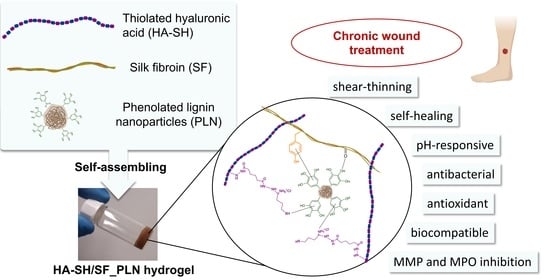
Lignin-Based Nanoparticles as Both Structural and Active Elements in Self-Assembling and Self-Healing Multifunctional Hydrogels for Chronic Wound Management
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Modification and Characterization of HA
2.2.1. Preparation of HA-ADH and HA-SH
2.2.2. FTIR Analysis
2.2.3. Determination of Amino and Thiol Groups
2.3. Preparation and Characterization of PLN
2.4. Synthesis of HA-SH/SF Hydrogels
2.5. Rheological Characterization
2.6. Cryogenic Scanning Electron Microscopy (Cryo-SEM)
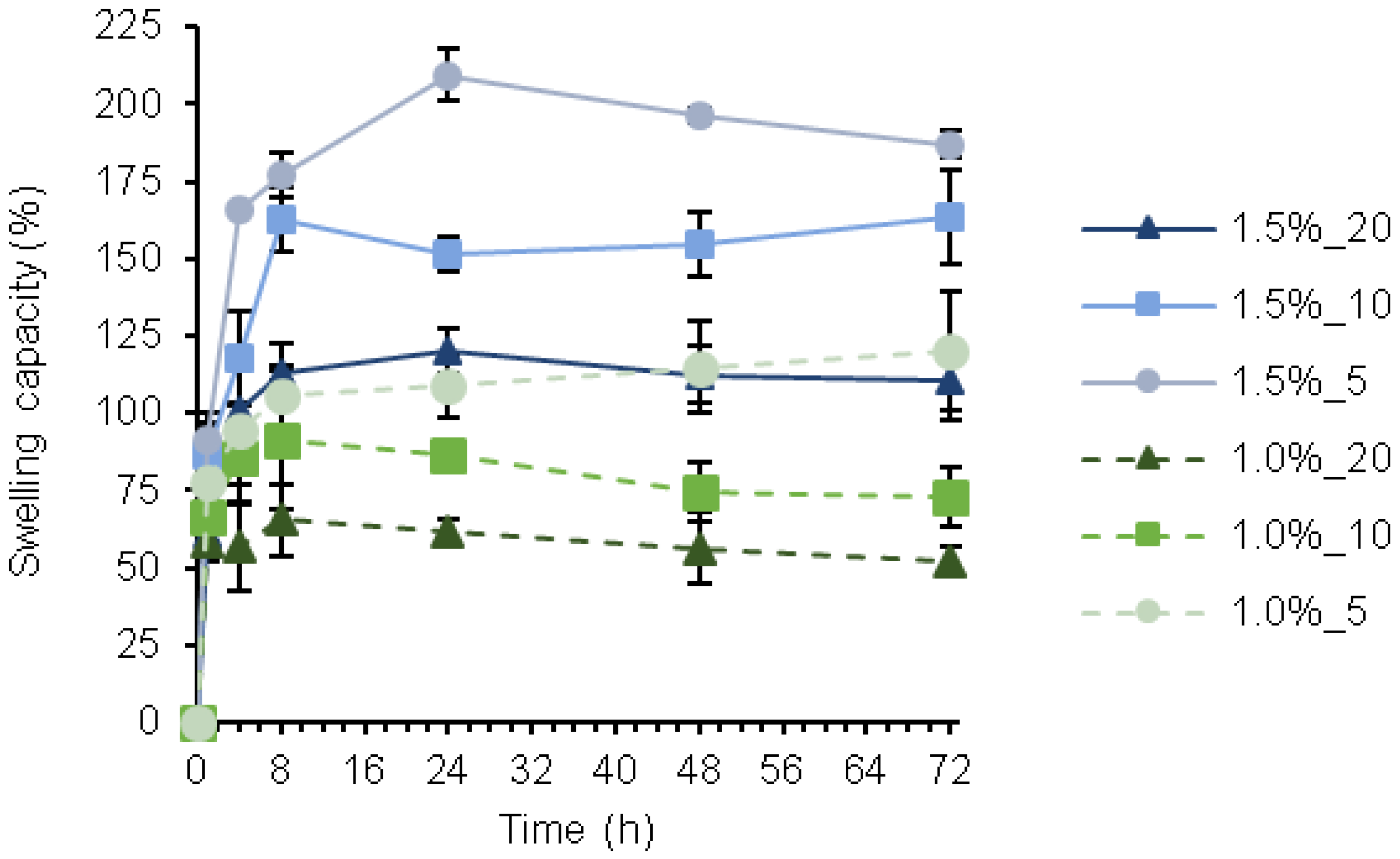
2.7. Swelling Capacity
2.8. Stability in PBS
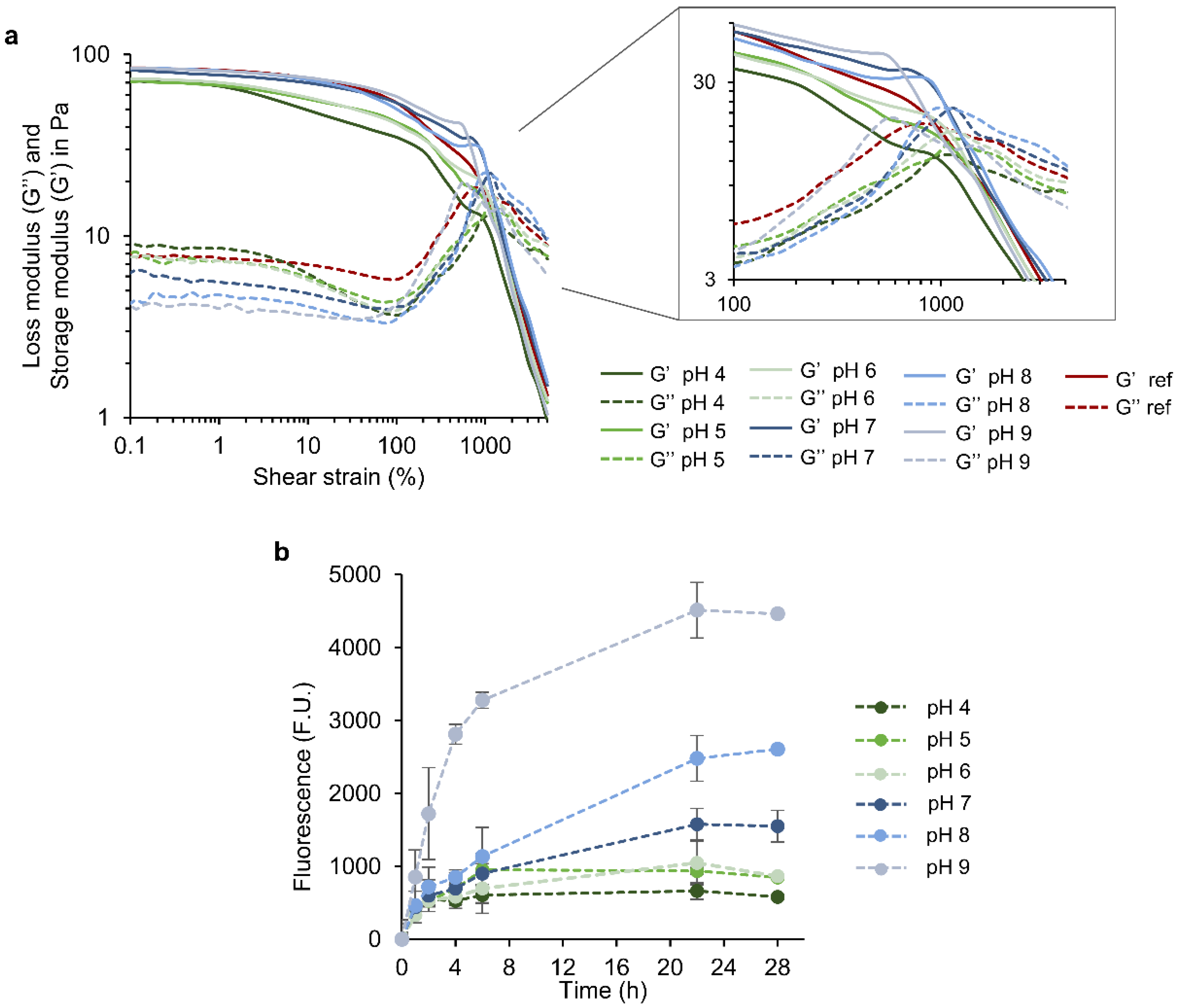
2.9. pH Responsiveness
2.10. Biodegradability and PLN Release in the Presence of Hyaluronidase
2.11. Antioxidant Activity
2.12. Antibacterial Activity
2.13. Morphology of Bacterial Cells
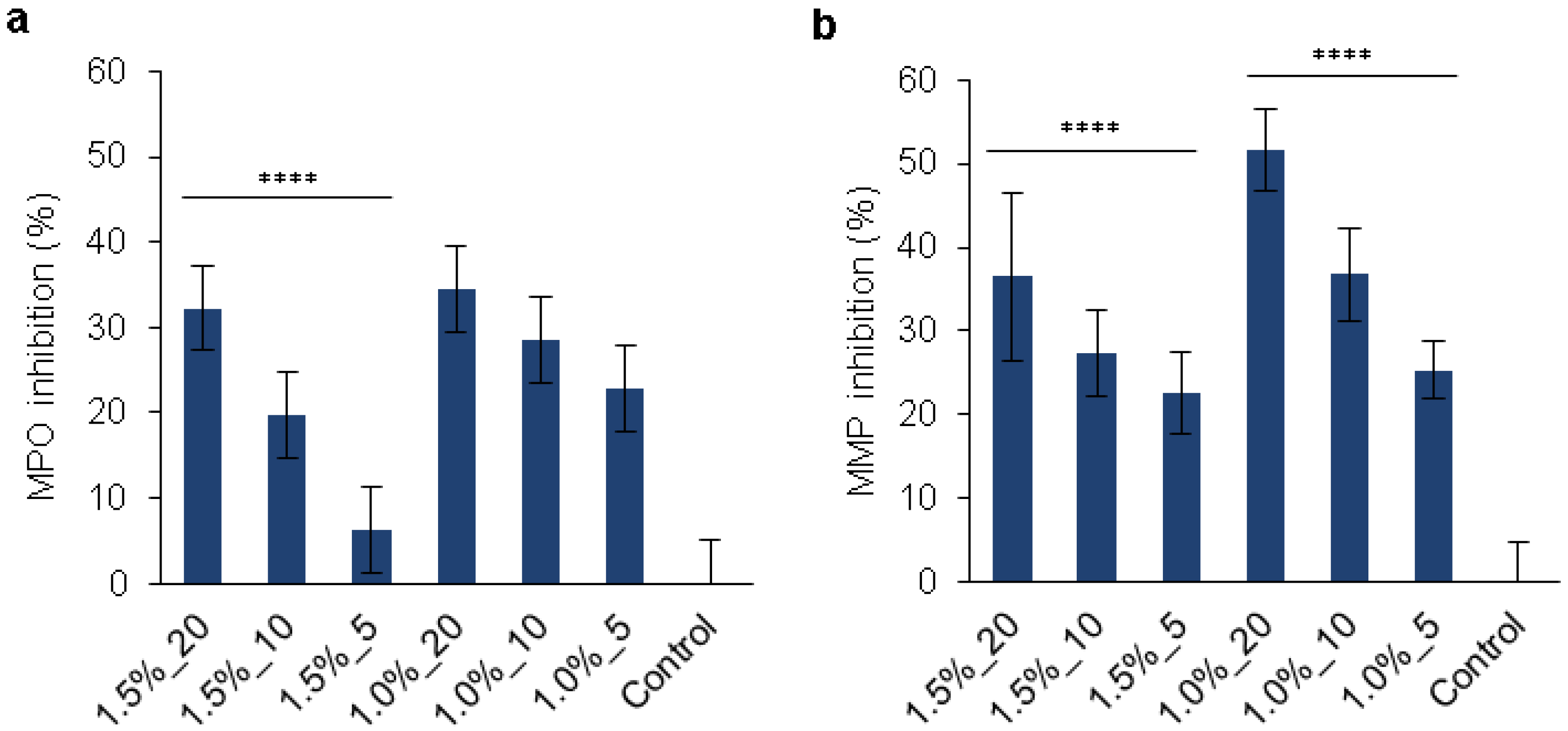
2.14. MPO and MMPs Inhibition
2.15. Cytotoxicity toward Human Cells
2.16. Data Analysis
3. Results and Discussion
3.1. Synthesis of Self-Assembling HA-SH/SF_PLN Hydrogels
3.2. Rheological Properties of HA-SH/SF_PLN Hydrogels
3.3. Swelling Capacity
3.4. Stability
3.5. Release of PLN and Hydrogel Stability in Response to pH and Hyaluronidase
3.6. Multiple Features of the Hydrogels for Promoting Wound Healing
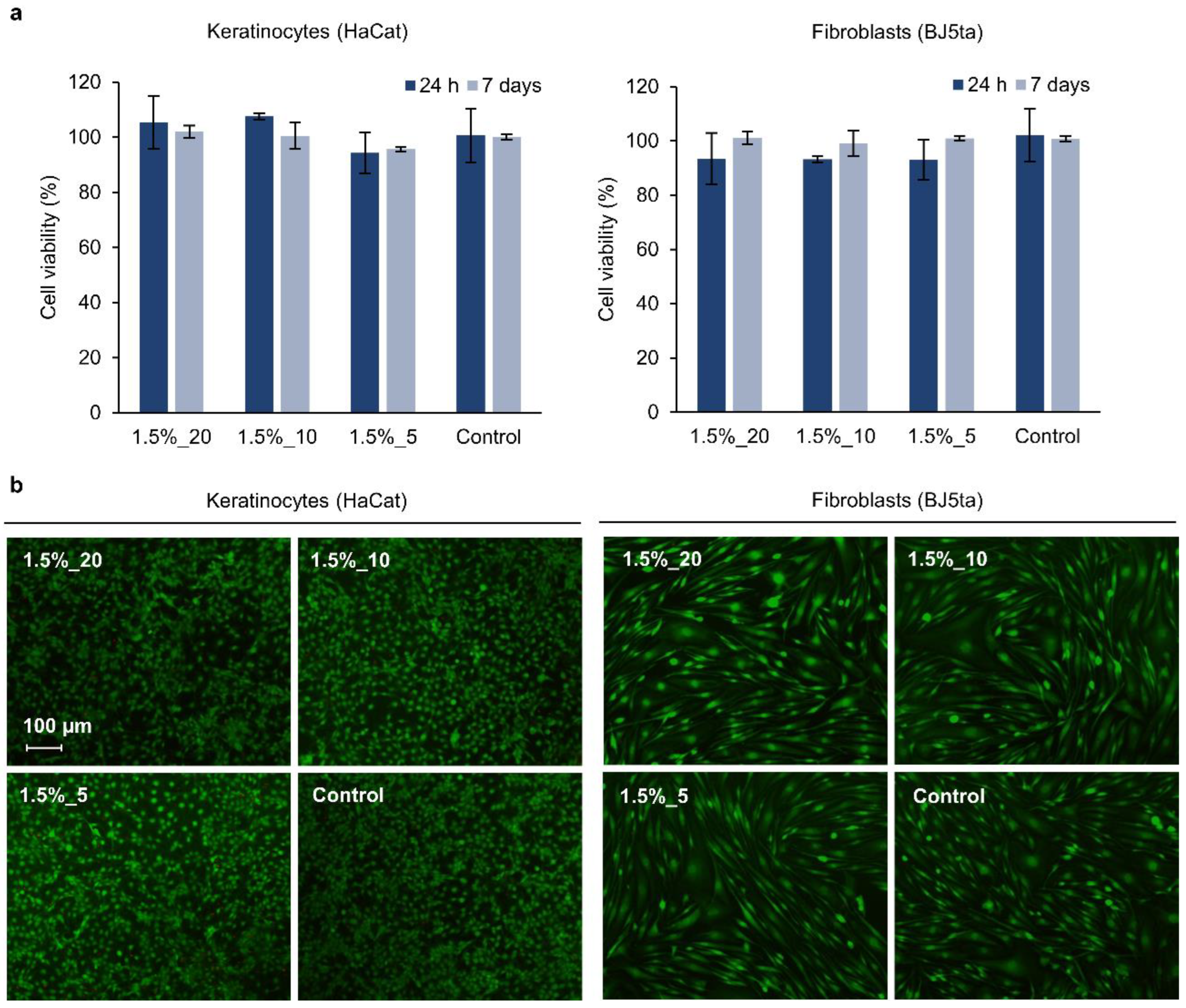
3.7. Cytotoxicity Evaluation of the Hydrogels
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Stefanov, I.; Pérez-Rafael, S.; Hoyo, J.; Cailloux, J.; Santana Pérez, O.O.; Hinojosa-Caballero, D.; Tzanov, T. Multifunctional Enzymatically Generated Hydrogels for Chronic Wound Application. Biomacromolecules 2017, 18, 1544–1555. [Google Scholar] [CrossRef]
- Haske-Cornelius, O.; Bischof, S.; Beer, B.; Jimenez Bartolome, M.; Olatunde Olakanmi, E.; Mokoba, M.; Guebitz, G.M.; Nyanhongo, G.S. Enzymatic Synthesis of Highly Flexible Lignin Cross-Linked Succinyl-Chitosan Hydrogels Reinforced with Reed Cellulose Fibres. Eur. Polym. J. 2019, 120, 109201. [Google Scholar] [CrossRef]
- Hu, W.; Wang, Z.; Xiao, Y.; Zhang, S.; Wang, J. Advances in Crosslinking Strategies of Biomedical Hydrogels. Biomater. Sci. 2019, 7, 843–855. [Google Scholar] [CrossRef] [PubMed]
