Biomedicine Innovations and Its Nanohydrogel Classifications
Abstract
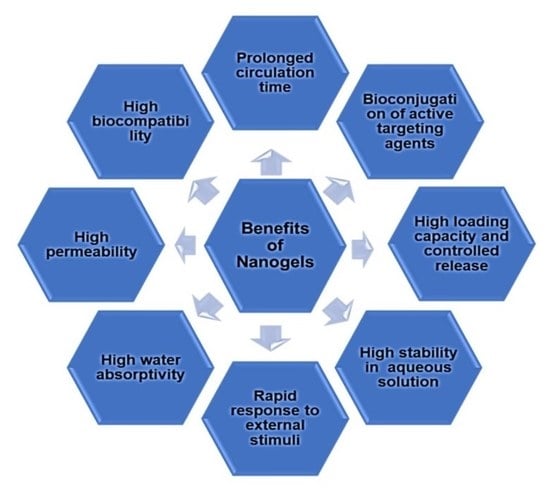
1. Introduction
2. Different Synthetic Approaches to the Design of Nanogels
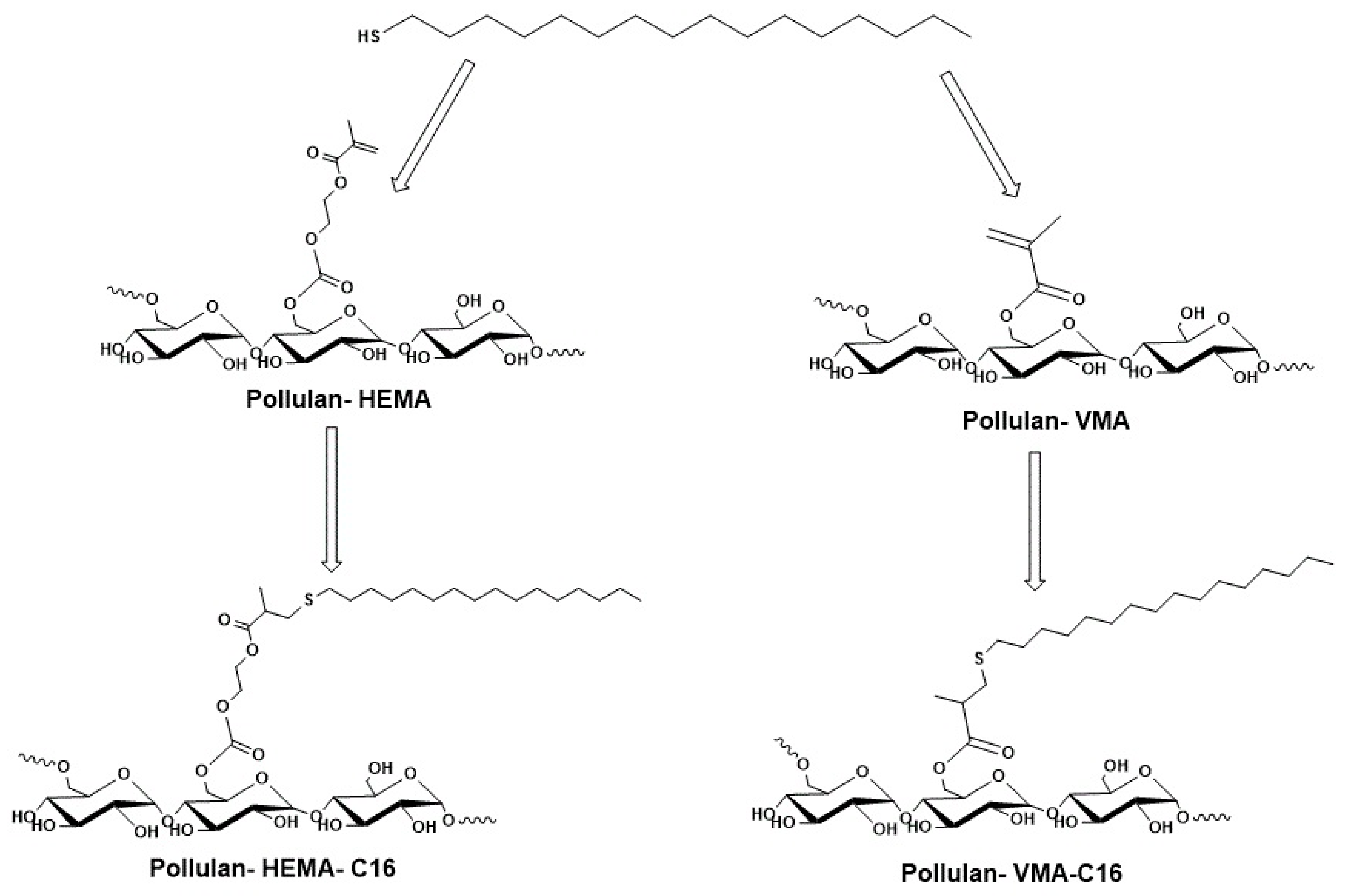
2.1. Physical Crosslinking
2.2. Chemical Crosslinking
2.2.1. Inverse Emulsion Polymerization (IEP)
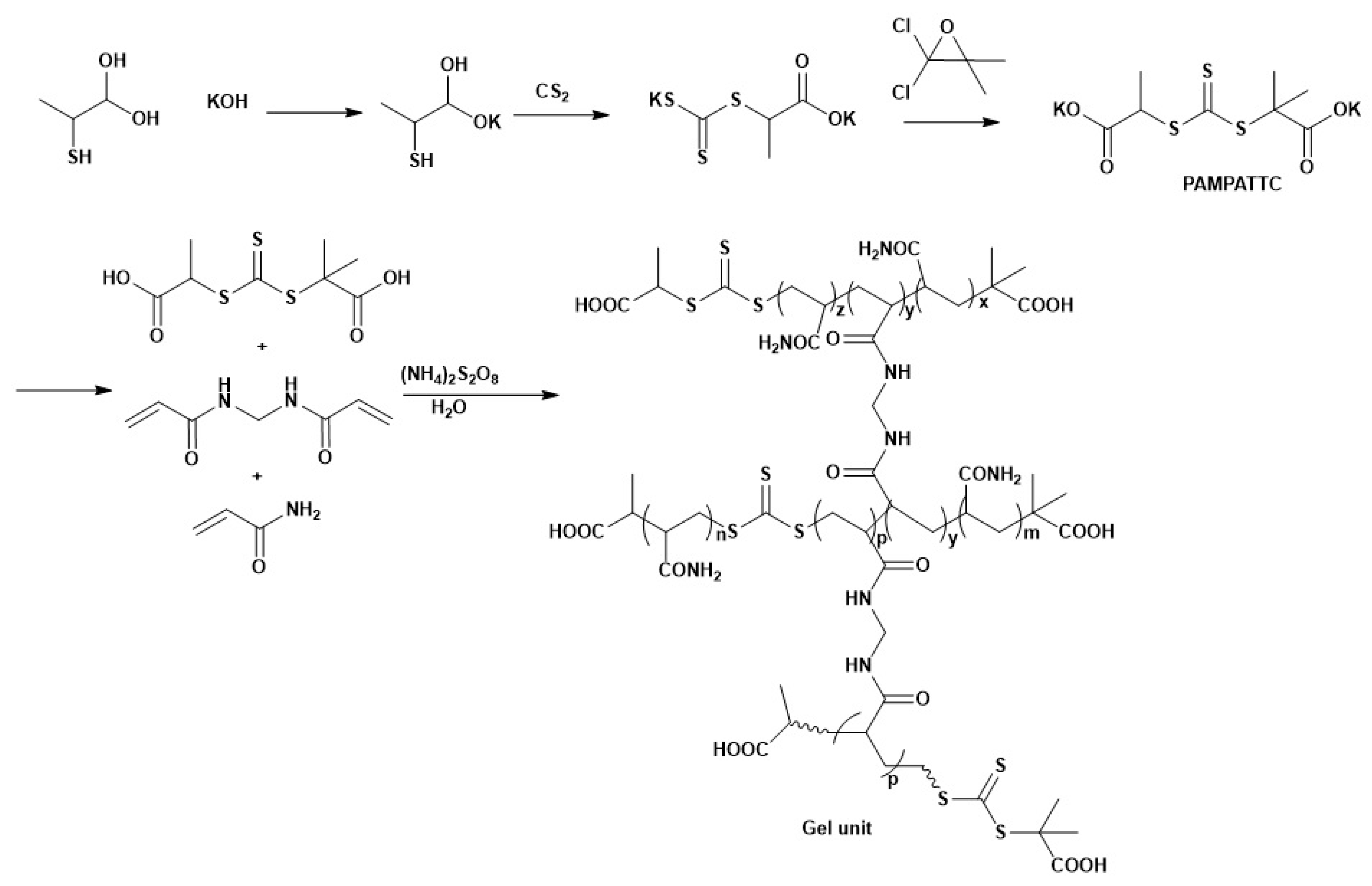
2.2.2. Reversible Addition–Fragmentation Chain Transfer (RAFT) Polymerization
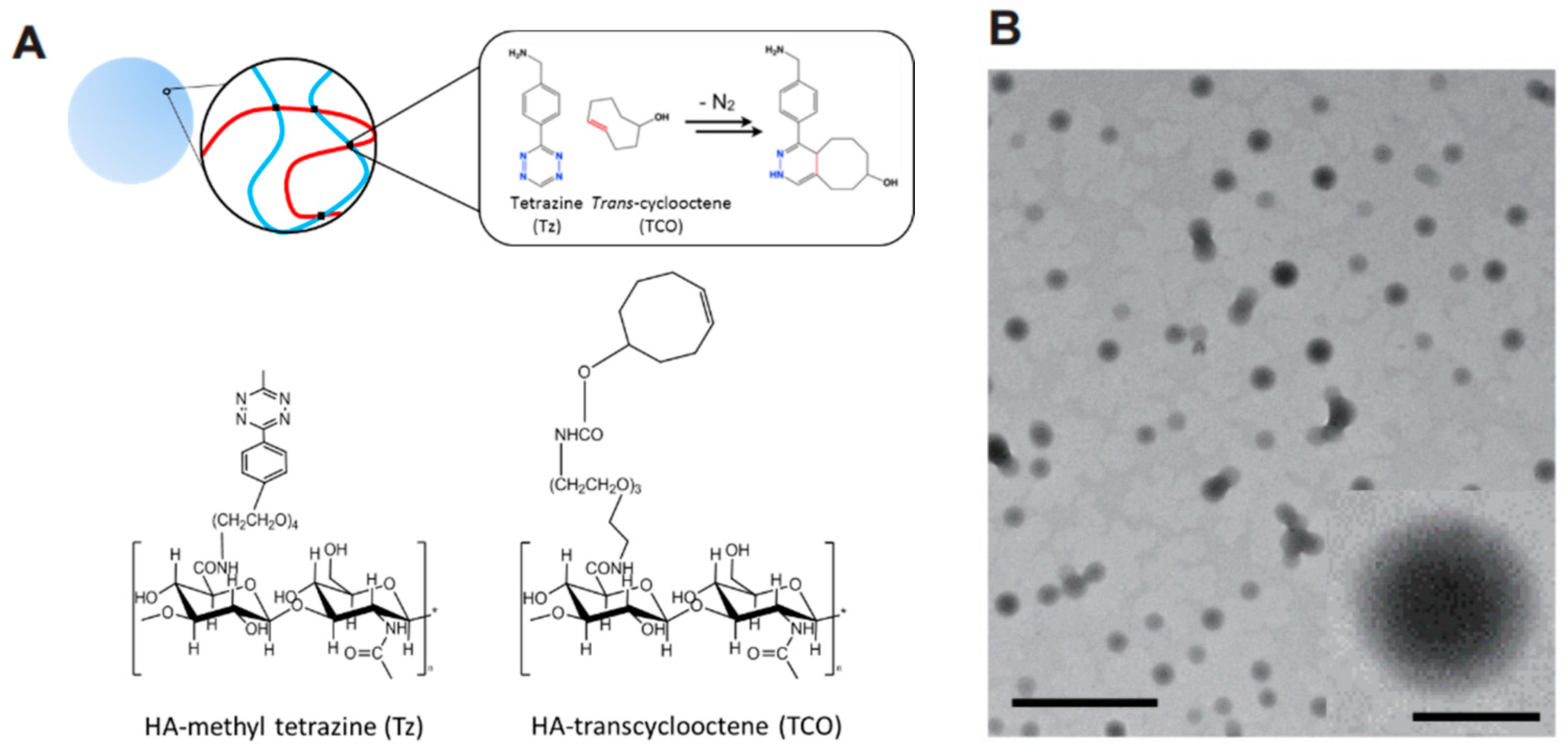
2.2.3. Click Chemistry Crosslinking Polymerization
2.2.4. Photo-Induced Crosslinking Polymerization
2.2.5. Disulfide Based Crosslinking
2.2.6. Amine Based Crosslinking
3. Fabrication of Nanogels Using 3D Printing Technologies
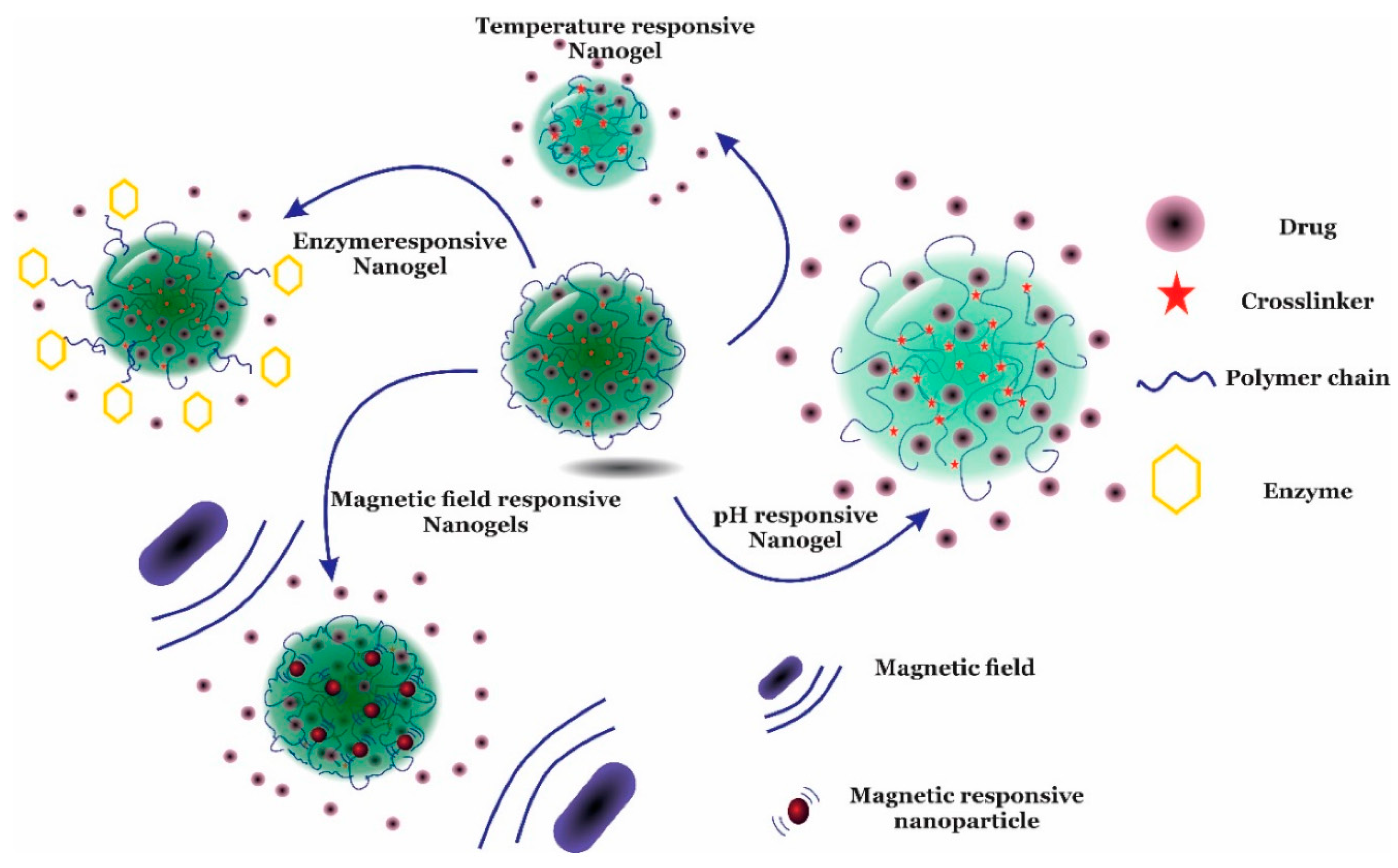
4. Stimuli-Responsive Drug Release Mechanisms of Nanogels
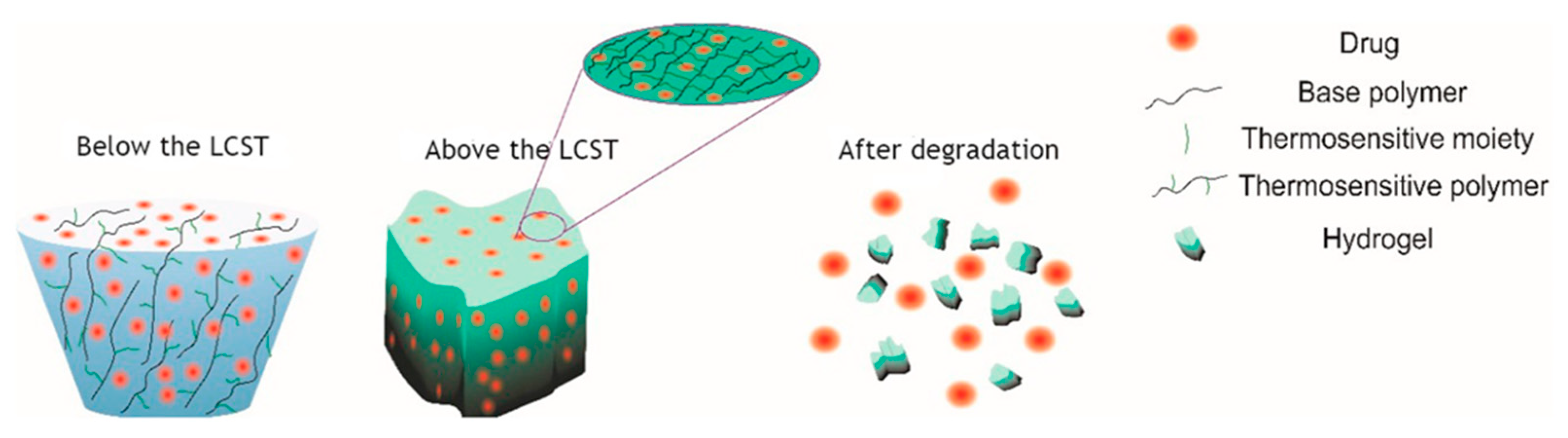
4.1. Thermo-Responsive Nanogels
4.2. pH-Responsive Nanogels
4.3. Photo-Responsive Nanogels
4.4. Magnetic-Responsive Nanogels
4.5. Ultrasound-Responsive Nanogels
4.6. Multi-Stimuli-Responsive Nanogels
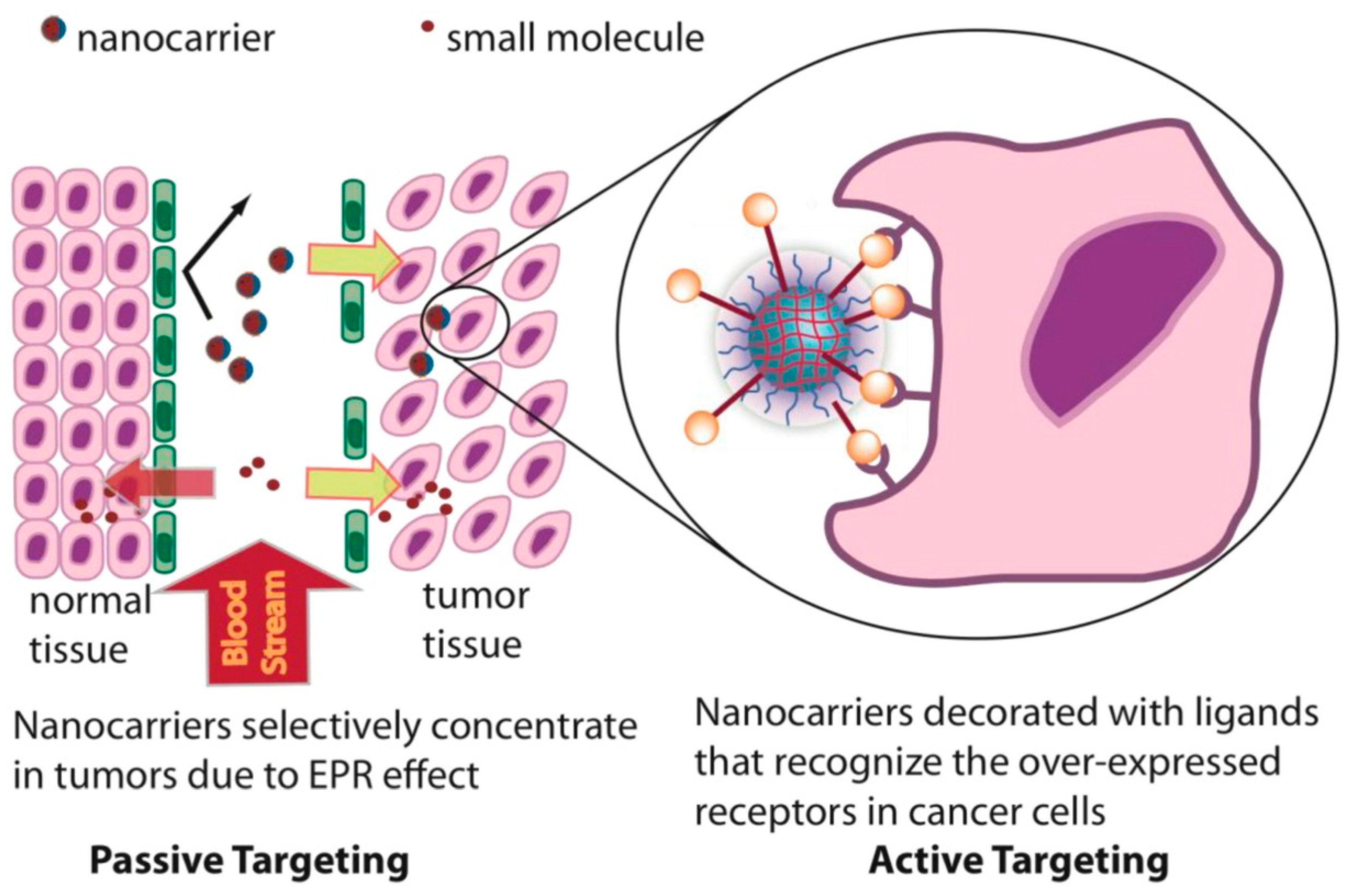
5. Passive Targeting of Nanogel
6. Active Targeting and Corresponding Modification of Nanogels
6.1. Small-Molecule Conjugation
6.2. Peptide Conjugation
6.3. Antibody Conjugation
6.4. Bio-Membrane Camouflaged
7. Application of Nanogels for the Delivery of Low and High-Molecular-Weight Chemotherapeutic Agents
7.1. Small-Molecule Delivery
7.2. Bio-Macromolecule Delivery
7.2.1. Proteins Delivery
7.2.2. Nucleic Acid Delivery
8. Nanogel in Combinational Chemotherapy
8.1. Photo Induced Chemotherapy
8.1.1. Photothermal Chemotherapy
8.1.2. Photodynamic Chemotherapy
8.2. Combinatorial Chemo-Immunotherapy
9. Nanogel Toxicity and Nanotoxicology
10. Conclusions and Future Perspective
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Suhail, M.; Rosenholm, J.M.; Minhas, M.U.; Badshah, S.F.; Naeem, A.; Khan, K.U.; Fahad, M. Nanogels as drug-delivery systems: A comprehensive overview. Ther. Deliv. 2019, 10, 697–717. [Google Scholar] [CrossRef] [PubMed]
- Mauri, E.; Giannitelli, S.M.; Trombetta, M.; Rainer, A. Synthesis of Nanogels: Current Trends and Future Outlook. Gels 2021, 7, 36. [Google Scholar] [CrossRef] [PubMed]
- Pinelli, F.; Ortolà, Ó.F.; Makvandi, P.; Perale, G.; Rossi, F. In vivo drug delivery applications of nanogels: A review. Nanomedicine 2020, 15, 2707–2727. [Google Scholar] [CrossRef] [PubMed]
- Sabir, F.; Asad, M.I.; Qindeel, M.; Afzal, I.; Dar, M.J.; Shah, K.U.; Zeb, A.; Khan, G.M.; Ahmed, N.; Din, F.-u. Polymeric nanogels as versatile nanoplatforms for biomedical applications. J. Nanomater. 2019, 2019, 1526186. [Google Scholar] [CrossRef]
- Khoee, S.; Asadi, H. Nanogels: Chemical approaches to preparation. Encycl. Biomed. Polym. Polym. Biomater. 2016, 27, 5266–5293. [Google Scholar]
- Oh, J.K.; Bencherif, S.A.; Matyjaszewski, K. Atom transfer radical polymerization in inverse miniemulsion: A versatile route toward preparation and functionalization of microgels/nanogels for targeted drug delivery applications. Polymer 2009, 50, 4407–4423. [Google Scholar] [CrossRef]
- Yin, Y.; Hu, B.; Yuan, X.; Cai, L.; Gao, H.; Yang, Q. Nanogel: A versatile nano-delivery system for biomedical applications. Pharmaceutics 2020, 12, 290. [Google Scholar] [CrossRef]
- Soni, K.S.; Desale, S.S.; Bronich, T.K. Nanogels: An overview of properties, biomedical applications and obstacles to clinical translation. J. Control. Release 2016, 240, 109–126. [Google Scholar] [CrossRef]
- Kabanov, A.V.; Vinogradov, S.V. Nanogels as pharmaceutical carriers: Finite networks of infinite capabilities. Angew. Chem. Int. Ed. 2009, 48, 5418–5429. [Google Scholar] [CrossRef]
- Hajebi, S.; Rabiee, N.; Bagherzadeh, M.; Ahmadi, S.; Rabiee, M.; Roghani-Mamaqani, H.; Tahriri, M.; Tayebi, L.; Hamblin, M.R. Stimulus-responsive polymeric nanogels as smart drug delivery systems. Acta Biomater. 2019, 92, 1–18. [Google Scholar] [CrossRef]
- Hamidi, M.; Azadi, A.; Rafiei, P. Hydrogel nanoparticles in drug delivery. Adv. Drug Deliv. Rev. 2008, 60, 1638–1649. [Google Scholar] [CrossRef] [PubMed]
- Egrilmez, S.; Yildirim-Theveny, Ş. Treatment-resistant bacterial keratitis: Challenges and solutions. Clin. Ophthalmol. 2020, 14, 287. [Google Scholar] [CrossRef] [PubMed]
- Amamoto, Y.; Otsuka, H.; Takahara, A. Synthesis and characterization of polymeric nanogels. Nanotechnologies Life Sci. Online 2012, 4, 601–620. [Google Scholar]
- Sood, N.; Bhardwaj, A.; Mehta, S.; Mehta, A. Stimuli-responsive hydrogels in drug delivery and tissue engineering. Drug Deliv. 2016, 23, 748–770. [Google Scholar] [CrossRef] [PubMed]
- Neamtu, I.; Rusu, A.G.; Diaconu, A.; Nita, L.E.; Chiriac, A.P. Basic concepts and recent advances in nanogels as carriers for medical applications. Drug Deliv. 2017, 24, 539–557. [Google Scholar] [CrossRef]
- Zhang, X.; Malhotra, S.; Molina, M.; Haag, R. Micro-and nanogels with labile crosslinks–from synthesis to biomedical applications. Chem. Soc. Rev. 2015, 44, 1948–1973. [Google Scholar] [CrossRef]
- Kharkar, P.M.; Kiick, K.L.; Kloxin, A.M. Designing degradable hydrogels for orthogonal control of cell microenvironments. Chem. Soc. Rev. 2013, 42, 7335–7372. [Google Scholar] [CrossRef]
- Rajput, R.; Narkhede, J.; Naik, J. Nanogels as nanocarriers for drug delivery: A review. ADMET DMPK 2020, 8, 1. [Google Scholar] [CrossRef]
- Sasaki, Y.; Akiyoshi, K. Nanogel engineering for new nanobiomaterials: From chaperoning engineering to biomedical applications. Chem. Rec. 2010, 10, 366–376. [Google Scholar] [CrossRef]
- Akiyoshi, K.; Kang, E.-C.; Kurumada, S.; Sunamoto, J.; Principi, T.; Winnik, F.M. Controlled association of amphiphilic polymers in water: Thermosensitive nanoparticles formed by self-assembly of hydrophobically modified pullulans and poly (N-isopropylacrylamides). Macromolecules 2000, 33, 3244–3249. [Google Scholar] [CrossRef]
- Hirokawa, Y.; Tanaka, T. Volume phase transition in a non-ionic gel. In Proceedings of the AIP Conference Proceedings, American Institute of Physics, New York, NY, USA, 1 January 1984; pp. 203–208. [Google Scholar]
