Analysis of Time-Dependent Pharmacokinetics Using In Vitro–In Vivo Extrapolation and Physiologically Based Pharmacokinetic Modeling
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Rat In Vivo PK Studies
2.3. Non-Compartmental Analysis and Related PK Parameters
2.4. Determination of Fraction Unbound in Rat Plasma (fu,p), Hepatocyte Suspension (fu,inc,hepa), and Intrahepatocyte (fu,hepa)
2.5. Assessment of Intrinsic Metabolic Clearance (CLint,met), Hepatic Uptake Clearance (PSinf), and Liver-to-Plasma Unbound Drug Concentration Ratio at Steady State (Kpuu,ss) Using Isolated Hepatocytes
2.6. Calculation of In Vivo Overall Intrinsic Clearance (CLint,all) and Overall Hepatic Clearance (CLh)
2.7. Determination of Fraction Unbound in Rat Plasma (fu,p), Hepatocyte Suspension (fu,inc,hepa), and Intrahepatocyte (fu,hepa)
2.8. Accuracy, Precision, and Bias Assessments of the Model Predictions
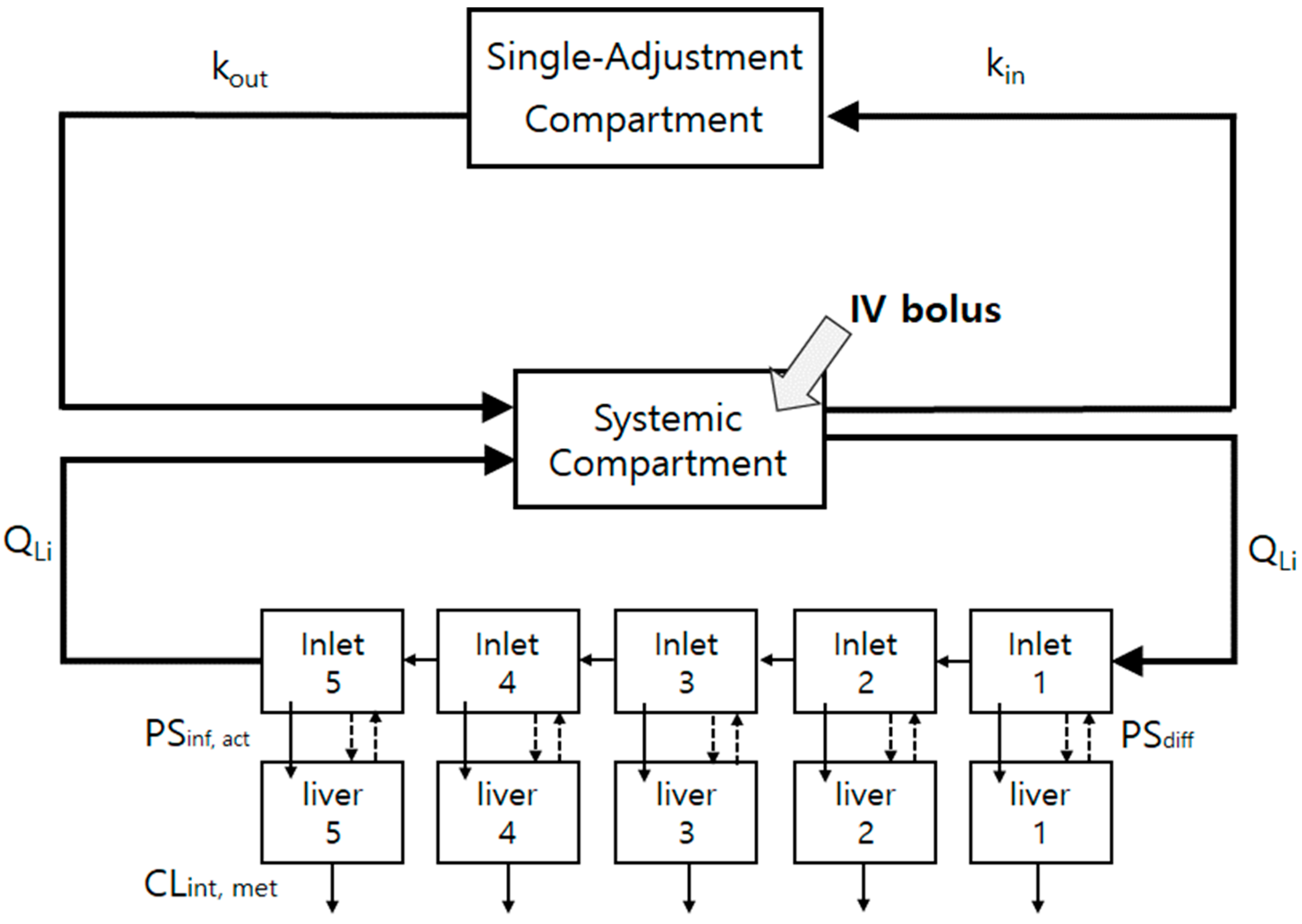
2.9. The Minimal PBPK (mPBPK) Model Construction for the Control and Repeated-Dosing Groups
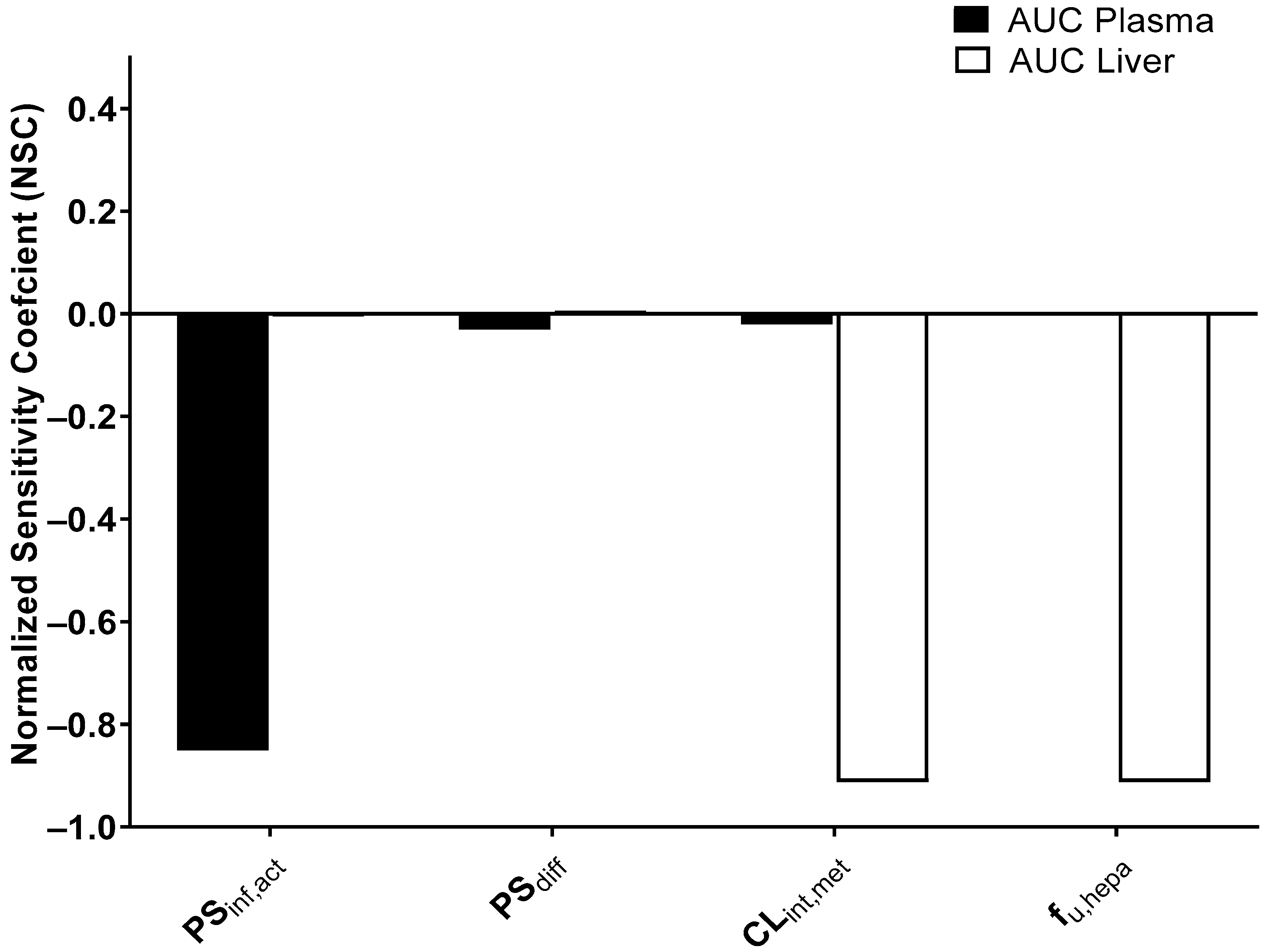
2.10. Sensitivity Analysis
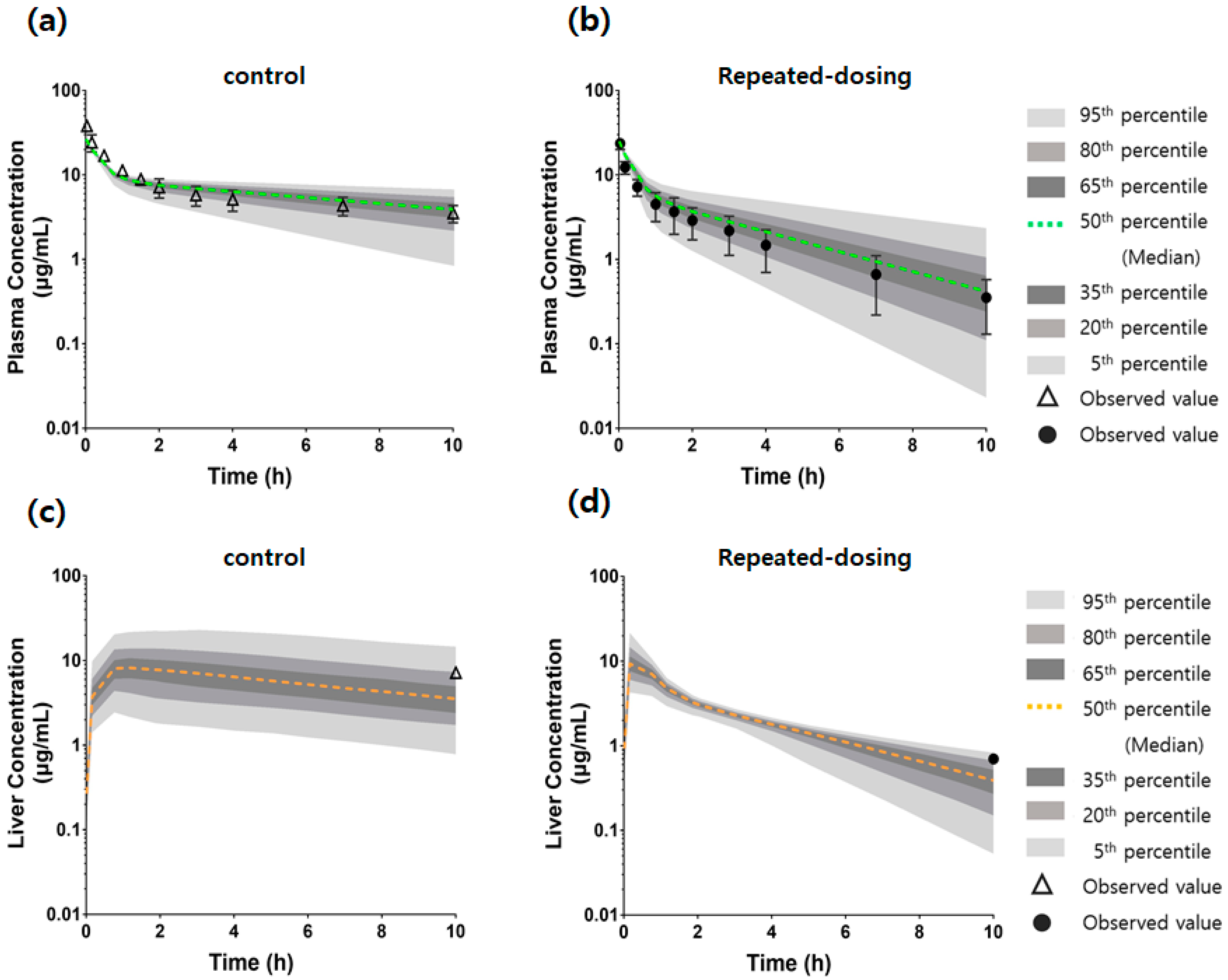
2.11. Comparison of the Model Predictions Using Monte Carlo Simulation and Observed Data of Plasma and Liver Concentrations
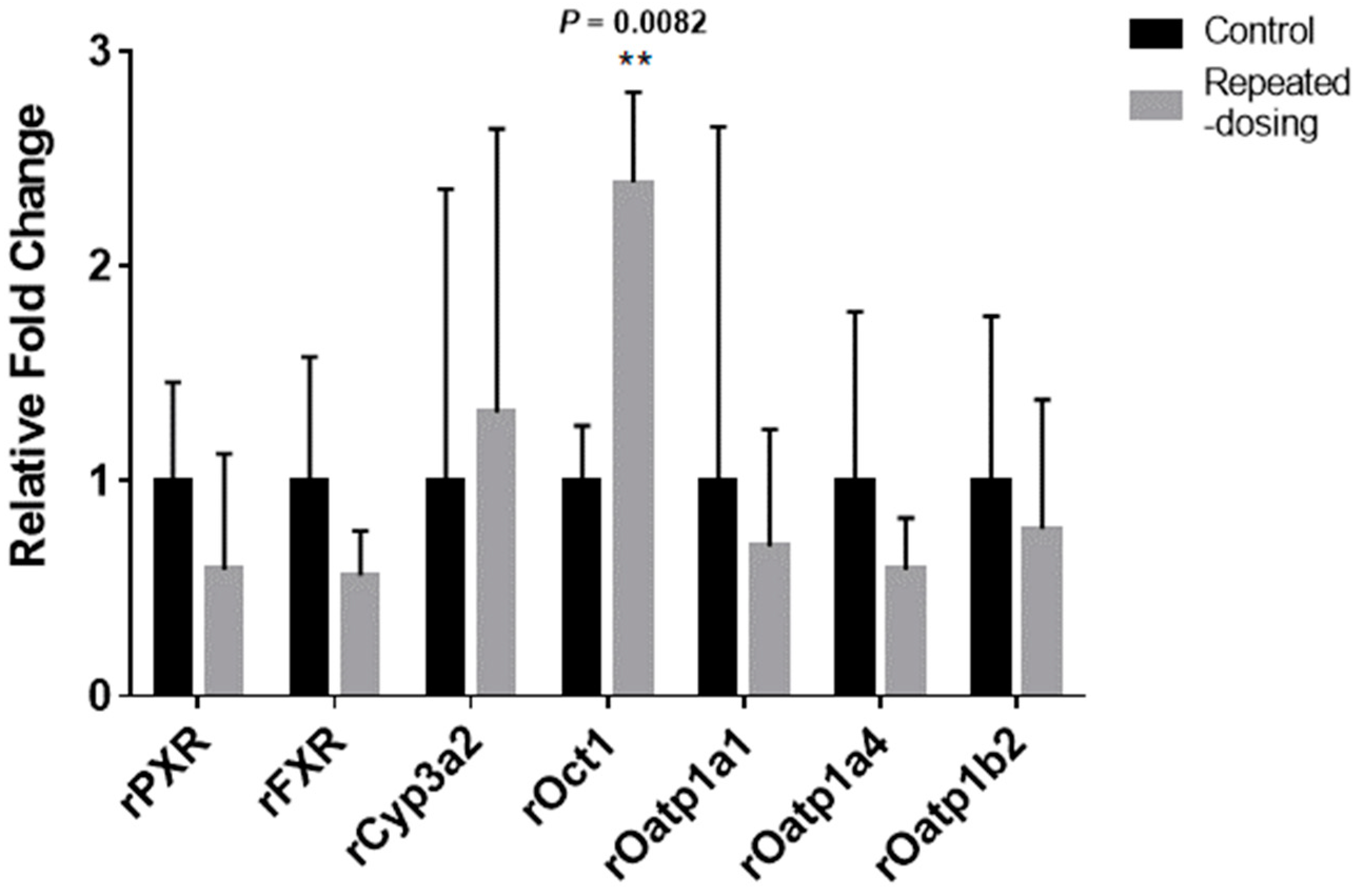
2.12. Gene Expression Analysis Using Quantitative PCR (qPCR)
3. Results
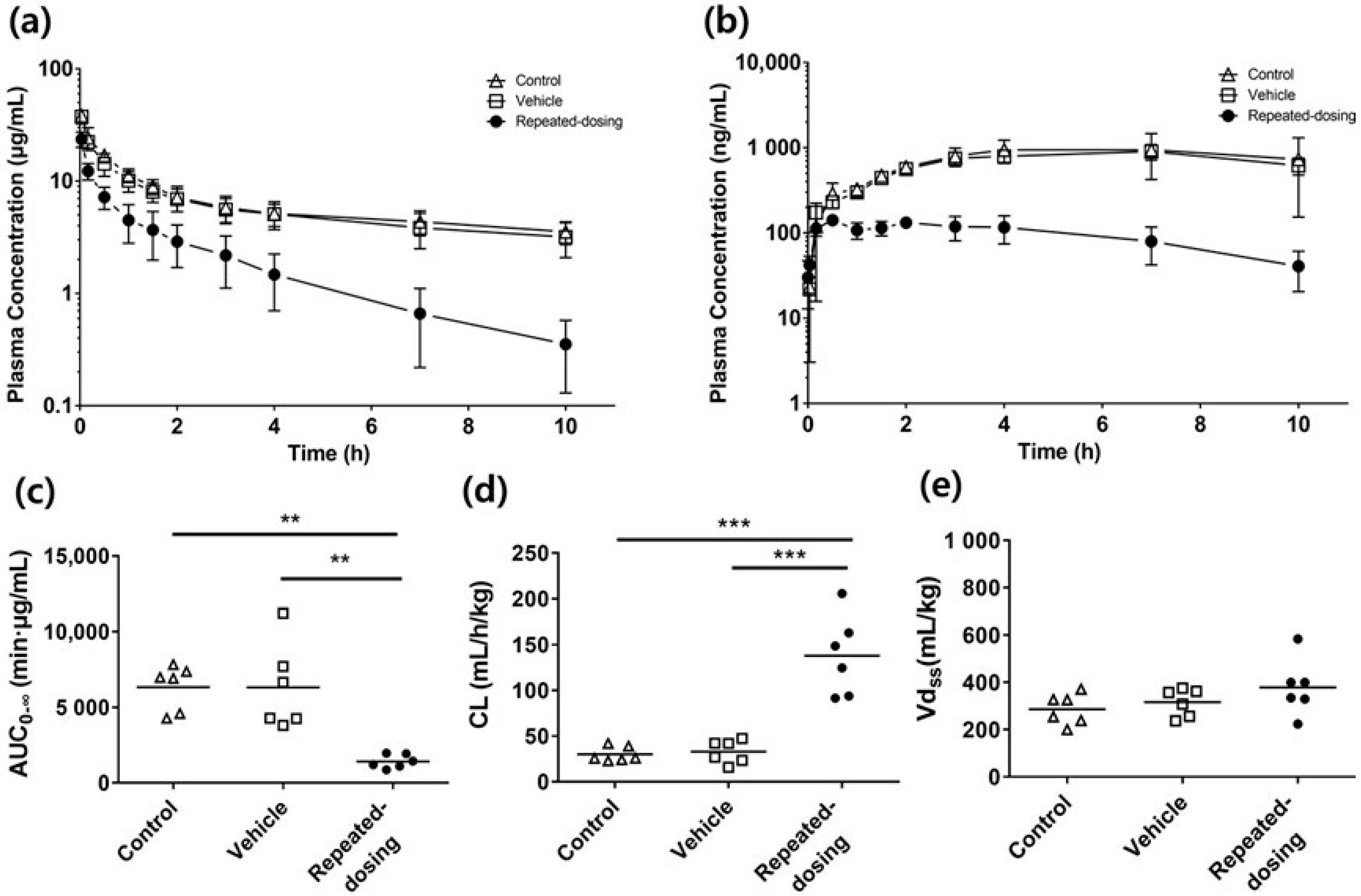
3.1. TDPK of SCR430
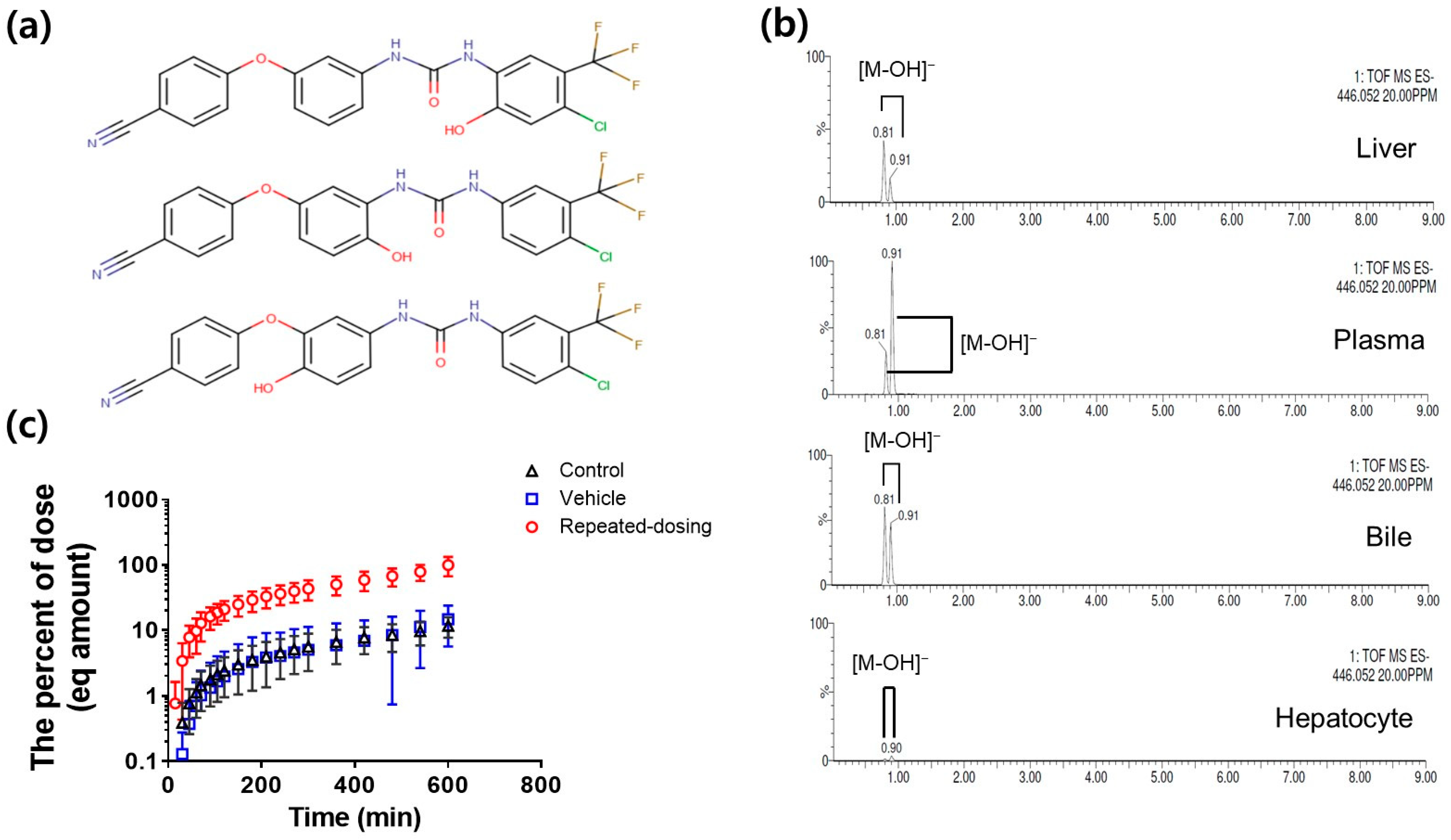
3.2. Metabolite Identification and Quantification of SCR430
3.3. Low Fraction Unbound of SCR430 in the Plasma and Liver
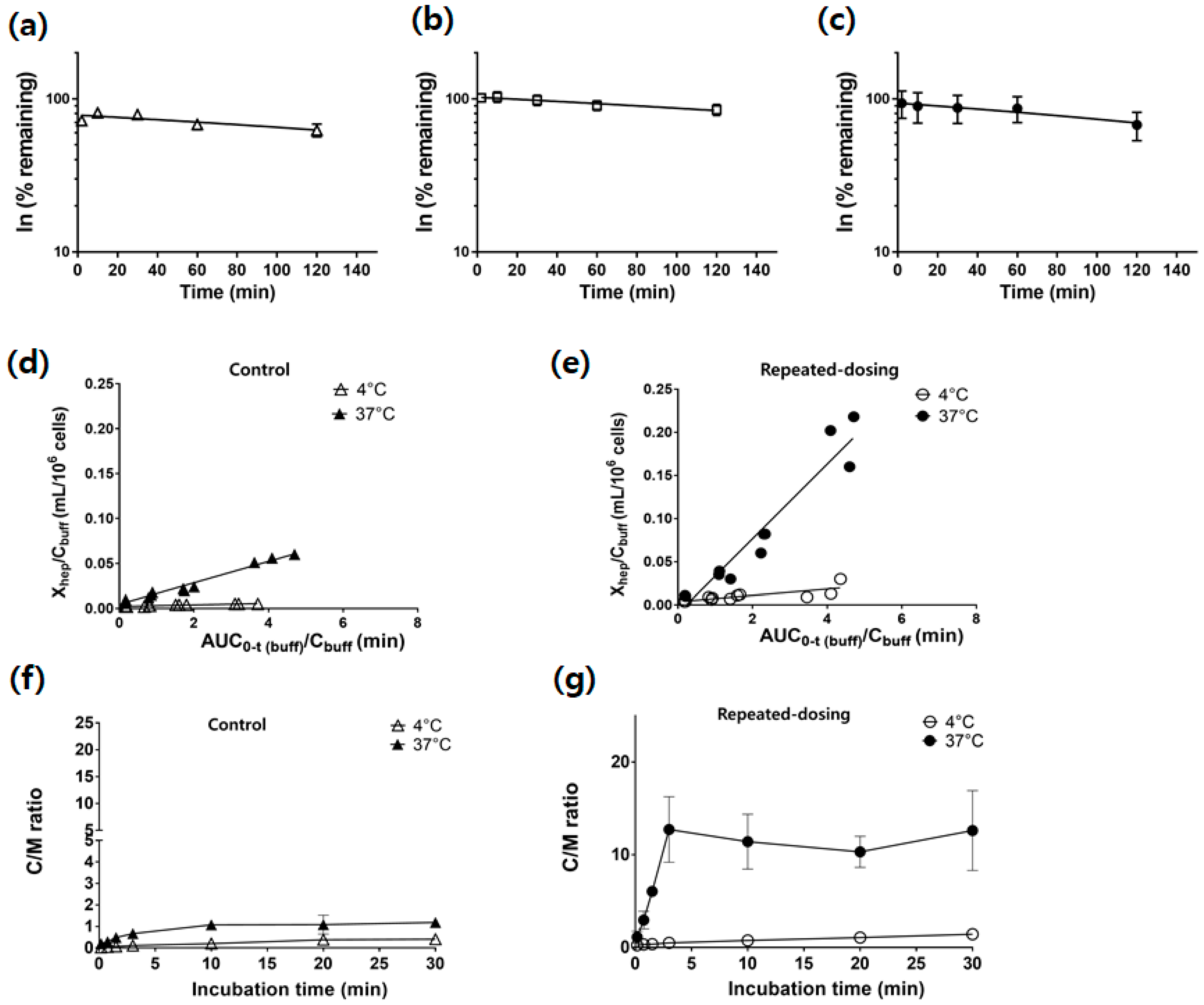
