Abstract
Corneal transplantation is considered a convenient strategy for various types of corneal disease needs. Even though it has been applied as a suitable solution for most corneal disorders, patients still face several issues due to a lack of healthy donor corneas, and rejection is another unknown risk of corneal transplant tissue. Corneal tissue engineering (CTE) has gained significant consideration as an efficient approach to developing tissue-engineered scaffolds for corneal healing and regeneration. Several approaches are tested to develop a substrate with equal transmittance and mechanical properties to improve the regeneration of cornea tissue. In this regard, bioprinted scaffolds have recently received sufficient attention in simulating corneal structure, owing to their spectacular spatial control which produces a three-cell-loaded-dimensional corneal structure. In this review, the anatomy and function of different layers of corneal tissue are highlighted, and then the potential of the 3D bioprinting technique for promoting corneal regeneration is also discussed.
1. Introduction
The cornea, which is located in the anterior part of the eye, is a transparent layer and acts as the window of the eye [1,2,3]. The corneal structure contains three transparent layers, and two membranes [2]. The corneal structure transfers light into the eye’s environment and protects the eye’s structure from mechanical or chemical environmental injuries, UV light, and infection [4]. Corneal dysfunction causes corneal visual loss [5].
Corneal surgery and corneal transplantation are well-known therapies for corneal blindness [5]. According to the World Health Organization (WHO), about 10 million patients globally need healthy corneal donation [1]. Additionally, over 40,000 corneal transplantations are carried out in the United States annually [6]. However, corneal transplantation displayed several drawbacks including shortness of high-quality donor corneas, expensive surgery, and rejected tissue due to the immune system and weakness for long-term transplantation [7]. In addition, because aging diminishes the function of endothelial cells, the quality of the transplanted cornea is of utmost importance [8]. Furthermore, the tissue becomes ineligible for corneal transplantation by therapies that alter the corneal structure to improve vision, such as LASIK [9]. Scientists are utilizing stem cells and tissue-engineering techniques to generate bioengineered cornea, or even individual corneal layers, to address the shortage of eligible corneas for donation [10,11,12].
Tissue engineering utilizes cells, bioactive macromolecules, and scaffolds, or a blend of the mentioned factors [13,14,15,16,17]. Human corneal cell keratoplasty (HCCK) was recently chosen as an advanced corneal surgery technique. The HCCK technique includes transparent carriers to improve human corneal cell behavior [18,19,20]. These lamellar keratoplasties and tissue-engineered full-thickness are recognized as successful transplantations [5]. Although donor corneas are used in these approaches, they still possess some challenges such as allograft tissue availability and rejection [21]. Results have shown that the proliferative ability of cultured human corneal cells can be preserved; thus, cornea tissue engineering (CTE) is recognized as a suitable approach for reconstructing corneal damage [22]. A recent study revealed that human corneal cells (HCCs) have adequate efficacy for cell propagation, but they might show low biocompatibility, weak light transmittance, and poor mechanical properties [23,24,25]. There are several methods of producing tissue-engineered scaffolds that completely resemble corneal structures [9,26,27,28,29]. Among them, 3D bioprinting technology is one of the potential approaches for producing artificial target tissue scaffolds. For example, the advantage of choosing this method in scaffold construction is the induction of the natural process during embryogenetic tissue formation and imitation [30,31,32]. Overall, 3D printing is attractive due to its high spatial resolution, and the simultaneous processing of cells and materials [33]. The conventional 3D printer consists of a classic inkjet, nozzles, and printer heads with material loaded into the cartridges as bioinks [34,35,36,37,38,39]. Thus, this review paper will highlight the corneal anatomy and different corneal layers’ functions, ocular disorders, and a summary of different approaches in scaffold constructions with a specific emphasis on 3D printed corneal tissue-engineered scaffolds.
2. Corneal Anatomy and Physiology
The cornea, known as the window of the eye, is optically transparent, including a special structure that is avascular anatomically. This dome-shaped and specialized tissue is located in the anterior part of the eye. Two major roles of the cornea are protecting the eye from harsh environments, and transmitting over 80% of light to inner portions (Figure 1A) [23]. As is evident in Figure 1B, the cornea is composed of three arranged and transparent layers, and two membranes: The cornea includes the outermost layer of epithelium, stroma, and the innermost layer of endothelium. Additionally, the epithelium and stroma are separated by Bowman’s membrane. However, the stroma and endothelium are separated by Descemet’s membrane (Figure 1B) [22]. Furthermore, the cornea acts as the last superficial barrier of the eye, providing safety from external potential dangers, and infections [40]. Moreover, to maintain and protect the integrity of the eye surface, corneal nerves play a vital role [23]. Consequently, corneal regeneration is obtained by nerve density, and corneal sensation factors after transplantation [4].
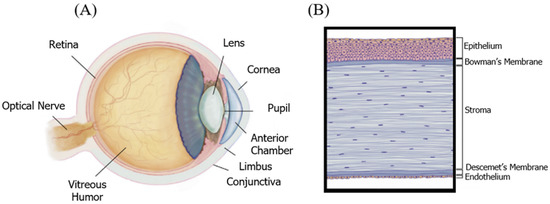
Figure 1.
(A) The anatomy of the eye, cornea. (B) The cornea is an optically transparent multilayered structure consisting of three cell layers and two membranes. Adopted and modified from [1] (Chapter 67) with permission.
In addition, the aqueous humor is located at the eye’s surface, and the function of the cornea depends on its malleability [41]. Moreover, it should be noted that the tear film is placed in the outermost portion of the eye, and acts as a reservoir for antibacterial and growth factors [9]. Additionally, one of the most critical roles of tear film in maintaining homeostasis, proliferation, and repair is covering the corneal surface. The anatomical importance of the cornea, which includes five transparent and arranged layers, corresponds to a wide-angle lens [13].
2.1. Corneal Epithelium
The epithelium is the outermost layer of the corneal tissue, and acts critically in the refraction of light into the eye [42,43,44]. The epithelium is a highly innervated tissue with nerve endings terminating at corneal epithelial layers [45]. The epithelium is a multilayered tissue and has five cell layers which occupy 10% of the corneal structure, and is about 50 μm thick [22]. The epithelium, a biological barrier, is responsible for the transfer of all soluble constituents and water out or into the stroma to maintain proper corneal light transparency, providing a smooth layer [23]. There are three cell types in the epithelial layer of the cornea. These cell types consist of 3–4 layers of flattened squamous cells, 1–3 layers of wing cells, and a single layer of columnar basal cells. It should be noted that these cells are held together by tight junctions [1]. These cell types are regenerated every 7–10 days continuously by the limbus stem cells (LSCs) [46].
There are some challenges in the regeneration of the epithelial layer by tissue engineering approaches, such as mimicking its arranged complexity, maintaining integrity as a sufficient barrier, and replacing epithelial cells continually [47,48,49]. In general, the epithelial layer, as the outermost layer, can keep the eye safe from mechanical damage, infection, and injuries [4]. In addition, it has a role in protecting the retina from UV damage [4].
2.2. Corneal Stroma
The stroma occupies 90% of the corneal tissue and 5% of corneal keratocyte cells (CKCs), and is an acellular layer but also a dense connective layer derived from neural crest cells [40]. The stroma comprises over 200 noncellular collagenous lamellae that are fully uniform, small, and aligned collagen fibers [7]. When injuries occur, flattened fibroblasts are activated. These lie quiescently, typically to produce collagen, then stabilize collagenous lamellae, and secrete the stromal components [2]. There are two important properties of a healthy stroma layer: optical transparency, and suitable mechanical strength [12,13,14]. Optical transparency is needed for biophysical properties, and suitable mechanical strength can be decreased when this organized structure is disturbed. Light transmittance can be reduced as a result of stromal damage and disruption. The stroma expresses two major challenges for the tissue engineer: equal mechanical stability, and high optical transparency [50].
2.3. Corneal Endothelium
Although the endothelium is the thinnest layer of the corneal tissue, it is important for maintaining function, and the ability to maintain corneal reproduction [1]. It is necessary to maintain dehydration by keeping optimal optical clarity [51]. Originally, the human endothelial cells (HECs) consist of about 5000 cells/mm2, while the number of HECs shows loss with increasing age. In general, the major challenge for tissue-engineered transplantation is the HECs cell number of out 2500 cells/mm2 [52]. The endothelium functions as a leaking pump of the corneal structure by leaking from the stroma layer in the presence of excessive stromal hydration (above 80%) [53]. The pumping-leak function process contains Na+ and K+-ATPase pumps that occupy the basolateral membrane. The main function of pumping-leak is to maintain stromal relative dehydration through transporting ions and water from the stroma to the tear film and aqueous humor [54,55,56]. The main characterization challenge is the efficiency measurement of the transplanted HECs [57]. There are some selective glucose transporters in this layer, permitting nutrition transformation from the aqueous humor to feed the epithelial and CKCs. Therefore, the main function of the endothelial layer is optical transparency with regulated hydrophilic proteoglycan and collagen interfibrillar spacing. In addition, endothelial distortion might lead to a loss in pump function [58].
