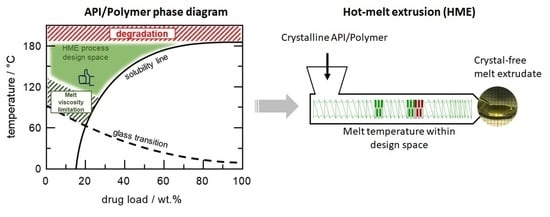
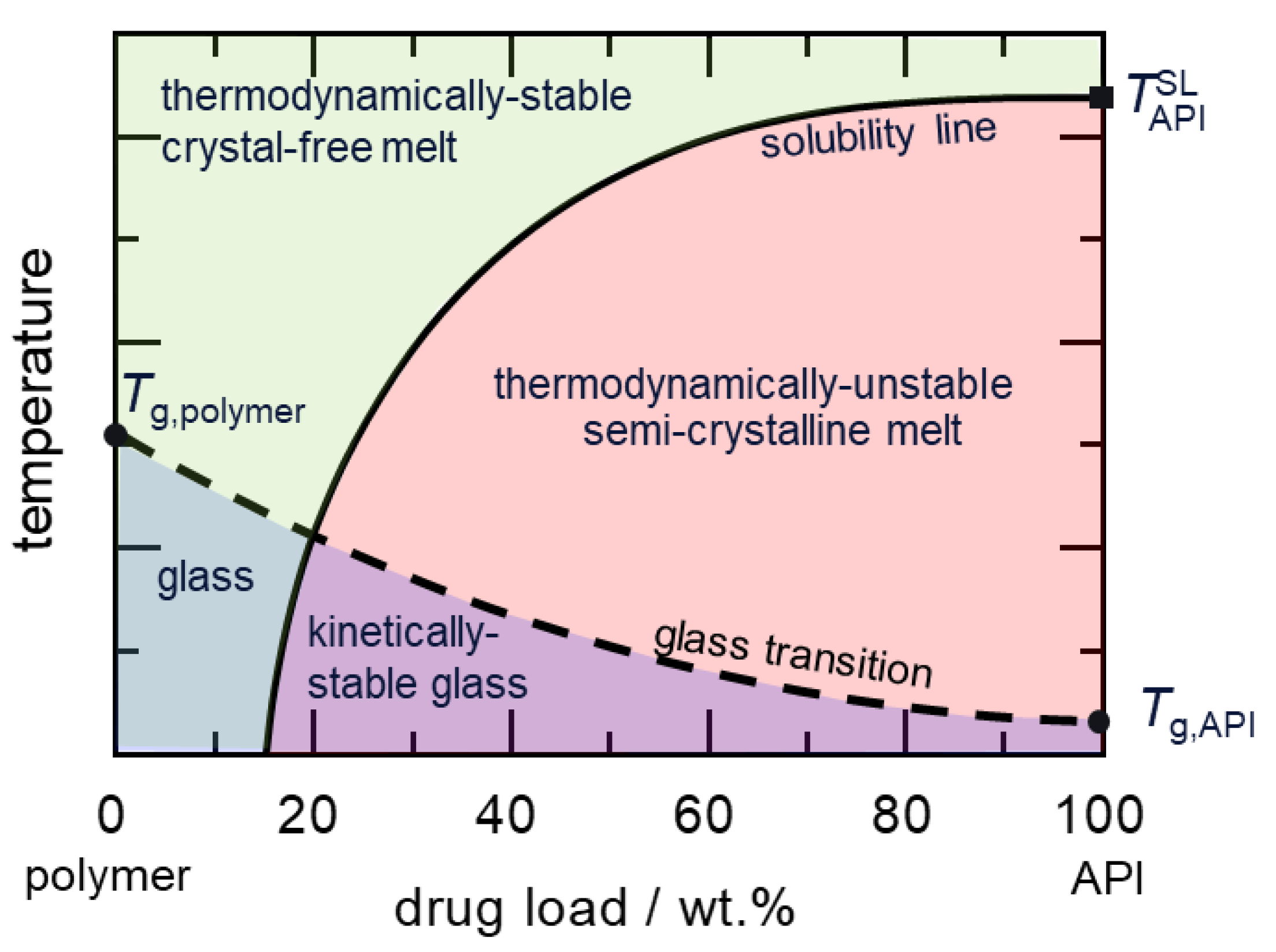
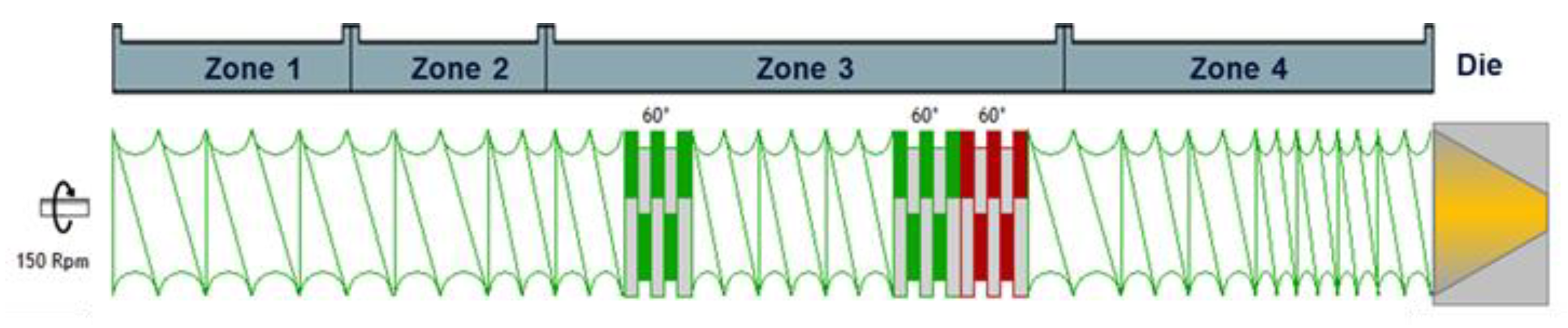
A Hot-Melt Extrusion Risk Assessment Classification System for Amorphous Solid Dispersion Formulation Development
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of API–Polymer Physical Mixtures for Thermal Analysis
2.3. Differential Scanning Calorimetry (DSC)
2.4. Thermogravimetric Analysis (TGA)
2.5. HME
2.6. Polarized Light Microscopy (PLM)
2.7. X-Ray Powder Diffraction (XRPD)
2.8. Advanced Polymer Chromatography™ (APC™)
3. Modeling
3.1. Modeling API Solubility in Polymer
3.2. Modeling Glass Transition Temperature
4. Results and Discussions
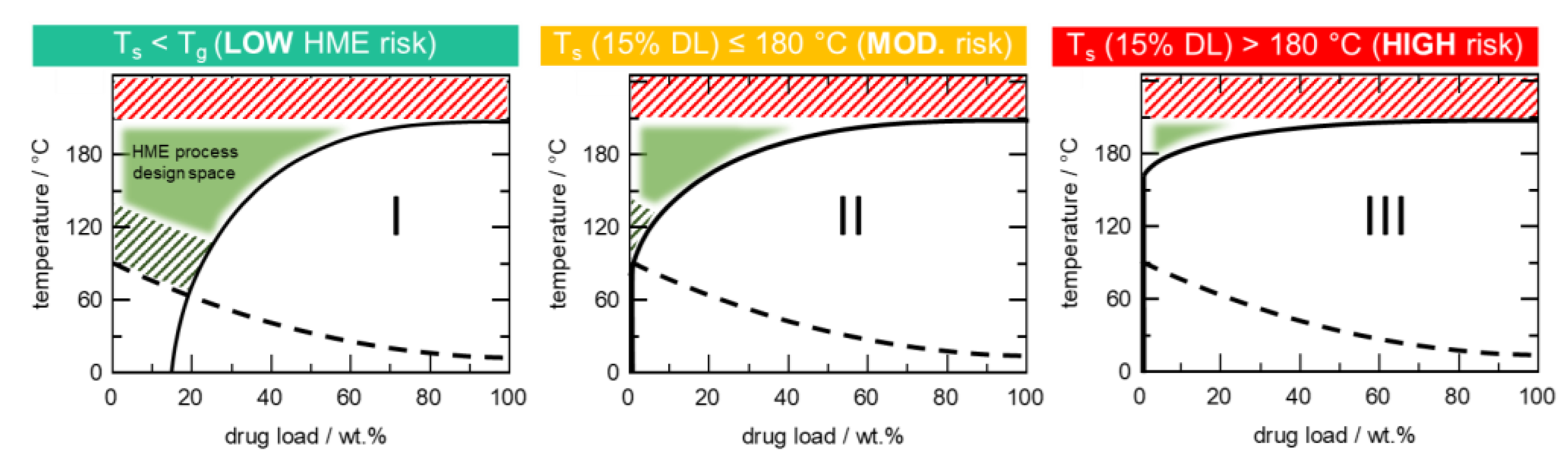
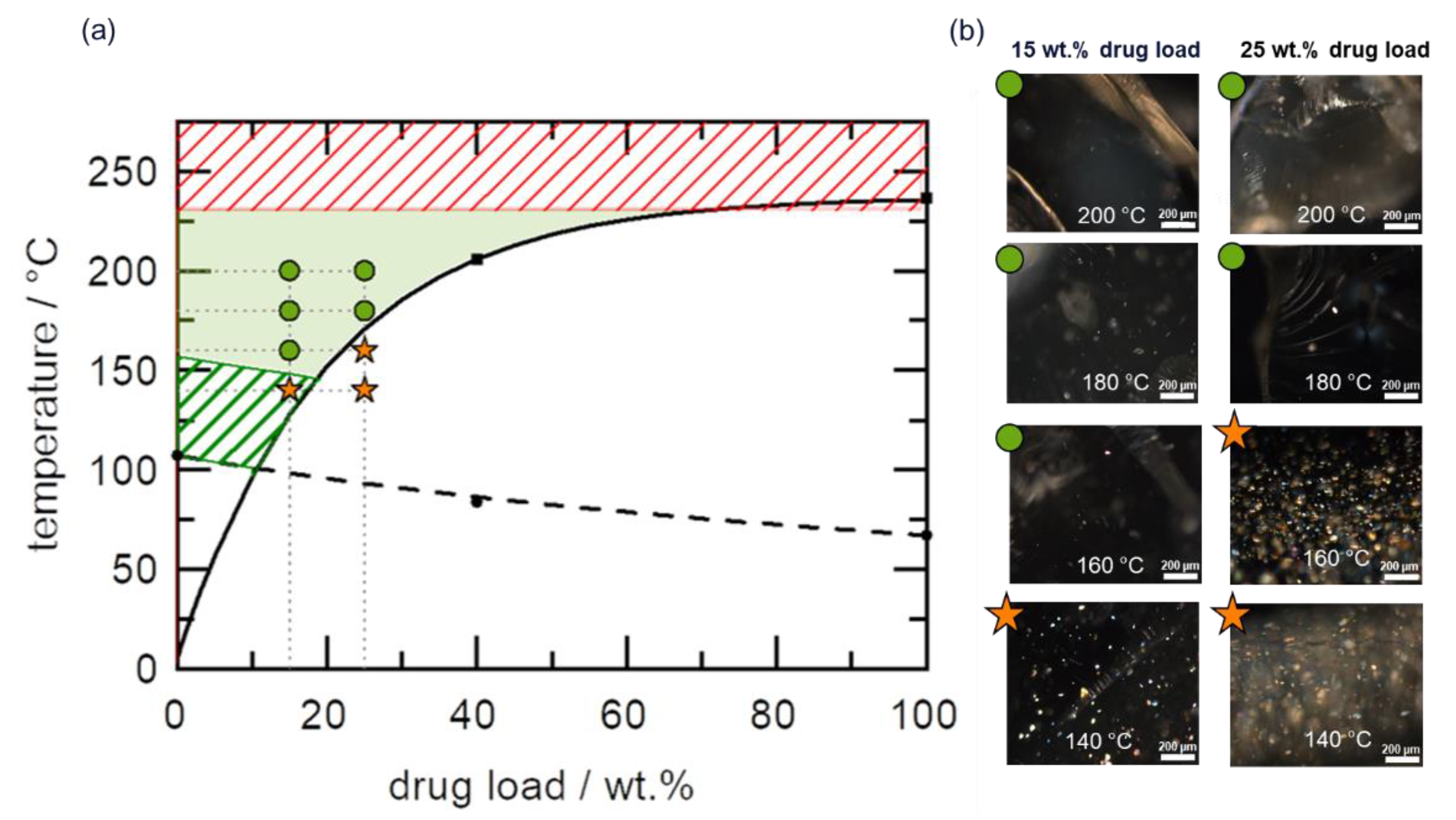
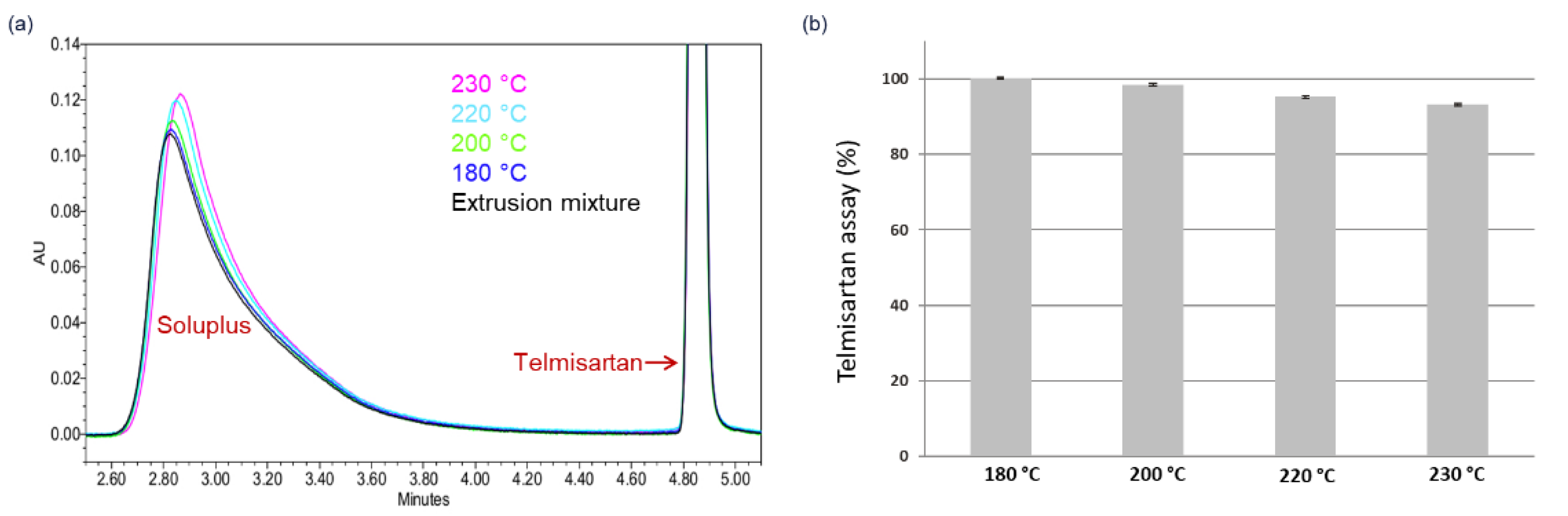
4.1. HCS Class I System
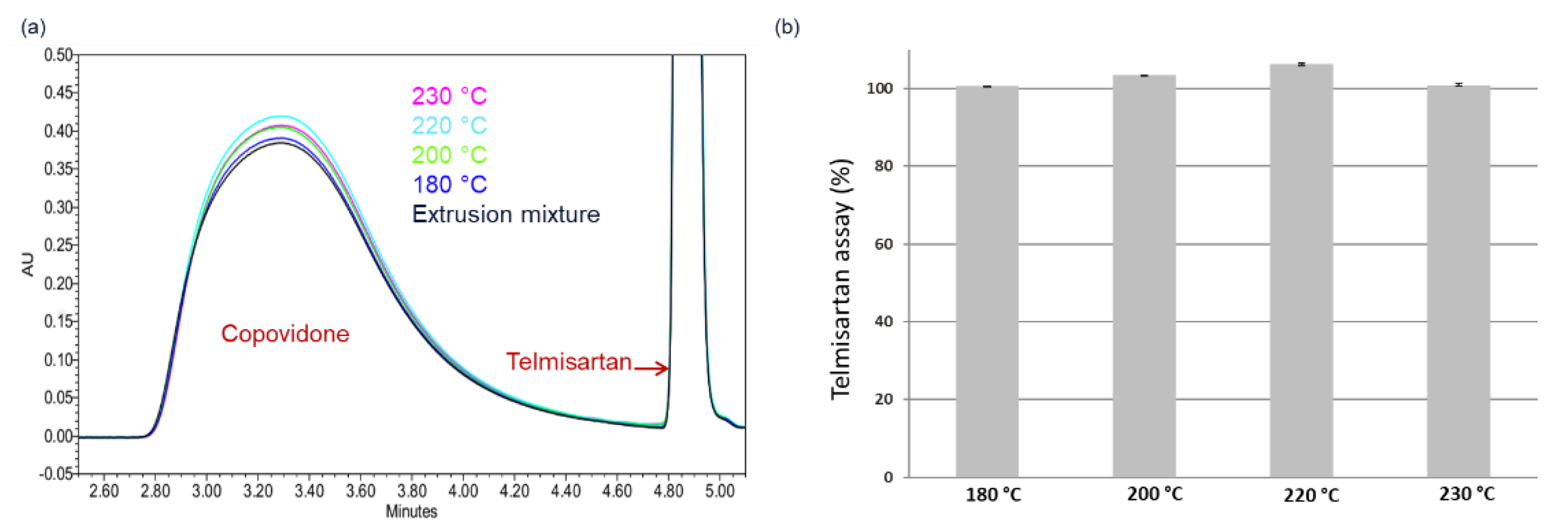
4.2. HCS Class II System
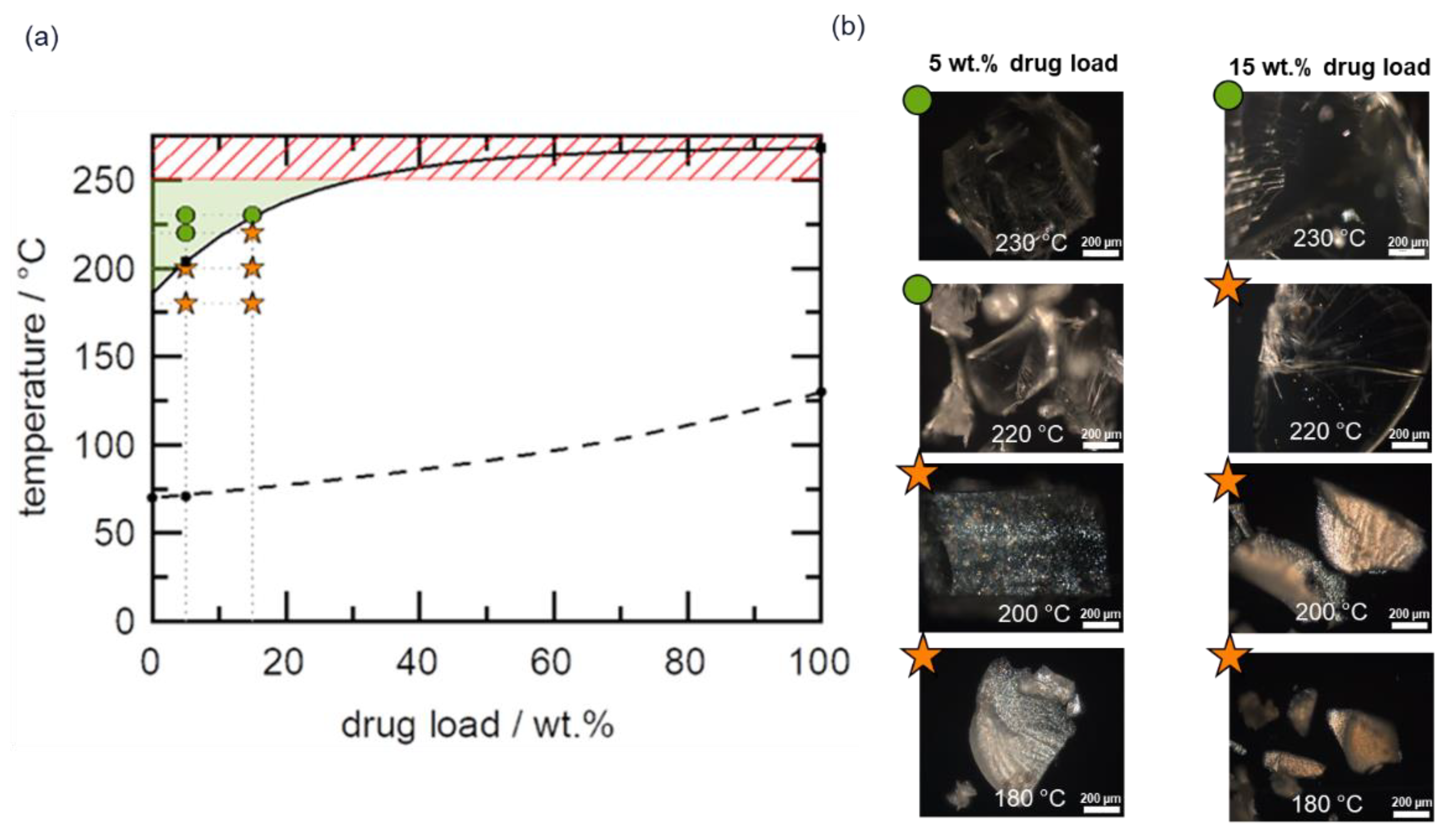
4.3. HCS Class III System
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| A | Dimensionless fitting parameter |
| APC™ | Advanced Polymer Chromatography™ |
| API | Active pharmaceutical ingredient |
| ASD | Amorphous solid dispersion |
| C | Dimensionless fitting parameter |
| CQA | Critical quality attribute |
| DL | Drug load |
| HCS | HME risk classification system |
| HME | Hot-melt extrusion |
| K | Kelvin |
| PLM | Polarized light microscopy |
| QbD | Quality-by-design |
| Solubility temperature | |
| Tg | Glass transition temperature |
| API melting temperature | |
| wAPI | API load |
| XRPD | X-ray powder diffraction |
References