- Dannert, C.; Stokke, B.T.; Dias, R.S. Nanoparticle-Hydrogel Composites: From Molecular Interactions to Macroscopic Behavior. Polymers 2019, 11, 275. [Google Scholar] [CrossRef] [PubMed]
- Pereira, K.A.B.; Oliveira, P.F.; Chaves, I.; Pedroni, L.G.; Oliveira, L.A.; Mansur, C.R.E. Rheological Properties of Nanocomposite Hydrogels Containing Aluminum and Zinc Oxides with Potential Application for Conformance Control. Colloid Polym. Sci. 2022, 300, 609–624. [Google Scholar] [CrossRef]
- Tang, Y.; Zhang, X.; Li, X.; Ma, C.; Chu, X.; Wang, L.; Xu, W. A Review on Recent Advances of Protein-Polymer Hydrogels. Eur. Polym. J. 2022, 162, 110881. [Google Scholar] [CrossRef]
- Chai, Q.; Jiao, Y.; Yu, X. Hydrogels for Biomedical Applications: Their Characteristics and the Mechanisms behind Them. Gels 2017, 3, 6. [Google Scholar] [CrossRef]
- Han, G.; Ceilley, R. Chronic Wound Healing: A Review of Current Management and Treatments. Adv. Ther. 2017, 34, 599–610. [Google Scholar] [CrossRef]
- Mauri, E.; Negri, A.; Rebellato, E.; Masi, M.; Perale, G.; Rossi, F. Hydrogel-Nanoparticles Composite System for Controlled Drug Delivery. Gels 2018, 4, 74. [Google Scholar] [CrossRef]
- Liu, B.; Li, J.; Lei, X.; Miao, S.; Zhang, S.; Cheng, P.; Song, Y.; Wu, H.; Gao, Y.; Bi, L.; et al. Cell-Loaded Injectable Gelatin/Alginate/LAPONITE® Nanocomposite Hydrogel Promotes Bone Healing in a Critical-Size Rat Calvarial Defect Model. RSC Adv. 2020, 10, 25652–25661. [Google Scholar] [CrossRef] [PubMed]
- Baranwal, J.; Barse, B.; Fais, A.; Delogu, G.L.; Kumar, A. Biopolymer: A Sustainable Material for Food and Medical Applications. Polymers 2022, 14, 983. [Google Scholar] [CrossRef] [PubMed]
- Koehler, J.; Brandl, F.P.; Goepferich, A.M. Hydrogel Wound Dressings for Bioactive Treatment of Acute and Chronic Wounds. Eur. Polym. J. 2018, 100, 1–11. [Google Scholar] [CrossRef]
- Tavakoli, S.; Klar, A.S. Advanced Hydrogels as Wound Dressings. Biomolecules 2020, 10, 1169. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, H.N.; Hardman, M.J. Wound Healing: Cellular Mechanisms and Pathological Outcomes: Cellular Mechanisms of Wound Repair. Open Biol. 2020, 10, 200223. [Google Scholar] [CrossRef] [PubMed]