3. Cells
3.1. Epithelium Cells
The corneal epithelial cells function as a physical barrier that resists the outer environment to maintain a healthy stroma layer. This effective corneal cell layer has a continuous turnover, with a lifespan of approximately 7 to 10 days (Figure 2). This turnover function is well described by the XYZ hypothesis [1]: X, the basal epithelial cells form the layer capable of proliferation properties; Y, migration centripetally of peripheral cells of new basal cells from the limbus to the cornea; and Z, loss of the epithelial cells from the surface. Generally, the epithelial cells shed constantly, and are substituted by a new cell sheet [59]. X + Y = Z describes the corneal epithelium’s maintenance function: cell loss and replacement. These three stages describe the complete corneal wound healing process: Z represents the epithelial cell loss from the limbus, step Y describes the covering of the surface by the wound surface, and lastly, in the final step X, proliferation provides cells with the ability to replace the epithelial tissue. As a result, the intensity of the centripetal movement and enhancement of proliferation ability are reasons to promote corneal wound healing [60].
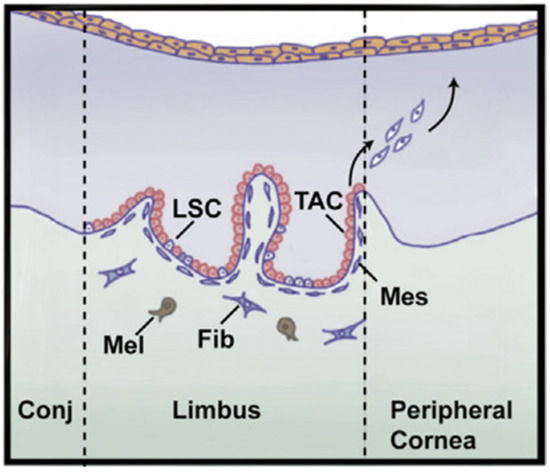
Figure 2.
The major role of the limbus is to regenerate epithelium where the limbal stem cells (LSCs) reside. LSCs produce transient amplifying cells (TACs) that have a significant proliferation potential. Then, TACs migrate to epithelium which is responsible for producing epithelial cells and is replaced. Mes = mesenchymal cell, Mel = melanocyte, Fib = fibroblast, Conj = conjunctiva. Adopted and modified from [1] (Chapter 67) with permission.
3.2. Stroma Cells
The corneal stroma consists of both extracellular and cellular components [61]. Cellular components of the mature corneal stroma are CKCs. CKCs have a dendritic morphology and are responsible for the maintenance of the ECM of the stroma. CKCs generate keratocan and lumican, and they are the key factors in maintaining the shape and transparency of the stroma. These small leucine-rich protein family members (keratocan and lumican) are the most important keratan sulfate proteoglycans in the corneal stroma [62,63,64]. Keratocan is solely found as a proteoglycan in the cornea, while lumican may also exist in various tissues as a glycosylated protein [33]. Both keratocan and lumican interact with collagen fibrils, and regulate the structure of this tissue to fit within their limits for specific properties [4]. Based on previous evidence, keratocan plays a crucial role in preserving the corneal structure [9].
Wound healing processes change the dendritic morphology of CKCs to be fibroblastic in appearance [65]. Two important functions of the keratocyte—the expression of keratocan and keratan sulfate synthesis—are decreased during the fibroblast/myofibroblast transformation [9]. Both isolated keratocytes from the corneal stroma and cultured keratocytes exhibit fibroblastic/myofibroblast phenotypes, and, meanwhile, show decreased keratocan expression and keratan sulfate synthesis, similar to in vivo wound healing [66]. This demonstrates that keratocan can be regarded as an indication of the native keratocyte phenotype [2].
3.3. Endothelium Cells
The key function of the endothelial cells is to pump excess fluid from the stroma and epithelium into the superficial layer of the cornea to maintain optimum corneal nutrition, and hydration [67]. This is recognized as the “pump-leak hypothesis”, preserving the cornea in a dehydrated state. It is worth mentioning that the hydration stage plays an important role in optical transparency (Figure 3) [60]. Endothelial cells are responsible for transporting proteins from inner layers using Na/K ATPase pumps. Thus, this gradient provides an osmotic pressure to maintain corneal stroma hydration, which is essential for endothelial cell growth [68].
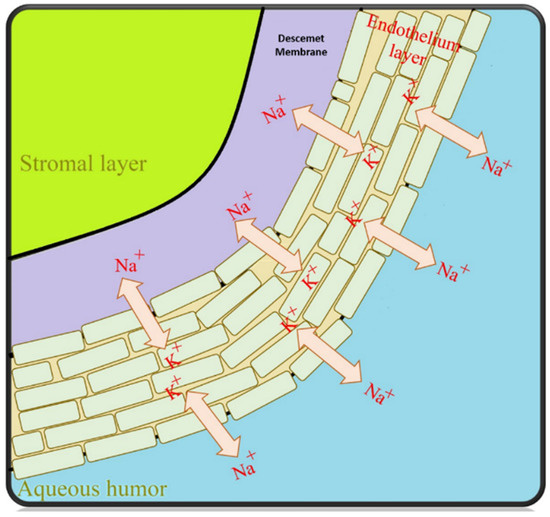
Figure 3.
The corneal endothelial “pump-leak” hypothesis illustrated—basic principles [68].
4. Corneal Scarring
Understanding the processes of deficiency or disease in almost any aspect of the visual system necessitates an intensive investigation of the structural foundations of the cornea, hence necessitating a considerable emphasis on individualized medical and surgical regeneration therapy. Vision impairment and obstruction of light to the eye have revealed a lot of biological information about ophthalmic diseases, ranging from damaged superficial layers and limbal cells to corneal injuries [4].
4.1. Keratoconus
Overall, keratoconus involves a general weakness of the connective tissue of the cornea. It is a progressive, noninflammatory corneal dystrophy resulting in thinning and protrusion of the cornea, changing it from a dome shape to a conical shape with gradual bulging [69,70,71]. Initially, patients experience blurred vision with the same symptoms as irregular astigmatism and refractive defect [72,73,74]. Vision is obscured as keratoconus progresses. The extent of vision impairment is subject to the degree of progression.
As keratoconus progresses it can be more easily diagnosed, as patients experience impaired night vision, photophobia, severe headaches due to eye strain, and eye itching. Usually, the condition is bilateral, and begins in the early teenage years. Corneal scarring is seen in advanced keratoconus stages, and can contribute to further vision loss until it eventually progresses to the point that corneal transplantation is critical to repair vision [75,76,77]. Keratoconus is responsible for stromal scarring, axial thinning, the disintegration of the epithelial basement membrane, and breaks in the Bowman’s membrane. According to the reported clinical case studies, the progression of keratoconus typically alters inevitable astigmatism from regular to irregular [4].
4.2. Dry Eye Disease
Dry eye has a wide range of eye surface diseases. According to a study reported in the 2007 international dry eye workshop, dry eye is a multidimensional disease, and its symptoms include tear instability, visual disturbance, eye discomfort, and potentially ocular surface damage [78]. According to data from previous studies, approximately 4.91 million Americans suffer from dry eye disease. Furthermore, there are tens of millions of less severe symptoms that can lead to dry eye failure if they are not followed up, which can trigger irritation—such as extended use of visual display terminals, or contact lens wear [79]. The pathophysiology involves either increased tear evaporation, decreased tear secretion, or both, resulting in hyperosmolarity of the tear film, and ocular surface inflammation. Corneal epithelial integrity can be seen in dry eye disease in its moderate to severe forms disrupted with punctate epithelial erosions; these erosions are detectable with fluorescein staining. The most common treatment for moderate to severe dry eyes is tearing supplementation, anti-inflammatory drops, eyelid hygiene, punctual plugs, and oral tetracycline [80].
4.3. Bacterial Keratitis
Bacterial keratitis is well-known as a devastating infection of the cornea, which can occur when the ocular defense is damaged. As a result, its spread causes inflammation, and gradual loss of vision. It is important to note that epithelial defects and decreased corneal sensitivity are prompting factors for severe ulcers, stromal necrosis, and bacterial growth. Failure in the protective mechanism and lack of ocular surface integrity causes the penetration of bacterial microbes into the cornea [81]. The most common causative organisms of bacterial keratitis include Staphylococcus aureus, Streptococcus pneumonia, and Staphylococcus epidermidis [82].
4.4. Light and Chemical Injuries
Clinically, exposure to UV light from a light source can damage the corneal epithelium, and cause fluorescein staining. Snow blindness, tanning bed use, direct lightening, and direct observation of the sun are some of the major causes of lesions on the corneal surface. Other important factors (such as chemical burns) can cause weakness in the cells of the corneal surface and affect the epithelial regeneration, even leading to potential blindness [83]. There are currently common treatments for superficial diseases that increase the ability of the corneal epithelium to recover. For extreme cases, cell-based restorative and repopulation treatments are necessary [21], which include techniques such as stem cell transplants, allografts, and limbal autografts to promote re-epithelialization [84].
4.5. Corneal Abrasion and Foreign Body
Corneal abrasions are more common in patients with symptoms in the epithelial layer, as they are more susceptible to injury. Abrasions typically occur with a range of symptoms, such as foreign body sensation, pain, tearing, sensitivity to light, and decreased vision, and it should be also noted that patients typically present with a history of trauma [85]. The presence of a foreign body within the corneal calls for immediate action to avoid permanent scarring, and serious loss of the epithelial cell surface. A deep wound with infected foreign material is likely to result in severe complications, initiating traumatic iritis, recurrent erosion syndrome, bacterial keratitis, and corneal ulcers [86].
