An Overview of siRNA Delivery Strategies for Urological Cancers
Abstract
1. Introduction
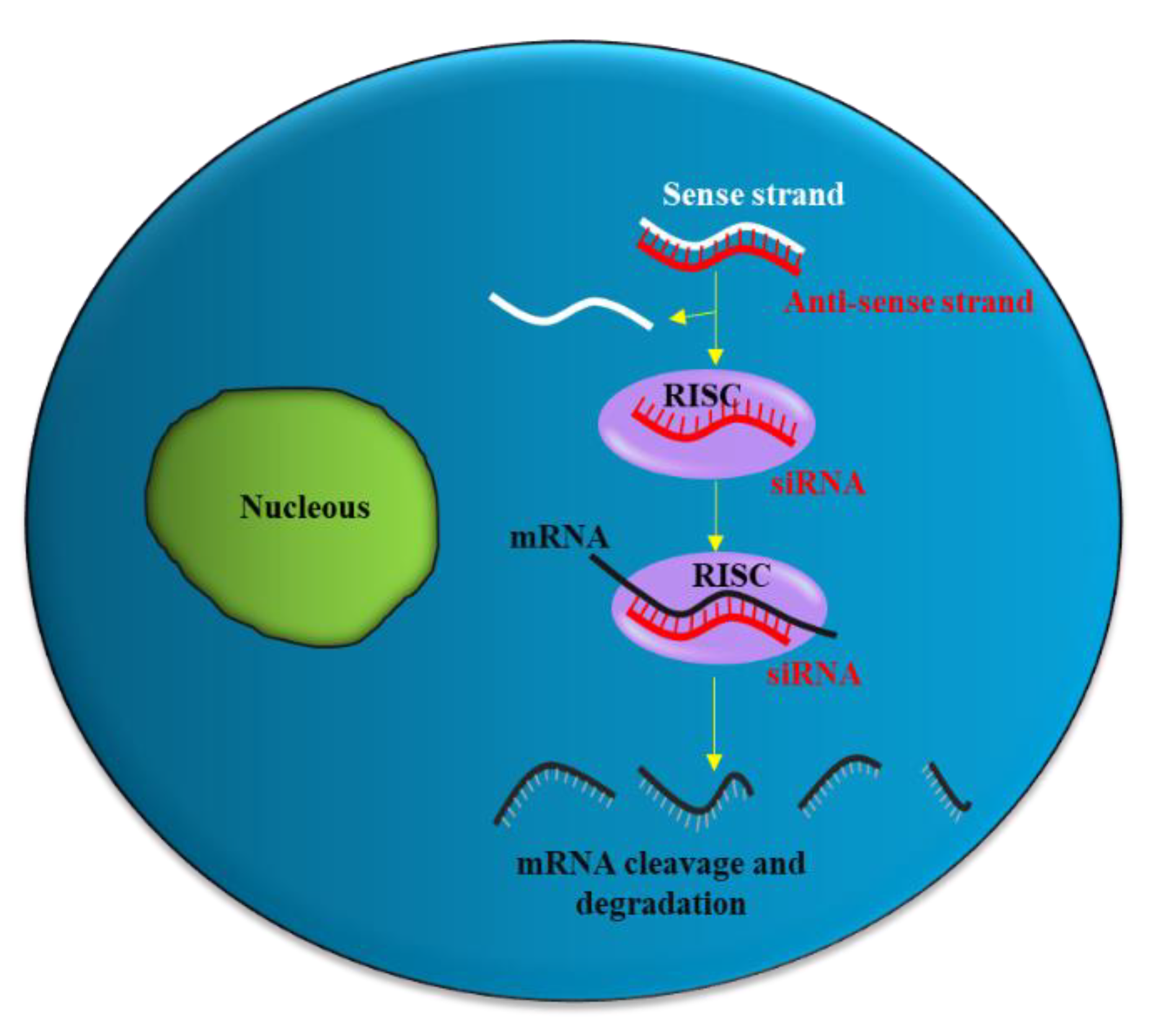
2. siRNA Structure, Function and Delivery
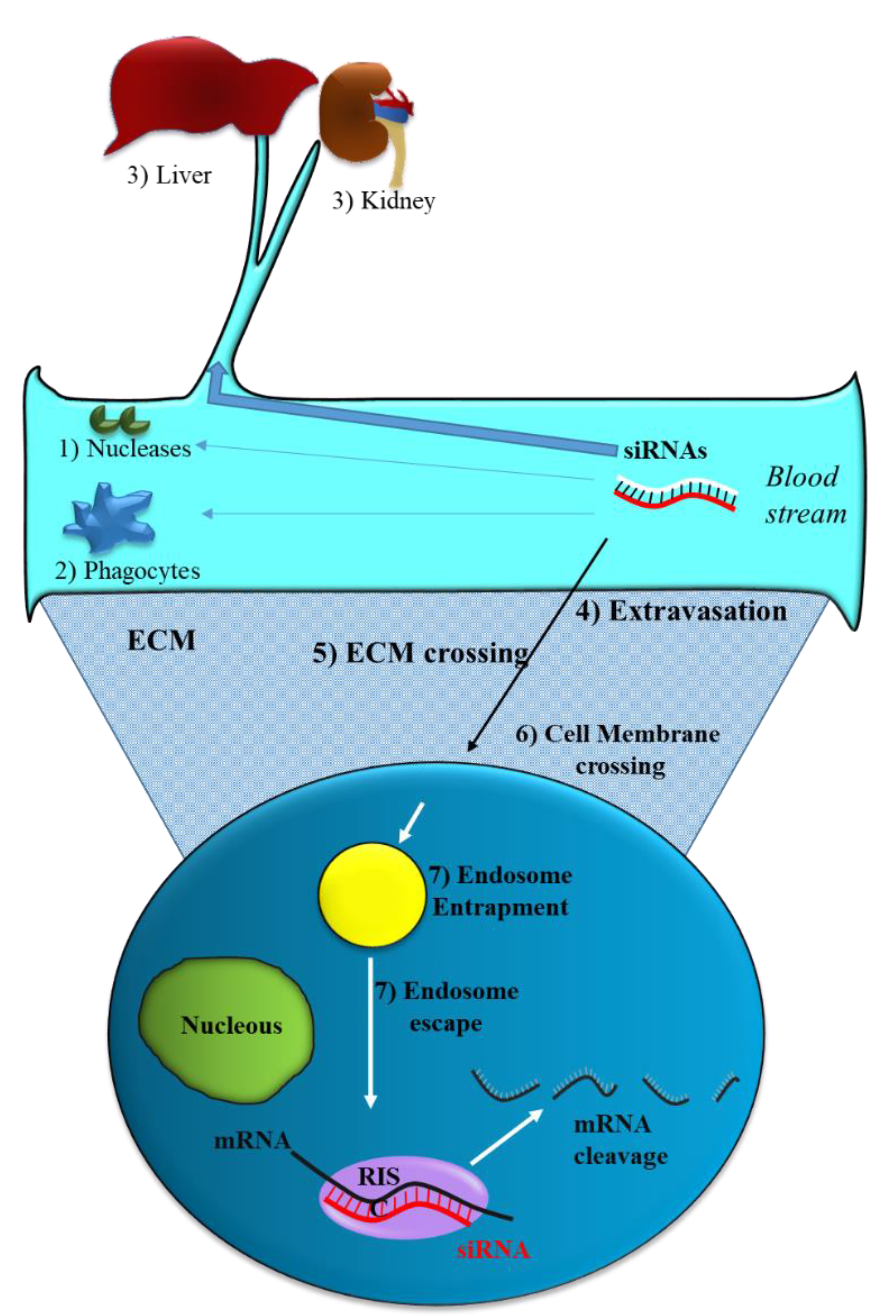
2.1. siRNA Delivery Problems
2.2. Strategies to Optimize siRNA Delivery
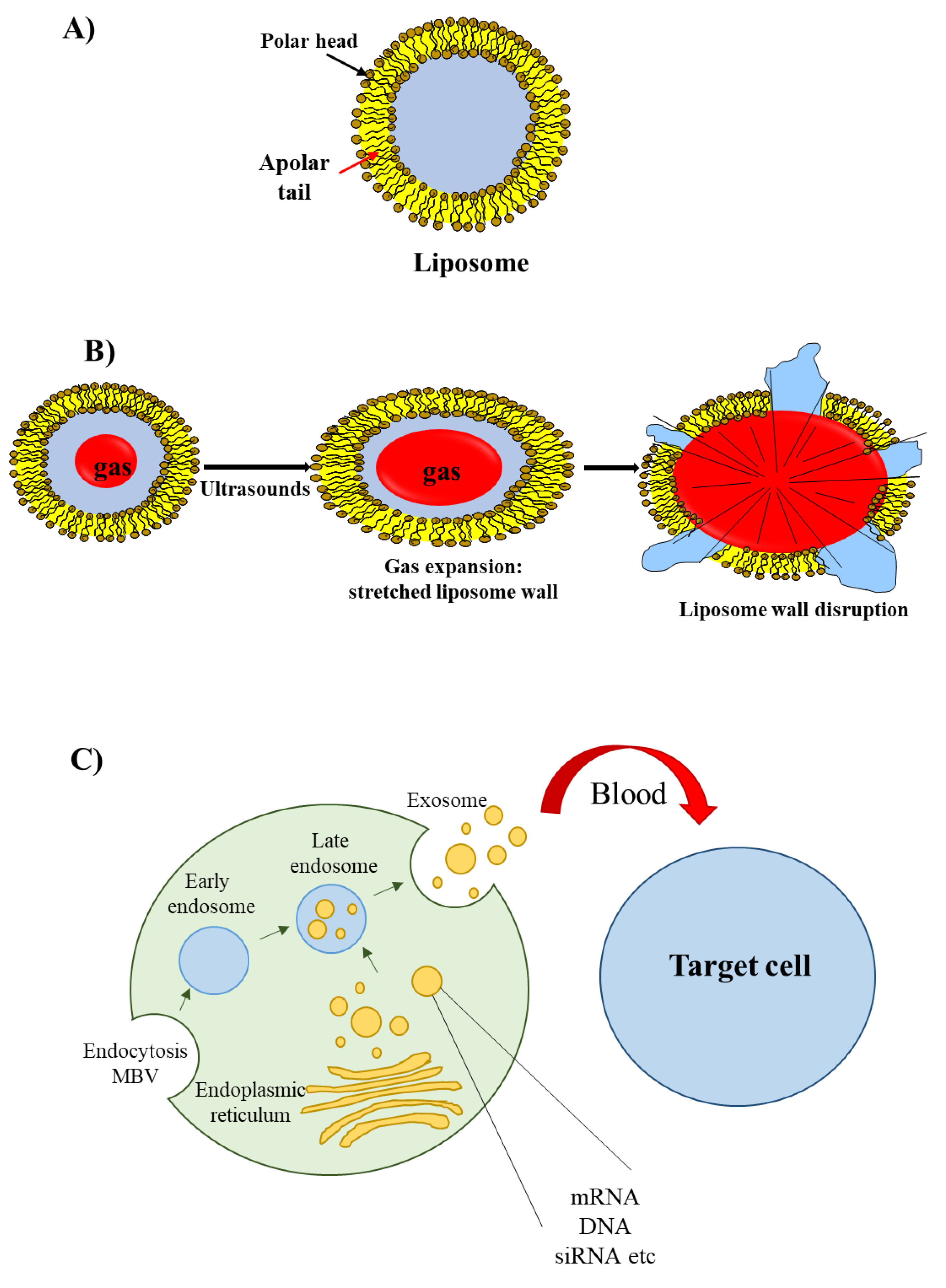
2.3. Lipid-Based Delivery Materials
2.4. Polymer-Based Delivery Materials
2.5. Other Delivery Materials
3. siRNA Delivery in Urological Cancers
3.1. siRNA Delivery in BC
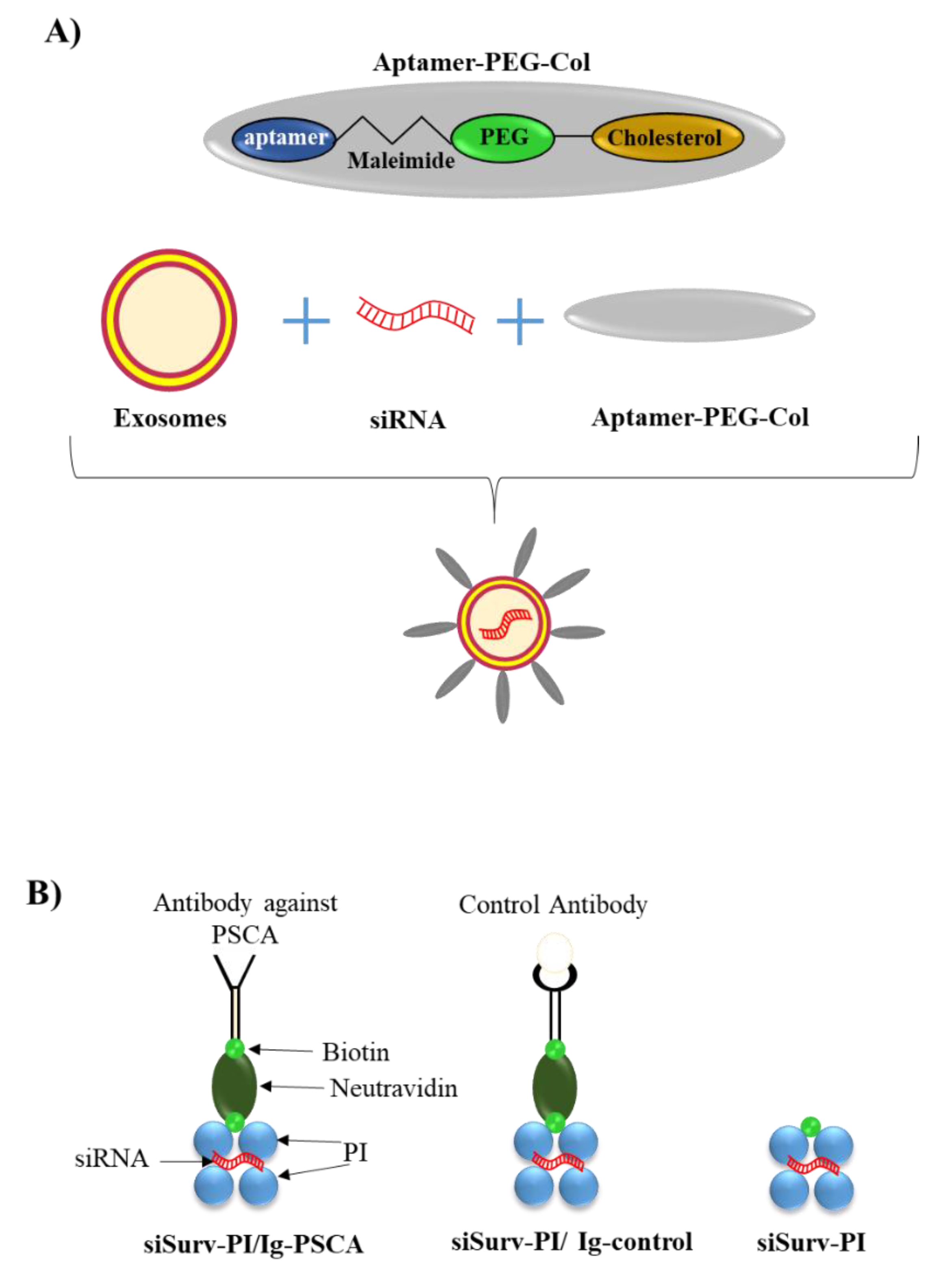
3.1.1. Lipid-Based Delivery Approaches
| Target mRNA | Delivery System | Disease Model | Reference |
|---|---|---|---|
| PLK-1 | exosomes | UMUC3 and SW780 cell line | [67] |
| Survivin | pegylated lipid | RT4 cell line | [68] |
| Mouse model | |||
| Nrf2 | dendrimer | T24, 253J B-VC-r and HK-2 cell lines | [71] |
| Survivin | PLGA-Chitosan | Human ureter model and in vivo mouse bladder; UM-UC-3 cell line and xenograft mouse model | [72] |
| Bcl2 | Chitosan-hyaluronic acid dialdehyde able to bind CD44 | T24 cell line, xenograft subcutaneous mouse model | [73] |
| EIF5A2 | Catechin (Mg(II) | T24 cell line, subcutaneous mouse model, Rat in situ model | [74] |
| RIPK4 | halloysite nanotube | in-situ bladder model | [75] |
| SPAG5 | chrysotile nanotubes | T24 cell line, | [76] |
| xenograft subcutaneous mouse model, lung metastasis model, in situ rat model |
3.1.2. Polymeric-Based Delivery Approaches
3.1.3. Other Delivery Approaches
3.2. siRNA Delivery in PC
3.2.1. Lipid-Based Delivery Approaches
| Target mRNA | Delivery System | Disease Model | Reference |
|---|---|---|---|
| Survivin | Microbubble-liposome | LNCaP, PC3 cell lines, Xenograft mouse model | [86] |
| CDH2 | Commercial liposome | LNCaP cell line | [87] |
| EphA2 | Cationi solid nanoparticles | PC3, DU145 cell lines | [88] |
| Cldn4 | Commercial liposome | LNCaP PC3 cell lines, | [89] |
| Survivin | PEI conjugated exsosomes | PC3 cell lines | [90] |
| SIRT6 | Exososomes conjugated with A3 aptamer | DU145, PC3, BPH-1 cell lines, xenograft mouse model | [91] |
| FoxM1 | Microbubble-liposome conjugated with A10-3.2 aptamer | LNCaP, PC3 cell lines, xenograft mouse model | [92] |
| DLX | Commercial liposome | VCaP cell lines, | [93] |
| Survivin | Poly(propylene) Imine conjugated with an antibody Against PSCA | 293TPSCA/ffluc PC3PSCA cell lines, Xenograft mouse model | [94] |
| Hsp27 | Dendrimer like delivery system | PC3 cell lines Xenograft mouse model | [95] |
| GRP78 | CaP-DESP-PEG-RGD | PC3 cell lines Xenograft mouse model | [96] |
| Metalloproteinase 10 | Fe3O4 nanoparticles conjugated with PEI/PEG | NIH-3T3, PC3 cell lines | [97] |
| FOAX1 | Electroporation | Patients derived organoid | [98] |
3.2.2. Polymer-Based Delivery Approaches
3.2.3. Other Delivery Approaches
3.3. siRNA Delivery in RC
3.3.1. Lipid-Based Delivery Approaches
| Target mRNA | Delivery System | Disease Model | Reference |
|---|---|---|---|
| PLK1 | Liposome containing the cationic lipid YSK05 | OS-RC-2 cell line, Xenograft mouse model | [124] |
| HMGA2 | Commercial liposome | ACHN cell line | [125] |
| KSP/VEGF | Lipidi particle | Clinical trial | [126,127] |
| Lim-1 | Polydiacetylenic nanofibers | 786-O, cell line, xenograft mouse model | [128] |
| Survival gene | Glycogen | MDA-MB-231-luc2 HK2 cell lines | [129] |
3.3.2. Polymer-Based Delivery Approaches
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- He, M.-H.; Chen, L.; Zheng, T.; Tu, Y.; He, Q.; Fu, H.-L.; Lin, J.-C.; Zhang, W.; Shu, G.; He, L.; et al. Potential applications of nanotechnology in urological cancer. Front. Pharmacol. 2018, 9, 745. [Google Scholar] [CrossRef] [PubMed]
- Witjes, J.A.; Bruins, H.M.; Cathomas, R.; Compérat, E.M.; Cowan, N.C.; Gakis, G.; Hernández, V.; Espinós, E.L.; Lorch, A.; Neuzillet, Y.; et al. European association of urology guidelines on muscle-invasive and metastatic bladder cancer: Summary of the 2020 guidelines. Eur. Urol. 2021, 79, 82–104. [Google Scholar] [CrossRef] [PubMed]
- Freedman, N.D.; Silverman, D.T.; Hollenbeck, A.R.; Schatzkin, A.; Abnet, C.C. Association between smoking and risk of bladder cancer among men and women. JAMA 2011, 306, 737–745. [Google Scholar] [CrossRef] [PubMed]
- Cumberbatch, M.G.K.; Jubber, I.; Black, P.C.; Esperto, F.; Figueroa, J.D.; Kamat, A.M.; Kiemeney, L.; Lotan, Y.; Pang, K.; Silverman, D.T.; et al. Epidemiology of bladder cancer: A systematic review and contemporary update of risk factors in 2018. Eur. Urol. 2018, 74, 784–795. [Google Scholar] [CrossRef] [PubMed]
- Laukhtina, E.; Shim, S.R.; Mori, K.; D‘Andrea, D.; Soria, F.; Rajwa, P.; Mostafaei, H.; Compérat, E.; Cimadamore, A.; Moschini, M.; et al. Diagnostic accuracy of novel urinary biomarker tests in non-muscle-invasive bladder cancer: A systematic review and network meta-analysis. Eur. Urol. Oncol. 2021, 4, 927–942. [Google Scholar] [CrossRef] [PubMed]