- Bessa, L.J.; Fazii, P.; Di Giulio, M.; Cellini, L. Bacterial Isolates from Infected Wounds and Their Antibiotic Susceptibility Pattern: Some Remarks about Wound Infection. Int. Wound J. 2015, 12, 47–52. [Google Scholar] [CrossRef]
- Gjødsbøl, K.; Christensen, J.J.; Karlsmark, T.; Jørgensen, B.; Klein, B.M.; Krogfelt, K.A. Multiple Bacterial Species Reside in Chronic Wounds: A Longitudinal Study. Int. Wound J. 2006, 3, 225–231. [Google Scholar] [CrossRef]
- Jiang, T.; Li, Q.; Qiu, J.; Chen, J.; Du, S.; Xu, X.; Yang, X.; Chen, Z.; Chen, T.; Wu, Z. Nanobiotechnology: Applications in Chronic Wound Healing. Int. J. Nanomed. 2022, 17, 3125–3145. [Google Scholar] [CrossRef]
- Li, Y.; Cummins, E. Hazard Characterization of Silver Nanoparticles for Human Exposure Routes. J. Environ. Sci. Health-Part A Toxic/Hazard. Subst. Environ. Eng. 2020, 55, 704–725. [Google Scholar] [CrossRef]
- Cattoir, V.; Felden, B. Future Antibacterial Strategies: From Basic Concepts to Clinical Challenges. J. Infect. Dis. 2019, 220, 350–360. [Google Scholar] [CrossRef]
- Morena, A.G.; Tzanov, T. Antibacterial Lignin-Based Nanoparticles and Their Use in Composite Materials. Nanoscale Adv. 2022, 4, 4447–4469. [Google Scholar] [CrossRef]
- Lizundia, E.; Sipponen, M.H.; Greca, L.G.; Balakshin, M.; Tardy, B.L.; Rojas, O.J.; Puglia, D. Multifunctional Lignin-Based Nanocomposites and Nanohybrids. Green Chem. 2021, 23, 6698–6760. [Google Scholar] [CrossRef] [PubMed]
- Dhall, S.; Do, D.C.; Garcia, M.; Kim, J.; Mirebrahim, S.H.; Lyubovitsky, J.; Lonardi, S.; Nothnagel, E.A.; Schiller, N.; Martins-Green, M. Generating and Reversing Chronic Wounds in Diabetic Mice by Manipulating Wound Redox Parameters. J. Diabetes Res. 2014, 2014, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.; Kassim, S.Y.; Parks, W.C.; Heinecke, J.W. Hypochlorous Acid Oxygenates the Cysteine Switch Domain of Pro-Matrilysin (MMP-7): A Mechanism for Matrix Metalloproteinase Activation and Atherosclerotic Plaque Rupture by Myeloperoxidase. J. Biol. Chem. 2001, 276, 41279–41287. [Google Scholar] [CrossRef] [PubMed]
