Drugs for the Quorum Sensing Inhibition of Oral Biofilm: New Frontiers and Insights in the Treatment of Periodontitis
Abstract
1. Introduction
2. Materials and Methods
3. Quorum Sensing Signaling in Microbial Biofilm
4. Quorum Sensing in the Transition from Eubiotic to Dysbiotic Oral Biofilm
5. Antimicrobial Drugs Used for the Prevention and Treatment of Periodontal Disease
6. Quorum Sensing Inhibition Strategies
- Lactonases, by hydrolyzing the ester bond of the lactone ring, cause the consequent degradation of AHLs [107]. Several classes of lactonases have been identified in a large variety, both in bacteria but also in archaea and eukaryotes; some of these prefer AHLs with long acyl chains (e.g., the phosphotriesterase-like lactonases (PLLs) and the paraoxonases (PONs)), while others present a wider spectrum of action (e.g., the metal-β-lactamase-like lactonases (MLLs) and the α/β hydrolase fold lactonases). These lactonases were selected as a model for engineering so that they could be modified to confer enhanced AHL lactonase activity [101];
- Acylases are the other class of QQ enzymes and hydrolyse the amide bond of AHL. In particular, a QQ acylase is represented by PvdQ, which, thanks to its hydrophobic binding pocket, has specificity for long-chain AHLs, and thanks to a targeted engineering approach has changed the range of action also toward shorter AHLs [108,109];
- Oxidoreductases do not degrade the AHL but modify its activity, leading to the inability to bind to the respective receptor [110].
7. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tonetti, M.S.; Greenwell, H.; Kornman, K.S. Staging and grading of periodontitis: Framework and proposal of a new classification and case definition. J. Periodontol. 2018, 89 (Suppl. 1), S159–S172. [Google Scholar] [CrossRef] [PubMed]
- Petersen, P.E.; Ogawa, H. The global burden of periodontal disease: Towards integration with chronic disease prevention and control. Periodontology 2000 2012, 60, 15–39. [Google Scholar] [CrossRef] [PubMed]
- Papapanou, P.N. Epidemiology of periodontal diseases: An update. J. Int. Acad. Periodontol. 1999, 1, 110–116. [Google Scholar] [PubMed]
- Genco, R.J.; Sanz, M. Clinical and public health implications of periodontal and systemic diseases: An overview. Periodontology 2000 2020, 83, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Sanz, M.; Lang, N.P.; Kinane, D.F.; Berglundh, T.; Chapple, I.; Tonetti, M.S. Seventh European Workshop on Periodontology of the European Academy of Periodontology at the Parador at la Granja, Segovia, Spain. J. Clin. Periodontol. 2011, 38 (Suppl. 11), 1–2. [Google Scholar] [CrossRef] [PubMed]
- Bui, F.Q.; Almeida-da-Silva, C.L.C.; Huynh, B.; Trinh, A.; Liu, J.; Woodward, J.; Asadi, H.; Ojcius, D.M. Association between periodontal pathogens and systemic disease. Biomed. J. 2019, 42, 27–35. [Google Scholar] [CrossRef]
- Sanz, M.; Kornman, K. Periodontitis and adverse pregnancy outcomes: Consensus report of the Joint EFP/AAP Workshop on Periodontitis and Systemic Diseases. J. Periodontol. 2013, 84, S164–S169. [Google Scholar] [CrossRef]
- Matarese, G.; Isola, G.; Anastasi, G.P.; Cutroneo, G.; Cordasco, G.; Favaloro, A.; Vita, G.; Vermiglio, G.; Milardi, D.; Zizzari, V.L.; et al. Transforming growth factor beta 1 and vascular endothelial growth factor levels in the pathogenesis of periodontal disease. Eur. J. Inflamm. 2013, 11, 479–488. [Google Scholar] [CrossRef]
- Lertpimonchai, A.; Rattanasiri, S.; Arj-Ong Vallibhakara, S.; Attia, J.; Thakkinstian, A. The association between oral hygiene and periodontitis: A systematic review and meta-analysis. Int. Dent. J. 2017, 67, 332–343. [Google Scholar] [CrossRef]
- Cekici, A.; Kantarci, A.; Hasturk, H.; Van Dyke, T.E. Inflammatory and immune pathways in the pathogenesis of periodontal disease. Periodontology 2000 2014, 64, 57–80. [Google Scholar] [CrossRef]
- Socransky, S.S.; Haffajee, A.D. Periodontal microbial ecology. Periodontology 2000 2005, 38, 135–187. [Google Scholar] [CrossRef]
- Socransky, S.S.; Haffajee, A.D.; Cugini, M.A.; Smith, C.; Kent, R.L., Jr. Microbial complexes in subgingival plaque. J. Clin. Periodontol. 1998, 25, 134–144. [Google Scholar] [CrossRef]
- Bagavad Gita, J.; George, A.V.; Pavithra, N.; Chandrasekaran, S.C.; Latchumanadhas, K.; Gnanamani, A. Dysregulation of miR-146a by periodontal pathogens: A risk for acute coronary syndrome. J. Periodontol. 2019, 90, 756–765. [Google Scholar] [CrossRef]
- Preshaw, P.M.; Alba, A.L.; Herrera, D.; Jepsen, S.; Konstantinidis, A.; Makrilakis, K.; Taylor, R. Periodontitis and diabetes: A two-way relationship. Diabetologia 2012, 55, 21–31. [Google Scholar] [CrossRef]
- Bianconi, V.; Sahebkar, A.; Atkin, S.L.; Pirro, M. The regulation and importance of monocyte chemoattractant protein-1. Curr. Opin. Hematol. 2018, 25, 44–51. [Google Scholar] [CrossRef]