- Mottet, N.; van den Bergh, R.C.N.; Briers, E.; Van den Broeck, T.; Cumberbatch, M.G.; De Santis, M.; Fanti, S.; Fossati, N.; Gandaglia, G.; Gillessen, S.; et al. EAU-EANM-ESTRO-ESUR-SIOG guidelines on prostate cancer-2020 update. Part 1: Screening, diagnosis, and local treatment with curative intent. Eur. Urol. 2021, 79, 243–262. [Google Scholar] [CrossRef] [PubMed]
- Arnold, M.; Karim-Kos, H.E.; Coebergh, J.W.; Byrnes, G.; Antilla, A.; Ferlay, J.; Renehan, A.G.; Forman, D.; Soerjomataram, I. Recent trends in incidence of five common cancers in 26 European countries since 1988: Analysis of the European cancer observatory. Eur. J. Cancer 2015, 51, 1164–1187. [Google Scholar] [CrossRef] [PubMed]
- Albright, F.; Stephenson, R.A.; Agarwal, N.; Teerlink, C.C.; Lowrance, W.T.; Farnham, J.M.; Albright, L.A. Prostate cancer risk prediction based on complete prostate cancer family history. Prostate 2015, 75, 390–398. [Google Scholar] [CrossRef] [PubMed]
- Leitzmann, M.F.; Rohrmann, S. Risk factors for the onset of prostatic cancer: Age, location, and behavioral correlates. Clin. Epidemiol. 2012, 4, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Tan, D.S.; Mok, T.S.; Rebbeck, T.R. Cancer genomics: Diversity and disparity across ethnicity and geography. J. Clin. Oncol. 2016, 34, 91–101. [Google Scholar] [CrossRef] [PubMed]
- Cornford, P.; Bellmunt, J.; Bolla, M.; Briers, E.; De, S.M.; Gross, T.; Henry, A.M.; Joniau, S.; Lam, T.B.; Mason, M.D. EAU-ESTRO-SIOG guidelines on prostate cancer. Part II: Treatment of relapsing, metastatic, and castration-resistant prostate cancer. Eur. Urol. 2017, 71, 630–642. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, A.J.; Garrett-Mayer, E.; de Wit, R.; Tannock, I.; Eisenberger, M. Prediction of survival following first-line chemotherapy in men with castration-resistant metastatic prostate cancer. Clin. Cancer Res. 2010, 16, 203–211. [Google Scholar] [CrossRef]
- Halabi, S.; Lin, C.Y.; Small, E.J.; Armstrong, A.J.; Kaplan, E.B.; Petrylak, D.; Sternberg, C.N.; Shen, L.; Oudard, S.; de Bono, J.; et al. Prognostic model predicting metastatic castration-resistant prostate cancer survival in men treated with second-line chemotherapy. J. Natl. Cancer Inst. 2013, 105, 1729–1737. [Google Scholar] [CrossRef]
- Capitanio, U.; Montorsi, F. Renal cancer. Lancet 2016, 387, 894–906. [Google Scholar] [CrossRef]
- Levi, F.; Ferlay, J.; Galeone, C.; Lucchini, F.; Negri, E.; Boyle, P.; La Vecchia, C. The changing pattern of kidney cancer incidence and mortality in Europe. BJU Int. 2008, 101, 949–958. [Google Scholar] [CrossRef]
- Theis, R.P.; Dolwick Grieb, S.M.; Burr, D.; Siddiqui, T.; Asal, N.R. Smoking, environmental tobacco smoke, and risk of renal cell cancer: A population-based case-control study. BMC Cancer 2008, 8, 387. [Google Scholar] [CrossRef] [PubMed]
- Deckers, I.A.; van den Brandt, P.A.; van Engeland, M.; van Schooten, F.J.; Godschalk, R.W.; Keszei, A.P.; Schouten, L.J. Polymorphisms in genes of the renin-angiotensin-aldosterone system and renal cell cancer risk: Interplay with hypertension and intakes of sodium, potassium and fluid. Int. J. Cancer 2015, 136, 1104–1116. [Google Scholar] [CrossRef]
- Gati, A.; Kouidhi, S.; Marrakchi, R.; El, G.A.; Kourda, N.; Derouiche, A.; Chebil, M.; Caignard, A.; Perier, A. Obesity and renal cancer: Role of adipokines in the tumor-immune system conflict. Oncoimmunology 2014, 3, e27810. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, R.; Ricketts, C.J.; Sourbier, C.; Linehan, W.M. New strategies in renal cell carcinoma: Targeting the genetic and metabolic basis of disease. Clin. Cancer Res. 2015, 21, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Palapattu, G.S.; Kristo, B.; Rajfer, J. Paraneoplastic syndromes in urologic malignancy: The many faces of renal cell carcinoma. Rev. Urol. 2002, 4, 163–170. [Google Scholar] [PubMed]
- Rassweiler, J.; Rassweiler, M.C.; Kenngott, H.; Frede, T.; Michel, M.S.; Alken, P.; Clayman, R. The past, present and future of minimally invasive therapy in urology: A review and speculative outlook. Minim. Invasive Ther. Allied Technol. 2013, 22, 200–209. [Google Scholar] [CrossRef] [PubMed]
- Tran, J.; Ornstein, M.C. Clinical review on the management of metastatic renal cell carcinoma. JCO Oncol. Pract. 2022, 18, 187–196. [Google Scholar] [CrossRef] [PubMed]
- Crouzet, S.; Rouviere, O.; Martin, X.; Gelet, A. High-intensity focused ultrasound as focal therapy of prostate cancer. Curr. Opin. Urol. 2014, 24, 225–230. [Google Scholar] [CrossRef] [PubMed]
- Czerwinska, M.; Bilewicz, A.; Kruszewski, M.; Wegierek-Ciuk, A.; Lankoff, A. Targeted radionuclide therapy of prostate cancer-from basic research to clinical perspectives. Molecules 2020, 25, 1743. [Google Scholar] [CrossRef]
- Cattrini, C.; Castro, E.; Lozano, R.; Zanardi, E.; Rubagotti, A.; Boccardo, F.; Olmos, D. Current treatment options for metastatic hormone-sensitive prostate cancer. Cancers 2019, 11, 1355. [Google Scholar] [CrossRef] [PubMed]
- Carthon, B.C. Clinical considerations and challenges in treating patients with oligometastatic prostate cancer. J. Oncol. Pract. 2017, 13, 19–20. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Chen, C.H.; Chan, T.M.; Wu, Y.J.; Chen, J.J. Review: Application of nanoparticles in urothelial cancer of the urinary bladder. J. Med. Biol. Eng. 2015, 35, 419–427. [Google Scholar] [CrossRef]