5. Three-Dimensional Bioprinting
In general, the created scaffold structure is similar for all 3D printed models (Figure 4). First, the creation of a high-quality 3D model from the desired object is required. Then, the 3D structure should be printed in 2D layers of thickness, defined by the 3D image. The data will form the structure for layer-by-layer printing by transferring the command to the printer’s desktop. The flexible manufacturing process allows it to be provided to the targeted tissue. In addition, graphical methods can be designed, including computer-aided design (CAD), and magnetic resonance imaging (MRI) of a structure similar to the data received from patients [33].
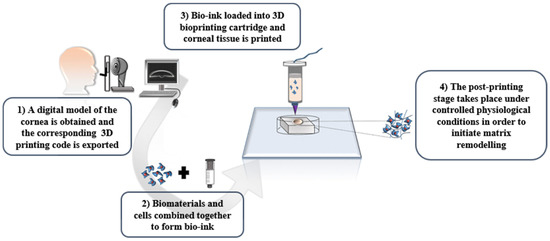
Figure 4.
The path of making patient-specific devices via 3D printing [33] with permission.
Another consideration is bioprinting, which uses cellular encapsulated biological materials as bioinks [87]. Scaffolds printed with a cell are produced in situ. In this circumstance, the printing process must be carried out in disinfected conditions, and be compatible with the cell. The importance of maintaining structure and having mechanical properties in the printed structure are factors that limit the selection of cell-compatible materials [88]. It is also important to select the appropriate rheological parameter to reduce the shear pressure, which is required for the printing parameters [89]. Nevertheless, cell-loaded bioprinting reduces the resolution of the printed substrate. Secondly, the need to increase the cell density ratio relative to the surface area is of critical importance [90]. It should be noted that a healthy threshold of cell density in solid organs is considered to be about 109 to 1010 cells per cell culture well. Up until now, bioprinted hydrogel scaffolds only had a cell density ranging between 105 and 107 cells per cell culture well [91].
Bioprinting methods have successfully been applied by several researchers, and have demonstrated the reliable properties of bioprinting techniques to generate ex vivo constructs and membranes [92]. For example, scientists have obtained noticeable features by a microextrusion approach to produce a proper replacement for neural studies, generating 3D models of interacting human endothelial cells (HECs), and cancer studies by using a laser-based method, or even recreation of native ECM of cartilage by a droplet-based technique [93,94,95]. As a top-down approach, bioprinted scaffolds are known as a biofabrication technology for fabricating several types of ex vivo membranes and tissues artificially by consecutive deposition of cell-loaded layers [96,97,98,99]. Different approaches can be applied for fabricating bioprinted scaffolds such as laser-based, droplet-based, and extrusion-based techniques (Figure 5) [100]. These bioprinting techniques are compatible with several kinds of bioinks which can be crosslinked in different ways. However, optimizing bioink, according to the requirements of each of these bioprinting techniques, is associated with different challenges. An extrusion-based method is the most popular in comparison with other types of bioprinting approaches [101,102,103], since it is compatible with most injectable hydrogel platforms for biomedical engineering, and regeneration medicine applications [93,94,104]. In brief, this method contains pre-polymerized bioink which is extruded through a single nozzle under pressure. The pressurized air can be applied to the printer head to produce a 3D construct by extruding printing material layer by layer.
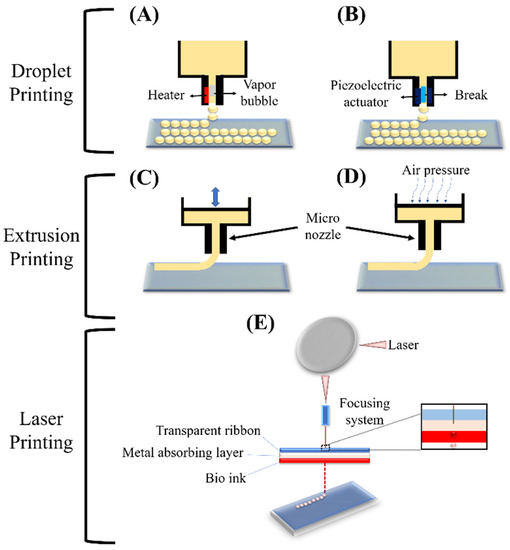
Figure 5.
Schematic of the basic techniques of 3D printing: Droplet printing (thermal (A), piezoelectric (B)), Extrusion printing (piston (C), pneumatic (D)), and Laser printing (stereolithographic (E)) [100].
In addition to these three bioprinting methods, recent studies have demonstrated two more techniques that have illustrated interesting results. These techniques can be categorized into two major categories. Among these techniques, one involves using photocurable gels, and another is based on applying thermosensitive and natural (such as collagen) bioinks [105,106,107,108]. As a photocurable gel, both poly (ethylene glycol) diacrylate (PEGDA) and gelatin methacrylate (GelMA) can be crosslinked using lithium phenyl (2,4,6-trimethyl benzoyl) phosphinate (LAP) as a crosslinker under visible light. Bernal et al. [105] fabricated a GelMA-LAP bioink system to produce 3D printed osteogenic models by employing the volumetric method, setting 2D light with rotating and synchronously irradiated patterns. It should be noted that the volumetric bioprinting technique enables geometrically constructed production which aims to create centimeter-scale constructs at an unprecedented printing rate, opening new possibilities for upscaling the creation of the hydrogel-based structure. The result showed that the polymer was not cured evenly and only in some parts of the structure, preventing the gelation threshold as a result of increased absorption. Looking on the bright side, after fourteen days of continuous cell culturing, the tissue-engineered construct revealed enhanced alkaline phosphatase (ALP) expression, and mineral deposition. Another study by Grigoryan et al. [106] generated multi-vascular and intravascular structures via photopolymerizable gel with the addition of food dye for the stereolithography approach. Both studies illustrated that it is possible to fabricate a complex 3D construct via these methods, and demonstrate new possibilities for fabricating corneal tissue suitable for tissue transplant applications. The second category of bioprinting methods involves applying thermosensitive and natural bioinks [107,108]. Skylar-Scott et al. [107] generated a 3D printing scaffold with the sacrificial writing into functional tissue (SWIFT) approach. In the SWIFT technique, a high volume of cells are transferred into the engineered ECM during the bioprinting procedure. This technique contains a sacrificial gel that contains the cells. This gel is printed and after printing it is liquified by melting at room temperature; thus, the gel is removed creating a path for the medium to flow. The results have shown that after eight days of cell seeding cardiomyocyte cells showed a beating function, proving the successful functional application. Another study which was designed in the reverse order, in comparison to SWIFT, utilized freeform reversible embedding of suspended hydrogels (FRESH), supporting the 3D printed structure during the process which revealed positive results after 14 days of cell seeding [108]. Thus, the obtained results can be used for human corneal generation.
Corneal Bioprinting
In general, corneal bioprinting offers a wide range of possibilities to address current challenges and requirements of corneal tissue regeneration (i.e., controllable structure and properties, similar mechanical strength to withstand environmental as well as structural pressure, and fabricating a fully-organized corneal construct) [100]. As was addressed in Section 2 and Section 3, the corneal structure consists of three transparent layers. The thickest layer of the cornea is the stromal layer (~500 µm), and the epithelium and endothelium are both delicate in structure (<50 µm) [109]. The stromal layer occupied over 90% of the corneal structure, and functionally is the most important tissue in CTE due to its transparency (as a result of aligned collagen lamellae and proteoglycan expressions) and mechanical performance (due to cross-linked collagen fibrils) [110]. In addition, the peripheral and central sections of the stromal layer show different mechanical properties, which play a crucial role in the orientation of collagen content, and corneal cell differentiation and alignment [111]. Therefore, to enhance the functionality of the stromal part, it is crucial to simulate the micro and macrostructure since the physical, mechanical, and chemical properties of the tissue-engineered structure directly and noticeably affect biological factors [9].
According to current studies, various bioprinting techniques for CTE have been considered (Table 1) [33,67,112,113,114,115,116,117,118,119,120,121]. Sorkio et al. [112] analyzed the feasibility of a laser-based bioprinting method to generate corneal tissue. In brief, the collagen type-I cell loaded enhanced LSCs cell attachment and proliferation. Although it is possible to print a tissue-like stromal layer, the tissue-engineered structure may not be appropriate in its structure and mechanical properties because of the very sensitive nature of CKCs. Additionally, the fabricated scaffold did not have proper transparency for corneal demands. Another study by Isaacson et al. [33] reported preparing bioink consisting of alginate-collagen type-I and CKCs. The prepared ink was injected into a 3D mold made by acrylonitrile butadiene styrene (ABS), and using the FRESH method. The final result was a structure similar to the 3D structure of corneal, with corneal tissue. Even though the produced construct showed suitable transparency, it could not support CKCs properly and the cells could not reach a dendritic shape.

Table 1.
Summary of different experimental studies based on 3D printing techniques.
