Developing Novel Hydroxypropyl-β-Cyclodextrin-Based Nanosponges as Carriers for Anticancer Hydrophobic Agents: Overcoming Limitations of Host–Guest Complexes in a Comparative Evaluation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Phase Solubility Study
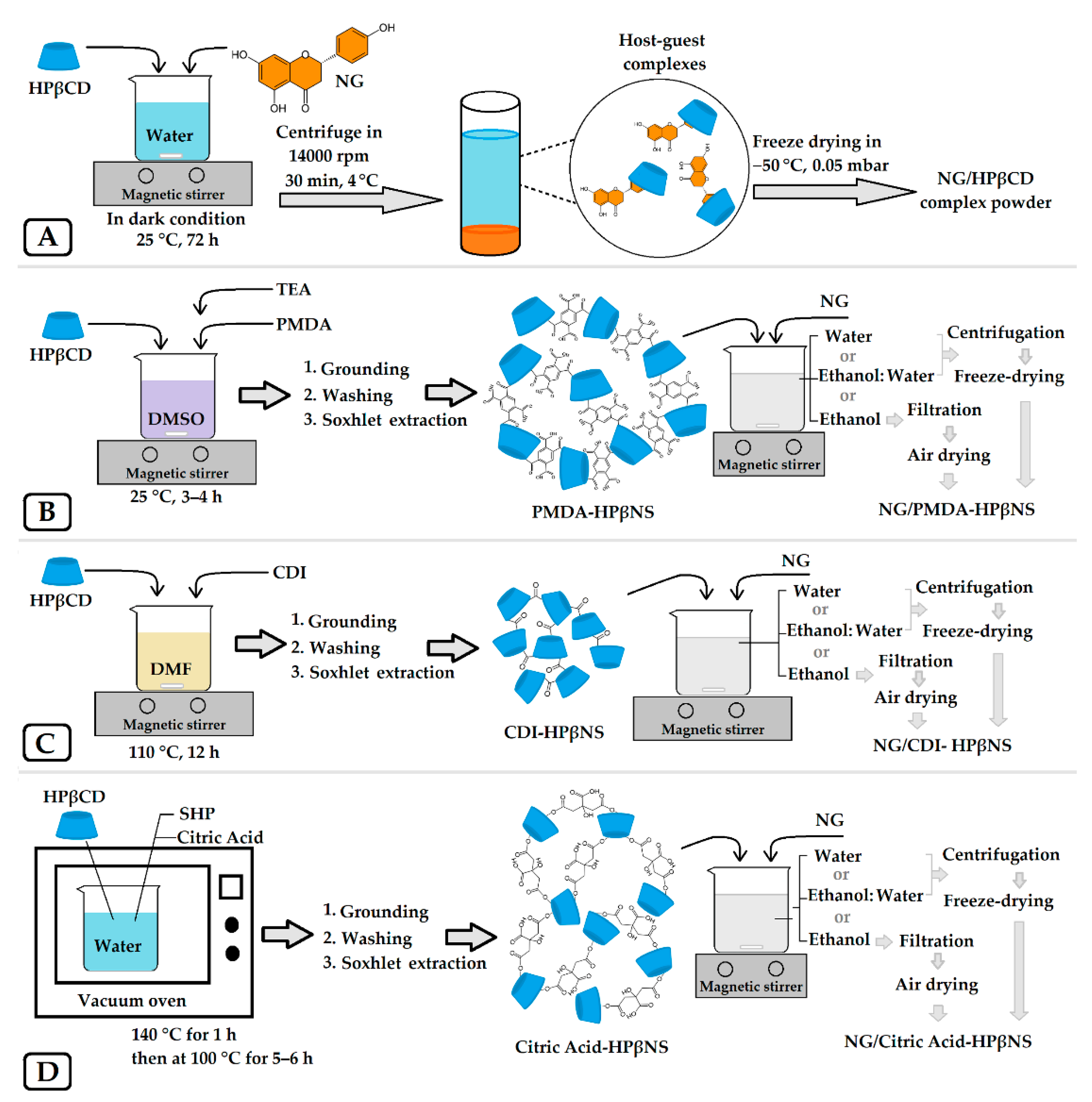
2.3. Preparation of the NG/HPβCD Complex
2.4. Fabrication of HPβCD-Based Nanosponges and NG Loading
2.5. Determination of NG Loading Capacity in NG/HPβCD Complex and NG-Loaded HPβNS
2.6. Preparation of the Physical Mixture
2.7. Physicochemical Characterization
2.7.1. Thermogravimetric Analysis
2.7.2. Determination of Particle Size, Polydispersity Index, and Zeta Potential
2.7.3. Fourier Transform Infrared Spectroscopy
2.7.4. 1H NMR and 13C Cross-Polarization Magic Angle Spinning Solid-State NMR
