Changes in Disposition of Ezetimibe and Its Active Metabolites Induced by Impaired Hepatic Function: The Influence of Enzyme and Transporter Activities
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Construction of CCl4-Induced Hepatic Failure Rat Model
2.3. Biochemistry and Histopathologic Study
2.4. Pharmacokinetic Experiments
2.5. Liver S9 Fraction Isolation
2.6. Human and Rat Liver S9 Fraction Incubation
2.7. Hepatocyte Isolation and Transporter-Mediated Uptake Assays
2.8. Liver Slices
2.9. Cell Culture and Uptake Studies Using Transporter-Expressing HEK293 Cells
2.10. mRNA Analysis
2.11. Transport Studies with Human MDR1-, MRP2-, and MRP3-Expressing Membrane Vesicles
2.12. Determination of EZE, EZE-Ph, and EZE-Hy
2.13. Data Analysis
3. Results
3.1. Biochemistry Parameters and Histopathologic Sections of Control and CCl4-Induced Rats
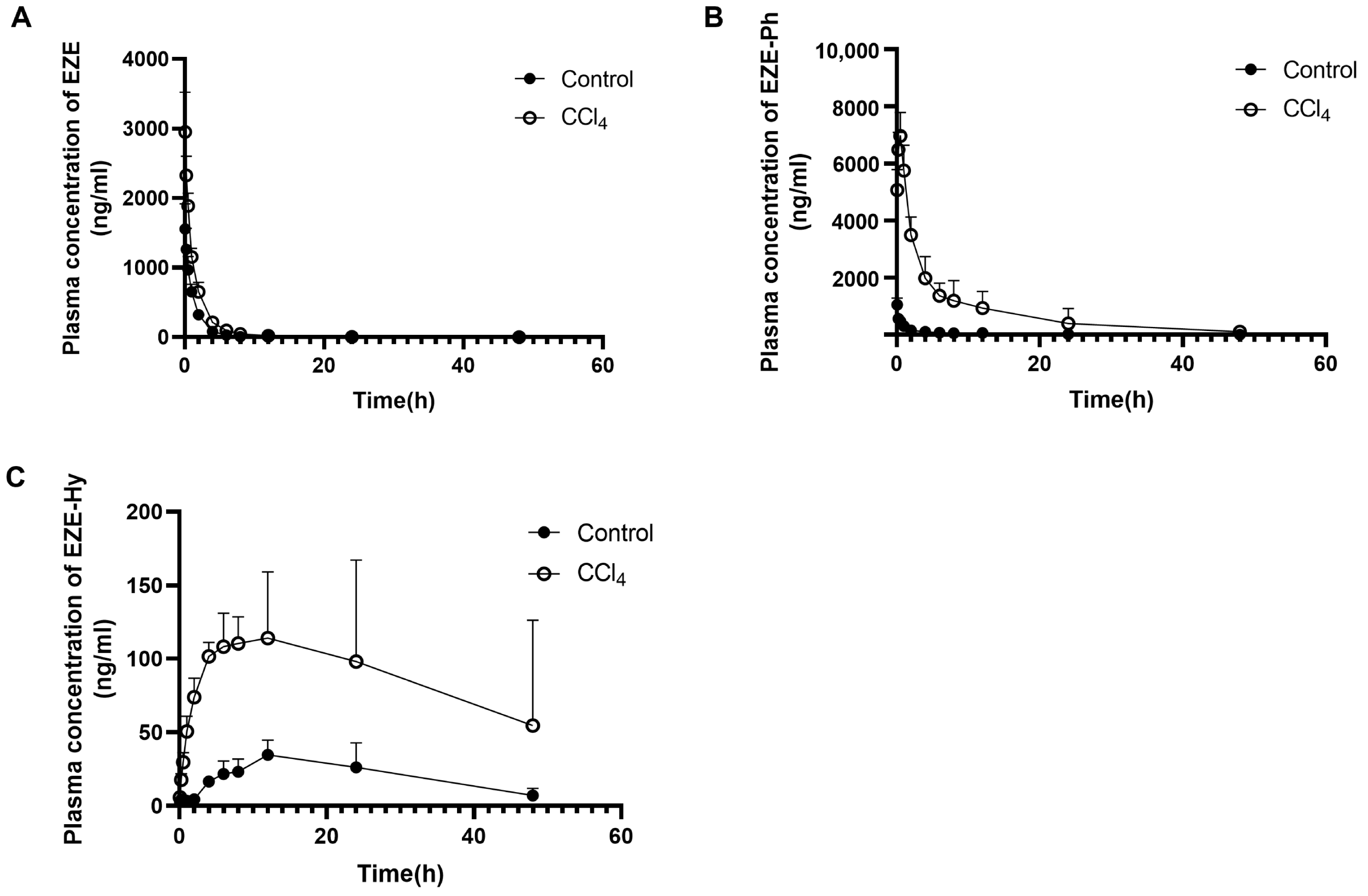
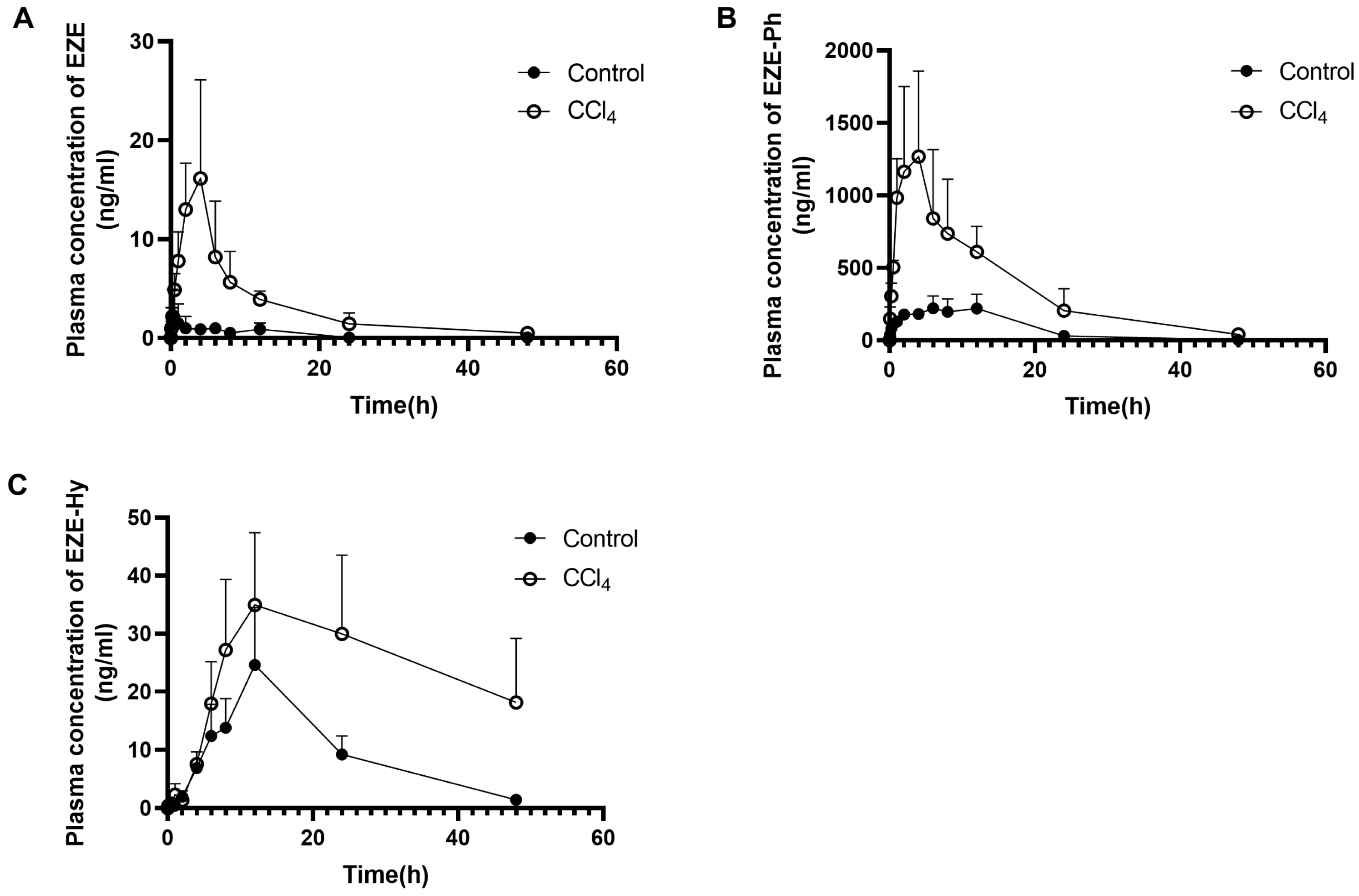
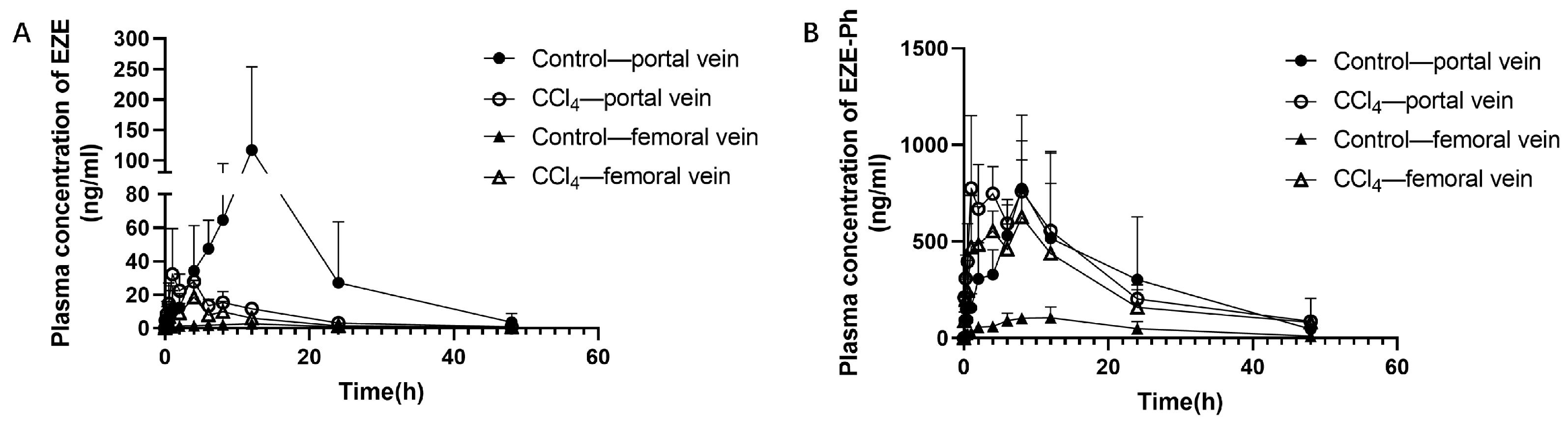
3.2. Pharmacokinetic Study
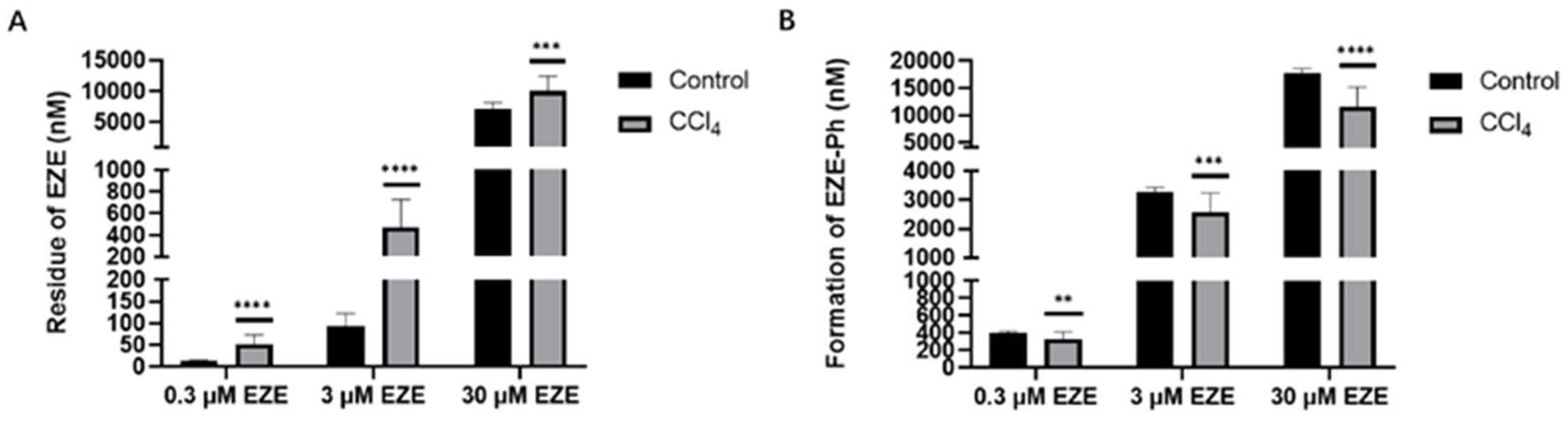
3.3. Incubation of EZE with Rat Liver S9 Fractions and Inhibition of EZE Glucuronidation by Bile Acids
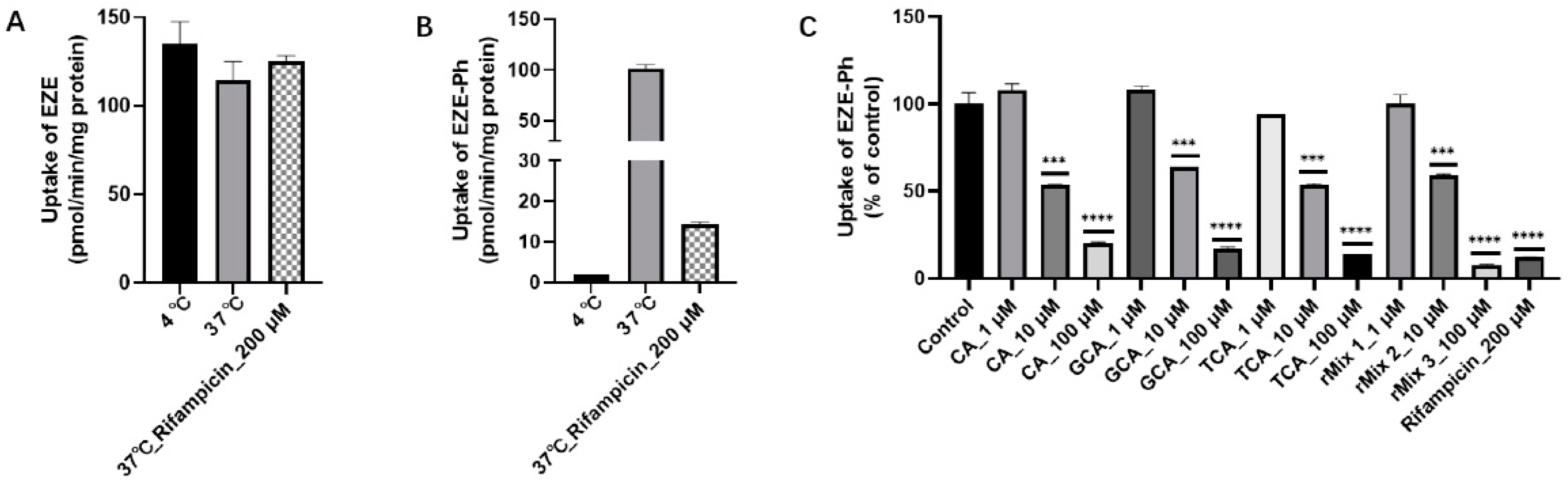
