Evaluation of the Anti-Histoplasma capsulatum Activity of Indole and Nitrofuran Derivatives and Their Pharmacological Safety in Three-Dimensional Cell Cultures
Abstract
1. Introduction
2. Materials and Methods
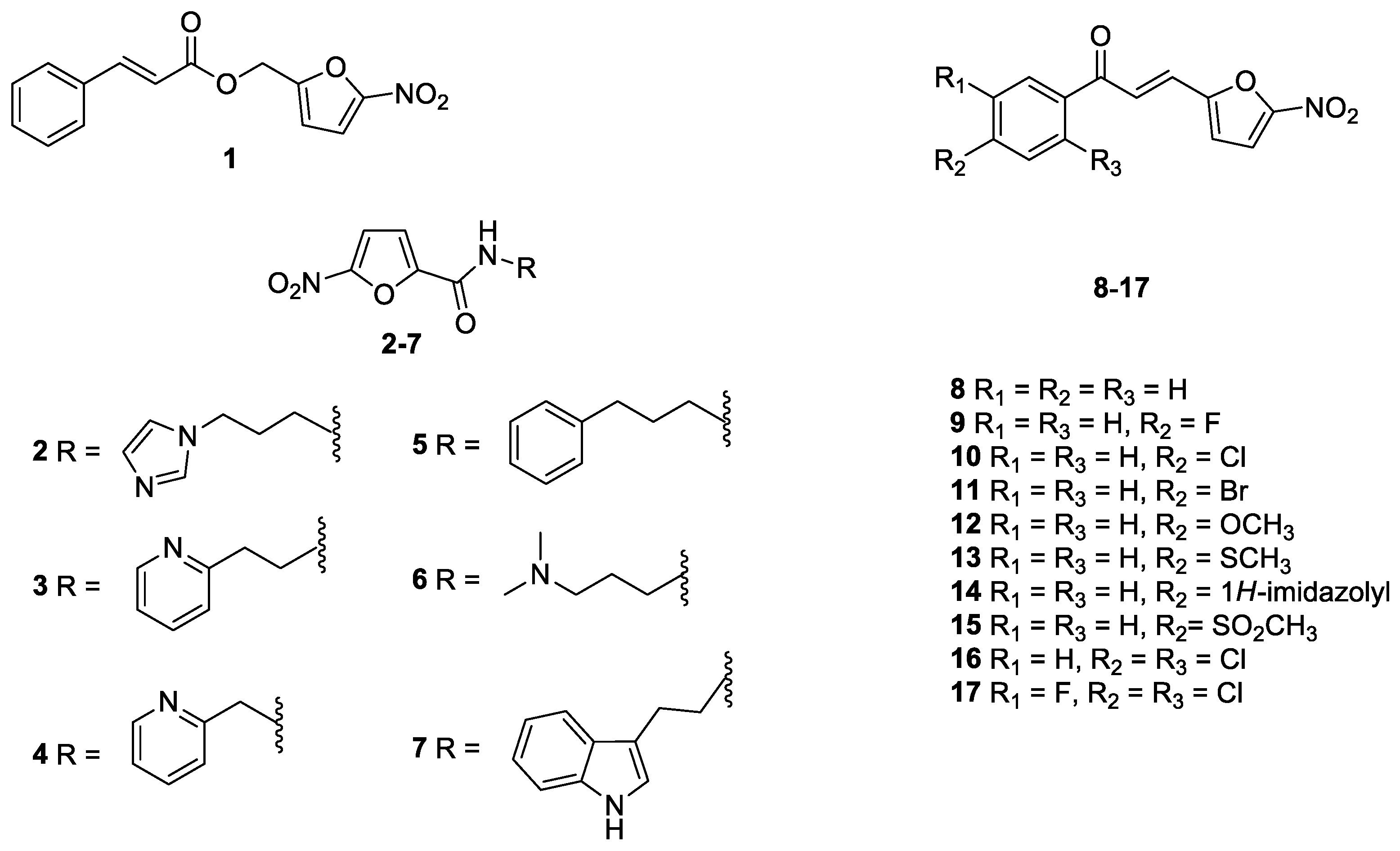
2.1. Antifungal Drugs, Indole and Nitrofuran Derivatives
2.2. Microorganisms and Culture Conditions
2.3. Susceptibility of H. capsulatum to Nitrofuran and Indole Derivates and Antifungal Drugs and Determination of Minimum Fungicide Concentration (MFC)
2.3.1. Determination of Minimum Inhibitory Concentration (MIC90)
2.3.2. Determination of Minimum Fungicide Concentration (MFC)
2.4. Cell Lines Mantainance
2.4.1. Characterization of Three-Dimensional Cell Culture (3D)
Diameter Establishment
Cell Quantification and Viability Using Trypan Blue
Cell Viability—Qualitative
External Cell Morphology of Spheroids
Confocal Fluorescence Microscopy
2.4.2. Cytotoxicity Assay in Three-Dimensional (3D) and Monolayer (2D) Models by Resazurin Colorimetric Method
2.5. Determination of the Mechanisms of Action
2.5.1. Ergosterol Dosage
2.5.2. Evaluation of Morphology and Cell Wall Damage Using Calcofluor White Staining and Laser Scanning Confocal Microscopy
2.5.3. Quantification of Reactive Oxygen Species (ROS)
2.5.4. Apoptosis/Necrosis Assay
2.6. Statistical Analysis
3. Results
3.1. Determination of MIC90 and MFC
3.2. Three-Dimensional Cell Culture
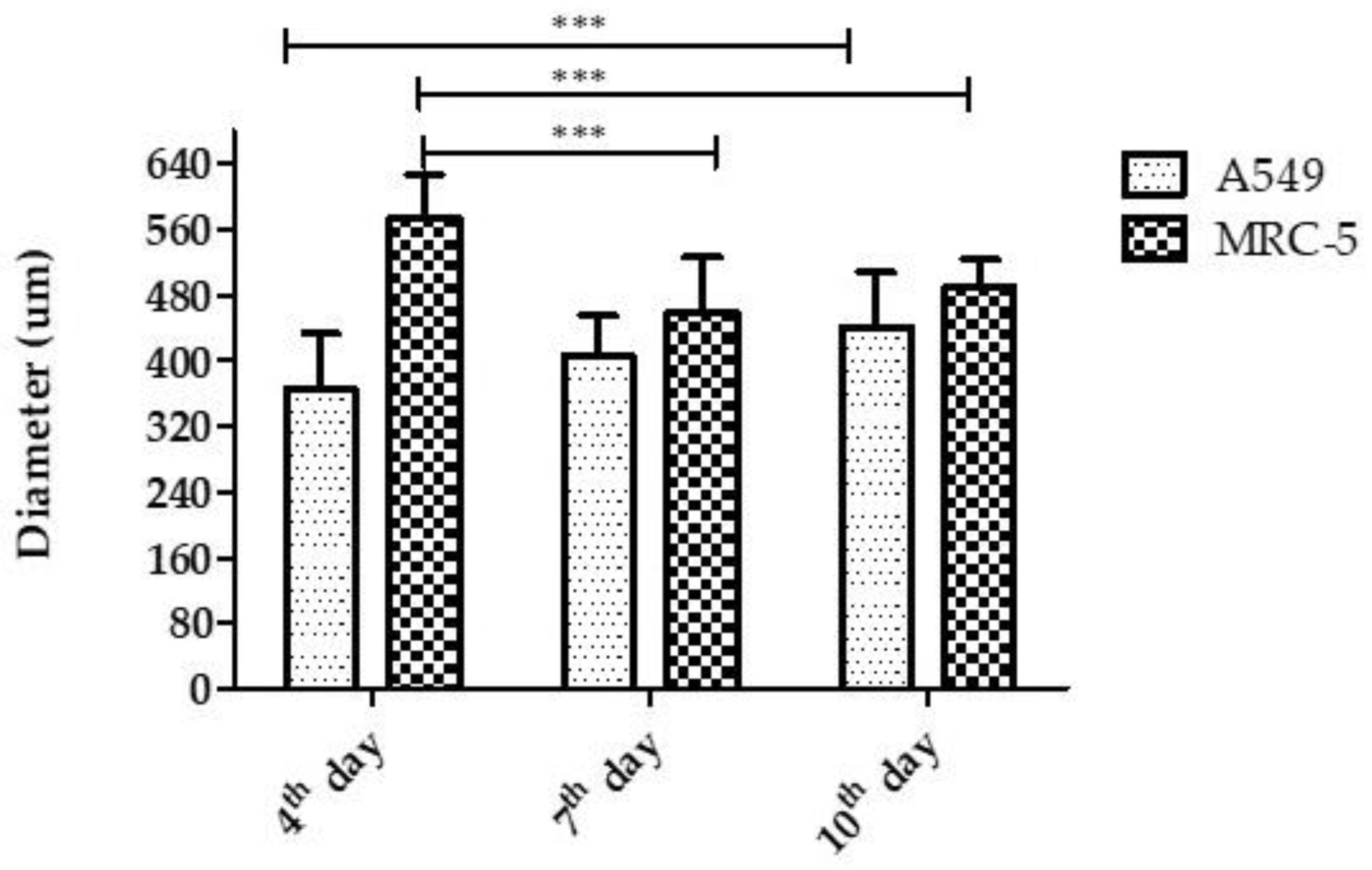
3.2.1. Diameter
3.2.2. Quantification and Cell Viability Using Trypan Blue
3.2.3. Cell Viability Using the Resazurin Colorimetric Method
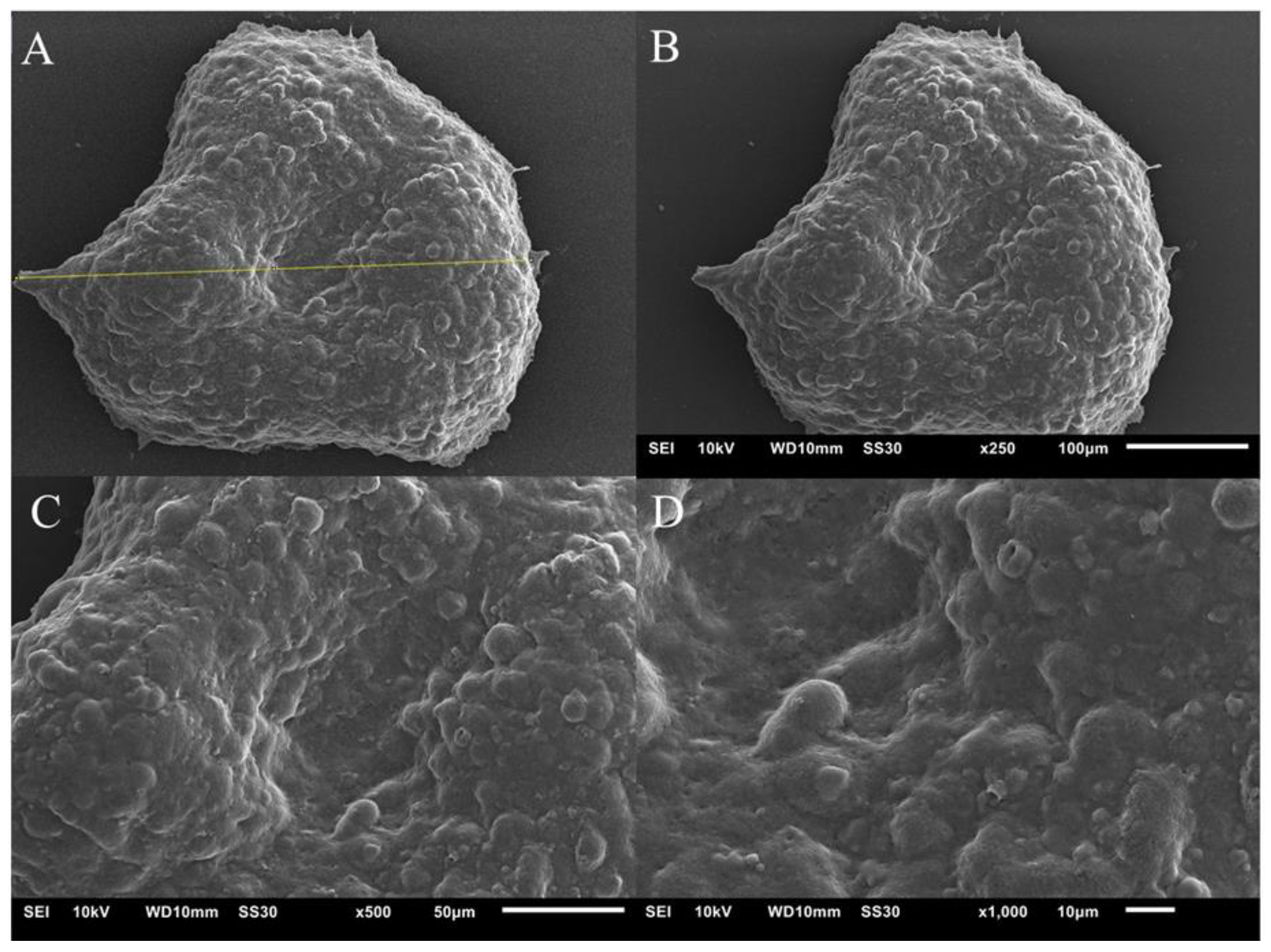
3.2.4. Scanning Electron Microscopy
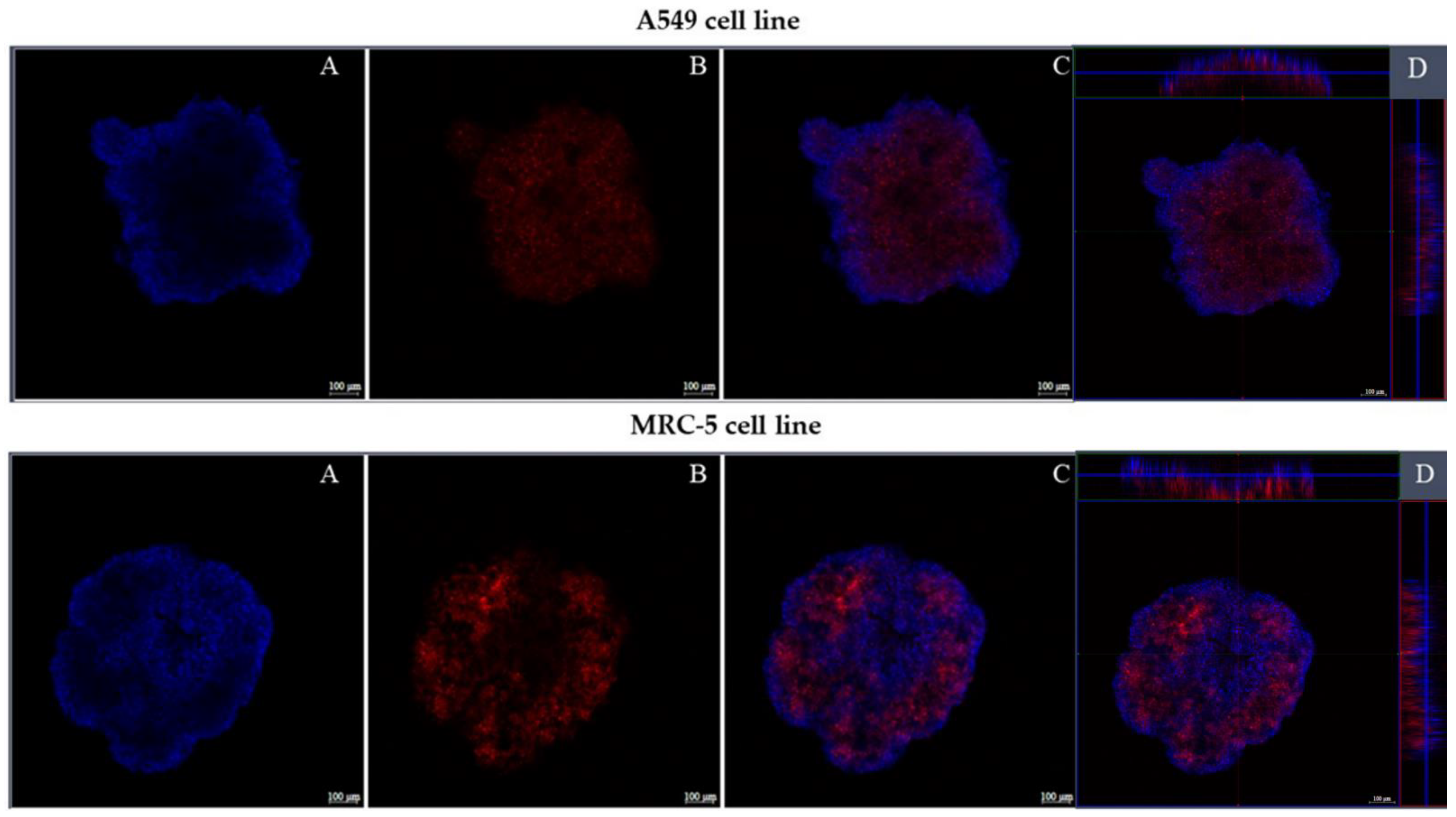
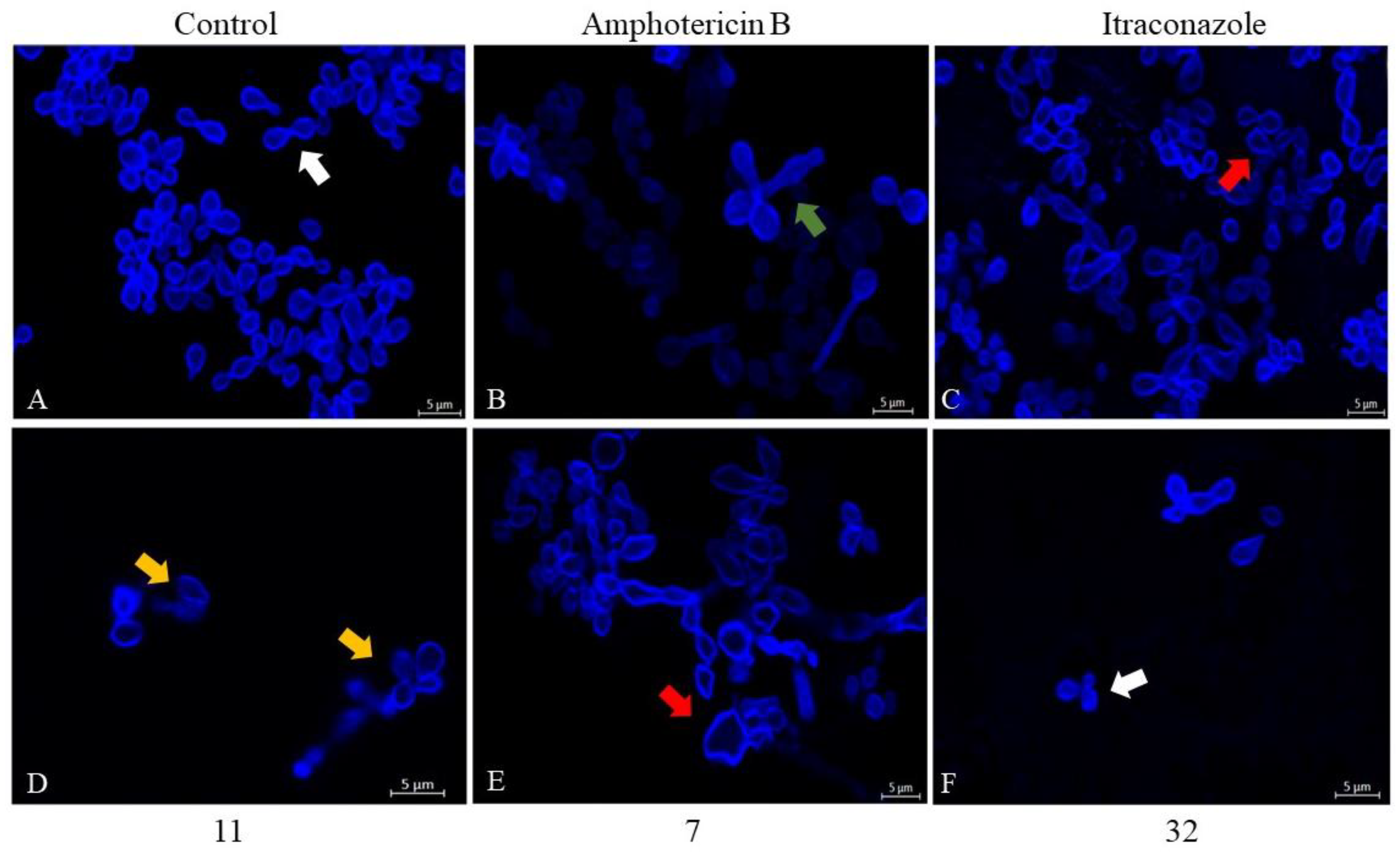
3.2.5. Confocal Fluorescence Microscopy
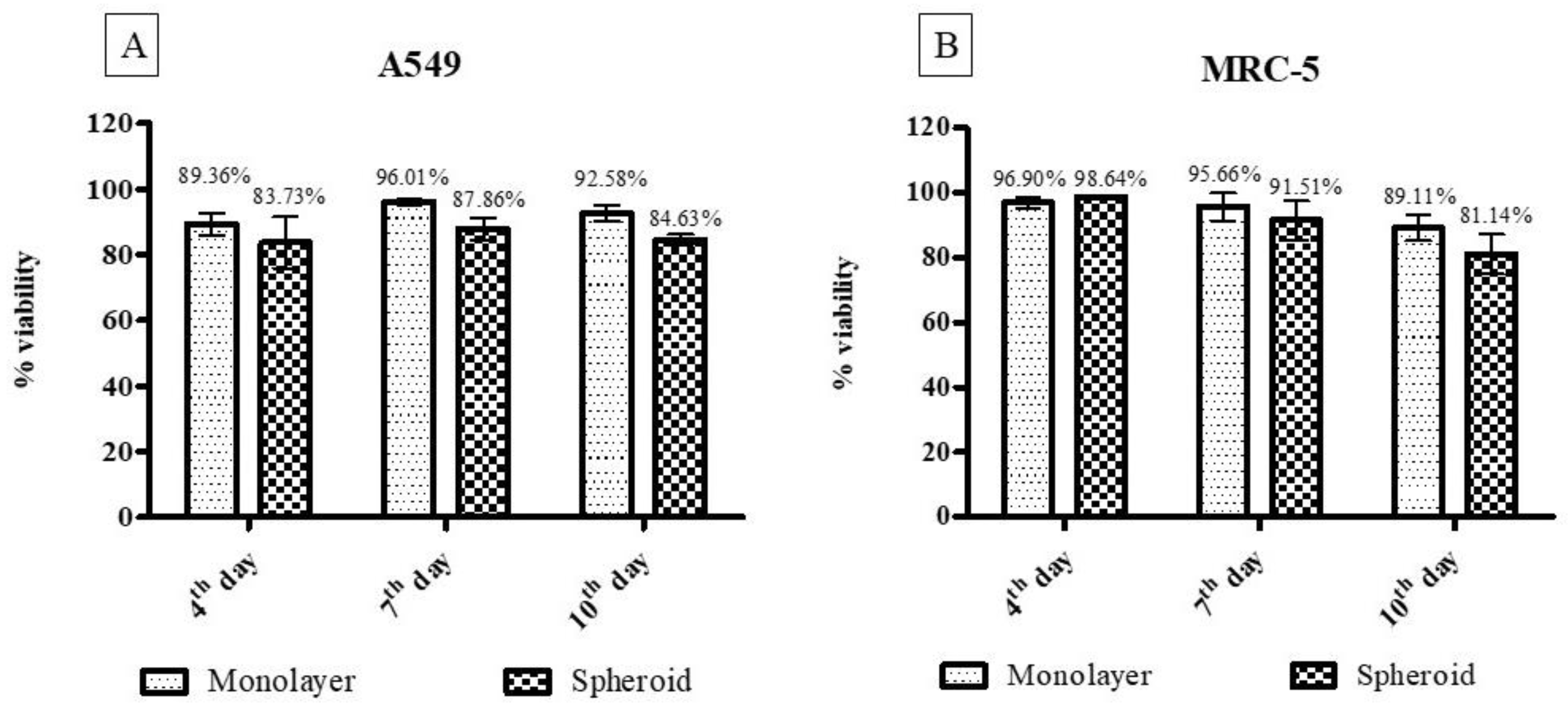
3.2.6. Cytotoxicity Assay for Three-Dimensional and Monolayer Cell Culture
3.3. Determination of the Mechanisms of Action
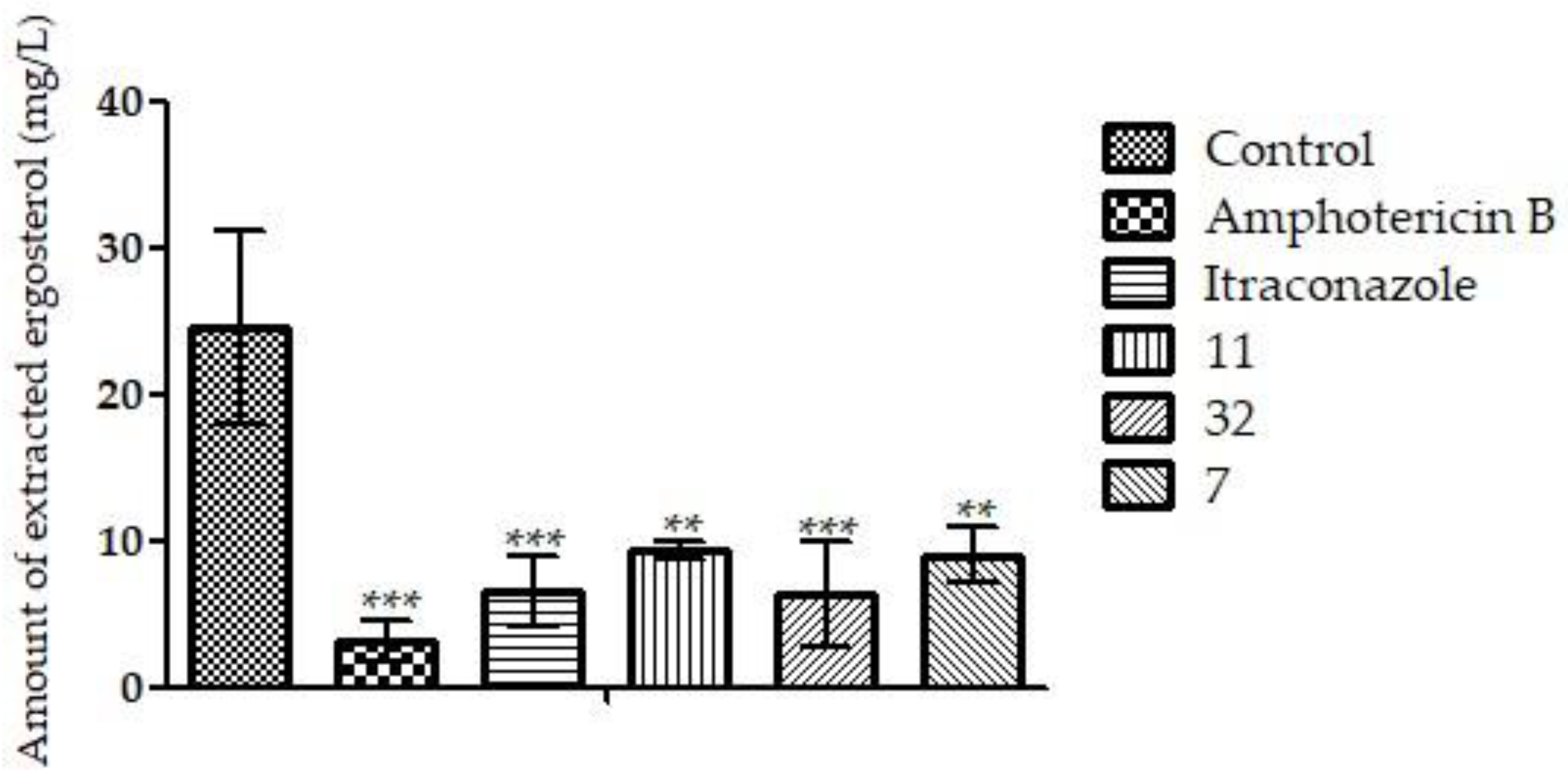
3.3.1. Ergosterol Dosage
3.3.2. Evaluation of Morphology and Wall Damage
3.3.3. Reactive Oxygen Species (ROS)
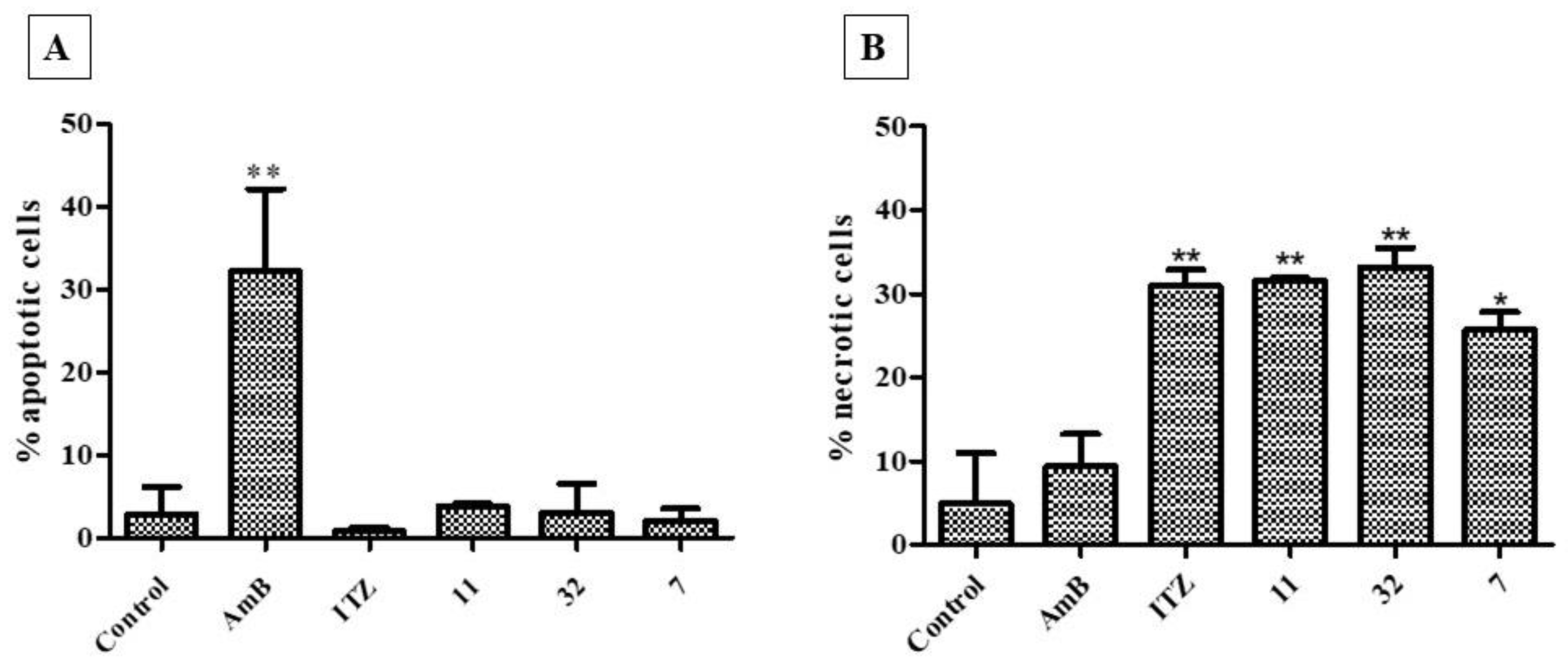
3.3.4. Cell Death Assay (Necrosis–Apoptosis)
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wheat, L.J.; Azar, M.M.; Bahr, N.C.; Spec, A.; Relich, R.F.; Hage, C. Histoplasmosis. Infect. Dis. Clin. North. Am. 2016, 30, 207–227. [Google Scholar] [CrossRef] [PubMed]
- Bongomin, F.; Kwizera, R.; Denning, D.W. Getting Histoplasmosis on the Map of International Recommendations for Patients with Advanced HIV Disease. J. Fungi. 2019, 5, 80. [Google Scholar] [CrossRef] [PubMed]
- Bongomin, F.; Gago, S.; Oladele, R.O.; Denning, D.W. Global and Multi-National Prevalence of Fungal Diseases—Estimate Precision. J. Fungi. 2017, 3, 57. [Google Scholar] [CrossRef] [PubMed]
- Goughenour, K.D.; Rappleye, C.A. Antifungal therapeutics for dimorphic fungal pathogens. Virulence 2016, 8, 211–221. [Google Scholar] [CrossRef]
- Ashraf, N.; Kubat, R.C.; Poplin, V.; Adenis, A.A.; Denning, D.W.; Wright, L.; McCotter, O.; Schwartz, I.S.; Jackson, B.R.; Chiller, T.; et al. Re-drawing the Maps for Endemic Mycoses. Mycopathologia 2020, 185, 843–865. [Google Scholar] [CrossRef]
- Ferreira, M.S.; Borges, A.S. Histoplasmose. Rev. Soc. Bras. Med. Trop. 2009, 42, 192–198. [Google Scholar] [CrossRef]
- Araúz, A.B.; Papineni, P. Histoplasmosis. Infect. Dis. Clin. North. Am. 2021, 35, 471–491. [Google Scholar] [CrossRef]
- Kauffman, C.A. Histoplasmosis. Clin. Chest Med. 2009, 30, 217–225. [Google Scholar] [CrossRef]
- Wheat, L.J.; Connolly, P.; Smedema, M.; Durkin, M.; Brizendine, E.; Mann, P.; Patel, R.; McNicholas, P.M.; Goldman, M. Activity of newer triazoles against Histoplasma capsulatum from patients with AIDS who failed fluconazole. J. Antimicrob. Chemother. 2006, 57, 1235–1239. [Google Scholar] [CrossRef]
- Azar, M.M.; Hage, C.A. Clinical Perspectives in the Diagnosis and Management of Histoplasmosis. Clin. Chest Med. 2017, 38, 403–415. [Google Scholar] [CrossRef]
- Bates, D.W.; Su, L.; Yu, D.T.; Chertow, G.M.; Seger, D.L.; Gomes, D.R.J.; Dasbach, E.J.; Platt, R. Mortality and Costs of Acute Renal Failure Associated with Amphotericin B Therapy. Clin. Infect. Dis. 2001, 32, 686–693. [Google Scholar] [CrossRef]
