E.; Albayrak, A.; Halici, Z. Nimesulide is a selective COX-2 inhibitory, atypical non-steroidal anti-inflammatory drug. Curr. Med. Chem. 2008, 15, 278–283. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Yang, K.G.B.; Wang, Y.; Pu, X.; Li, S.; Li, H. Enlarged pore size chiral mesoporous silica nanoparticles loaded poorly water-soluble drug perform superior delivery effect. Molecules 2019, 24, 3552. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huskisson, E.C. Nimesulide, a balanced drug for the treatment of osteoarthritis. Clin. Exp. Rheumatol. 2001, 19, S21–S25. [Google Scholar] [PubMed]
- Wu, L.; Gou, K.; Guo, X.; Guo, Y.; Chen, M.; Hou, J.; Li, S.; Li, H. Dual response to pH and chiral microenvironments for the release of a flurbiprofen-loaded chiral self-assembled mesoporous silica drug delivery system. Colloids Surf. B 2021, 199, 111501. [Google Scholar] [CrossRef]
- Tokunaga, M.; Ohuchi, K.; Yoshizawa, S.; Tsurufuji, S.; Rikimaru, A.; Wakamatsu, E. Change of prostaglandin E level in joint fluids after treatment with flurbiprofen in patients with rheumatoid arthritis and osteoarthritis. Ann. Rheum. Dis. 1981, 40, 462–465. [Google Scholar] [CrossRef] [Green Version]
- Brogden, R.N.; Heel, W.C.; Speight, T.M.; Avery, G.S. Flurbiprofen: A review of its pharmacological properties and therapeutic use in rheumatic diseases. Drugs 1979, 18, 417–438. [Google Scholar] [CrossRef]
- Fietta, P.; Fietta, P.; Delsante, G. Central nervous system effects of natural and synthetic glucocorticoids. Psychiatry Clin. Neurosci. 2009, 63, 613–622. [Google Scholar] [CrossRef]
- Stahn, C.; Buttgereit, F. Genomic and nongenomic effects of glucocorticosteroids. Nat. Clin. Pract. Rheumatol. 2008, 4, 525–533. [Google Scholar] [CrossRef]
- Vegiopoulos, A.; Herzig, S. Glucocorticosterids, metabolism and metabolic diseases. Mol. Cell. Endocrinol. 2007, 275, 43–61. [Google Scholar] [CrossRef] [Green Version]
- Cain, D.; Cidlowski, J. Immune regulation by glucocorticoids. Nat. Rev. Immunol. 2017, 17, 233–247. [Google Scholar] [CrossRef]
- Dygai, A.M.; Shakhov, V.P.; Goldberg, E.D. Role of glucocorticoids in the regulation of bone marrow hemopoiesis in stress reaction. Biomed. Pharmacother. 1991, 45, 9–14. [Google Scholar] [CrossRef]
- Stahn, C.; Löwenberg, M.; Hommes, D.W.; Buttgereit, F. Molecular mechanisms of glucocorticoid action and selective glucocorticoid receptor agonists. Mol. Cell. Endocrinol. 2007, 275, 71–78. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Michels, A.; Michels, N. Addison Disease: Early detection and treatment principles. Am. Fam. Physician 2014, 89, 563–568. [Google Scholar]
- Paragliola, R.M.; Papi, G.; Pontecorvi, A.; Corsello, S.M. Treatment with synthetic glucocorticosteroids and the hypothalamus-pituitary-adrenal axis. Int. J. Mol. Sci. 2017, 18, 2201. [Google Scholar] [CrossRef]
- Broersen, L.H.A.; Pereira, A.M.; Jørgensen, J.O.L.; Dekkers, O.M. Adrenal insufficiency in corticosteroids use: Systematic review and meta-analysis. J. Clin. Endocrinol. Metab. 2015, 100, 2171–2180. [Google Scholar] [CrossRef] [PubMed]
- Amr, N.H.; Mahmoud, R.A.A.; Youssef, O.; Toaima, N.N.; Elsedfy, H. Effect of long-term glucocorticoid therapy on cardiac functions in children with congenital adrenal hyperplasia. Clin. Endocrinol. 2021, 94, 210–218. [Google Scholar] [CrossRef]
