Recent Advances in the Therapeutic Efficacy of Artesunate
Abstract
:1. Introduction
2. Malaria and Its Parasite Life Cycle
2.1. Mechanism of Action of Artesunate on Malaria and Its Structure–Activity Relationship
2.2. The Efficacy of ART-Based Formulations on Malaria
2.3. The Therapeutic Effects of Artesunate on Cancer
2.4. Artesunate Efficacy in Viral Infections, Skin Diseases, and Diabetes
2.5. Other Recent Reports on the Biological Activities of ART
3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ART | artesunate |
| DIBAL | diisobutylaluminum hydride |
| RBCs | red blood cells |
| ROS | reactive oxygen species |
| NLCs | nanostructured lipid carriers |
| SMA | severe malaria anaemia |
| Hb | haemoglobin |
| PMIF | plasmodium macrophage migration inhibitory factor |
| ED50 | effective dose 50 |
| IC50 | half-maximal inhibitory concentration |
| Cmax | maximum serum concentration |
| Qpcr | quantitative polymerase chain reaction |
| AQ | amodiaquine |
| HCC | hepatocellular carcinoma |
| HPV | human papillomavirus |
| DNA | deoxyribonucleic acid |
| RCC | renal cell carcinoma |
| RNA | ribonucleic acid |
| HCMV | renal cell carcinoma |
| ACT | artesunate combination therapy |
| AD | atopic dermatitis |
| HSCs | hepatic stellate cells |
| DMSO | dimethyl sulfoxide |
| MDA | 3,4-methylenedioxyamphetamine |
| SOD | superoxide dismutase |
| LPS | liposaccharide |
| LPL | lipoprotein lipase |
| ATP | adenosine triphosphate |
| VSMCs | vascular smooth muscle cells |
| ALP | alkaline phosphatase |
| RA | rheumatoid arthritis |
References
- Aprioku, J.S.; Obianime, A.W. Structure-Activity-Relationship (SAR) of Artemisinins on Some Biological Systems in Male Guinea Pigs. Insight Pharm. Sci. 2011, 1, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Song, L.; Ge, T.; Li, Z.; Sun, J.; Li, G.; Sun, Y.; Fang, L.; Ma, Y.J.; Garred, P. Artesunate: A Natural Product-Based Immunomodulator Involved in Human Complement. Biomed. Pharmacother. 2021, 136, 111234. [Google Scholar] [CrossRef] [PubMed]
- Ismail, M.; Ling, L.; Du, Y.; Yao, C.; Li, X. Liposomes of Dimeric Artesunate Phospholipid: A Combination of Dimerization and Self-Assembly to Combat Malaria. Biomaterials 2018, 163, 76–87. [Google Scholar] [CrossRef] [PubMed]
- Kouakou, Y.I.; Tod, M.; Leboucher, G.; Lavoignat, A.; Bonnot, G.; Bienvenu, A.L.; Picot, S. Systematic Review of Artesunate Pharmacokinetics: Implication for Treatment of Resistant Malaria. Int. J. Infect. Dis. 2019, 89, 30–44. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kannan, D.; Yadav, N.; Ahmad, S.; Namdev, P.; Bhattacharjee, S.; Lochab, B.; Singh, S. Pre-Clinical Study of Iron Oxide Nanoparticles Fortified Artesunate for Efficient Targeting of Malarial Parasite. EBioMedicine 2019, 45, 261–277. [Google Scholar] [CrossRef] [Green Version]
- Adebayo, J.O.; Tijjani, H.; Adegunloye, A.P.; Ishola, A.A.; Balogun, E.A.; Malomo, S.O. Enhancing the Antimalarial Activity of Artesunate. Parasitol. Res. 2020, 119, 2749–2764. [Google Scholar] [CrossRef]
- Ismail, M.; Du, Y.; Ling, L.; Li, X. Artesunate-Heparin Conjugate Based Nanocapsules with Improved Pharmacokinetics to Combat Malaria. Int. J. Pharm. 2019, 162–171. [Google Scholar] [CrossRef]
- Zhang, D.; Zhou, C.; Lv, P.; Zhao, Y.; Liang, J.; Liao, X.; Yang, B. Preparation and Characterization of a Novel Host-Guest Complex Based on Folate-Modified β-Cyclodextrin and Artesunate. Mater. Sci. Eng. C 2018, 86, 48–55. [Google Scholar] [CrossRef]
- Cai, L.; Tang, H.; Zhou, M.; Ding, Y.; Li, X.; Shi, Z. Artesunate Attenuated the Progression of Abdominal Aortic Aneurysm in a Mouse Model. J. Surg. Res. 2021, 267, 404–413. [Google Scholar] [CrossRef]
