New and Old Horizons for an Ancient Drug: Pharmacokinetics, Pharmacodynamics, and Clinical Perspectives of Dimethyl Fumarate
Abstract
1. Introduction
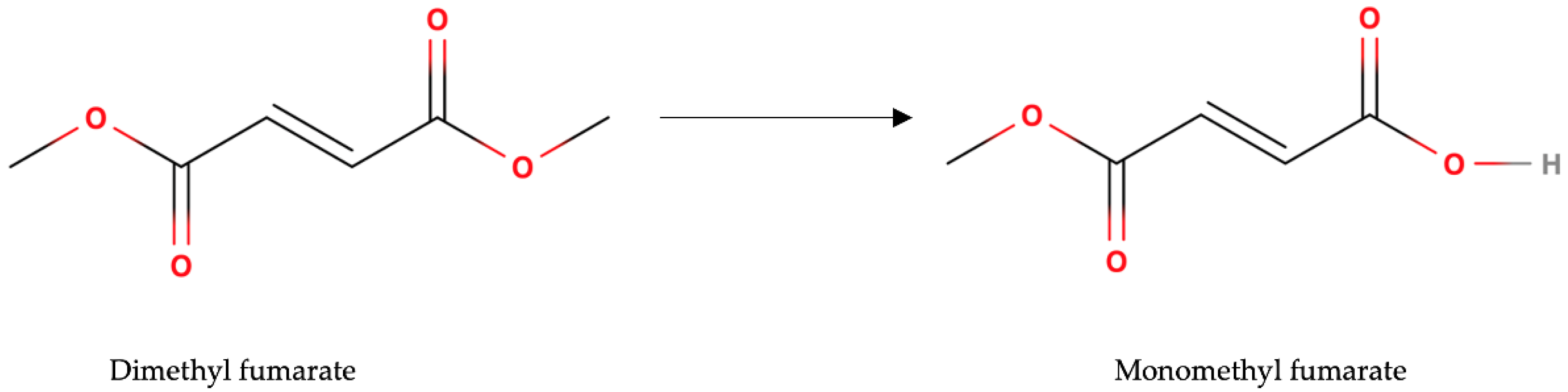
2. Pharmacokinetics
3. Pharmacodynamics
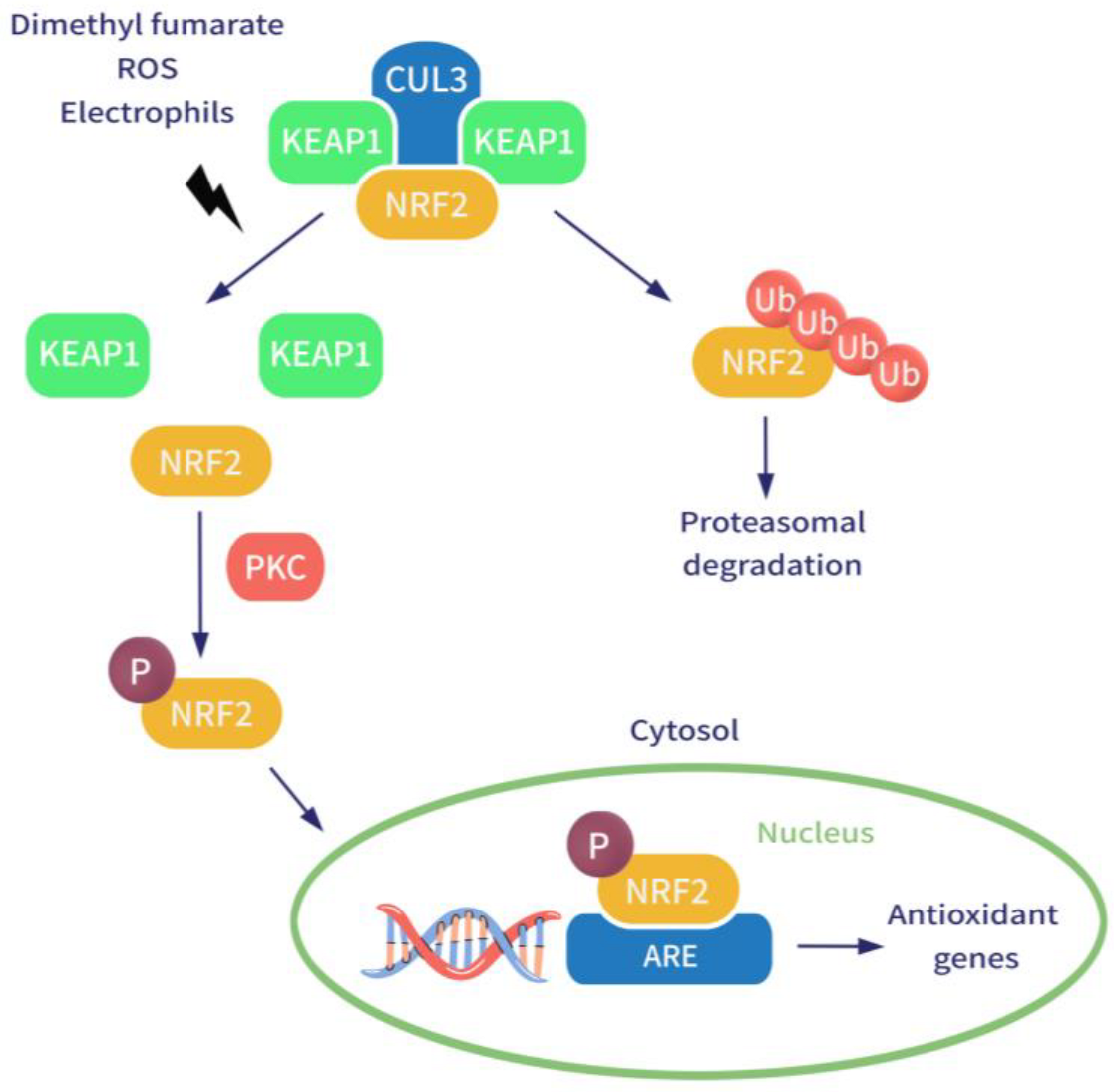
3.1. Enhancing Nuclear Factor 2 Pathway
3.2. Effects on the Hydroxycarboxylic Acid Receptor 2 (HCAR2)
3.3. Modulation of GSH
3.4. Inhibition of NF-κB
3.5. Effects on the Immune System
3.6. Regulation of Iron Metabolism in the Brain
3.7. Antimicrobial Effects and Modulation of Gut Microbiota
4. Adverse Effects
4.1. Gastrointestinal Adverse Effects
4.2. Hematological Disorders
4.3. Progressive Multiple Leukoencephalopathy
4.4. Fanconi Syndrome
4.5. Flushing
5. Indications for DMF
5.1. Psoriasis
5.2. Multiple Sclerosis
6. Special Issues and Possible Future Indications
6.1. Pregnancy and Breastfeeding
6.2. SARS-CoV-2 Infection
6.3. Cancer
6.4. Cardiovascular Diseases
6.5. Solid Lipid Nanoparticles for DMF Administration
7. Conclusions and Expert Opinion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Schweckendiek, W. Treatment of psoriasis vulgaris. Med. Monatsschr. 1959, 13, 103–104. [Google Scholar] [PubMed]