3.4. Similar CLint,met between the Control, Vehicle, and Repeated-Dosing Groups
3.5. Increased PSinf,hep and Kpuu,ss in the Repeated-Dosing Group
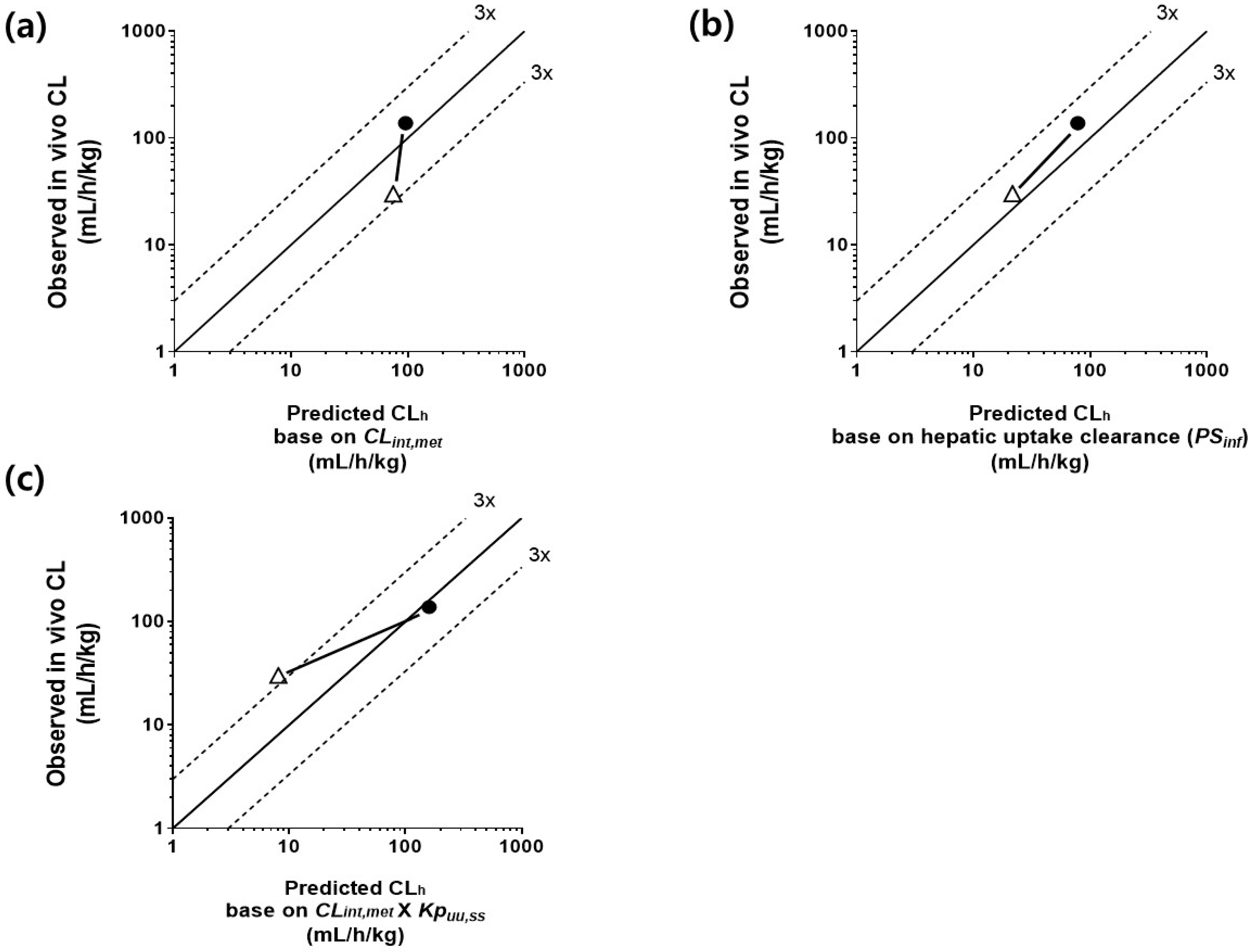
3.6. Comparison of CLh Determined by Three IVIVE Methods and In Vivo Clearance in the Control and Repeated-Dosing Groups
3.7. An mPBPK Model Could Predict the Differences in Plasma and Liver Concentrations in Control and Repeated-Dosing Groups
3.8. Sensitivity Analysis
3.9. The mRNA Expression Differences between the Three Groups
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
Appendix A
Appendix A.1. Analytical Method
Appendix A.2. The Mass-Balanced Differential Equation for the Liver Compartments
Appendix B
| Gene | Forward Primer (5′-3′) | Reverse Primer (5′-3′) | Product Size (bp) | PCR Conditions |
|---|---|---|---|---|
| rGapdh | AACGACCCCTTCATTGAC | TCCACGACATACTCAGCAC | 191 | D: 95 °C for 10 s, A: 57 °C for 30 s, E: 72 °C for 30 s |
| rPXR | TCCACTGCATGCTGAAGAAG | AACCTGTGTGCAGGATAGGG | 187 | D: 95 °C for 10 s, A: 56 °C for 30 s, E: 72 °C for 40 s |
| rFXR | CCAACCTGGGTTTCTACCC | CACACAGCTCATCCCCTTT | 183 | D: 95 °C for 10 s, A: 56 °C for 30 s, E: 72 °C for 40 s |
| rCyp3a2 | CGACTTGGAACCCATAGAC | CATGTCAAATCTCCCTAAGCC | 117 | D: 95 °C for 10 s, A: 58 °C for 30 s, E: 72 °C for 30 s |
| rOatp1a1 (Oatp1) | ACCTGGAACAGCAGTATGGAAAA | ACCGATAGGCAAAATGCTAGGTAT | 163 | D: 95 °C-5 to 10 s, A: 56 °C for 6 s, E: 72 °C for 6 s |
| rOatp1a4 (Oatp2) | TGTGATGACCGTTGATAATTTTCCA | TTCTCCACATATAGTTGGTGCTGAA | 81 | D: 95 °C-5 to 10 s, A: 56 °C for 10 s, E: 72 °C for 6 s |
| rOct1 | CTCTGGCTACAGGAGAACGAC | CCTGGTACAGCACAGCACAA | 401 | D: 94 °C for 30 s, A: 60 °C for 30 s, E: 72 °C for 30 s |