- Fuentefria, A.; Pippi, B.; Lana, D.D.; Donato, K.; Andrade, S. Antifungals discovery: An insight into new strategies to combat antifungal resistance. Lett. Appl. Microbiol. 2017, 66, 2–13. [Google Scholar] [CrossRef]
- Chang, Y.-L.; Yu, S.-J.; Heitman, J.; Wellington, M.; Chen, Y.-L. New facets of antifungal therapy. Virulence 2016, 8, 222–236. [Google Scholar] [CrossRef]
- Campoy, S.; Adrio, J.L. Antifungals. Biochem. Pharmacol. 2017, 133, 86–96. [Google Scholar] [CrossRef]
- Krasavin, M.; Parchinsky, V.; Kantin, G.; Manicheva, O.; Dogonadze, M.; Vinogradova, T.; Karge, B.; Brönstrup, M. New nitrofurans amenable by isocyanide multicomponent chemistry are active against multidrug-resistant and poly-resistant Mycobacterium tuberculosis. Bioorganic. Med. Chem. 2017, 25, 1867–1874. [Google Scholar] [CrossRef]
- Zhao, H.; Guo, W.; Quan, W.; Jiang, J.; Qu, B. Occurrence and levels of nitrofuran metabolites in sea cucumber from Dalian, China. Food Addit. Contam. Part A 2016, 33, 1672–1677. [Google Scholar] [CrossRef]
- Falk, N.; Berenstein, A.J.; Moscatelli, G.; Moroni, S.; González, N.; Ballering, G.; Freilij, H.; Altcheh, J. Effectiveness of Nifurtimox in the Treatment of Chagas Disease: A Long-Term Retrospective Cohort Study in Children and Adults. Antimicrob. Agents Chemother. 2022, e02021. [Google Scholar] [CrossRef]
- Abbott, A.; Montgomery, S.P.; Chancey, R.J. Characteristics and Adverse Events of Patients for Whom Nifurtimox Was Released Through CDC-Sponsored Investigational New Drug Program for Treatment of Chagas Disease—United States, 2001–2021. MMWR. Morb. Mortal. Wkly. Rep. 2022, 71, 371–374. [Google Scholar] [CrossRef]
- Aldeek, F.; Hsieh, K.C.; Ugochukwu, O.N.; Gerard, G.; Hammack, W. Accurate Quantitation and Analysis of Nitrofuran Metabolites, Chloramphenicol, and Florfenicol in Seafood by Ultrahigh-Performance Liquid Chromatography–Tandem Mass Spectrometry: Method Validation and Regulatory Samples. J. Agric. Food Chem. 2017, 66, 5018–5030. [Google Scholar] [CrossRef]
- Bs, K.C.; Stocks, E.; Bhat, D.; Fish, J.; Rubin, C.D. How Common are Pulmonary and Hepatic Adverse Effects in Older Adults Prescribed Nitrofurantoin? J. Am. Geriatr. Soc. 2017, 65, 1316–1320. [Google Scholar] [CrossRef]
- Behrouzi-Fardmoghadam, M.; Pourrajab, F.; Ardestani, S.K.; Emami, S.; Shafiee, A.; Foroumadi, A. Synthesis and in vitro anti-leishmanial activity of 1-[5-(5-nitrofuran-2-yl)-1,3,4-thiadiazol-2-yl]- and 1-[5-(5-nitrothiophen-2-yl)-1,3,4-thiadiazol-2-yl]-4-aroylpiperazines. Bioorganic Med. Chem. 2008, 16, 4509–4515. [Google Scholar] [CrossRef]
- Maya, J.; Bollo, S.; Nuñez-Vergara, L.J.; Squella, J.A.; Repetto, Y.; Morello, A.; Périé, J.; Chauvière, G. Trypanosoma cruzi: Effect and mode of action of nitroimidazole and nitrofuran derivatives. Biochem. Pharmacol. 2003, 65, 999–1006. [Google Scholar] [CrossRef]
- Zuma, N.H.; Aucamp, J.; N’Da, D.D. An update on derivatisation and repurposing of clinical nitrofuran drugs. Eur. J. Pharm. Sci. 2019, 140, 105092. [Google Scholar] [CrossRef]
- De Vita, D.; Friggeri, L.; D’Auria, F.D.; Pandolfi, F.; Piccoli, F.; Panella, S.; Palamara, A.T.; Simonetti, G.; Scipione, L.; Di Santo, R.; et al. Activity of caffeic acid derivatives against Candida albicans biofilm. Bioorganic Med. Chem. Lett. 2014, 24, 1502–1505. [Google Scholar] [CrossRef]
- De Vita, D.; Simonetti, G.; Pandolfi, F.; Costi, R.; Di Santo, R.; D’Auria, F.D.; Scipione, L. Exploring the anti-biofilm activity of cinnamic acid derivatives in Candida albicans. Bioorganic Med. Chem. Lett. 2016, 26, 5931–5935. [Google Scholar] [CrossRef]
- Kamal, A.; Hussaini, S.M.A.; Sucharitha, M.L.; Poornachandra, Y.; Sultana, F.; Kumar, C.G. Synthesis and antimicrobial potential of nitrofuran–triazole congeners. Org. Biomol. Chem. 2015, 13, 9388–9397. [Google Scholar] [CrossRef]
- Trefzger, O.S.; Barbosa, N.V.; Scapolatempo, R.L.; Das Neves, A.R.; Ortale, M.L.F.S.; Carvalho, D.B.; Honorato, A.M.; Fragoso, M.R.; Shuiguemoto, C.Y.K.; Perdomo, R.T.; et al. Design, synthesis, antileishmanial, and antifungal biological evaluation of novel 3,5-disubstituted isoxazole compounds based on 5-nitrofuran scaffolds. Arch. der Pharm. 2019, 353, e1900241. [Google Scholar] [CrossRef]