- Landeck, L.; Asadullah, K.; Amasuno, A.; Pau-Charles, I.; Mrowietz, U. Dimethyl fumarate (DMF) vs. monoethyl fumarate (MEF) salts for the treatment of plaque psoriasis: A review of clinical data. Arch. Dermatol. Res. 2018, 310, 475–483. [Google Scholar] [CrossRef] [PubMed]
- Werdenberg, D.; Joshi, R.; Wolffram, S.; Merkle, H.; Langguth, P. Presystemic metabolism and intestinal absorption of antipsoriatic fumaric acid esters. Biopharm. Drug Dispos. 2003, 24, 259–273. [Google Scholar] [CrossRef] [PubMed]
- Litjens, N.; Burggraaf, J.; Van Strijen, E.; Van Gulpen, C.; Mattie, H.; Schoemaker, R.C.; Van Dissel, J.T.; Thio, H.B.; Nibbering, P.H. Pharmacokinetics of oral fumarates in healthy subjects. Br. J. Clin. Pharmacol. 2004, 58, 429–432. [Google Scholar] [CrossRef]
- Linker, R.A.; Haghikia, A. Dimethyl fumarate in multiple sclerosis: Latest developments, evidence and place in therapy. Ther. Adv. Chronic. Dis. 2016, 7, 198–207. [Google Scholar] [CrossRef]
- Skilarence Summary of Products Characteristics. Almirall. May 2018. Available online: https://www.ema.europa.eu/en/documents/product-information/skilarence-epar-product-information_en.pdf (accessed on 29 November 2022).