- Kondylis, F.I.; Demirci, S.; Ladaga, L.; Kolm, P.; Schellhammer, P.F. Outcomes after intravesical bacillus Calmette-Guerin are not affected by substaging of high grade T1 transitional cell carcinoma. J. Urol. 2000, 163, 1120–1123. [Google Scholar] [CrossRef]
- Dobruch, J.; Oszczudlowski, M. Bladder cancer: Current challenges and future directions. Medicina 2021, 57, 749. [Google Scholar] [CrossRef] [PubMed]
- Ashrafizadeh, M.; Hushmandi, K.; Rahmani, M.E.; Zarrin, V.; Hosseinzadeh, K.S.; Bokaie, S.; Najafi, M.; Tavakol, S.; Mohammadinejad, R.; Nabavi, N.; et al. Progress in delivery of siRNA-based therapeutics employing nano-vehicles for treatment of prostate cancer. Bioengineering 2020, 7, 91. [Google Scholar] [CrossRef] [PubMed]
- Ashrafizadeh, M.; Delfi, M.; Hashemi, F.; Zabolian, A.; Saleki, H.; Bagherian, M.; Azami, N.; Farahani, M.V.; Sharifzadeh, S.O.; Hamzehlou, S.; et al. Biomedical application of chitosan-based nanoscale delivery systems: Potential usefulness in siRNA delivery for cancer therapy. Carbohydr. Polym. 2021, 260, 117809. [Google Scholar] [CrossRef] [PubMed]
- Tatiparti, K.; Sau, S.; Kashaw, S.K.; Iyer, A.K. siRNA delivery strategies: A comprehensive review of recent developments. Nanomaterials 2017, 7, 77. [Google Scholar] [CrossRef]
- Grassi, M.; Cavallaro, G.; Scirè, S.; Scaggiante, B.; Daps, B.; Farra, R.; Baiz, D.; Giansante, C.; Guarnieri, G.; Perin, D.; et al. Current strategies to improve the efficacy and the delivery of nucleic acid based drugs. Curr. Signal Transduct. Ther. 2010, 5, 92–120. [Google Scholar] [CrossRef]
- Barba, A.A.; Cascone, S.; Caccavo, D.; Lamberti, G.; Chiarappa, G.; Abrami, M.; Grassi, G.; Grassi, M.; Tomaiuolo, G.; Guido, S.; et al. Engineering approaches in siRNA delivery. Int. J. Pharm. 2017, 525, 343–358. [Google Scholar] [CrossRef] [PubMed]
- Scaggiante, B.; Dapas, B.; Farra, R.; Grassi, M.; Pozzato, G.; Giansante, C.; Fiotti, N.; Grassi, G. Improving siRNA bio-distribution and minimizing side effects. Curr. Drug Metab. 2011, 12, 11–23. [Google Scholar] [CrossRef] [PubMed]
- Grassi, G.; Dawson, P.; Guarnieri, G.; Kandolf, R.; Grassi, M. Therapeutic potential of hammerhead ribozymes in the treatment of hyper-proliferative diseases. Curr. Pharm. Biotechnol. 2004, 5, 369–386. [Google Scholar] [CrossRef]
- Grassi, G.; Marini, J.C. Ribozymes: Structure, function, and potential therapy for dominant genetic disorders. Ann. Med. 1996, 28, 499–510. [Google Scholar] [CrossRef] [PubMed]
- Farra, R.; Musiani, F.; Perrone, F.; Cemazar, M.; Kamensek, U.; Tonon, F.; Abrami, M.; Rucigaj, A.; Grassi, M.; Pozzato, G.; et al. Polymer-mediated delivery of siRNAs to hepatocellular carcinoma: Variables affecting specificity and effectiveness. Molecules 2018, 23, 777. [Google Scholar] [CrossRef]
- Scarabel, L.; Perrone, F.; Garziera, M.; Farra, R.; Grassi, M.; Musiani, F.; Russo, S.C.; Salis, B.; De, S.L.; Toffoli, G.; et al. Strategies to optimize siRNA delivery to hepatocellular carcinoma cells. Expert Opin. Drug Deliv. 2017, 14, 797–810. [Google Scholar] [CrossRef] [PubMed]
- Farra, R.; Maruna, M.; Perrone, F.; Grassi, M.; Benedetti, F.; Maddaloni, M.; El, B.M.; Parisi, S.; Rizzolio, F.; Forte, G.; et al. Strategies for delivery of siRNAs to ovarian cancer cells. Pharmaceutics 2019, 11, 547. [Google Scholar] [CrossRef]
- Barba, A.A.; Lamberti, G.; Sardo, C.; Dapas, B.; Abrami, M.; Grassi, M.; Farra, R.; Tonon, F.; Forte, G.; Musiani, F.; et al. Novel lipid and polymeric materials as delivery systems for nucleic acid based drugs. Curr. Drug Metab. 2015, 16, 427–452. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Hong, J.; Zheng, S.; Ding, Y.; Guo, S.; Zhang, H.; Zhang, X.; Du, Q.; Liang, Z. Elimination pathways of systemically delivered siRNA. Mol. Ther. 2011, 19, 381–385. [Google Scholar] [CrossRef] [PubMed]
- Kaneda, Y. Gene therapy: A battle against biological barriers. Curr. Mol. Med. 2001, 1, 493–499. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Li, J.; Li, X.; Mu, H.; Zhang, X.; Shi, Y.; Chu, Y.; Wang, A.; Wu, Z.; Sun, K. Magnetically and pH dual responsive dendrosomes for tumor accumulation enhanced folate-targeted hybrid drug delivery. J. Control. Release 2016, 232, 161–174. [Google Scholar] [CrossRef]
- Huang, S.L. Liposomes in ultrasonic drug and gene delivery. Adv. Drug Deliv. Rev. 2008, 60, 1167–1176. [Google Scholar] [CrossRef]
- Husseini, G.A.; Pitt, W.G. Micelles and nanoparticles for ultrasonic drug and gene delivery. Adv. Drug Deliv. Rev. 2008, 60, 1137–1152. [Google Scholar] [CrossRef] [PubMed]
- Nyborg, W.L. Ultrasound, contrast agents and biological cells; a simplified model for their interaction during in vitro experiments. Ultrasound Med. Biol. 2006, 32, 1557–1568. [Google Scholar] [CrossRef] [PubMed]
- Kalluri, R.; LeBleu, V.S. The biology, function, and biomedical applications of exosomes. Science 2020, 367, eaau6977. [Google Scholar] [CrossRef] [PubMed]