- Shenoy, R.K.; Gokarn, A.; Toshniwal, A.; Kalantri, S.A.; Chichra, A.; Punatar, S.; Bonda, A.; Nayak, L.; Mathew, L.J.; Bhat, V.; et al. Efficacy of Artesunate for Treatment of Cytomegalovirus Reactivations in Allogeneic Haematopoietic Stem Cell Transplant Recipients Who Are Intolerant/Unsuitable for Ganciclovir Therapy. Blood 2019, 134, 4506. [Google Scholar] [CrossRef]
- Kumaran, U.; Gaonkar, S.; Chaudhuri, M.; Sheriff, A.K.; Rao, N.; Raja, M.; Shenoi, A. Chromosomally Integrated Human Herpes Virus 6A-Associated Myocarditis in a Neonate Treated with Artesunate. J. Paediatr. Child Health 2021, 1, 6–8. [Google Scholar] [CrossRef]
- Li, Z.; Shi, X.; Liu, J.; Shao, F.; Huang, G.; Zhou, Z.; Zheng, P. Artesunate Prevents Type 1 Diabetes in NOD Mice Mainly by Inducing Protective IL-4—Producing T Cells and Regulatory T Cells. FASEB J. 2019, 33, 8241–8248. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Li, Y.; Zhao, D.; Li, H.; Zhang, W.; Xu, J.; Hou, J.; Feng, X.; Wang, H. Dihydroartemisinin suppresses glycolysis of LNCaP cells by inhibiting PI3K/AKT pathway and downregulating HIF-1α expression. Life Sci. 2019, 233, 116730. [Google Scholar] [CrossRef] [PubMed]
- Ooko, E.; Saeed, M.E.; Kadioglu, O.; Sarvi, S.; Colak, M.; Elmasaoudi, K.; Janah, R.; Greten, H.J.; Efferth, T. Artemisinin derivatives induce iron-dependent cell death (ferroptosis) in tumor cells. Phytomedicine 2015, 22, 1045–1054. [Google Scholar] [CrossRef]
- Shi, X.; Wang, L.; Li, X.; Bai, J.; Li, J.; Li, S.; Wang, Z.; Zhou, M. Dihydroartemisinin induces autophagy-dependent death in human tongue squamous cell carcinoma cells through DNA double-strand break-mediated oxidative stress. Oncotarget 2017, 8, 45981. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, F.; Zhou, J.Y.; Zhang, D.; Liu, M.H.; Chen, Y.G. Artesunate induces apoptosis and autophagy in HCT116 colon cancer cells, and autophagy inhibition enhances the artesunateinduced apoptosis. Int. J. Mol. Med. 2018, 42, 1295–1304. [Google Scholar] [PubMed] [Green Version]
- Xu, C.; Zhang, H.; Mu, L.; Yang, X. Artemisinins as anticancer drugs: Novel therapeutic approaches, molecular mechanisms, and clinical trials. Front. Pharmacol. 2020, 11, 1608. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.; Wang, H.; Kunda, P.; Chen, X.; Liu, Q.L.; Liu, T. Artesunate exerts specific cytotoxicity in retinoblastoma cells via CD71. Oncol. Rep. 2013, 30, 1473–1482. [Google Scholar] [CrossRef] [PubMed]
- Hahn, F.; Niesar, A.; Wangen, C.; Wild, M.; Grau, B.; Herrmann, L.; Capci, A.; Adrait, A.; Couté, Y.; Tsogoeva, S.B.; et al. Target Verification of Artesunate-Related Antiviral Drugs: Assessing the Role of Mitochondrial and Regulatory Proteins by Click Chemistry and Fluorescence Labeling. Antiviral Res. 2020, 180, 104861. [Google Scholar] [CrossRef]
- Hahn, F.; Fröhlich, T.; Frank, T.; Bertzbach, L.D.; Kohrt, S.; Kaufer, B.B.; Stamminger, T.; Tsogoeva, S.B.; Marschall, M. Artesunate-Derived Monomeric, Dimeric and Trimeric Experimental Drugs—Their Unique Mechanistic Basis and Pronounced Antiherpesviral Activity. Antiviral Res. 2018, 152, 104–110. [Google Scholar] [CrossRef]
- He, L.H.; Gao, J.H.; Yu, X.H.; Wen, F.J.; Luo, J.J.; Qin, Y.S.; Chen, M.X.; Zhang, D.W.; Wang, Z.B.; Tang, C.K. Artesunate Inhibits Atherosclerosis by Upregulating Vascular Smooth Muscle Cells-Derived LPL Expression via the KLF2/NRF2/TCF7L2 Pathway. Eur. J. Pharmacol. 2020, 884, 173408. [Google Scholar] [CrossRef] [PubMed]
- Su, X.; Guo, W.; Yuan, B.; Wang, D.; Liu, L.; Wu, X.; Zhang, Y.; Kong, X.; Lin, N. Artesunate Attenuates Bone Erosion in Rheumatoid Arthritis by Suppressing Reactive Oxygen Species via Activating P62/Nrf2 Signaling. Biomed. Pharmacother. 2021, 137, 111382. [Google Scholar] [CrossRef] [PubMed]
- Zhang, E.; Wang, J.; Chen, Q.; Wang, Z.; Li, D.; Jiang, N.; Ju, X. Artesunate Ameliorates Sepsis-Induced Acute Lung Injury by Activating the MTOR/AKT/PI3K Axis. Gene 2020, 759, 144969. [Google Scholar] [CrossRef] [PubMed]