- Shibli-Rahhal, A.; Beek, M.V.; Schlechte, J.A. Cushing’s syndrome. Clin. Dermatol. 2006, 24, 260–265. [Google Scholar] [CrossRef]
- Barnes, P.J. Glucocorticosteroids: Current and future directions. Brit. J. Pharmacol. 2011, 163, 29–43. [Google Scholar] [CrossRef] [Green Version]
- Gold, R.; Buttgereit, F.; Toyka, K.V. Mechanism of action of glucocorticosteoid hormones: Possible implications for therapy of neuroimmunological disorders. J. Neuroimmunol. 2001, 117, 1–8. [Google Scholar] [CrossRef]
- Shimada, T.; Hiwatashi, N.; Yamazaki, H. Relationship between glucocorticoid receptor and response to glucocorticoid therapy in ulcerative colitis. Dis. Colon Rectum 1997, 40, S54–S58. [Google Scholar] [CrossRef]
- Gao, R.; Li, Y.; Cao, Y.; Zheng, R.; Tang, L.; Yang, J.; Lu, X. Glucocorticoid versus traditional therapy for hepatitis B virus-related acute-on-chronic liver failure. A systematic review and meta-analysis. Medicine 2020, 99, e20604. [Google Scholar] [CrossRef]
- Niewoehner, D.E.; Erbland, M.L.; Deupree, R.H.; Collins, D.; Gross, N.J.; Light, R.W.; Anderson, P.; Morgan, N.A. Effect of systemic glucocorticoids on exacerbations of chronic obstructive pulmonary disease. N. Eng. J. Med. 1999, 340, 1941–1947. [Google Scholar] [CrossRef]
- Kaneda, T.; Iwai, S.; Suematsu, T.; Yamamoto, R.; Takata, M.; Higashikata, T.; Ino, H.; Tsujibata, A. Acute necrotizing eosinophilic myocarditis complicated by complete atriventricular block promptly responded to glucocorticoid therapy. J. Cardiol. Cases 2017, 16, 5–8. [Google Scholar] [CrossRef] [Green Version]
- Ahluwalia, A. Topical glucocorticoids and the skin-mechanisms of action: An uptade. Mediat. Inflamm. 1998, 7, 183–193. [Google Scholar] [CrossRef]
- Hua, C.; Buttgereit, F.; Combe, B. Glucocorticosteroids in rheumatoid arthritis: Current status and future studies. RMD Open 2020, 6, e000536. [Google Scholar] [CrossRef] [Green Version]
- Sadowska, A.M.; Klebe, B.; Germonpré, P.; De Backer, W.A. Glucocorticosteroids as antioxidants in treatment of asthma and COPD: New application for an old medication? Steroids 2007, 72, 1–6. [Google Scholar] [CrossRef]
- Shapiro, A.G. Corticosteroids in the treatment of allergic disease: Principles and practise. Pediatric Clin. North Am. 1983, 30, 955–971. [Google Scholar] [CrossRef]
- Gaynon, P.S.; Lustig, R.H. The use of glucocorticoids in accute lymphoblastic leukemia of childhood. Molecular, cellular, and clinical considerations. J. Pediatric Hematol. Oncol. 1995, 17, 1–12. [Google Scholar] [CrossRef]
- Weller, M. Glucocorticoid treatment of primary CNS lymphoma. J. Neuro-Oncol. 1999, 43, 237–239. [Google Scholar] [CrossRef]
- Fricke, L.; Klüter, H.; Feddersen, A.; Doehn, C.; Steinhoff, J.; Hoyer, J.; Sack, K. Preoperative application of glucocorticosteroids efficaciously reduces the primary immunological response in kidney transplantation. Clin. Transplant. 1996, 10, 432–436. [Google Scholar] [PubMed]
- Choo, K.J.L.; Simons, F.E.R.; Sheikh, A. Glucocorticoids for the treatment of anaphylaxis (Review). Evid.-Based Child Health 2013, 8, 1276–1294. [Google Scholar] [CrossRef] [PubMed]
- Kaal, E.C.A.; Vecht, C.J. The management of brain endema in brain tumors. Curr. Opin. Oncol. 2004, 16, 593–600. [Google Scholar] [CrossRef] [PubMed]