- Sekiguchi, K.; Obi, N. Studies on Absorption of Eutectic Mixture. I. A Comparison of the Behavior of Eutectic Mixture of Sulfathiazole and that of Ordinary Sulfathiazole in Man. Chem. Pharm. Bull. 1961, 9, 866–872. [Google Scholar] [CrossRef] [Green Version]
- Ford, J.L. The current status of solid dispersions. Pharma. Acta Helv. 1986, 61, 69–88. [Google Scholar]
- Alam, M.A.; Ali, R.; Al-Jenoobi, F.I.; Al-Mohizea, A.M. Solid dispersions: A strategy for poorly aqueous soluble drugs and technology updates. Expert Opin. Drug Deliv. 2012, 9, 1419–1440. [Google Scholar] [CrossRef] [PubMed]
- Allawadi, D.; Singh, N.; Singh, S.; Arora, S. Solid dispersions: A review on drug delivery system and solubility enhancement. IJPSR 2013, 4, 2094–2105. [Google Scholar]
- Mendonsa, N.; Almutairy, B.; Kallakunta, V.R.; Sarabu, S.; Thipsay, P.; Bandari, S.; Repka, M.A. Manufacturing strategies to develop amorphous solid dispersions: An overview. J. Drug Deliv. Sci. Technol. 2020, 55, 101459. [Google Scholar] [CrossRef]
- Schittny, A.; Huwyler, J.; Puchkow, M. Mechanisms of increased bioavailability through amorphous solid dispersions: A review. Drug Deliv. 2020, 27, 110–127. [Google Scholar] [CrossRef]
- Tran, P.; Pyo, Y.-C.; Kim, D.-H.; Lee, S.-E.; Kim, J.-K.; Park, J.-S. Overview of the Manufacturing Methods of Solid Dispersion Technology for Improving the Solubility of Poorly Water-Soluble Drugs and Application to Anticancer Drugs. Pharmaceutics 2019, 11, 132. [Google Scholar] [CrossRef] [Green Version]
- Vasconcelos, T.; Marques, S.; das Neves, J.; Sarmento, B. Amorphous solid dispersions: Rational selection of a manufacturing process. Adv. Drug Deliv. Rev. 2016, 100, 85–101. [Google Scholar] [CrossRef]