- Vaso, C.O.; Pandolfi, F.; Bila, N.M.; De Vita, D.; Bortolami, M.; Mendes-Giannini, M.J.S.; Tudino, V.; Costi, R.; Costa-Orlandi, C.B.; Fusco-Almeida, A.M.; et al. Synthesis and Evaluation of the Antifungal and Toxicological Activity of Nitrofuran Derivatives. Pharmaceutics 2022, 14, 593. [Google Scholar] [CrossRef]
- Karimabad, M.N.; Mahmoodi, M.; Jafarzadeh, A.; Darekordi, A.; Hajizadeh, M.R.; Hassanshahi, G. Molecular Targets, Anti-cancer Properties and Potency of Synthetic Indole-3-carbinol Derivatives. Mini. Reviews Med. Chem. 2019, 19, 540–554. [Google Scholar] [CrossRef]
- Nieto, M.J. Indole and Indoline Scaffolds in Antimicrobials: Overview, Synthesis and Recent Advances in Antimicrobial Research. Curr. Med. Chem. 2021, 28, 4828–4844. [Google Scholar] [CrossRef]
- Crucitti, G.C.; Pescatori, L.; Messore, A.; Madia, V.N.; Pupo, G.; Saccoliti, F.; Scipione, L.; Tortorella, S.; Di Leva, F.S.; Cosconati, S.; et al. Discovery of N-aryl-naphthylamines as in vitro inhibitors of the interaction between HIV integrase and the cofactor LEDGF/p75. Eur. J. Med. Chem. 2015, 101, 288–294. [Google Scholar] [CrossRef] [PubMed]
- Guerra, A.S.H.D.S.; Malta, D.J.D.N.; Laranjeira, L.P.M.; Maia, M.B.S.; Colaço, N.C.; de Lima, M.D.C.A.; Galdino, S.L.; Pitta, I.D.R.; Gonçalves-Silva, T. Anti-inflammatory and antinociceptive activities of indole–imidazolidine derivatives. Int. Immunopharmacol. 2011, 11, 1816–1822. [Google Scholar] [CrossRef]
- Pandolfi, F.; D’Acierno, F.; Bortolami, M.; De Vita, D.; Gallo, F.; De Meo, A.; Di Santo, R.; Costi, R.; Simonetti, G.; Scipione, L. Searching for new agents active against Candida albicans biofilm: A series of indole derivatives, design, synthesis and biological evaluation. Eur. J. Med. Chem. 2019, 165, 93–106. [Google Scholar] [CrossRef] [PubMed]
- Vila, T.; Lopez-Ribot, J.L. Screening the Pathogen Box for Identification of Candida albicans Biofilm Inhibitors. Antimicrob. Agents Chemother. 2017, 61, e02006-16. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.; Go, G.; Mylonakis, E.; Kim, Y. The bacterial signalling molecule indole attenuates the virulence of the fungal pathogen Candida albicans. J. Appl. Microbiol. 2012, 113, 622–628. [Google Scholar] [CrossRef]
- Grässer, U.; Bubel, M.; Sossong, D.; Oberringer, M.; Pohlemann, T.; Metzger, W. Dissociation of mono- and co-culture spheroids into single cells for subsequent flow cytometric analysis. Ann. Anat. Anat. Anz. 2018, 216, 1–8. [Google Scholar] [CrossRef]
- Friedrich, J.; Seidel, C.; Ebner, R.; Kunz-Schughart, L.A. Spheroid-based drug screen: Considerations and practical approach. Nat. Protoc. 2009, 4, 309–324. [Google Scholar] [CrossRef]
- Doke, S.K.; Dhawale, S.C. Alternatives to animal testing: A review. Saudi Pharm. J. 2013, 23, 223–229. [Google Scholar] [CrossRef]
- Bin Kim, J. Three-dimensional tissue culture models in cancer biology. Semin. Cancer Biol. 2005, 15, 365–377. [Google Scholar] [CrossRef]
- Edmondson, R.; Broglie, J.J.; Adcock, A.F.; Yang, L. Three-Dimensional Cell Culture Systems and Their Applications in Drug Discovery and Cell-Based Biosensors. ASSAY Drug Dev. Technol. 2014, 12, 207–218. [Google Scholar] [CrossRef]
- Jensen, C.; Teng, Y. Is It Time to Start Transitioning From 2D to 3D Cell Culture? Front. Mol. Biosci. 2020, 7, 33. [Google Scholar] [CrossRef]
- Ravi, M.; Paramesh, V.; Kaviya, S.R.; Anuradha, E.; Solomon, F.D.P. 3D Cell Culture Systems: Advantages and Applications. J. Cell. Physiol. 2015, 230, 16–26. [Google Scholar] [CrossRef]
- Teixeira, M.D.M.; Patané, J.S.L.; Taylor, M.L.; Gomez, B.L.; Theodoro, R.; De Hoog, S.; Engelthaler, D.M.; Zancopé-Oliveira, R.M.; Felipe, M.S.S.; Barker, B.M. Worldwide Phylogenetic Distributions and Population Dynamics of the Genus Histoplasma. PLOS Neglected Trop. Dis. 2016, 10, e0004732. [Google Scholar] [CrossRef]
- Baltazar, L.M.; Zamith-Miranda, D.; Burnet, M.C.; Choi, H.; Nimrichter, L.; Nakayasu, E.S.; Nosanchuk, J.D. Concentration-dependent protein loading of extracellular vesicles released by Histoplasma capsulatum after antibody treatment and its modulatory action upon macrophages. Sci. Rep. 2018, 8, 8065. [Google Scholar] [CrossRef]