2.7.5. Particle Morphology by Scanning Electron Microscopy
2.8. In Vitro Release Study
2.9. Cytotoxicity Studies
2.10. Statistical Analysis
3. Results and Discussion
3.1. Phase Solubility Studies
3.2. Determination of NG Loading Capacity in NG/HPβCD Complex and NG-Loaded HPβNS
3.3. Physicochemical Characterization
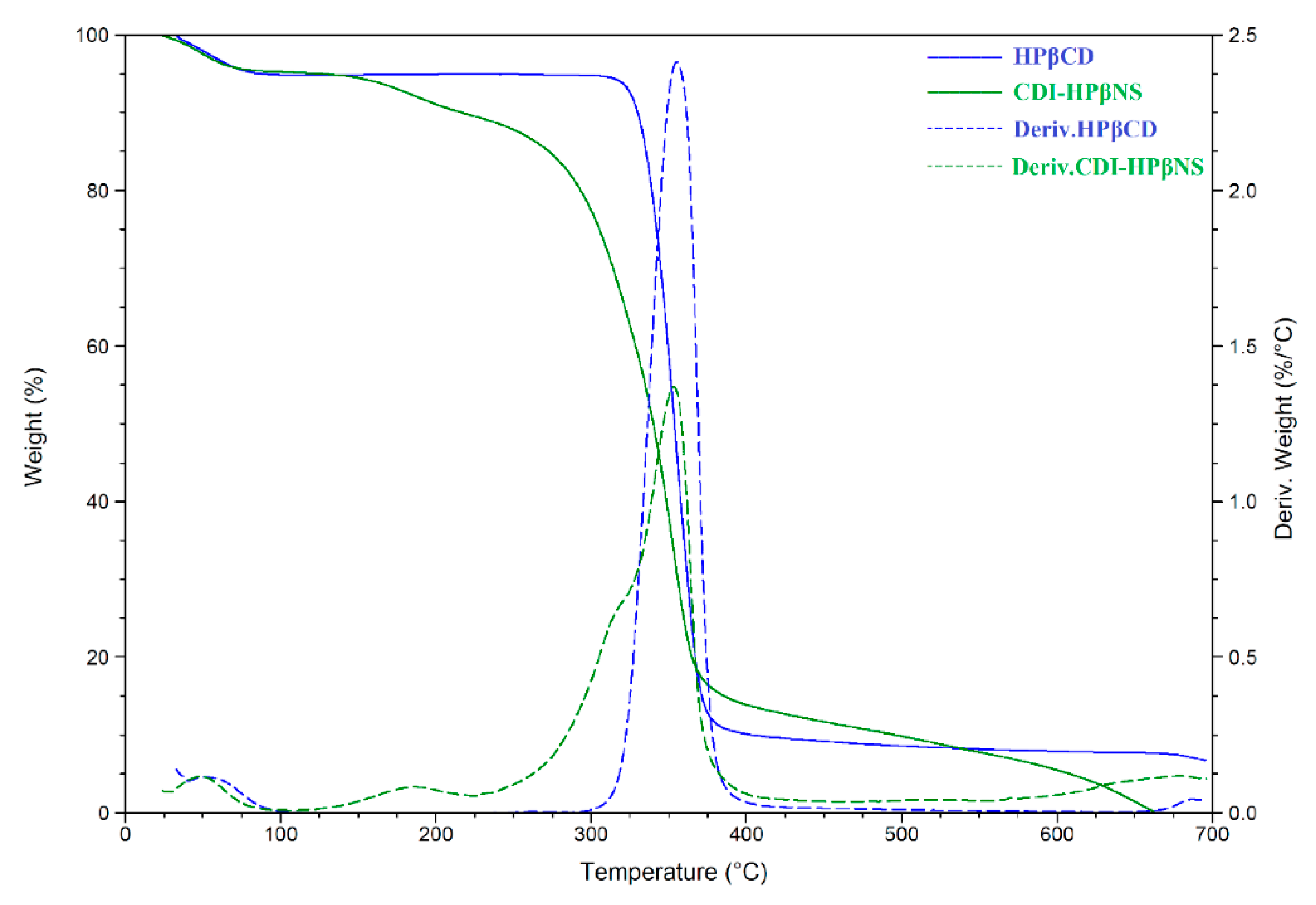
3.3.1. Thermogravimetric Analysis
3.3.2. Determination of Particle Size, Polydispersity Index, and Zeta Potential
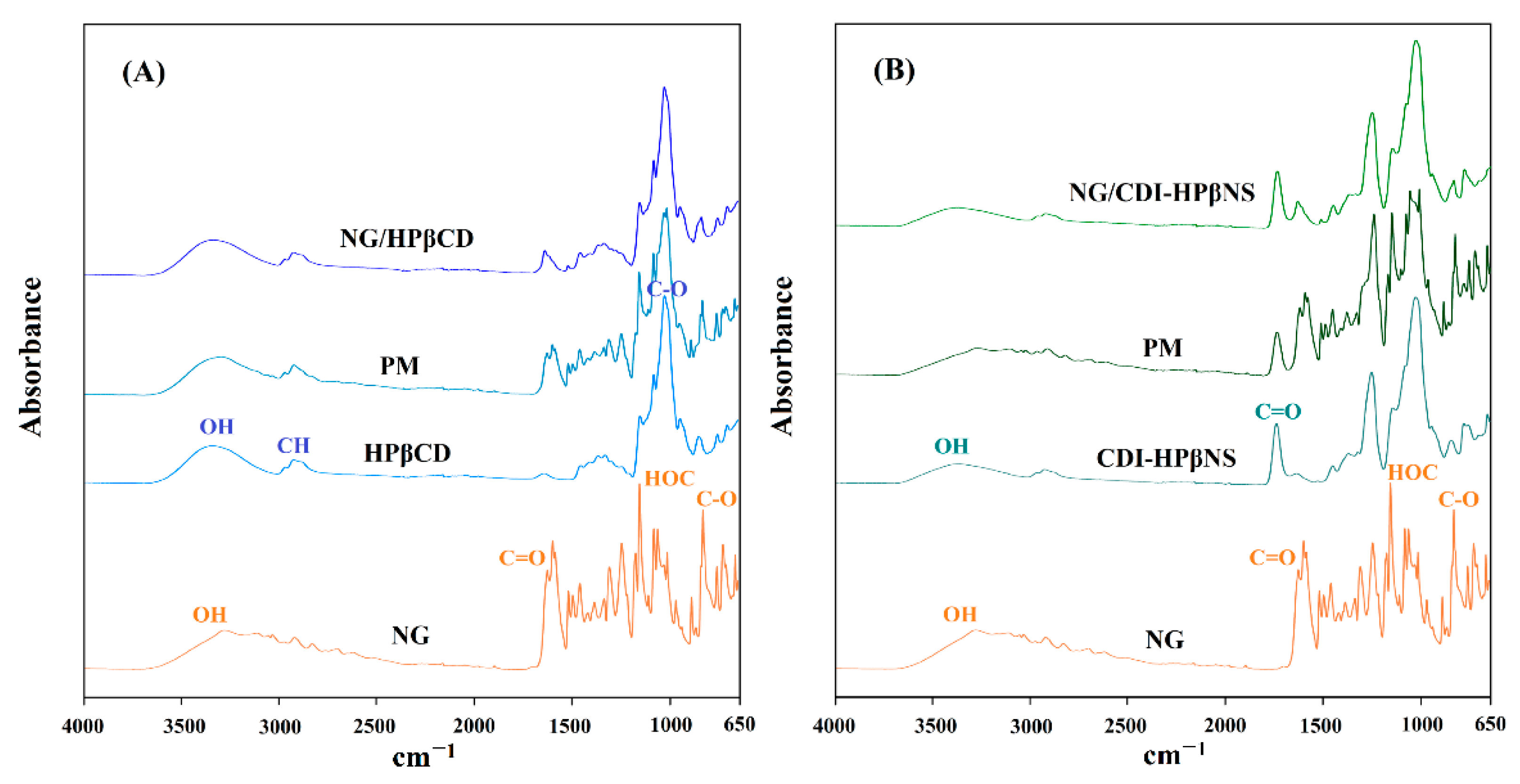
3.3.3. Fourier Transform Infrared Spectroscopy
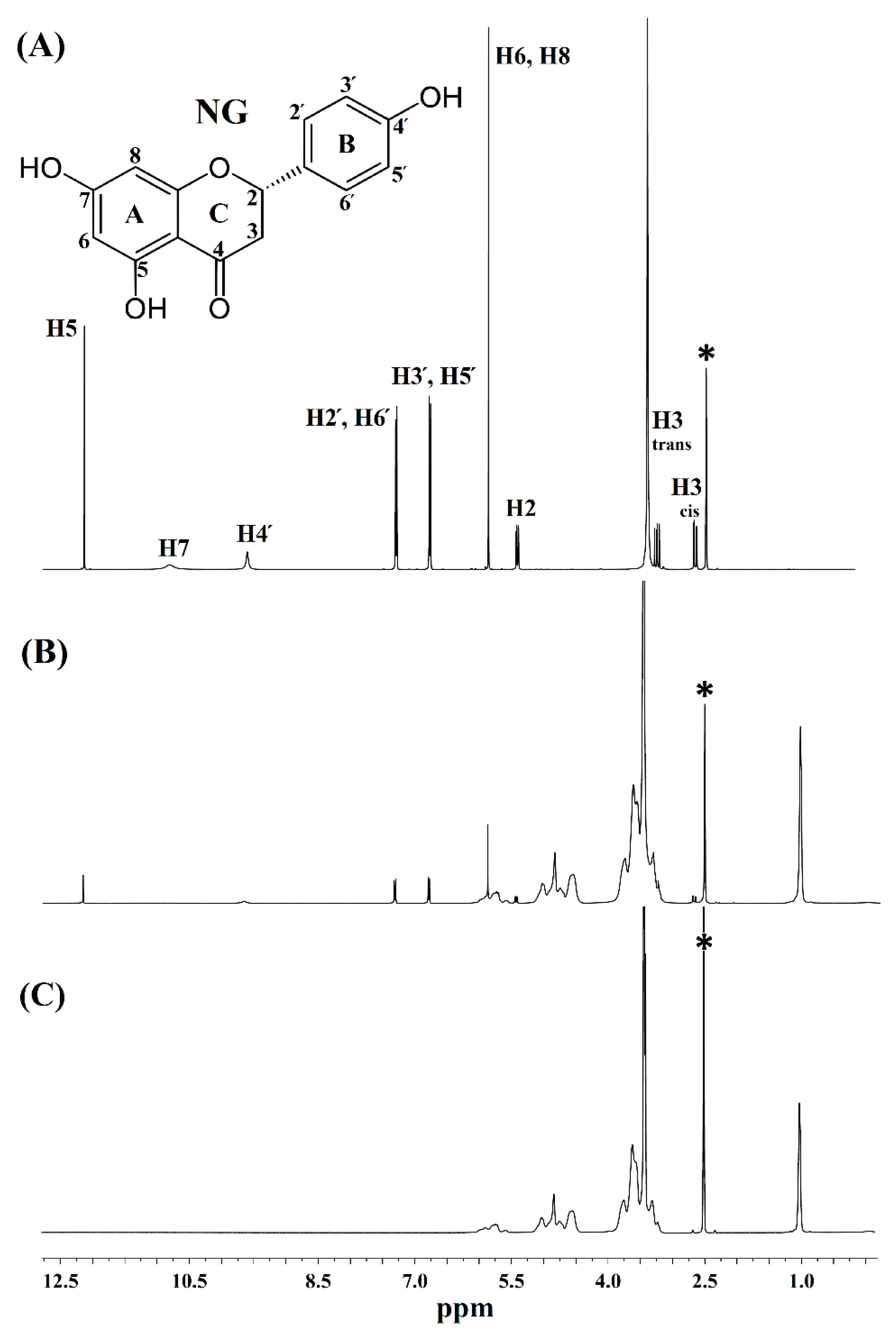
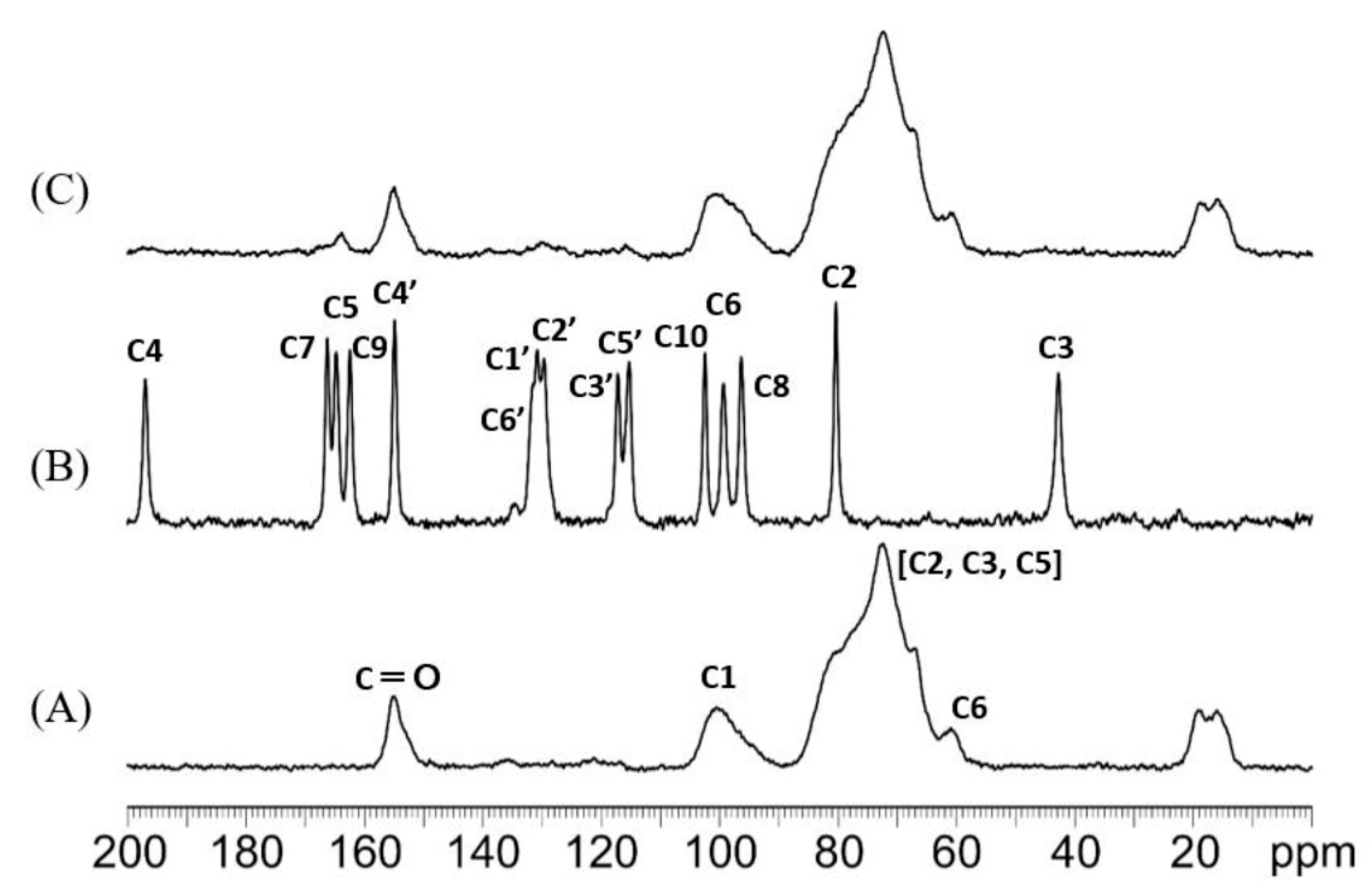
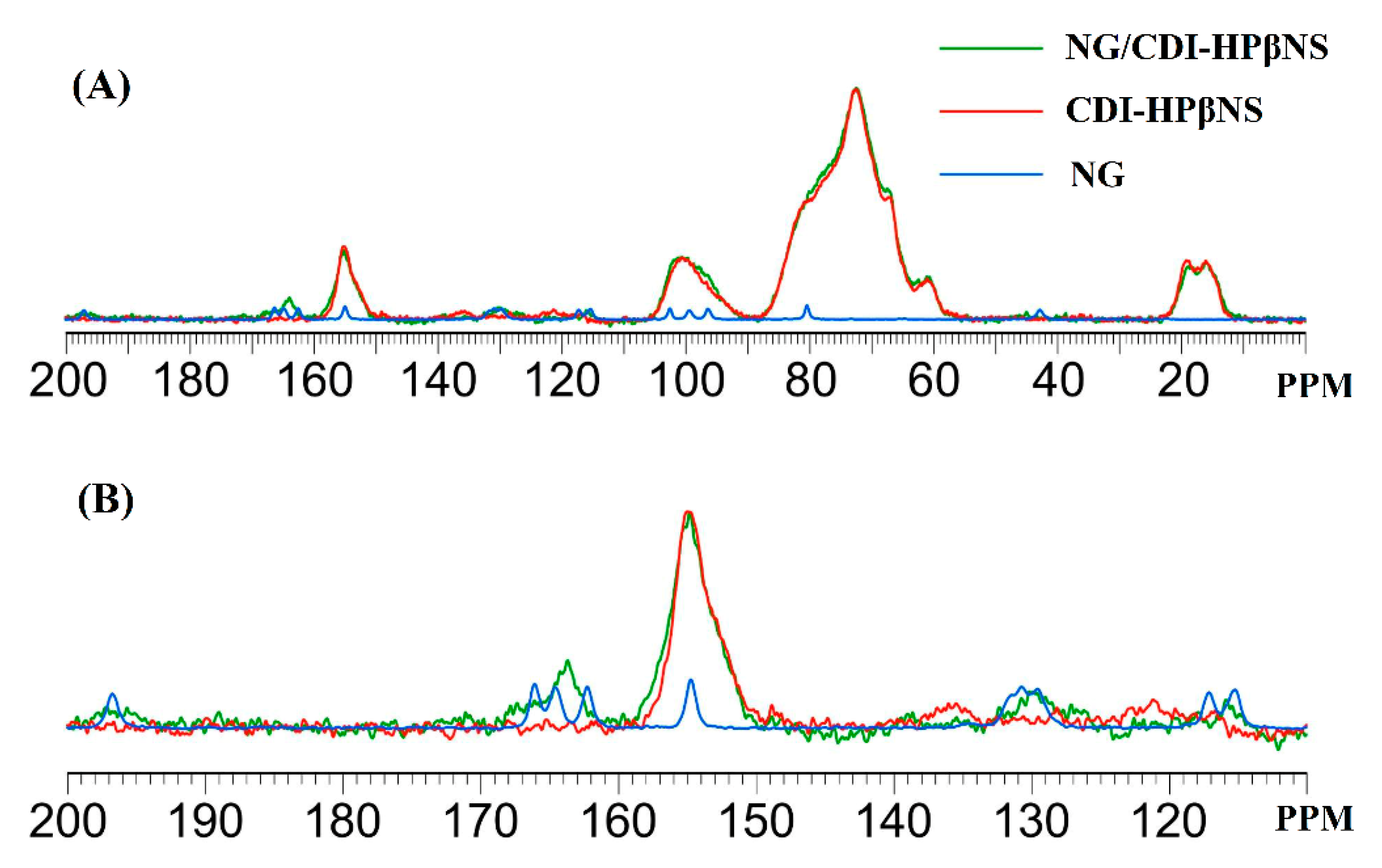
3.3.4. H NMR and 13C Cross-Polarization Magic Angle Spinning Solid-State NMR
3.3.5. Particle Morphology by Scanning Electron Microscopy