- Vo, C.L.-N.; Park, C.; Lee, B.-J. Current trends and future perspectives of solid dispersions containing poorly water-soluble drugs. Eur. J. Pharm. Biopharm. 2013, 85, 799–813. [Google Scholar] [CrossRef]
- Zhang, J.; Han, R.; Chen, W.; Zhang, W.; Ji, Y.; Chen, L.; Pan, H.; Yang, X.; Pan, W.; Ouyang, D. Analysis of the Literature and Patents on Solid Dispersions from 1980 to 2015. Molecules 2018, 23, 1697. [Google Scholar] [CrossRef] [Green Version]
- Ingkatawornwong, S.; Kaewnopparat, N.; Tantishaiyakul, V. Studies on aging piroxicam-polyvinylpyrrolidone solid dispersions. Die Pharm. 2001, 56, 227–230. [Google Scholar]
- Appel, L.; Curatolo, W.J.; Herbig, S.M.; Nightingale, J.; Thombre, A. Osmotic System for Delivery of Solid Amorphous Dispersions of Drugs. European Patent EP 102788A2 20000816, 26 January 2000. [Google Scholar]
- Tantishaiyakul, V.; Kaewnopparat, N.; Ingkatawornwong, S. Properties of solid dispersions of piroxicam in polyvinylpyrrolidone. Int. J. Pharm. 1999, 181, 143–151. [Google Scholar] [CrossRef]
- Prudic, A.; Ji, Y.; Sadowski, G. Thermodynamic Phase Behavior of API/Polymer Solid Dispersions. Mol. Pharm. 2014, 11, 2294–2304. [Google Scholar] [CrossRef] [PubMed]
- Breitenbach, J. Melt extrusion: From process to drug delivery technology. Eur. J. Pharm. Biopharm. 2002, 54, 107–117. [Google Scholar] [CrossRef]
- Evans, R.C.; Bochmann, E.S.; Kyeremateng, S.O.; Gryczke, A.; Wagner, K.G. Holistic QbD approach for hot-melt extrusion process design space evaluation: Linking materials science, experimentation and process modeling. Eur. J. Pharm. Biopharm. 2019, 141, 149–160. [Google Scholar] [CrossRef]