- Siewe, N.; Friedman, A. Increase Hemoglobin Level in Severe Malarial Anemia While Controlling Parasitemia: A Mathematical Model. Math. Biosci. 2020, 326, 108374. [Google Scholar] [CrossRef]
- Mahdi, A.S.; Molai, M.; Chandwani, J.; Al Khalili, H.; Ibrahim, H.; Pandak, N.; Khamis, F.; Petersen, E. Late Onset Acute Pancreatitis in P. Falciparum Malaria—An Adverse Reaction to Intravenous Artesunate? IDCases 2018, 12, 124–126. [Google Scholar] [CrossRef]
- Mina, P.R.; Kumar, S.; Agarwal, K.; Kumar, R.; Pal, A.; Tandon, S.; Yadav, S.K.; Yadav, S.; Darokar, M.P. 4-Chloro Eugenol Interacts Synergistically with Artesunate against Drug Resistant P. Falciparum Inducing Oxidative Stress. Biomed. Pharmacother. 2021, 137, 111311. [Google Scholar] [CrossRef]
- Shehu, A.M.; Miko, A.M.; Iliya, I.A.; Ihunwo, A.O.; Alawa, J.N.; Adebisi, S.S. Effects of Prenatal Exposure to Artesunate on the Developing Cerebral Cortex in Wistar Rat Fetuses (Rattus Norvegicus). IBRO Rep. 2019, 7, 36. [Google Scholar] [CrossRef]
- Agbo, C.P.; Ugwuanyi, T.C.; Ugwuoke, W.I.; McConville, C.; Attama, A.A.; Ofokansi, K.C. Intranasal Artesunate-Loaded Nanostructured Lipid Carriers: A Convenient Alternative to Parenteral Formulations for the Treatment of Severe and Cerebral Malaria. J. Control. Release 2021, 334, 224–236. [Google Scholar] [CrossRef]
- Kone, A.; Sissoko, S.; Fofana, B.; Sangare, C.O.; Dembele, D.; Haidara, A.S.; Diallo, N.; Coulibaly, A.; Traore, A.; Toure, S.; et al. Different Plasmodium Falciparum Clearance Times in Two Malian Villages Following Artesunate Monotherapy. Int. J. Infect. Dis. 2020, 95, 399–405. [Google Scholar] [CrossRef]
- Wilson, M.E. Parenteral Artesunate for Treatment of Severe Malaria. Curr. Infect. Dis. Rep. 2006, 8, 33–34. [Google Scholar] [CrossRef]
- Deshpande, S.; Kuppast, B. 4-Aminoquinolines: An Overview of Antimalarial Chemotherapy. Med. Chem. 2016, 6, 1–11. [Google Scholar] [CrossRef]
- Soulard, V.; Bosson-Vanga, H.; Lorthiois, A.; Roucher, C.; Franetich, J.F.; Zanghi, G.; Bordessoulles, M.; Tefit, M.; Thellier, M.; Morosan, S.; et al. Plasmodium falciparum Full Life Cycle and Plasmodium Ovale Liver Stages in Humanized Mice. Nat. Commun. 2015, 6, 7690. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Varo, R.; Chaccour, C.; Bassat, Q. Update on Malaria. Med. Clín. 2020, 155, 395–402. [Google Scholar] [CrossRef]
- Biamonte, M.A.; Wanner, J.; Le Roch, K.G. Recent Advances in Malaria Drug Discovery. Bioorganic Med. Chem. Lett. 2013, 23, 2829–2843. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, F.; Zuo, Q.; Jiang, T.; Song, H.; Zhou, J. A Newly Synthesized Oleanolic Acid Derivative Inhibits the Growth of Osteosarcoma Cells in Vitro and in Vivo by Decreasing C-MYC-Dependent Glycolysis. J. Cell. Biochem. 2019, 120, 9264–9276. [Google Scholar] [CrossRef] [PubMed]
- Asano, M.; Iwahashi, H. Determination of the Structures of Radicals Formed in the Reaction of Antimalarial Drug Artemisinin with Ferrous Ions. Eur. J. Med. Chem. 2017, 127, 740–747. [Google Scholar] [CrossRef]
- Fereja, T.H.; Kitte, S.A.; Gao, W.; Yuan, F.; Snizhko, D.; Qi, L.; Nsabimana, A.; Liu, Z.; Xu, G. Artesunate-Luminol Chemiluminescence System for the Detection of Hemin. Talanta 2019, 204, 379–385. [Google Scholar] [CrossRef]
- Ahirrao, P.; Batra, D.; Jain, U.K. Artemisinin, a Potential Antimalarial Drug: Current Status. J. Chem. Pharm. Res. 2016, 8, 624–636. [Google Scholar]
- Cosmas, S.; Ekpo, D.E.; Asomadu, R.O.; Assor, J.O.; Nnamani, V.I.; Durojaye, O.A. Review on Structure-Activity Relationship (SAR) Using Antimalarial Drug Design as a Case Study. Int. J. Sci. Eng. Res. 2018, 9, 1743–1752. [Google Scholar]