- Natasha, G.; Gundogan, B.; Tan, A.; Farhatnia, Y.; Wu, W.; Rajadas, J.; Seifalian, A.M. Exosomes as immunotheranostic nanoparticles. Clin. Ther. 2014, 36, 820–829. [Google Scholar] [CrossRef] [PubMed]
- Nayar, R.; Schroit, A.J. Generation of pH-sensitive liposomes: Use of large unilamellar vesicles containing N-succinyldioleoylphosphatidylethanolamine. Biochemistry 1985, 24, 5967–5971. [Google Scholar] [CrossRef] [PubMed]
- Yoshizaki, Y.; Yuba, E.; Komatsu, T.; Udaka, K.; Harada, A.; Kono, K. Improvement of peptide-based tumor immunotherapy using pH-sensitive fusogenic polymer-modified liposomes. Molecules 2016, 21, 1284. [Google Scholar] [CrossRef] [PubMed]
- Park, T.G.; Jeong, J.H.; Kim, S.W. Current status of polymeric gene delivery systems. Adv. Drug Deliv. Rev. 2006, 58, 467–486. [Google Scholar] [CrossRef] [PubMed]
- York, A.W.; Kirkland, S.E.; McCormick, C.L. Advances in the synthesis of amphiphilic block copolymers via RAFT polymerization: Stimuli-responsive drug and gene delivery. Adv. Drug Deliv. Rev. 2008, 60, 1018–1036. [Google Scholar] [CrossRef] [PubMed]
- Roberts, M.J.; Bentley, M.D.; Harris, J.M. Chemistry for peptide and protein PEGylation. Adv. Drug Deliv. Rev. 2002, 54, 459–476. [Google Scholar] [CrossRef]
- Makadia, H.K.; Siegel, S.J. Poly Lactic-co-glycolic acid (PLGA) as biodegradable controlled drug delivery carrier. Polymers 2011, 3, 1377–1397. [Google Scholar] [CrossRef]
- Serrano-Sevilla, I.; Artiga, A.; Mitchell, S.G.; De, M.L.; de la Fuente, J.M. Natural polysaccharides for siRNA delivery: Nanocarriers based on chitosan, hyaluronic acid, and their derivatives. Molecules 2019, 24, 2570. [Google Scholar] [CrossRef] [PubMed]
- Dufes, C.; Uchegbu, I.F.; Schatzlein, A.G. Dendrimers in gene delivery. Adv. Drug Deliv. Rev. 2005, 57, 2177–2202. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.; Zhao, W.; Yu, J.; Li, Y.; Zhao, C. Recent development of pH-responsive polymers for cancer nanomedicine. Molecules 2018, 24, 4. [Google Scholar] [CrossRef] [PubMed]
- Scaggiante, B.; Dapas, B.; Grassi, M.; Zanconati, F.; Farra, R.; Tonon, F.; Fiorentino, S.M.; Abrami, M.; Grassi, G. Nucleic acid-based aptamers and their applications. Future Med. 2013, 55–71. [Google Scholar]
- Dapas, B.; Pozzato, G.; Zorzet, S.; Capolla, S.; Paolo, M.; Scaggiante, B.; Coan, M.; Guerra, C.; Gnan, C.; Gattei, V.; et al. Effects of eEF1A1 targeting by aptamer/siRNA in chronic lymphocytic leukaemia cells. Int. J. Pharm. 2020, 574, 118895. [Google Scholar] [CrossRef]
- Nedyalkova, M.; Donkova, B.; Romanova, J.; Tzvetkov, G.; Madurga, S.; Simeonov, V. Iron oxide nanoparticles—In vivo/in vitro biomedical applications and in silico studies. Adv. Colloid Interface Sci. 2017, 249, 192–212. [Google Scholar] [CrossRef]
- Zare, H.; Ahmadi, S.; Ghasemi, A.; Ghanbari, M.; Rabiee, N.; Bagherzadeh, M.; Karimi, M.; Webster, T.J.; Hamblin, M.R.; Mostafavi, E. Carbon nanotubes: Smart drug/gene delivery carriers. Int. J. Nanomed. 2021, 16, 1681–1706. [Google Scholar] [CrossRef] [PubMed]
- Nogawa, M.; Yuasa, T.; Kimura, S.; Tanaka, M.; Kuroda, J.; Sato, K.; Yokota, A.; Segawa, H.; Toda, Y.; Kageyama, S.; et al. Intravesical administration of small interfering RNA targeting PLK-1 successfully prevents the growth of bladder cancer. J. Clin. Investig. 2005, 115, 978–985. [Google Scholar] [CrossRef] [PubMed]
- Fristrup, N.; Ulhoi, B.P.; Birkenkamp-Demtroder, K.; Mansilla, F.; Sanchez-Carbayo, M.; Segersten, U.; Malmstrom, P.U.; Hartmann, A.; Palou, J.; Alvarez-Mugica, M.; et al. Cathepsin E, maspin, Plk1, and survivin are promising prognostic protein markers for progression in non-muscle invasive bladder cancer. Am. J. Pathol. 2012, 180, 1824–1834. [Google Scholar] [CrossRef] [PubMed]
- Brassesco, M.S.; Pezuk, J.A.; Morales, A.G.; de Oliveira, J.C.; Roberto, G.M.; da Silva, G.N.; Francisco de, O.H.; Scrideli, C.A.; Tone, L.G. In vitro targeting of Polo-like kinase 1 in bladder carcinoma: Comparative effects of four potent inhibitors. Cancer Biol. Ther. 2013, 14, 648–657. [Google Scholar] [CrossRef] [PubMed]
- Greco, K.A.; Franzen, C.A.; Foreman, K.E.; Flanigan, R.C.; Kuo, P.C.; Gupta, G.N. PLK-1 silencing in bladder cancer by siRNA delivered with exosomes. Urology 2016, 91, 241–247. [Google Scholar] [CrossRef] [PubMed]
- Cui, M.; Au, J.L.; Wientjes, M.G.; O’Donnell, M.A.; Loughlin, K.R.; Lu, Z. Intravenous siRNA silencing of survivin enhances activity of mitomycin c in human bladder RT4 xenografts. J. Urol. 2015, 194, 230–237. [Google Scholar] [CrossRef][Green Version]
- Akhtar, M.; Gallagher, L.; Rohan, S. Survivin: Role in diagnosis, prognosis, and treatment of bladder cancer. Adv. Anat. Pathol. 2006, 13, 122–126. [Google Scholar] [CrossRef] [PubMed]
- Jeon, C.; Kim, M.; Kwak, C.; Kim, H.H.; Ku, J.H. Prognostic role of survivin in bladder cancer: A systematic review and meta-analysis. PLoS ONE. 2013, 8, e76719. [Google Scholar] [CrossRef] [PubMed]