- Yan, K.; Lin, Q.; Tang, K.; Liu, S.; Du, Y.; Yu, X.; Li, S. Substance P participates in periodontitis by upregulating HIF-1alpha and RANKL/OPG ratio. BMC Oral Health 2020, 20, 27. [Google Scholar] [CrossRef]
- Ye, D.; Gajendra, S.; Lawyer, G.; Jadeja, N.; Pishey, D.; Pathagunti, S.; Lyons, J.; Veazie, P.; Watson, G.; McIntosh, S.; et al. Inflammatory biomarkers and growth factors in saliva and gingival crevicular fluid of e-cigarette users, cigarette smokers, and dual smokers: A pilot study. J. Periodontol. 2020, 91, 1274–1283. [Google Scholar] [CrossRef]
- Mittal, M.; Siddiqui, M.R.; Tran, K.; Reddy, S.P.; Malik, A.B. Reactive oxygen species in inflammation and tissue injury. Antioxid. Redox Signal. 2014, 20, 1126–1167. [Google Scholar] [CrossRef]
- Eick, S.; Nydegger, J.; Bürgin, W.; Salvi, G.E.; Sculean, A.; Ramseier, C. Microbiological analysis and the outcomes of periodontal treatment with or without adjunctive systemic antibiotics—A retrospective study. Clin. Oral Investig. 2018, 22, 3031–3041. [Google Scholar] [CrossRef]
- Franco, C.; Patricia, H.R.; Timo, S.; Claudia, B.; Marcela, H. Matrix Metalloproteinases as Regulators of Periodontal Inflammation. Int. J. Mol. Sci. 2017, 18, 440. [Google Scholar] [CrossRef]
- Becerra-Ruiz, J.S.; Guerrero-Velázquez, C.; Martínez-Esquivias, F.; Martínez-Pérez, L.A.; Guzmán-Flores, J.M. Innate and adaptive immunity of periodontal disease. From etiology to alveolar bone loss. Oral Dis. 2021, 28, 1441–1447. [Google Scholar] [CrossRef] [PubMed]
- Federle, M.J.; Bassler, B.L. Interspecies communication in bacteria. J. Clin. Investig. 2003, 112, 1291–1299. [Google Scholar] [CrossRef] [PubMed]
- Muras, A.; Mallo, N.; Otero-Casal, P.; Pose-Rodríguez, J.M.; Otero, A. Quorum sensing systems as a new target to prevent biofilm-related oral diseases. Oral Dis. 2022, 28, 307–313. [Google Scholar] [CrossRef] [PubMed]
- Souza, E.Q.M.; da Rocha, T.E.; Toro, L.F.; Guiati, I.Z.; Ervolino, E.; Garcia, V.G.; Wainwright, M.; Theodoro, L.H. Antimicrobial photodynamic therapy compared to systemic antibiotic therapy in non-surgical treatment of periodontitis: Systematic review and meta-analysis. Photodiagnosis Photodyn. Ther. 2020, 31, 101808. [Google Scholar] [CrossRef] [PubMed]
- Isola, G.; Polizzi, A.; Iorio-Siciliano, V.; Alibrandi, A.; Ramaglia, L.; Leonardi, R. Effectiveness of a nutraceutical agent in the non-surgical periodontal therapy: A randomized, controlled clinical trial. Clin. Oral Investig. 2021, 25, 1035–1045. [Google Scholar] [CrossRef]
- Mummolo, S.; Marchetti, E.; Di Martino, S.; Scorzetti, L.; Marzo, G. Aggressive periodontitis: Laser Nd:YAG treatment versus conventional surgical therapy. Eur. J. Paediatr. Dent. 2008, 9, 88–92. [Google Scholar]
- Khattri, S.; Nagraj, S.K.; Arora, A.; Eachempati, P.; Kusum, C.K.; Bhat, K.G.; Johnson, T.M.; Lodi, G. Adjunctive systemic antimicrobials for the non-surgical treatment of periodontitis. Cochrane Database Syst. Rev. 2020, 2020, CD012568. [Google Scholar]
- Morozumi, T.; Yashima, A.; Gomi, K.; Ujiie, Y.; Izumi, Y.; Akizuki, T.; Mizutani, K.; Takamatsu, H.; Minabe, M.; Miyauchi, S. Increased systemic levels of inflammatory mediators following one-stage full-mouth scaling and root planing. J. Periodontal Res. 2018, 53, 536–544. [Google Scholar] [CrossRef]
- Basavaraju, M.; Sisnity, V.S.; Palaparthy, R.; Addanki, P.K. Quorum quenching: Signal jamming in dental plaque biofilms. J. Dent. Sci. 2016, 11, 349–352. [Google Scholar] [CrossRef]
- Czaczyk, K.; Myszka, K. Biosynthesis of extracellular polymeric substances (EPS) and its role in microbial biofilm formation. Pol. J. Environ. Stud. 2007, 16, 799–806. [Google Scholar]
- Hao, Y.; Winans, S.C.; Glick, B.R.; Charles, T.C. Identification and characterization of new LuxR/LuxI-type quorum sensing systems from metagenomic libraries. Environ. Microbiol. 2010, 12, 105–117. [Google Scholar] [CrossRef]
- Plančak, D.; Musić, L.; Puhar, I. Quorum sensing of periodontal pathogens. Acta Stomatol. Croat. 2015, 49, 234. [Google Scholar] [CrossRef]
- Papenfort, K.; Bassler, B.L. Quorum sensing signal–response systems in Gram-negative bacteria. Nat. Rev. Microbiol. 2016, 14, 576–588. [Google Scholar] [CrossRef]
- Reading, N.C.; Sperandio, V. Quorum sensing: The many languages of bacteria. FEMS Microbiol. Lett. 2006, 254, 1–11. [Google Scholar] [CrossRef]
- Nealson, K.H.; Hastings, J.W. Bacterial bioluminescence: Its control and ecological significance. Microbiol. Rev. 1979, 43, 496–518. [Google Scholar] [CrossRef]
- Bassler, B.L.; Wright, M.; Showalter, R.E.; Silverman, M.R. Intercellular signalling in Vibrio harveyi: Sequence and function of genes regulating expression of luminescence. Mol. Microbiol. 1993, 9, 773–786. [Google Scholar] [CrossRef]
- Bassler, B.L.; Losick, R. Bacterially speaking. Cell 2006, 125, 237–246. [Google Scholar] [CrossRef]