- Evans, R.C.; Kyeremateng, S.O.; Asmus, L.; Degenhardt, M.; Rosenberg, J.; Wagner, K.G. Development and Performance of a Highly Sensitive Model Formulation Based on Torasemide to Enhance Hot-Melt Extrusion Process Understanding and Process Development. AAPS PharmSciTech 2018, 19, 1592–1605. [Google Scholar] [CrossRef]
- Maniruzzaman, M.; Boateng, J.S.; Snowden, M.J.; Douroumis, D. A review of hot-melt extrusion: Process technology to pharmaceutical products. ISRN Pharm. 2012, 2012, 436763. [Google Scholar] [CrossRef] [Green Version]
- Kolter, K.; Karl, M.; Gryczke, A. Hot-Melt Extrusion with BASF Pharma Polymers, 2nd ed.; BASF: Ludwigshafen, Germany, 2012; pp. 18–30. [Google Scholar]
- Bhujbal, S.V.; Mitra, B.; Jain, U.; Gong, Y.; Agrawal, A.; Karki, S.; Taylor, L.S.; Kumar, S.; Tony Zhou, Q. Pharmaceutical amorphous solid dispersion: A review of manufacturing strategies. Acta Pharm. Sinica. B 2021, 11, 2505–2536. [Google Scholar] [CrossRef]
- Thompson, S.A.; Davis, D.A.; Moon, C.; Williams, R.O. Increasing Drug Loading of Weakly Acidic Telmisartan in Amorphous Solid Dispersions through pH Modification during Hot-Melt Extrusion. Mol. Pharm. 2022, 19, 318–331. [Google Scholar] [CrossRef]
- Tiwari, R.V.; Patil, H.; Repka, M.A. Contribution of hot-melt extrusion technology to advance drug delivery in the 21st century. Expert Opin. Drug Deliv. 2016, 13, 451–464. [Google Scholar] [CrossRef]
- Lin, D.; Huang, Y. A thermal analysis method to predict the complete phase diagram of drug-polymer solid dispersions. Int. J. Pharm. 2010, 399, 109–115. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Booth, J.; Meehan, E.; Jones, D.S.; Li, S.; Andrews, G.P. Construction of drug-polymer thermodynamic phase diagrams using Flory-Huggins interaction theory: Identifying the relevance of temperature and drug weight fraction to phase separation within solid dispersions. Mol. Pharm. 2013, 10, 236–248. [Google Scholar] [CrossRef] [PubMed]