The vitality and proliferation of CKCs are challenging, since this sensitive type of cell simply converts to scar-inducing stromal fibroblasts at non-desirable conditions. Recently, this challenge was overcome with the droplet-based printing technique, since it is more compatible with cells relating to laser-based or extrusion-based printing techniques. Campos et al. [113] printed collagen-type I-agarose bioink corneal construct with CKCs encapsulation to produce a dome-shaped structure in a layer-by-layer manner. The obtained results illustrated cell vitality and proliferation similar to the control sample, and showed positive expression for both lumican and keratocan markers.
From an anatomical standpoint, the stromal layer is difficult to regenerate with current techniques due to its highly complex microstructure, and it being made of randomly oriented collagen lamellae [120]. Additionally, the stromal mechanical strength and light transmittance performance are completely related to the stromal unique structure, which is fabricated from randomly oriented collagen fibrils [2]. Current research based on the fabrication of aligned PCL-PEG fibers with the incorporation of limbal stem cells (LSCs) and 15% GelMA gel to fabricate corneal tissue-engineered construct showed better mechanical strength with improved suture-ability, as well as improvement in expression of CKCs markers. Moreover, the scaffold illustrated high transparency and similar mechanical strength, in comparison with the native cornea [120]. Other studies have also been based on the advancement of bioprinting techniques for CTE by employing decellularized cornea gel or GelMA, showing improvement in CKCs differentiation, and better tensile strength. The printed scaffold improved filopodial elongations and phenotype maintenance similar to CKCs in vivo. The collected results motivated researchers to apply the bioprinting technique for the generation of CTE scaffolds due to their impact on architecture, transparency, mechanical strength, and cell/scaffold interactions [118,121].
After examining the aforementioned studies, it can be challenging to introduce the most promising technique to simulate corneal structure. For instance, enhanced mechanical features are possible with an extrusion-based approach, or improved elastic modulus can be achieved with a droplet-based technique; however, mechanical properties with a laser-based technique are not discussed yet. As reported in recent papers, the corneal stroma has about a 150–700 kPa elastic modulus [7]. Thus, the extrusion-based method would be the best candidate if mechanical properties have been chosen as the most important parameter. However, other properties such as microstructure and geometrical curvature are also prominent, which can be better satisfied using the droplet-based method. Furthermore, bioprinting considerably removes the possibility of human error, and it is superior to casting gels into molds in simulating native curvature of the corneal. Additionally, according to the microstructure, although it is not addressed by recent studies, future studies may be focused on the CKCs migration and orientation by employing smart fiber alignment [123].
6. Nanotechnology in CTE
Nanotechnology can be employed for the development of corneal scaffolds to enhance their physicochemical characteristics [9]. Nano scaffolds offer unique mechanical aspects that promote cell adhesion, proliferation, and differentiation, in addition to facilitating gas and nutrient exchange and waste removal [4]. For instance, dendrimers (~10 nm) are high-contrast polymers with a 3D ionic form, and many end groups [9]. The greatest benefits of dendritic systems are their high density of functional side chains, their capacity to manage network crosslinks, and their scalability across a broad range of sizes. It has been demonstrated that dendrimer-based hydrogels enhance the efficient healing of corneal fractures, without scarring or inflammation. Due to the ability to modulate the crosslinking process and alter the chemistry of crosslinking, it is feasible to influence the duration of resorption, and hence control the wound healing process over a longer period [124]. Thus, dendrimers are labile “smart” nanomaterials that can be employed for wound healing during long recovery periods, with a minimal likelihood of triggering an inflammatory reaction [124,125]. Combining nanotechnology and corneal tissue engineering with natural biomaterials could be a potential approach for reaching the current goal in this category [126]. For instance, to create biomaterials with the appropriate attributes, metal nanoparticles, graphene oxide, carbon nanotubes, and nanoliposomes can be combined. Enhancing the proliferation and functionality of additional stem cells is facilitated by their in situ transformation from sol to gel. Soft nanoparticles can interact with polymer chains and contribute to the hydrogel grid’s subsequent crosslinking, hence enhancing its mechanical aspects [126,127,128]. In a study, Tayebi et al. [129] produced chitosan nanoparticles into chitosan/polycaprolactone membranes yielding a biodegradable, transparent scaffold for cultivating corneal endothelial cells. The chitosan nanoparticles/polycaprolactone, which have the lowest wettability, exhibited transparency comparable to human stromal tissue. The scaffold was non-cytotoxic, and enhanced the proliferation of CECs. The biophysical results revealed that CECs adhered to the scaffold, and formed a dense monolayer. Thus, the created scaffold appears to be appropriate for corneal endothelium regeneration. In another study, Chang et al. [130] developed a novel ophthalmic formulation based on moxifloxacin and dexamethasone-loaded nanostructured lipid carriers mixed with collagen/gelatin/alginate for the treatment of a corneal disorder, particularly bacterial keratitis. The nanoparticles had the following characteristics: average size: 132.1 ± 73.58 nm; zeta potential: 6.27 ± 4.95 mV; entrapment efficiency: 91.5 ± 3.5%; and drug content: 18.1 ± 1.7%. The findings indicated that the nanoparticles could release an effective working concentration in 60 min, and sustain the drug release for a minimum of 12 h. While the samples did not show any toxicities, the substrate enhanced the cell numbers of CEpCs. An animal study confirmed that it inhibits the growth of pathogen microorganisms, and promotes corneal wound healing. The results suggest that the nanoparticle formulation may be an effective anti-inflammatory agent for CTE. The application of nanoparticles, through using the bioprinting technique, permits tailored therapy for more precise and successful disease treatment [100]. Nanotechnology is predicted to be employed in the future to personalize regenerative medicine utilizing human stem cells, and to provide therapeutic tools to maintain a healthy environment for the growth and maturation of stem cells in the damaged area [131]. However, nanotechnology research in CTE is still in its infancy, and only limited in vivo investigations are reported. The behavior of corneal cells in tissue engineering constructions in corneal injury has been widely proven in vitro, but in vivo proof-of-concept investigations are lacking, leaving many concerns unanswered.
7. Conclusions and Future Progress
Recently, different studies on the advancement of CTE replacements are focused on analyzing various biomaterials, and fabrication methods. Although there are several studies on this subject, the prior studies displayed a lack of understanding of the corneal function and its structure; therefore, there is still significant room for progress in mimicking the native corneal properties, such as corneal physicochemical properties. Even though different studies have shown that biomaterials might have similar mechanical, optical, and physical properties to the natural cornea, it is challenging to arrange these biomaterials into the same well-organized structures as the natural cornea. Bioprinted tissue engineering scaffolds with proper orthogonal lamellae architecture can be a crucial step for the successful fabrication of CTE scaffolds. In this regard, the fabrication of a successful CTE scaffold will be mostly dependent on generating necessary features, such as releasing important functional biomolecules to improve corneal components, cells, and nerve regeneration. Furthermore, to produce a tissue-engineered scaffold adjusting mechanical and optical features is crucial as well. Research shows that bioprinted scaffolds equipped with nanotechnology components and nanoscale characteristics can improve the potential of CTE. The ultimate aim of CTE is to improve, preserve, and restore vision by developing nanotechnology-enabled regenerative therapies to heal damaged corneal tissues based on unique patient needs.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
A.O.M.S., S.H.K., S.A.P., A.Z., F.S., N.M., M.J.B. and M.R. declare that they have no conflict of interest. This article does not contain any studies with human or animal subjects performed by any of the authors.
Abbreviations
Corneal keratocyte cells (CKCs), collagen (COL), limbal epithelial cells (LECs), alginate (ALG), agarose (AG), methacrylated gelatin (GelMa), limbal stromal stem cells (LSSCs), decellularized cornea (dC), corneal endothelial cells (CECs), corneal epithelial cells (CEpCs), human turbinate-derived mesenchymal stem cells (hTMSC).