- Gonçalves, L.N.C.; Costa-Orlandi, C.B.; Bila, N.M.; Vaso, C.O.; Da Silva, R.A.M.; Mendes-Giannini, M.J.S.; Taylor, M.L.; Fusco-Almeida, A.M. Biofilm Formation by Histoplasma capsulatum in Different Culture Media and Oxygen Atmospheres. Front. Microbiol. 2020, 11, 1455. [Google Scholar] [CrossRef]
- Clinical and Laboratory Standards Institute (CLSI). Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts 27, (M27-A3); Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2008. [Google Scholar]
- Li, R.-K.; Ciblak, M.A.; Nordoff, N.; Pasarell, L.; Warnock, D.W.; McGinnis, M.R. In Vitro Activities of Voriconazole, Itraconazole, and Amphotericin B against Blastomyces dermatitidis, Coccidioides immitis, and Histoplasma capsulatum. Antimicrob. Agents Chemother. 2000, 44, 1734–1736. [Google Scholar] [CrossRef]
- Wheat, L.J.; Connolly, P.; Smedema, M.; Brizendine, E.; Hafner, R.; AIDS Clinical Trials Group and the Mycoses Study Group of the National Institute of Allergy and Infectious Diseases. Emergence of Resistance to Fluconazole as a Cause of Failure during Treatment of Histoplasmosis in Patients with Acquired Immunodeficiency Disease Syndrome. Clin. Infect. Dis. 2001, 33, 1910–1913. [Google Scholar] [CrossRef]
- Kathuria, S.; Singh, P.K.; Meis, J.F.; Chowdhary, A. In Vitro Antifungal Susceptibility Profile and Correlation of Mycelial and Yeast Forms of Molecularly Characterized Histoplasma capsulatum Strains from India. Antimicrob. Agents Chemother. 2014, 58, 5613–5616. [Google Scholar] [CrossRef]
- Silva, A.C.A.D.P.E.; Oliveira, H.C.; Silva, J.F.; Sangalli-Leite, F.; Scorzoni, L.; Fusco-Almeida, A.M.; Mendes-Giannini, M.J.S. Microplate alamarBlue Assay for Paracoccidioides Susceptibility Testing. J. Clin. Microbiol. 2013, 51, 1250–1252. [Google Scholar] [CrossRef]
- Bila, N.M.; Costa-Orlandi, C.B.; Vaso, C.O.; Bonatti, J.L.C.; de Assis, L.R.; Regasini, L.O.; Fontana, C.R.; Fusco-Almeida, A.M.; Mendes-Giannini, M.J.S. 2-Hydroxychalcone as a Potent Compound and Photosensitizer Against Dermatophyte Biofilms. Front. Cell. Infect. Microbiol. 2021, 11, 399. [Google Scholar] [CrossRef]
- Costa-Orlandi, C.B.; Serafim-Pinto, A.; Da Silva, P.B.; Bila, N.M.; Bonatti, J.L.D.C.; Scorzoni, L.; Singulani, J.D.L.; Dos Santos, C.T.; Nazaré, A.C.; Chorilli, M.; et al. Incorporation of Nonyl 3,4-Dihydroxybenzoate Into Nanostructured Lipid Systems: Effective Alternative for Maintaining Anti-Dermatophytic and Antibiofilm Activities and Reducing Toxicity at High Concentrations. Front. Microbiol. 2020, 11, 1154. [Google Scholar] [CrossRef] [PubMed]
- Nakano, N.; Fukuhara-Takaki, K.; Jono, T.; Nakajou, K.; Eto, N.; Horiuchi, S.; Takeya, M.; Nagai, R. Association of Advanced Glycation End Products with A549 Cells, a Human Pulmonary Epithelial Cell Line, Is Mediated by a Receptor Distinct from the Scavenger Receptor Family and RAGE. J. Biochem. 2006, 139, 821–829. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Zhang, Y.; Wang, J.; Yu, W.; Wang, W.; Ma, X. Monitoring of Cell Viability and Proliferation in Hydrogel-Encapsulated System by Resazurin Assay. Appl. Biochem. Biotechnol. 2010, 162, 1996–2007. [Google Scholar] [CrossRef] [PubMed]
- Kirk, S.; Skepper, J.; Donald, A. Application of environmental scanning electron microscopy to determine biological surface structure. J. Microsc. 2009, 233, 205–224. [Google Scholar] [CrossRef]
- Scorzoni, L.; Sangalli-Leite, F.; de Lacorte Singulani, J.; de Paula e Silva Silva, A.C.A.; Costa-Orlandi, C.B.; Fusco-Almeida, A.M.; Mendes-Giannini, M.J.S. Searching new antifungals: The use of in vitro and in vivo methods for evaluation of natural compounds. J. Microbiol. Methods 2016, 123, 68–78. [Google Scholar] [CrossRef]
- Arthington-Skaggs, B.; Jradi, H.; Desai, T.; Morrison, C.J. Quantitation of Ergosterol Content: Novel Method for Determination of Fluconazole Susceptibility of Candida albicans. J. Clin. Microbiol. 1999, 37, 3332–3337. [Google Scholar] [CrossRef]