- Ferreira, S.A.; Coutinho, P.J.; Gama, F.M. Synthesis and characterization of self-assembled nanogels made of pullulan. Materials 2011, 4, 601–620. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Chen, Q.; Zhou, S. Carbon-based hybrid nanogels: A synergistic nanoplatform for combined biosensing, bioimaging, and responsive drug delivery. Chem. Soc. Rev. 2018, 47, 4198–4232. [Google Scholar] [CrossRef] [PubMed]
- Peres, L.B.; dos Anjos, R.S.; Tappertzhofen, L.C.; Feuser, P.E.; de Araújo, P.H.; Landfester, K.; Sayer, C.; Munoz-Espi, R. pH-responsive physically and chemically cross-linked glutamic-acid-based hydrogels and nanogels. Eur. Polym. J. 2018, 101, 341–349. [Google Scholar] [CrossRef]
- Hajebi, S.; Abdollahi, A.; Roghani-Mamaqani, H.; Salami-Kalajahi, M. Hybrid and hollow Poly (N, N-dimethylaminoethyl methacrylate) nanogels as stimuli-responsive carriers for controlled release of doxorubicin. Polymer 2019, 180, 121716. [Google Scholar] [CrossRef]
- Gao, F.; Wu, X.; Wu, D.; Yu, J.; Yao, J.; Qi, Q.; Cao, Z.; Cui, Q.; Mi, Y. Preparation of degradable magnetic temperature-and redox-responsive polymeric/Fe3O4 nanocomposite nanogels in inverse miniemulsions for loading and release of 5-fluorouracil. Colloids Surf. A Physicochem. Eng. Asp. 2020, 587, 124363. [Google Scholar] [CrossRef]
- Tran, T.N.; Piogé, S.; Fontaine, L.; Pascual, S. Hydrogen-Bonding UCST-Thermosensitive Nanogels by Direct Photo-RAFT Polymerization-Induced Self-Assembly in Aqueous Dispersion. Macromol. Rapid Commun. 2020, 41, 2000203. [Google Scholar] [CrossRef]
- Takada, K.; Matsumoto, A. Reversible addition-fragmentation chain transfer polymerization of diisopropyl fumarate using various dithiobenzoates as chain transfer agents. J. Polym. Sci. Part A Polym. Chem. 2017, 55, 3266–3275. [Google Scholar] [CrossRef]
- Yang, Z.; Dai, Y.; Zhang, F.; Zhang, Y.; Zheng, H.; Zhang, P.; Zhou, C. Synthesis of a hydrosoluble reversible addition-fragmentation chain transfer agent and application in the preparation of micro/nano-polyacrylamide gel dispersions. J. Appl. Polym. Sci. 2021, 138, 50930. [Google Scholar] [CrossRef]
- Nieswandt, K.; Georgopanos, P.; Held, M.; Sperling, E.; Abetz, V. RAFT Emulsion Polymerization of Styrene Using a Poly ((N, N-dimethyl acrylamide)-co-(N-isopropyl acrylamide)) mCTA: Synthesis and Thermosensitivity. Polymers 2021, 14, 62. [Google Scholar] [CrossRef]
- Moses, J.E.; Moorhouse, A.D. The growing applications of click chemistry. Chem. Soc. Rev. 2007, 36, 1249–1262. [Google Scholar] [CrossRef]
- Hein, C.D.; Liu, X.-M.; Wang, D. Click chemistry, a powerful tool for pharmaceutical sciences. Pharm. Res. 2008, 25, 2216–2230. [Google Scholar] [CrossRef] [PubMed]
- Phan, Q.T.; Patil, M.P.; Tu, T.T.; Kim, G.-D.; Lim, K.T. Synthesis of zwitterionic redox-responsive nanogels by one-pot amine-thiol-ene reaction for anticancer drug release application. React. Funct. Polym. 2020, 147, 104463. [Google Scholar] [CrossRef]
- Choi, H.; Kwon, M.; Choi, H.E.; Hahn, S.K.; Kim, K.S. Non-Invasive Topical Drug-Delivery System Using Hyaluronate Nanogels Crosslinked via Click Chemistry. Materials 2021, 14, 1504. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Ding, J.; Li, M.; Chen, X.; Xiao, C.; Zhuang, X.; Huang, Y.; Chen, X. One-step “click chemistry”-synthesized cross-linked prodrug nanogel for highly selective intracellular drug delivery and upregulated antitumor efficacy. ACS Appl. Mater. Interfaces 2016, 8, 10673–10682. [Google Scholar] [CrossRef] [PubMed]
- Preston, G.W.; Wilson, A.J. Photo-induced covalent cross-linking for the analysis of biomolecular interactions. Chem. Soc. Rev. 2013, 42, 3289–3301. [Google Scholar] [CrossRef]
- Eckmann, D.; Composto, R.; Tsourkas, A.; Muzykantov, V. Nanogel carrier design for targeted drug delivery. J. Mater. Chem. B 2014, 2, 8085–8097. [Google Scholar] [CrossRef]
- He, J.; Yan, B.; Tremblay, L.; Zhao, Y. Both core-and shell-cross-linked nanogels: Photoinduced size change, intraparticle LCST, and interparticle UCST thermal behaviors. Langmuir 2011, 27, 436–444. [Google Scholar] [CrossRef]
- He, J.; Tong, X.; Zhao, Y. Photoresponsive nanogels based on photocontrollable cross-links. Macromolecules 2009, 42, 4845–4852. [Google Scholar] [CrossRef]
- Kang, M.G.; Lee, M.Y.; Cha, J.M.; Lee, J.K.; Lee, S.C.; Kim, J.; Hwang, Y.-S.; Bae, H. Nanogels derived from fish gelatin: Application to drug delivery system. Mar. Drugs 2019, 17, 246. [Google Scholar] [CrossRef]
- Williams, C.G.; Malik, A.N.; Kim, T.K.; Manson, P.N.; Elisseeff, J.H. Variable cytocompatibility of six cell lines with photoinitiators used for polymerizing hydrogels and cell encapsulation. Biomaterials 2005, 26, 1211–1218. [Google Scholar] [CrossRef]
- Sun, K.H.; Sohn, Y.S.; Jeong, B. Thermogelling poly (ethylene oxide-b-propylene oxide-b-ethylene oxide) disulfide multiblock copolymer as a thiol-sensitive degradable polymer. Biomacromolecules 2006, 7, 2871–2877. [Google Scholar] [CrossRef]
- Castellani, O.F.; Martínez, E.N.; Añón, M.C. Role of disulfide bonds upon the structural stability of an amaranth globulin. J. Agric. Food Chem. 1999, 47, 3001–3008. [Google Scholar] [CrossRef]
- Oh, J.K.; Siegwart, D.J.; Lee, H.-i.; Sherwood, G.; Peteanu, L.; Hollinger, J.O.; Kataoka, K.; Matyjaszewski, K. Biodegradable nanogels prepared by atom transfer radical polymerization as potential drug delivery carriers: Synthesis, biodegradation, in vitro release, and bioconjugation. J. Am. Chem. Soc. 2007, 129, 5939–5945. [Google Scholar] [CrossRef]
- Ryu, J.-H.; Chacko, R.T.; Jiwpanich, S.; Bickerton, S.; Babu, R.P.; Thayumanavan, S. Self-cross-linked polymer nanogels: A versatile nanoscopic drug delivery platform. J. Am. Chem. Soc. 2010, 132, 17227–17235. [Google Scholar] [CrossRef] [PubMed]
- Jiwpanich, S.; Ryu, J.-H.; Bickerton, S.; Thayumanavan, S. Noncovalent encapsulation stabilities in supramolecular nanoassemblies. J. Am. Chem. Soc. 2010, 132, 10683–10685. [Google Scholar] [CrossRef] [PubMed]