- Ambrosio, L.; Argenziano, M.; Cucci, M.A.; Grattarola, M.; de Graaf, I.A.M.; Dianzani, C.; Barrera, G.; Sanchez, N.J.; Gomez, R.; Cavalli, R.; et al. Carbosilane dendrimers loaded with siRNA targeting Nrf2 as a tool to overcome cisplatin chemoresistance in bladder cancer cells. Antioxidants 2020, 9, 993. [Google Scholar] [CrossRef] [PubMed]
- Martin, D.T.; Steinbach, J.M.; Liu, J.; Shimizu, S.; Kaimakliotis, H.Z.; Wheeler, M.A.; Hittelman, A.B.; Mark, S.W.; Weiss, R.M. Surface-modified nanoparticles enhance transurothelial penetration and delivery of survivin siRNA in treating bladder cancer. Mol. Cancer Ther. 2014, 13, 71–81. [Google Scholar] [CrossRef]
- GuhaSarkar, S.; Banerjee, R. Intravesical drug delivery: Challenges, current status, opportunities and novel strategies. J. Control. Release 2010, 148, 147–159. [Google Scholar] [CrossRef]
- Park, J.H.; Cho, H.J.; Yoon, H.Y.; Yoon, I.S.; Ko, S.H.; Shim, J.S.; Cho, J.H.; Park, J.H.; Kim, K.; Kwon, I.C.; et al. Hyaluronic acid derivative-coated nanohybrid liposomes for cancer imaging and drug delivery. J. Control. Release 2014, 174, 98–108. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Yu, T.; Zhou, B.; Wei, J.; Fang, Y.; Lu, J.; Guo, L.; Chen, W.; Liu, Z.P.; Luo, J. Mg(II)-Catechin nanoparticles delivering siRNA targeting EIF5A2 inhibit bladder cancer cell growth in vitro and in vivo. Biomaterials 2016, 81, 125–134. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhang, Y.; Zeng, Q.; Zeng, H.; Liu, X.; Wu, P.; Xie, H.; He, L.; Long, Z.; Lu, X.; et al. Delivery of RIPK4 small interfering RNA for bladder cancer therapy using natural halloysite nanotubes. Sci. Adv. 2019, 5, eaaw6499. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhang, Y.; Zeng, H.; Wang, L.; Zhang, Q.; Wu, P.; Liu, X.; Xie, H.; Xiang, W.; Liu, B.; et al. Fe-doped chrysotile nanotubes containing siRNAs to silence SPAG5 to treat bladder cancer. J. Nanobiotechnol. 2021, 19, 189. [Google Scholar] [CrossRef]
- Liang, Y.; Wang, Y.; Wang, L.; Liang, Z.; Li, D.; Xu, X.; Chen, Y.; Yang, X.; Zhang, H.; Niu, H. Self-crosslinkable chitosan-hyaluronic acid dialdehyde nanoparticles for CD44-targeted siRNA delivery to treat bladder cancer. Bioact. Mater. 2021, 6, 433–446. [Google Scholar] [CrossRef] [PubMed]
- Posocco, B.; Dreussi, E.; de Santa, J.; Toffoli, G.; Abrami, M.; Musiani, F.; Grassi, M.; Farra, R.; Tonon, F.; Grassi, G.; et al. Polysaccharides for the delivery of antitumor drugs. Materials 2015, 8, 2569–2615. [Google Scholar] [CrossRef]
- Kim, S.H.; Ho, J.N.; Jin, H.; Lee, S.C.; Lee, S.E.; Hong, S.K.; Lee, J.W.; Lee, E.S.; Byun, S.S. Upregulated expression of BCL2, MCM7, and CCNE1 indicate cisplatin-resistance in the set of two human bladder cancer cell lines: T24 cisplatin sensitive and T24R2 cisplatin resistant bladder cancer cell lines. Investig. Clin. Urol. 2016, 57, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Guan, X.Y.; Sham, J.S.; Tang, T.C.; Fang, Y.; Huo, K.K.; Yang, J.M. Isolation of a novel candidate oncogene within a frequently amplified region at 3q26 in ovarian cancer. Cancer Res. 2001, 61, 3806–3809. [Google Scholar]
- Tang, D.J.; Dong, S.S.; Ma, N.F.; Xie, D.; Chen, L.; Fu, L.; Lau, S.H.; Li, Y.; Li, Y.; Guan, X.Y. Overexpression of eukaryotic initiation factor 5A2 enhances cell motility and promotes tumor metastasis in hepatocellular carcinoma. Hepatology 2010, 51, 1255–1263. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Luo, J.H.; Hua, W.F.; Zhou, F.J.; Lin, M.C.; Kung, H.F.; Zeng, Y.X.; Guan, X.Y.; Xie, D. Overexpression of EIF-5A2 is an independent predictor of outcome in patients of urothelial carcinoma of the bladder treated with radical cystectomy. Cancer Epidemiol. Biomark. Prev. 2009, 18, 400–408. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.Y.; Zeng, Q.H.; Cao, P.G.; Xie, D.; Chen, X.; Yang, F.; He, L.Y.; Dai, Y.B.; Li, J.J.; Liu, X.M.; et al. RIPK4 promotes bladder urothelial carcinoma cell aggressiveness by upregulating VEGF-A through the NF-kappaB pathway. Br. J. Cancer 2018, 118, 1617–1627. [Google Scholar] [CrossRef]
- He, J.; Green, A.R.; Li, Y.; Chan, S.Y.T.; Liu, D.X. SPAG5: An emerging oncogene. Trends Cancer 2020, 6, 543–547. [Google Scholar] [CrossRef] [PubMed]
- Scaggiante, B.; Dapas, B.; Bonin, S.; Grassi, M.; Zennaro, C.; Farra, R.; Cristiano, L.; Siracusano, S.; Zanconati, F.; Giansante, C.; et al. Dissecting the expression of EEF1A1/2 genes in human prostate cancer cells: The potential of EEF1A2 as a hallmark for prostate transformation and progression. Br. J. Cancer 2012, 106, 166–173. [Google Scholar] [CrossRef] [PubMed]
- Bae, Y.J.; Yoon, Y.I.; Yoon, T.J.; Lee, H.J. Ultrasound-guided delivery of siRNA and a chemotherapeutic drug by using microbubble complexes: In vitro and in vivo evaluations in a prostate cancer model. Korean J. Radiol. 2016, 17, 497–508. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lu, C.H.; Wu, C.H.; Hsieh, P.F.; Wu, C.Y.; Kuo, W.W.; Ou, C.H.; Lin, V.C.H. Small interfering RNA targeting N-cadherin regulates cell proliferation and migration in enzalutamide-resistant prostate cancer. Oncol. Lett. 2022, 23, 90. [Google Scholar] [CrossRef]