- Petersen, F.C.; Pecharki, D.; Scheie, A.A. Biofilm mode of growth of Streptococcus intermedius favored by a competence-stimulating signaling peptide. J. Bacteriol. 2004, 186, 6327–6331. [Google Scholar] [CrossRef]
- Waters, C.M.; Bassler, B.L. Quorum sensing: Cell-to-cell communication in bacteria. Annu. Rev. Cell Dev. Biol. 2005, 21, 319–346. [Google Scholar] [CrossRef]
- Determinants of pulse wave velocity in healthy people and in the presence of cardiovascular risk factors: ‘Establishing normal and reference values’. Eur. Heart J. 2010, 31, 2338–2350. [CrossRef]
- Li, Y.H.; Lau, P.C.; Lee, J.H.; Ellen, R.P.; Cvitkovitch, D.G. Natural genetic transformation of Streptococcus mutans growing in biofilms. J. Bacteriol. 2001, 183, 897–908. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.H.; Tang, N.; Aspiras, M.B.; Lau, P.C.; Lee, J.H.; Ellen, R.P.; Cvitkovitch, D.G. A quorum-sensing signaling system essential for genetic competence in Streptococcus mutans is involved in biofilm formation. J. Bacteriol. 2002, 184, 2699–2708. [Google Scholar] [CrossRef]
- Ishii, S.; Fukui, K.; Yokoshima, S.; Kumagai, K.; Beniyama, Y.; Kodama, T.; Fukuyama, T.; Okabe, T.; Nagano, T.; Kojima, H.; et al. High-throughput Screening of Small Molecule Inhibitors of the Streptococcus Quorum-sensing Signal Pathway. Sci. Rep. 2017, 7, 4029. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.H.; Lau, P.C.; Tang, N.; Svensäter, G.; Ellen, R.P.; Cvitkovitch, D.G. Novel two-component regulatory system involved in biofilm formation and acid resistance in Streptococcus mutans. J. Bacteriol. 2002, 184, 6333–6342. [Google Scholar] [CrossRef] [PubMed]
- Xie, Z.; Okinaga, T.; Niu, G.; Qi, F.; Merritt, J. Identification of a novel bacteriocin regulatory system in Streptococcus mutans. Mol. Microbiol. 2010, 78, 1431–1447. [Google Scholar] [CrossRef]
- Shanker, E.; Federle, M.J. Quorum Sensing Regulation of Competence and Bacteriocins in Streptococcus pneumoniae and mutans. Genes 2017, 8, 15. [Google Scholar] [CrossRef]
- Rued, B.E.; Covington, B.C.; Bushin, L.B.; Szewczyk, G.; Laczkovich, I.; Seyedsayamdost, M.R.; Federle, M.J. Quorum Sensing in Streptococcus mutans Regulates Production of Tryglysin, a Novel RaS-RiPP Antimicrobial Compound. mBio 2021, 12, e02688-20. [Google Scholar] [CrossRef]
- Bassler, B.L. How bacteria talk to each other: Regulation of gene expression by quorum sensing. Curr. Opin. Microbiol. 1999, 2, 582–587. [Google Scholar] [CrossRef]
- Wright, P.P.; Ramachandra, S.S. Quorum Sensing and Quorum Quenching with a Focus on Cariogenic and Periodontopathic Oral Biofilms. Microorganisms 2022, 10, 1783. [Google Scholar] [CrossRef]
- Frias, J.; Olle, E.; Alsina, M. Periodontal pathogens produce quorum sensing signal molecules. Infect. Immun. 2001, 69, 3431–3434. [Google Scholar] [CrossRef]
- Wang, B.Y.; Alvarez, P.; Hong, J.; Kuramitsu, H.K. Periodontal pathogens interfere with quorum-sensing-dependent virulence properties in Streptococcus mutans. J. Periodontal. Res. 2011, 46, 105–110. [Google Scholar] [CrossRef]
- Bachtiar, E.W.; Bachtiar, B.M.; Jarosz, L.M.; Amir, L.R.; Sunarto, H.; Ganin, H.; Meijler, M.M.; Krom, B.P. AI-2 of Aggregatibacter actinomycetemcomitans inhibits Candida albicans biofilm formation. Front. Cell. Infect. Microbiol. 2014, 4, 94. [Google Scholar] [CrossRef]
- Wang, X.; Li, X.; Ling, J. Streptococcus gordonii LuxS/autoinducer-2 quorum-sensing system modulates the dual-species biofilm formation with Streptococcus mutans. J. Basic Microbiol. 2017, 57, 605–616. [Google Scholar] [CrossRef]
- Koo, H.; Andes, D.R.; Krysan, D.J. Candida-streptococcal interactions in biofilm-associated oral diseases. PLoS Pathog. 2018, 14, e1007342. [Google Scholar] [CrossRef]
- Kumari, A.; Pasini, P.; Daunert, S. Detection of bacterial quorum sensing N-acyl homoserine lactones in clinical samples. Anal. Bioanal. Chem. 2008, 391, 1619–1627. [Google Scholar] [CrossRef]
- Kumari, A.; Pasini, P.; Deo, S.K.; Flomenhoft, D.; Shashidhar, H.; Daunert, S. Biosensing systems for the detection of bacterial quorum signaling molecules. Anal. Chem. 2006, 78, 7603–7609. [Google Scholar] [CrossRef]
- Muras, A.; Mayer, C.; Otero-Casal, P.; Exterkate, R.A.M.; Brandt, B.W.; Crielaard, W.; Otero, A.; Krom, B.P. Short-Chain N-Acylhomoserine Lactone Quorum-Sensing Molecules Promote Periodontal Pathogens in In Vitro Oral Biofilms. Appl. Environ. Microbiol. 2020, 86, e01941-19. [Google Scholar] [CrossRef]
- Hajishengallis, G.; Darveau, R.P.; Curtis, M.A. The keystone-pathogen hypothesis. Nat. Rev. Microbiol. 2012, 10, 717–725. [Google Scholar] [CrossRef]
- Kolenbrander, P.E.; Palmer, R.J., Jr.; Rickard, A.H.; Jakubovics, N.S.; Chalmers, N.I.; Diaz, P.I. Bacterial interactions and successions during plaque development. Periodontology 2000 2006, 42, 47–79. [Google Scholar] [CrossRef]