References
- Lanza, R.; Langer, R.; Vacanti, J.P.; Atala, A. Principles of Tissue Engineering; Academic Press: Cambridge, MA, USA, 2020. [Google Scholar]
- Salehi, A.O.M.; Keshel, S.H.; Sefat, F.; Tayebi, L. Use of Polycaprolactone in Corneal Tissue Engineering: A Review. Mater. Today Commun. 2021, 27, 102402. [Google Scholar] [CrossRef]
- Goodarzi, H.; Jadidi, K.; Pourmotabed, S.; Sharifi, E.; Aghamollaei, H. Preparation and in vitro characterization of cross-linked collagen–gelatin hydrogel using EDC/NHS for corneal tissue engineering applications. Int. J. Biol. Macromol. 2019, 126, 620–632. [Google Scholar] [CrossRef] [PubMed]
- Yousaf, S.; Keshel, S.H.; Farzi, G.A.; Momeni-Moghadam, M.; Ahmadi, E.D.; Asencio, I.O.; Mozafari, M.; Sefat, F. Scaffolds for corneal tissue engineering. In Handbook of Tissue Engineering Scaffolds; Elsevier: Amsterdam, The Netherlands, 2021; Volume 2, pp. 649–672. [Google Scholar]
- Chen, Z.; You, J.; Liu, X.; Cooper, S.; Hodge, C.; Sutton, G.; Crook, J.M.; Wallace, G.G. Biomaterials for corneal bioengineering. Biomed. Mater. 2018, 13, 032002. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Yan, C.; Zhu, M.; Yao, Q.; Shao, C.; Lu, W.; Wang, J.; Mo, X.; Gu, P.; Fu, Y. Electrospun nanofibrous SF/P (LLA-CL) membrane: A potential substratum for endothelial keratoplasty. Int. J. Nanomed. 2015, 10, 3337. [Google Scholar]
- Wu, Z.; Kong, B.; Liu, R.; Sun, W.; Mi, S. Engineering of corneal tissue through an aligned PVA/collagen composite nanofibrous electrospun scaffold. Nanomaterials 2018, 8, 124. [Google Scholar] [CrossRef]
- Akter, F. Tissue Engineering Made Easy; Academic Press: Cambridge, MA, USA, 2016. [Google Scholar]
- Ahearne, M.; Fernández-Pérez, J.; Masterton, S.; Madden, P.W.; Bhattacharjee, P. Designing Scaffolds for Corneal Regeneration. Adv. Funct. Mater. 2020, 30, 1908996. [Google Scholar] [CrossRef]
- Koons, G.L.; Diba, M.; Mikos, A.G. Materials design for bone-tissue engineering. Nat. Rev. Mater. 2020, 5, 584–603. [Google Scholar] [CrossRef]
- Guérin, L.-P.; Le-Bel, G.; Desjardins, P.; Couture, C.; Gillard, E.; Boisselier, É.; Bazin, R.; Germain, L.; Guérin, S.L. The Human Tissue-Engineered Cornea (hTEC): Recent Progress. Int. J. Mol. Sci. 2021, 22, 1291. [Google Scholar] [CrossRef]
- Nosrati, H.; Abpeikar, Z.; Mahmoudian, Z.G.; Zafari, M.; Majidi, J.; Alizadeh, A.; Moradi, L.; Asadpour, S. Corneal epithelium tissue engineering: Recent advances in regeneration and replacement of the corneal surface. Regen. Med. 2020, 15, 2029–2044. [Google Scholar] [CrossRef]
- Salehi, A.O.M.; Nourbakhsh, M.S.; Rafienia, M.; Baradaran-Rafii, A.; Keshel, S.H. Corneal stromal regeneration by hybrid oriented poly (ε-caprolactone)/lyophilized silk fibroin electrospun scaffold. Int. J. Biol. Macromol. 2020, 161, 377–388. [Google Scholar] [CrossRef]
- Salehi, A.O.M.; Keshel, S.H.; Rafienia, M.; Nourbakhsh, M.S.; Baradaran-Rafii, A. Promoting keratocyte stem like cell proliferation and differentiation by aligned polycaprolactone-silk fibroin fibers containing Aloe vera. Biomater. Adv. 2022, 137, 212840. [Google Scholar] [CrossRef]
- Janmohammadi, M.; Nazemi, Z.; Salehi, A.O.M.; Seyfoori, A.; John, J.V.; Nourbakhsh, M.S.; Akbari, M. Cellulose-based composite scaffolds for bone tissue engineering and localized drug delivery. Bioact. Mater. 2023, 20, 137–163. [Google Scholar] [CrossRef] [PubMed]
- Movahedi, M.; Salehi, A.O.M.; Moezi, D.; Yarahmadian, R. In vitro and in vivo study of aspirin loaded, electrospun polycaprolactone–maltodextrin membrane for enhanced skin tissue regeneration. Int. J. Polym. Mater. Polym. Biomater. 2021, 71, 1334–1344. [Google Scholar] [CrossRef]
- Movahedi, M.; Salehi, A.O.M.; Etemad, S. Casein release and characterization of electrospun nanofibres for cartilage tissue engineering. Bull. Mater. Sci. 2022, 45, 76. [Google Scholar] [CrossRef]
- Biazar, E.; Baradaran-Rafii, A.; Heidari-Keshel, S.; Tavakolifard, S. Oriented nanofibrous silk as a natural scaffold for ocular epithelial regeneration. J. Biomater. Sci. Polym. Ed. 2015, 26, 1139–1151. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.H.; Jeng, B.H. Indications for keratoplasty in management of corneal ectasia. Curr. Opin. Ophthalmol. 2022, 33, 318–323. [Google Scholar] [CrossRef]
- El Zarif, M.; Alió, J.L.; Alió del Barrio, J.L.; De Miguel, M.P.; Abdul Jawad, K.; Makdissy, N. Corneal stromal regeneration: A review of human clinical studies in keratoconus treatment. Front. Med. 2021, 8, 650724. [Google Scholar] [CrossRef]
- Kumar, A.; Yun, H.; Funderburgh, M.L.; Du, Y. Regenerative therapy for the Cornea. Prog. Retin. Eye Res. 2021, 87, 101011. [Google Scholar] [CrossRef]
- Ghezzi, C.E.; Rnjak-Kovacina, J.; Kaplan, D.L. Corneal tissue engineering: Recent advances and future perspectives. Tissue Eng. Part B Rev. 2015, 21, 278–287. [Google Scholar] [CrossRef]
- Kong, B.; Mi, S. Electrospun scaffolds for corneal tissue engineering: A review. Materials 2016, 9, 614. [Google Scholar] [CrossRef]
- Wicklein, V.J.; Singer, B.B.; Scheibel, T.; Salehi, S. Nanoengineered Biomaterials for Corneal Regeneration. In Nanoengineered Biomaterials for Regenerative Medicine; Elsevier: Amsterdam, The Netherlands, 2021; pp. 379–415. [Google Scholar]
- Nosrati, H.; Alizadeh, Z.; Nosrati, A.; Ashrafi-Dehkordi, K.; Banitalebi-Dehkordi, M.; Sanami, S.; Khodaei, M. Stem cell-based therapeutic strategies for corneal epithelium regeneration. Tissue Cell 2021, 68, 101470. [Google Scholar] [CrossRef] [PubMed]
- Bigham, A.; Salehi, A.O.M.; Rafienia, M.; Salamat, M.R.; Rahmati, S.; Raucci, M.G.; Ambrosio, L. Zn-substituted Mg2SiO4 nanoparticles-incorporated PCL-silk fibroin composite scaffold: A multifunctional platform towards bone tissue regeneration. Mater. Sci. Eng. C 2021, 127, 112242. [Google Scholar] [CrossRef] [PubMed]
- Ulag, S.; Uysal, E.; Bedir, T.; Sengor, M.; Ekren, N.; Ustundag, C.B.; Midha, S.; Kalaskar, D.M.; Gunduz, O. Recent developments and characterization techniques in 3D printing of corneal stroma tissue. Polym. Adv. Technol. 2021, 32, 3287–3296. [Google Scholar] [CrossRef]
- Mahdavi, S.S.; Abdekhodaie, M.J.; Mashayekhan, S.; Baradaran-Rafii, A.; Djalilian, A.R. Bioengineering approaches for corneal regenerative medicine. Tissue Eng. Regen. Med. 2020, 17, 567–593. [Google Scholar] [CrossRef] [PubMed]
- Reddy, R.; Reddy, N. Biomimetic approaches for tissue engineering. J. Biomater. Sci. Polym. Ed. 2021, 29, 1667–1685. [Google Scholar] [CrossRef] [PubMed]
- Sanie-Jahromi, F.; Eghtedari, M.; Mirzaei, E.; Jalalpour, M.H.; Asvar, Z.; Nejabat, M.; Javidi-Azad, F. Propagation of limbal stem cells on polycaprolactone and polycaprolactone/gelatin fibrous scaffolds and transplantation in animal model. BioImpacts BI 2020, 10, 45. [Google Scholar] [CrossRef] [PubMed]
- Mahdavi, S.S.; Abdekhodaie, M.J.; Kumar, H.; Mashayekhan, S.; Baradaran-Rafii, A.; Kim, K. Stereolithography 3D bioprinting method for fabrication of human corneal stroma equivalent. Ann. Biomed. Eng. 2020, 48, 1955–1970. [Google Scholar] [CrossRef] [PubMed]
- Miotto, M.; Gouveia, R.M.; Ionescu, A.M.; Figueiredo, F.; Hamley, I.W.; Connon, C.J. 4D corneal tissue engineering: Achieving time-dependent tissue self-Curvature through localized control of cell actuators. Adv. Funct. Mater. 2021, 29, 1807334. [Google Scholar] [CrossRef]