3.4. Rat Hepatocyte Uptake Studies and Inhibition of EZE-Ph Uptake by Bile Acids
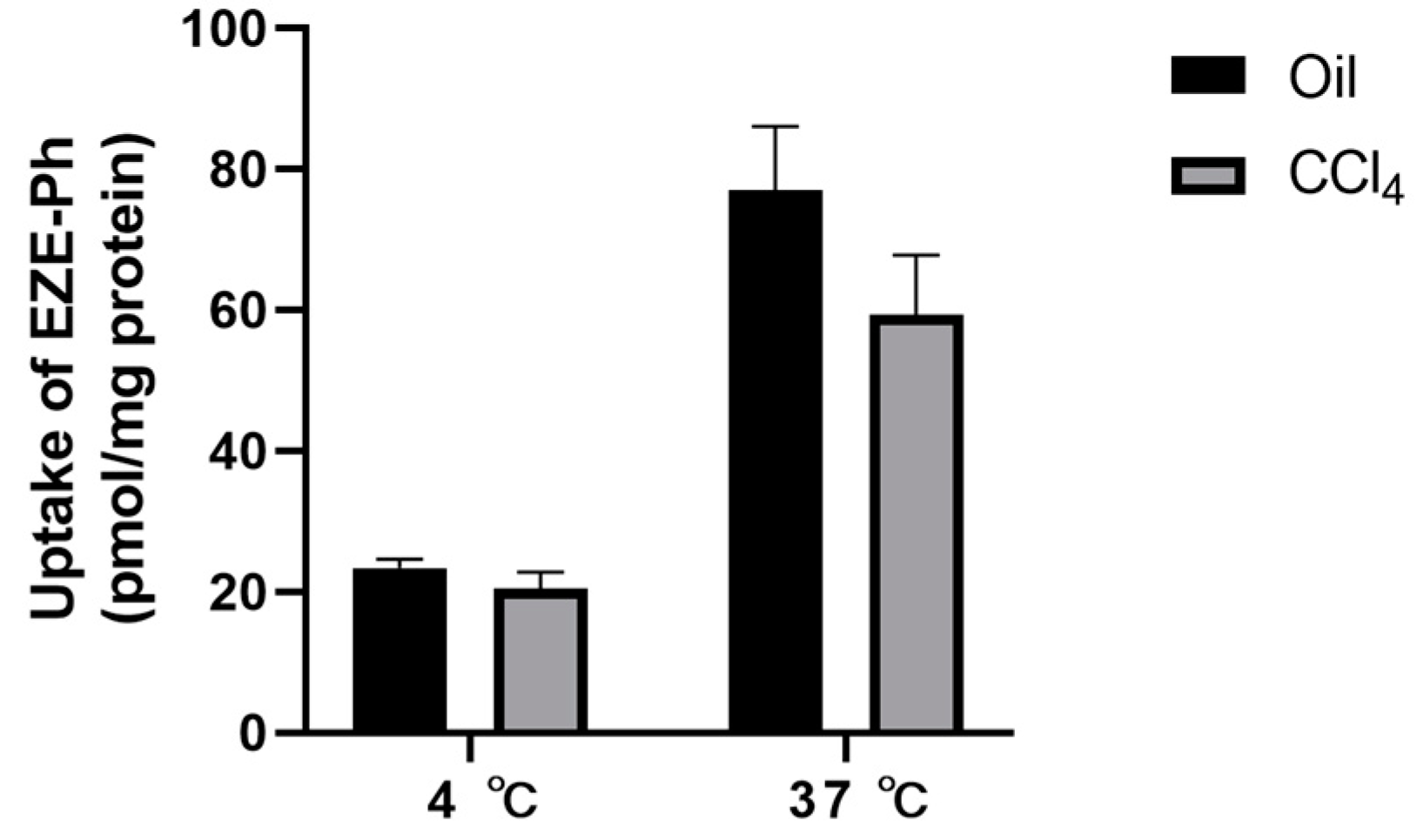
3.5. Uptake of EZE-Ph in Liver Slices
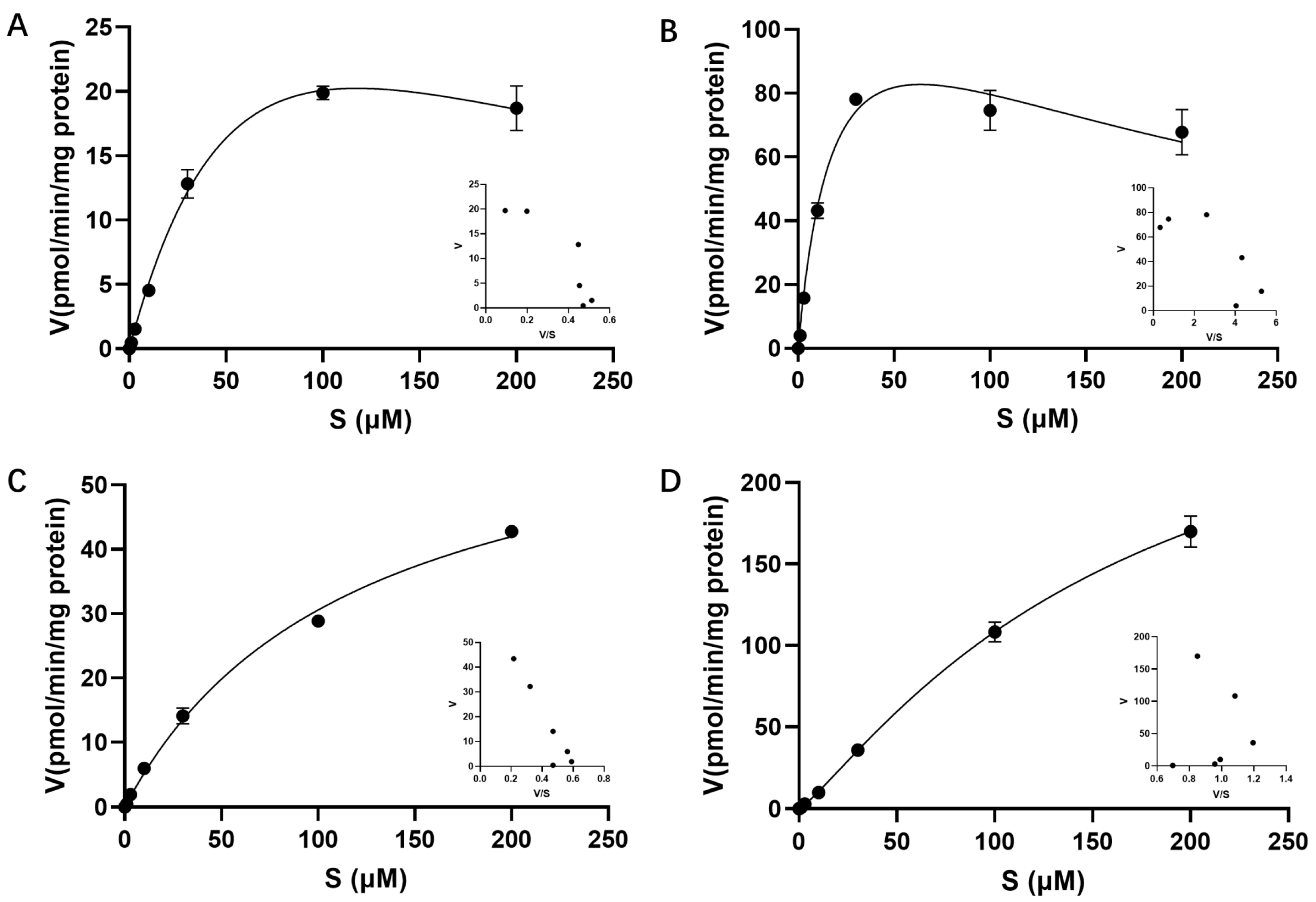
3.6. Uptake of EZE-Ph into Transfected HEK293 Cells
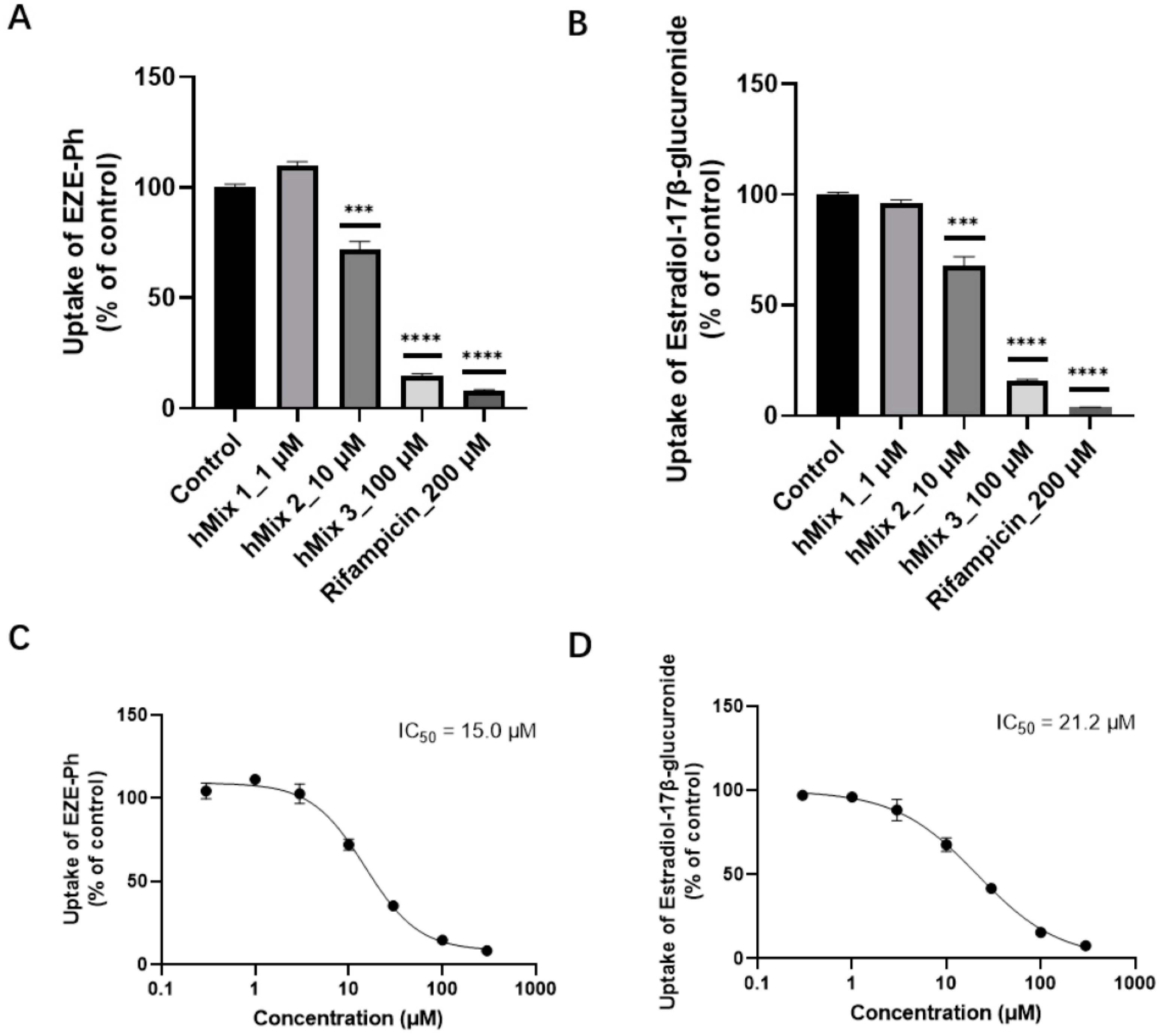
3.7. Inhibition of Bile Acids for the Uptake of EZE-Ph in OATP1B3-Transfected HEK293 Cells