- Kolenbrander, P.E.; Andersen, R.N.; Blehert, D.S.; Egland, P.G.; Foster, J.S.; Palmer, R.J., Jr. Communication among oral bacteria. Microbiol. Mol. Biol. Rev. 2002, 66, 486–505. [Google Scholar] [CrossRef]
- Rickard, A.H.; Palmer, R.J., Jr.; Blehert, D.S.; Campagna, S.R.; Semmelhack, M.F.; Egland, P.G.; Bassler, B.L.; Kolenbrander, P.E. Autoinducer 2: A concentration-dependent signal for mutualistic bacterial biofilm growth. Mol. Microbiol. 2006, 60, 1446–1456. [Google Scholar] [CrossRef] [PubMed]
- Shao, H.; Lamont, R.J.; Demuth, D.R. Autoinducer 2 is required for biofilm growth of Aggregatibacter (Actinobacillus) actinomycetemcomitans. Infect. Immun. 2007, 75, 4211–4218. [Google Scholar] [CrossRef] [PubMed]
- James, D.; Shao, H.; Lamont, R.J.; Demuth, D.R. The Actinobacillus actinomycetemcomitans ribose binding protein RbsB interacts with cognate and heterologous autoinducer 2 signals. Infect. Immun. 2006, 74, 4021–4029. [Google Scholar] [CrossRef] [PubMed]
- Shao, H.; James, D.; Lamont, R.J.; Demuth, D.R. Differential interaction of Aggregatibacter (Actinobacillus) actinomycetemcomitans LsrB and RbsB proteins with autoinducer 2. J. Bacteriol. 2007, 189, 5559–5565. [Google Scholar] [CrossRef] [PubMed]
- Fine, D.H.; Velliyagounder, K.; Furgang, D.; Kaplan, J.B. The Actinobacillus actinomycetemcomitans autotransporter adhesin Aae exhibits specificity for buccal epithelial cells from humans and old world primates. Infect. Immun. 2005, 73, 1947–1953. [Google Scholar] [CrossRef]
- Rupani, D.; Izano, E.A.; Schreiner, H.C.; Fine, D.H.; Kaplan, J.B. Aggregatibacter actinomycetemcomitans serotype f O-polysaccharide mediates coaggregation with Fusobacterium nucleatum. Oral Microbiol. Immunol. 2008, 23, 127–130. [Google Scholar] [CrossRef]
- Szafrański, S.P.; Deng, Z.L.; Tomasch, J.; Jarek, M.; Bhuju, S.; Rohde, M.; Sztajer, H.; Wagner-Döbler, I. Quorum sensing of Streptococcus mutans is activated by Aggregatibacter actinomycetemcomitans and by the periodontal microbiome. BMC Genom. 2017, 18, 238. [Google Scholar] [CrossRef]
- Fong, K.P.; Gao, L.; Demuth, D.R. luxS and arcB control aerobic growth of Actinobacillus actinomycetemcomitans under iron limitation. Infect. Immun. 2003, 71, 298–308. [Google Scholar] [CrossRef]
- James, C.E.; Hasegawa, Y.; Park, Y.; Yeung, V.; Tribble, G.D.; Kuboniwa, M.; Demuth, D.R.; Lamont, R.J. LuxS involvement in the regulation of genes coding for hemin and iron acquisition systems in Porphyromonas gingivalis. Infect. Immun. 2006, 74, 3834–3844. [Google Scholar] [CrossRef]
- McNab, R.; Ford, S.K.; El-Sabaeny, A.; Barbieri, B.; Cook, G.S.; Lamont, R.J. LuxS-based signaling in Streptococcus gordonii: Autoinducer 2 controls carbohydrate metabolism and biofilm formation with Porphyromonas gingivalis. J. Bacteriol. 2003, 185, 274–284. [Google Scholar] [CrossRef]
- Yuan, L.; Hillman, J.D.; Progulske-Fox, A. Microarray analysis of quorum-sensing-regulated genes in Porphyromonas gingivalis. Infect. Immun. 2005, 73, 4146–4154. [Google Scholar] [CrossRef]
- Jang, Y.J.; Choi, Y.J.; Lee, S.H.; Jun, H.K.; Choi, B.K. Autoinducer 2 of Fusobacterium nucleatum as a target molecule to inhibit biofilm formation of periodontopathogens. Arch. Oral Biol. 2013, 58, 17–27. [Google Scholar] [CrossRef]
- Jang, Y.J.; Sim, J.; Jun, H.K.; Choi, B.K. Differential effect of autoinducer 2 of Fusobacterium nucleatum on oral streptococci. Arch. Oral Biol. 2013, 58, 1594–1602. [Google Scholar] [CrossRef]
- Azakami, H.; Teramura, I.; Matsunaga, T.; Akimichi, H.; Noiri, Y.; Ebisu, S.; Kato, A. Characterization of autoinducer 2 signal in Eikenella corrodens and its role in biofilm formation. J. Biosci. Bioeng. 2006, 102, 110–117. [Google Scholar] [CrossRef]
- Muras, A.; Otero-Casal, P.; Blanc, V.; Otero, A. Acyl homoserine lactone-mediated quorum sensing in the oral cavity: A paradigm revisited. Sci. Rep. 2020, 10, 9800. [Google Scholar] [CrossRef]
- Baehni, P.C.; Takeuchi, Y. Anti-plaque agents in the prevention of biofilm-associated oral diseases. Oral Dis. 2003, 9 (Suppl. 1), 23–29. [Google Scholar] [CrossRef]
- Mouthrinses and periodontal disease. Int. Dent. J. 2002, 52, 346–352. [CrossRef]
- Herrera, D.; Sanz, M.; Jepsen, S.; Needleman, I.; Roldán, S. A systematic review on the effect of systemic antimicrobials as an adjunct to scaling and root planing in periodontitis patients. J. Clin. Periodontol. 2002, 29 (Suppl. 3), 136–159; discussion 160–132. [Google Scholar] [CrossRef]
- Ehmke, B.; Moter, A.; Beikler, T.; Milian, E.; Flemmig, T.F. Adjunctive antimicrobial therapy of periodontitis: Long-term effects on disease progression and oral colonization. J. Periodontol. 2005, 76, 749–759. [Google Scholar] [CrossRef]
- Mombelli, A.; Cionca, N.; Almaghlouth, A. Does adjunctive antimicrobial therapy reduce the perceived need for periodontal surgery? Periodontology 2000 2011, 55, 205–216. [Google Scholar] [CrossRef]