- Gulin-Sarfraz, T.; Jonasson, S.; Wigenstam, E.; Haartman, E.v.; Bucht, A.; Rosenholm, J.M. Feasibility study of mesoporous silica particles for pulmonary drug delivery: Therapeutic treatment with dexamethasone in a mouse model of airway inflammation. Pharmaceutics 2019, 11, 149. [Google Scholar] [CrossRef] [Green Version]
- García-Fernández, A.; Sancho, M.; Bisbal, V.; Amorós, P.; Marcos, M.D.; Orzáez, M.; Sancenón, F.; Martínez-Máñez, R. Targeted-lung delivery of dexamethasone using gated mesoporous silica nanoparticles. A new therapeutic approach for acute lung injury treatment. J. Control. Release 2021, 337, 14–26. [Google Scholar] [CrossRef]
- Hashemikia, S.; Hemmatinejad, N.; Ahmadi, E.; Montazer, M. Antibacterial and anti-inflammatory drug delivery properties on cotton fabric using betamethasone-loaded mesoporous silica particles stabilized with chitosan and silicone softener. Drug Deliv. 2015, 23, 2946–2955. [Google Scholar] [CrossRef] [Green Version]
- Dibas, A.; Yorio, T. Glucocorticoid therapy and ocular hypertension. Eur. J. Pharmacol. 2016, 787, 57–71. [Google Scholar] [CrossRef] [Green Version]
- Vandewalle, J.; Luypaert, A.; De Bosscher, K.; Libert, C. Therapeutic mechanisms of glucocorticoids. Trends Endocrinol. Metab. 2018, 29, 42–54. [Google Scholar] [CrossRef]
- Leung, K.W.; Wong, A.S.-T. Pharmacology of ginsenosides: A literature review. Chin. Med. 2010, 5, 20. [Google Scholar] [CrossRef] [Green Version]
- Chen, C.-Y.; Shie, M.-Y.; Lee, A.-X.; Chou, Y.-T.; Chiang, C.; Lin, C.-P. 3D-Printed ginsenoside Rb1-loaded mesoporous calcium silicate/calcium sulfate scaffolds for inflammation inhibition and bone regeneration. Biomedicines 2021, 9, 907. [Google Scholar] [CrossRef]
- He, M.; Qin, Z.; Liang, X.; He, X.; Zhu, B.; Lu, Z.; Wei, Q.; Zheng, L. A pH-responsive mesoporous silica nanoparticles-based drug delivery system with controlled release of andrographolide for OA treatment. Regener. Biomater. 2021, 8, rbab020. [Google Scholar] [CrossRef] [PubMed]
- Mussard, E.; Cesaro, A.; Lespessailles, E.; Legrain, B.; Berteina-Raboin, S.; Toumi, H. Andrographolide, a natural antioxidant: An uptade. Antioxidants 2019, 8, 571. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dadej, A.; Woźniak-Braszak, A.; Bilski, P.; Piotrowska-Kempisty, H.; Józkowiak, M.; Geszke-Moritz, M.; Moritz, M.; Dadej, D.; Jelińska, A. Modification of the release of poorly soluble sulindac with the APTES-modified SBA-15 mesoporous silica. Pharmaceutics 2021, 13, 1693. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhang, S.; Zhang, X.; Hou, Y.; Meng, X.; Li, G.; Xu, F.; Teng, L.; Qi, Y.; Sun, F.; et al. Folate receptor-targeting semiconducting polymer dots hybrid mesoporous silica nanoparticles against rheumatoid arthritis through synergistic photothermal therapy, photodynamic therapy, and chemotherapy. Int. J. Pharm. 2021, 607, 120947. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Zheng, N.; Chen, L.; Xie, L.; Cui, M.; Li, S.; Xu, L. Effect of shape on mesoporous silica nanoparticles for oral delivery of indomethacin. Pharmaceutics 2019, 11, 4. [Google Scholar] [CrossRef] [Green Version]
- Kurtuldu, F.; Kaňková, H.; Beltrán, A.M.; Liverani, L.; Galusek, D.; Boccaccini, A. Anti-inflammatory and antibacterial activities of cerium-containing mesoporous bioactive glass nanoparticles for drug-free biomedical applications. Mater. Today Bio 2021, 12, 100150. [Google Scholar] [CrossRef]