| Parameter | Value |
|---|---|
| Physicochemical properties | |
| Molecular weight (g/mol) | 431.8 |
| pKa | 6.27 a |
| clogD7.4 | 5.95 b |
| clogP | 6.15 b |
| Name | Q1 Mass | Q3 Mass | Dwell Time (s) | Cone (V) | Collision (V) |
|---|---|---|---|---|---|
| SCR430 | 432.1 | 196.1 | 0.15 | 40 | 20 |
| Hydroxylated metabolite | 448.1 | 212.1 | 0.15 | 55 | 25 |
| Sorafenib (IS) | 464.9 | 252.2 | 0.15 | 55 | 38 |
References
- Levy, R.H. Time-dependent pharmacokinetics. Pharmacol. Ther. 1982, 17, 383–397. [Google Scholar] [CrossRef]
- Hu, M.H.; Chen, L.J.; Chen, Y.L.; Tsai, M.S.; Shiau, C.W.; Chao, T.I.; Liu, C.Y.; Kao, J.H.; Chen, K.F. Targeting SHP-1-STAT3 signaling: A promising therapeutic approach for the treatment of cholangiocarcinoma. Oncotarget 2017, 8, 65077–65089. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.Y.; Tseng, L.M.; Su, J.C.; Chang, K.C.; Chu, P.Y.; Tai, W.T.; Shiau, C.W.; Chen, K.F. Novel sorafenib analogues induce apoptosis through SHP-1 dependent STAT3 inactivation in human breast cancer cells. Breast Cancer Res. 2013, 15, R63. [Google Scholar] [CrossRef]
- Iwanaga, K.; Honjo, T.; Miyazaki, M.; Kakemi, M. Time-dependent changes in hepatic and intestinal induction of cytochrome P450 3A after administration of dexamethasone to rats. Xenobiotica 2013, 43, 765–773. [Google Scholar] [CrossRef]
- Niemi, M.; Backman, J.T.; Fromm, M.F.; Neuvonen, P.J.; Kivisto, K.T. Pharmacokinetic interactions with rifampicin: Clinical relevance. Clin. Pharm. 2003, 42, 819–850. [Google Scholar] [CrossRef] [PubMed]