- Griffiths, G.S.; Ayob, R.; Guerrero, A.; Nibali, L.; Suvan, J.; Moles, D.R.; Tonetti, M.S. Amoxicillin and metronidazole as an adjunctive treatment in generalized aggressive periodontitis at initial therapy or re-treatment: A randomized controlled clinical trial. J. Clin. Periodontol. 2011, 38, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Kaner, D.; Christan, C.; Dietrich, T.; Bernimoulin, J.P.; Kleber, B.M.; Friedmann, A. Timing affects the clinical outcome of adjunctive systemic antibiotic therapy for generalized aggressive periodontitis. J. Periodontol. 2007, 78, 1201–1208. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro Edel, P.; Bittencourt, S.; Zanin, I.C.; Bovi Ambrosano, G.M.; Sallum, E.A.; Nociti, F.H.; Gonçalves, R.B.; Casati, M.Z. Full-mouth ultrasonic debridement associated with amoxicillin and metronidazole in the treatment of severe chronic periodontitis. J. Periodontol. 2009, 80, 1254–1264. [Google Scholar] [CrossRef] [PubMed]
- Kalsi, R.; Vandana, K.L.; Prakash, S. Effect of local drug delivery in chronic periodontitis patients: A meta-analysis. J. Indian Soc. Periodontol. 2011, 15, 304–309. [Google Scholar] [CrossRef] [PubMed]
- Lang, N.P.; Tan, W.C.; Krähenmann, M.A.; Zwahlen, M. A systematic review of the effects of full-mouth debridement with and without antiseptics in patients with chronic periodontitis. J. Clin. Periodontol. 2008, 35, 8–21. [Google Scholar] [CrossRef]
- Berglundh, T.; Giannobile, W.V.; Sanz, M.; Lang, N.P. (Eds.) Lindhe’s Clinical Periodontology and Implant Dentistry; John Wiley & Sons: Hoboken, NJ, USA, 2021. [Google Scholar]
- James, P.; Worthington, H.V.; Parnell, C.; Harding, M.; Lamont, T.; Cheung, A.; Whelton, H.; Riley, P. Chlorhexidine mouthrinse as an adjunctive treatment for gingival health. Cochrane Database Syst. Rev. 2017, 3, Cd008676. [Google Scholar] [CrossRef]
- Zhao, H.; Hu, J.; Zhao, L. Adjunctive subgingival application of Chlorhexidine gel in nonsurgical periodontal treatment for chronic periodontitis: A systematic review and meta-analysis. BMC Oral Health 2020, 20, 34. [Google Scholar] [CrossRef]
- Brookes, Z.L.S.; Bescos, R.; Belfield, L.A.; Ali, K.; Roberts, A. Current uses of chlorhexidine for management of oral disease: A narrative review. J. Dent. 2020, 103, 103497. [Google Scholar] [CrossRef]
- Stoeken, J.E.; Paraskevas, S.; van der Weijden, G.A. The long-term effect of a mouthrinse containing essential oils on dental plaque and gingivitis: A systematic review. J. Periodontol. 2007, 78, 1218–1228. [Google Scholar] [CrossRef]
- Ciancio, S.G. Antiseptics and antibiotics as chemotherapeutic agents for periodontitis management. Compend. Contin. Educ. Dent. 2000, 21, 59–62+64+66+78. [Google Scholar]
- Gunsolley, J.C. A meta-analysis of six-month studies of antiplaque and antigingivitis agents. J. Am. Dent. Assoc. 2006, 137, 1649–1657. [Google Scholar] [CrossRef]
- Chee, B.; Park, B.; Fitzsimmons, T.; Coates, A.M.; Bartold, P.M. Omega-3 fatty acids as an adjunct for periodontal therapy-a review. Clin. Oral Investig. 2016, 20, 879–894. [Google Scholar] [CrossRef]
- Kruse, A.B.; Kowalski, C.D.; Leuthold, S.; Vach, K.; Ratka-Krüger, P.; Woelber, J.P. What is the impact of the adjunctive use of omega-3 fatty acids in the treatment of periodontitis? A systematic review and meta-analysis. Lipids Health Dis. 2020, 19, 100. [Google Scholar] [CrossRef]
- Heo, H.; Bae, J.H.; Amano, A.; Park, T.; Choi, Y.H. Supplemental or dietary intake of omega-3 fatty acids for the treatment of periodontitis: A meta-analysis. J. Clin. Periodontol. 2022, 49, 362–377. [Google Scholar] [CrossRef]
- Mayanagi, G.; Kimura, M.; Nakaya, S.; Hirata, H.; Sakamoto, M.; Benno, Y.; Shimauchi, H. Probiotic effects of orally administered Lactobacillus salivarius WB21-containing tablets on periodontopathic bacteria: A double-blinded, placebo-controlled, randomized clinical trial. J. Clin. Periodontol. 2009, 36, 506–513. [Google Scholar] [CrossRef]
- Nguyen, T.; Brody, H.; Radaic, A.; Kapila, Y. Probiotics for periodontal health—Current molecular findings. Periodontology 2000 2021, 87, 254–267. [Google Scholar] [CrossRef]
- Rehman, Z.U.; Leiknes, T. Quorum-Quenching Bacteria Isolated From Red Sea Sediments Reduce Biofilm Formation by Pseudomonas aeruginosa. Front. Microbiol. 2018, 9, 1354. [Google Scholar] [CrossRef]
- Murugayah, S.A.; Gerth, M.L. Engineering quorum quenching enzymes: Progress and perspectives. Biochem. Soc. Trans. 2019, 47, 793–800. [Google Scholar] [CrossRef]
- Lade, H.; Paul, D.; Kweon, J.H. Quorum quenching mediated approaches for control of membrane biofouling. Int. J. Biol. Sci. 2014, 10, 550–565. [Google Scholar] [CrossRef]
- Sikdar, R.; Elias, M. Quorum quenching enzymes and their effects on virulence, biofilm, and microbiomes: A review of recent advances. Expert Rev. Anti. Infect. Ther. 2020, 18, 1221–1233. [Google Scholar] [CrossRef]