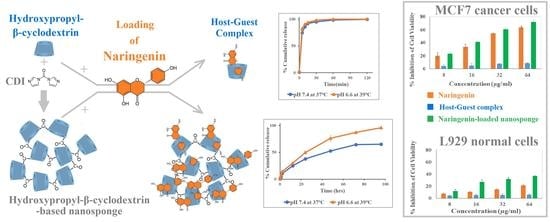
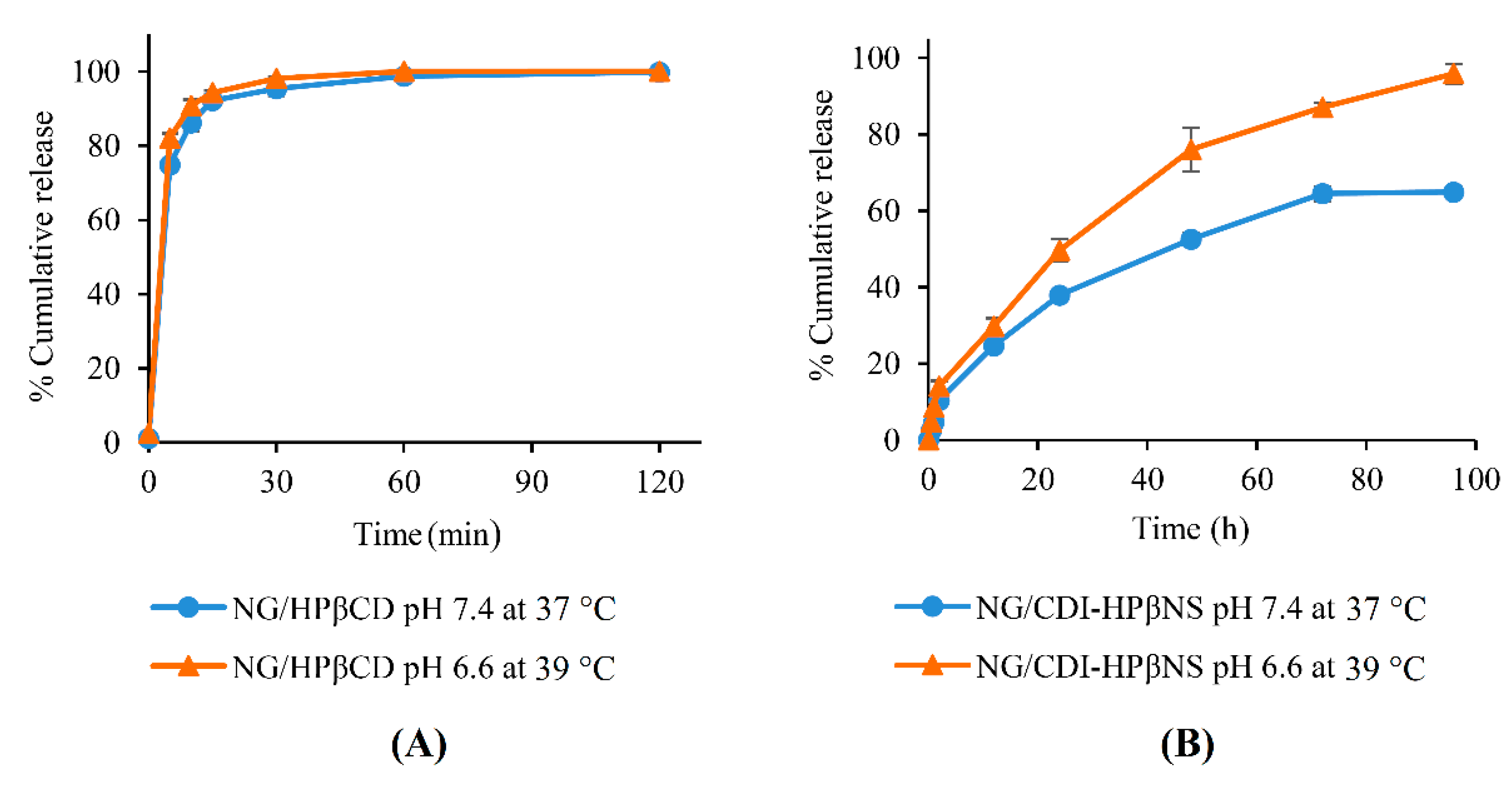
3.4. In Vitro Release Study
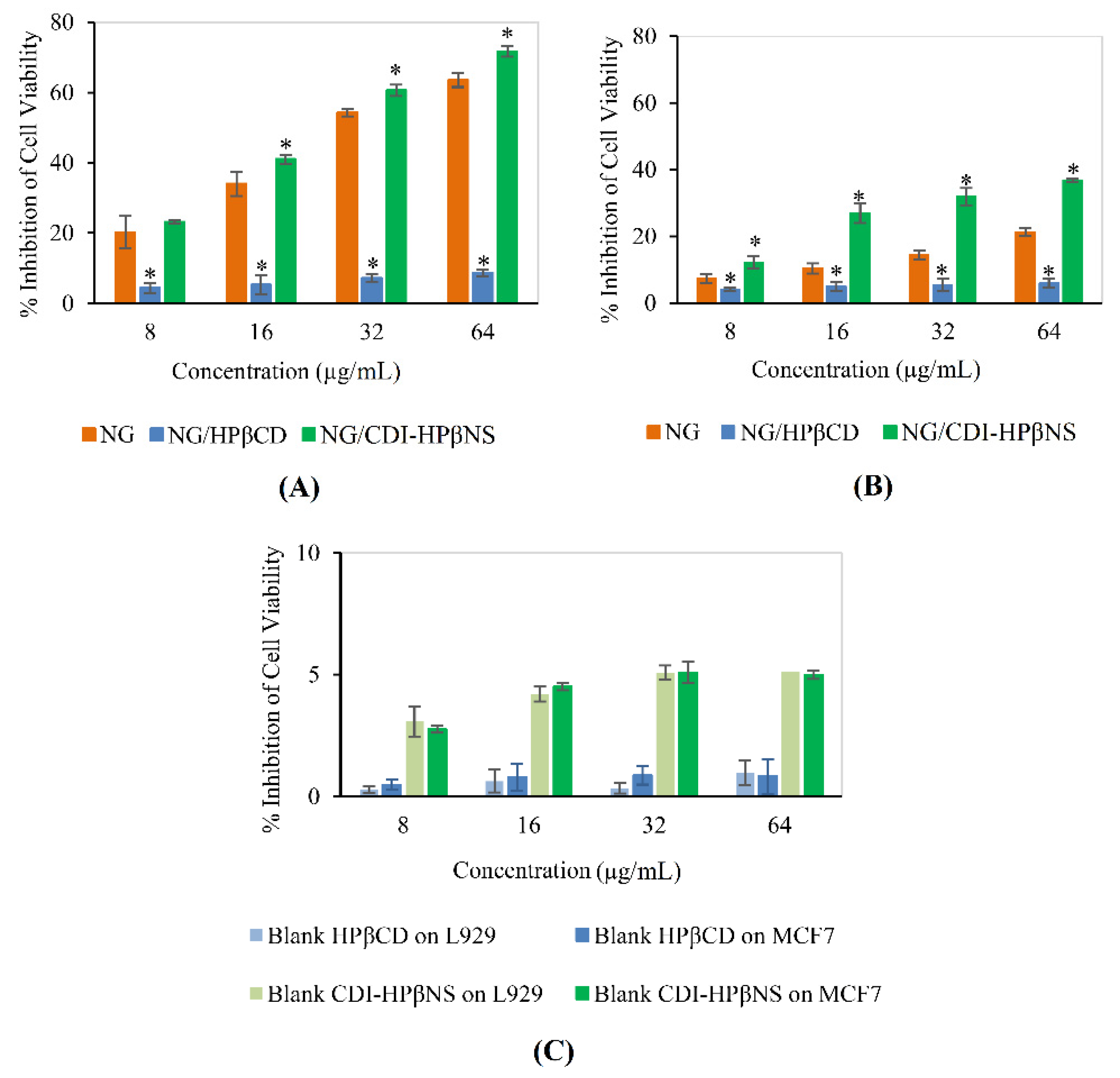
3.5. Cytotoxicity Studies
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Choudhari, A.S.; Mandave, P.C.; Deshpande, M.; Ranjekar, P.; Prakash, O. Phytochemicals in Cancer Treatment: From Preclinical Studies to Clinical Practice. Front. Pharmacol. 2020, 10, 1614. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahamad, M.S.; Siddiqui, S.; Jafri, A.; Ahmad, S.; Afzal, M.; Arshad, M. Induction of Apoptosis and Antiproliferative Activity of Naringenin in Human Epidermoid Carcinoma Cell through ROS Generation and Cell Cycle Arrest. PLoS ONE 2014, 9, e110003. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mir, I.A.; Tiku, A.B. Chemopreventive and Therapeutic Potential of “Naringenin,” a Flavanone Present in Citrus Fruits. Nutr. Cancer 2015, 67, 27–42. [Google Scholar] [CrossRef] [PubMed]
- Arafah, A.; Rehman, M.U.; Mir, T.M.; Wali, A.F.; Ali, R.; Qamar, W.; Khan, R.; Ahmad, A.; Aga, S.S.; Alqahtani, S.; et al. Multi-Therapeutic Potential of Naringenin (4′,5,7-Trihydroxyflavonone): Experimental Evidence and Mechanisms. Plants 2020, 9, 1784. [Google Scholar] [CrossRef] [PubMed]
- Kanno, S.; Tomizawa, A.; Hiura, T.; Osanai, Y.; Shouji, A.; Ujibe, M.; Ohtake, T.; Kimura, K.; Ishikawa, M. Inhibitory Effects of Naringenin on Tumor Growth in Human Cancer Cell Lines and Sarcoma S-180-Implanted Mice. Biol. Pharm. Bull. 2005, 28, 527–530. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kanaze, F.I.; Bounartzi, M.I.; Georgarakis, M.; Niopas, I. Pharmacokinetics of the Citrus Flavanone Aglycones Hesperetin and Naringenin after Single Oral Administration in Human Subjects. Eur. J. Clin. Nutr. 2007, 61, 472–477. [Google Scholar] [CrossRef] [Green Version]
- Brewster, M.E.; Loftsson, T. Cyclodextrins as Pharmaceutical Solubilizers. Adv. Drug Deliv. Rev. 2007, 59, 645–666. [Google Scholar] [CrossRef]
- Praphanwittaya, P.; Saokham, P.; Jansook, P.; Loftsson, T. Aqueous Solubility of Kinase Inhibitors: I the Effect of Hydrophilic Polymers on Their γ-Cyclodextrin Solubilization. J. Drug Deliv. Sci. Technol. 2020, 55, 101462. [Google Scholar] [CrossRef]
- Sohajda, T. Pharmaceutical Applications of Cyclodextrins. Available online: https://cyclolab.hu/userfiles/CDformulation.pdf (accessed on 14 March 2022).