- Baneyx, G.; Parrott, N.; Meille, C.; Iliadis, A.; Lave, T. Physiologically based pharmacokinetic modeling of CYP3A4 induction by rifampicin in human: Influence of time between substrate and inducer administration. Eur. J. Pharm. Sci. 2014, 56, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Fahrmayr, C.; Fromm, M.F.; Konig, J. Hepatic OATP and OCT uptake transporters: Their role for drug-drug interactions and pharmacogenetic aspects. Drug Metab. Rev. 2010, 42, 380–401. [Google Scholar] [CrossRef]
- Niu, C.R.; Smith, B.; Lai, Y.R. Transporter Gene Regulation in Sandwich Cultured Human Hepatocytes Through the Activation of Constitutive Androstane Receptor (CAR) or Aryl Hydrocarbon Receptor (AhR). Front. Pharmacol. 2021, 11, 620197. [Google Scholar] [CrossRef]
- Patel, M.; Taskar, K.S.; Zamek-Gliszczynski, M.J. Importance of Hepatic Transporters in Clinical Disposition of Drugs and Their Metabolites. J. Clin. Pharmacol. 2016, 56, S23–S39. [Google Scholar] [CrossRef]
- Kusuhara, H.; Sugiyama, Y. In vitro-in vivo extrapolation of transporter-mediated clearance in the liver and kidney. Drug Metab. Pharm. 2009, 24, 37–52. [Google Scholar] [CrossRef]
- Varma, M.V.; Steyn, S.J.; Allerton, C.; El-Kattan, A.F. Predicting Clearance Mechanism in Drug Discovery: Extended Clearance Classification System (ECCS). Pharm. Res. 2015, 32, 3785–3802. [Google Scholar] [CrossRef]
- Zhang, Y.; Huo, M.; Zhou, J.; Xie, S. PKSolver: An add-in program for pharmacokinetic and pharmacodynamic data analysis in Microsoft Excel. Comput. Methods Programs Biomed. 2010, 99, 306–314. [Google Scholar] [CrossRef]
- Li, R.; Ghosh, A.; Maurer, T.S.; Kimoto, E.; Barton, H.A. Physiologically based pharmacokinetic prediction of telmisartan in human. Drug Metab. Dispos. 2014, 42, 1646–1655. [Google Scholar] [CrossRef]
