A Promising Ultra-Small Unilamellar Carrier System for Enhanced Skin Delivery of α-Mangostin as an Anti-Age-Spot Serum
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Experimental Design
2.3. USUC Base Formula Optimization
Preparation of α-Mangostin USUC
2.4. Characterization of a α-Mangostin-Loaded USUC
2.4.1. Physicochemical Properties
2.4.2. Determination of Encapsulation Efficiency (EE)
2.4.3. Microscopic Analysis via Transmission Electron Microscope (TEM)
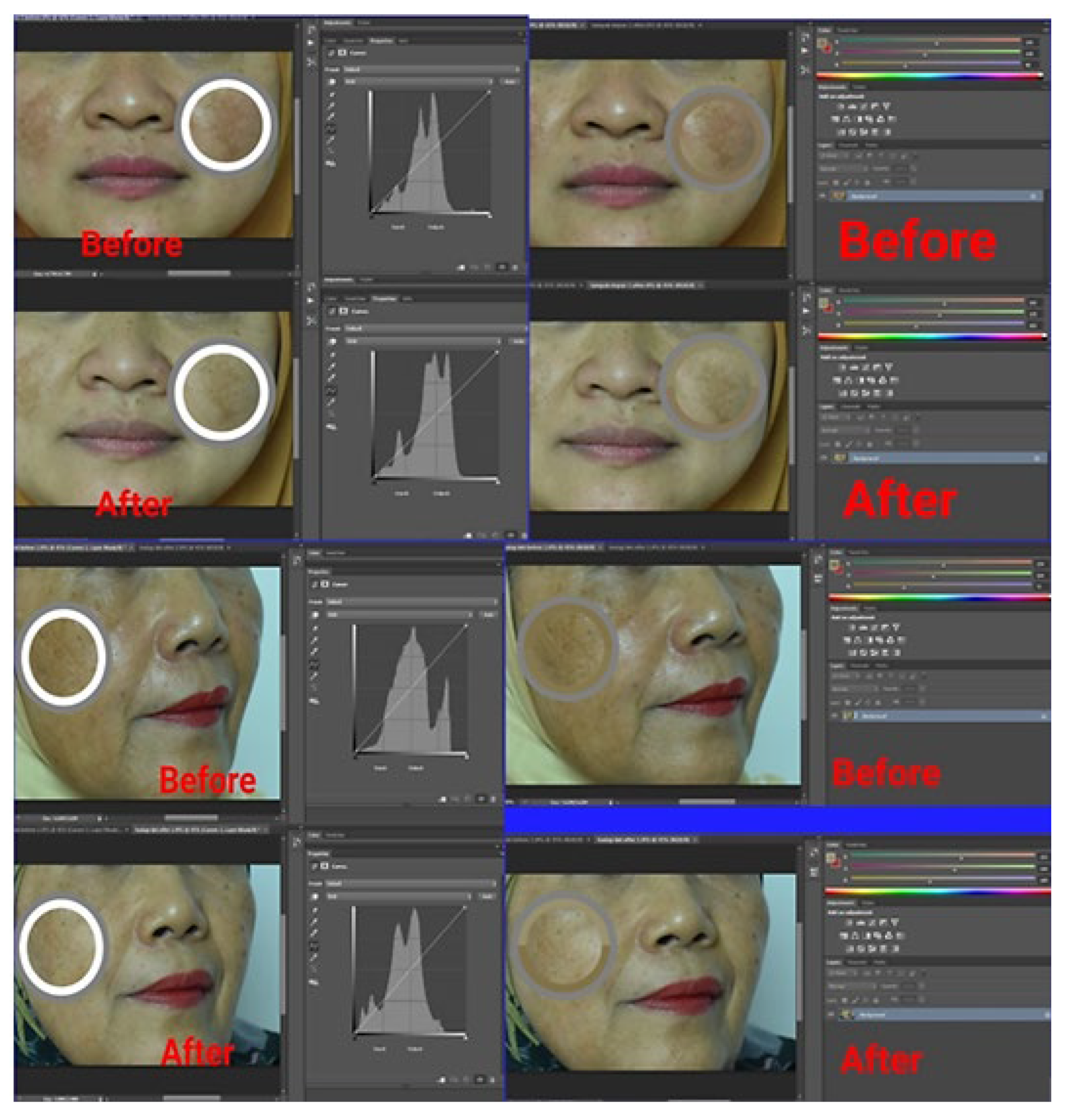
2.4.4. Visual Evaluation of an α-Mangostin-Loaded USUC in Human Volunteers
2.5. Statistical Analysis
3. Results
3.1. Base Formula Optimization
3.2. Response (Y): Effect of Independent Variables on Particle Size
9514.47 X1X4 − 11,365.72 X1X5 + 5081.28 X2X3 + 5741.47 X2X4 + 17,683.78 X2X5 + 17,052.72 X3X4 + 2258.53 X3X5 +
3296.47 X4X5 + 4112.72 X1X2X3 + 10,829.28 X1X2X4 + 10,746.97 X1X2X5 − 2854.22 X1X3X4 + 2572.47 X1X3X5 −