- Arthington-Skaggs, B.A.; Lee-Yang, W.; Ciblak, M.A.; Frade, J.P.; Brandt, M.E.; Hajjeh, R.A.; Harrison, L.H.; Sofair, A.N.; Warnock, A.D.W. Comparison of Visual and Spectrophotometric Methods of Broth Microdilution MIC End Point Determination and Evaluation of a Sterol Quantitation Method for In Vitro Susceptibility Testing of Fluconazole and Itraconazole against Trailing and Nontrailing Candida Isolates. Antimicrob. Agents Chemother. 2002, 46, 2477–2481. [Google Scholar] [CrossRef]
- Arthington-Skaggs, B.A.; Warnock, D.W.; Morrison, C.J. Quantitation of Candida albicans Ergosterol Content Improves the Correlation between In Vitro Antifungal Susceptibility Test Results and In Vivo Outcome after Fluconazole Treatment in a Murine Model of Invasive Candidiasis. Antimicrob. Agents Chemother. 2000, 44, 2081–2085. [Google Scholar] [CrossRef]
- Jothi, R.; Sangavi, R.; Kumar, P.; Pandian, S.K.; Gowrishankar, S. Catechol thwarts virulent dimorphism in Candida albicans and potentiates the antifungal efficacy of azoles and polyenes. Sci. Rep. 2021, 11, 21049. [Google Scholar] [CrossRef]
- Bvumbi, C.; Chi, G.F.; Stevens, M.Y.; Mombeshora, M.; Mukanganyama, S. The Effects of Tormentic Acid and Extracts from Callistemon citrinus on Candida albicans and Candida tropicalis Growth and Inhibition of Ergosterol Biosynthesis in Candida albicans. Sci. World J. 2021, 2021, 8856147. [Google Scholar] [CrossRef]
- de Lacorte Singulani, J.; Galeane, M.C.; Ramos, M.D.; Gomes, P.C.; Dos Santos, C.T.; De Souza, B.M.; Palma, M.S.; Almeida, A.M.F.; Giannini, M.J.S.M. Antifungal Activity, Toxicity, and Membranolytic Action of a Mastoparan Analog Peptide. Front. Cell. Infect. Microbiol. 2019, 9, 419. [Google Scholar] [CrossRef]
- Mesa-Arango, A.C.; Trevijano-Contador, N.; Román, E.; Sánchez-Fresneda, R.; Casas, C.; Herrero, E.; Argüelles, J.C.; Pla, J.; Cuenca-Estrella, M.; Zaragoza, O. The Production of Reactive Oxygen Species Is a Universal Action Mechanism of Amphotericin B against Pathogenic Yeasts and Contributes to the Fungicidal Effect of This Drug. Antimicrob. Agents Chemother. 2014, 58, 6627–6638. [Google Scholar] [CrossRef]
- Sangalli-Leite, F.; Scorzoni, L.; Mesa-Arango, A.C.; Casas, C.; Herrero, E.; Gianinni, M.J.S.M.; Rodríguez-Tudela, J.L.; Cuenca-Estrella, M.; Zaragoza, O. Amphotericin B mediates killing in Cryptococcus neoformans through the induction of a strong oxidative burst. Microbes Infect. 2011, 13, 457–467. [Google Scholar] [CrossRef]
- Edwards, J.A.; Kemski, M.M.; Rappleye, C.A. Identification of an Aminothiazole with Antifungal Activity against Intracellular Histoplasma capsulatum. Antimicrob. Agents Chemother. 2013, 57, 4349–4359. [Google Scholar] [CrossRef]
- Brilhante, R.S.N.; Malaquias, D.M.; Caetano, P.; Castelo-Branco, D.; De Lima, R.A.C.; Marques, F.J.D.F.; Silva, N.F.; De Alencar, L.P.; Monteiro, A.J.; de Carmargo, Z.P.; et al. In vitro inhibitory effect of miltefosine against strains of Histoplasma capsulatum var. capsulatum and Sporothrix spp. Med. Mycol. 2014, 52, 320–325. [Google Scholar] [CrossRef][Green Version]
- Cordeiro, R.D.A.; Marques, F.J.D.F.; Cordeiro, R.D.A.; da Silva, M.R.; Malaquias, A.D.M.; de Melo, C.V.S.; Mafezoli, J.; Oliveira, M.D.C.F.D.; Brilhante, R.S.N.; Rocha, M.F.G.; et al. Synthesis and Antifungal Activity In Vitro of Isoniazid Derivatives against Histoplasma capsulatum var. capsulatum. Antimicrob. Agents Chemother. 2014, 58, 2504–2511. [Google Scholar] [CrossRef]
- Melo, W.C.; dos Santos, M.B.; Marques, B.D.C.; Regasini, L.O.; Giannini, M.J.S.M.; Almeida, A.M.F. Selective photoinactivation of Histoplasma capsulatum by water-soluble derivatives chalcones. Photodiagnosis Photodyn. Ther. 2017, 18, 232–235. [Google Scholar] [CrossRef]
- Wheat, L.J.; Freifeld, A.G.; Kleiman, M.B.; Baddley, J.W.; McKinsey, D.S.; Loyd, J.; Kauffman, C.A. Clinical Practice Guidelines for the Management of Patients with Histoplasmosis: 2007 Update by the Infectious Diseases Society of America. Clin. Infect. Dis. 2007, 45, 807–825. [Google Scholar] [CrossRef]
- Azar, M.M.; Loyd, J.L.; Relich, R.F.; Wheat, L.J.; Hage, C.A. Current Concepts in the Epidemiology, Diagnosis, and Management of Histoplasmosis Syndromes. Semin. Respir. Crit. Care Med. 2020, 41, 13–30. [Google Scholar] [CrossRef]
- Carlsson, J.; Yuhas, J.M. Liquid-Overlay Culture of Cellular Spheroids. Recent Results Cancer Res. 1984, 95, 1–23. [Google Scholar] [CrossRef]