- Lazaro, J.L.; Izzo, V.; Meaume, S.; Davies, A.H.; Lobmann, R.; Uccioli, L. Elevated Levels of Matrix Metalloproteinases and Chronic Wound Healing: An Updated Review of Clinical Evidence. J. Wound Care 2016, 25, 277–287. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Rafael, S.; Ivanova, K.; Stefanov, I.; Puiggalí, J.; del Valle, L.J.; Todorova, K.; Dimitrov, P.; Hinojosa-Caballero, D.; Tzanov, T. Nanoparticle-Driven Self-Assembling Injectable Hydrogels Provide a Multi-Factorial Approach for Chronic Wound Treatment. Acta Biomater. 2021, 134, 131–143. [Google Scholar] [CrossRef]
- Stefanov, I.; Hinojosa-Caballero, D.; Maspoch, S.; Hoyo, J.; Tzanov, T. Enzymatic Synthesis of a Thiolated Chitosan-Based Wound Dressing Crosslinked with Chicoric Acid. J. Mater. Chem. B 2018, 6, 7943–7953. [Google Scholar] [CrossRef]
- Petkova, P.; Francesko, A.; Tzanov, T. Enzyme-Assisted Formation of Hybrid Biopolymer Hydrogels Incorporating Active Phenolic Nanospheres. Eng. Life Sci. 2015, 15, 416–424. [Google Scholar] [CrossRef]
- Morena, A.G.; Bassegoda, A.; Natan, M.; Jacobi, G.; Banin, E.; Tzanov, T. Antibacterial Properties and Mechanisms of Action of Sonoenzymatically Synthesized Lignin-Based Nanoparticles. ACS Appl. Mater. Interfaces 2022, 14, 37270–37279. [Google Scholar] [CrossRef]
- Morena, A.G.; Stefanov, I.; Ivanova, K.; Pérez-Rafael, S.; Sánchez-Soto, M.; Tzanov, T. Antibacterial Polyurethane Foams with Incorporated Lignin-Capped Silver Nanoparticles for Chronic Wound Treatment. Ind. Eng. Chem. Res. 2020, 59, 4504–4514. [Google Scholar] [CrossRef]
- Becker, L.C.; Bergfeld, W.F.; Belsito, D.V.; Klaassen, C.D.; Marks, J.G.; Shank, R.C.; Slaga, T.J.; Snyder, P.W.; Andersen, F.A. Final Report of the Safety Assessment of Hyaluronic Acid, Potassium Hyaluronate, and Sodium Hyaluronate. Int. J. Toxicol. 2009, 28, 5–67. [Google Scholar] [CrossRef]
- Pollini, M.; Paladini, F. Bioinspired Materials for Wound Healing Application: The Potential of Silk Fibroin. Materials 2020, 13, 3361. [Google Scholar] [CrossRef] [PubMed]
- Roy, N.; Saha, N.; Saha, P. Stability Study of Novel Medicated Hydrogel Wound Dressings. Int. J. Polym. Mater. Polym. Biomater. 2013, 62, 150–156. [Google Scholar] [CrossRef]