- Park, C.W.; Yang, H.-M.; Lee, H.J.; Kim, J.-D. Core–shell nanogel of PEG–poly (aspartic acid) and its pH-responsive release of rh-insulin. Soft Matter 2013, 9, 1781–1788. [Google Scholar] [CrossRef]
- Pujana, M.A.; Pérez-Álvarez, L.; Iturbe, L.C.C.; Katime, I. Water dispersible pH-responsive chitosan nanogels modified with biocompatible crosslinking-agents. Polymer 2012, 53, 3107–3116. [Google Scholar] [CrossRef]
- Cho, H.; Jammalamadaka, U.; Tappa, K. Nanogels for pharmaceutical and biomedical applications and their fabrication using 3D printing technologies. Materials 2018, 11, 302. [Google Scholar] [CrossRef] [PubMed]
- Kondiah, P.P.; Rants’o, T.A.; Makhathini, S.S.; Mdanda, S.; Choonara, Y.E. An Oral 3D Printed PLGA-Tocopherol PEG Succinate Nanocomposite Hydrogel for High-Dose Methotrexate Delivery in Maintenance Chemotherapy. Biomedicines 2022, 10, 1470. [Google Scholar] [CrossRef]
- Chen, Z.; Zhao, D.; Liu, B.; Nian, G.; Li, X.; Yin, J.; Qu, S.; Yang, W. 3D printing of multifunctional hydrogels. Adv. Funct. Mater. 2019, 29, 1900971. [Google Scholar] [CrossRef]
- Cho, H.; Jammalamadaka, U.; Tappa, K.; Egbulefu, C.; Prior, J.; Tang, R.; Achilefu, S. 3D printing of poloxamer 407 nanogel discs and their applications in adjuvant ovarian cancer therapy. Mol. Pharm. 2019, 16, 552–560. [Google Scholar] [CrossRef] [PubMed]
- Bhardwaj, A.; Kumar, L.; Mehta, S.; Mehta, A. Stimuli-sensitive Systems-an emerging delivery system for drugs. Artif. Cells Nanomed. Biotechnol. 2015, 43, 299–310. [Google Scholar] [CrossRef]
- García, M.C.; Cuggino, J.C. Stimulus-responsive nanogels for drug delivery. In Stimuli Responsive Polymeric Nanocarriers for Drug Delivery Applications; Elsevier: Amsterdam, The Netherlands, 2018; Volume 1, pp. 321–341. [Google Scholar]
- Jiang, L.; Zhou, Q.; Mu, K.; Xie, H.; Zhu, Y.; Zhu, W.; Zhao, Y.; Xu, H.; Yang, X. pH/temperature sensitive magnetic nanogels conjugated with Cy5. 5-labled lactoferrin for MR and fluorescence imaging of glioma in rats. Biomaterials 2013, 34, 7418–7428. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.; Tan, H.; Zhao, L.; Sun, W.; Zhu, L.; Sun, Y.; Hao, H.; Xing, H.; Liu, L.; Qu, X. Ultrasound-triggered thrombolysis using urokinase-loaded nanogels. Int. J. Pharm. 2012, 434, 384–390. [Google Scholar] [CrossRef]
- Jin, S.; Li, D.; Yang, P.; Guo, J.; Lu, J.Q.; Wang, C. Redox/pH stimuli-responsive biodegradable PEGylated P (MAA/BACy) nanohydrogels for controlled releasing of anticancer drugs. Colloids Surf. A Physicochem. Eng. Asp. 2015, 484, 47–55. [Google Scholar] [CrossRef]
- Kim, Y.K.; Kim, E.-J.; Lim, J.H.; Cho, H.K.; Hong, W.J.; Jeon, H.H.; Chung, B.G. Dual stimuli-triggered nanogels in response to temperature and pH changes for controlled drug release. Nanoscale Res. Lett. 2019, 14, 77. [Google Scholar] [CrossRef]
- Li, M.-H.; Keller, P. Stimuli-responsive polymer vesicles. Soft Matter 2009, 5, 927–937. [Google Scholar] [CrossRef]
- Ma, X.; Yang, S.; Zhang, T.; Wang, S.; Yang, Q.; Xiao, Y.; Shi, X.; Xue, P.; Kang, Y.; Liu, G. Bioresponsive immune-booster-based prodrug nanogel for cancer immunotherapy. Acta Pharm. Sin. B 2021, 12, 451–466. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Chu, X.; Hou, Y. Stimuli-responsive cancer therapy based on nanoparticles. Chem. Commun. 2014, 50, 11614–11630. [Google Scholar] [CrossRef]
- Zha, L.; Banik, B.; Alexis, F. Stimulus responsive nanogels for drug delivery. Soft Matter 2011, 7, 5908–5916. [Google Scholar] [CrossRef]
- Smeets, N.M.; Hoare, T. Designing responsive microgels for drug delivery applications. J. Polym. Sci. Part A Polym. Chem. 2013, 51, 3027–3043. [Google Scholar] [CrossRef]
- Preman, N.K.; Barki, R.R.; Vijayan, A.; Sanjeeva, S.G.; Johnson, R.P. Recent developments in stimuli-responsive polymer nanogels for drug delivery and diagnostics: A Review. Eur. J. Pharm. Biopharm. 2020, 157, 121–153. [Google Scholar] [CrossRef] [PubMed]
- Ghaeini-Hesaroeiye, S.; Razmi Bagtash, H.; Boddohi, S.; Vasheghani-Farahani, E.; Jabbari, E. Thermoresponsive nanogels based on different polymeric moieties for biomedical applications. Gels 2020, 6, 20. [Google Scholar] [CrossRef] [PubMed]
- Seo, S.; Lee, C.-S.; Jung, Y.-S.; Na, K. Thermo-sensitivity and triggered drug release of polysaccharide nanogels derived from pullulan-g-poly (L-lactide) copolymers. Carbohydr. Polym. 2012, 87, 1105–1111. [Google Scholar] [CrossRef]
- Fathi, M.; Entezami, A.A.; Ebrahimi, A.; Safa, K.D. Synthesis of thermosensitive nanohydrogels by crosslinker free method based on N-isopropylacrylamide: Applicable in the naltrexone sustained release. Macromol. Res. 2013, 21, 17–26. [Google Scholar] [CrossRef]
- Ngadaonye, J.I.; Geever, L.M.; Cloonan, M.O.; Higginbotham, C.L. Photopolymerised thermo-responsive poly (N, N-diethylacrylamide)-based copolymer hydrogels for potential drug delivery applications. J. Polym. Res. 2012, 19, 9822. [Google Scholar] [CrossRef]
- Pelton, R. Temperature-sensitive aqueous microgels. Adv. Colloid Interface Sci. 2000, 85, 1–33. [Google Scholar] [CrossRef]
- Chen, Y.; Ballard, N.; Bon, S.A. Moldable high internal phase emulsion hydrogel objects from non-covalently crosslinked poly (N-isopropylacrylamide) nanogel dispersions. Chem. Commun. 2013, 49, 1524–1526. [Google Scholar] [CrossRef]
- Wang, G.; Xie, R.; Ju, X.J.; Chu, L.Y. Thermo-Responsive Polyethersulfone Composite Membranes Blended with Poly (N-isopropylacrylamide) Nanogels. Chem. Eng. Technol. 2012, 35, 2015–2022. [Google Scholar] [CrossRef]
- González-Ayón, M.A.; Licea-Claverie, A.; Sañudo-Barajas, J.A. Different Strategies for the Preparation of Galactose-Functionalized Thermo-Responsive Nanogels with Potential as Smart Drug Delivery Systems. Polymers 2020, 12, 2150. [Google Scholar] [CrossRef]
- Qureshi, M.A.; Khatoon, F. Different types of smart nanogel for targeted delivery. J. Sci. Adv. Mater. Devices 2019, 4, 201–212. [Google Scholar] [CrossRef]