117.22 X1X4X5 + 15,366.03 X2X3X4 + 2897.72 X2X3X5 + 2009.78 X2X4X5 − 1123.09 X3X4X5 − 4539.53 X1X2X3X4 +
3196.28 X1X2X3X5 − 1439.03 X1X2X4X5 + 3396.59 X1X3X4X5 + 591.84 X2X3X4X5 + 5084.66 X1X2X3X4X5
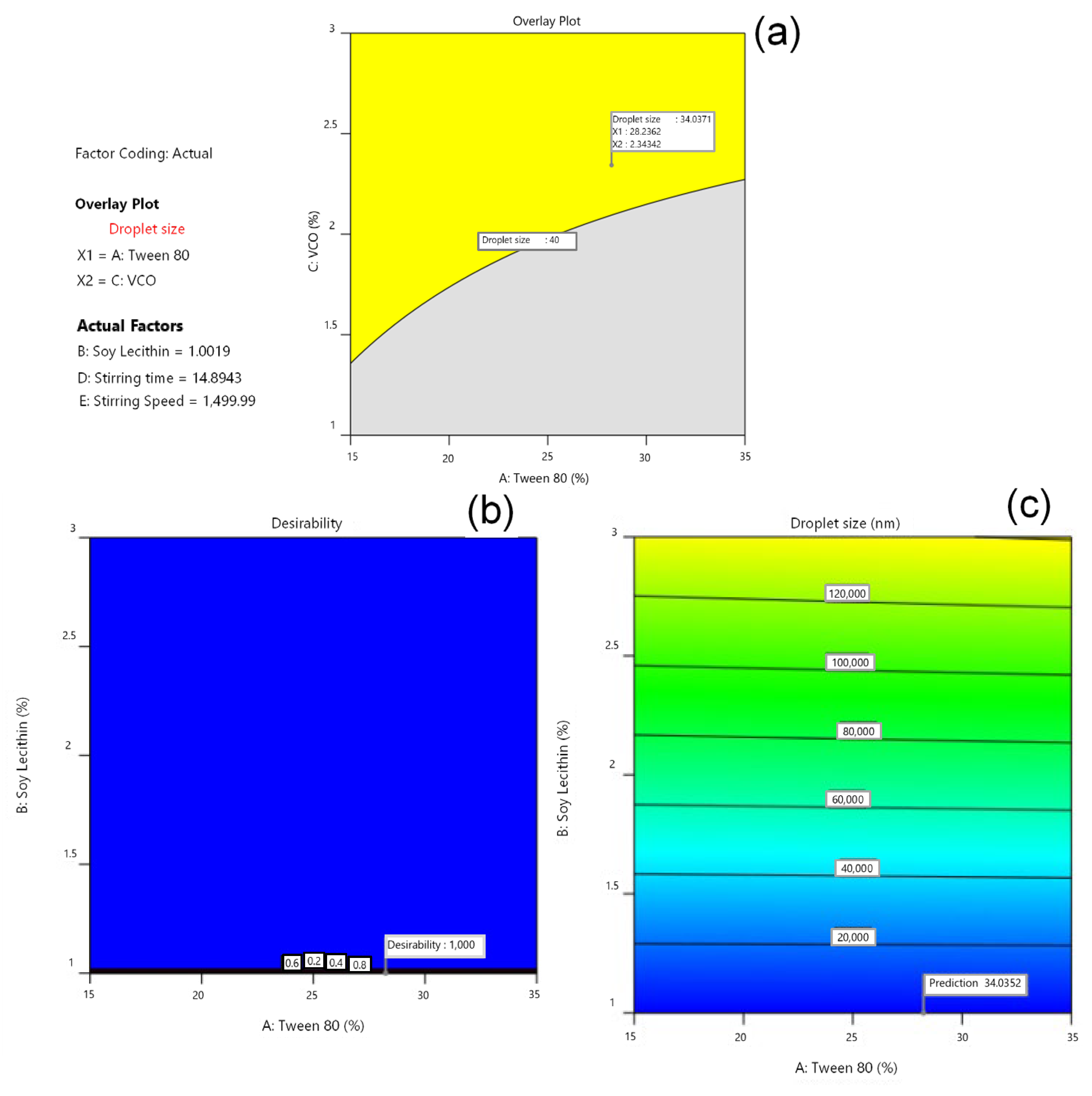
3.3. Determination of Optimal Formula by Software Design Expert®
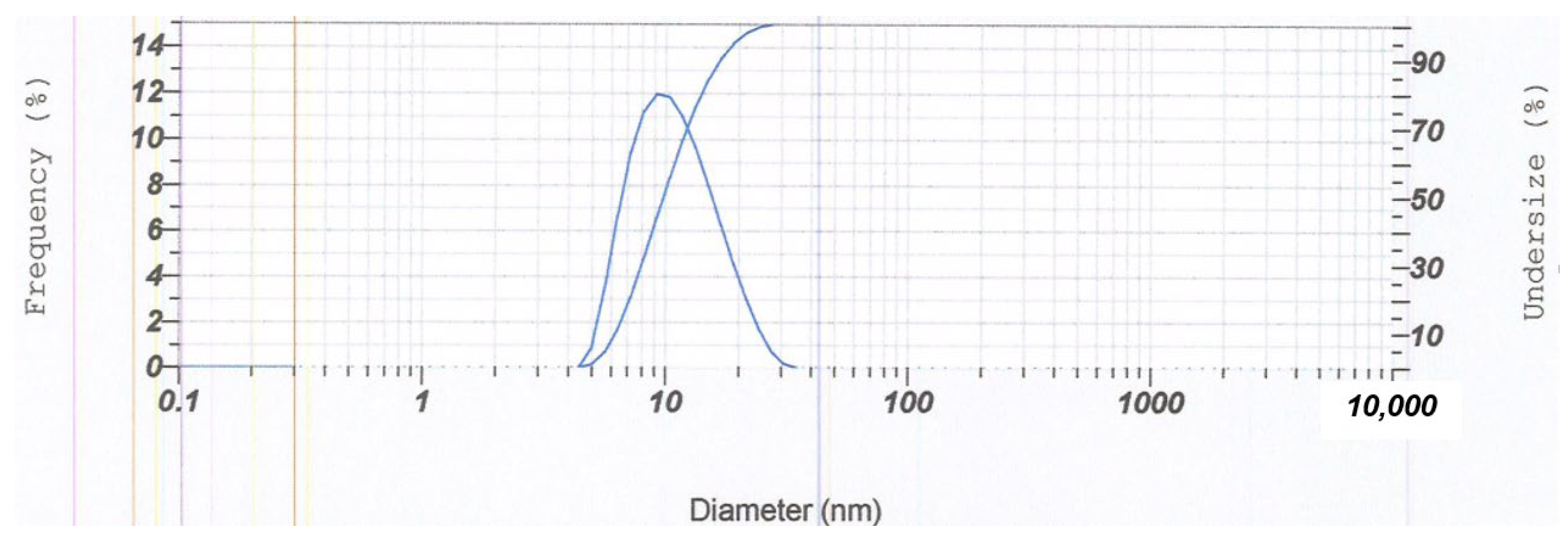
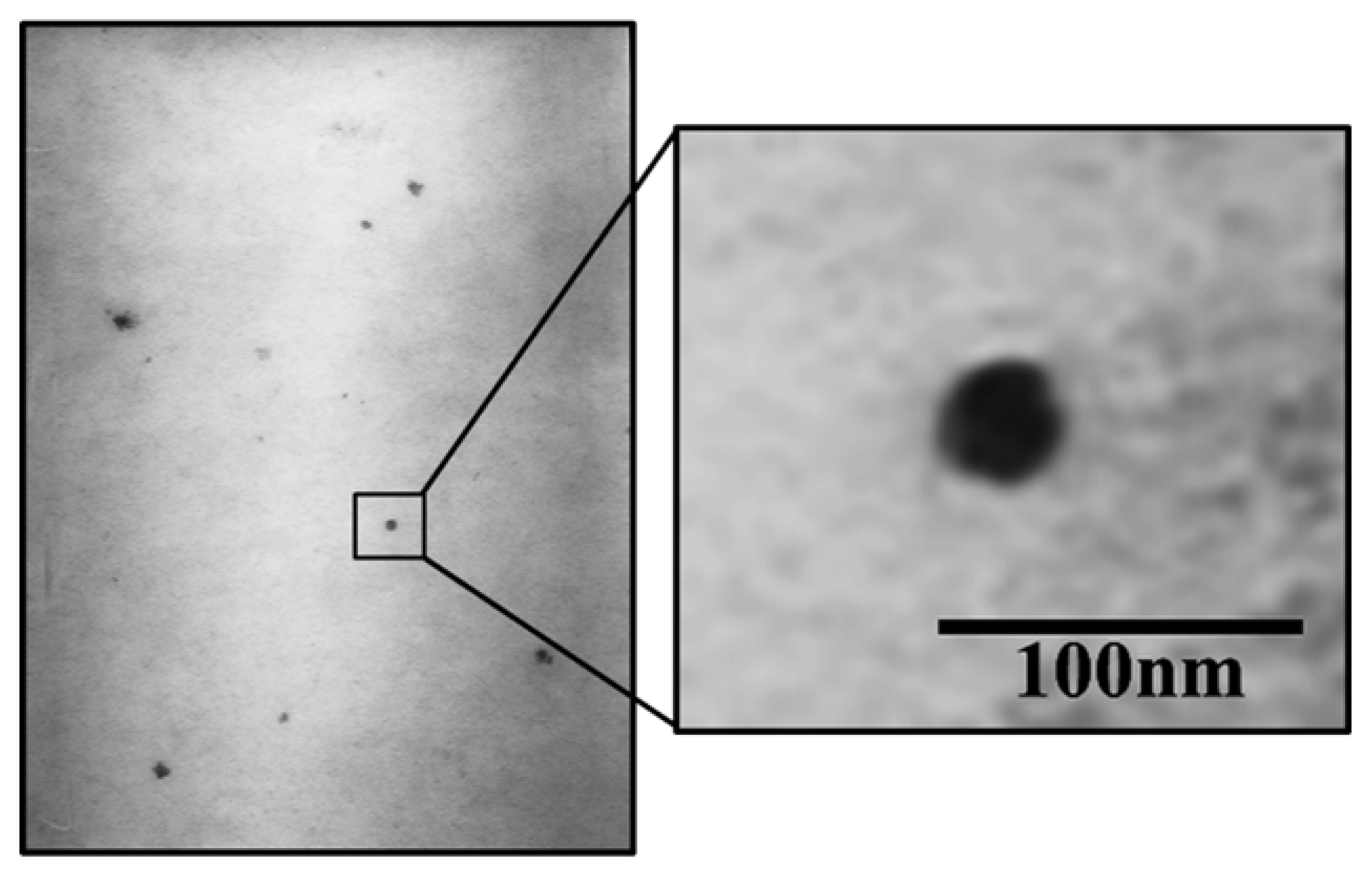
3.4. Characterization of the Optimum Base USUC Formula
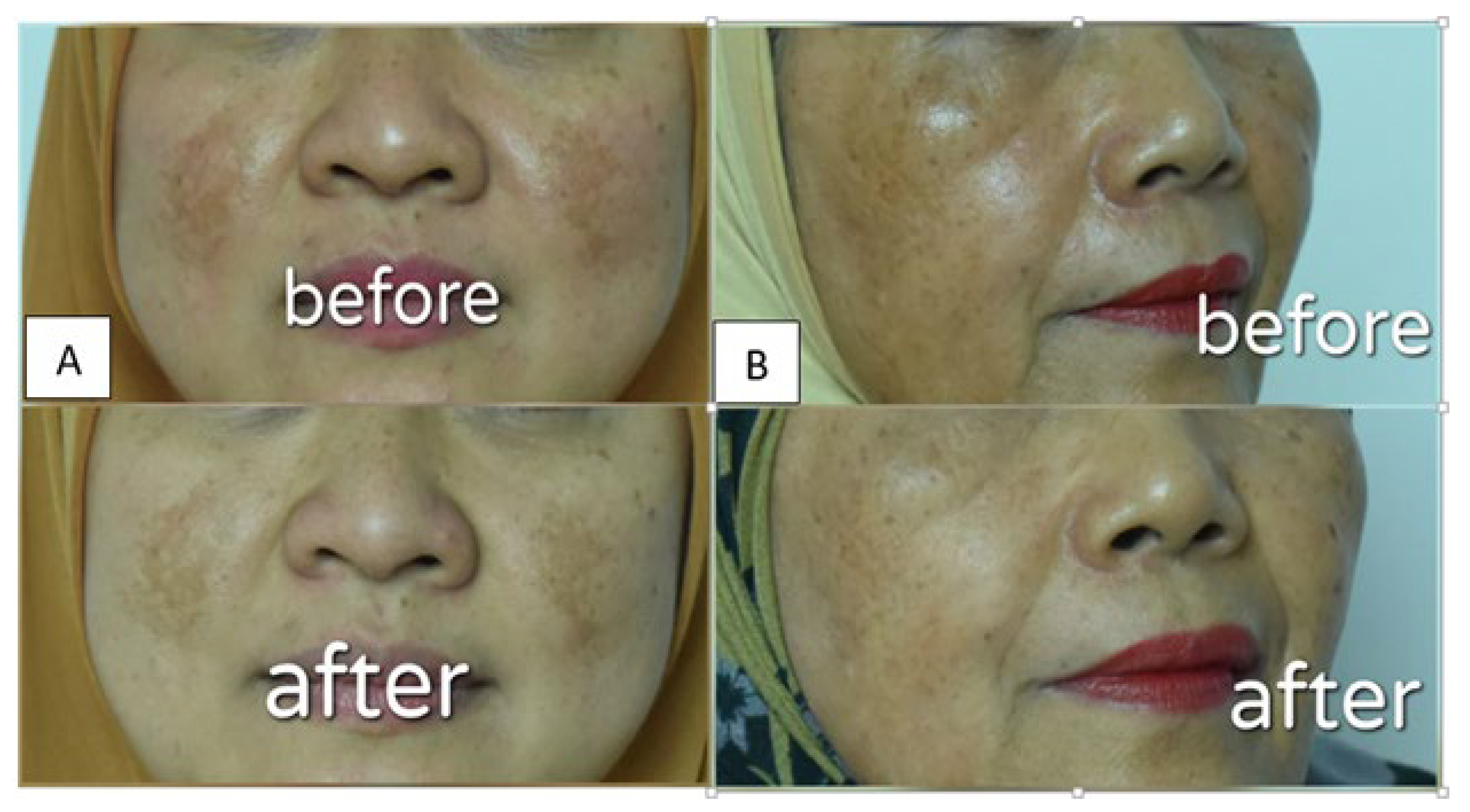
3.5. Visual Evaluation of α-Mangostin-Loaded USUCs in Human Volunteers
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Roberts, M.S.; Cheruvu, H.S.; Mangion, S.E.; Alinaghi, A.; Benson, H.A.E.; Mohammed, Y.; Holmes, A.; van der Hoek, J.; Pastore, M.; Grice, J.E. Topical Drug Delivery: History, Percutaneous Absorption, and Product Development. Adv. Drug Deliv. Rev. 2021, 177, 113929. [Google Scholar] [CrossRef] [PubMed]