- Fan, H.; Wang, J.; Gong, J.P. Barnacle Cement Proteins-Inspired Tough Hydrogels with Robust, Long-Lasting, and Repeatable Underwater Adhesion. Adv. Funct. Mater. 2020, 31, 2009334. [Google Scholar] [CrossRef]
- Krogsgaard, M.; Nue, V.; Birkedal, H. Mussel-Inspired Materials: Self-Healing through Coordination Chemistry. Chem.-A Eur. J. 2016, 22, 844–857. [Google Scholar] [CrossRef] [PubMed]
- Le Bourvellec, C.; Renard, C.M.G.C. Interactions between Polyphenols and Macromolecules: Quantification Methods and Mechanisms. Crit. Rev. Food Sci. Nutr. 2012, 52, 213–248. [Google Scholar] [CrossRef]
- Hausken, K.G.; Frevol, R.L.; Dowdle, K.P.; Young, A.N.; Talusig, J.M.; Holbrook, C.C.; Rubin, B.K.; Murphy, A.R. Quantitative Functionalization of the Tyrosine Residues in Silk Fibroin through an Amino-Tyrosine Intermediate. Macromol. Chem. Phys. 2022, 223, 2200119. [Google Scholar] [CrossRef]
- Zhang, L.; Lu, H.; Chu, J.; Ma, J.; Fan, Y.; Wang, Z.; Ni, Y. Lignin-Directed Control of Silver Nanoparticles with Tunable Size in Porous Lignocellulose Hydrogels and Their Application in Catalytic Reduction. ACS Sustain. Chem. Eng. 2020, 8, 12655–12663. [Google Scholar] [CrossRef]
- Guerritore, M.; Castaldo, R.; Silvestri, B.; Avolio, R.; Cocca, M.; Errico, M.E.; Avella, M.; Gentile, G.; Ambrogi, V. Hyper-Crosslinked Polymer Nanocomposites Containing Mesoporous Silica Nanoparticles with Enhanced Adsorption towards Polar Dyes. Polymers 2020, 12, 1388. [Google Scholar] [CrossRef]
- Del Saz-Orozco, B.; Oliet, M.; Alonso, M.V.; Rojo, E.; Rodríguez, F. Formulation Optimization of Unreinforced and Lignin Nanoparticle-Reinforced Phenolic Foams Using an Analysis of Variance Approach. Compos. Sci. Technol. 2012, 72, 667–674. [Google Scholar] [CrossRef]
- Appel, E.A.; Tibbitt, M.W.; Webber, M.J.; Mattix, B.A.; Veiseh, O.; Langer, R. Self-Assembled Hydrogels Utilizing Polymer-Nanoparticle Interactions. Nat. Commun. 2015, 6, 1–9. [Google Scholar] [CrossRef]
- Kostina, N.Y.; Sharifi, S.; De Los Santos Pereira, A.; Michálek, J.; Grijpma, D.W.; Rodriguez-Emmenegger, C. Novel Antifouling Self-Healing Poly(Carboxybetaine Methacrylamide-Co-HEMA) Nanocomposite Hydrogels with Superior Mechanical Properties. J. Mater. Chem. B 2013, 1, 5644–5650. [Google Scholar] [CrossRef] [PubMed]