- Yu, S.; Li, S.; Yang, H.; Lee, F.; Wu, J.T.; Qian, M.G. A novel liquid chromatography/tandem mass spectrometry based depletion method for measuring red blood cell partitioning of pharmaceutical compounds in drug discovery. Rapid Commun. Mass Spectrom. 2005, 19, 250–254. [Google Scholar] [CrossRef]
- Srivastava, A.; Pike, A.; Williamson, B.; Fenner, K. A Novel Method for Preventing Non-specific Binding in Equilibrium Dialysis Assays Using Solutol® as an Additive. J. Pharm. Sci. 2021, 110, 1412–1417. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Badrinarayanan, A.; Li, X.; Roberts, J.; Hayashi, M.; Virk, M.; Gupta, A. Comparison of In Vitro to In Vivo Extrapolation Approaches for Predicting Transporter-Mediated Hepatic Uptake Clearance Using Suspended Rat Hepatocytes. Drug Metab. Dispos. 2020, 48, 861–872. [Google Scholar] [CrossRef] [PubMed]
- Yoshikado, T.; Toshimoto, K.; Nakada, T.; Ikejiri, K.; Kusuhara, H.; Maeda, K.; Sugiyama, Y. Comparison of Methods for Estimating Unbound Intracellular-to-Medium Concentration Ratios in Rat and Human Hepatocytes Using Statins. Drug Metab. Dispos. 2017, 45, 779–789. [Google Scholar] [CrossRef] [PubMed]
- Seglen, P.O. Preparation of isolated rat liver cells. Methods Cell Biol. 1976, 13, 29–83. [Google Scholar] [CrossRef]
- Soars, M.G.; Grime, K.; Sproston, J.L.; Webborn, P.J.; Riley, R.J. Use of hepatocytes to assess the contribution of hepatic uptake to clearance in vivo. Drug Metab. Dispos. 2007, 35, 859–865. [Google Scholar] [CrossRef] [PubMed]