- Oner, E.; Kotmakci, M.; Baird, A.M.; Gray, S.G.; Debelec, B.B.; Bozkurt, E.; Kantarci, A.G.; Finn, S.P. Development of EphA2 siRNA-loaded lipid nanoparticles and combination with a small-molecule histone demethylase inhibitor in prostate cancer cells and tumor spheroids. J. Nanobiotechnol. 2021, 19, 71. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Shen, H.; Naguib, A.; Weiss, R.M.; Martin, D.T. Knocking down claudin receptors leads to a decrease in prostate cancer cell migration, cell growth, cell viability and clonogenic cell survival. Mol. Biomed. 2021, 2, 31. [Google Scholar] [CrossRef] [PubMed]
- Zhupanyn, P.; Ewe, A.; Buch, T.; Malek, A.; Rademacher, P.; Muller, C.; Reinert, A.; Jaimes, Y.; Aigner, A. Extracellular vesicle (ECV)-modified polyethylenimine (PEI) complexes for enhanced siRNA delivery in vitro and in vivo. J. Control. Release 2020, 319, 63–76. [Google Scholar] [CrossRef] [PubMed]
- Han, Q.; Xie, Q.R.; Li, F.; Cheng, Y.; Wu, T.; Zhang, Y.; Lu, X.; Wong, A.S.T.; Sha, J.; Xia, W. Targeted inhibition of SIRT6 via engineered exosomes impairs tumorigenesis and metastasis in prostate cancer. Theranostics 2021, 11, 6526–6541. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Zhao, H.; Guo, L.; Wang, Y.; Song, J.; Zhao, X.; Li, C.; Hao, L.; Wang, D.; Tang, J. Ultrasound-mediated nanobubble destruction (UMND) facilitates the delivery of A10-3.2 aptamer targeted and siRNA-loaded cationic nanobubbles for therapy of prostate cancer. Drug Deliv. 2018, 25, 226–240. [Google Scholar] [CrossRef] [PubMed]
- Goel, S.; Bhatia, V.; Kundu, S.; Biswas, T.; Carskadon, S.; Gupta, N.; Asim, M.; Morrissey, C.; Palanisamy, N.; Ateeq, B. Transcriptional network involving ERG and AR orchestrates Distal-less homeobox-1 mediated prostate cancer progression. Nat. Commun. 2021, 12, 5325. [Google Scholar] [CrossRef]
- Jugel, W.; Aigner, A.; Michen, S.; Hagstotz, A.; Ewe, A.; Appelhans, D.; Schackert, G.; Temme, A.; Tietze, S. Targeted RNAi of BIRC5/survivin using antibody-conjugated poly(propylene imine)-based polyplexes inhibits growth of PSCA-positive tumors. Pharmaceutics 2021, 13, 676. [Google Scholar] [CrossRef]
- Dong, Y.; Chen, Y.; Zhu, D.; Shi, K.; Ma, C.; Zhang, W.; Rocchi, P.; Jiang, L.; Liu, X. Self-assembly of amphiphilic phospholipid peptide dendrimer-based nanovectors for effective delivery of siRNA therapeutics in prostate cancer therapy. J. Control. Release 2020, 322, 416–425. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; He, Z.; Xiang, L.; Li, L.; Zhang, H.; Lin, F.; Cao, H. Codelivery of GRP78 siRNA and docetaxel via RGD-PEG-DSPE/DOPA/CaP nanoparticles for the treatment of castration-resistant prostate cancer. Drug Des. Devel. Ther. 2019, 13, 1357–1372. [Google Scholar] [CrossRef] [PubMed]
- Panday, R.; Abdalla, A.M.E.; Yu, M.; Li, X.; Ouyang, C.; Yang, G. Functionally modified magnetic nanoparticles for effective siRNA delivery to prostate cancer cells in vitro. J. Biomater Appl. 2020, 34, 952–964. [Google Scholar] [CrossRef] [PubMed]
- Baca, S.C.; Takeda, D.Y.; Seo, J.H.; Hwang, J.; Ku, S.Y.; Arafeh, R.; Arnoff, T.; Agarwal, S.; Bell, C.; O’Connor, E.; et al. Reprogramming of the FOXA1 cistrome in treatment-emergent neuroendocrine prostate cancer. Nat. Commun. 2021, 12, 1979. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, H.; Kono, E.; Tran, C.P.; Miyazaki, H.; Yamashiro, J.; Shimomura, T.; Fazli, L.; Wada, R.; Huang, J.; Vessella, R.L.; et al. Monoclonal antibody targeting of N-cadherin inhibits prostate cancer growth, metastasis and castration resistance. Nat. Med. 2010, 16, 1414–1420. [Google Scholar] [CrossRef]
- Tandon, M.; Vemula, S.V.; Mittal, S.K. Emerging strategies for EphA2 receptor targeting for cancer therapeutics. Expert Opin. Ther. Targets. 2011, 15, 31–51. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Chang, J.; Varghese, D.; Dellinger, M.; Kumar, S.; Best, A.M.; Ruiz, J.; Bruick, R.; Pena-Llopis, S.; Xu, J.; et al. A small molecule modulates Jumonji histone demethylase activity and selectively inhibits cancer growth. Nat. Commun. 2013, 4, 2035. [Google Scholar] [CrossRef] [PubMed]
- Landers, K.A.; Samaratunga, H.; Teng, L.; Buck, M.; Burger, M.J.; Scells, B.; Lavin, M.F.; Gardiner, R.A. Identification of claudin-4 as a marker highly overexpressed in both primary and metastatic prostate cancer. Br. J. Cancer 2008, 99, 491–501. [Google Scholar] [CrossRef]
- Bartholow, T.L.; Chandran, U.R.; Becich, M.J.; Parwani, A.V. Immunohistochemical profiles of claudin-3 in primary and metastatic prostatic adenocarcinoma. Diagn. Pathol. 2011, 6, 12. [Google Scholar] [CrossRef] [PubMed]
- Song, Z.; Yao, X.; Wu, M. Direct interaction between survivin and Smac/DIABLO is essential for the anti-apoptotic activity of survivin during taxol-induced apoptosis. J. Biol. Chem. 2003, 278, 23130–23140. [Google Scholar] [CrossRef] [PubMed]
- Wiedemuth, R.; Klink, B.; Topfer, K.; Schrock, E.; Schackert, G.; Tatsuka, M.; Temme, A. Survivin safeguards chromosome numbers and protects from aneuploidy independently from p53. Mol. Cancer 2014, 13, 107. [Google Scholar] [CrossRef] [PubMed]