- Isaacson, A.; Swioklo, S.; Connon, C.J. 3D bioprinting of a corneal stroma equivalent. Exp. Eye Res. 2021, 173, 188–193. [Google Scholar] [CrossRef]
- Murphy, S.V.; Atala, A. 3D bioprinting of tissues and organs. Nat. Biotechnol. 2014, 32, 773–785. [Google Scholar] [CrossRef]
- Kang, H.-W.; Lee, S.J.; Ko, I.K.; Kengla, C.; Yoo, J.J.; Atala, A. A 3D bioprinting system to produce human-scale tissue constructs with structural integrity. Nat. Biotechnol. 2016, 34, 312–319. [Google Scholar] [CrossRef] [PubMed]
- Jeong, C.G.; Atala, A. 3D printing and biofabrication for load bearing tissue engineering. Eng. Miner. Load Bear. Tissue 2015, 881, 3–14. [Google Scholar]
- Shafiee, A.; Atala, A. Printing technologies for medical applications. Trends Mol. Med. 2016, 22, 254–265. [Google Scholar] [CrossRef] [PubMed]
- Colaco, M.; Igel, D.A.; Atala, A. The potential of 3D printing in urological research and patient care. Nat. Rev. Urol. 2021, 15, 213. [Google Scholar] [CrossRef] [PubMed]
- Gillispie, G.J.; Han, A.; Uzun-Per, M.; Fisher, J.; Mikos, A.G.; Niazi, M.K.K.; Yoo, J.J.; Lee, S.J.; Atala, A. The Influence of Printing Parameters and Cell Density on Bioink Printing Outcomes. Tissue Eng. Part A 2020, 26, 1349–1358. [Google Scholar] [CrossRef]
- Akter, F. Principles of Tissue Engineering. In Tissue Engineering Made Easy; Elsevier: Amsterdam, The Netherlands, 2016; pp. 3–16. [Google Scholar]
- Sridhar, M.S. Anatomy of the cornea and ocular surface. Ind. J. Ophthalmol. 2021, 66, 190. [Google Scholar] [CrossRef]
- Baradaran-Rafii, A.; Biazar, E.; Heidari-Keshel, S. Cellular response of limbal stem cells on polycaprolactone nanofibrous scaffolds for ocular epithelial regeneration. Curr. Eye Res. 2016, 41, 326–333. [Google Scholar] [CrossRef]
- Palchesko, R.N.; Carrasquilla, S.D.; Feinberg, A.W. Natural biomaterials for corneal tissue engineering, repair, and regeneration. Adv. Healthc. Mater. 2021, 7, 1701434. [Google Scholar] [CrossRef]
- Islam, M.M.; Sharifi, R.; Gonzalez-Andrades, M. Corneal Tissue Engineering. In Corneal Regeneration; Springer: Berlin/Heidelberg, Germany, 2019; pp. 23–37. [Google Scholar]
- Stepp, M.A.; Tadvalkar, G.; Hakh, R.; Pal-Ghosh, S. Corneal epithelial cells function as surrogate Schwann cells for their sensory nerves. Glia 2017, 65, 851–863. [Google Scholar] [CrossRef]
- Campbell, J.D.; Ahmad, S.; Agrawal, A.; Bienek, C.; Atkinson, A.; Mcgowan, N.W.; Kaye, S.; Mantry, S.; Ramaesh, K.; Glover, A. Allogeneic Ex Vivo Expanded Corneal Epithelial Stem Cell Transplantation: A Randomized Controlled Clinical Trial. Stem Cells Transl. Med. 2021, 8, 323–331. [Google Scholar] [CrossRef]
- Arabpour, Z.; Baradaran-Rafii, A.; Bakhshaiesh, N.L.; Ai, J.; Ebrahimi-Barough, S.; Esmaeili Malekabadi, H.; Nazeri, N.; Vaez, A.; Salehi, M.; Sefat, F. Design and characterization of biodegradable multi layered electrospun nanofibers for corneal tissue engineering applications. J. Biomed. Mater. Res. Part A 2021, 107, 2340–2349. [Google Scholar] [CrossRef] [PubMed]
- Hancox, Z.; Keshel, S.H.; Yousaf, S.; Saeinasab, M.; Shahbazi, M.-A.; Sefat, F. The progress in corneal translational medicine. Biomater. Sci. 2020, 8, 6469–6504. [Google Scholar] [CrossRef] [PubMed]
- Kianersi, S.; Solouk, A.; Saber-Samandari, S.; Keshel, S.H.; Pasbakhsh, P. Alginate nanoparticles as ocular drug delivery carriers. J. Drug Deliv. Sci. Technol. 2021, 66, 102889. [Google Scholar] [CrossRef]
- Fernández-Pérez, J.; Kador, K.E.; Lynch, A.P.; Ahearne, M. Characterization of extracellular matrix modified poly (ε-caprolactone) electrospun scaffolds with differing fiber orientations for corneal stroma regeneration. Mater. Sci. Eng. C 2020, 108, 110415. [Google Scholar] [CrossRef]
- Kostenko, A.; Swioklo, S.; Connon, C.J. Alginate in corneal tissue engineering. Biomed. Mater. 2022, 17, 022004. [Google Scholar] [CrossRef]
- Delaey, J.; De Vos, L.; Koppen, C.; Dubruel, P.; Van Vlierberghe, S.; Van den Bogerd, B. Tissue engineered scaffolds for corneal endothelial regeneration: A material’s perspective. Biomater. Sci. 2022, 10, 2440–2461. [Google Scholar] [CrossRef]
- Bosch, B.M.; Bosch-Rue, E.; Perpiñan-Blasco, M.; Perez, R.A. Design of functional biomaterials as substrates for corneal endothelium tissue engineering. Regen. Biomater. 2022, 9, rbac052. [Google Scholar] [CrossRef]
- Takahashi, H. Corneal endothelium and phacoemulsification. Cornea 2016, 35, S3–S7. [Google Scholar] [CrossRef]
- Eghrari, A.O.; Riazuddin, S.A.; Gottsch, J.D. Overview of the cornea: Structure, function, and development. In Progress in Molecular Biology and Translational Science; Elsevier: Amsterdam, The Netherlands, 2015; pp. 7–23. [Google Scholar]
- El Zarif, M.; del Barrio, J.L.A.; Arnalich-Montiel, F.; De Miguel, M.P.; Makdissy, N.; Alió, J.L. Corneal stroma regeneration: A new approach for the treatment of cornea disease. Asia-Pac. J. Ophthalmol. 2020, 9, 571–579. [Google Scholar] [CrossRef]
- Zhao, J.; Tian, M.; Li, Y.; Su, W.; Fan, T. Construction of tissue-engineered human corneal endothelium for corneal endothelial regeneration using a crosslinked amniotic membrane scaffold. Acta Biomater. 2022, 147, 185–197. [Google Scholar] [CrossRef]
- Kim, D.K.; Sim, B.R.; Khang, G. Nature-derived aloe vera gel blended silk fibroin film scaffolds for cornea endothelial cell regeneration and transplantation. ACS Appl. Mater. Interfaces 2016, 8, 15160–15168. [Google Scholar] [CrossRef] [PubMed]
- Sang, S.; Yan, Y.; Shen, Z.; Cao, Y.; Duan, Q.; He, M.; Zhang, Q. Photo-crosslinked hydrogels for tissue engineering of corneal epithelium. Exp. Eye Res. 2022, 218, 109027. [Google Scholar] [CrossRef] [PubMed]
- Gandhi, S.; Jain, S. The Anatomy and Physiology of Cornea. In Keratoprostheses and Artificial Corneas; Springer: Berlin/Heidelberg, Germany, 2015; pp. 19–25. [Google Scholar]
- Binte, M.; Yusoff, N.Z.; Riau, A.K.; Yam, G.H.; Binte Halim, N.S.H.; Mehta, J.S. Isolation and propagation of human corneal stromal keratocytes for tissue engineering and cell therapy. Cells 2022, 11, 178. [Google Scholar] [CrossRef]
- Lagali, N. Corneal stromal regeneration: Current status and future therapeutic potential. Curr. Eye Res. 2020, 45, 278–290. [Google Scholar] [CrossRef]
- Matthyssen, S.; Van den Bogerd, B.; Dhubhghaill, S.N.; Koppen, C.; Zakaria, N. Corneal regeneration: A review of stromal replacements. Acta Biomater. 2021, 69, 31–41. [Google Scholar] [CrossRef]
- Del Barrio, J.L.A.; Arnalich-Montiel, F.; De Miguel, M.P.; El Zarif, M.; Alió, J.L. Corneal stroma regeneration: Preclinical studies. Exp. Eye Res. 2021, 202, 108314. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, L.; Zhao, J.; Liang, J.; Zhang, Z.; Li, Q.; Zhang, J.; Wan, P.; Wu, Z. Reconstructing auto tissue engineering lamellar cornea with aspartic acid modified acellular porcine corneal stroma and preconditioned limbal stem cell for corneal regeneration. Biomaterials 2022, 289, 121745. [Google Scholar] [CrossRef] [PubMed]
- Formisano, N.; van der Putten, C.; Grant, R.; Sahin, G.; Truckenmüller, R.K.; Bouten, C.V.; Kurniawan, N.A.; Giselbrecht, S. Mechanical Properties of Bioengineered Corneal Stroma. Adv. Healthc. Mater. 2021, 10, 2100972. [Google Scholar] [CrossRef] [PubMed]
- Khalili, M.; Asadi, M.; Kahroba, H.; Soleyman, M.R.; Andre, H.; Alizadeh, E. Corneal endothelium tissue engineering: An evolution of signaling molecules, cells, and scaffolds toward 3D bioprinting and cell sheets. J. Cell. Physiol. 2021, 236, 3275–3303. [Google Scholar] [CrossRef] [PubMed]