- Zhao, H.; Eguchi, S.; Alam, A.; Ma, D. The role of nuclear factor-erythroid 2 related factor 2 (Nrf-2) in the protection against lung injury. Am. J. Physiol. Lung Cell. Mol. Physiol. 2017, 312, L155–L162. [Google Scholar] [CrossRef]
- Ruggieri, S.; Tortorella, C.; Gasperini, C. Pharmacology and clinical efficacy of dimethyl fumarate (BG-12) for treatment of relapsing-remitting multiple sclerosis. Ther. Clin. Risk Manag. 2014, 10, 229–239. [Google Scholar]
- Kobayashi, A.; Kang, M.-I.; Okawa, H.; Ohtsuji, M.; Zenke, Y.; Chiba, T.; Igarashi, K.; Yamamoto, M. Oxidative stress sensor Keap1 functions as an adaptor for Cul3-based E3 ligase to regulate proteasomal degradation of Nrf2. Mol. Cell. Biol. 2004, 24, 7130–7139. [Google Scholar] [CrossRef]
- Itoh, K.; Chiba, T.; Takahashi, S.; Ishii, T.; Igarashi, K.; Katoh, Y.; Oyake, T.; Hayashi, N.; Satoh, K.; Hatayama, I.; et al. An Nrf2/small Maf heterodimer mediates the induction of phase II detoxifying enzyme genes through antioxidant response elements. Biochem. Biophys. Res. Commun. 1997, 236, 313–322. [Google Scholar] [CrossRef]
- Di Nuzzo, L.; Orlando, R.; Nasca, C.; Nicoletti, F. Molecular pharmacodynamics of new oral drugs used in the treatment of multiple sclerosis. Drug Des. Devel. Ther. 2014, 8, 555–568. [Google Scholar]
- Brennan, M.S.; Matos, M.F.; Li, B.; Hronowski, X.; Gao, B.; Juhasz, P.; Rhodes, K.J.; Scannevin, R.H. Dimethyl fumarate and monoethyl fumarate exhibit differential effects on KEAP1, NRF2 activation, and glutathione depletion in vitro. PLoS ONE 2015, 10, e0120254. [Google Scholar] [CrossRef] [PubMed]
- Cuadrado, A.; Kuügler, S.; Lastres-Becker, I. Pharmacological targeting of GSK-3 and NRF2 provides neuroprotection in a preclinical model of tauopathy. Redox Biol. 2018, 14, 522–534. [Google Scholar] [CrossRef] [PubMed]
- Piroli, G.G.; Manuel, A.M.; Patel, T.; Walla, M.D.; Shi, L.; Lanci, S.A.; Wang, J.; Galloway, A.; Ortinski, P.I.; Smith, D.S.; et al. Identification of Novel Protein Targets of Dimethyl Fumarate Modification in Neurons and Astrocytes Reveals Actions Independent of Nrf2 Stabilization. Mol. Cell. Proteom. 2019, 18, 504–519. [Google Scholar] [CrossRef] [PubMed]
- Rosito, M.; Testi, C.; Parisi, G.; Cortese, B.; Baiocco, P.; Di Angelantonio, S. Exploring the Use of Dimethyl Fumarate as Microglia Modulator for Neurodegenerative Diseases Treatment. Antioxidants 2020, 9, 700. [Google Scholar] [CrossRef] [PubMed]
- Cuadrado, A.; Martín-Moldes, Z.; Ye, J.; Lastres-Becker, I. Transcription factors NRF2 and NF-κB are coordinated effectors of the Rho family, GTP-binding protein RAC1 during inflammation. J. Biol. Chem. 2014, 289, 15244–15258. [Google Scholar] [CrossRef] [PubMed]
- Parodi, B.; Rossi, S.; Morando, S.; Cordano, C.; Bragoni, A.; Motta, C.; Usai, C.; Wipke, B.T.; Scannevin, R.H.; Mancardi, G.L.; et al. Fumarates modulate microglia activation through a novel HCAR2 signaling pathway and rescue synaptic dysregulation in inflamed CNS. Acta Neuropathol. 2015, 130, 279–295. [Google Scholar] [CrossRef]
- Chen, H.; Assmann, J.C.; Krenz, A.; Rahman, M.; Grimm, M.; Karsten, C.M.; Kohl, J.; Offermanns, S.; Wettschureck, N.; Schwaninger, M. Hydroxycarboxylic acid receptor 2 mediates dimethyl fumarate’s protective effect in EAE. J. Clin. Investig. 2014, 124, 2188–2192. [Google Scholar] [CrossRef]
- Hanson, J.; Gille, A.; Offermanns, S. Role of HCA₂ (GPR109A) in nicotinic acid and fumaric acid ester-induced effects on the skin. Pharmacol. Ther. 2012, 136, 1–7. [Google Scholar] [CrossRef]