- Sim, T.; Lim, C.; Hoang, N.H.; Oh, K.T. Recent advance of pH-sensitive nanocarriers targeting solid tumors. J. Pharm. Investig. 2017, 47, 383–394. [Google Scholar] [CrossRef]
- Du, J.Z.; Sun, T.M.; Song, W.J.; Wu, J.; Wang, J. A tumor-acidity-activated charge-conversional nanogel as an intelligent vehicle for promoted tumoral-cell uptake and drug delivery. Angew. Chem. 2010, 122, 3703–3708. [Google Scholar] [CrossRef]
- Liechty, W.B.; Scheuerle, R.L.; Peppas, N.A. Tunable, responsive nanogels containing t-butyl methacrylate and 2-(t-butylamino) ethyl methacrylate. Polymer 2013, 54, 3784–3795. [Google Scholar] [CrossRef]
- Wei, P.; Gangapurwala, G.; Pretzel, D.; Leiske, M.N.; Wang, L.; Hoeppener, S.; Schubert, S.; Brendel, J.C.; Schubert, U.S. Smart pH-sensitive nanogels for controlled release in an acidic environment. Biomacromolecules 2018, 20, 130–140. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Bui, Q.N.; Duy, L.T.M.; Yang, H.Y.; Lee, D.S. One-step preparation of pH-responsive polymeric nanogels as intelligent drug delivery systems for tumor therapy. Biomacromolecules 2018, 19, 2062–2070. [Google Scholar] [CrossRef]
- Noh, G.J.; Lim, S.A.; Lee, E.S. pH-responsive squeezing polysaccharidic nanogels for efficient docetaxel delivery. Polym. Adv. Technol. 2019, 30, 2067–2074. [Google Scholar] [CrossRef]
- Cuggino, J.C.; Molina, M.; Wedepohl, S.; Igarzabal, C.I.A.; Calderón, M.; Gugliotta, L.M. Responsive nanogels for application as smart carriers in endocytic pH-triggered drug delivery systems. Eur. Polym. J. 2016, 78, 14–24. [Google Scholar] [CrossRef]
- Farazi, S.; Chen, F.; Foster, H.; Boquiren, R.; McAlpine, S.R.; Chapman, R. Real time monitoring of peptide delivery in vitro using high payload pH responsive nanogels. Polym. Chem. 2020, 11, 425–432. [Google Scholar] [CrossRef]
- Nguyen, N.T.; Milani, A.H.; Jennings, J.; Adlam, D.J.; Freemont, A.J.; Hoyland, J.A.; Saunders, B.R. Highly compressive and stretchable poly (ethylene glycol) based hydrogels synthesised using pH-responsive nanogels without free-radical chemistry. Nanoscale 2019, 11, 7921–7930. [Google Scholar] [CrossRef]
- Pan, G.; Mou, Q.; Ma, Y.; Ding, F.; Zhang, J.; Guo, Y.; Huang, X.; Li, Q.; Zhu, X.; Zhang, C. pH-Responsive and Gemcitabine-Containing DNA Nanogel To Facilitate the Chemodrug Delivery. ACS Appl. Mater. Interfaces 2019, 11, 41082–41090. [Google Scholar] [CrossRef]
- Maiz-Fernández, S.; Pérez-Álvarez, L.; Ruiz-Rubio, L.; Pérez González, R.; Sáez-Martínez, V.; Ruiz Pérez, J.; Vilas-Vilela, J.L. Synthesis and characterization of covalently crosslinked pH-responsive hyaluronic acid nanogels: Effect of synthesis parameters. Polymers 2019, 11, 742. [Google Scholar] [CrossRef]
- Timko, B.P.; Arruebo, M.; Shankarappa, S.A.; McAlvin, J.B.; Okonkwo, O.S.; Mizrahi, B.; Stefanescu, C.F.; Gomez, L.; Zhu, J.; Zhu, A. Near-infrared–actuated devices for remotely controlled drug delivery. Proc. Natl. Acad. Sci. USA 2014, 111, 1349–1354. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Lorenzo, C.; Bromberg, L.; Concheiro, A. Light-sensitive intelligent drug delivery systems. Photochem. Photobiol. 2009, 85, 848–860. [Google Scholar] [CrossRef] [PubMed]
- Sherman, A.I.; Ter-Pogossian, M. Lymph-node concentration of radioactive colloidal gold following interstitial injection. Cancer 1953, 6, 1238–1240. [Google Scholar] [CrossRef]
- Daniel, M.-C.; Astruc, D. Gold nanoparticles: Assembly, supramolecular chemistry, quantum-size-related properties, and applications toward biology, catalysis, and nanotechnology. Chem. Rev. 2004, 104, 293–346. [Google Scholar] [CrossRef] [PubMed]
- Shan, J.; Tenhu, H. Recent advances in polymer protected gold nanoparticles: Synthesis, properties and applications. Chem. Commun. 2007, 44, 4580–4598. [Google Scholar] [CrossRef]
- Nakamura, T.; Tamura, A.; Murotani, H.; Oishi, M.; Jinji, Y.; Matsuishi, K.; Nagasaki, Y. Large payloads of gold nanoparticles into the polyamine network core of stimuli-responsive PEGylated nanogels for selective and noninvasive cancer photothermal therapy. Nanoscale 2010, 2, 739–746. [Google Scholar] [CrossRef]
- Kawano, T.; Niidome, Y.; Mori, T.; Katayama, Y.; Niidome, T. PNIPAM gel-coated gold nanorods for targeted delivery responding to a near-infrared laser. Bioconjugate Chem. 2009, 20, 209–212. [Google Scholar] [CrossRef]
- Wu, W.; Shen, J.; Banerjee, P.; Zhou, S. Core–shell hybrid nanogels for integration of optical temperature-sensing, targeted tumor cell imaging, and combined chemo-photothermal treatment. Biomaterials 2010, 31, 7555–7566. [Google Scholar] [CrossRef]
- Sun, H.; Yu, J.; Gong, P.; Xu, D.; Zhang, C.; Yao, S. Novel core–shell magnetic nanogels synthesized in an emulsion-free aqueous system under UV irradiation for targeted radiopharmaceutical applications. J. Magn. Magn. Mater. 2005, 294, 273–280. [Google Scholar] [CrossRef]
- Adriane, K.; Huang, J.; Ding, G.; Chen, J.; Liu, Y. Self assembled magnetic PVP/PVA hydrogel microspheres; magnetic drug targeting of VX2 auricular tumours using pingyangmycin. J. Drug Target. 2006, 14, 243–253. [Google Scholar] [CrossRef]
- Cazares-Cortes, E.; Espinosa, A.; Guigner, J.-M.; Michel, A.; Griffete, N.; Wilhelm, C.; Ménager, C. Doxorubicin intracellular remote release from biocompatible oligo (ethylene glycol) methyl ether methacrylate-based magnetic nanogels triggered by magnetic hyperthermia. ACS Appl. Mater. Interfaces 2017, 9, 25775–25788. [Google Scholar] [CrossRef]
- Chen, Z.; Wu, C.; Zhang, Z.; Wu, W.; Wang, X.; Yu, Z. Synthesis, functionalization, and nanomedical applications of functional magnetic nanoparticles. Chin. Chem. Lett. 2018, 29, 1601–1608. [Google Scholar] [CrossRef]
- Pan, J.; Hu, P.; Guo, Y.; Hao, J.; Ni, D.; Xu, Y.; Bao, Q.; Yao, H.; Wei, C.; Wu, Q. Combined magnetic hyperthermia and immune therapy for primary and metastatic tumor treatments. ACS Nano 2020, 14, 1033–1044. [Google Scholar] [CrossRef] [PubMed]
- Shen, N.; Lei, B.; Wang, Y.; Xu, S.; Liu, H. Redox/ultrasound dual stimuli-responsive nanogel for precisely controllable drug release. New J. Chem. 2018, 42, 9472–9481. [Google Scholar] [CrossRef]