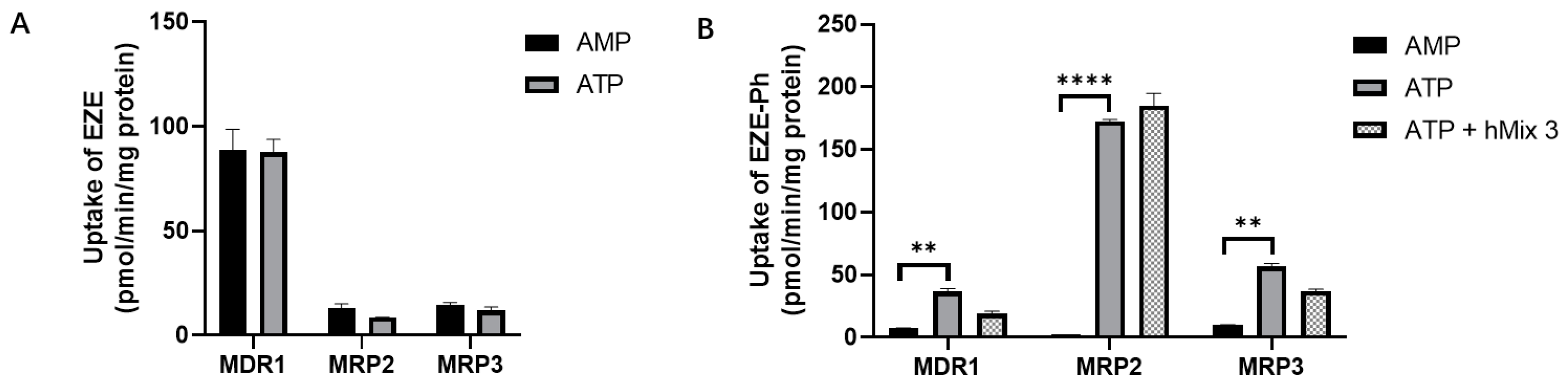
3.8. Efflux Transport Studies with Membrane Vesicles
3.9. Intestinal and Hepatic Gene Expression of Efflux Transporters in Rats
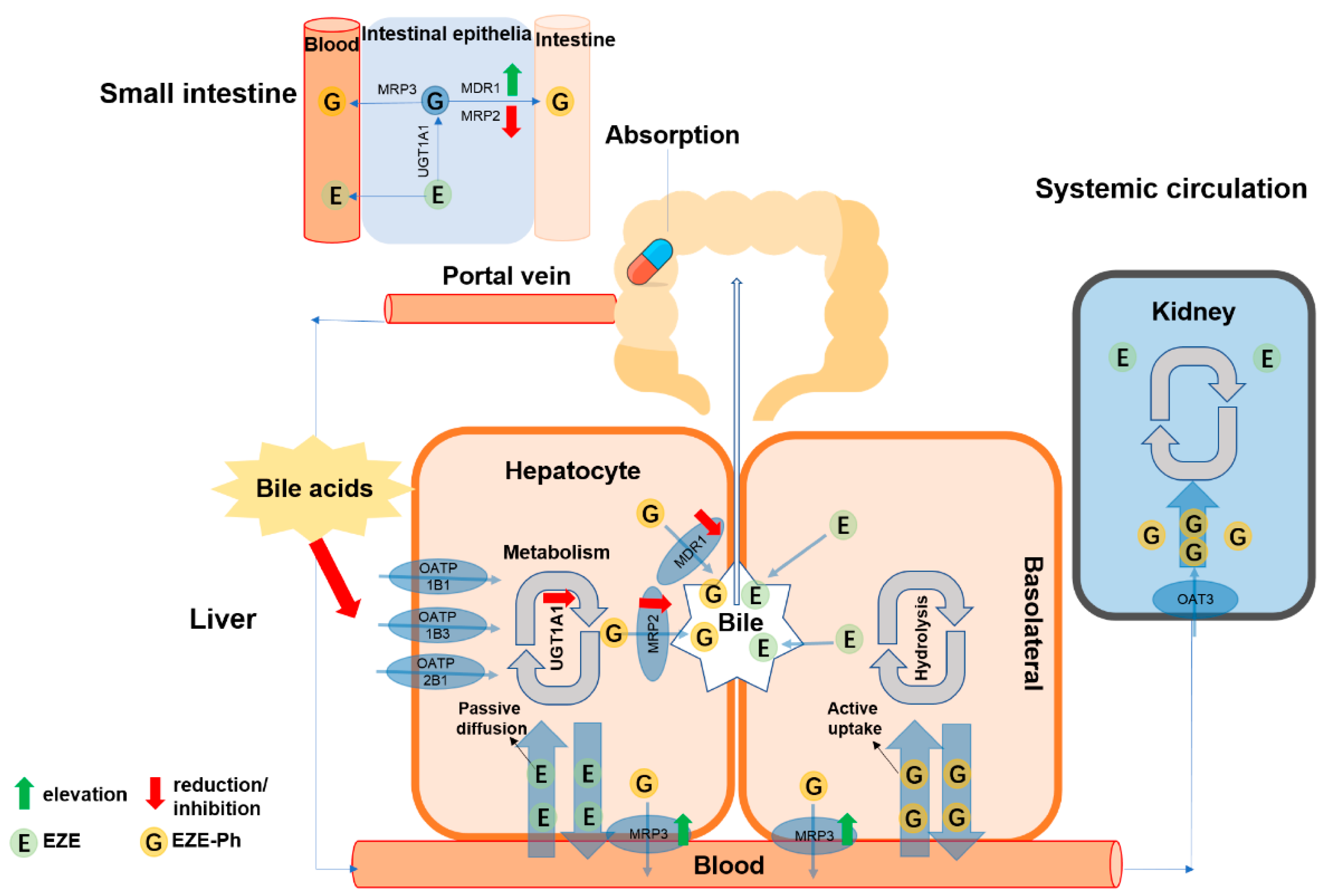
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Roser, H.R.a.M. Causes of Death. 2018. Available online: https://ourworldindata.org/causes-of-death (accessed on 1 December 2019).