- Hoffmann, C.; Dietrich, M.; Herrmann, A.K.; Schacht, T.; Albrecht, P.; Methner, A. Dimethyl Fumarate Induces Glutathione Recycling by Upregulation of Glutathione Reductase. Oxidative Med. Cell. Longev. 2017, 2017, 6093903. [Google Scholar] [CrossRef]
- Gillard, G.O.; Collette, B.; Anderson, J.; Chao, J.; Scannevin, R.H.; Huss, D.J.; Fontenot, J.D. DMF, but not other fumarates, inhibits NF-κB activity in vitro in an Nrf2-independent manner. J. Neuroimmunol. 2015, 283, 74–85. [Google Scholar] [CrossRef]
- Kastrati, I.; Siklos, M.I.; Calderon-Gierszal, E.L.; El-Shennawy, L.; Georgieva, G.; Thayer, E.N.; Thatcher, G.R.; Frasor, J. Dimethyl Fumarate Inhibits the Nuclear Factor κB Pathway in Breast Cancer Cells by Covalent Modification of p65 Protein. J. Biol. Chem. 2016, 291, 3639–3647. [Google Scholar] [CrossRef] [PubMed]
- Treumer, F.; Zhu, K.; Gläser, R.; Mrowietz, U. Dimethylfumarate is a potent inducer of apoptosis in human T cells. J. Investig. Dermatol. 2003, 121, 1383–1388. [Google Scholar] [CrossRef] [PubMed]
- De Jong, R.; Bezemer, A.C.; Zomerdijk, T.P.; Pouw-Kraan, T.; Ottenhoff, T.H.; Nibbering, P.H. Selective stimulation of T helper 2 cytokine responses by the anti-psoriasis agent monomethylfumarate. Eur. J. Immunol. 1996, 26, 2067–2074. [Google Scholar] [CrossRef] [PubMed]
- Vandermeeren, M.; Janssens, S.; Borgers, M.; Geysen, J. Dimethylfumarate is an inhibitor of cytokine-induced E-selectin, VCAM-1, and ICAM-1 expression in human endothelial cells. Biochem. Biophys. Res. Commun. 1997, 234, 19–23. [Google Scholar] [CrossRef] [PubMed]
- Foster, C.A.; Dreyfuss, M.; Mandak, B.; Meingassner, J.G.; Naegeli, H.U.; Nussbaumer, A.; Oberer, L.; Scheel, G.; Swoboda, E.M. Pharmacological modulation of endothelial cell-associated adhesion molecule expression: Implications for future treatment of dermatological diseases. J. Dermatol. 1994, 21, 847–854. [Google Scholar] [CrossRef]
- Connor, J.R.; Menzies, S.L. Relationship of iron to oligodendrocytes and myelination. Glia 1996, 17, 83–93. [Google Scholar] [CrossRef]
- Hallgren, B.; Sourander, P. The effect of age on the non-haemin iron in the human brain. J. Neurochem. 1958, 3, 41–51. [Google Scholar] [CrossRef]
- Todorich, B.; Zhang, X.; Connor, J.R. H-ferritin is the major source of iron for oligodendrocytes. Glia 2011, 59, 927–935. [Google Scholar] [CrossRef]
- Craelius, W.; Migdal, M.W.; Luessenhop, C.P.; Sugar, A.; Mihalakis, I. Iron deposits surrounding multiple sclerosis plaques. Arch. Pathol. Lab. Med. 1982, 106, 397–399. [Google Scholar]
- Haider, L.; Simeonidou, C.; Steinberger, G.; Hametner, S.; Grigoriadis, N.; Deretzi, G.; Kovacs, G.G.; Kutzelnigg, A.; Lassmann, H.; Frischer, J.M. Multiple sclerosis deep grey matter: The relation between demyelination, neurodegeneration, inflammation and iron. J. Neurol. Neurosurg. Psychiatry 2014, 85, 1386–1395. [Google Scholar] [CrossRef]
- Kronenberg, J.; Pars, K.; Brieskorn, M.; Prajeeth, C.K.; Heckers, S.; Schwenkenbecher, P.; Skripuletz, T.; Pul, R.; Pavlou, A.; Stangel, M. Fumaric Acids Directly Influence Gene Expression of Neuroprotective Factors in Rodent Microglia. Int. J. Mol. Sci. 2019, 20, 325. [Google Scholar] [CrossRef] [PubMed]
- Barnes, R.H.; Karatzas, K.A.G. Investigation into the antimicrobial activity of fumarate against Listeria monocytogenes and its mode of action under acidic conditions. Int. J. Food Microbiol. 2020, 324, 108614. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.H.; Sun, D.W.; Kuang, R. Inhibition of Escherichia coli by dimethyl fumarate. Int. J. Food. Microbiol. 2001, 65, 125–130. [Google Scholar] [CrossRef]