- Musther, H.; Harwood, M.D.; Yang, J.; Turner, D.B.; Rostami-Hodjegan, A.; Jamei, M. The Constraints, Construction, and Verification of a Strain-Specific Physiologically Based Pharmacokinetic Rat Model. J. Pharm. Sci. 2017, 106, 2826–2838. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, T.; Kusuhara, H.; Sugiyama, Y. Application of physiologically based pharmacokinetic modeling and clearance concept to drugs showing transporter-mediated distribution and clearance in humans. J. Pharm. Pharm. 2010, 37, 575–590. [Google Scholar] [CrossRef]
- Izumi, S.; Nozaki, Y.; Komori, T.; Takenaka, O.; Maeda, K.; Kusuhara, H.; Sugiyama, Y. Comparison of the predictability of human hepatic clearance for organic anion transporting polypeptide substrate drugs between different in vitro–in vivo extrapolation approaches. J. Pharm. Sci. 2017, 106, 2678–2687. [Google Scholar] [CrossRef] [PubMed]
- Nozaki, Y.; Izumi, S. Recent advances in preclinical in vitro approaches towards quantitative prediction of hepatic clearance and drug-drug interactions involving organic anion transporting polypeptide (OATP) 1B transporters. Drug Metab. Pharm. 2020, 35, 56–70. [Google Scholar] [CrossRef] [PubMed]
- Koyanagi, T.; Yano, K.; Kim, S.; Murayama, N.; Yamazaki, H.; Tamai, I. In vivo hepatic clearance of lipophilic drugs predicted by in vitro uptake data into cryopreserved hepatocytes suspended in sera of rats, guinea pigs, monkeys and humans. Xenobiotica 2019, 49, 887–894. [Google Scholar] [CrossRef]
- Shitara, Y.; Maeda, K.; Ikejiri, K.; Yoshida, K.; Horie, T.; Sugiyama, Y. Clinical significance of organic anion transporting polypeptides (OATPs) in drug disposition: Their roles in hepatic clearance and intestinal absorption. Biopharm. Drug Dispos. 2013, 34, 45–78. [Google Scholar] [CrossRef]