- Wu, D.; Wan, M. A Novel Fluoride Anion Modified Gelatin Nanogel System. J. Pharm. Pharm. Sci. 2008, 11, 32–45. [Google Scholar] [CrossRef][Green Version]
- Bardajee, G.R.; Khamooshi, N.; Nasri, S.; Vancaeyzeele, C. Multi-stimuli responsive nanogel/hydrogel nanocomposites based on κ-carrageenan for prolonged release of levodopa as model drug. Int. J. Biol. Macromol. 2020, 153, 180–189. [Google Scholar] [CrossRef]
- Cao, Z.; Zhou, X.; Wang, G. Selective release of hydrophobic and hydrophilic cargos from multi-stimuli-responsive nanogels. ACS Appl. Mater. Interfaces 2016, 8, 28888–28896. [Google Scholar] [CrossRef]
- Xu, W.; Ledin, P.A.; Iatridi, Z.; Tsitsilianis, C.; Tsukruk, V.V. Multicompartmental Microcapsules with Orthogonal Programmable Two-Way Sequencing of Hydrophobic and Hydrophilic Cargo Release. Angew. Chem. Int. Ed. 2016, 55, 4908–4913. [Google Scholar] [CrossRef]
- Cao, Z.; Wu, H.; Dong, J.; Wang, G. Quadruple-stimuli-sensitive polymeric nanocarriers for controlled release under combined stimulation. Macromolecules 2014, 47, 8777–8783. [Google Scholar] [CrossRef]
- Gao, W.; Hu, Y.; Xu, L.; Liu, M.; Wu, H.; He, B. Dual pH and glucose sensitive gel gated mesoporous silica nanoparticles for drug delivery. Chin. Chem. Lett. 2018, 29, 1795–1798. [Google Scholar] [CrossRef]
- Samah, N.H.A.; Heard, C.M. Enhanced in vitro transdermal delivery of caffeine using a temperature-and pH-sensitive nanogel, poly (NIPAM-co-AAc). Int. J. Pharm. 2013, 453, 630–640. [Google Scholar] [CrossRef] [PubMed]
- Nita, L.E.; Chiriac, A.P.; Diaconu, A.; Tudorachi, N.; Mititelu-Tartau, L. Multifunctional nanogels with dual temperature and pH responsiveness. Int. J. Pharm. 2016, 515, 165–175. [Google Scholar] [CrossRef]
- Xia, X.; Hu, Z. Synthesis and light scattering study of microgels with interpenetrating polymer networks. Langmuir 2004, 20, 2094–2098. [Google Scholar] [CrossRef] [PubMed]
- Chiang, W.-H.; Hsu, Y.-H.; Tang, F.-F.; Chern, C.-S.; Chiu, H.-C. Temperature/pH-induced morphological regulations of shell cross-linked graft copolymer assemblies. Polymer 2010, 51, 6248–6257. [Google Scholar] [CrossRef]
- Salehi, R.; Rasouli, S.; Hamishehkar, H. Smart thermo/pH responsive magnetic nanogels for the simultaneous delivery of doxorubicin and methotrexate. Int. J. Pharm. 2015, 487, 274–284. [Google Scholar] [CrossRef]
- Su, S.; Wang, H.; Liu, X.; Wu, Y.; Nie, G. iRGD-coupled responsive fluorescent nanogel for targeted drug delivery. Biomaterials 2013, 34, 3523–3533. [Google Scholar] [CrossRef]
- Chen, D.; Yu, H.; Sun, K.; Liu, W.; Wang, H. Dual thermoresponsive and pH-responsive self-assembled micellar nanogel for anticancer drug delivery. Drug Deliv. 2014, 21, 258–264. [Google Scholar] [CrossRef]
- Qu, Y.; Chu, B.; Wei, X.; Lei, M.; Hu, D.; Zha, R.; Zhong, L.; Wang, M.; Wang, F.; Qian, Z. Redox/pH dual-stimuli responsive camptothecin prodrug nanogels for “on-demand” drug delivery. J. Control. Release 2019, 296, 93–106. [Google Scholar] [CrossRef]
- Wu, H.; Jin, H.; Wang, C.; Zhang, Z.; Ruan, H.; Sun, L.; Yang, C.; Li, Y.; Qin, W.; Wang, C. Synergistic cisplatin/doxorubicin combination chemotherapy for multidrug-resistant cancer via polymeric nanogels targeting delivery. ACS Appl. Mater. Interfaces 2017, 9, 9426–9436. [Google Scholar] [CrossRef]
- Yang, H.; Wang, Q.; Huang, S.; Xiao, A.; Li, F.; Gan, L.; Yang, X. Smart pH/redox dual-responsive nanogels for on-demand intracellular anticancer drug release. ACS Appl. Mater. Interfaces 2016, 8, 7729–7738. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Achazi, K.; Haag, R. Boronate Cross-linked ATP-and pH-Responsive Nanogels for Intracellular Delivery of Anticancer Drugs. Adv. Healthc. Mater. 2015, 4, 585–592. [Google Scholar] [CrossRef] [PubMed]
- Lamprecht, A.; Ubrich, N.; Yamamoto, H.; Schäfer, U.; Takeuchi, H.; Maincent, P.; Kawashima, Y.; Lehr, C.-M. Biodegradable nanoparticles for targeted drug delivery in treatment of inflammatory bowel disease. J. Pharmacol. Exp. Ther. 2001, 299, 775–781. [Google Scholar]
- Chytil, P.; Etrych, T.; Koňák, Č.; Šírová, M.; Mrkvan, T.; Bouček, J.; Říhová, B.; Ulbrich, K. New HPMA copolymer-based drug carriers with covalently bound hydrophobic substituents for solid tumour targeting. J. Control. Release 2008, 127, 121–130. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Li, Q.; Zhang, Y.; Xia, Y.; Yun, L.; Zhang, Q.; Zhang, T.; Chen, X.; Chen, H.; Li, W. A nanogel with passive targeting function and adjustable polyplex surface properties for efficient anti-tumor gene therapy. RSC Adv. 2016, 6, 84445–84456. [Google Scholar] [CrossRef]
- Tabatabaei Mirakabad, F.S.; Nejati-Koshki, K.; Akbarzadeh, A.; Yamchi, M.R.; Milani, M.; Zarghami, N.; Zeighamian, V.; Rahimzadeh, A.; Alimohammadi, S.; Hanifehpour, Y. PLGA-based nanoparticles as cancer drug delivery systems. Asian Pac. J. Cancer Prev. 2014, 15, 517–535. [Google Scholar] [CrossRef]
- Sanità, G.; Carrese, B.; Lamberti, A. Nanoparticle Surface Functionalization: How to Improve Biocompatibility and Cellular Internalization. Front. Mol. Biosci. 2020, 7, 381. [Google Scholar] [CrossRef]
- Kousalová, J.; Etrych, T. Polymeric nanogels as drug delivery systems. Physiol. Res. 2018, 67 (Suppl. 2), S305–S317. [Google Scholar] [CrossRef]
- Yoo, J.; Park, C.; Yi, G.; Lee, D.; Koo, H. Active targeting strategies using biological ligands for nanoparticle drug delivery systems. Cancers 2019, 11, 640. [Google Scholar] [CrossRef]
- Steichen, S.D.; Caldorera-Moore, M.; Peppas, N.A. A review of current nanoparticle and targeting moieties for the delivery of cancer therapeutics. Eur. J. Pharm. Sci. 2013, 48, 416–427. [Google Scholar] [CrossRef] [PubMed]
- de Gracia Lux, C.; Joshi-Barr, S.; Nguyen, T.; Mahmoud, E.; Schopf, E.; Fomina, N.; Almutairi, A. Biocompatible polymeric nanoparticles degrade and release cargo in response to biologically relevant levels of hydrogen peroxide. J. Am. Chem. Soc. 2012, 134, 15758–15764. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Yoon, J.; Bae, S.; Park, M.; Kang, C.; Ke, Q.; Lee, D.; Kang, P.M. Therapeutic use of H2O2-responsive anti-oxidant polymer nanoparticles for doxorubicin-induced cardiomyopathy. Biomaterials 2014, 35, 5944–5953. [Google Scholar] [CrossRef] [PubMed]