- Ni, N.; Li, M.; Wang, J.; Wang, B. Inhibitors and antagonists of bacterial quorum sensing. Med. Res. Rev. 2009, 29, 65–124. [Google Scholar] [CrossRef] [PubMed]
- Rampioni, G.; Leoni, L.; Williams, P. The art of antibacterial warfare: Deception through interference with quorum sensing-mediated communication. Bioorg. Chem. 2014, 55, 60–68. [Google Scholar] [CrossRef]
- Watson, W.T.; Minogue, T.D.; Val, D.L.; von Bodman, S.B.; Churchill, M.E. Structural basis and specificity of acyl-homoserine lactone signal production in bacterial quorum sensing. Mol. Cell 2002, 9, 685–694. [Google Scholar] [CrossRef] [PubMed]
- Rutherford, S.T.; Bassler, B.L. Bacterial quorum sensing: Its role in virulence and possibilities for its control. Cold Spring Harb. Perspect. Med. 2012, 2, a012427. [Google Scholar] [CrossRef]
- Asahi, Y.; Noiri, Y.; Igarashi, J.; Asai, H.; Suga, H.; Ebisu, S. Effects of N-acyl homoserine lactone analogues on Porphyromonas gingivalis biofilm formation. J. Periodontal Res. 2010, 45, 255–261. [Google Scholar] [CrossRef]
- Uroz, S.; Oger, P.M.; Chapelle, E.; Adeline, M.T.; Faure, D.; Dessaux, Y. A Rhodococcus qsdA-encoded enzyme defines a novel class of large-spectrum quorum-quenching lactonases. Appl. Environ. Microbiol. 2008, 74, 1357–1366. [Google Scholar] [CrossRef]
- Leadbetter, J.R.; Greenberg, E.P. Metabolism of acyl-homoserine lactone quorum-sensing signals by Variovorax paradoxus. J. Bacteriol. 2000, 182, 6921–6926. [Google Scholar] [CrossRef]
- Koch, G.; Nadal-Jimenez, P.; Reis, C.R.; Muntendam, R.; Bokhove, M.; Melillo, E.; Dijkstra, B.W.; Cool, R.H.; Quax, W.J. Reducing virulence of the human pathogen Burkholderia by altering the substrate specificity of the quorum-quenching acylase PvdQ. Proc. Natl. Acad. Sci. USA 2014, 111, 1568–1573. [Google Scholar] [CrossRef]
- Bijtenhoorn, P.; Mayerhofer, H.; Müller-Dieckmann, J.; Utpatel, C.; Schipper, C.; Hornung, C.; Szesny, M.; Grond, S.; Thürmer, A.; Brzuszkiewicz, E.B.; et al. A Novel Metagenomic Short-Chain Dehydrogenase/Reductase Attenuates Pseudomonas aeruginosa Biofilm Formation and Virulence on Caenorhabditis elegans. PLoS ONE 2011, 6, e26278. [Google Scholar] [CrossRef] [PubMed]
- Dwivedi, D.; Singh, V. Effects of the natural compounds embelin and piperine on the biofilm-producing property of Streptococcus mutans. J. Tradit. Complement. Med. 2016, 6, 57–61. [Google Scholar] [CrossRef] [PubMed]
- Fournier-Larente, J.; Morin, M.P.; Grenier, D. Green tea catechins potentiate the effect of antibiotics and modulate adherence and gene expression in Porphyromonas gingivalis. Arch. Oral. Biol. 2016, 65, 35–43. [Google Scholar] [CrossRef]
- Yada, S.; Kamalesh, B.; Sonwane, S.; Guptha, I.; Swetha, R.K. Quorum sensing inhibition, relevance to periodontics. J. Int. Oral Health 2015, 7, 67–69. [Google Scholar]
- Amin, A.; Hanif, M.; Abbas, K.; Ramzan, M.; Rasheed, A.; Zaman, A.; Pieters, L. Studies on effects of umbelliferon derivatives against periodontal bacteria; antibiofilm, inhibition of quorum sensing and molecular docking analysis. Microb. Pathog. 2020, 144, 104184. [Google Scholar] [CrossRef]
- Asfour, H.Z. Anti-Quorum Sensing Natural Compounds. J. Microsc. Ultrastruct. 2018, 6, 1–10. [Google Scholar] [CrossRef]
- Dong, Y.H.; Wang, L.Y.; Zhang, L.H. Quorum-quenching microbial infections: Mechanisms and implications. Philos. Trans. R Soc. Lond. B Biol. Sci. 2007, 362, 1201–1211. [Google Scholar] [CrossRef]
- González, J.E.; Keshavan, N.D. Messing with Bacterial Quorum Sensing. Microbiol. Mol. Biol. Rev. 2006, 70, 859–875. [Google Scholar] [CrossRef]
- Park, J.S.; Ryu, E.J.; Li, L.; Choi, B.K.; Kim, B.M. New bicyclic brominated furanones as potent autoinducer-2 quorum-sensing inhibitors against bacterial biofilm formation. Eur. J. Med. Chem. 2017, 137, 76–87. [Google Scholar] [CrossRef]
- Ponnusamy, K.; Paul, D.; Sam Kim, Y.; Kweon, J.H. 2(5H)-Furanone: A Prospective strategy for biofouling-control in membrane biofilm bacteria by quorum sensing inhibition. Braz. J. Microbiol. 2010, 41, 227–234. [Google Scholar] [CrossRef]
- Cho, Y.J.; Song, H.Y.; Ben Amara, H.; Choi, B.K.; Eunju, R.; Cho, Y.A.; Seol, Y.; Lee, Y.; Ku, Y.; Rhyu, I.C.; et al. In Vivo Inhibition of Porphyromonas gingivalis Growth and Prevention of Periodontitis With Quorum-Sensing Inhibitors. J. Periodontol. 2016, 87, 1075–1082. [Google Scholar] [CrossRef]
- Ryu, E.J.; Sim, J.; Sim, J.; Lee, J.; Choi, B.K. D-Galactose as an autoinducer 2 inhibitor to control the biofilm formation of periodontopathogens. J. Microbiol. 2016, 54, 632–637. [Google Scholar] [CrossRef]
- Guendouze, A.; Plener, L.; Bzdrenga, J.; Jacquet, P.; Rémy, B.; Elias, M.; Lavigne, J.-P.; Daudé, D.; Chabrière, E. Effect of Quorum Quenching Lactonase in Clinical Isolates of Pseudomonas aeruginosa and Comparison with Quorum Sensing Inhibitors. Front. Microbiol. 2017, 8, 227. [Google Scholar] [CrossRef] [PubMed]