- Ramírez-Gamboa, D.; Díaz-Zamorano, A.L.; Meléndez-Sánchez, E.R.; Reyes-Pardo, H.; Villaseñor-Zepeda, K.R.; López-Arellanes, M.E.; Sosa-Hernández, J.E.; Coronado-Apodaca, K.G.; Gámez-Méndez, A.; Afewerki, S.; et al. Photolyase Production and Current Applications: A Review. Molecules 2022, 27, 5998. [Google Scholar] [CrossRef] [PubMed]
- Montenegro, L. Nanocarriers for Skin Delivery of Cosmetic Antioxidants. J. Pharm. Pharmacogn. Res. 2014, 2, 73–92. [Google Scholar]
- Mu, L.; Sprando, R.L. Application of Nanotechnology in Cosmetics. Pharm. Res. 2010, 27, 1746–1749. [Google Scholar] [CrossRef]
- Nastiti, C.M.R.R.; Ponto, T.; Mohammed, Y.; Roberts, M.S.; Benson, H.A.E. Novel Nanocarriers for Targeted Topical Skin Delivery of the Antioxidant Resveratrol. Pharmaceutics 2020, 12, 108. [Google Scholar] [CrossRef]
- Roberts, M.S.; Mohammed, Y.; Pastore, M.N.; Namjoshi, S.; Yousef, S.; Alinaghi, A.; Haridass, I.N.; Abd, E.; Leite-Silva, V.R.; Benson, H.A.E. Topical and Cutaneous Delivery Using Nanosystems. J. Control. Release 2017, 247, 86–105. [Google Scholar] [CrossRef]
- Nastiti, C.M.R.R.; Ponto, T.; Abd, E.; Grice, J.E.; Benson, H.A.E.; Roberts, M.S. Topical Nano and Microemulsions for Skin Delivery. Pharmaceutics 2017, 9, 37. [Google Scholar] [CrossRef] [PubMed]
- Baschong, W.; Herzog, B.; Artmann, C.W.; Mendrok, C.; Mongiat, S.; Lupia, J.A. NanotopesTM: A Novel Ultra-Small Unilamellar Carrier System for Cosmetic Actives; William Andrew Inc.: Norwich, NY, USA, 2005; ISBN 9780815516828. [Google Scholar]
- Georg, H.; Ag, V. Verwendung von Nanodispersionen in kosmetischen Endformulierungen (Use of Nanotopes in Cosmetic Products). European Patent EP 0 956 851 B1, 1 2006. Available online: https://patents.google.com/patent/EP0956851B1/en (accessed on 1 December 2022).