- Lehmkemper, K.; Kyeremateng, S.O.; Heinzerling, O.; Degenhardt, M.; Sadowski, G. Impact of Polymer Type and Relative Humidity on the Long-Term Physical Stability of Amorphous Solid Dispersions. Mol. Pharm. 2017, 14, 4374–4386. [Google Scholar] [CrossRef] [PubMed]
- Lehmkemper, K.; Kyeremateng, S.O.; Heinzerling, O.; Degenhardt, M.; Sadowski, G. Long-Term Physical Stability of PVP- and PVPVA-Amorphous Solid Dispersions. Mol. Pharm. 2017, 14, 157–171. [Google Scholar] [CrossRef] [PubMed]
- Hancock, B.C.; Shamblin, S.L.; Zografi, G. Molecular Mobility of Amorphous Pharmaceutical Solids Below Their Glass Transition Temperatures. Pharm. Res. 1995, 12, 799–806. [Google Scholar] [CrossRef]
- Moseson, D.E.; Taylor, L.S. The application of temperature-composition phase diagrams for hot melt extrusion processing of amorphous solid dispersions to prevent residual crystallinity. Int. J. Pharm. 2018, 553, 454–466. [Google Scholar] [CrossRef]
- Thakore, S.D.; Akhtar, J.; Jain, R.; Paudel, A.; Bansal, A.K. Analytical and Computational Methods for the Determination of Drug-Polymer Solubility and Miscibility. Mol. Pharm. 2021, 18, 2835–2866. [Google Scholar] [CrossRef]
- Kyeremateng, S.O.; Pudlas, M.; Woehrle, G.H. A fast and reliable empirical approach for estimating solubility of crystalline drugs in polymers for hot melt extrusion formulations. J. Pharm. Sci. 2014, 103, 2847–2858. [Google Scholar] [CrossRef]