- Rumah, K.R.; Vartanian, T.K.; Fischetti, V.A. Oral Multiple Sclerosis Drugs Inhibit the in vitro Growth of Epsilon Toxin Producing Gut Bacterium, Clostridium perfringens. Front. Cell. Infect. Microbiol. 2017, 7, 11. [Google Scholar] [CrossRef] [PubMed]
- Jones, S.A.; Gibson, T.; Maltby, R.; Chowdhury, F.Z.; Stewart, V.; Cohen, P.S.; Conway, T. Anaerobic respiration of Escherichia coli in the mouse intestine. Infect. Immun. 2011, 79, 4218–4226. [Google Scholar] [CrossRef]
- Storm-Larsen, C.; Myhr, K.M.; Farbu, E.; Midgard, R.; Nyquist, K.; Broch, L.; Berg-Hansen, P.; Buness, A.; Holm, K.; Ueland, T.; et al. Gut microbiota composition during a 12-week intervention with delayed-release dimethyl fumarate in multiple sclerosis—A pilot trial. Mult. Scler. J. Exp. Transl. Clin. 2019, 5, 2055217319888767. [Google Scholar] [CrossRef]
- Phillips, J.T.; Erwin, A.A.; Agrella, S.; Kremenchutzky, M.; Kramer, J.F.; Darkes, M.J.; Kendter, J.; Abourjaily, H.; Rana, J.; Fox, R.J. Consensus Management of Gastrointestinal Events Associated with Delayed-Release Dimethyl Fumarate: A Delphi Study. Neurol. Ther. 2015, 4, 137–146. [Google Scholar] [CrossRef]
- Makhani, N.; Schreiner, T. Oral dimethyl fumarate in children with multiple sclerosis: A dual-center study. Pediatr. Neurol. 2016, 57, 101–104. [Google Scholar] [CrossRef]
- Lucchini, M.; Prosperini, L.; Buscarinu, M.C.; Centonze, D.; Conte, A.; Cortese, A.; Elia, G.; Fantozzi, R.; Ferraro, E.; Gasperini, C.; et al. Predictors of lymphocyte count recovery after dimethyl fumarate-induced lymphopenia in people with multiple sclerosis. J. Neurol. 2021, 268, 2238–2245. [Google Scholar] [CrossRef]
- Mills, E.A.; Ogrodnik, M.A.; Plave, A.; Mao-Draayer, Y. Emerging Understanding of the Mechanism of Action for Dimethyl Fumarate in the Treatment of Multiple Sclerosis. Front. Neurol. 2018, 9, 5. [Google Scholar] [CrossRef]
- Nieuwkamp, D.J.; Murk, J.L.; van Oosten, B.W.; Cremers, C.H.; Killestein, J.; Viveen, M.C.; Van Hecke, W.; Frijlink, D.W.; Wattjes, M.P. PML in Dutch MS Patients Consortium. PML in a patient without severe lymphocytopenia receiving dimethyl fumarate. N. Engl. J. Med. 2015, 372, 1474–1476. [Google Scholar] [CrossRef] [PubMed]
- Shackelton, L.A.; Rambaut, A.; Pybus, O.G.; Holmes, E.C. JC Virus evolution and its association with human populations. J. Virol. 2006, 80, 9928–9933. [Google Scholar] [CrossRef]
- Jordan, A.L.; Yang, J.; Fisher, C.J.; Racke, M.K.; Mao-Draayer, Y. Progressive multifocal leukoencephalopathy in dimethyl fumarate-treated multiple sclerosis patients. Mult. Scler. 2022, 28, 7–15. [Google Scholar] [CrossRef]
- Bharat, A.; Xie, F.; Baddley, J.W.; Beukelman, T.; Chen, L.; Calabrese, L.; Curtis, J.R. Incidence and risk factors for progressive multifocal leukoencephalopathy among patients with selected rheumatic diseases. Arthritis. Care Res. 2012, 64, 612–615. [Google Scholar] [CrossRef] [PubMed]
- Vola, E.A.; Petracca, M.; Cocozza, S.; De Angelis, M.; Carotenuto, A.; Pontillo, G.; Morra, V.B.; Tedeschi, E.; Lanzillo, R. Possible progressive multifocal leukoencephalopathy and active multiple sclerosis under dimethyl fumarate: The central role of MRI in informing therapeutic decisions. BMC Neurol. 2021, 21, 146. [Google Scholar] [CrossRef] [PubMed]
- Wan, E.R.; Siew, K.; Heptinstall, L.; Walsh, S.B. Fumaric acid ester-induced renal Fanconi syndrome: Evidence of mitochondrial toxicity. Clin. Kidney J. 2021, 14, 2085–2089. [Google Scholar] [CrossRef]
- O’Gorman, J.; Russell, H.K.; Li, J.; Phillips, G.; Kurukulasuriya, N.C.; Viglietta, V. Effect of Aspirin Pretreatment or Slow Dose Titration on Flushing and Gastrointestinal Events in Healthy Volunteers Receiving Delayed-release Dimethyl Fumarate. Clin. Ther. 2015, 37, 1402–1419.e5. [Google Scholar] [CrossRef]