- Erdő, F.; Bors, L.A.; Farkas, D.; Bajza, Á.; Gizurarson, S. Evaluation of Intranasal Delivery Route of Drug Administration for Brain Targeting. Brain Res. Bull. 2018, 14, 155–170. [Google Scholar] [CrossRef]
- Marco-Hernández, J.; Camprubí, D.; Aylagas, C.; Gupta, H.; Castro, P. Failure of Intravenous Artesunate Treatment for Plasmodium Falciparum Malaria in a Splenectomized Traveller: A Diagnostic Challenge. Travel Med. Infect. Dis. 2019, 30, 142–143. [Google Scholar] [CrossRef] [PubMed]
- Driscoll, J.; Maqbool, M.G.; Latimer, M. Drug-Induced Autoimmune Haemolytic Anaemia in a Patient Treated with Artesunate for Malaria Infection. Pathology 2020, 52, S110. [Google Scholar] [CrossRef]
- Kurth, F.; Lingscheid, T.; Steiner, F.; Stegemann, M.S.; Bélard, S.; Menner, N.; Pongratz, P.; Kim, J.; von Bernuth, H.; Mayer, B.; et al. Hemolysis after Oral Artemisinin Combination Therapy for Uncomplicated Plasmodium Falciparum Malaria. Emerg. Infect. Dis. 2016, 22, 1381–1386. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- White, N.J. Anaemia and Malaria. Malar. J. 2018, 17, 371. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jauréguiberry, S.; Ndour, P.A.; Roussel, C.; Ader, F.; Safeukui, I.; Nguyen, M.; Biligui, S.; Ciceron, L.; Mouri, O.; Kendjo, E.; et al. Post-artesunate Delayed Hemolysis Is a Predictable Event Related to the Lifesaving Effect of Artemisinins. Blood 2014, 124, 167–175. [Google Scholar] [CrossRef] [Green Version]
- Guyatt, H.L.; Snow, R.W. Impact of Malaria during Pregnancy on Low Birth Weight in Sub-Saharan Africa. Clin. Microbiol. Rev. 2004, 17, 760–769. [Google Scholar] [CrossRef] [Green Version]
- Sagara, I.; Beavogui, A.H.; Zongo, I.; Soulama, I.; Borghini-Fuhrer, I.; Fofana, B.; Traore, A.; Diallo, N.; Diakite, H.; Togo, A.H.; et al. Pyronaridine–Artesunate or Dihydroartemisinin–Piperaquine versus Current First-Line Therapies for Repeated Treatment of Uncomplicated Malaria: A Randomised, Multicentre, Open-Label, Longitudinal, Controlled, Phase 3b/4 Trial. Lancet 2018, 391, 1378–1390. [Google Scholar] [CrossRef] [Green Version]
- Osei, S.A.; Biney, R.P.; Obese, E.; Agbenyeku, M.A.P.; Attah, I.Y.; Ameyaw, E.O.; Boampong, J.N. Xylopic Acid-Amodiaquine and Xylopic Acid-Artesunate Combinations Are Effective in Managing Malaria in Plasmodium berghei-Infected Mice. Malar. J. 2021, 20, 113. [Google Scholar] [CrossRef]
- Lantero, E.; Aláez-Versón, C.R.; Romero, P.; Sierra, T.; Fernàndez-Busquets, X. Repurposing Heparin as Antimalarial: Evaluation of Multiple Modifications toward in Vivo Application. Pharmaceutics 2020, 12, 825. [Google Scholar] [CrossRef]
- Bartlett, A.H.; Park, P.W. Glycans in Diseases and Therapeutics; Springer: Berlin/Heidelberg, Germany, 2011. [Google Scholar] [CrossRef]
- Raffray, L.; Receveur, M.C.; Beguet, M.; Lauroua, P.; Pistone, T.; Malvy, D. Severe Delayed Autoimmune Haemolytic Anaemia Following Artesunate Administration in Severe Malaria: A Case Report. Malar. J. 2014, 13, 398. [Google Scholar] [CrossRef]
- Corpolongo, A.; De Nardo, P.; Ghirga, P.; Gentilotti, E.; Bellagamba, R.; Tommasi, C.; Paglia, M.G.; Nicastri, E.; Narciso, P. Haemolytic Anaemia in an HIV-Infected Patient with Severe Falciparum Malaria after Treatment with Oral Artemether-Lumefantrine. Malar. J. 2012, 11, 2–5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rabaneda-Gutiérrez, L.; Alcalá-Minagorre, P.J.; Sánchez-Bautista, A. Hemolytic Anemia in Pediatric Patients Treated with Artesunate for Severe Malaria. Enfermedades Infecc. Microbiol. Clin. 2020, 38, 139–140. [Google Scholar] [CrossRef] [PubMed]
- Landre, S.; Bienvenu, A.L.; Miailhes, P.; Abraham, P.; Simon, M.; Becker, A.; Conrad, A.; Bonnot, G.; Kouakou, Y.I.; Chidiac, C.; et al. Recrudescence of a High Parasitaemia, Severe Plasmodium Falciparum Malaria Episode, Treated by Artesunate Monotherapy. Int. J. Infect. Dis. 2021, 105, 345–348. [Google Scholar] [CrossRef] [PubMed]