- Iemtsev, A.; Hassouna, F.; Mathers, A.; Klajmon, M.; Dendisová, M.; Malinová, L.; Školáková, T.; Fulem, M. Physical stability of hydroxypropyl methylcellulose-based amorphous solid dispersions: Experimental and computational study. Int. J. Pharm. 2020, 589, 119845. [Google Scholar] [CrossRef]
- Theil, F.; Anantharaman, S.; Kyeremateng, S.O.; van Lishaut, H.; Dreis-Kühne, S.H.; Rosenberg, J.; Mägerlein, M.; Woehrle, G.H. Frozen in Time: Kinetically Stabilized Amorphous Solid Dispersions of Nifedipine Stable after a Quarter Century of Storage. Mol. Pharm. 2017, 14, 183–192. [Google Scholar] [CrossRef]
- Ojo, A.T.; Lee, P.I. A Mechanistic Model for Predicting the Physical Stability of Amorphous Solid Dispersions. J. Pharm. Sci. 2021, 110, 1495–1512. [Google Scholar] [CrossRef] [PubMed]
- Mathers, A.; Hassouna, F.; Klajmon, M.; Fulem, M. Comparative Study of DSC-Based Protocols for API-Polymer Solubility Determination. Mol. Pharm. 2021, 18, 1742–1757. [Google Scholar] [CrossRef] [PubMed]
- Mathers, A.; Hassouna, F.; Malinová, L.; Merna, J.; Růžička, K.; Fulem, M. Impact of Hot-Melt Extrusion Processing Conditions on Physicochemical Properties of Amorphous Solid Dispersions Containing Thermally Labile Acrylic Copolymer. J. Pharm. Sci. 2020, 109, 1008–1019. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Huang, S.; Lowinger, M.B.; Liu, X.; Lu, X.; Su, Y.; Williams, R.O. Influence of mechanical and thermal energy on nifedipine amorphous solid dispersions prepared by hot melt extrusion: Preparation and physical stability. Int. J. Pharm. 2019, 561, 324–334. [Google Scholar] [CrossRef] [PubMed]
- Fox, T.G. Influence of Diluent and of Copolymer Composition on the Glass Temperature of a Polymer System. Bull. Am. Phys. Soc. 1956, 1, 123. [Google Scholar]