- Klyce, S.D. 12. Endothelial pump and barrier function. Exp. Eye Res. 2020, 198, 108068. [Google Scholar] [CrossRef] [PubMed]
- Atalay, E.; Özalp, O.; Yıldırım, N. Advances in the diagnosis and treatment of keratoconus. Ther. Adv. Ophthalmol. 2021, 13, 25158414211012796. [Google Scholar] [CrossRef] [PubMed]
- Tafti, M.F.; Aghamollaei, H.; Moghaddam, M.M.; Jadidi, K.; Alio, J.L.; Faghihi, S. Emerging tissue engineering strategies for the corneal regeneration. J. Tissue Eng. Regen. Med. 2022, 16, 683–706. [Google Scholar] [CrossRef] [PubMed]
- Niazi, S.; Niknejad, H.; Niazi, F.; Doroodgar, F.; Sanginabadi, A. Tissue-engineered recombinant human collagen-based corneal substitutes in end-stage keratoconus. Investig. Ophthalmol. Vis. Sci. 2021, 60, 5105. [Google Scholar]
- Sharif, R.; Priyadarsini, S.; Rowsey, T.G.; Ma, J.-X.; Karamichos, D. Corneal tissue engineering: An in vitro model of the stromal-nerve interactions of the human cornea. JoVE J. Vis. Exp. 2021, 131, e56308. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharjee, P.; Ahearne, M. Significance of crosslinking approaches in the development of next generation hydrogels for corneal tissue engineering. Pharmaceutics 2021, 13, 319. [Google Scholar] [CrossRef]
- Qin, J.; Yin, N. Tobramycin Collagen Fast Dissolving Ocular Films for Corneal Tissue Engineering of Keratoconus. J. Biomater. Tissue Eng. 2021, 9, 804–809. [Google Scholar] [CrossRef]
- Alió del Barrio, J.L.; Alió, J.L. Cellular therapy of the corneal stroma: A new type of corneal surgery for keratoconus and corneal dystrophies. Eye Vis. 2021, 5, 1–10. [Google Scholar] [CrossRef]
- Jadidi, K.; Mosavi, S.A.; Nejat, F.; Aghamolaei, H.; Pirhadi, S. Innovative intra-corneal ring-supported graft surgery for treatment of keratoconus and cornea regeneration: Surgical technique and case report. Ind. J. Ophthalmol. 2022, 70, 3412–3415. [Google Scholar]
- Teo, A.W.J.; Mansoor, H.; Sim, N.; Lin, M.T.-Y.; Liu, Y.-C. In Vivo Confocal Microscopy Evaluation in Patients with Keratoconus. J. Clin. Med. 2022, 11, 393. [Google Scholar] [CrossRef]
- Veernala, I.; Jaffet, J.; Fried, J.; Mertsch, S.; Schrader, S.; Basu, S.; Vemuganti, G.; Singh, V. Lacrimal gland regeneration: The unmet challenges and promise for dry eye therapy. Ocul. Surf. 2022, 25, 129–141. [Google Scholar] [CrossRef]
- Gong, Q.; Zhang, S.; Jiang, L.; Lin, M.; Xu, Z.; Yu, Y.; Wang, Q.; Lu, F.; Hu, L. The effect of nerve growth factor on corneal nerve regeneration and dry eye after LASIK. Exp. Eye Res. 2021, 203, 108428. [Google Scholar] [CrossRef] [PubMed]
- Lu, Q.; Al-Sheikh, O.; Elisseeff, J.H.; Grant, M.P. Biomaterials and tissue engineering strategies for conjunctival reconstruction and dry eye treatment. Middle East Afr. J. Ophthalmol. 2015, 22, 428. [Google Scholar] [PubMed]
- Hill, L.J.; Moakes, R.J.; Vareechon, C.; Butt, G.; Ng, A.; Brock, K.; Chouhan, G.; Vincent, R.C.; Abbondante, S.; Williams, R.L. Sustained release of decorin to the surface of the eye enables scarless corneal regeneration. NPJ Regen. Med. 2021, 3, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Tabbara, K.F.; El-Asrar, A.M.A.; Khairallah, M. Ocular Infections; Springer: Berlin/Heidelberg, Germany, 2014. [Google Scholar]
- Xenoulis, P.G.; Steiner, J.M. Lipid metabolism and hyperlipidemia in dogs. Vet. J. 2010, 183, 12–21. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.; Wang, Y.; Zhang, H.; Gao, N.; Hu, A. Limbal allografting from living-related donors to treat partial limbal deficiency secondary to ocular chemical burns. Arch. Ophthalmol. 2011, 129, 1267–1273. [Google Scholar] [CrossRef] [PubMed]
- Wipperman, J.; Dorsch, J.N. Evaluation and management of corneal abrasions. Am. Fam. Physician 2013, 87, 114–120. [Google Scholar] [PubMed]
- Shahid, S.M.; Harrison, N. Corneal abrasion: Assessment and management. InnovAiT 2013, 6, 551–554. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, J.; Ji, Z.; Yan, W.; Zhao, H.; Huang, W.; Liu, H. Application of Bioprinting in Ophthalmology. Int. J. Bioprinting 2022, 8, 552. [Google Scholar] [CrossRef]
- Song, Y.; Hua, S.; Sayyar, S.; Chen, Z.; Chung, J.; Liu, X.; Yue, Z.; Angus, C.; Filippi, B.; Beirne, S. Corneal bioprinting using a high concentration pure collagen I transparent bioink. Bioprinting 2022, 28, e00235. [Google Scholar] [CrossRef]
- Stafiej, P.; Küng, F.; Thieme, D.; Czugala, M.; Kruse, F.E.; Schubert, D.W.; Fuchsluger, T.A. Adhesion and metabolic activity of human corneal cells on PCL based nanofiber matrices. Mater. Sci. Eng. C 2017, 71, 764–770. [Google Scholar] [CrossRef]
- Zhang, M.; Yang, F.; Han, D.; Zhang, S.Y.; Dong, Y.; Li, X.; Ling, L.; Deng, Z.; Cao, X.; Tian, J. 3D Bioprinting of Corneal Decellularized Extracellular Matrix (CECM): GelMA Composite Hydrogel for Corneal Stroma Engineering. 2022. Available online: https://ssrn.com/abstract=4246348 (accessed on 20 October 2022).
- Kim, J.I.; Kim, J.Y.; Park, C.H. Fabrication of transparent hemispherical 3D nanofibrous scaffolds with radially aligned patterns via a novel electrospinning method. Sci. Rep. 2021, 8, 3424. [Google Scholar] [CrossRef] [PubMed]
- Chameettachal, S.; Pati, F. Preparation and Characterization of Decellularized Corneal Matrix Hydrogel for Different Clinical Indications and 3D Bioprinting Applications; RAIITH: Kirkcaldy, UK, 2022. [Google Scholar]
- Lindsay, C.D.; Roth, J.G.; LeSavage, B.L.; Heilshorn, S.C. Bioprinting of stem cell expansion lattices. Acta Biomater. 2021, 95, 225–235. [Google Scholar] [CrossRef] [PubMed]
- Phamduy, T.B.; Sweat, R.S.; Azimi, M.S.; Burow, M.E.; Murfee, W.L.; Chrisey, D.B. Printing cancer cells into intact microvascular networks: A model for investigating cancer cell dynamics during angiogenesis. Integr. Biol. 2015, 7, 1068–1078. [Google Scholar] [CrossRef] [PubMed]
- Betsch, M.; Cristian, C.; Lin, Y.Y.; Blaeser, A.; Schöneberg, J.; Vogt, M.; Buhl, E.M.; Fischer, H.; Duarte Campos, D.F. Incorporating 4D into bioprinting: Real-time magnetically directed collagen fiber alignment for generating complex multilayered tissues. Adv. Healthc. Mater. 2021, 7, 1800894. [Google Scholar] [CrossRef] [PubMed]
- Leberfinger, A.N.; Dinda, S.; Wu, Y.; Koduru, S.V.; Ozbolat, V.; Ravnic, D.J.; Ozbolat, I.T. Bioprinting functional tissues. Acta Biomater. 2021, 95, 32–49. [Google Scholar] [CrossRef] [PubMed]
- Atala, A. Introduction: 3D Printing for Biomaterials; ACS Publications: Washington, DC, USA, 2020. [Google Scholar]
- Murphy, S.V.; De Coppi, P.; Atala, A. Opportunities and challenges of translational 3D bioprinting. Nat. Biomed. Eng. 2020, 4, 370–380. [Google Scholar] [CrossRef] [PubMed]
- Smith, B.T.; Bittner, S.M.; Watson, E.; Smoak, M.M.; Diaz-Gomez, L.; Molina, E.R.; Kim, Y.S.; Hudgins, C.D.; Melchiorri, A.J.; Scott, D.W. Multimaterial dual gradient three-dimensional printing for osteogenic differentiation and spatial segregation. Tissue Eng. Part A 2020, 26, 239–252. [Google Scholar] [CrossRef]
- Zhang, B.; Xue, Q.; Li, J.; Ma, L.; Yao, Y.; Ye, H.; Cui, Z.; Yang, H. 3D bioprinting for artificial cornea: Challenges and perspectives. Med. Eng. Phys. 2021, 71, 68–78. [Google Scholar] [CrossRef]