- Ivanova, K.; Fernandes, M.M.; Mendoza, E.; Tzanov, T. Enzyme multilayer coatings inhibit Pseudomonas aeruginosa biofilm formation on urinary catheters. Appl. Microbiol. Biotechnol. 2015, 99, 4373–4385. [Google Scholar] [CrossRef] [PubMed]
- Schwab, M.; Bergonzi, C.; Sakkos, J.; Staley, C.; Zhang, Q.; Sadowsky, M.J.; Aksan, A.; Elias, M. Signal Disruption Leads to Changes in Bacterial Community Population. Front. Microbiol. 2019, 10, 611. [Google Scholar] [CrossRef] [PubMed]
- Simonetti, O.; Cirioni, O.; Cacciatore, I.; Baldassarre, L.; Orlando, F.; Pierpaoli, E.; Lucarini, G.; Orsetti, E.; Provinciali, M.; Fornasari, E.; et al. Efficacy of the Quorum Sensing Inhibitor FS10 Alone and in Combination with Tigecycline in an Animal Model of Staphylococcal Infected Wound. PLoS ONE 2016, 11, e0151956. [Google Scholar] [CrossRef]
- Paluch, E.; Rewak-Soroczyńska, J.; Jędrusik, I.; Mazurkiewicz, E.; Jermakow, K. Prevention of biofilm formation by quorum quenching. Appl. Microbiol. Biotechnol. 2020, 104, 1871–1881. [Google Scholar] [CrossRef]
- Abranches, J.; Zeng, L.; Kajfasz, J.K.; Palmer, S.R.; Chakraborty, B.; Wen, Z.T.; Richards, V.P.; Brady, L.J.; Lemos, J.A. Biology of Oral Streptococci. Microbiol. Spectr. 2018, 6. [Google Scholar] [CrossRef]
- Park, O.J.; Kwon, Y.; Park, C.; So, Y.J.; Park, T.H.; Jeong, S.; Im, J.; Yun, C.H.; Han, S.H. Streptococcus gordonii: Pathogenesis and Host Response to Its Cell Wall Components. Microorganisms 2020, 8, 1852. [Google Scholar] [CrossRef]
- Loo, C.Y.; Corliss, D.A.; Ganeshkumar, N. Streptococcus gordonii biofilm formation: Identification of genes that code for biofilm phenotypes. J. Bacteriol. 2000, 182, 1374–1382. [Google Scholar] [CrossRef]
- Park, T.; Im, J.; Kim, A.R.; Lee, D.; Jeong, S.; Yun, C.H.; Han, S.H. Short-chain fatty acids inhibit the biofilm formation of Streptococcus gordonii through negative regulation of competence-stimulating peptide signaling pathway. J. Microbiol. 2021, 59, 1142–1149. [Google Scholar] [CrossRef]
- OmerOglou, E.; Karaca, B.; Kibar, H.; Haliscelik, O.; Kiran, F. The role of microbiota-derived postbiotic mediators on biofilm formation and quorum sensing-mediated virulence of Streptococcus mutans: A perspective on preventing dental caries. Microb. Pathog. 2022, 164, 105390. [Google Scholar] [CrossRef]
- Kaspar, J.R.; Walker, A.R. Expanding the Vocabulary of Peptide Signals in Streptococcus mutans. Front. Cell. Infect. Microbiol. 2019, 9, 194. [Google Scholar] [CrossRef]
- Vijayakumar, A.; Sarveswari, H.B.; Vasudevan, S.; Shanmugam, K.; Solomon, A.P.; Neelakantan, P. Baicalein Inhibits Streptococcus mutans Biofilms and Dental Caries-Related Virulence Phenotypes. Antibiotics 2021, 10, 215. [Google Scholar] [CrossRef]
- Balasubramanian, A.R.; Vasudevan, S.; Shanmugam, K.; Lévesque, C.M.; Solomon, A.P.; Neelakantan, P. Combinatorial effects of trans-cinnamaldehyde with fluoride and chlorhexidine on Streptococcus mutans. J. Appl. Microbiol. 2021, 130, 382–393. [Google Scholar] [CrossRef]
- Ogawa, A.; Furukawa, S.; Fujita, S.; Mitobe, J.; Kawarai, T.; Narisawa, N.; Sekizuka, T.; Kuroda, M.; Ochiai, K.; Ogihara, H.; et al. Inhibition of Streptococcus mutans biofilm formation by Streptococcus salivarius FruA. Appl. Environ. Microbiol. 2011, 77, 1572–1580. [Google Scholar] [CrossRef]
- Wasfi, R.; Abd El-Rahman, O.A.; Zafer, M.M.; Ashour, H.M. Probiotic Lactobacillus sp. inhibit growth, biofilm formation and gene expression of caries-inducing Streptococcus mutans. J. Cell. Mol. Med. 2018, 22, 1972–1983. [Google Scholar] [CrossRef]
- Kasper, S.H.; Samarian, D.; Jadhav, A.P.; Rickard, A.H.; Musah, R.A.; Cady, N.C. S-aryl-L-cysteine sulphoxides and related organosulphur compounds alter oral biofilm development and AI-2-based cell-cell communication. J. Appl. Microbiol. 2014, 117, 1472–1486. [Google Scholar] [CrossRef]
- Elani, H.W.; Starr, J.R.; Da Silva, J.D.; Gallucci, G.O. Trends in Dental Implant Use in the U.S., 1999-2016, and Projections to 2026. J. Dent. Res. 2018, 97, 1424–1430. [Google Scholar] [CrossRef]
- Ramachandra, S.S.; Rana, R.; Reetika, S.; Jithendra, K.D. Options to avoid the second surgical site: A review of literature. Cell Tissue Bank 2014, 15, 297–305. [Google Scholar] [CrossRef]
- Heitz-Mayfield, L.J.A.; Salvi, G.E. Peri-implant mucositis. J. Clin. Periodontol. 2018, 45 (Suppl. 20), S237–S245. [Google Scholar] [CrossRef]
- Renvert, S.; Persson, G.R.; Pirih, F.Q.; Camargo, P.M. Peri-implant health, peri-implant mucositis, and peri-implantitis: Case definitions and diagnostic considerations. J. Periodontol. 2018, 89 (Suppl. 1), S304–S312. [Google Scholar] [CrossRef]