- Kulik, L.; El-Serag, H.B. Epidemiology and Management of Hepatocellular Carcinoma. Gastroenterology 2019, 156, 477–491.e471. [Google Scholar] [CrossRef] [PubMed]
- Massarweh, N.N.; El-Serag, H.B. Epidemiology of Hepatocellular Carcinoma and Intrahepatic Cholangiocarcinoma. Cancer Control 2017, 24, 1073274817729245. [Google Scholar] [CrossRef]
- Pellicoro, A.; Ramachandran, P.; Iredale, J.P.; Fallowfield, J.A. Liver fibrosis and repair: Immune regulation of wound healing in a solid organ. Nat. Rev. Immunol. 2014, 14, 181–194. [Google Scholar] [CrossRef] [PubMed]
- Sherman, M.; Klein, A. AASLD single-topic research conference on hepatocellular carcinoma: Conference proceedings. Hepatology 2004, 40, 1465–1473. [Google Scholar] [CrossRef]
- Nasser, A.F.; Heidbreder, C.; Liu, Y.; Fudala, P.J. Pharmacokinetics of Sublingual Buprenorphine and Naloxone in Subjects with Mild to Severe Hepatic Impairment (Child-Pugh Classes A, B, and C), in Hepatitis C Virus-Seropositive Subjects, and in Healthy Volunteers. Clin. Pharm. 2015, 54, 837–849. [Google Scholar] [CrossRef] [PubMed]
- Zgheib, N.K.; Branch, R.A. Drug metabolism and liver disease: A drug-gene-environment interaction. Drug Metab. Rev. 2017, 49, 35–55. [Google Scholar] [CrossRef]
- Fisher, C.D.; Lickteig, A.J.; Augustine, L.M.; Ranger-Moore, J.; Jackson, J.P.; Ferguson, S.S.; Cherrington, N.J. Hepatic cytochrome P450 enzyme alterations in humans with progressive stages of nonalcoholic fatty liver disease. Drug Metab. Dispos. 2009, 37, 2087–2094. [Google Scholar] [CrossRef]
- Frye, R.F.; Zgheib, N.K.; Matzke, G.R.; Chaves-Gnecco, D.; Rabinovitz, M.; Shaikh, O.S.; Branch, R.A. Liver disease selectively modulates cytochrome P450—mediated metabolism. Clin. Pharmacol. Ther. 2006, 80, 235–245. [Google Scholar] [CrossRef]
- Okushin, K.; Tsutsumi, T.; Enooku, K.; Fujinaga, H.; Kado, A.; Shibahara, J.; Fukayama, M.; Moriya, K.; Yotsuyanagi, H.; Koike, K. The intrahepatic expression levels of bile acid transporters are inversely correlated with the histological progression of nonalcoholic fatty liver disease. J. Gastroenterol. 2016, 51, 808–818. [Google Scholar] [CrossRef]
- Ramachandran, P.; Iredale, J.P. Reversibility of liver fibrosis. Ann. Hepatol. 2009, 8, 283–291. [Google Scholar] [CrossRef]
- Yamamura, Y.; Kotaki, H.; Tanaka, N.; Aikawa, T.; Sawada, Y.; Iga, T. The pharmacokinetics of glycyrrhizin and its restorative effect on hepatic function in patients with chronic hepatitis and in chronically carbon-tetrachloride-intoxicated rats. Biopharm. Drug Dispos. 1997, 18, 717–725. [Google Scholar] [CrossRef]
- Piotrowska-Kempisty, H.; Nowicki, M.; Jodynis-Liebert, J.; Kurpik, M.; Ewertowska, M.; Adamska, T.; Oszmianski, J.; Kujawska, M. Assessment of Hepatoprotective Effect of Chokeberry Juice in Rats Treated Chronically with Carbon Tetrachloride. Molecules 2020, 25, 1268. [Google Scholar] [CrossRef] [PubMed]
- Ravichandra, A.; Schwabe, R.F. Mouse Models of Liver Fibrosis. Methods Mol. Biol. 2021, 2299, 339–356. [Google Scholar] [CrossRef] [PubMed]
- Sugiyama, T.; Nagata, J.; Yamagishi, A.; Endoh, K.; Saito, M.; Yamada, K.; Yamada, S.; Umegaki, K. Selective protection of curcumin against carbon tetrachloride-induced inactivation of hepatic cytochrome P450 isozymes in rats. Life Sci. 2006, 78, 2188–2193. [Google Scholar] [CrossRef] [PubMed]
- Song, I.-S.; Lee, Y.-M.; Chung, S.-J.; Shim, C.-K. Multiple alterations of canalicular membrane transport activities in rats with CCl(4)-induced hepatic injury. Drug Metab. Dispos. 2003, 31, 482–490. [Google Scholar] [CrossRef]
- Khemawoot, P.; Maruyama, C.; Tsukada, H.; Noda, H.; Ishizaki, J.; Yokogawa, K.; Miyamoto, K. Influence of chronic hepatic failure on disposition kinetics of valproate excretion through a phase II reaction in rats treated with carbon tetrachloride. Biopharm. Drug Dispos. 2007, 28, 331–338. [Google Scholar] [CrossRef]
- Taguchi, K.; Miyasato, M.; Watanabe, H.; Sakai, H.; Tsuchida, E.; Horinouchi, H.; Kobayashi, K.; Maruyama, T.; Otagiri, M. Alteration in the pharmacokinetics of hemoglobin-vesicles in a rat model of chronic liver cirrhosis is associated with Kupffer cell phagocyte activity. J. Pharm. Sci. 2011, 100, 775–783. [Google Scholar] [CrossRef]
- Golder, M.R.; Liu, J.; Andersen, J.N.; Shipitsin, M.V.; Vohidov, F.; Nguyen, H.V.; Ehrlich, D.C.; Huh, S.J.; Vangamudi, B.; Economides, K.D.; et al. Reduction of liver fibrosis by rationally designed macromolecular telmisartan prodrugs. Nat. Biomed. Eng. 2018, 2, 822–830. [Google Scholar] [CrossRef]
- Yuan, Z.W.; Li, Y.Z.; Liu, Z.Q.; Feng, S.L.; Zhou, H.; Liu, C.X.; Liu, L.; Xie, Y. Role of tangeretin as a potential bioavailability enhancer for silybin: Pharmacokinetic and pharmacological studies. Pharmacol. Res. 2018, 128, 153–166. [Google Scholar] [CrossRef]
- Luo, L.; Aubrecht, J.; Li, D.; Warner, R.L.; Johnson, K.J.; Kenny, J.; Colangelo, J.L. Assessment of serum bile acid profiles as biomarkers of liver injury and liver disease in humans. PLoS ONE 2018, 13, e0193824. [Google Scholar] [CrossRef]
- Barre, L.; Fournel-Gigleux, S.; Finel, M.; Netter, P.; Magdalou, J.; Ouzzine, M. Substrate specificity of the human UDP-glucuronosyltransferase UGT2B4 and UGT2B7. Identification of a critical aromatic amino acid residue at position 33. FEBS J. 2007, 274, 1256–1264. [Google Scholar] [CrossRef] [PubMed]
- Gall, W.E.; Zawada, G.; Mojarrabi, B.; Tephly, T.R.; Green, M.D.; Coffman, B.L.; Mackenzie, P.I.; Radominska-Pandya, A. Differential glucuronidation of bile acids, androgens and estrogens by human UGT1A3 and 2B7. J. Steroid Biochem. Mol. Biol. 1999, 70, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Trauner, M.; Boyer, J.L. Bile salt transporters: Molecular characterization, function, and regulation. Physiol. Rev. 2003, 83, 633–671. [Google Scholar] [CrossRef] [PubMed]
- Choudhuri, S.; Klaassen, C.D. Molecular Regulation of Bile Acid Homeostasis. Drug Metab. Dispos. 2022, 50, 425–455. [Google Scholar] [CrossRef]
- Garcia-Calvo, M.; Lisnock, J.M.; Bull, H.G.; Hawes, B.E.; Burnett, D.A.; Braun, M.P.; Crona, J.H.; Davis, H.R., Jr.; Dean, D.C.; Detmers, P.A.; et al. The target of ezetimibe is Niemann-Pick C1-Like 1 (NPC1L1). Proc. Natl. Acad. Sci. USA 2005, 102, 8132–8137. [Google Scholar] [CrossRef] [PubMed]
- Ghosal, A.; Hapangama, N.; Yuan, Y.; Achanfuo-Yeboah, J.; Iannucci, R.; Chowdhury, S.; Alton, K.; Patrick, J.E.; Zbaida, S. Identification of human UDP-glucuronosyltransferase enzyme(s) responsible for the glucuronidation of ezetimibe (Zetia). Drug Metab. Dispos. 2004, 32, 314–320. [Google Scholar] [CrossRef] [PubMed]
- Van Heek, M.; Farley, C.; Compton, D.S.; Hoos, L.; Alton, K.B.; Sybertz, E.J.; Davis, H.R., Jr. Comparison of the activity and disposition of the novel cholesterol absorption inhibitor, SCH58235, and its glucuronide, SCH60663. Br. J. Pharmacol. 2000, 129, 1748–1754. [Google Scholar] [CrossRef]
- Patrick, J.E.; Kosoglou, T.; Stauber, K.L.; Alton, K.B.; Maxwell, S.E.; Zhu, Y.; Statkevich, P.; Iannucci, R.; Chowdhury, S.; Affrime, M.; et al. Disposition of the Selective Cholesterol Absorption Inhibitor Ezetimibe in Healthy Male Subjects. Drug Metab. Dispos. 2002, 30, 430–437. [Google Scholar] [CrossRef]
- Oswald, S.; König, J.; Lütjohann, D.; Giessmann, T.; Kroemer, H.K.; Rimmbach, C.; Rosskopf, D.; Fromm, M.F.; Siegmund, W. Disposition of ezetimibe is influenced by polymorphisms of the hepatic uptake carrier OATP1B1. Pharm. Genom. 2008, 18, 559–568. [Google Scholar] [CrossRef]
- Oswald, S.; Haenisch, S.; Fricke, C.; Sudhop, T.; Remmler, C.; Giessmann, T.; Jedlitschky, G.; Adam, U.; Dazert, E.; Warzok, R.; et al. Intestinal expression of P-glycoprotein (ABCB1), multidrug resistance associated protein 2 (ABCC2), and uridine diphosphate-glucuronosyltransferase 1A1 predicts the disposition and modulates the effects of the cholesterol absorption inhibitor ezetimibe in humans. Clin. Pharmacol. Ther. 2006, 79, 206–217. [Google Scholar] [CrossRef]
- Zetia Clinical Pharmacology Biopharmaceutic Review. 2002. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2002/21445_Zetia_biopharmr_P2.pdf (accessed on 25 October 2002).