- Britalan, E.; Hoelig, P.; Lindley, D.J.; Sanzgiri, Y.D.; Tong, P. Melt-Extruded Solid Dispersions Containing an Apoptosis-Inducing Agent. U.S. Patent US20120108590A1, 5 October 2010. [Google Scholar]
- Breitenbach, J.; Alani, L.; Berndl, G.; Gosh, S.; Liepold, B.; Reinhold, U.; Rosenberg, J. Abbott Laboratories assignee. Solid dosage pharmaceutical form. U.S. Patent US20050048112A1, 28 August 2003. [Google Scholar]
- Harmon, P.A.; Variankaval, N. Solid Dosage Formulations of an Orexin Receptor Antagonist. U.S. Patent US10098892 B2, 29 May 2013. [Google Scholar]
- Rosenberg, J.; Berndl, G.; Neumann, J.; Breitenbach, J. Compositions and Dosage Forms for Application in the Oral Cavity in the Treatment of Mykoses. WIPO Patent WO 2002011694 A2, 14 February 2002. [Google Scholar]
- Sever, N.; Westedt, U.; Lander, U.; Schneider, K.; Steitz, B.; Mueller, T.; Reul, R.; Obermiller, C.; Jayasankar, A.; Simon, M.; et al. Solid Pharmaceutical Compositions for Treating HCV. WIPO Patent WO 2016210273, 29 December 2016. [Google Scholar]
- Lu, J.; Obara, S.; Ioannidis, N.; Suwardie, J.; Gogos, C.; Kikuchi, S. Understanding the Processing Window of Hypromellose Acetate Succinate for Hot-Melt Extrusion, Part I: Polymer Characterization and Hot-Melt Extrusion. Adv. Polym. Technol. 2018, 37, 154–166. [Google Scholar] [CrossRef]
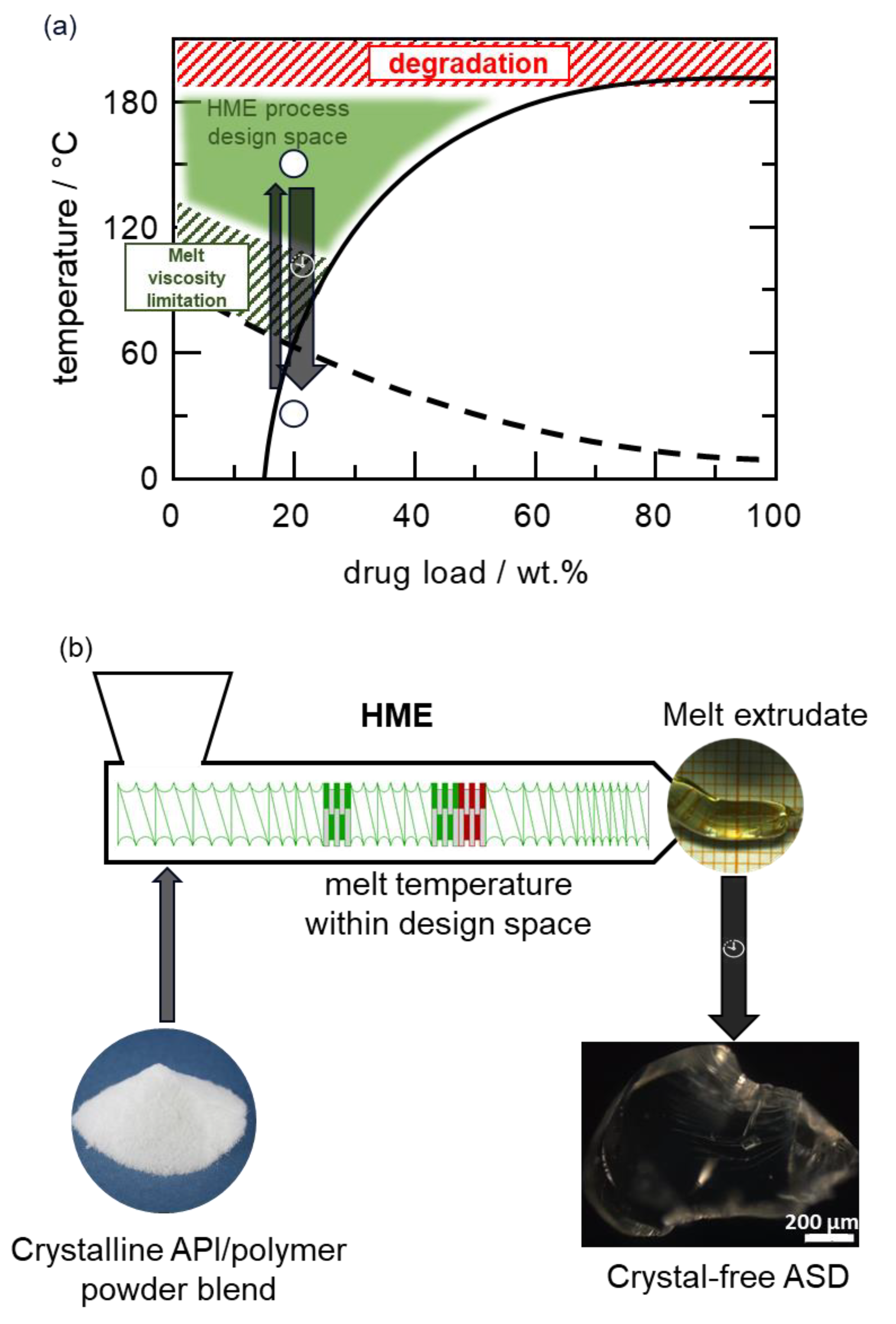


| Melting Temperature/°C | Thermal Degradation Temperature/°C | Tg/°C | |
|---|---|---|---|
| Telmisartan | 269 | 280 | 129 |
| Sulfamerazine | 236 | 246 | 129 |
| Copovidone | - | 230 | 107 |
| Soluplus | - | 250 | 70 |