- Wyss, K.; Wångdahl, A.; Vesterlund, M.; Hammar, U.; Dashti, S.; Naucler, P.; Färnert, A. Obesity and Diabetes as Risk Factors for Severe Plasmodium Falciparum Malaria: Results from a Swedish Nationwide Study. Clin. Infect. Dis. 2017, 65, 949–958. [Google Scholar] [CrossRef]
- McEwen, J. Artesunate- and Amodiaquine-Associated Extrapyramidal Reactions. Drug Saf. 2012, 35, 667–675. [Google Scholar] [CrossRef] [PubMed]
- Vreden, S.G.S.; Bansie, R.D.; Jitan, J.K.; Adhin, M.R. Assessing Parasite Clearance during Uncomplicated Plasmodium Falciparum Infection Treated with Artesunate Monotherapy in Suriname. Infect. Drug Resist. 2016, 9, 261–267. [Google Scholar] [CrossRef] [Green Version]
- Mohammed, A.O.; Tewolde, S.; Estifanos, D.; Tekeste, Y.; Osman, M.H. Therapeutic Efficacy of Artesunate—Amiodaquine for Treating Uncomplicated Falciparum Malaria at Ghindae Zonal Referral Hospital, Eritrea. Acta Trop. 2018, 177, 94–96. [Google Scholar] [CrossRef]
- Zodda, D.; Procopio, G.; Hewitt, K.; Parrish, A.; Balani, B.; Feldman, J. Severe Malaria Presenting to the ED: A Collaborative Approach Utilizing Exchange Transfusion and Artesunate. Am. J. Emerg. Med. 2018, 36, 1126.e1–1126.e4. [Google Scholar] [CrossRef]
- Thera, M.A.; Kone, A.K.; Tangara, B.; Diarra, E.; Niare, S.; Dembele, A.; Sissoko, M.S.; Doumbo, O.K. School-Aged Children Based Seasonal Malaria Chemoprevention Using Artesunate-Amodiaquine in Mali. Parasite Epidemiol. Control 2018, 3, 96–105. [Google Scholar] [CrossRef]
- Varo, R.; Quintó, L.; Sitoe, A.; Madrid, L.; Acácio, S.; Vitorino, P.; Valente, A.M.; Mayor, A.; Camprubí, D.; Muñoz, J.; et al. Post-Malarial Anemia in Mozambican Children Treated with Quinine or Artesunate: A Retrospective Observational Study. Int. J. Infect. Dis. 2020, 96, 655–662. [Google Scholar] [CrossRef]
- Mancuso, R.I.; Castillo, A.C.; Saad, S.T.O. Artesunate Leads to Endoplasmic Reticulum Stress Via Eif2A-Atf4 Pathway in Leukemic Cells. Hematol. Transfus. Cell Ther. 2020, 42, 142. [Google Scholar] [CrossRef]
- Pirali, M.; Taheri, M.; Zarei, S.; Majidi, M.; Ghafouri, H. Artesunate, as a HSP70 ATPase Activity Inhibitor, Induces Apoptosis in Breast Cancer Cells. Int. J. Biol. Macromol. 2020, 164, 3369–3375. [Google Scholar] [CrossRef] [PubMed]
- Fei, Z.; Gu, W.; Xie, R.; Su, H.; Jiang, Y. Artesunate Enhances Radiosensitivity of Esophageal Cancer Cells by Inhibiting the Repair of DNA Damage. J. Pharmacol. Sci. 2018, 138, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Mota, T.C.; Garcia, T.B.; Bonfim, L.T.; Portilho, A.J.S.; Pinto, C.A.; Burbano, R.M.R.; de Oliveira Bahia, M. Markers of Oxidative-Nitrosative Stress Induced by Artesunate Are Followed by Clastogenic and Aneugenic Effects and Apoptosis in Human Lymphocytes. J. Appl. Toxicol. 2019, 39, 1405–1412. [Google Scholar] [CrossRef] [PubMed]
- Juengel, E.; Markowitsch, S.; Schupp, P.; Schunke, J.; Efferth, T.; Mager, R.; Haferkamp, A. Artesunate Reduces Cell Growth and Induces Ferroptosis in Therapy-Resistant Renal Cell Carcinoma Cells. Eur. Urol. Suppl. 2019, 18, e3044. [Google Scholar] [CrossRef]
- Zhang, J.; Zhou, L.; Xiang, J.D.; Jin, C.S.; Li, M.Q.; He, Y.Y. Artesunate-Induced ATG5-Related Autophagy Enhances the Cytotoxicity of NK92 Cells on Endometrial Cancer Cells via Interactions between CD155 and CD226/TIGIT. Int. Immunopharmacol. 2021, 97, 107705. [Google Scholar] [CrossRef]
- Ranieri, G.; Gadaleta-Caldarola, G.; Goffredo, V.; Patruno, R.; Mangia, A.; Rizzo, A.; Sciorsci, R.L.; Gadaleta, C.D. Sorafenib (BAY 43-9006) in Hepatocellular Carcinoma Patients: From Discovery to Clinical Development. Curr. Med. Chem. 2012, 19, 938–944. [Google Scholar] [CrossRef]