- Low, P.S.; Kularatne, S.A. Folate-targeted therapeutic and imaging agents for cancer. Curr. Opin. Chem. Biol. 2009, 13, 256–262. [Google Scholar] [CrossRef] [PubMed]
- Bueno, R.; Appasani, K.; Mercer, H.; Lester, S.; Sugarbaker, D. The α folate receptor is highly activated in malignant pleural mesothelioma. J. Thorac. Cardiovasc. Surg. 2001, 121, 225–233. [Google Scholar] [CrossRef]
- Parker, N.; Turk, M.J.; Westrick, E.; Lewis, J.D.; Low, P.S.; Leamon, C.P. Folate receptor expression in carcinomas and normal tissues determined by a quantitative radioligand binding assay. Anal. Biochem. 2005, 338, 284–293. [Google Scholar] [CrossRef] [PubMed]
- Low, P.S.; Henne, W.A.; Doorneweerd, D.D. Discovery and development of folic-acid-based receptor targeting for imaging and therapy of cancer and inflammatory diseases. Acc. Chem. Res. 2008, 41, 120–129. [Google Scholar] [CrossRef]
- Nukolova, N.V.; Oberoi, H.S.; Cohen, S.M.; Kabanov, A.V.; Bronich, T.K. Folate-decorated nanogels for targeted therapy of ovarian cancer. Biomaterials 2011, 32, 5417–5426. [Google Scholar] [CrossRef] [PubMed]
- Spicer, C.D.; Jumeaux, C.; Gupta, B.; Stevens, M.M. Peptide and protein nanoparticle conjugates: Versatile platforms for biomedical applications. Chem. Soc. Rev. 2018, 47, 3574–3620. [Google Scholar] [CrossRef]
- Pangburn, T.O.; Petersen, M.A.; Waybrant, B.; Adil, M.M.; Kokkoli, E. Peptide-and aptamer-functionalized nanovectors for targeted delivery of therapeutics. J. Biomech. Eng. 2009, 131, 074005. [Google Scholar] [CrossRef] [PubMed]
- Battigelli, A.; Russier, J.; Venturelli, E.; Fabbro, C.; Petronilli, V.; Bernardi, P.; Da Ros, T.; Prato, M.; Bianco, A. Peptide-based carbon nanotubes for mitochondrial targeting. Nanoscale 2013, 5, 9110–9117. [Google Scholar] [CrossRef] [PubMed]
- Ning, Q.; Liu, Y.-F.; Ye, P.-J.; Gao, P.; Li, Z.-P.; Tang, S.-Y.; He, D.-X.; Tang, S.-S.; Wei, H.; Yu, C.-Y. Delivery of liver-specific miRNA-122 using a targeted macromolecular prodrug toward synergistic therapy for hepatocellular carcinoma. ACS Appl. Mater. Interfaces 2019, 11, 10578–10588. [Google Scholar] [CrossRef] [PubMed]
- Blackburn, W.H.; Dickerson, E.B.; Smith, M.H.; McDonald, J.F.; Lyon, L.A. Peptide-functionalized nanogels for targeted siRNA delivery. Bioconjugate Chem. 2009, 20, 960–968. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Peng, J.; Chen, C.; Xiao, Y.; Tan, L.; Xie, X.; Xu, X.; Qian, Z. Targeting delivery of rapamycin with anti-collagen IV peptide conjugated Fe3O4@ nanogels system for vascular restenosis therapy. J. Biomed. Nanotechnol 2018, 14, 1208–1224. [Google Scholar] [CrossRef]
- Mi, P.; Cabral, H.; Kataoka, K. Ligand-installed nanocarriers toward precision therapy. Adv. Mater. 2020, 32, 1902604. [Google Scholar] [CrossRef] [PubMed]
- Heath, T.D.; Fraley, R.T.; Papahdjopoulos, D. Antibody targeting of liposomes: Cell specificity obtained by conjugation of F (ab’) 2 to vesicle surface. Science 1980, 210, 539–541. [Google Scholar] [CrossRef]
- Nukolova, N.V.; Yang, Z.; Kim, J.O.; Kabanov, A.V.; Bronich, T.K. Polyelectrolyte nanogels decorated with monoclonal antibody for targeted drug delivery. React. Funct. Polym. 2011, 71, 315–323. [Google Scholar] [CrossRef] [PubMed]
- Panowski, S.; Bhakta, S.; Raab, H.; Polakis, P.; Junutula, J.R. Site-Specific Antibody Drug Conjugates for Cancer Therapy; mabs, Taylor & Francis: Oxford, UK, 2014; pp. 34–45. [Google Scholar]
- Juan, A.; Cimas, F.J.; Bravo, I.; Pandiella, A.; Ocaña, A.; Alonso-Moreno, C. An Overview of Antibody Conjugated Polymeric Nanoparticles for Breast Cancer Therapy. Pharmaceutics 2020, 12, 802. [Google Scholar] [CrossRef]
- Canakci, M.; Singh, K.; Munkhbat, O.; Shanthalingam, S.; Mitra, A.; Gordon, M.; Osborne, B.A.; Thayumanavan, S. Targeting CD4+ Cells with Anti-CD4 Conjugated Mertansine-Loaded Nanogels. Biomacromolecules 2020, 21, 2473–2481. [Google Scholar] [CrossRef]
- Chai, Z.; Hu, X.; Lu, W. Cell membrane-coated nanoparticles for tumor-targeted drug delivery. Sci. China Mater. 2017, 60, 504–510. [Google Scholar] [CrossRef]
- Li, R.; He, Y.; Zhang, S.; Qin, J.; Wang, J. Cell membrane-based nanoparticles: A new biomimetic platform for tumor diagnosis and treatment. Acta Pharm. Sin. B 2018, 8, 14–22. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Wang, S.; Wang, Y.; Wang, L. Stem cell membrane–camouflaged bioinspired nanoparticles for targeted photodynamic therapy of lung cancer. J. Nanoparticle Res. 2020, 22, 176. [Google Scholar] [CrossRef]
- Luan, S.; Zhu, Y.; Wu, X.; Wang, Y.; Liang, F.; Song, S. Hyaluronic-acid-based pH-sensitive nanogels for tumor-targeted drug delivery. ACS Biomater. Sci. Eng. 2017, 3, 2410–2419. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Zhao, X. Glutathione-induced structural transform of double-cross-linked PEGylated nanogel for efficient intracellular anticancer drug delivery. Mol. Pharm. 2019, 16, 2826–2837. [Google Scholar] [CrossRef]
- Ma, W.; Chen, Q.; Xu, W.; Yu, M.; Yang, Y.; Zou, B.; Zhang, Y.S.; Ding, J.; Yu, Z. Self-targeting visualizable hyaluronate nanogel for synchronized intracellular release of doxorubicin and cisplatin in combating multidrug-resistant breast cancer. Nano Res. 2021, 14, 846–857. [Google Scholar] [CrossRef]
- Zhang, W.; Ai, S.; Ji, P.; Liu, J.; Li, Y.; Zhang, Y.; He, P. Photothermally enhanced chemotherapy delivered by graphene oxide-based multiresponsive nanogels. ACS Appl. Bio Mater. 2018, 2, 330–338. [Google Scholar] [CrossRef]
- Chang, R.; Tsai, W.-B. Fabrication of photothermo-responsive drug-loaded nanogel for synergetic cancer therapy. Polymers 2018, 10, 1098. [Google Scholar] [CrossRef]