- Lin, R.-Z.; Chang, H.-Y. Recent advances in three-dimensional multicellular spheroid culture for biomedical research. Biotechnol. J. 2008, 3, 1172–1184. [Google Scholar] [CrossRef]
- Owen, M.R.; Byrne, H.M.; Lewis, C.E. Mathematical modelling of the use of macrophages as vehicles for drug delivery to hypoxic tumour sites. J. Theor. Biol. 2004, 226, 377–391. [Google Scholar] [CrossRef]
- Byrne, H.M. Dissecting cancer through mathematics: From the cell to the animal model. Nat. Cancer 2010, 10, 221–230. [Google Scholar] [CrossRef]
- Fiorentzis, M.; Katopodis, P.; Kalirai, H.; Seitz, B.; Viestenz, A.; Coupland, S.E. Conjunctival melanoma and electrochemotherapy: Preliminary results using 2D and 3D cell culture models in vitro. Acta. Ophthalmol. 2018, 97, e632–e640. [Google Scholar] [CrossRef]
- Gurski, L.A.; Petrelli, N.J.; Jia, X.; Farach-Carson, M.C. 3D Matrices for Anti-Cancer Drug Testing and Development. Oncol. Issues 2010, 25, 20–25. [Google Scholar] [CrossRef]
- Xu, X.; Gurski, L.A.; Zhang, C.; Harrington, D.A.; Farach-Carson, M.C.; Jia, X. Recreating the tumor microenvironment in a bilayer, hyaluronic acid hydrogel construct for the growth of prostate cancer spheroids. Biomaterials 2012, 33, 9049–9060. [Google Scholar] [CrossRef]
- Bonnier, F.; Keating, M.; Wróbel, T.; Majzner, K.; Baranska, M.; Garcia-Munoz, A.; Blanco, A.; Byrne, H. Cell viability assessment using the Alamar blue assay: A comparison of 2D and 3D cell culture models. Toxicol. Vitr. 2014, 29, 124–131. [Google Scholar] [CrossRef]
- Schreiber-Brynzak, E.; Klapproth, E.; Unger, C.; Lichtscheidl, I.; Göschl, S.; Schweighofer, S.V.; Trondl, R.; Dolznig, H.; Jakupec, M.A.; Keppler, B. Three-dimensional and co-culture models for preclinical evaluation of metal-based anticancer drugs. Investig. New Drugs 2015, 33, 835–847. [Google Scholar] [CrossRef] [PubMed]
- Proctor, W.R.; Foster, A.J.; Vogt, J.; Summers, C.; Middleton, B.; Pilling, M.; Shienson, D.; Kijanska, M.; Ströbel, S.; Kelm, J.M.; et al. Utility of spherical human liver microtissues for prediction of clinical drug-induced liver injury. Arch. Toxicol. 2017, 91, 2849–2863. [Google Scholar] [CrossRef] [PubMed]
- Scorzoni, L.; de Paula, E.S.A.C.; Marcos, C.M.; Assato, P.A.; de Melo, W.C.; de Oliveira, H.C.; Costa-Orlandi, C.B.; Mendes-Giannini, M.J.; Fusco-Almeida, A.M. Antifungal Therapy: New Advances in the Understanding and Treatment of Mycosis. Front. Microbiol. 2017, 8, 36. [Google Scholar] [CrossRef] [PubMed]
- Arranz, J.C.E.; Ochoa-Pacheco, A.; Beaven, M.; Peres-Roses, R.; Gámez, Y.M.; Camacho-Pozo, I.M.; Maury, G.L.; de Macedo, M.B.; Cos, P.; Tavares, J.F.; et al. Bioassay-guided In vitro study of the antimicrobial and cytotoxic properties of the leaves from Excoecaria lucida Sw. Pharmacogn. Res. 2017, 9, 396–400. [Google Scholar] [CrossRef]
- Sanglard, D. Emerging Threats in Antifungal-Resistant Fungal Pathogens. Front. Med. 2016, 3, 11. [Google Scholar] [CrossRef]
- Limper, A.H.; Adenis, A.; Le, T.; Harrison, T.S. Fungal infections in HIV/AIDS. Lancet Infect. Dis. 2017, 17, e334–e343. [Google Scholar] [CrossRef]
- Stass, H.; Feleder, E.; Garcia-Bournissen, F.; Nagelschmitz, J.; Weimann, B.; Yerino, G.; Altcheh, J. Biopharmaceutical Characteristics of Nifurtimox Tablets for Age- and Body Weight-Adjusted Dosing in Patients with Chagas Disease. Clin. Pharmacol. Drug Dev. 2020, 10, 542–555. [Google Scholar] [CrossRef]
- Gerpe, A.; Álvarez, G.; Benítez, D.; Boiani, L.; Quiroga, M.; Hernández, P.; Sortino, M.; Zacchino, S.; González, M.; Cerecetto, H. 5-Nitrofuranes and 5-nitrothiophenes with anti-Trypanosoma cruzi activity and ability to accumulate squalene. Bioorganic Med. Chem. 2009, 17, 7500–7509. [Google Scholar] [CrossRef]
- Gerpe, A.; Odreman-Nuñez, I.; Draper, P.; Boiani, L.; Urbina, J.A.; González, M.; Cerecetto, H. Heteroallyl-containing 5-nitrofuranes as new anti-Trypanosoma cruzi agents with a dual mechanism of action. Bioorganic Med. Chem. 2008, 16, 569–577. [Google Scholar] [CrossRef]
- Pagniez, F.; Lebouvier, N.; Na, Y.M.; Ourliac-Garnier, I.; Picot, C.; Le Borgne, M.; Le Pape, P. Biological exploration of a novel 1,2,4-triazole-indole hybrid molecule as antifungal agent. J. Enzym. Inhib. Med. Chem. 2020, 35, 398–403. [Google Scholar] [CrossRef]