- Hardwick, R.N.; Fisher, C.D.; Street, S.M.; Canet, M.J.; Cherrington, N.J. Molecular mechanism of altered ezetimibe disposition in nonalcoholic steatohepatitis. Drug Metab. Dispos. 2012, 40, 450–460. [Google Scholar] [CrossRef] [PubMed]
- Berry, M.N.; Friend, D.S. High-yield preparation of isolated rat liver parenchymal cells: A biochemical and fine structural study. J. Cell. Biol. 1969, 43, 506–520. [Google Scholar] [CrossRef] [PubMed]
- Obatomi, D.; Brant, S.; Anthonypillai, V.; Early, D.; Bach, P. Optimizing preincubation conditions for precision-cut rat kidney and liver tissue slices: Effect of culture media and antioxidants. Toxicol. In Vitro 1998, 12, 725–737. [Google Scholar] [CrossRef]
- Rio, D.C.; Ares, M., Jr.; Hannon, G.J.; Nilsen, T.W. Purification of RNA using TRIzol (TRI reagent). Cold Spring Harb. Protoc. 2010, 2010, pdb.prot5439. [Google Scholar] [CrossRef]
- Yang, L.; Xiong, A.; He, Y.; Wang, Z.; Wang, C.; Wang, Z.; Li, W.; Yang, L.; Hu, Z. Bile acids metabonomic study on the CCl4- and alpha-naphthylisothiocyanate-induced animal models: Quantitative analysis of 22 bile acids by ultraperformance liquid chromatography-mass spectrometry. Chem. Res. Toxicol. 2008, 21, 2280–2288. [Google Scholar] [CrossRef] [PubMed]
- Bai, C.-l.; Canfield, P.J.; Stacey, N.H. Individual serum bile acids as early indicators of carbon tetrachloride- and chloroform-induced liver injury. Toxicology 1992, 75, 221–234. [Google Scholar] [PubMed]
- Wang, R.; Xiong, A.-Z.; Teng, Z.-Q.; Yang, Q.-W.; Shi, Y.-H.; Yang, L. Radix Paeoniae Rubra and Radix Paeoniae Alba Attenuate CCl4-Induced Acute Liver Injury: An Ultra-Performance Liquid Chromatography-Mass Spectrometry (UPLC-MS) Based Metabolomic Approach for the Pharmacodynamic Study of Traditional Chinese Medicines (TCMs). Int. J. Mol. Sci. 2012, 13, 14634–14647. [Google Scholar] [CrossRef]
- Sang, C.; Wang, X.; Zhou, K.; Sun, T.; Bian, H.; Gao, X.; Wang, Y.; Zhang, H.; Jia, W.; Liu, P.; et al. Bile Acid Profiles Are Distinct among Patients with Different Etiologies of Chronic Liver Disease. J. Proteome Res. 2021, 20, 2340–2351. [Google Scholar] [CrossRef]
- Schneider, H.; Fiander, H.; Latta, R.K.; Ross, N.W. Bile acid inhibition of xenobiotic-metabolizing enzymes is a factor in the mechanism of colon carcinogenesis: Tests of aspects of the concept with glucuronosyltransferase. Eur. J. Cancer Prev. 1993, 2, 393–400. [Google Scholar] [CrossRef]
- Hagenbuch, B.; Meier, P.J. Organic anion transporting polypeptides of the OATP/SLC21 family: Phylogenetic classification as OATP/SLCO superfamily, new nomenclature and molecular/functional properties. Pflug. Arch. 2004, 447, 653–665. [Google Scholar] [CrossRef]
- De Waart, D.R.; Vlaming, M.L.; Kunne, C.; Schinkel, A.H.; Oude Elferink, R.P. Complex pharmacokinetic behavior of ezetimibe depends on abcc2, abcc3, and abcg2. Drug Metab. Dispos. 2009, 37, 1698–1702. [Google Scholar] [CrossRef] [PubMed]

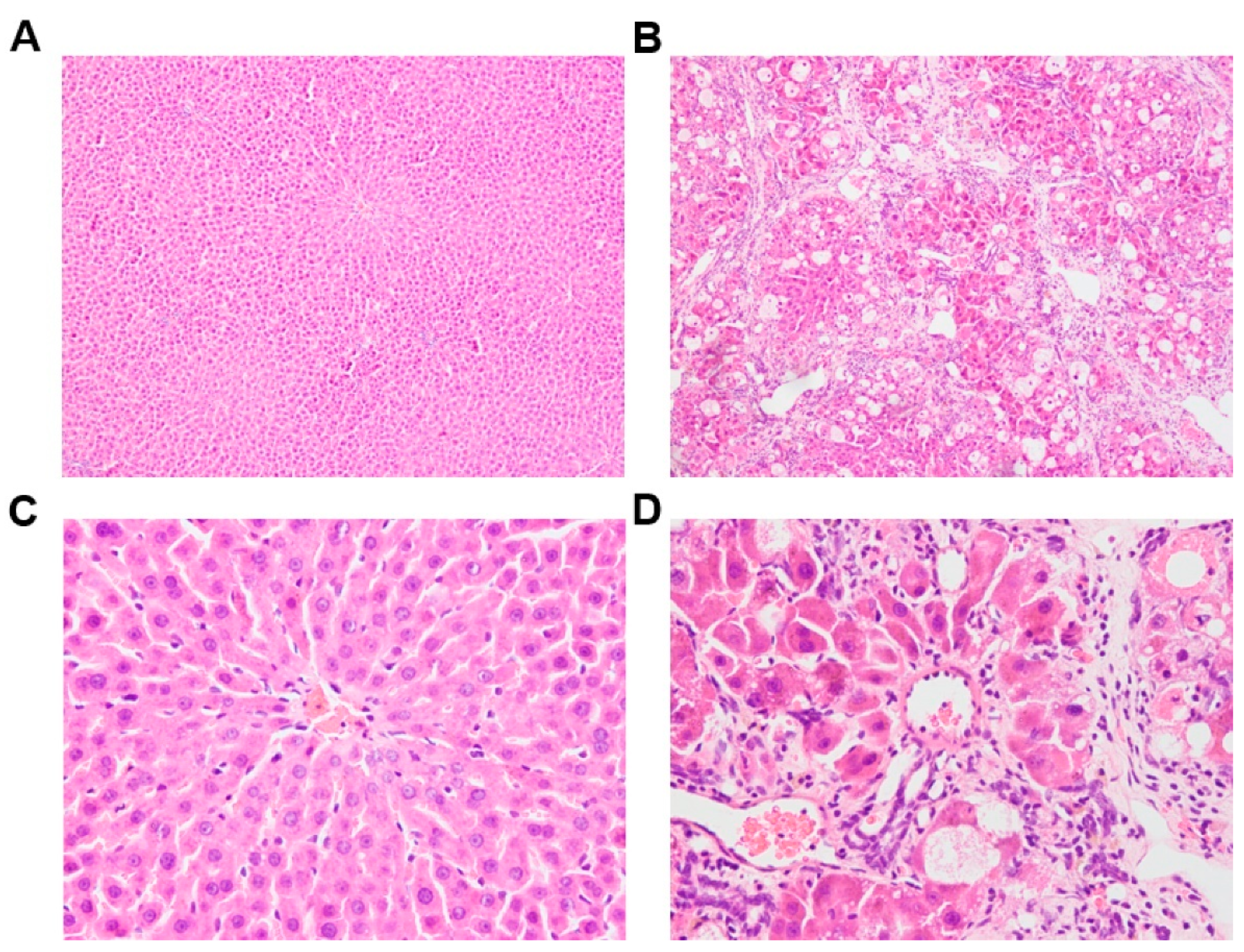