- Eleftheriadis, G.K.; Filippousi, M.; Tsachouridou, V.; Darda, M.-A.; Sygellou, L.; Kontopoulou, I.; Bouropoulos, N.; Steriotis, T.; Charalambopoulou, G.; Vizirianakis, I.S.; et al. Evaluation of mesoporous carbon aerogels as carriers of the non-steroidal anti-inflammatory drug ibuprofen. Int. J. Pharm. 2016, 515, 262–270. [Google Scholar] [CrossRef]
- Du, X.; Kleitz, F.; Li, X.; Huang, H.; Zhang, X.; Qiao, S.-Z. Disulfide-bridged organosilica frameworks: Designed, synthesis, redox-triggered biodegradation, and nanobiomedical applications. Adv. Funct. Mater. 2018, 28, 1707325. [Google Scholar] [CrossRef] [Green Version]
- Bithi, K.A.; Minami, H.; Hossain, M.K.; Rahman, M.M.; Rahman, M.A.; Gafur, M.A.; Ahmad, H. Cationic polyelectrolyte grafted mesoporous magnetic silica composite particles for targeted drug delivery and thrombolysis. Materialia 2020, 11, 100676. [Google Scholar] [CrossRef]
- Beñová, E.; Hornebecq, V.; Zeleňák, V.; Huntošová, V.; Almáši, M.; Máčajová, M.; Bergé-Lefranc, D. pH-responsive mesoporous silica drug delivery system, its biocompatibility and co-adsorption/co-release of 5-fluorouracil and naproxen. Appl. Surf. Sci. 2021, 561, 150011. [Google Scholar] [CrossRef]
- Zhou, J.; Zhu, F.; Li, J.; Wang, Y. Concealed body mesoporous silica nanoparticles for orally delivering indometacin with chiral recognition function. Mater. Sci. Eng. C 2018, 90, 314–324. [Google Scholar] [CrossRef] [PubMed]
- Gou, K.; Guo, X.; Wang, Y.; Wang, Y.; Sang, Z.; Ma, S.; Guo, Y.; Xie, L.; Li, S.; Li, H. Chiral microenvironment-responsive mesoporous silica nanoparticles for delivering indometacin with chiral recognition function. Mater. Des. 2022, 214, 110359. [Google Scholar] [CrossRef]
- Zeleňáková, A.; Szűcsová, J.; Nagy, L.; Girman, V.; Zeleňák, V.; Huntošová, V. Magnetic characterization and moderate cytotoxicity of magnetic mesoporous silica nanocomposite for drug delivery of naproxen. Nanomaterials 2021, 11, 901. [Google Scholar] [CrossRef] [PubMed]
- Lau, M.; Sealy, B.; Combes, V.; Morsch, M.; Garcia-Bennett, A.E. Enhanced antioxidant effects of the anti-inflammatory compound probucol when released from mesoporous silica particles. Pharmaceutics 2022, 14, 502. [Google Scholar] [CrossRef]
- Yan, X.; Zhang, L.; Zhang, Y.; Qiao, K.; Yan, Z.; Komarneni, S. Amine-modified mesocellular silica foams for CO2 capture. Chem. Eng. J. 2011, 193, 918–924. [Google Scholar] [CrossRef]
- Lin, Y.-S.; Hurley, K.R.; Haynes, C.L. Critical considerations in the biomedical use of mesoporous silica nanoparticles. J. Phys. Chem. Lett. 2012, 3, 364–374. [Google Scholar] [CrossRef]
- Chen, W.; Wang, J.; Cheng, L.; Du, W.; Wang, J.; Pan, W.; Qiu, S.; Song, L.; Ma, X.; Hu, Y. Polypyrrole-coated mesoporous TiO2 nanocomposites simultaneously loading DOX and aspirin prodrugs for a synergistic theranostic and anti-inflammatory effect. ACS Appl. Bio Mater. 2021, 4, 1483–1492. [Google Scholar] [CrossRef]
- De Almeida Junior, S.; Pereira, P.M.; de Sousa Tótoli, V.; Neves, E.S.; Monochio, M.; Alvarenga, A.W.O.; Hori, J.I.; Braz, W.R.; Rocha, L.A.; Nassar, E.J.; et al. Incorporation of indomethacin into a mesoporous silica nanoparticle enhances the anti-inflammatory effect indomethacin into a mesoporous silica. Eur. J. Pharm. Sci. 2021, 157, 105601. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).