- Powell, G.B.; Kelly, L.; Ahrens, D.P.; Barry, A.P.; Kratschmer, C.; Levy, M.; Sullenger, B.A. Tunable cytotoxic aptamer-drug conjugates for the treatment of prostate cancer. Proc. Natl. Acad. Sci. USA 2018, 115, 4761–4766. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Xie, Q.R.; Wang, B.; Shao, J.; Zhang, T.; Liu, T.; Huang, G.; Xia, W. Inhibition of SIRT6 in prostate cancer reduces cell viability and increases sensitivity to chemotherapeutics. Protein Cell 2013, 4, 702–710. [Google Scholar] [CrossRef] [PubMed]
- Farokhzad, O.C.; Jon, S.; Khademhosseini, A.; Tran, T.N.; Lavan, D.A.; Langer, R. Nanoparticle-aptamer bioconjugates: A new approach for targeting prostate cancer cells. Cancer Res. 2004, 64, 7668–7672. [Google Scholar] [CrossRef]
- Wang, Y.; Yao, B.; Wang, Y.; Zhang, M.; Fu, S.; Gao, H.; Peng, R.; Zhang, L.; Tang, J. Increased FoxM1 expression is a target for metformin in the suppression of EMT in prostate cancer. Int. J. Mol. Med. 2014, 33, 1514–1522. [Google Scholar] [CrossRef]
- Abate-Shen, C. Deregulated homeobox gene expression in cancer: Cause or consequence? Nat. Rev. Cancer 2002, 2, 777–785. [Google Scholar] [CrossRef]
- Van, N.L.; Hendriks, R.J.; Dijkstra, S.; Trooskens, G.; Cornel, E.B.; Jannink, S.A.; de Jong, H.; Hessels, D.; Smit, F.P.; Melchers, W.J.; et al. Detection of high-grade prostate cancer using a urinary molecular biomarker-based risk score. Eur. Urol. 2016, 70, 740–748. [Google Scholar]
- Korenchuk, S.; Lehr, J.E.; MClean, L.; Lee, Y.G.; Whitney, S.; Vessella, R.; Lin, D.L.; Pienta, K.J. VCaP, a cell-based model system of human prostate cancer. Vivo 2001, 15, 163–168. [Google Scholar]
- Reiter, R.E.; Gu, Z.; Watabe, T.; Thomas, G.; Szigeti, K.; Davis, E.; Wahl, M.; Nisitani, S.; Yamashiro, J.; Le Beau, M.M.; et al. Prostate stem cell antigen: A cell surface marker overexpressed in prostate cancer. Proc. Natl. Acad. Sci. USA 1998, 95, 1735–1740. [Google Scholar] [CrossRef] [PubMed]
- Bosutti, A.; Kalaja, O.; Zanconati, F.; Dapas, B.; Grassi, G.; Passamonti, S.; Scaggiante, B. A rapid and specific method to simultaneously quantify eukaryotic elongation factor 1A1 and A2 protein levels in cancer cells. J. Pharm. BioMed. Anal. 2019, 176, 112814. [Google Scholar] [CrossRef]
- Rocchi, P.; Beraldi, E.; Ettinger, S.; Fazli, L.; Vessella, R.L.; Nelson, C.; Gleave, M. Increased Hsp27 after androgen ablation facilitates androgen-independent progression in prostate cancer via signal transducers and activators of transcription 3-mediated suppression of apoptosis. Cancer Res. 2005, 65, 11083–11093. [Google Scholar] [CrossRef]
- Lima, T.S.; Iglesias-Gato, D.; Souza, L.D.O.; Stenvang, J.; Lima, D.S.; Roder, M.A.; Brasso, K.; Moreira, J.M.A. Molecular profiling of docetaxel-resistant prostate cancer cells identifies multiple mechanisms of therapeutic resistance. Cancers 2021, 13, 1290. [Google Scholar] [CrossRef]
- Sun, Y.; Kang, C.; Liu, F.; Zhou, Y.; Luo, L.; Qiao, H. RGD Peptide-based target drug delivery of doxorubicin nanomedicine. Drug Dev. Res. 2017, 78, 283–291. [Google Scholar] [CrossRef]
- Pootrakul, L.; Datar, R.H.; Shi, S.R.; Cai, J.; Hawes, D.; Groshen, S.G.; Lee, A.S.; Cote, R.J. Expression of stress response protein Grp78 is associated with the development of castration-resistant prostate cancer. Clin. Cancer Res. 2006, 12, 5987–5993. [Google Scholar] [CrossRef]
- Arima, T.; Enokida, H.; Kubo, H.; Kagara, I.; Matsuda, R.; Toki, K.; Nishimura, H.; Chiyomaru, T.; Tatarano, S.; Idesako, T.; et al. Nuclear translocation of ADAM-10 contributes to the pathogenesis and progression of human prostate cancer. Cancer Sci. 2007, 98, 1720–1726. [Google Scholar] [CrossRef]
- Titomirov, A.V.; Sukharev, S.; Kistanova, E. In vivo electroporation and stable transformation of skin cells of newborn mice by plasmid DNA. Biochim. Biophys. Acta. 1991, 1088, 131–134. [Google Scholar] [CrossRef]
- Teng, M.; Zhou, S.; Cai, C.; Lupien, M.; He, H.H. Pioneer of prostate cancer: Past, present and the future of FOXA1. Protein Cell. 2021, 12, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Gerhardt, J.; Montani, M.; Wild, P.; Beer, M.; Huber, F.; Hermanns, T.; Muntener, M.; Kristiansen, G. FOXA1 promotes tumor progression in prostate cancer and represents a novel hallmark of castration-resistant prostate cancer. Am. J. Pathol. 2012, 180, 848–861. [Google Scholar] [CrossRef] [PubMed]
- Puca, L.; Bareja, R.; Prandi, D.; Shaw, R.; Benelli, M.; Karthaus, W.R.; Hess, J.; Sigouros, M.; Donoghue, A.; Kossai, M.; et al. Patient derived organoids to model rare prostate cancer phenotypes. Nat. Commun. 2018, 9, 2404. [Google Scholar] [CrossRef] [PubMed]
- Sakurai, Y.; Hatakeyama, H.; Akita, H.; Harashima, H. Improvement of doxorubicin efficacy using liposomal anti-polo-like kinase 1 siRNA in human renal cell carcinomas. Mol. Pharm. 2014, 11, 2713–2719. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Fu, Q.; Pu, L.; Liu, X.; Liu, Y. Effects of HMGA2 gene silencing on cell cycle and apoptosis in the metastatic renal carcinoma cell line ACHN. J. Int. Med. Res. 2022, 50, 3000605221075511. [Google Scholar] [CrossRef] [PubMed]