- Kurkov, S.V.; Loftsson, T. Cyclodextrins. Int. J. Pharm. 2013, 453, 167–180. [Google Scholar] [CrossRef]
- Stella, V.J.; He, Q. Cyclodextrins. Toxicol. Pathol. 2008, 36, 30–42. [Google Scholar] [CrossRef]
- Loftsson, T.; Másson, M.; Sigurjónsdóttir, J.F. Methods to Enhance the Complexation Efficiency of Cyclodextrins. S.T.P. Pharma Sci. 1999, 9, 237–242. [Google Scholar]
- Saokham, P.; Muankaew, C.; Jansook, P.; Loftsson, T. Solubility of Cyclodextrins and Drug/Cyclodextrin Complexes. Molecules 2018, 23, 1161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caldera, F.; Tannous, M.; Cavalli, R.; Zanetti, M.; Trotta, F. Evolution of Cyclodextrin Nanosponges. Int. J. Pharm. 2017, 531, 470–479. [Google Scholar] [CrossRef] [PubMed]
- Ansari, K.A.; Vavia, P.R.; Trotta, F.; Cavalli, R. Cyclodextrin-Based Nanosponges for Delivery of Resveratrol: In Vitro Characterisation, Stability, Cytotoxicity and Permeation Study. AAPS PharmSciTech 2011, 12, 279–286. [Google Scholar] [CrossRef] [Green Version]
- Torne, S.; Darandale, S.; Vavia, P.; Trotta, F.; Cavalli, R. Cyclodextrin-Based Nanosponges: Effective Nanocarrier for Tamoxifen Delivery. Pharm. Dev. Technol. 2013, 18, 619–625. [Google Scholar] [CrossRef]
- Mognetti, B.; Barberis, A.; Marino, S.; Berta, G.; De Francia, S.; Trotta, F.; Cavalli, R. In Vitro Enhancement of Anticancer Activity of Paclitaxel by a Cremophor Free Cyclodextrin-Based Nanosponge Formulation. J. Incl. Phenom. Macrocycl. Chem. 2012, 74, 201–210. [Google Scholar] [CrossRef]
- Clemente, N.; Argenziano, M.; Gigliotti, C.L.; Ferrara, B.; Boggio, E.; Chiocchetti, A.; Caldera, F.; Trotta, F.; Benetti, E.; Annaratone, L.; et al. Paclitaxel-Loaded Nanosponges Inhibit Growth and Angiogenesis in Melanoma Cell Models. Front. Pharmacol. 2019, 10, 776. [Google Scholar] [CrossRef] [Green Version]
- Gigliotti, C.L.; Ferrara, B.; Occhipinti, S.; Boggio, E.; Barrera, G.; Pizzimenti, S.; Giovarelli, M.; Fantozzi, R.; Chiocchetti, A.; Argenziano, M.; et al. Enhanced Cytotoxic Effect of Camptothecin Nanosponges in Anaplastic Thyroid Cancer Cells in Vitro and in Vivo on Orthotopic Xenograft Tumors. Drug Deliv. 2017, 24, 670–680. [Google Scholar] [CrossRef] [Green Version]
- Anandam, S.; Selvamuthukumar, S. Fabrication of Cyclodextrin Nanosponges for Quercetin Delivery: Physicochemical Characterization, Photostability, and Antioxidant Effects. J. Mater. Sci. 2014, 49, 8140–8153. [Google Scholar] [CrossRef]
- Darandale, S.S.; Vavia, P.R. Cyclodextrin-Based Nanosponges of Curcumin: Formulation and Physicochemical Characterization. J. Incl. Phenom. Macrocycl. Chem. 2013, 75, 315–322. [Google Scholar] [CrossRef]
- Argenziano, M.; Gigliotti, C.L.; Clemente, N.; Boggio, E.; Ferrara, B.; Trotta, F.; Pizzimenti, S.; Barrera, G.; Boldorini, R.; Bessone, F.; et al. Improvement in the Anti-Tumor Efficacy of Doxorubicin Nanosponges in In Vitro and in Mice Bearing Breast Tumor Models. Cancers 2020, 12, 162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krabicová, I.; Appleton, S.L.; Tannous, M.; Hoti, G.; Caldera, F.; Rubin Pedrazzo, A.; Cecone, C.; Cavalli, R.; Trotta, F. History of Cyclodextrin Nanosponges. Polymers 2020, 12, 1122. [Google Scholar] [CrossRef] [PubMed]
- Cecone, C.; Hoti, G.; Krabicová, I.; Appleton, S.L.; Caldera, F.; Bracco, P.; Zanetti, M.; Trotta, F. Sustainable Synthesis of Cyclodextrin-Based Polymers by Exploiting Natural Deep Eutectic Solvents. Green Chem. 2020, 22, 5806–5814. [Google Scholar] [CrossRef]
- Garcia-Fernandez, M.J.; Tabary, N.; Chai, F.; Cazaux, F.; Blanchemain, N.; Flament, M.-P.; Martel, B. New Multifunctional Pharmaceutical Excipient in Tablet Formulation Based on Citric Acid-Cyclodextrin Polymer. Int. J. Pharm. 2016, 511, 913–920. [Google Scholar] [CrossRef]
- Higuchi, T.; Connors, K.A. Phase Solubility Techniques. In Advances in Analytical Chemistry and Instrumentation; Reilly, C.N., Ed.; Interscience: Hoboken, NJ, USA, 1965; Volume 4, pp. 117–212. [Google Scholar]
- Loftsson, T. Cyclodextrins in Parenteral Formulations. J. Pharm. Sci. 2021, 110, 654–664. [Google Scholar] [CrossRef] [PubMed]
- Kfoury, M.; Landy, D.; Ruellan, S.; Auezova, L.; Greige-Gerges, H.; Fourmentin, S. Determination of Formation Constants and Structural Characterization of Cyclodextrin Inclusion Complexes with Two Phenolic Isomers: Carvacrol and Thymol. Beilstein J. Org. Chem. 2016, 12, 29–42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shende, P.K.; Trotta, F.; Gaud, R.S.; Deshmukh, K.; Cavalli, R.; Biasizzo, M. Influence of Different Techniques on Formulation and Comparative Characterization of Inclusion Complexes of ASA with β-Cyclodextrin and Inclusion Complexes of ASA with PMDA Cross-Linked β-Cyclodextrin Nanosponges. J. Incl. Phenom. Macrocycl. Chem. 2012, 74, 447–454. [Google Scholar] [CrossRef]