- Li, N.; Badrinarayanan, A.; Ishida, K.; Li, X.; Roberts, J.; Wang, S.; Hayashi, M.; Gupta, A. Albumin-mediated uptake improves human clearance prediction for hepatic uptake transporter substrates aiding a mechanistic in vitro-in vivo extrapolation (IVIVE) strategy in discovery research. AAPS J. 2021, 23, 1. [Google Scholar] [CrossRef] [PubMed]
- Roberts, M.S.; Rowland, M. A dispersion model of hepatic elimination: 1. Formulation of the model and bolus considerations. J Pharm. Biopharm. 1986, 14, 227–260. [Google Scholar] [CrossRef]
- Poulin, P.; Theil, F.P. Prediction of pharmacokinetics prior to in vivo studies. II. Generic physiologically based pharmacokinetic models of drug disposition. J. Pharm. Sci. 2002, 91, 1358–1370. [Google Scholar] [CrossRef]
- Yoshikado, T.; Yoshida, K.; Kotani, N.; Nakada, T.; Asaumi, R.; Toshimoto, K.; Maeda, K.; Kusuhara, H.; Sugiyama, Y. Quantitative Analyses of Hepatic OATP-Mediated Interactions Between Statins and Inhibitors Using PBPK Modeling with a Parameter Optimization Method. Clin Pharm. 2016, 100, 513–523. [Google Scholar] [CrossRef]
- Sweeney, L.M.; Gargas, M.L.; Strother, D.E.; Kedderis, G.L. Physiologically based pharmacokinetic model parameter estimation and sensitivity and variability analyses for acrylonitrile disposition in humans. Toxicol. Sci. 2003, 71, 27–40. [Google Scholar] [CrossRef][Green Version]
- Evans, M.V.; Andersen, M.E. Sensitivity analysis of a physiological model for 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD): Assessing the impact of specific model parameters on sequestration in liver and fat in the rat. Toxicol. Sci. 2000, 54, 71–80. [Google Scholar] [CrossRef] [PubMed]