- Qiao, H.; Chen, X.; Chen, E.; Zhang, J.; Huang, D.; Yang, D.; Ding, Y.; Qian, H.; Feijen, J.; Chen, W. Folated pH-degradable nanogels for the simultaneous delivery of docetaxel and an IDO1-inhibitor in enhancing cancer chemo-immunotherapy. Biomater. Sci. 2019, 7, 2749–2758. [Google Scholar] [CrossRef]
- Senthilkumar, T.; Lv, F.; Zhao, H.; Liu, L.; Wang, S. Conjugated Polymer Nanogel Binding Anticancer Drug through Hydrogen Bonds for Sustainable Drug Delivery. ACS Appl. Bio Mater. 2019, 2, 6012–6020. [Google Scholar] [CrossRef]
- Molinos, M.; Carvalho, V.; Silva, D.M.; Gama, F.M. Development of a hybrid dextrin hydrogel encapsulating dextrin nanogel as protein delivery system. Biomacromolecules 2012, 13, 517–527. [Google Scholar] [CrossRef]
- Morimoto, N.; Hirano, S.; Takahashi, H.; Loethen, S.; Thompson, D.H.; Akiyoshi, K. Self-assembled pH-sensitive cholesteryl pullulan nanogel as a protein delivery vehicle. Biomacromolecules 2013, 14, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Itani, R.; Al Faraj, A. siRNA Conjugated Nanoparticles—A Next Generation Strategy to Treat Lung Cancer. Int. J. Mol. Sci. 2019, 20, 6088. [Google Scholar] [CrossRef] [PubMed]
- de Carvalho Vicentini, F.T.M.; Borgheti-Cardoso, L.N.; Depieri, L.V.; de Macedo Mano, D.; Abelha, T.F.; Petrilli, R.; Bentley, M.V.L.B. Delivery systems and local administration routes for therapeutic siRNA. Pharm. Res. 2013, 30, 915–931. [Google Scholar] [CrossRef] [PubMed]
- Xue, H.; Ding, F.; Zhang, J.; Guo, Y.; Gao, X.; Feng, J.; Zhu, X.; Zhang, C. DNA tetrahedron-based nanogels for siRNA delivery and gene silencing. Chem. Commun. 2019, 55, 4222–4225. [Google Scholar] [CrossRef] [PubMed]
- Jackson, R.A.; Chen, E.S. Synthetic lethal approaches for assessing combinatorial efficacy of chemotherapeutic drugs. Pharmacol. Ther. 2016, 162, 69–85. [Google Scholar] [CrossRef]
- Parhi, P.; Mohanty, C.; Sahoo, S.K. Nanotechnology-based combinational drug delivery: An emerging approach for cancer therapy. Drug Discov. Today 2012, 17, 1044–1052. [Google Scholar] [CrossRef]
- Li, B.; Shao, H.; Gao, L.; Li, H.; Sheng, H.; Zhu, L. Nano-drug co-delivery system of natural active ingredients and chemotherapy drugs for cancer treatment: A review. Drug Deliv. 2022, 29, 2130–2161. [Google Scholar] [CrossRef]
- Grzybowski, A.; Pietrzak, K. From patient to discoverer—Niels Ryberg Finsen (1860–1904)—The founder of phototherapy in dermatology. Clin. Dermatol. 2012, 30, 451–455. [Google Scholar] [CrossRef]
- Celli, J.P.; Spring, B.Q.; Rizvi, I.; Evans, C.L.; Samkoe, K.S.; Verma, S.; Pogue, B.W.; Hasan, T. Imaging and photodynamic therapy: Mechanisms, monitoring, and optimization. Chem. Rev. 2010, 110, 2795–2838. [Google Scholar] [CrossRef]
- Lal, S.; Clare, S.E.; Halas, N.J. Nanoshell-enabled photothermal cancer therapy: Impending clinical impact. Acc. Chem. Res. 2008, 41, 1842–1851. [Google Scholar] [CrossRef]
- Bao, T.; Yin, W.; Zheng, X.; Zhang, X.; Yu, J.; Dong, X.; Yong, Y.; Gao, F.; Yan, L.; Gu, Z. One-pot synthesis of PEGylated plasmonic MoO3–x hollow nanospheres for photoacoustic imaging guided chemo-photothermal combinational therapy of cancer. Biomaterials 2016, 76, 11–24. [Google Scholar] [CrossRef] [PubMed]
- Luo, D.; Carter, K.A.; Miranda, D.; Lovell, J.F. Chemophototherapy: An emerging treatment option for solid tumors. Adv. Sci. 2017, 4, 1600106. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhang, J.; Wang, Y.; Wang, C.; Xiao, J.; Zhang, Q.; Cheng, Y. Multi-responsive photothermal-chemotherapy with drug-loaded melanin-like nanoparticles for synergetic tumor ablation. Biomaterials 2016, 81, 114–124. [Google Scholar] [CrossRef] [PubMed]
- Theune, L.E.; Buchmann, J.; Wedepohl, S.; Molina, M.; Laufer, J.; Calderón, M. NIR-and thermo-responsive semi-interpenetrated polypyrrole nanogels for imaging guided combinational photothermal and chemotherapy. J. Control. Release 2019, 311, 147–161. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Bhattarai, P.; Dai, Z.; Chen, X. Photothermal therapy and photoacoustic imaging via nanotheranostics in fighting cancer. Chem. Soc. Rev. 2019, 48, 2053–2108. [Google Scholar] [CrossRef]
- Chen, Y.-C.; Chiu, W.-T.; Chang, C.; Wu, P.-C.; Tu, T.-Y.; Lin, H.-P.; Chang, H.-C. Chemo-photothermal effects of doxorubicin/silica–carbon hollow spheres on liver cancer. RSC Adv. 2018, 8, 36775–36784. [Google Scholar] [CrossRef]
- Li, Z.; Chen, Y.; Yang, Y.; Yu, Y.; Zhang, Y.; Zhu, D.; Yu, X.; Ouyang, X.; Xie, Z.; Zhao, Y. Recent advances in nanomaterials-based chemo-photothermal combination therapy for improving cancer treatment. Front. Bioeng. Biotechnol. 2019, 7, 293. [Google Scholar] [CrossRef]
- dos Santos, A. l. F.; de Almeida, D.R.Q.; Terra, L.F.; Baptista, M. c. S.; Labriola, L. Photodynamic therapy in cancer treatment-an update review. J. Cancer Metastasis Treat. 2019, 5, 25. [Google Scholar] [CrossRef]
- Pinto da Silva, L.; Magalhães, C.M.; Núñez-Montenegro, A.; Ferreira, P.J.; Duarte, D.; Rodríguez-Borges, J.E.; Vale, N.; Esteves da Silva, J.C. Study of the combination of self-activating photodynamic therapy and chemotherapy for cancer treatment. Biomolecules 2019, 9, 384. [Google Scholar] [CrossRef]
- Chizenga, E.P.; Abrahamse, H. Nanotechnology in Modern Photodynamic Therapy of Cancer: A Review of Cellular Resistance Patterns Affecting the Therapeutic Response. Pharmaceutics 2020, 12, 632. [Google Scholar] [CrossRef]
- Yao, X.; Chen, L.; Chen, X.; Xie, Z.; Ding, J.; He, C.; Zhang, J.; Chen, X. pH-responsive metallo-supramolecular nanogel for synergistic chemo-photodynamic therapy. Acta Biomater. 2015, 25, 162–171. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Xu, W.; Liu, J.; Li, D.; Li, G.; Ding, J.; Chen, X. Polypeptide nanoformulation-induced immunogenic cell death and remission of immunosuppression for enhanced chemoimmunotherapy. Sci. Bull. 2021, 66, 362–373. [Google Scholar] [CrossRef]
- Lockhart, J.N.; Beezer, D.B.; Stevens, D.M.; Spears, B.R.; Harth, E. One-pot polyglycidol nanogels via liposome master templates for dual drug delivery. J. Control. Release 2016, 244, 366–374. [Google Scholar] [CrossRef] [PubMed]