- Tabernero, J.; Shapiro, G.I.; LoRusso, P.M.; Cervantes, A.; Schwartz, G.K.; Weiss, G.J.; Paz-Ares, L.; Cho, D.C.; Infante, J.R.; Alsina, M.; et al. First-in-humans trial of an RNA interference therapeutic targeting VEGF and KSP in cancer patients with liver involvement. Cancer Discov. 2013, 3, 406–417. [Google Scholar] [CrossRef]
- Hattab, D.; Gazzali, A.M.; Bakhtiar, A. Clinical advances of siRNA-based nanotherapeutics for cancer treatment. Pharmaceutics 2021, 13, 1009. [Google Scholar] [CrossRef]
- Neuberg, P.; Hamaidi, I.; Danilin, S.; Ripoll, M.; Lindner, V.; Nothisen, M.; Wagner, A.; Kichler, A.; Massfelder, T.; Remy, J.S. Polydiacetylenic nanofibers as new siRNA vehicles for in vitro and in vivo delivery. Nanoscale 2018, 10, 1587–1590. [Google Scholar] [CrossRef]
- Racaniello, G.F.; Laquintana, V.; Vergnaud, J.; Lopedota, A.; Cutrignelli, A.; Lopalco, A.; Leonetti, F.; Franco, M.; Fiume, M.; Pontrelli, P.; et al. Development of purified glycogen derivatives as siRNA nanovectors. Int. J. Pharm. 2021, 608, 121128. [Google Scholar] [CrossRef]
- Unachukwu, U.; Chada, K.; D’Armiento, J. High Mobility Group AT-Hook 2 (HMGA2) oncogenicity in mesenchymal and epithelial neoplasia. Int. J. Mol. Sci. 2020, 21, 3151. [Google Scholar] [CrossRef]
- Liu, Y.; Fu, Q.Z.; Pu, L.; Meng, Q.G.; Liu, X.F.; Dong, S.F.; Yang, J.X.; Lv, G.Y. HMGA2 expression in renal carcinoma and its clinical significance. J. Med. Biochem. 2015, 34, 338–343. [Google Scholar] [CrossRef] [PubMed]
- Farra, R.; Grassi, G.; Tonon, F.; Abrami, M.; Grassi, M.; Pozzato, G.; Fiotti, N.; Forte, G.; Dapas, B. The role of the transcription factor E2F1 in hepatocellular carcinoma. Curr. Drug Deliv. 2017, 14, 272–281. [Google Scholar]
- Dormoy, V.; Beraud, C.; Lindner, V.; Thomas, L.; Coquard, C.; Barthelmebs, M.; Jacqmin, D.; Lang, H.; Massfelder, T. LIM-class homeobox gene Lim1, a novel oncogene in human renal cell carcinoma. Oncogene 2011, 30, 1753–1763. [Google Scholar] [CrossRef] [PubMed]
- Mizrahy, S.; Peer, D. Polysaccharides as building blocks for nanotherapeutics. Chem. Soc. Rev. 2012, 41, 2623–2640. [Google Scholar] [CrossRef] [PubMed]
- Grassi, M.; Grassi, G. Application of mathematical modeling in sustained release delivery systems. Expert Opin. Drug Deliv. 2014, 11, 1299–1321. [Google Scholar] [CrossRef] [PubMed]

| Delivery Material | Advantages | Disadvantages |
|---|---|---|
| Liposomes | Easy siRNA loading Minor toxicity Structure easily tunable | No targeting specificity unless equipped with targeting moieties |
| Echogenic liposomes | As above with the possibility to induce ultrasound controlled delivery | Not applicable in deep tissue |
| Exosomes | As above with excellent biodistribution and the possibility to escape clearance by the mononuclear phagocyte system | No targeting specificity |
| Polymers | Production/isolation non expensive Structure easily tunable In general non toxic Possibility to escape endosome (PEI) In general easy siRNA loading Targeting ability to CD44 (HA) | Described toxicity for PEI Low solubility for CH Electrostatic repulsion of siRNA due to polyanionic nature for HA |
| Aptamers | Non toxic Able to target any desired molecule For DNA aptamers low production cost Can easily be stored for very long periods without losing their activity | RNA aptamers may be unstable in the biological environment. The selection procedure may be complex |
| Magnetic nanoparticles | Large surface area which can be functionalized with smart functional groups Magnetic behavior allows targeting to a defined tisse following application of an external magnetic field | Need functional groups on their surface for siRNA loading |
| Carbon nanotubes | High drug loading capacity Cellular uptake can be modulated varying the dimension | Poorly biodegradable |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Halib, N.; Pavan, N.; Trombetta, C.; Dapas, B.; Farra, R.; Scaggiante, B.; Grassi, M.; Grassi, G. An Overview of siRNA Delivery Strategies for Urological Cancers. Pharmaceutics 2022, 14, 718. https://doi.org/10.3390/pharmaceutics14040718
Halib N, Pavan N, Trombetta C, Dapas B, Farra R, Scaggiante B, Grassi M, Grassi G. An Overview of siRNA Delivery Strategies for Urological Cancers. Pharmaceutics. 2022; 14(4):718. https://doi.org/10.3390/pharmaceutics14040718
Chicago/Turabian StyleHalib, Nadia, Nicola Pavan, Carlo Trombetta, Barbara Dapas, Rossella Farra, Bruna Scaggiante, Mario Grassi, and Gabriele Grassi. 2022. "An Overview of siRNA Delivery Strategies for Urological Cancers" Pharmaceutics 14, no. 4: 718. https://doi.org/10.3390/pharmaceutics14040718
APA StyleHalib, N., Pavan, N., Trombetta, C., Dapas, B., Farra, R., Scaggiante, B., Grassi, M., & Grassi, G. (2022). An Overview of siRNA Delivery Strategies for Urological Cancers. Pharmaceutics, 14(4), 718. https://doi.org/10.3390/pharmaceutics14040718