- Trimble, C.L.; Levinson, K.; Maldonado, L.; Donovan, M.; Clark, K.T.; Fu, J.; Shay, M.E.; Sauter, M.E.; Sanders, S.; Frantz, P.S.; et al. A First-in-Human Proof-of-Concept Trial of Intravaginal Artesunate to Treat Cervical Intraepithelial Neoplasia (CIN2/3). Gynecol. Oncol. 2020, 159, 37. [Google Scholar] [CrossRef]
- Xi, J.; Huang, Y.; Chen, J.; Zhang, J.; Gao, L.; Fan, L.; Qian, X. Artesunate-Loaded Poly (Lactic-Co-Glycolic Acid)/Polydopamine-Manganese Oxides Nanoparticles as an Oxidase Mimic for Tumor Chemo-Catalytic Therapy. Int. J. Biol. Macromol. 2021, 181, 72–81. [Google Scholar] [CrossRef]
- Xiong, B.; Chen, Y.; Liu, Y.; Hu, X.; Han, H.; Li, Q. Artesunate-Loaded Porous PLGA Microsphere as a Pulmonary Delivery System for the Treatment of Non-Small Cell Lung Cancer. Colloids Surfaces B Biointerfaces 2021, 206, 111937. [Google Scholar] [CrossRef]
- Wei, S.; Liu, L.; Chen, Z.; Yin, W.; Liu, Y.; Ouyang, Q.; Zeng, F.; Nie, Y.; Chen, T. Artesunate Inhibits the Mevalonate Pathway and Promotes Glioma Cell Senescence. J. Cell. Mol. Med. 2020, 24, 276–284. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, V.L.; Verma, S.; Das, P. Artesunate Suppresses Inflammation and Oxidative Stress in a Rat Model of Colorectal Cancer. Drug Dev. Res. 2019, 80, 1089–1097. [Google Scholar] [CrossRef] [PubMed]
- Li, X.Y.; Das, I.; Lepletier, A.; Addala, V.; Bald, T.; Stannard, K.; Barkauskas, D.; Liu, J.; Aguilera, A.R.; Takeda, K.; et al. CD155 Loss Enhances Tumor Suppression via Combined Host and Tumor-Intrinsic Mechanisms. J. Clin. Investig. 2018, 128, 2613–2625. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wild, M.; Bertzbach, L.D.; Tannig, P.; Wangen, C.; Müller, R.; Herrmann, L.; Fröhlich, T.; Tsogoeva, S.B.; Kaufer, B.B.; Marschall, M.; et al. The Trimeric Artesunate Derivative TF27 Exerts Strong Anti-Cytomegaloviral Efficacy: Focus on Prophylactic Efficacy and Oral Treatment of Immunocompetent Mice. Antiviral Res. 2020, 178, 104788. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Zhang, Y.; He, H.; Liu, Q.; Huang, S.; Guo, X. Artesunate Enhances the Immune Response of Rabies Vaccine as an Adjuvant. Vaccine 2019, 37, 7478–7481. [Google Scholar] [CrossRef] [PubMed]
- Kong, Z.; Liu, R.; Cheng, Y. Artesunate Alleviates Liver Fibrosis by Regulating Ferroptosis Signaling Pathway. Biomed. Pharmacother. 2019, 109, 2043–2053. [Google Scholar] [CrossRef]
- Bai, X.Y.; Liu, P.; Chai, Y.W.; Wang, Y.; Ren, S.H.; Li, Y.Y.; Zhou, H. Artesunate Attenuates 2, 4-Dinitrochlorobenzene-Induced Atopic Dermatitis by down-Regulating Th17 Cell Responses in BALB/c Mice. Eur. J. Pharmacol. 2020, 874, 173020. [Google Scholar] [CrossRef]
- Wan, Q.; Chen, H.; Xiong, G.; Jiao, R.; Liu, Y.; Li, X.; Sun, Y.; Wang, J.; Yan, L. Artesunate Protects against Surgery-Induced Knee Arthrofibrosis by Activating Beclin-1-Mediated Autophagy via Inhibition of MTOR Signaling. Eur. J. Pharmacol. 2019, 854, 149–158. [Google Scholar] [CrossRef]
- Alagbonsi, A.I.; Salman, T.M.; Sulaiman, S.O.; Adedini, K.A.; Kebu, S. Possible Mechanisms of the Hypoglycaemic Effect of Artesunate: Gender Implication. Metab. Open 2021, 10, 100087. [Google Scholar] [CrossRef]
- Thum, T. MicroRNA Therapeutics in Cardiovascular Medicine. EMBO Mol. Med. 2011, 2, 3–14. [Google Scholar] [CrossRef]
- Zhou, Y.; Gilmore, K.; Ramirez, S.; Settels, E.; Gammeltoft, K.A.; Pham, L.V.; Fahnøe, U.; Feng, S.; Offersgaard, A.; Trimpert, J.; et al. In Vitro Efficacy of Artemisinin-Based Treatments against SARS-CoV-2. Sci. Rep. 2021, 11, 14571. [Google Scholar] [CrossRef]
- Gendrot, M.; Duflot, I.; Boxberger, M.; Delandre, O.; Jardot, P.; Le Bideau, M.; Andreani, J.; Fonta, I.; Mosnier, J.; Rolland, C.; et al. Antimalarial Artemisinin-Based Combination Therapies (ACT) and COVID-19 in Africa: In Vitro Inhibition of SARS-CoV-2 Replication by Mefloquine-Artesunate. Int. J. Infect. Dis. 2020, 99, 437–440. [Google Scholar] [CrossRef] [PubMed]