- van der Weijden, J.; Paulis, L.E.; Verdoes, M.; van Hest, J.C.; Figdor, C.G. The right touch: Design of artificial antigen-presenting cells to stimulate the immune system. Chem. Sci. 2014, 5, 3355–3367. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, L.; Shi, Q.; Zhao, Q.; Ma, H. Tumor Microenvironment–Responsive Polypeptide Nanogels for Controlled Antitumor Drug Delivery. Front. Pharmacol. 2021, 12, 2744. [Google Scholar] [CrossRef]
- Patra, J.K.; Das, G.; Fraceto, L.F.; Campos, E.V.R.; del Pilar Rodriguez-Torres, M.; Acosta-Torres, L.S.; Diaz-Torres, L.A.; Grillo, R.; Swamy, M.K.; Sharma, S. Nano based drug delivery systems: Recent developments and future prospects. J. Nanobiotechnol. 2018, 16, 71. [Google Scholar] [CrossRef]
- De Jong, W.H.; Borm, P.J. Drug delivery and nanoparticles: Applications and hazards. Int. J. Nanomed. 2008, 3, 133. [Google Scholar] [CrossRef]
- Hossain, S.; Chowdhury, E.H.; Akaike, T. Nanoparticles and toxicity in therapeutic delivery: The ongoing debate. Ther. Deliv. 2011, 2, 125–132. [Google Scholar] [CrossRef]
- Zielińska, A.; Costa, B.; Ferreira, M.V.; Miguéis, D.; Louros, J.; Durazzo, A.; Lucarini, M.; Eder, P.; Chaud, M.; Morsink, M. Nanotoxicology and nanosafety: Safety-by-design and testing at a glance. Int. J. Environ. Res. Public Health 2020, 17, 4657. [Google Scholar] [CrossRef]
- Bhattacharjee, S.; Brayden, D.J. Development of nanotoxicology: Implications for drug delivery and medical devices. Nanomedicine 2015, 10, 2289–2305. [Google Scholar] [CrossRef]
- Gonçalves, C.; Pereira, P.; Gama, M. Self-assembled hydrogel nanoparticles for drug delivery applications. Materials 2010, 3, 1420–1460. [Google Scholar] [CrossRef]


| Nanogel Type | Synthesis Process | Drug | Stimuli Responsiveness | Application | Ref |
|---|---|---|---|---|---|
| Paramagnetic iron oxide nanogels composed of paramagnetic iron oxide nanogels composed | Free radical polymerization | Doxorubicin | Novel dual temperature/pH-sensitive | Chemotherapy | [109] |
| PNIPAM-co-AAc nanogel | Surfactant free emulsion polymerization | β-lipoprotein(β-LP) | Temperature- and pH responsive | Intestine-specific drug delivery | [58] |
| Poly (N-isopropyl acrylamide-co-acrylic acid) nanogels | Free radical precipitation polymerization | Doxorubicin | Thermo- and pH responsive | Anti-tumor drug delivery | [110] |
| Ketal derivative, mPEG2000-Isopropylideneglycerol (mPEG-IS, PI) polymer | Self-assembled micellar nanogel | Paclitaxel (PTX) | Dual thermoresponsive and pH-responsive | Cancer therapy | [111] |
| P(CPT-MAA) prodrug nanogels | Distillation-precipitation polymerization | Camptothecin | pH/redox dual-responsive | Anti-tumor drug delivery | [112] |
| PAA-based nanogels | Reflux-precipitationpolymerization (RPP) | Cisplatin/Doxorubicin | GSH/pH dual stimuli-responsiveness | Combination Chemotherapy | [113] |
| P(NIPAM-ss-AA) nanogel | Precipitation polymerization | Doxorubicin | pH/redox dual responsive | Intracellular anticancer drug release | [114] |
| PEGylated PMAA (PEG-PMAA) nanohydrogels | Facile reflux-precipitation polymerization | Doxorubicin/ Paclitaxel | Redox/pH dual stimuli-responsive | Anti-cancer therapy | [57] |
| Dendritic polyglycerol (dPG) nanogel | Surfactant-free inverse nanoprecipitation | methotrexate (MTX) | ATP and pH dual-responsive | Anti-cancer therapy | [115] |
| Nanogel | Description | Drug Delivered | Application | Pre-Clinical/Clinical Study | Ref |
|---|---|---|---|---|---|
| DMMA-modified nanogel | Acidity-activated charge-conversional nanogel as an intelligent vehicle for promoted tumoral-cell uptake and Dox delivery | Dox | Chemotherapy | In vitro cell viability of MDA-MB-435s cells | [75] |
| polysaccharide-based nanogel | Hyaluronic-Acid-Based pH-Sensitive Nanogels | Dox | Chemotherapy | In vitro cell viability and In vivo mice model tumor volume evaluation | [146] |
| PEGylated PMAA-based nanogel | Glutathione-sensitive nanogel | Dox | Chemotherapy | In vitro cell viability of HepG2 cells | [147] |
| HA-based nanogel | Self-targeting hyaluronate (HA) nanogels (CDDPHANG/Dox) | Dox and Cisplatin | Chemotherapy | In vitro cell viability of MCF-7/ADR breast cancer cells | [113,148] |
| pNIPAAm nanogels | Photothermo-Responsive Nanogel | 5-fluorouracil | Chemo-photothermal synergistic therapy | In vitro L929 cell mortality | |
| GO-hybridized pNIPAM nanogels | Photothermally Enhanced Chemotherapy Delivered by Graphene Oxide-based Multi-responsive Nanogels | Dox | Chemo-photothermal synergistic therapy | In vitro cell viability of HeLa cells | [149,150] |
| Folated PVA-based nanogels | Folated pH degradable PVA nanogels | Docetaxel | Chemo-immunotherapy | In vitro cell viability of 4T1 breast cancer cells | [151] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Makhathini, S.S.; Mdanda, S.; Kondiah, P.J.; Kharodia, M.E.; Rumbold, K.; Alagidede, I.; Pathak, Y.; Bulbulia, Z.; Rants’o, T.A.; Kondiah, P.P.D. Biomedicine Innovations and Its Nanohydrogel Classifications. Pharmaceutics 2022, 14, 2839. https://doi.org/10.3390/pharmaceutics14122839
Makhathini SS, Mdanda S, Kondiah PJ, Kharodia ME, Rumbold K, Alagidede I, Pathak Y, Bulbulia Z, Rants’o TA, Kondiah PPD. Biomedicine Innovations and Its Nanohydrogel Classifications. Pharmaceutics. 2022; 14(12):2839. https://doi.org/10.3390/pharmaceutics14122839
Chicago/Turabian StyleMakhathini, Sifiso S., Sipho Mdanda, Pariksha J. Kondiah, Moosa E. Kharodia, Karl Rumbold, Imhotep Alagidede, Yashwant Pathak, Zain Bulbulia, Thankhoe A. Rants’o, and Pierre P. D. Kondiah. 2022. "Biomedicine Innovations and Its Nanohydrogel Classifications" Pharmaceutics 14, no. 12: 2839. https://doi.org/10.3390/pharmaceutics14122839
APA StyleMakhathini, S. S., Mdanda, S., Kondiah, P. J., Kharodia, M. E., Rumbold, K., Alagidede, I., Pathak, Y., Bulbulia, Z., Rants’o, T. A., & Kondiah, P. P. D. (2022). Biomedicine Innovations and Its Nanohydrogel Classifications. Pharmaceutics, 14(12), 2839. https://doi.org/10.3390/pharmaceutics14122839