- Corazza, M.; Odorici, G.; Conti, A.; Di Lernia, V.; Motolese, A.; Bardazzi, F.; Di Nuzzo, S.; Monti, A.; Arginelli, F.; Filippi, F.; et al. Dimethyl fumarate treatment for psoriasis in a real-life setting: A multicentric retrospective study. Dermatol. Ther. 2021, 34, e15066. [Google Scholar] [CrossRef]
- Mrowietz, U.; Van De Kerkhof, P.; Schoenenberger, A.; Ryzhkova, A.; Pau-Charles, I.; Llamas-Velasco, M.; Daudén, E.; Carrascosa, J.M.; Cueva, P.; Salgado-Boquete, L.; et al. Efficacy of dimethyl fumarate treatment for moderate-to-severe plaque psoriasis: Presentation extracts from the 29th EADV virtual congress, 29–31 October 2020. Expert. Rev. Clin. Immunol. 2021, 17, 1–11. [Google Scholar] [CrossRef]
- Bardazzi, F.; Magnano, M.; Campanati, A.; Loconsole, F.; Carpentieri, A.; Potenza, C.; Bernardini, N.; Di Lernia, V.; Carrera, C.; Raone, B.; et al. Biologic Therapies in HIV-infected Patients with Psoriasis: An Italian Experience. Acta Derm. Venereol. 2017, 97, 989–990. [Google Scholar] [CrossRef]
- Gisondi, P.; Cazzaniga, S.; Chimenti, S.; Maccarone, M.; Picardo, M.; Girolomoni, G.; Naldi, L.; Psocare Study Group. Latent tuberculosis infection in patients with chronic plaque psoriasis: Evidence from the Italian Psocare Registry. Br. J. Dermatol. 2015, 172, 1613–1620, Erratum in: Br. J. Dermatol. 2017, 176, 1415–1416.. [Google Scholar] [CrossRef]
- Naval, S.M.; Casas, L.R.; Spackman, E. Cost effectiveness of dimethyl fumarate (DMF) in the treatment of moderate to severe plaque psoriasis: A uk perspective. Value Health 2018, 21, S1eS481. [Google Scholar]
- Schimrigk, S.; Brune, N.; Hellwig, K.; Lukas, C.; Bellenberg, B.; Rieks, M.; Hoffmann, V.; Pohlau, D.; Przuntek, H. Oral fumaric acid esters for the treatment of active multiple sclerosis: An open-label, baseline-controlled pilot study. Eur. J. Neurol. 2006, 13, 604–610. [Google Scholar] [CrossRef] [PubMed]
- Kappos, L.; Gold, R.; Miller, D.H.; MacManus, D.G.; Havrdova, E.; Limmroth, V.; Polman, C.H.; Schmierer, K.; Yousry, T.A.; Yang, M.; et al. Efficacy and safety of oral fumarate in patients with relapsing-remitting multiple sclerosis: A multicentre, randomised, double-blind, placebo-controlled phase IIb study. Lancet 2008, 372, 1463–1472. [Google Scholar] [CrossRef] [PubMed]
- Fernández, Ó.; Giovannoni, G.; Fox, R.J.; Gold, R.; Phillips, J.T.; Potts, J.; Okwuokenye, M.; Marantz, J.L. Efficacy and Safety of Delayed-release Dimethyl Fumarate for Relapsing-remitting Multiple Sclerosis in Prior Interferon Users: An Integrated Analysis of DEFINE and CONFIRM. Clin. Ther. 2017, 39, 1671–1679. [Google Scholar] [CrossRef] [PubMed]
- Phillips, J.T.; Selmaj, K.; Gold, R.; Fox, R.J.; Havrdova, E.; Giovannoni, G.; Abourjaily, H.; Pace, A.; Novas, M.; Hotermans, C.; et al. Clinical Significance of Gastrointestinal and Flushing Events in Patients with Multiple Sclerosis Treated with Delayed-Release Dimethyl Fumarate. Int. J. MS Care 2015, 17, 236–243. [Google Scholar] [CrossRef]
- Hellwig, K.; Rog, D.; McGuigan, C.; Houtchens, M.K.; Bruen, D.R.; Mokliatchouk, O.; Branco, F.; Peng, X.; Everage, N.J. Interim Analysis of Pregnancy Outcomes After Exposure to Dimethyl Fumarate in a Prospective International Registry. Neurol. Neuroimmunol. Neuroinflamm. 2021, 9, e1114. [Google Scholar] [CrossRef]
- Drugs and Lactation Database (LactMed); National Library of Medicine: Bethesda, MD, USA, 2006; Dimethyl Fumarate. [Updated 19 September 2022].