- Zolghadri, S.; Bahrami, A.; Hassan Khan, M.T.; Munoz-Munoz, J.; Garcia-Molina, F.; Garcia-Canovas, F.; Saboury, A.A. A Comprehensive Review on Tyrosinase Inhibitors. J. Enzyme Inhib. Med. Chem. 2019, 34, 279–309. [Google Scholar] [CrossRef]
- Zhou, S.; Yotsumoto, H.; Tian, Y.; Sakamoto, K. α-Mangostin Suppressed Melanogenesis in B16F10 Murine Melanoma Cells through GSK3β and ERK Signaling Pathway. Biochem. Biophys. Rep. 2021, 26, 100949. [Google Scholar] [CrossRef]
- Ganesan, P.; Choi, D.K. Current Application of Phytocompound-Based Nanocosmeceuticals for Beauty and Skin Therapy. Int. J. Nanomed. 2016, 11, 1987–2007. [Google Scholar] [CrossRef]
- Chen, Z.L.; Huang, M.; Wang, X.R.; Fu, J.; Han, M.; Shen, Y.Q.; Xia, Z.; Gao, J.Q. Transferrin-Modified Liposome Promotes α-Mangostin to Penetrate the Blood-Brain Barrier. Nanomed. Nanotechnol. Biol. Med. 2016, 12, 421–430. [Google Scholar] [CrossRef] [PubMed]
- Limphapayom, W.; Loylerd, K.; Leabwan, N.; Sukhasem, S. Encapsulation of Alpha-Mangostin in Cosmetic Production by Using Nanotechnology. Acta Hortic. 2017, 1186, 189–191. [Google Scholar] [CrossRef]
- Chin, G.S.; Todo, H.; Kadhum, W.R.; Hamid, M.A.; Sugibayashi, K. In Vitro Permeation and Skin Retention of α-Mangostin Proniosome. Chem. Pharm. Bull. 2016, 64, 1666–1673. [Google Scholar] [CrossRef][Green Version]
- Barel, A.H.; Paye, M.; Maibach, H.I. Handbook of Cosmetic Science and Technology; Informa Healthcare: New York, NY, USA, 2018; ISBN 0824702921. [Google Scholar]
- Hidayat, I.R.; Zuhrotun, A.; Sopyan, I. Design-Expert Software Sebagai Alat Optimasi Formulasi Sediaan Farmasi. Maj. Farmasetika 2020, 6, 99–120. [Google Scholar] [CrossRef]
- Bolton, S.; Bon, C. Pharmaceutical Statistics: Practical and Clinical Applications, 4th ed.; Revised and Expanded; Marcel Dekker: New York, NY, USA, 2004; ISBN 9780203912799. [Google Scholar]
- Khanam, N.; Alam, M.I.; Md Yusuf Ali, Q.M.A.I.; Siddiqui, A.U.R. A Review on Optimization of Drug Delivery System with Experimental Designs. Int. J. Appl. Pharm. 2018, 10, 7–12. [Google Scholar] [CrossRef]
- Damayanti, H.; Wikarsa, S.; Jafar, G. Formulasi Nanoemulgel Ekstrak Kulit Manggis (Garcinia Mangostana L.). J. Ris. Kefarmasian Indones. 2019, 1, 166–176. [Google Scholar] [CrossRef]
- Ciba Inc. Ciba® TINODERM™. Available online: https://vdocuments.mx/ciba-tinoderm-please-note-that-ciba-patents-of-the-nan-maximal-skin.html?page=1 (accessed on 6 December 2021).