- Trotta, F.; Zanetti, M.; Cavalli, R. Cyclodextrin-Based Nanosponges as Drug Carriers. Beilstein J. Org. Chem. 2012, 8, 2091–2099. [Google Scholar] [CrossRef]
- Dhakar, N.K.; Caldera, F.; Bessone, F.; Cecone, C.; Pedrazzo, A.R.; Cavalli, R.; Dianzani, C.; Trotta, F. Evaluation of Solubility Enhancement, Antioxidant Activity, and Cytotoxicity Studies of Kynurenic Acid Loaded Cyclodextrin Nanosponge. Carbohydr. Polym. 2019, 224, 115–168. [Google Scholar] [CrossRef]
- Wallace, S.J.; Li, J.; Nation, R.L.; Boyd, B.J. Drug Release from Nanomedicines: Selection of Appropriate Encapsulation and Release Methodology. Drug Deliv. Transl. Res. 2012, 2, 284–292. [Google Scholar] [CrossRef] [Green Version]
- Dhakar, N.K.; Matencio, A.; Caldera, F.; Argenziano, M.; Cavalli, R.; Dianzani, C.; Zanetti, M.; López-Nicolás, J.M.; Trotta, F. Comparative Evaluation of Solubility, Cytotoxicity and Photostability Studies of Resveratrol and Oxyresveratrol Loaded Nanosponges. Pharmaceutics 2019, 11, 545. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Loftsson, T.; Hreinsdóttir, D.; Másson, M. Evaluation of Cyclodextrin Solubilization of Drugs. Int. J. Pharm. 2005, 302, 18–28. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Cheng, X.; Li, N.; Wang, H.; Chen, H. Nanocarriers and Their Loading Strategies. Adv. Healthc. Mater. 2019, 8, 1801002. [Google Scholar] [CrossRef] [PubMed]
- Al-Qubaisi, M.S.; Rasedee, A.; Flaifel, M.H.; Eid, E.E.M.; Hussein-Al-Ali, S.; Alhassan, F.H.; Salih, A.M.; Hussein, M.Z.; Zainal, Z.; Sani, D.; et al. Characterization of Thymoquinone/Hydroxypropyl-β-Cyclodextrin Inclusion Complex: Application to Anti-Allergy Properties. Eur. J. Pharm. Sci. 2019, 133, 167–182. [Google Scholar] [CrossRef] [PubMed]
- Pedrazzo, A.R.; Caldera, F.; Zanetti, M.; Appleton, S.L.; Dhakar, N.K.; Trotta, F. Mechanochemical Green Synthesis of Hyper-Crosslinked Cyclodextrin Polymers. Beilstein J. Org. Chem. 2020, 16, 1554–1563. [Google Scholar] [CrossRef] [PubMed]
- Sá Couto, A.R.; Ryzhakov, A.; Loftsson, T. 2-Hydroxypropyl-β-Cyclodextrin Aggregates: Identification and Development of Analytical Techniques. Materials 2018, 11, 1971. [Google Scholar] [CrossRef] [Green Version]
- Praphanwittaya, P.; Saokham, P.; Jansook, P.; Loftsson, T. Aqueous Solubility of Kinase Inhibitors: II the Effect of Hexadimethrine Bromide on the Dovitinib/γ-Cyclodextrin Complexation. J. Drug Deliv. Sci. Technol. 2020, 55, 101463. [Google Scholar] [CrossRef]
- Loftsson, T.; Másson, M.; Brewster, M.E. Self-Association of Cyclodextrins and Cyclodextrin Complexes. J. Pharm. Sci. 2004, 93, 1091–1099. [Google Scholar] [CrossRef]
- Unsalan, O.; Erdogdu, Y.; Gulluoglu, M.T. FT-Raman and FT-IR Spectral and Quantum Chemical Studies on Some Flavonoid Derivatives: Baicalein and Naringenin. J. Raman Spectrosc. 2009, 40, 562–570. [Google Scholar] [CrossRef]
- Yuan, C.; Liu, B.; Liu, H. Characterization of Hydroxypropyl-β-Cyclodextrins with Different Substitution Patterns via FTIR, GC-MS, and TG-DTA. Carbohydr. Polym. 2015, 118, 36–40. [Google Scholar] [CrossRef]
- Malanga, M.; Szemán, J.; Fenyvesi, É.; Puskás, I.; Csabai, K.; Gyémánt, G.; Fenyvesi, F.; Szente, L. “Back to the Future”: A New Look at Hydroxypropyl Beta-Cyclodextrins. J. Pharm. Sci. 2016, 105, 2921–2931. [Google Scholar] [CrossRef] [Green Version]
- Venuti, V.; Crupi, V.; Fazio, B.; Majolino, D.; Acri, G.; Testagrossa, B.; Stancanelli, R.; De Gaetano, F.; Gagliardi, A.; Paolino, D.; et al. Physicochemical Characterization and Antioxidant Activity Evaluation of Idebenone/Hydroxypropyl-β-Cyclodextrin Inclusion Complex. Biomolecules 2019, 9, 531. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Castiglione, F.; Crupi, V.; Majolino, D.; Mele, A.; Panzeri, W.; Rossi, B.; Trotta, F.; Venuti, V. Vibrational Dynamics and Hydrogen Bond Properties of β-CD Nanosponges: An FTIR-ATR, Raman and Solid-State NMR Spectroscopic Study. J. Incl. Phenom. Macrocycl. Chem. 2013, 75, 247–254. [Google Scholar] [CrossRef]
- Wawer, I.; Zielinska, A. 13C CP/MAS NMR Studies of Flavonoids. Magn. Reson. Chem. 2001, 39, 374–380. [Google Scholar] [CrossRef]