| Parameter | Control Rats | CCl4-Induced Rats |
|---|---|---|
| Liver weight/body weight | 31.1 ± 3.4 | 37.9 ± 5.9 c |
| ALT (U/L) | 13.0 ± 5.4 | 112 ± 33 d |
| AST (U/L) | 21.7 ± 6.2 | 125 ± 34 d |
| AKP (U/L) | 2562 ± 674 | 9887 ± 1633 d |
| Route of Administration | Group | Pharmacokinetic Parameters | EZE | EZE-Ph | EZE-Hy |
|---|---|---|---|---|---|
| i.v. | Control rats | Cmax (ng/mL) | 1558 ± 361 * | 1047 ± 240 | 36.9 ± 9.6 |
| tmax (h) | - | 0.083 ± 0.00 | 16.8 ± 6.6 | ||
| t1/2 (h) | 6.76 ± 3.31 | 8.96 ± 2.19 | 16.1 ± 7.6 | ||
| AUC0–t (ng·h/mL) | 2181 ± 304 | 2467 ± 418 | 992 ± 392 | ||
| CCl4-induced rats | Cmax (ng/mL) | 2952 ± 571 *,b | 6972 ± 807 d | 146 ± 42 c | |
| tmax (h) | - | 0.45 ± 0.11 b | 11.2 ± 7.6 | ||
| t1/2 (h) | 7.67 ± 3.08 | 9.58 ± 6.20 | 15.3 ± 2.4 | ||
| AUC0–t (ng·h/mL) | 4612 ± 781 c | 40450 ± 1810 b | 4247 ± 2051 b | ||
| p.o. | Control rats | Cmax (ng/mL) | 2.63 ± 2.68 | 276 ± 69 | 24.6 ± 9.9 |
| tmax (h) | 4.95 ± 4.84 | 8.80 ± 4.60 | 12.0 ± 0.0 | ||
| t1/2 (h) | 5.56 ± 3.78 | 5.50 ± 0.36 | - | ||
| AUC0–t (ng·h/mL) | 17.0 ± 10.0 | 4174 ± 1140 | 463 ± 160 | ||
| CCl4-induced rats | Cmax (ng/mL) | 20.4 ± 8.1 b | 1342 ± 585 b | 38.1 ± 10.1 | |
| tmax (h) | 5.20 ± 3.90 | 3.00 ± 1.41 | 14.4 ± 5.4 | ||
| t1/2 (h) | 12.3 ± 5.3 | 8.98 ± 2.10 | - | ||
| AUC0–t (ng·h/mL) | 189 ± 66 c | 18255 ± 6993 b | 1172 ± 418 b |
| Transporter | Kinetic Model | Km (μM) | Vmax (pmol/min/mg Protein) | CLint (μL/min/mg Protein) | h |
|---|---|---|---|---|---|
| OATP1B1 | Substrate inhibition | 91.5 | 51.7 | 0.56 | |
| OATP1B3 | Substrate inhibition | 19.9 | 134 | 6.73 | |
| OATP2B1 | Michaelis–Menten | 119 | 66.9 | 0.56 | |
| OAT3 | Allosteric sigmoidal | 166 | 306 | 1.84 | 1.19 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xie, N.; Wang, H.; Qin, H.; Guo, Z.; Xue, H.; Hu, J.; Chen, X. Changes in Disposition of Ezetimibe and Its Active Metabolites Induced by Impaired Hepatic Function: The Influence of Enzyme and Transporter Activities. Pharmaceutics 2022, 14, 2743. https://doi.org/10.3390/pharmaceutics14122743
Xie N, Wang H, Qin H, Guo Z, Xue H, Hu J, Chen X. Changes in Disposition of Ezetimibe and Its Active Metabolites Induced by Impaired Hepatic Function: The Influence of Enzyme and Transporter Activities. Pharmaceutics. 2022; 14(12):2743. https://doi.org/10.3390/pharmaceutics14122743
Chicago/Turabian StyleXie, Ningjie, Hong Wang, Hua Qin, Zitao Guo, Hao Xue, Jiafeng Hu, and Xiaoyan Chen. 2022. "Changes in Disposition of Ezetimibe and Its Active Metabolites Induced by Impaired Hepatic Function: The Influence of Enzyme and Transporter Activities" Pharmaceutics 14, no. 12: 2743. https://doi.org/10.3390/pharmaceutics14122743
APA StyleXie, N., Wang, H., Qin, H., Guo, Z., Xue, H., Hu, J., & Chen, X. (2022). Changes in Disposition of Ezetimibe and Its Active Metabolites Induced by Impaired Hepatic Function: The Influence of Enzyme and Transporter Activities. Pharmaceutics, 14(12), 2743. https://doi.org/10.3390/pharmaceutics14122743