- Pooja; Prasher, P.; Singh, P.; Pawar, K.; Vikramdeo, K.S.; Mondal, N.; Komath, S.S. Synthesis of amino acid appended indoles: Appreciable anti-fungal activity and inhibition of ergosterol biosynthesis as their probable mode of action. Eur. J. Med. Chem. 2014, 80, 325–339. [Google Scholar] [CrossRef]
- Saccoliti, F.; Madia, V.N.; Tudino, V.; De Leo, A.; Pescatori, L.; Messore, A.; De Vita, D.; Scipione, L.; Brun, R.; Kaiser, M.; et al. Design, Synthesis, and Biological Evaluation of New 1-(Aryl-1H-pyrrolyl)(phenyl)methyl-1H-imidazole Derivatives as Antiprotozoal Agents. J. Med. Chem. 2019, 62, 1330–1347. [Google Scholar] [CrossRef]
- Anderson, T.M.; Clay, M.C.; Cioffi, A.G.; Diaz, K.A.; Hisao, G.S.; Tuttle, M.D.; Nieuwkoop, A.; Comellas, G.; Maryum, N.; Wang, S.; et al. Amphotericin forms an extramembranous and fungicidal sterol sponge. Nat. Chem. Biol. 2014, 10, 400–406. [Google Scholar] [CrossRef]
- Lewandowska, A.; Soutar, C.P.; Greenwood, A.I.; Nimerovsky, E.; De Lio, A.M.; Holler, J.T.; Hisao, G.S.; Khandelwal, A.; Zhang, J.; SantaMaria, A.M.; et al. Fungicidal amphotericin B sponges are assemblies of staggered asymmetric homodimers encasing large void volumes. Nat. Struct. Mol. Biol. 2021, 28, 972–981. [Google Scholar] [CrossRef]
- Guo, X.; Zhang, J.; Li, X.; Xiao, E.; Lange, J.D.; Rienstra, C.M.; Burke, M.D.; Mitchell, D.A. Sterol Sponge Mechanism is Conserved for Glycosylated Polyene Macrolides. ACS Central Sci. 2021, 7, 781–791. [Google Scholar] [CrossRef]
- Harrington, B.J.; Hageage, J.G.J. Calcofluor White: A Review of its Uses and Applications in Clinical Mycology and Parasitology. Lab. Med. 2003, 34, 361–367. [Google Scholar] [CrossRef]
- Hwang, I.-S.; Lee, J.; Lee, D.G. Indole-3-carbinol Generates Reactive Oxygen Species and Induces Apoptosis. Biol. Pharm. Bull. 2011, 34, 1602–1608. [Google Scholar] [CrossRef]
- Elmore, S. Apoptosis: A review of programmed cell death. Toxicol. Pathol. 2007, 35, 495–516. [Google Scholar] [CrossRef]
- Proskuryakov, S.; Gabai, S.Y.P.A.V.L. Mechanisms of Tumor Cell Necrosis. Curr. Pharm. Des. 2010, 16, 56–68. [Google Scholar] [CrossRef]
- Tower, J. Programmed cell death in aging. Ageing Res. Rev. 2015, 23, 90–100. [Google Scholar] [CrossRef]

| Compound | Concentration (µg/mL) | Compound | Concentration (µg/mL) | Compound | Concentration (µg/mL) |
|---|---|---|---|---|---|
| 1 | ≥125 | 20 | 7.8 | 39 | 7.8 |
| 2 | 15.6 | 21 | 7.8 | 40 | 62.5 |
| 3 | 1.95 | 22 | 31.25 | 41 | 7.8 |
| 4 | 0.98 | 23 | 31.25 | 42 | 31.25 |
| 5 | 0.25 | 24 | 62.5 | 43 | 62.5 |
| 6 | 62.5 | 25 | ≥125 | 44 | 125 |
| 7 | 0.122 | 26 | ≥125 | 45 | 62.5 |
| 8 | 31.25 | 27 | ≥125 | 46 | 31.25 |
| 9 | 0.98 | 28 | 31.25 | 47 | 125 |
| 10 | 7.8 | 29 | 31.25 | 48 | ≥125 |
| 11 | 0.25 | 30 | 15.6 | 49 | 125 |
| 12 | 7.8 | 31 | 3.9 | 50 | 3.9 |
| 13 | 62.5 | 32 | 3.9 | 51 | 31.25 |
| 14 | 3.9 | 33 | 1.95 | 52 | ≥125 |
| 15 | 7.8 | 34 | ≥125 | 53 | ≥125 |
| 16 | 31.25 | 35 | 125 | 54 | ≥125 |
| 17 | ≥125 | 36 | ≥125 | ||
| 18 | 31.25 | 37 | 15.6 | ||
| 19 | 15.6 | 38 | ≥125 |
| EH-315 (µg/mL) | ATCC G217-B (µg/mL) | ||||
|---|---|---|---|---|---|
| Compounds | Derivate | MIC90 | MFC | MIC90 | MFC |
| 3 | Nitrofuran | 1.95 | 1.95 | 3.9 | 3.9 |
| 4 | Nitrofuran | 0.98 | 0.98 | 3.9 | 3.9 |
| 5 | Nitrofuran | 0.24 | 0.24 | 1.95 | 1.95 |
| 7 | Nitrofuran/Indole | 0.122 | 0.122 | 0.98 | 0.98 |
| 9 | Nitrofuran | 0.98 | 0.98 | 3.9 | 3.9 |
| 10 | Nitrofuran | 7.81 | 7.81 | 7.81 | 15.62 |
| 11 | Nitrofuran | 0.24 | 0.24 | 0.48 | 0.48 |
| 12 | Nitrofuran | 7.81 | 7.81 | 7.81 | 15.62 |
| 14 | Nitrofuran | 3.90 | 7.81 | 7.81 | 7.81 |
| 15 | Nitrofuran | 7.81 | 15.62 | >250 | >250 |
| 20 | Indole | 7.81 | 7.81 | 7.81 | 7.81 |
| 21 | Indole | 7.81 | 7.81 | 7.81 | 7.81 |
| 31 | Nitrofuran/indole | 3.90 | 3.90 | 31.25 | 31.25 |
| 32 | Indole | 3.90 | 3.90 | 3.90 | 3.90 |
| 33 | Indole | 1.95 | 1.95 | 3.90 | 3.90 |
| 39 | Indole | 7.81 | 7.81 | 15.62 | 15.62 |
| 41 | Indole | 7.81 | 7.81 | 61.25 | 61.25 |
| 50 | Indole | 3.90 | 3.90 | 31.25 | 31.25 |
| AmB | Polyene | 0.06 | - | 0.03 | - |
| ITZ | Azole | 0.125 | - | 0.007 | - |