- Byette, F.; Bouchard, F.; Pellerin, C.; Paquin, J.; Marcotte, I.; Mateescu, M.A. Cell-Culture Compatible Silk Fibroin Scaffolds Concomitantly Patterned by Freezing Conditions and Salt Concentration. Polym. Bull. 2011, 67, 159–175. [Google Scholar] [CrossRef]
- Kowalski, G.; Kijowska, K.; Witczak, M.; Kuterasiński, L.; Lukasiewicz, M. Synthesis and Effect of Structure on Swelling Properties of Hydrogels Based on High Methylated Pectin and Acrylic Polymers. Polymers 2019, 11, 114. [Google Scholar] [CrossRef] [PubMed]
- Jones, E.M.; Cochrane, C.A.; Percival, S.L. The Effect of PH on the Extracellular Matrix and Biofilms. Adv. Wound Care 2015, 4, 431–439. [Google Scholar] [CrossRef] [PubMed]
- Lambers, H.; Piessens, S.; Bloem, A.; Pronk, H.; Finkel, P. Natural Skin Surface PH Is on Average below 5, Which Is Beneficial for Its Resident Flora. Int. J. Cosmet. Sci. 2006, 28, 359–370. [Google Scholar] [CrossRef] [PubMed]
- Wallace, L.A.; Gwynne, L.; Jenkins, T. Challenges and Opportunities of PH in Chronic Wounds. Ther. Deliv. 2019, 10, 719–735. [Google Scholar] [CrossRef] [PubMed]
- Percival, S.L.; McCarty, S.; Hunt, J.A.; Woods, E.J. The Effects of PH on Wound Healing, Biofilms, and Antimicrobial Efficacy. Wound Repair Regen. 2014, 22, 174–186. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, I.; Mishra, D.; Das, T.; Maiti, T.K. Wound PH-Responsive Sustained Release of Therapeutics from a Poly(NIPAAm-Co-AAc) Hydrogel. J. Biomater. Sci. Polym. Ed. 2012, 23, 111–132. [Google Scholar] [CrossRef]
- Kiaee, G.; Mostafalu, P.; Samandari, M.; Sonkusale, S. A PH-Mediated Electronic Wound Dressing for Controlled Drug Delivery. Adv. Healthc. Mater. 2018, 7, 1800396. [Google Scholar] [CrossRef]
- Mirani, B.; Pagan, E.; Currie, B.; Siddiqui, M.A.; Hosseinzadeh, R.; Mostafalu, P.; Zhang, Y.S.; Ghahary, A.; Akbari, M. An Advanced Multifunctional Hydrogel-Based Dressing for Wound Monitoring and Drug Delivery. Adv. Healthc. Mater. 2017, 6, 1700718. [Google Scholar] [CrossRef]
- Jia, Y.; Zhang, X.; Yang, W.; Lin, C.; Tao, B.; Deng, Z.; Gao, P.; Yang, Y.; Cai, K. A PH-Responsive Hyaluronic Acid Hydrogel for Regulating the Inflammation and Remodeling of the ECM in Diabetic Wounds. J. Mater. Chem. B 2022, 10, 2875–2888. [Google Scholar] [CrossRef] [PubMed]