- Campanati, A.; Brisigotti, V.; Diotallevi, F.; D’Agostino, G.M.; Paolinelli, M.; Radi, G.; Rizzetto, G.; Sapigni, C.; Tagliati, C.; Offidani, A. Active implications for dermatologists in ‘SARS-CoV-2 ERA’: Personal experience and review of literature. J. Eur. Acad. Dermatol. Venereol. 2020, 34, 1626–1632. [Google Scholar] [CrossRef]
- Olagnier, D.; Farahani, E.; Thyrsted, J.; Blay-Cadanet, J.; Herengt, A.; Idorn, M.; Hait, A.; Hernaez, B.; Knudsen, A.; Iversen, M.B.; et al. SARS-CoV-2-mediated suppression of NRF2-signaling reveals potent antiviral and anti-inflammatory activity of 4-octyl-itaconate and dimethyl fumarate. Nat. Commun. 2020, 11, 4938. [Google Scholar] [CrossRef]
- Mantero, V.; Abate, L.; Basilico, P.; Balgera, R.; Salmaggi, A.; Nourbakhsh, B.; Cordano, C. COVID-19 in dimethyl fumarate-treated patients with multiple sclerosis. J. Neurol. 2020, 25, 1–3. [Google Scholar] [CrossRef]
- Esposito, M.; Campanati, A.; Giunta, A.; Calianno, G.; Bianchi, L.; Diotallevi, F.; Offidani, A.M.; Fargnoli, M.C. Dimethyl Fumarate’s Effectiveness and Safety in Psoriasis: A Real-Life Experience During the COVID-19 Pandemic. Dermatol. Ther. 2022, 12, 671–681. [Google Scholar] [CrossRef]
- Timpani, C.A.; Rybalka, E. Calming the (cytokine) storm: Dimethyl fumarate as a therapeutic candidate for COVID-19. Pharmaceuticals 2020, 14, 15. [Google Scholar] [CrossRef] [PubMed]
- Capone, F.; Ferraro, E.; Motolese, F.; Di Lazzaro, V. COVID-19 in multiple sclerosis patients treated with dimethyl fumarate. J. Neurol. 2021, 268, 3132–3134. [Google Scholar] [CrossRef]
- Kourakis, S.; Timpani, C.A.; de Haan, J.B.; Gueven, N.; Fischer, D.; Rybalka, E. Dimethyl Fumarate and Its Esters: A Drug with Broad Clinical Utility? Pharmaceuticals 2020, 13, 306. [Google Scholar] [CrossRef]
- Loewe, R.; Valero, T.; Kremling, S.; Pratscher, B.; Kunstfeld, R.; Pehamberger, H.; Petzelbauer, P. Dimethylfumarate impairs melanoma growth and metastasis. Cancer Res. 2006, 66, 11888–11896. [Google Scholar] [CrossRef] [PubMed]
- Nicolay, J.P.; Albrecht, J.D.; Assaf, C.; Dippel, E.; Stadler, R.; Wehkamp, U.; Wobser, M.; Guelow, K.; Goerdt, S.; Krammer, P.H. Dimethyl fumarate (DMF) therapy in CTCL: Results from a clinical phase II study. Eur. J. Cancer 2021, 156, S21–S22. [Google Scholar] [CrossRef] [PubMed]
- Kaluzki, I.; Hailemariam-Jahn, T.; Doll, M.; Kaufmann, R.; Balermpas, P.; Zoöller, N.; Kippenberger, S.; Meissner, M.; Jahn, H. Doll Dimethylfumarate Inhibits Colorectal Carcinoma Cell Proliferation: Evidence for Cell Cycle Arrest, Apoptosis and Autophagy. Cells 2019, 8, 1329. [Google Scholar] [CrossRef]
- Han, G.; Zhou, Q. Dimethylfumarate induces cell cycle arrest and apoptosis via regulating intracellular redox systems in HeLa cells. Vitr. Cell. Dev. Biol. Anim. 2016, 52, 1034–1041. [Google Scholar] [CrossRef]
- Saidu, N.E.B.; Noeé, G.; Cerles, O.; Cabel, L.; Kavian-Tessler, N.; Chouzenoux, S. Dimethyl FumarateControls the NRF2/DJ-1 Axis in Cancer Cells: Therapeutic Applications. Mol. Cancer Ther. 2017, 16, 529. [Google Scholar] [CrossRef]