- Kang, M.; Kim, S.; Kim, H.; Song, Y.; Jung, D.; Kang, S.; Seo, J.H.; Nam, S.; Lee, Y. Calcium-Binding Polymer-Coated Poly(lactide- co-glycolide) Microparticles for Sustained Release of Quorum Sensing Inhibitors to Prevent Biofilm Formation on Hydroxyapatite Surfaces. ACS Appl. Mater. Interfaces 2019, 11, 7686–7694. [Google Scholar] [CrossRef]
- An, S.-J.; Namkung, J.-U.; Ha, K.-W.; Jun, H.-K.; Kim, H.Y.; Choi, B.-K. Inhibitory effect of d-arabinose on oral bacteria biofilm formation on titanium discs. Anaerobe 2022, 75, 102533. [Google Scholar] [CrossRef] [PubMed]
- Hemmati, F.; Rezaee, M.A.; Ebrahimzadeh, S.; Yousefi, L.; Nouri, R.; Kafil, H.S.; Gholizadeh, P. Novel Strategies to Combat Bacterial Biofilms. Mol. Biotechnol. 2021, 63, 569–586. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.; Soh, Y.; Heo, S.-M. Recent Advances of Therapeutic Targets for the Treatment of Periodontal Disease. Biomol. Ther. 2021, 29, 263–267. [Google Scholar] [CrossRef] [PubMed]
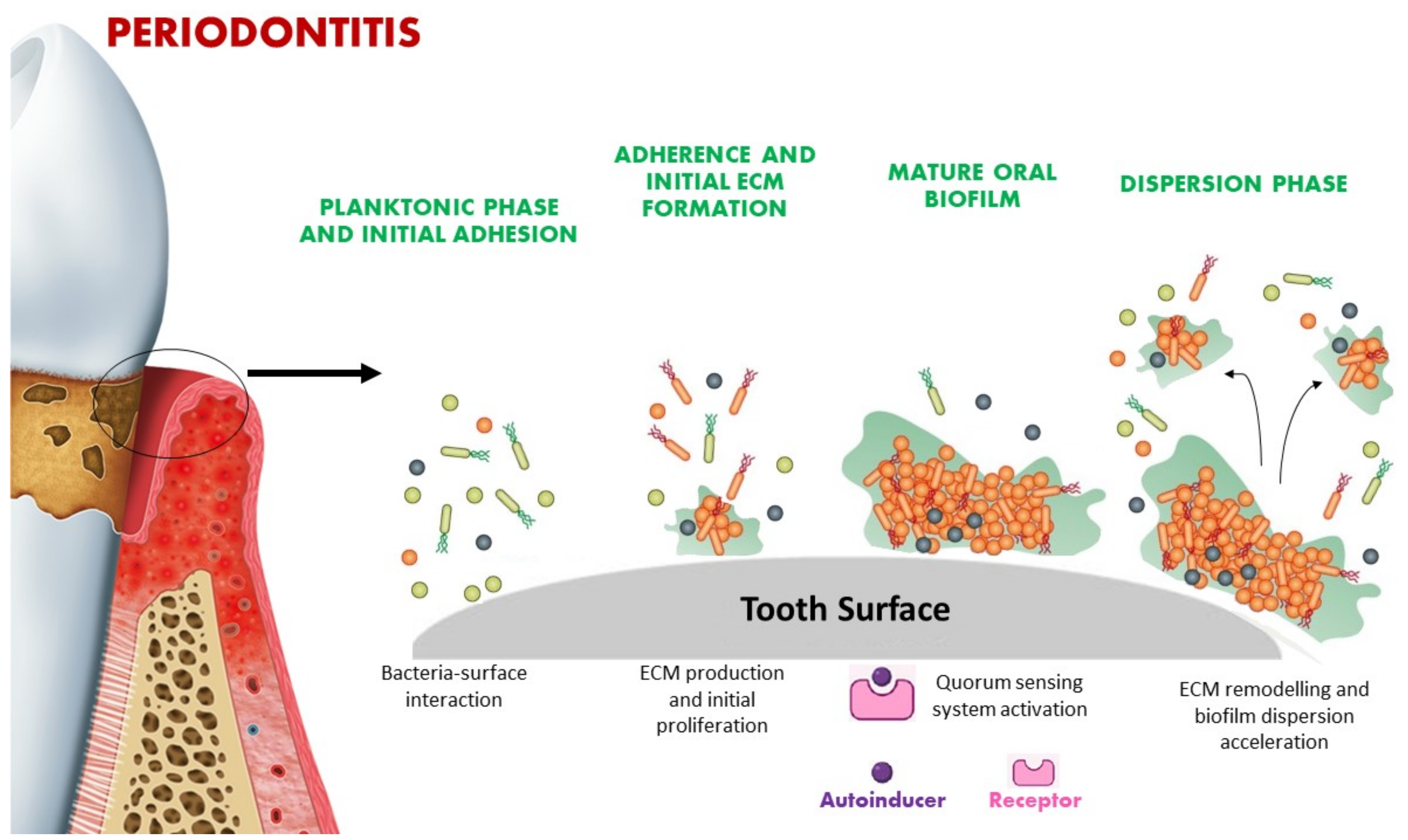
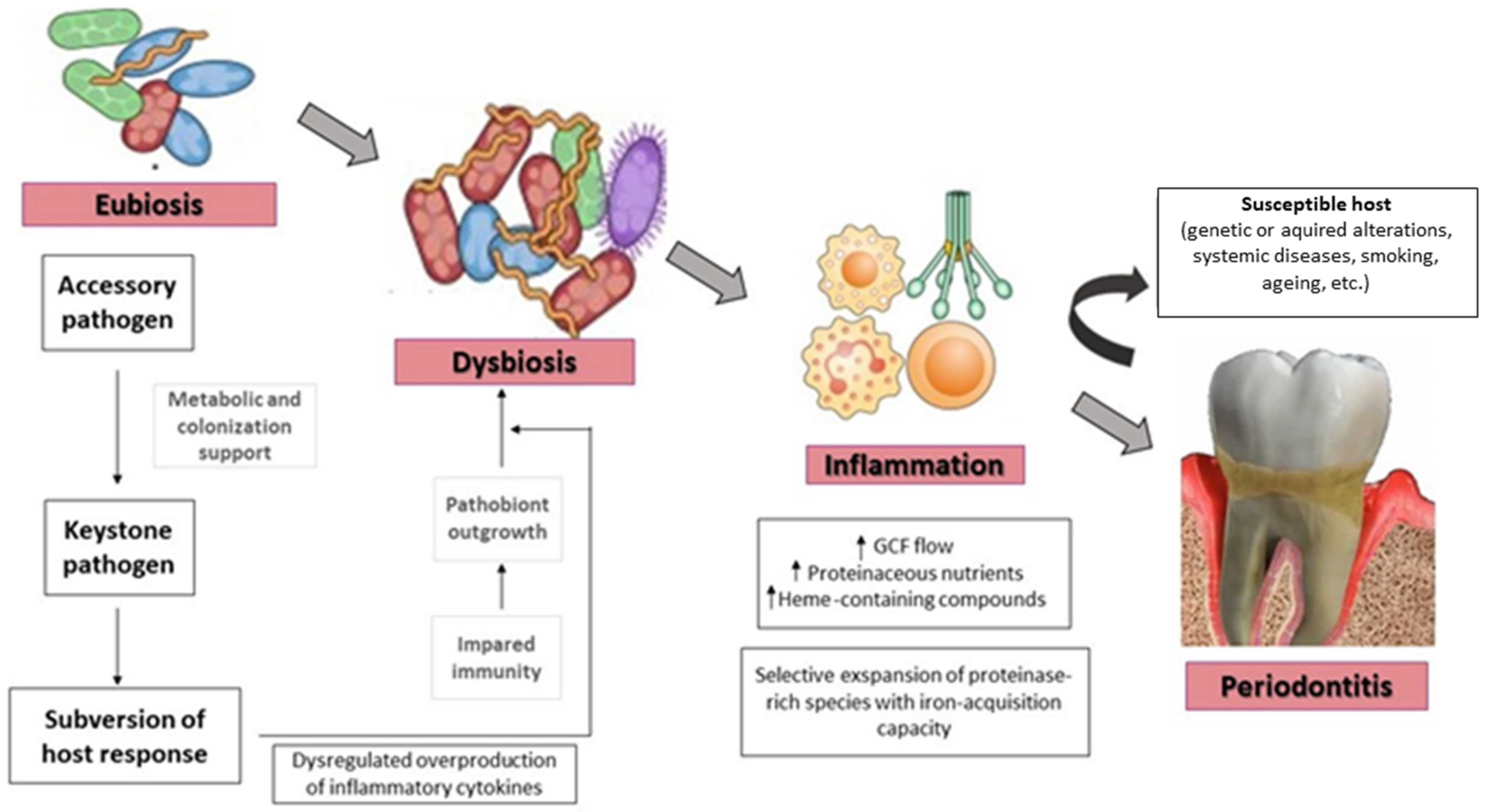
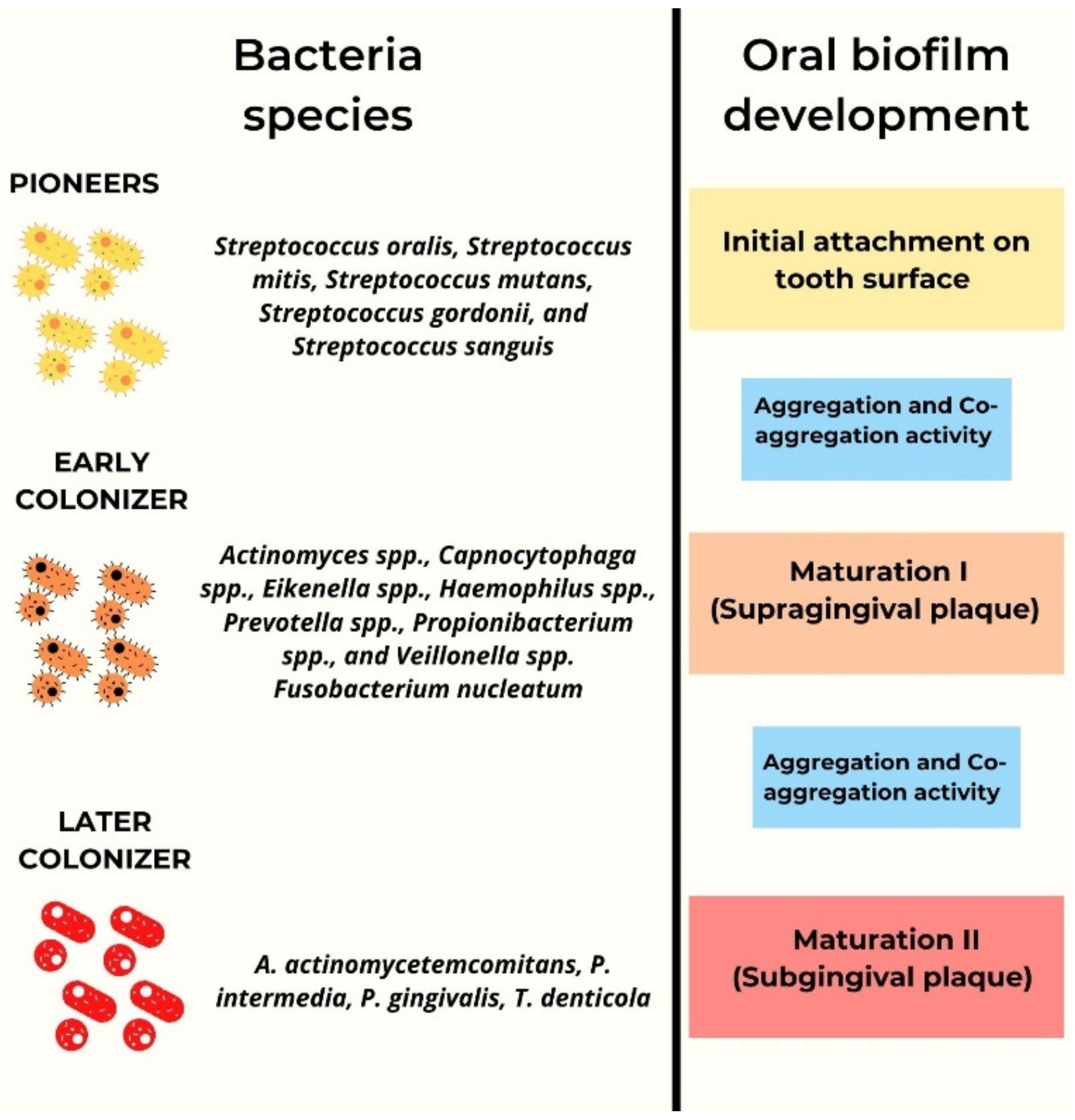
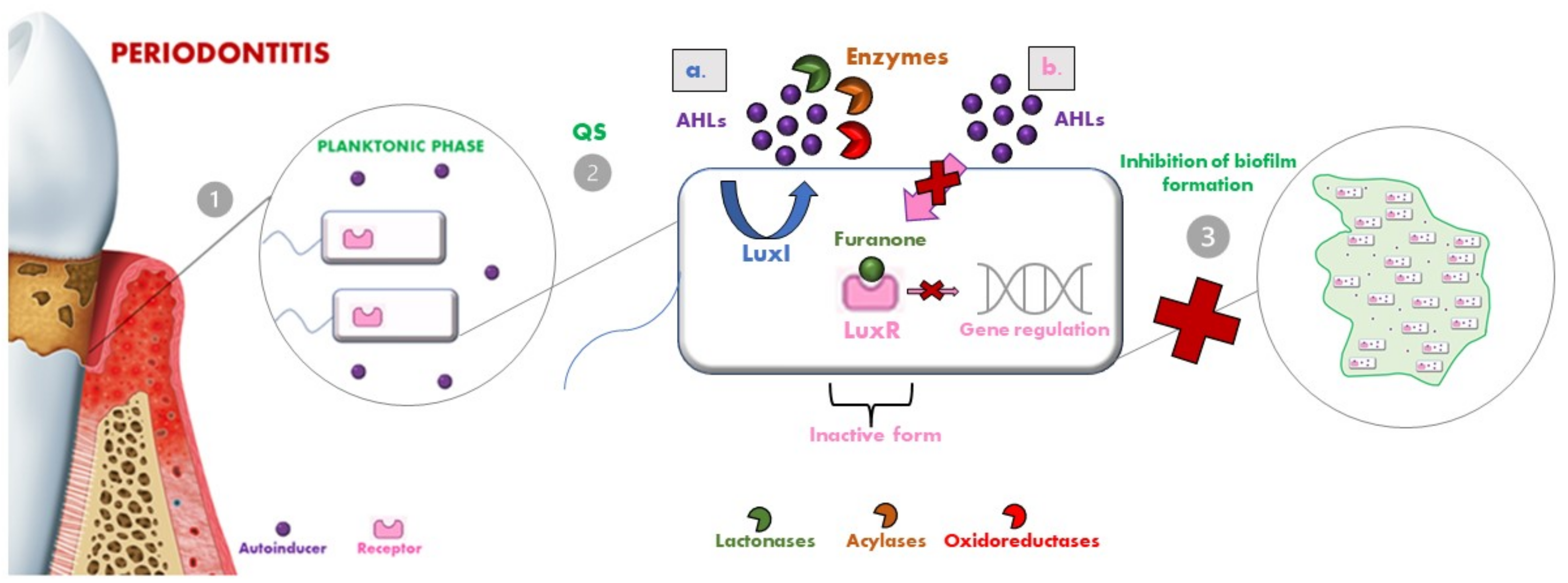
| System | Molecules | Bacteria Type | Oral Bacteria Species | Mechanism |
|---|---|---|---|---|
| Acyl homoserine lactone (AHL) | LuxR and LuxI | Gram-negative | Lack of consistent evidence [57] | Species-specific |
| Acyl homoserine lactone (AHL) | LuxM and LuxN | Gram-negative | Lack of consistent evidence [57] | Species-specific |
| Small peptides (AIP) | Small peptides | Gram-positive | S. mutans [40,41,42] | Species-specific |
| Furanosyl borate diester (AI-2) | LuxPQ and LuxS | Gram-negative Gram-positive | F. nucleatum, P. intermedia, P.gingivalis, T. denticola, A.a, S. mutans, S. gordonii [50,51,52,53] | Inter-species |
| QS Inhibitor | Mechanism of Action | Refs. |
|---|---|---|
| Synthetic analogues of AHL | Inhibition of a well-organized biofilm formation against P.gingivalis | [106] |
| Lactonases | Degradation of AHLs (hydrolysis of the ester bond of the lactone ring) | [107] |
| Acylases | Degradation of AHLs (hydrolysis of the amide bond) | [108] |
| Oxidoreductases | Inhibition of receptor binding (modification of AHL action) | [110] |
| Coumarin | Inhibition of the formation of P.gingivalis biofilm | [112] |
| Green tea extracts | Inhibition of P. gingivalis biofilm growth and adhesion | [112] |
| Umbelliferone derivatives | Moderate antibiofilm potential against clinical strains contained in the dental plaque of diabetic patients with periodontitis | [114] |
| Halogenated furanones | Increased turnover rate of the LuxR receptor (structural resemblance to AHLs) | [116,117] |
| Brominated bicyclic synthetic furanones | Reduction in biofilm biomass and thickness against P. gingivalis, F. nucleatum and T. forsythia | [118,119] |
| Brominated furanone and D-ribose | Reduction in P. gingivalis levels and bone loss | [120] |
| D-galactose | Reduction of P. gingivalis, F. nucleatum and T. forsythia biofilms formation and inhibition of the interaction of A. a. s serotypes b and f with F. nucleatum | [66,121] |
| Short-chain fatty acids (SCFAs) | Inhibition of biofilm formation of S. gordonii | [130] |
| Baicalein | Inhibition of S. mutans biofilm formation | [133] |
| CHX with fluoride and trans-cinnamaldehyde | Inhibition of S. mutans biofilm formation | [134] |
| Organosulfur compounds and S-Aryl-L-cysteine sulfoxides | Potential reduction in biofilm formation on S. mutans UA159, S. sanguis 10556, Actinomyces oris MG1 and inhibition of QS on V. harveyi | [137] |
| D-arabinose | Inhibition of biofilm formation of S. oralis, F. nucleatum and P. gingivalis (inhibitor of AI-2) | [143] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Polizzi, A.; Donzella, M.; Nicolosi, G.; Santonocito, S.; Pesce, P.; Isola, G. Drugs for the Quorum Sensing Inhibition of Oral Biofilm: New Frontiers and Insights in the Treatment of Periodontitis. Pharmaceutics 2022, 14, 2740. https://doi.org/10.3390/pharmaceutics14122740
Polizzi A, Donzella M, Nicolosi G, Santonocito S, Pesce P, Isola G. Drugs for the Quorum Sensing Inhibition of Oral Biofilm: New Frontiers and Insights in the Treatment of Periodontitis. Pharmaceutics. 2022; 14(12):2740. https://doi.org/10.3390/pharmaceutics14122740
Chicago/Turabian StylePolizzi, Alessandro, Martina Donzella, Giada Nicolosi, Simona Santonocito, Paolo Pesce, and Gaetano Isola. 2022. "Drugs for the Quorum Sensing Inhibition of Oral Biofilm: New Frontiers and Insights in the Treatment of Periodontitis" Pharmaceutics 14, no. 12: 2740. https://doi.org/10.3390/pharmaceutics14122740
APA StylePolizzi, A., Donzella, M., Nicolosi, G., Santonocito, S., Pesce, P., & Isola, G. (2022). Drugs for the Quorum Sensing Inhibition of Oral Biofilm: New Frontiers and Insights in the Treatment of Periodontitis. Pharmaceutics, 14(12), 2740. https://doi.org/10.3390/pharmaceutics14122740