- Bertzbach, L.D.; Conradie, A.M.; Hahn, F.; Wild, M.; Marschall, M.; Kaufer, B.B. Artesunate Derivative TF27 Inhibits Replication and Pathogenesis of an Oncogenic Avian Alphaherpesvirus. Antiviral Res. 2019, 171, 104606. [Google Scholar] [CrossRef] [PubMed]
- Jacquet, C.; Marschall, M.; Andouard, D.; El Hamel, C.; Chianea, T.; Tsogoeva, S.B.; Hantz, S.; Alain, S. A Highly Potent Trimeric Derivative of Artesunate Shows Promising Treatment Profiles in Experimental Models for Congenital HCMV Infection in Vitro and Ex Vivo. Antiviral Res. 2020, 175, 104700. [Google Scholar] [CrossRef]
- Karami, M.; Hemradj, V.V.; Ouweneel, D.M.; den Uil, C.A.; Limpens, J.; Otterspoor, L.C.; Vlaar, A.P.; Lagrand, W.K.; Henriques, J.P.S. Vasopressors and Inotropes in Acute Myocardial Infarction Related Cardiogenic Shock: A Systematic Review and Meta-Analysis. J. Clin. Med. 2020, 9, 2051. [Google Scholar] [CrossRef]
- Huang, Z.Z.; Xu, Y.; Xu, M.; Shi, Z.R.; Mai, S.Z.; Guo, Z.X.; Tang, Z.Q.; Luo, Y.J.; Guo, Q.; Xiong, H. Artesunate Alleviates Imiquimod-Induced Psoriasis-like Dermatitis in BALB/c Mice. Int. Immunopharmacol. 2019, 75, 105817. [Google Scholar] [CrossRef]
- Larson, S.A.; Dolivo, D.M.; Dominko, T. Artesunate Inhibits Myofibroblast Formation via Induction of Apoptosis and Antagonism of Pro-Fibrotic Gene Expression in Human Dermal Fibroblasts. Cell Biol. Int. 2019, 43, 1317–1322. [Google Scholar] [CrossRef]
- Shen, S.; Luo, J.; Ye, J. Artesunate Alleviates Schistosomiasis-Induced Liver Fibrosis by Downregulation of Mitochondrial Complex I Subunit NDUFB8 and Complex III Subunit UQCRC2 in Hepatic Stellate Cells. Acta Trop. 2021, 214, 105781. [Google Scholar] [CrossRef]
- Wan, Q.; Chen, H.; Li, X.; Yan, L.; Sun, Y.; Wang, J. Artesunate Inhibits Fibroblasts Proliferation and Reduces Surgery-Induced Epidural Fibrosis via the Autophagy-Mediated P53/P21waf1/Cip1 Pathway. Eur. J. Pharmacol. 2019, 842, 197–207. [Google Scholar] [CrossRef]
- Ackermann, A.M.; Moss, N.G.; Kaestner, K.H. GABA and Artesunate Do Not Induce Pancreatic α-to-β Cell Transdifferentiation In Vivo. Cell Metab. 2018, 28, 787–792. [Google Scholar] [CrossRef] [Green Version]
- Sun, Z.; Ma, Y.; Chen, F.; Wang, S.; Chen, B.; Shi, J. Artesunate Ameliorates High Glucose-Induced Rat Glomerular Mesangial Cell Injury by Suppressing the TLR4/NF-ΚB/NLRP3 Inflammasome Pathway. Chem. Biol. Interact. 2018, 293, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Shang, S.; Wu, J.; Li, X.; Liu, X.; Li, P.; Zheng, C.; Wang, Y.; Liu, S.; Zheng, J.; Zhou, H. Artesunate Interacts with the Vitamin D Receptor to Reverse Sepsis-Induced Immunosuppression in a Mouse Model via Enhancing Autophagy. Br. J. Pharmacol. 2020, 177, 4147–4165. [Google Scholar] [CrossRef] [PubMed]
- Zhan, T.; Wang, B.; Fu, J.; Shao, Y.; Ye, L.; Shi, H.; Zheng, L. Artesunate Inhibits Sjögren’s Syndrome-like Autoimmune Responses and BAFF-Induced B Cell Hyperactivation via TRAF6-Mediated NF-ΚB Signaling. Phytomedicine 2021, 80, 153381. [Google Scholar] [CrossRef] [PubMed]
- Zeng, H.B.; Dong, L.Q.; Xu, C.; Zhao, X.H.; Wu, L.G. Artesunate Promotes Osteoblast Differentiation through MiR-34a/DKK1 Axis. Acta Histochem. 2020, 122, 151601. [Google Scholar] [CrossRef] [PubMed]
- Dang, W.Z.; Li, H.; Jiang, B.; Nandakumar, K.S.; Liu, K.F.; Liu, L.X.; Yu, X.C.; Tan, H.J.; Zhou, C. Therapeutic Effects of Artesunate on Lupus-Prone MRL/Lpr Mice Are Dependent on T Follicular Helper Cell Differentiation and Activation of JAK2–STAT3 Signaling Pathway. Phytomedicine 2019, 62, 152965. [Google Scholar] [CrossRef]