- Dubbin, K.; Tabet, A.; Heilshorn, S.C. Quantitative criteria to benchmark new and existing bio-inks for cell compatibility. Biofabrication 2017, 9, 044102. [Google Scholar] [CrossRef]
- Cidonio, G.; Glinka, M.; Dawson, J.; Oreffo, R. The cell in the ink: Improving biofabrication by printing stem cells for skeletal regenerative medicine. Biomaterials 2021, 209, 10–24. [Google Scholar] [CrossRef]
- Liu, W.; Heinrich, M.A.; Zhou, Y.; Akpek, A.; Hu, N.; Liu, X.; Guan, X.; Zhong, Z.; Jin, X.; Khademhosseini, A. Extrusion bioprinting of shear-thinning gelatin methacryloyl bioinks. Adv. Healthc. Mater. 2017, 6, 1601451. [Google Scholar] [CrossRef] [PubMed]
- Foster, A.A.; Marquardt, L.M.; Heilshorn, S.C. The diverse roles of hydrogel mechanics in injectable stem cell transplantation. Curr. Opin. Chem. Eng. 2017, 15, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Bernal, P.N.; Delrot, P.; Loterie, D.; Li, Y.; Malda, J.; Moser, C.; Levato, R. Volumetric bioprinting of complex living-tissue constructs within seconds. Adv. Mater. 2021, 31, 1904209. [Google Scholar] [CrossRef] [PubMed]
- Grigoryan, B.; Paulsen, S.J.; Corbett, D.C.; Sazer, D.W.; Fortin, C.L.; Zaita, A.J.; Greenfield, P.T.; Calafat, N.J.; Gounley, J.P.; Ta, A.H. Multivascular networks and functional intravascular topologies within biocompatible hydrogels. Science 2021, 364, 458–464. [Google Scholar] [CrossRef] [PubMed]
- Skylar-Scott, M.A.; Uzel, S.G.; Nam, L.L.; Ahrens, J.H.; Truby, R.L.; Damaraju, S.; Lewis, J.A. Biomanufacturing of organ-specific tissues with high cellular density and embedded vascular channels. Sci. Adv. 2021, 5, eaaw2459. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.; Hudson, A.; Shiwarski, D.; Tashman, J.; Hinton, T.; Yerneni, S.; Bliley, J.; Campbell, P.; Feinberg, A. 3D bioprinting of collagen to rebuild components of the human heart. Science 2021, 365, 482–487. [Google Scholar] [CrossRef] [PubMed]
- Meek, K.M.; Knupp, C. Corneal structure and transparency. Prog. Retin. Eye Res. 2015, 49, 1–16. [Google Scholar] [CrossRef]
- Mohan, R.R.; Kempuraj, D.; D’Souza, S.; Ghosh, A. Corneal stromal repair and regeneration. Prog. Retin. Eye Res. 2022, 91, 101090. [Google Scholar] [CrossRef]
- Gouveia, R.M.; Lepert, G.; Gupta, S.; Mohan, R.R.; Paterson, C.; Connon, C.J. Assessment of corneal substrate biomechanics and its effect on epithelial stem cell maintenance and differentiation. Nat. Commun. 2021, 10, 1496. [Google Scholar] [CrossRef]
- Sorkio, A.; Koch, L.; Koivusalo, L.; Deiwick, A.; Miettinen, S.; Chichkov, B.; Skottman, H. Human stem cell based corneal tissue mimicking structures using laser-assisted 3D bioprinting and functional bioinks. Biomaterials 2021, 171, 57–71. [Google Scholar] [CrossRef]
- Duarte Campos, D.F.; Rohde, M.; Ross, M.; Anvari, P.; Blaeser, A.; Vogt, M.; Panfil, C.; Yam, G.H.F.; Mehta, J.S.; Fischer, H. Corneal bioprinting utilizing collagen-based bioinks and primary human keratocytes. J. Biomed. Mater. Res. Part A 2021, 107, 1945–1953. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Su, X.; Xu, Y.; Kong, B.; Sun, W.; Mi, S. Bioprinting three-dimensional cell-laden tissue constructs with controllable degradation. Sci. Rep. 2016, 6, 24474. [Google Scholar] [CrossRef] [PubMed]
- Kutlehria, S.; Dinh, T.C.; Bagde, A.; Patel, N.; Gebeyehu, A.; Singh, M. High-throughput 3D bioprinting of corneal stromal equivalents. J. Biomed. Mater. Res. Part B Appl. Biomater. 2020, 108, 2981–2994. [Google Scholar] [CrossRef] [PubMed]
- Osidak, E.O.; Kozhukhov, V.I.; Osidak, M.S.; Domogatsky, S.P. Collagen as Bioink for Bioprinting: A Comprehensive Review. Int. J. Bioprinting 2020, 6, 270. [Google Scholar]
- Zhang, B.; Xue, Q.; Hu, H.-Y.; Yu, M.-F.; Gao, L.; Luo, Y.-C.; Li, Y.; Li, J.-T.; Ma, L.; Yao, Y.-F. Integrated 3D bioprinting-based geometry-control strategy for fabricating corneal substitutes. J. Zhejiang Univ. Sci. B 2021, 20, 945–959. [Google Scholar] [CrossRef]
- Bektas, C.K.; Hasirci, V. Cell loaded 3D bioprinted GelMA hydrogels for corneal stroma engineering. Biomater. Sci. 2020, 8, 438–449. [Google Scholar] [CrossRef]
- Kim, K.W.; Lee, S.J.; Park, S.H.; Kim, J.C. Ex vivo functionality of 3D bioprinted corneal endothelium engineered with ribonuclease 5-overexpressing human corneal endothelial cells. Adv. Healthc. Mater. 2021, 7, 1800398. [Google Scholar] [CrossRef]
- Kong, B.; Chen, Y.; Liu, R.; Liu, X.; Liu, C.; Shao, Z.; Xiong, L.; Liu, X.; Sun, W.; Mi, S. Fiber reinforced GelMA hydrogel to induce the regeneration of corneal stroma. Nat. Commun. 2020, 11, 1435. [Google Scholar] [CrossRef]
- Kim, H.; Park, M.-N.; Kim, J.; Jang, J.; Kim, H.-K.; Cho, D.-W. Characterization of cornea-specific bioink: High transparency, improved in vivo safety. J. Tissue Eng. 2021, 10, 2041731418823382. [Google Scholar] [CrossRef]
- Kim, H.; Jang, J.; Park, J.; Lee, K.-P.; Lee, S.; Lee, D.-M.; Kim, K.H.; Kim, H.K.; Cho, D.-W. Shear-induced alignment of collagen fibrils using 3D cell printing for corneal stroma tissue engineering. Biofabrication 2021, 11, 035017. [Google Scholar] [CrossRef]
- Gouveia, R.M.; Koudouna, E.; Jester, J.; Figueiredo, F.; Connon, C.J. Template curvature influences cell alignment to create improved human corneal tissue equivalents. Adv. Biosyst. 2017, 1, 1700135. [Google Scholar] [CrossRef] [PubMed]
- Trujillo-de Santiago, G.; Sharifi, R.; Yue, K.; Sani, E.S.; Kashaf, S.S.; Alvarez, M.M.; Leijten, J.; Khademhosseini, A.; Dana, R.; Annabi, N. Ocular adhesives: Design, chemistry, crosslinking mechanisms, and applications. Biomaterials 2021, 197, 345–367. [Google Scholar] [CrossRef] [PubMed]
- Koppa Raghu, P.; Bansal, K.K.; Thakor, P.; Bhavana, V.; Madan, J.; Rosenholm, J.M.; Mehra, N.K. Evolution of nanotechnology in delivering drugs to eyes, skin, and wounds via topical route. Pharmaceuticals 2020, 13, 167. [Google Scholar] [CrossRef] [PubMed]
- Patra, H.K.; Azharuddin, M.; Islam, M.M.; Papapavlou, G.; Deb, S.; Osterrieth, J.; Zhu, G.H.; Romu, T.; Dhara, A.K.; Jafari, M.J.; et al. Rational Nanotoolbox with Theranostic Potential for Medicated Pro-Regenerative Corneal Implants. Adv. Funct. Mater. 2021, 29, 1903760. [Google Scholar] [CrossRef]
- Krishna, L.; Dhamodaran, K.; Jayadev, C.; Chatterjee, K.; Shetty, R.; Khora, S.S.; Das, D. Nanostructured scaffold as a determinant of stem cell fate. Stem Cell Res. Ther. 2016, 7, 188. [Google Scholar] [CrossRef]
- Motealleh, A.; Kehr, N.S. Nanocomposite hydrogels and their applications in tissue engineering. Adv. Healthc. Mater. 2017, 6, 1600938. [Google Scholar] [CrossRef] [PubMed]
- Tayebi, T.; Baradaran-Rafii, A.; Hajifathali, A.; Rahimpour, A.; Zali, H.; Shaabani, A.; Niknejad, H. Biofabrication of chitosan/chitosan nanoparticles/polycaprolactone transparent membrane for corneal endothelial tissue engineering. Sci. Rep. 2021, 11, 7060. [Google Scholar] [CrossRef]
- Chang, M.-C.; Kuo, Y.-J.; Hung, K.-H.; Peng, C.-L.; Chen, K.-Y.; Yeh, L.-K. Liposomal dexamethasone–moxifloxacin nanoparticle combinations with collagen/gelatin/alginate hydrogel for corneal infection treatment and wound healing. Biomed. Mater. 2020, 15, 055022. [Google Scholar] [CrossRef]
- Anitua, E.; Muruzabal, F.J.; De La Fuente, M.; Merayo, J.; Durán, J.; Orive, G. Plasma rich in growth factors for the treatment of ocular surface diseases. Curr. Eye Res. 2016, 41, 875–882. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).