- Pérez-Herrero, E.; Fernández-Medarde, A. The Reversed Intra- and Extracellular PH in Tumors as a Unified Strategy to Chemotherapeutic Delivery Using Targeted Nanocarriers. Acta Pharm. Sin. B 2021, 11, 2243–2264. [Google Scholar] [CrossRef]
- Seynhaeve, A.L.B.; Amin, M.; Haemmerich, D.; van Rhoon, G.C.; ten Hagen, T.L.M. Hyperthermia and Smart Drug Delivery Systems for Solid Tumor Therapy. Adv. Drug Deliv. Rev. 2020, 163–164, 125–144. [Google Scholar] [CrossRef]
- Shang, L.; Nienhaus, K.; Nienhaus, G. Engineered Nanoparticles Interacting with Cells: Size Matters. J. Nanobiotechnol. 2014, 12, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Kopustinskiene, D.M.; Jakstas, V.; Savickas, A.; Bernatoniene, J. Flavonoids as Anticancer Agents. Nutrients 2020, 12, 457. [Google Scholar] [CrossRef] [Green Version]
- Guo, D.; Wang, J.; Wang, X.; Luo, H.; Zhang, H.; Cao, D.; Chen, L.; Huang, N. Double Directional Adjusting Estrogenic Effect of Naringin from Rhizoma Drynariae (Gusuibu). J. Ethnopharmacol. 2011, 138, 451–457. [Google Scholar] [CrossRef]
- Kim, S.; Park, T.I. Naringenin: A Partial Agonist on Estrogen Receptor in T47D-KBluc Breast Cancer Cells. Int. J. Clin. Exp. Med. 2013, 6, 890–899. [Google Scholar]
- El-Kersh, D.M.; Ezzat, S.M.; Salama, M.M.; Mahrous, E.A.; Attia, Y.M.; Ahmed, M.S.; Elmazar, M.M. Anti-Estrogenic and Anti-Aromatase Activities of Citrus Peels Major Compounds in Breast Cancer. Sci. Rep. 2021, 11, 1–14. [Google Scholar] [CrossRef]


| Crosslinker | HPβCD (g) | Ratio * | Solvent (mL) | Catalyst | Conditions | Soxhlet Solvent | Ref. |
|---|---|---|---|---|---|---|---|
| PMDA | 4.886 | 1:4 | DMSO (20) | TEA | Magnet stirring at 25 ℃ for 3–4 h | Acetone | [29] |
| CDI | 6.5 | 1:8 | Anhydrous DMF (39) | - | Magnet stirring at 110 ℃ for about 12 h | Ethanol | [30] |
| Citric Acid | 4 | 1:4 | Deionized water (20) | SHP | in vacuum oven at 140 ℃ for 1 h then at 100 ℃ for 5–6 h | Acetone | [24] |
| NG Loading Capacity % | |||
|---|---|---|---|
| HPβNS Type | Water | Water/Ethanol 50:50 v/v | Ethanol |
| PMDA-HPβNS | 0.57 ± 0.27 | 2.17 ± 0.22 | ND a |
| CDI-HPβNS | 1.76 ± 0.26 | 3.85 ± 0.43 | 19.42 ± 0.49 |
| Citric Acid-HPβNS | 0.41 ± 0.2 | 0.79 ± 0.13 | 4.64 ± 0.33 |
| NG/HPβCD (0 h) | NG/HPβCD (7d) | NG/CDI-HPβNS (0 h) | NG/CDI-HPβNS (7d) | |
|---|---|---|---|---|
| Particle size (nm) | 42.33 ± 11.16 | 84.03 ± 13.46 | 428.33 ± 1.99 | 453.13 ± 20.26 |
| PDI | 0.29 ± 0.02 | 0.29 ± 0.03 | 0.26 ± 0.01 | 0.27 ± 0.03 |
| Zeta potential (mV) | 1.91 ± 0.29 | 5.01 ± 0.37 | −21.63 ± 2.14 | −26.03 ± 3.42 |
| 1H a Assignment | Chemical Shifts δ (ppm) | Δδ b (ppm) | |
|---|---|---|---|
| Free NG | NG/HPβCD Complex | ||
| H2 | 5.444 | 5.439 | −0.005 |
| H3 (Cis) | 2.688 | 2.682 | −0.006 |
| H3 (Trans) | 3.228 | 3.221 | −0.007 |
| H5 | 12.153 | 12.146 | −0.007 |
| H6 or H8 | 5.879 | 5.867 | −0.008 |
| H7 | 10.827 | - | - |
| H2′ or H6′ | 7.301 | 7.297 | −0.004 |
| H3′ or H5′ | 6.799 | 6.792 | −0.007 |
| H4′ | 9.623 | 9.646 | 0.023 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peimanfard, S.; Zarrabi, A.; Trotta, F.; Matencio, A.; Cecone, C.; Caldera, F. Developing Novel Hydroxypropyl-β-Cyclodextrin-Based Nanosponges as Carriers for Anticancer Hydrophobic Agents: Overcoming Limitations of Host–Guest Complexes in a Comparative Evaluation. Pharmaceutics 2022, 14, 1059. https://doi.org/10.3390/pharmaceutics14051059
Peimanfard S, Zarrabi A, Trotta F, Matencio A, Cecone C, Caldera F. Developing Novel Hydroxypropyl-β-Cyclodextrin-Based Nanosponges as Carriers for Anticancer Hydrophobic Agents: Overcoming Limitations of Host–Guest Complexes in a Comparative Evaluation. Pharmaceutics. 2022; 14(5):1059. https://doi.org/10.3390/pharmaceutics14051059
Chicago/Turabian StylePeimanfard, Shohreh, Ali Zarrabi, Francesco Trotta, Adrián Matencio, Claudio Cecone, and Fabrizio Caldera. 2022. "Developing Novel Hydroxypropyl-β-Cyclodextrin-Based Nanosponges as Carriers for Anticancer Hydrophobic Agents: Overcoming Limitations of Host–Guest Complexes in a Comparative Evaluation" Pharmaceutics 14, no. 5: 1059. https://doi.org/10.3390/pharmaceutics14051059
APA StylePeimanfard, S., Zarrabi, A., Trotta, F., Matencio, A., Cecone, C., & Caldera, F. (2022). Developing Novel Hydroxypropyl-β-Cyclodextrin-Based Nanosponges as Carriers for Anticancer Hydrophobic Agents: Overcoming Limitations of Host–Guest Complexes in a Comparative Evaluation. Pharmaceutics, 14(5), 1059. https://doi.org/10.3390/pharmaceutics14051059