- Wang, Y.; Liao, M.; Zhang, Y.; Deng, F.; Luo, J.; Wang, N.; Liu, M.; Ao, L.; Fang, Q.; Wang, Q.; et al. Artesunate Protects Immunosuppression Mice Induced by Glucocorticoids via Enhancing Pro-Inflammatory Cytokines Release and Bacterial Clearance. Eur. J. Pharmacol. 2021, 890, 173630. [Google Scholar] [CrossRef]
- Ghoneim, M.E.S.; Abdallah, D.M.; Shebl, A.M.; El-Abhar, H.S. The Interrupted Cross-Talk of Inflammatory and Oxidative Stress Trajectories Signifies the Effect of Artesunate against Hepatic Ischemia/Reperfusion-Induced Inflammasomopathy. Toxicol. Appl. Pharmacol. 2020, 409, 115309. [Google Scholar] [CrossRef]
- Pan, X.; Cen, Y.; Kuang, M.; Li, B.; Qin, R.; Zhou, H. Artesunate Interrupts the Self-Transcriptional Activation of MarA to Inhibit RND Family Pumps of Escherichia Coli. Int. J. Med. Microbiol. 2020, 310, 151465. [Google Scholar] [CrossRef]
- Feng, F.B.; Qiu, H.Y. Effects of Artesunate on Chondrocyte Proliferation, Apoptosis and Autophagy through the PI3K/AKT/MTOR Signaling Pathway in Rat Models with Rheumatoid Arthritis. Biomed. Pharmacother. 2018, 102, 1209–1220. [Google Scholar] [CrossRef]
- Zhang, J.; Zhou, S.; Xia, Z.; Peng, Z.; Luo, W.; Cheng, X.; Yang, R. Effectiveness of Artesunate Combined with Fractional CO2 Laser in a Hypertrophic Scar Model with Underlying Mechanism. Burns, 2021; in press. [Google Scholar] [CrossRef]
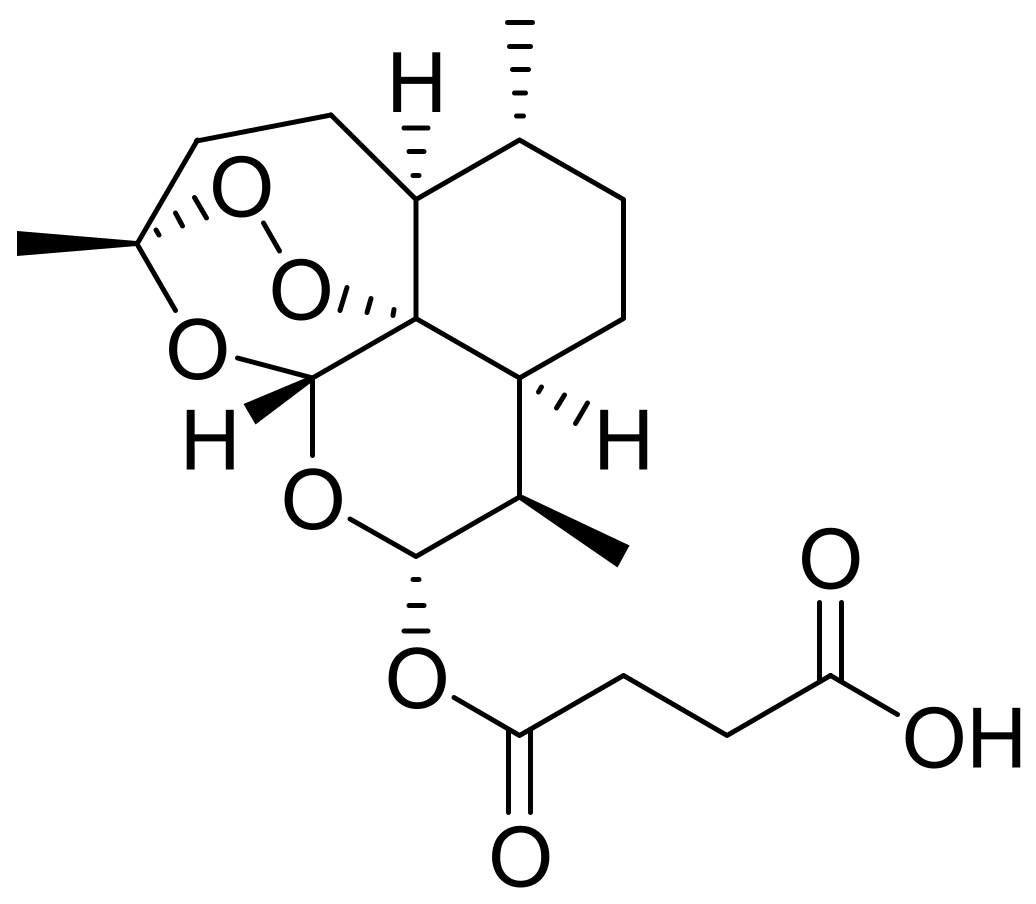
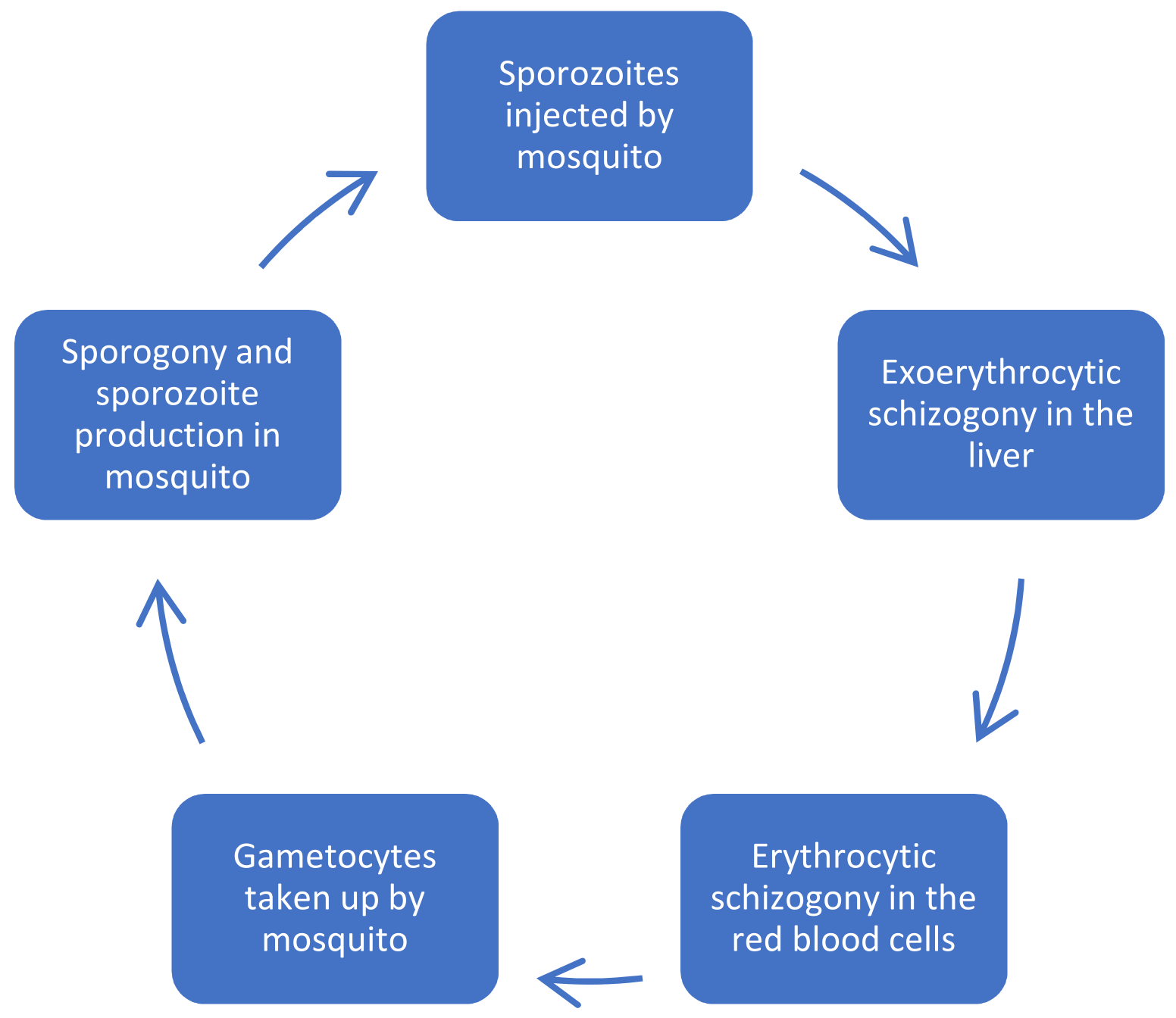
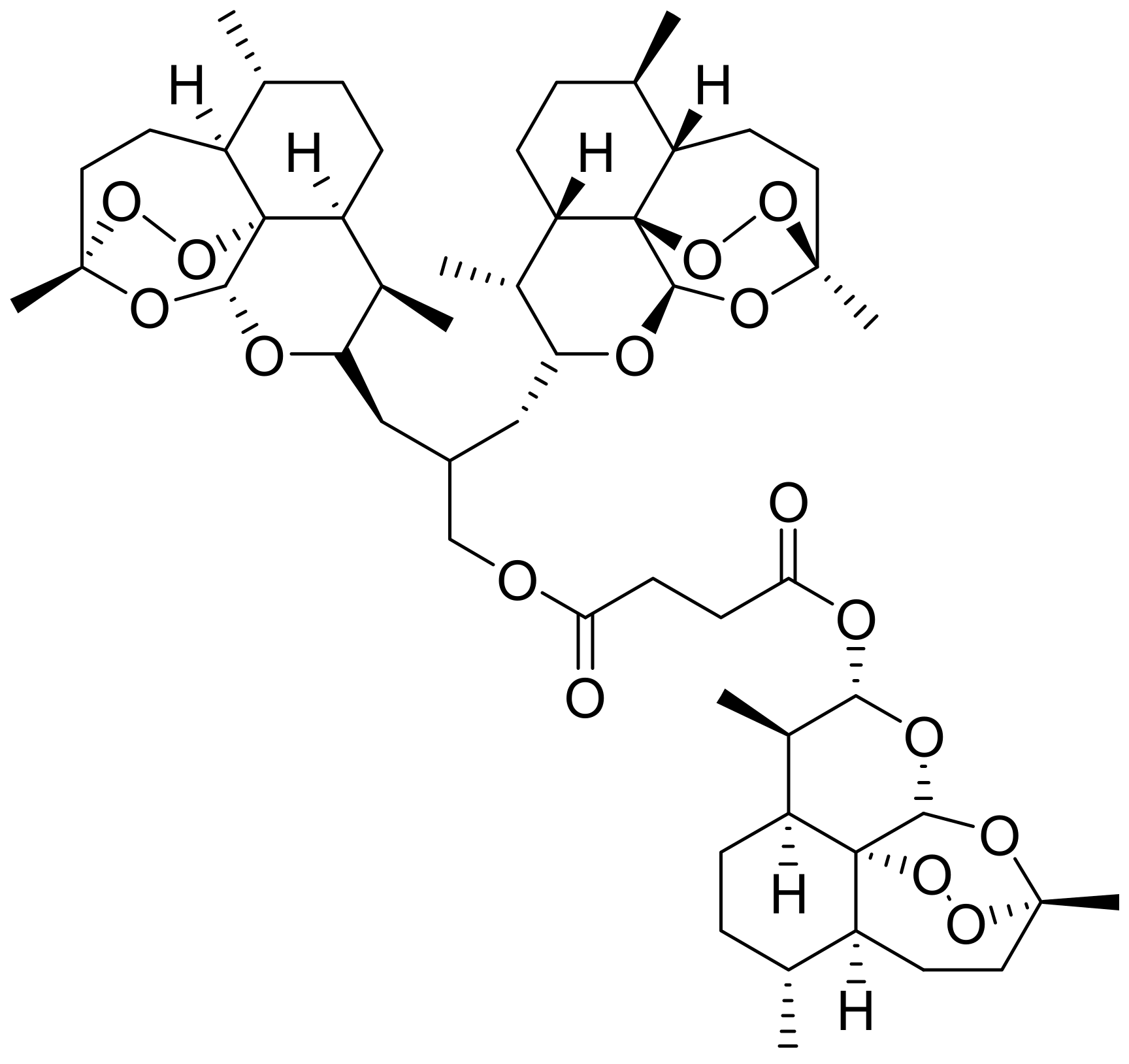
| Dose (mg/kg) | Chemo-Suppression (%) on Day 8 After Suppression | Mean Survival Time (Days) |
|---|---|---|
| Vehicle control | 0.0 ± 0.0 | 8.7 |
| 9.5 (ED50) ART | 55 ± 0.37 | 14.01 |
| 88 (ED50) 4CE | 47 ± 0.91 | 9.8 |
| ART + 4CE | 91.4 ± 0.64 | 16.3 |
| 100 ART | 99.2 ± 0.05 | ˃28 |
| Parameters (Unit) | Free ART | ART–HEP Nanocapsules |
|---|---|---|
| Cmax (µg/mL) | 14.13 | 18.12 |
| MRT0-t (h) | 2.44 | 9.39 |
| CL (L/h/kg) | 0.19 | 0.08 |
| Ke | 0.49 | 0.10 |
| t1/2 (h) | 1.39 | 4.51 |
| Classification | Number (%) | 95% CI (%) |
|---|---|---|
| Early treatment failure | 0 | 0–3.7 |
| Late clinical failure | 3 (3%) | 0.6–8.6 |
| Late parasitological failure | 1 (1%) | 0.0–5.5 |
| Adequate clinical and parasitological response | 95 (96%) | 90.0–98.9 |
| The cumulative success rate after survival analysis | 96.0% | 89.7–98.5 |
| Inhibition % | ||||||
|---|---|---|---|---|---|---|
| At 2× Plasma Cmax | At 1× Plasma Cmax | At 0.5× Plasma Cmax | ||||
| Combination | Conc. | Mean ± SD | Conc. | Mean ± SD | Conc. | Mean ± SD |
| Mefloquine–ART | 8.3−5 | 99.6 ± 0.7 | 4.1−2.5 | 72.1 ± 18.3 | 2.0−1.25 | 30.9 ± 14.1 |
| Amodiaquine–ART | 4.0–5.0 | 85.8 ± 9.9 | 2.0–2.5 | 32.3 ± 9.9 | 1.0–1.25 | 17.2± 6.5 |
| Pyronaridine–ART | 0.5–1.0 | 38.2 ± 5.7 | 0.25–0.5 | 34.1 ± 7.1 | 0.12–0.25 | 16.3± 5.0 |
| Lumefantrine–ART | 33.0−2.0 | 37.7 ± 3.4 | 16.5−0.5 | 27.1 ± 6.0 | 8.2−0.25 | 12.3± 4.4 |
| Piperaquine–ART | 1.0–3.1 | 29.7 ± 16.8 | 0.5–1.5 | 29.3 ± 4.6 | 0.25–0.75 | 14.0 ± 4.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ruwizhi, N.; Maseko, R.B.; Aderibigbe, B.A. Recent Advances in the Therapeutic Efficacy of Artesunate. Pharmaceutics 2022, 14, 504. https://doi.org/10.3390/pharmaceutics14030504
Ruwizhi N, Maseko RB, Aderibigbe BA. Recent Advances in the Therapeutic Efficacy of Artesunate. Pharmaceutics. 2022; 14(3):504. https://doi.org/10.3390/pharmaceutics14030504
Chicago/Turabian StyleRuwizhi, Ngonidzashe, Rejoice Bethusile Maseko, and Blessing Atim Aderibigbe. 2022. "Recent Advances in the Therapeutic Efficacy of Artesunate" Pharmaceutics 14, no. 3: 504. https://doi.org/10.3390/pharmaceutics14030504
APA StyleRuwizhi, N., Maseko, R. B., & Aderibigbe, B. A. (2022). Recent Advances in the Therapeutic Efficacy of Artesunate. Pharmaceutics, 14(3), 504. https://doi.org/10.3390/pharmaceutics14030504