| API–Polymer System | Drug Load (DL)/wt.% | Solubility Temperature 1/°C | Tg/°C | Parameter A | Parameter C |
|---|---|---|---|---|---|
| Sulfamerazine–Copovidone | 40 | 206.0 | 83.8 | 231.7 | 1 |
| Sulfamerazine–Soluplus | 40 | 217.0 | 66.0 | 150.4 | 1 |
| Telmisartan–Copovidone | 10 | 212.7 | 107.8 | 91.2 | 0 |
| Telmisartan–Soluplus | 5 | 203.7 | 70.7 | 82.5 | 0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kyeremateng, S.O.; Voges, K.; Dohrn, S.; Sobich, E.; Lander, U.; Weber, S.; Gessner, D.; Evans, R.C.; Degenhardt, M. A Hot-Melt Extrusion Risk Assessment Classification System for Amorphous Solid Dispersion Formulation Development. Pharmaceutics 2022, 14, 1044. https://doi.org/10.3390/pharmaceutics14051044
Kyeremateng SO, Voges K, Dohrn S, Sobich E, Lander U, Weber S, Gessner D, Evans RC, Degenhardt M. A Hot-Melt Extrusion Risk Assessment Classification System for Amorphous Solid Dispersion Formulation Development. Pharmaceutics. 2022; 14(5):1044. https://doi.org/10.3390/pharmaceutics14051044
Chicago/Turabian StyleKyeremateng, Samuel O., Kristin Voges, Stefanie Dohrn, Ekaterina Sobich, Ute Lander, Stefan Weber, David Gessner, Rachel C. Evans, and Matthias Degenhardt. 2022. "A Hot-Melt Extrusion Risk Assessment Classification System for Amorphous Solid Dispersion Formulation Development" Pharmaceutics 14, no. 5: 1044. https://doi.org/10.3390/pharmaceutics14051044
APA StyleKyeremateng, S. O., Voges, K., Dohrn, S., Sobich, E., Lander, U., Weber, S., Gessner, D., Evans, R. C., & Degenhardt, M. (2022). A Hot-Melt Extrusion Risk Assessment Classification System for Amorphous Solid Dispersion Formulation Development. Pharmaceutics, 14(5), 1044. https://doi.org/10.3390/pharmaceutics14051044