- Fiume, M.Z. Final Report on the Safety Assessment of Lecithin and Hydrogenated Lecithin. Int. J. Toxicol. 2001, 20, 21–45. [Google Scholar] [CrossRef]
- Handayani, F.S.; Nugroho, B.H.; Munawiroh, S.Z. Optimization of Low Energy Nanoemulsion of Grape Seed Oil Formulation Using D-Optimal Mixture Design (DMD) Optimasi Formulasi Nanoemulsi Minyak Biji Anggur Energi Rendah Dengan D-Optimal Mixture Design (DMD). J. Ilm. Farm. 2018, 14, 17–34. [Google Scholar]
- Gurpreet, K.; Singh, S.K. Review of Nanoemulsion Formulation and Characterization Techniques. Indian J. Pharm. Sci. 2018, 80, 781–789. [Google Scholar] [CrossRef]
- Kakran, M.; Shegokar, R.; Sahoo, N.G.; Al Shaal, L.; Li, L.; Müller, R.H. Fabrication of Quercetin Nanocrystals: Comparison of Different Methods. Eur. J. Pharm. Biopharm. 2012, 80, 113–121. [Google Scholar] [CrossRef]
- Ali, S.M.; Yosipovitch, G. Skin PH: From Basic Science to Basic Skin Care. Acta Derm. Venereol. 2013, 93, 261–267. [Google Scholar] [CrossRef] [PubMed]
- Bali, V.; Ali, M.; Ali, J. Study of Surfactant Combinations and Development of a Novel Nanoemulsion for Minimising Variations in Bioavailability of Ezetimibe. Colloids Surfaces B Biointerfaces 2010, 76, 410–420. [Google Scholar] [CrossRef] [PubMed]
- Thakkar, H.; Nangesh, J.; Parmar, M.; Patel, D. Formulation and Characterization of Lipid- Based Drug Delivery System of Raloxifene- Microemulsion and Self-Microemulsifying Drug Delivery System. J. Pharm. Bioallied Sci. 2011, 3, 442–449. [Google Scholar] [CrossRef] [PubMed]
- Kale, S.N.; Deore, S.L. Solubility Enhancement of Nebivolol by Micro Emulsion Technique. J. Young Pharm. 2016, 8, 356–367. [Google Scholar] [CrossRef]
- Ariviani, S.; Raharjo, S.; Anggrahini, S.; Naruki, S. Formulasi Dan Stabilitas Mikroemulsi O/W Dengan Metode Emulsifikasi Spontan Menggunakan Vco Dan Minyak Sawit Sebagai Fase Minyak: Pengaruh Rasio Surfaktan-Minyak. J. Agritech 2015, 35, 27. [Google Scholar] [CrossRef]
- Wathoni, N.; Rusdin, A.; Motoyama, K.; Joni, I.M.; Lesmana, R.; Muchtaridi, M. Nanoparticle Drug Delivery Systems for α-Mangostin. Nanotechnol. Sci. Appl. 2020, 13, 23–36. [Google Scholar] [CrossRef]
- Ghanbarzadeh, B.; Babazadeh, A.; Hamishehkar, H. Nano-Phytosome as a Potential Food-Grade Delivery System. Food Biosci. 2016, 15, 126–135. [Google Scholar] [CrossRef]
- Vander Haeghen, Y.; Naeyaert, J.M. Consistent Cutaneous Imaging with Commercial Digital Cameras. Arch. Dermatol. 2006, 142, 42–46. [Google Scholar] [CrossRef][Green Version]
- Jung, B.; Choi, B.; Shin, Y.; Durkin, A.J.; Nelson, J.S. Determination of Optimal View Angles for Quantitative Facial Image Analysis. J. Biomed. Opt. 2005, 10, 024002. [Google Scholar] [CrossRef][Green Version]
- Schindewolf, T.; Albert, R.; Harms, H. Evaluation of Different Image Acquisition Techniques for a Computer Vision System in the Diagnosis of Malignant Melanoma. J. Am. Acad. Dermatol. 1994, 31, 33–41. [Google Scholar] [CrossRef]