- Adepu, S.; Ramakrishna, S. Controlled Drug Delivery Systems: Current Status and Future Directions. Molecules 2021, 26, 5905. [Google Scholar] [CrossRef] [PubMed]
- Hendi, A.; Hassan, M.U.; Elsherif, M.; Alqattan, B.; Park, S.; Yetisen, A.K.; Butt, H. Healthcare Applications of PH-Sensitive Hydrogel-Based Devices: A Review. Int. J. Nanomed. 2020, 15, 3887–3901. [Google Scholar] [CrossRef] [PubMed]
- Umuhoza, D.; Yang, F.; Long, D.; Hao, Z.; Dai, J.; Zhao, A. Strategies for Tuning the Biodegradation of Silk Fibroin-Based Materials for Tissue Engineering Applications. ACS Biomater. Sci. Eng. 2020, 6, 1290–1310. [Google Scholar] [CrossRef]
- Castaneda-Arriaga, R.; Pérez-González, A.; Reina, M.; Alvarez-Idaboy, J.R.; Galano, A. Comprehensive Investigation of the Antioxidant and Pro-Oxidant Effects of Phenolic Compounds: A Double-Edged Sword in the Context of Oxidative Stress? J. Phys. Chem. B 2018, 122, 6198–6214. [Google Scholar] [CrossRef]
- Mohammed, A.A.; Niamah, A.K. Identification and Antioxidant Activity of Hyaluronic Acid Extracted from Local Isolates of Streptococcus Thermophilus. Mater. Today Proc. 2022, 60, 1523–1529. [Google Scholar] [CrossRef]
- Díaz-Gonzlez, M.; Rocasalbas, G.; Francesko, A.; Touriño, S.; Torres, J.L.; Tzanov, T. Inhibition of Deleterious Chronic Wound Enzymes with Plant Polyphenols. Biocatal. Biotransformation 2012, 30, 102–110. [Google Scholar] [CrossRef]
- Rocasalbas, G.; Touriño, S.; Torres, J.L.; Tzanov, T. A New Approach to Produce Plant Antioxidant-Loaded Chitosan for Modulating Proteolytic Environment and Bacterial Growth. J. Mater. Chem. B 2013, 1, 1241–1248. [Google Scholar] [CrossRef]
- Seczyk, L.; Swieca, M.; Kapusta, I.; Gawlik-Dziki, U. Protein–Phenolic Interactions as a Factor Affecting the Physicochemical Properties of White Bean Proteins. Molecules 2019, 24, 408. [Google Scholar] [CrossRef]
- Kim, Y.J.; Uyama, H.; Kobayashi, S. Inhibition Effects of (+)-Catechin–Aldehyde Polycondensates on Proteinases Causing Proteolytic Degradation of Extracellular Matrix. Biochem. Biophys. Res. Commun. 2004, 320, 256–261. [Google Scholar] [CrossRef]