| Cell Line | Model | Mean—Fourth Day | Mean—Seventh Day | Mean—Tenth Day |
|---|---|---|---|---|
| A549 | Monolayer | 5.23 × 104 ± 7.56 × 103 | 8.50 × 104 ± 2.73 × 104 | 1.03 × 105 ± 2.08 × 103 |
| Spheroid | 8.33 × 103 ± 2.36 × 103 | 1.23 × 104 ± 1.53 × 103 | 1.25 × 104 ± 2.08 × 103 | |
| MRC-5 | Monolayer | 1.91 × 105 ± 1.53 × 104 | 3.56 × 105 ± 3.96 × 104 (*) | 3.06 × 105 ± 3.58 × 104 |
| Spheroid | 2.69 × 103 ± 6.67 × 102 | 4.25 × 104 ± 3.75 × 103 (***) | 3.13 × 104 ± 7.60 × 103 (**) |
| A549 | ||||||
|---|---|---|---|---|---|---|
| Monolayer | Spheroid | |||||
| EH-315 | ATCC G217-B | EH-315 | ATCC G217-B | |||
| Compounds | CC50 (µg/mL) | SI | SI | CC50 (µg/mL) | SI | SI |
| 3 | 231.2 | 118.56 | 59.28 | >250 | >128.20 | >64.10 |
| 4 | 29.50 | 30.10 | 7.56 | 62.96 | 64.24 | 16.14 |
| 5 | 30.11 | 125.45 | 15.44 | 43.01 | 179.20 | 22.05 |
| 7 | 8.58 | 70.32 | 8.75 | >250 | >2049.18 | >255.1 |
| 9 | 23.41 | 23.88 | 6.00 | 17.25 | 17.60 | 4.42 |
| 10 | 38.67 | 4.95 | 4.95 | 32.97 | 4.22 | 4.22 |
| 11 | 12.24 | 50.16 | 25.50 | 56.06 | 229.75 | 116.79 |
| 12 | >250 | >32.01 | >32.01 | 104.8 | 13.41 | 13.41 |
| 14 | 33.44 | 8.83 | 4.4 | 27.61 | 7.07 | 3.53 |
| 20 | 81.41 | 10.42 | 10.42 | 81.41 | 10.42 | 10.42 |
| 21 | 40.00 | 5.12 | 5.12 | 40.00 | 5.12 | 5.12 |
| 32 | 81.41 | 20.87 | 20.87 | 81.41 | 20.76 | 20.76 |
| 33 | 12.26 | 6.28 | 3.14 | 13.22 | 6.77 | 1.69 |
| 39 | 42.79 | 5.47 | 2.73 | 60.5 | 7.74 | 3.87 |
| MRC-5 | ||||||
|---|---|---|---|---|---|---|
| Monolayer | Spheroid | |||||
| EH-315 | ATCC G217-B | EH-315 | ATCC G217-B | |||
| Compounds | CC50 (µg/mL) | SI | SI | CC50 (µg/mL) | SI | SI |
| 3 | >250 | >128.20 | >64.10 | >250 | >128.20 | >64.10 |
| 64 | 135.2 | 137.95 | 34.66 | >250 | > 255.10 | >64.10 |
| 5 | 39.76 | 165.66 | 20.38 | 81.41 | 339.20 | 41.74 |
| 7 | 71.24 | 583.93 | 72.69 | 73.50 | 602.45 | 75.00 |
| 9 | 47.23 | 48.19 | 12.11 | 169.6 | 173.06 | 43.48 |
| 10 | 215.4 | 27.58 | 27.58 | 57.92 | 7.41 | 7.41 |
| 11 | 62.56 | 256.39 | 130.33 | 67.79 | 277.82 | 141.22 |
| 12 | 228.0 | 29.19 | 29.19 | 76.12 | 9.74 | 9.74 |
| 14 | 26.8 | 6.87 | 3.43 | 7.68 | 1.96 | 0.98 |
| 20 | 20.39 | 2.61 | 2.61 | 25.35 | 3.57 | 3.57 |
| 21 | 33.77 | 4.32 | 4.32 | 52.34 | 6.70 | 6.70 |
| 32 | 24.7 | 6.33 | 6.33 | 26.14 | 6.70 | 6.70 |
| 33 | 18.74 | 9.56 | 4.78 | 14.03 | 7.19 | 3.59 |
| 39 | 40.00 | 5.12 | 2.56 | 40.00 | 5.12 | 2.56 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vaso, C.O.; Bila, N.M.; Pandolfi, F.; De Vita, D.; Bortolami, M.; Bonatti, J.L.C.; De Moraes Silva, R.A.; Gonçalves, L.N.C.; Tudino, V.; Costi, R.; et al. Evaluation of the Anti-Histoplasma capsulatum Activity of Indole and Nitrofuran Derivatives and Their Pharmacological Safety in Three-Dimensional Cell Cultures. Pharmaceutics 2022, 14, 1043. https://doi.org/10.3390/pharmaceutics14051043
Vaso CO, Bila NM, Pandolfi F, De Vita D, Bortolami M, Bonatti JLC, De Moraes Silva RA, Gonçalves LNC, Tudino V, Costi R, et al. Evaluation of the Anti-Histoplasma capsulatum Activity of Indole and Nitrofuran Derivatives and Their Pharmacological Safety in Three-Dimensional Cell Cultures. Pharmaceutics. 2022; 14(5):1043. https://doi.org/10.3390/pharmaceutics14051043
Chicago/Turabian StyleVaso, Carolina Orlando, Níura Madalena Bila, Fabiana Pandolfi, Daniela De Vita, Martina Bortolami, Jean Lucas Carvalho Bonatti, Rosângela Aparecida De Moraes Silva, Larissa Naiara Carvalho Gonçalves, Valeria Tudino, Roberta Costi, and et al. 2022. "Evaluation of the Anti-Histoplasma capsulatum Activity of Indole and Nitrofuran Derivatives and Their Pharmacological Safety in Three-Dimensional Cell Cultures" Pharmaceutics 14, no. 5: 1043. https://doi.org/10.3390/pharmaceutics14051043
APA StyleVaso, C. O., Bila, N. M., Pandolfi, F., De Vita, D., Bortolami, M., Bonatti, J. L. C., De Moraes Silva, R. A., Gonçalves, L. N. C., Tudino, V., Costi, R., Di Santo, R., Mendes-Giannini, M. J. S., Costa-Orlandi, C. B., Scipione, L., & Fusco-Almeida, A. M. (2022). Evaluation of the Anti-Histoplasma capsulatum Activity of Indole and Nitrofuran Derivatives and Their Pharmacological Safety in Three-Dimensional Cell Cultures. Pharmaceutics, 14(5), 1043. https://doi.org/10.3390/pharmaceutics14051043