- Schnuch, A.; Aberer, W.; Agathos, M.; Becker, D.; Brasch, J.; Elsner, P.; Frosch, P.J.; Fuchs, T.; Geier, J.; Hillen, U.; et al. Performing Patch Testing with Contact Allergens. JDDG-J. Ger. Soc. Dermatol. 2008, 6, 770–775. [Google Scholar] [CrossRef]
- Kallay, N.; Žalac, S. Stability of Nanodispersions: A Model for Kinetics of Aggregation of Nanoparticles. J. Colloid Interface Sci. 2002, 253, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Đorđević, S.M.; Santrač, A.; Cekić, N.D.; Marković, B.D.; Divović, B.; Ilić, T.M.; Savić, M.M.; Savić, S.D. Parenteral Nanoemulsions of Risperidone for Enhanced Brain Delivery in Acute Psychosis: Physicochemical and in Vivo Performances. Int. J. Pharm. 2017, 533, 421–430. [Google Scholar] [CrossRef] [PubMed]
- Muchtaridi, M.; Suryani, D.; Qosim, W.A.; Saptarini, N.M. Quantitative Analysis of A-Mangostin in Mangosteen (Garcinia Mangostana L.) Pericarp Extract from Four District of West Java by HPLC Method. Int. J. Pharm. Pharm. Sci. 2016, 8, 232–236. [Google Scholar]
- Suaniti, N.; Manurung, M.; Hartasiwi, N. Uji Sifat Virgin Coconut Oil (VCO) Hasil Ekstraksi Enzimatis Terhadap Berbagai Produk Minyak Kelapa Hasil Publikasi. J. Kim. 2014, 8, 171–177. [Google Scholar]



| No. | Factor | Level | |
|---|---|---|---|
| Low | High | ||
| 1 | X1: Tween 80 concentration (%) | 15 | 35 |
| 2 | X2: Soy lecithin concentration (%) | 1 | 3 |
| 3 | X3: VCO concentration (%) | 1 | 3 |
| 4 | X4: Stirring duration (minutes) | 10 | 15 |
| 5 | X5: Stirring speed (rpm) | 750 | 1500 |
| Formula | Tween 80 (%) | Soy Lecithin (%) | VCO (%) | Stirring Time (Minutes) | Stirring Speed (rpm) | Droplets Size (nm) |
|---|---|---|---|---|---|---|
| 1 | 15 | 1 | 1 | 10 | 750 | 11.3 |
| 2 | 15 | 1 | 1 | 15 | 750 | 51 |
| 3 | 15 | 1 | 3 | 10 | 750 | 36 |
| 4 | 15 | 1 | 3 | 15 | 750 | 189 |
| 5 | 15 | 3 | 1 | 10 | 750 | 165,100 |
| 6 | 15 | 3 | 1 | 15 | 750 | 33,400 |
| 7 | 15 | 3 | 3 | 10 | 750 | 72,600 |
| 8 | 15 | 3 | 3 | 15 | 750 | 136,200 |
| 9 | 15 | 1 | 1 | 10 | 1500 | 36 |
| 10 | 15 | 1 | 1 | 15 | 1500 | 43 |
| 11 | 15 | 1 | 3 | 10 | 1500 | 107 |
| 12 | 15 | 1 | 3 | 15 | 1500 | 12 |
| 13 | 15 | 3 | 1 | 10 | 1500 | 162,300 |
| 14 | 15 | 3 | 1 | 15 | 1500 | 94,100 |
| 15 | 15 | 3 | 3 | 10 | 1500 | 103,400 |
| 16 | 15 | 3 | 3 | 15 | 1500 | 158,400 |
| 17 | 35 | 1 | 1 | 10 | 750 | 98,000 |
| 18 | 35 | 1 | 1 | 15 | 750 | 74,000 |
| 19 | 35 | 1 | 3 | 10 | 750 | 89,500 |
| 20 | 35 | 1 | 3 | 15 | 750 | 92,600 |
| 21 | 35 | 3 | 1 | 10 | 750 | 43,000 |
| 22 | 35 | 3 | 1 | 15 | 750 | 62,400 |
| 23 | 35 | 3 | 3 | 10 | 750 | 26,300 |
| 24 | 35 | 3 | 3 | 15 | 750 | 114,000 |
| 25 | 35 | 1 | 1 | 10 | 1500 | 30 |
| 26 | 35 | 1 | 1 | 15 | 1500 | 76 |
| 27 | 35 | 1 | 3 | 10 | 1500 | 90 |
| 28 | 35 | 1 | 3 | 15 | 1500 | 12 |
| 29 | 35 | 3 | 1 | 10 | 1500 | 54,800 |
| 30 | 35 | 3 | 1 | 15 | 1500 | 57,400 |
| 31 | 35 | 3 | 3 | 10 | 1500 | 50,000 |
| 32 | 35 | 3 | 3 | 15 | 1500 | 184,500 |