- Schmieder, A.; Poppe, M.; Hametner, C.; Meyer-Schraml, H.; Schaarschmidt, M.-L.; Findeisen, P.; Benoit, S.; Bauer, B.; Schmid, S.; Goebeler, M.; et al. Impact of fumaric acid esters on cardiovascular risk factors and depression in psoriasis: A prospective pilot study. Arch. Dermatol. Res. 2015, 307, 413–424. [Google Scholar] [CrossRef]
- Kan, S.B.; Staun-Ram, E.; Golan, D.; Miller, A. HDL-cholesterol elevation associated with fingolimod and dimethyl fumarate therapies in multiple sclerosis. Mult. Scler. J. Exp. Transl. Clin. 2019, 5, 2055217319882720. [Google Scholar]
- Holzer, G.; Hoke, M.; Sabeti-Sandor, S.; Perkmann, T.; Rauscher, A.; Strassegger, B.; Radakovic, S.; Tanew, A. Disparate effects of adalimumab and fumaric acid esters on cardiovascular risk factors in psoriasis patients: Results from a prospective, randomized, observer-blinded head-to-head trial. J. Eur. Acad. Dermatol. Venereol. 2021, 35, 441–449. [Google Scholar] [CrossRef] [PubMed]
- Nour, O.A.; Shehatou, G.S.; Rahim, M.A.; El-Awady, M.S.; Suddek, G.M. Antioxidant and anti-inflammatory effects of dimethyl fumarate in hypercholesterolemic rabbits. Egypt. J. Basic. Appl. Sci. 2017, 4, 153–159. [Google Scholar] [CrossRef]
- Thomas, S.D.; Jha, N.K.; Sadek, B.; Ojha, S. Repurposing Dimethyl Fumarate for Cardiovascular Diseases: Pharmacological Effects, Molecular Mechanisms, and Therapeutic Promise. Pharmaceuticals 2022, 15, 497. [Google Scholar] [CrossRef]
- Esposito, E.; Cortesi, R.; Drechsler, M.; Fan, J.; Fu, B.M.; Calderan, L.; Mannucci, S.; Boschi, F.; Nastruzzi, C. Nanoformulations for dimethyl fumarate: Physicochemical characterization and in vitro/in vivo behavior. Eur. J. Pharm. Biopharm. 2017, 115, 285–296. [Google Scholar] [CrossRef]
| Adverse Effects | Possible Therapeutic Options |
|---|---|
| Diarrhea |
|
| Abdominal pain |
|
| Vomiting |
|
| Nausea |
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matteo, P.; Federico, D.; Emanuela, M.; Giulia, R.; Tommaso, B.; Alfredo, G.; Anna, C.; Annamaria, O. New and Old Horizons for an Ancient Drug: Pharmacokinetics, Pharmacodynamics, and Clinical Perspectives of Dimethyl Fumarate. Pharmaceutics 2022, 14, 2732. https://doi.org/10.3390/pharmaceutics14122732
Matteo P, Federico D, Emanuela M, Giulia R, Tommaso B, Alfredo G, Anna C, Annamaria O. New and Old Horizons for an Ancient Drug: Pharmacokinetics, Pharmacodynamics, and Clinical Perspectives of Dimethyl Fumarate. Pharmaceutics. 2022; 14(12):2732. https://doi.org/10.3390/pharmaceutics14122732
Chicago/Turabian StyleMatteo, Paolinelli, Diotallevi Federico, Martina Emanuela, Radi Giulia, Bianchelli Tommaso, Giacchetti Alfredo, Campanati Anna, and Offidani Annamaria. 2022. "New and Old Horizons for an Ancient Drug: Pharmacokinetics, Pharmacodynamics, and Clinical Perspectives of Dimethyl Fumarate" Pharmaceutics 14, no. 12: 2732. https://doi.org/10.3390/pharmaceutics14122732
APA StyleMatteo, P., Federico, D., Emanuela, M., Giulia, R., Tommaso, B., Alfredo, G., Anna, C., & Annamaria, O. (2022). New and Old Horizons for an Ancient Drug: Pharmacokinetics, Pharmacodynamics, and Clinical Perspectives of Dimethyl Fumarate. Pharmaceutics, 14(12), 2732. https://doi.org/10.3390/pharmaceutics14122732