- Monika, P.; Chandraprabha, M.N.; Murthy, K.N.C.; Rangarajan, A.; Waiker, P.V.; Sathish, M. Human Primary Chronic Wound Derived Fibroblasts Demonstrate Differential Pattern in Expression of Fibroblast Specific Markers, Cell Cycle Arrest and Reduced Proliferation. Exp. Mol. Pathol. 2022, 127, 104803. [Google Scholar] [CrossRef] [PubMed]
- Pastar, I.; Stojadinovic, O.; Yin, N.C.; Ramirez, H.; Nusbaum, A.G.; Sawaya, A.; Patel, S.B.; Khalid, L.; Isseroff, R.R.; Tomic-Canic, M. Epithelialization in Wound Healing: A Comprehensive Review. Adv. Wound Care 2014, 3, 445–464. [Google Scholar] [CrossRef] [PubMed]



| Composition | ||
|---|---|---|
| Hydrogel | Polymers | PLN (mg·mL−1) |
| 1.5%_20 | HA-SH (1.5 w/v %), SF (1.5 v/v %) | 20 |
| 1.5%_10 | HA-SH (1.5 w/v %), SF (1.5 v/v %) | 10 |
| 1.5%_5 | HA-SH (1.5 w/v %), SF (1.5 v/v %) | 5 |
| 1.0%_20 | HA-SH (1.0 w/v %), SF (1.0 v/v %) | 20 |
| 1.0%_10 | HA-SH (1.0 w/v %), SF (1.0 v/v %) | 10 |
| 1.0%_5 | HA-SH (1.0 w/v %), SF (1.0 v/v %) | 5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morena, A.G.; Pérez-Rafael, S.; Tzanov, T. Lignin-Based Nanoparticles as Both Structural and Active Elements in Self-Assembling and Self-Healing Multifunctional Hydrogels for Chronic Wound Management. Pharmaceutics 2022, 14, 2658. https://doi.org/10.3390/pharmaceutics14122658
Morena AG, Pérez-Rafael S, Tzanov T. Lignin-Based Nanoparticles as Both Structural and Active Elements in Self-Assembling and Self-Healing Multifunctional Hydrogels for Chronic Wound Management. Pharmaceutics. 2022; 14(12):2658. https://doi.org/10.3390/pharmaceutics14122658
Chicago/Turabian StyleMorena, A. Gala, Sílvia Pérez-Rafael, and Tzanko Tzanov. 2022. "Lignin-Based Nanoparticles as Both Structural and Active Elements in Self-Assembling and Self-Healing Multifunctional Hydrogels for Chronic Wound Management" Pharmaceutics 14, no. 12: 2658. https://doi.org/10.3390/pharmaceutics14122658
APA StyleMorena, A. G., Pérez-Rafael, S., & Tzanov, T. (2022). Lignin-Based Nanoparticles as Both Structural and Active Elements in Self-Assembling and Self-Healing Multifunctional Hydrogels for Chronic Wound Management. Pharmaceutics, 14(12), 2658. https://doi.org/10.3390/pharmaceutics14122658