| Opt 1 | 28.2 | 1 | 2.3 | 15 | 1500 | 34.04 |
| Parameters | Parameter Values with Graphs (Predictions) | Parameter Value for Confirmation (Determination) |
|---|---|---|
| Tween 80 | 28.24% | 28.3% |
| Soy Lecithin | 1% | 1% |
| VCO | 2.3% | 2.3% |
| Stirring Time | 14.99 min | 15 min |
| Stirring Speed | 1499 rpm | 1500 rpm |
| Droplet Size (PSA) | 34.04 nm | 36 nm |
| p-value | 0.977 | p ≥ 0.05 |
| Formula | pH 1 | Transmittance 1 (%) | Viscosity 1 (cP) | Freeze-and-Thaw Test | Specific Gravity (g/mL) | Droplet Size (nm) |
|---|---|---|---|---|---|---|
| 1 | 6.23 ± 0.15 | 87.65 ± 0.00 | 17.33 ± 0.58 | Stable | 1.03 | 11.3 |
| 3 | 6.20 ± 0.10 | 61.12 ± 0.05 | 15.00 ± 1.00 | Stable | 1.02 | 36 |
| 9 | 6.37 ± 0.06 | 35.80 ± 0.10 | 17.33 ± 0.58 | Stable | 1.03 | 36 |
| 12 | 6.37 ± 0.06 | 73.02 ± 0.02 | 16.00 ± 0.00 | Stable | 1.03 | 12 |
| 25 | 6.57 ± 0.06 | 89.68 ± 0.50 | 154.67 ± 1.15 | Stable | 1.04 | 30 |
| 28 | 6.50 ± 0.06 | 88.73 ± 0.80 | 217.67 ± 0.58 | Stable | 1.04 | 12 |
| F-opt | 6.50 ± 0.06 | 99.62 ± 0.67 | 37.33 ± 0.58 | Stable | 1.04 | 36 |
| Fα-mangostin USUC | 6.57 ± 0.06 | 90.95 ± 0.05 | 38.00 ± 0.000 | Stable | 1.04 | 16.5 |
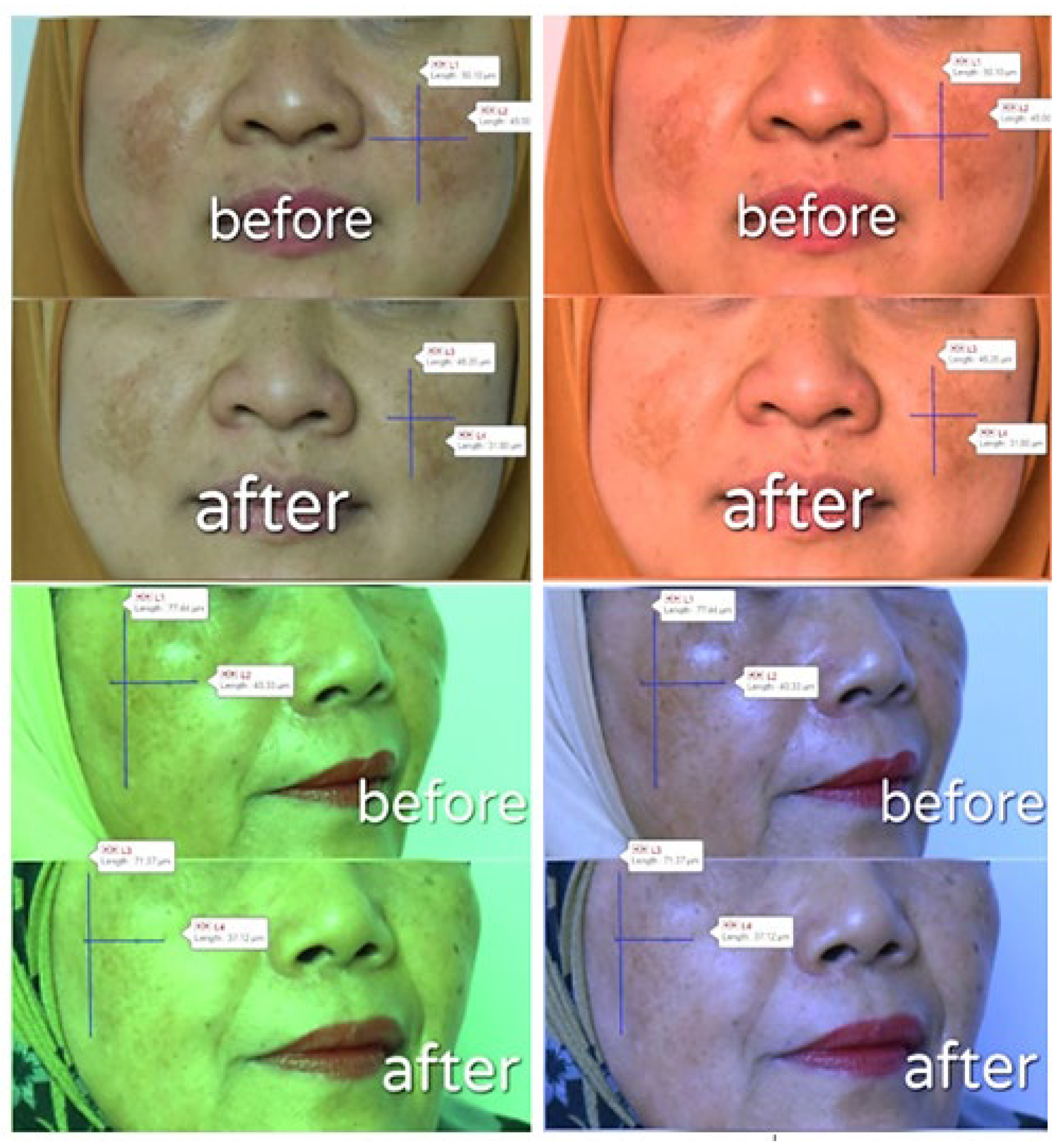
| Response | Volunteer 1 | Volunteer 2 | ||||
|---|---|---|---|---|---|---|
| Before | After | p-Value | Before | After | p-Value | |
| Red | 135 | 142 | 0.002 | 129 | 153 | 0.001 |
| Green | 118 | 135 | 0.000 | 114 | 144 | 0.001 |
| Blue | 90 | 102 | 0.006 | 72 | 109 | 0.000 |
| Length of Spot (μm) | 50.10 | 46.20 | 0.007 | 77.44 | 71.37 | 0.005 |
| Width of Spot (μm) | 45.00 | 31.80 | 0.011 | 40.33 | 37.12 | 0.013 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chairunisa, U.; Rustini; Nastiti, C.M.R.R.; Riswanto, F.D.O.; Benson, H.A.E.; Lucida, H. A Promising Ultra-Small Unilamellar Carrier System for Enhanced Skin Delivery of α-Mangostin as an Anti-Age-Spot Serum. Pharmaceutics 2022, 14, 2741. https://doi.org/10.3390/pharmaceutics14122741
Chairunisa U, Rustini, Nastiti CMRR, Riswanto FDO, Benson HAE, Lucida H. A Promising Ultra-Small Unilamellar Carrier System for Enhanced Skin Delivery of α-Mangostin as an Anti-Age-Spot Serum. Pharmaceutics. 2022; 14(12):2741. https://doi.org/10.3390/pharmaceutics14122741
Chicago/Turabian StyleChairunisa, Ully, Rustini, Christofori Maria Ratna Rini Nastiti, Florentinus Dika Octa Riswanto, Heather A. E. Benson, and Henny Lucida. 2022. "A Promising Ultra-Small Unilamellar Carrier System for Enhanced Skin Delivery of α-Mangostin as an Anti-Age-Spot Serum" Pharmaceutics 14, no. 12: 2741. https://doi.org/10.3390/pharmaceutics14122741
APA StyleChairunisa, U., Rustini, Nastiti, C. M. R. R., Riswanto, F. D. O., Benson, H. A. E., & Lucida, H. (2022). A Promising Ultra-Small Unilamellar Carrier System for Enhanced Skin Delivery of α-Mangostin as an Anti-Age-Spot Serum. Pharmaceutics, 14(12), 2741. https://doi.org/10.3390/pharmaceutics14122741