- Mirfazaelian, A.; Kim, K.B.; Anand, S.S.; Kim, H.J.; Tornero-Velez, R.; Bruckner, J.V.; Fisher, J.W. Development of a physiologically based pharmacokinetic model for deltamethrin in the adult male Sprague-Dawley rat. Toxicol. Sci. 2006, 93, 432–442. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Fisher, J.W.; Ross, M.K.; Filipov, N.M. A physiologically based pharmacokinetic model for atrazine and its main metabolites in the adult male C57BL/6 mouse. Toxicol. Appl. Pharm. 2011, 251, 16–31. [Google Scholar] [CrossRef] [PubMed]
- Yoon, M.; Nong, A.; Clewell, H.J., 3rd; Taylor, M.D.; Dorman, D.C.; Andersen, M.E. Evaluating placental transfer and tissue concentrations of manganese in the pregnant rat and fetuses after inhalation exposures with a PBPK model. Toxicol. Sci. 2009, 112, 44–58. [Google Scholar] [CrossRef]
- Li, M.; Gehring, R.; Riviere, J.E.; Lin, Z. Development and application of a population physiologically based pharmacokinetic model for penicillin G in swine and cattle for food safety assessment. Food Chem. Toxicol. 2017, 107, 74–87. [Google Scholar] [CrossRef]
- Gujer, W. Systems Analysis for Water Technology; Springer Science & Business Media: Berlin, Germany, 2008. [Google Scholar]
- Oh, J.H.; Lee, J.H.; Lee, Y.J. Evaluation of the Mrp2-mediated flavonoid-drug interaction potential of quercetin in rats and in vitro models. Asian J. Pharm. Sci. 2019, 14, 621–630. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Kilford, P.J.; Gertz, M.; Houston, J.B.; Galetin, A. Hepatocellular binding of drugs: Correction for unbound fraction in hepatocyte incubations using microsomal binding or drug lipophilicity data. Drug Metab. Dispos. 2008, 36, 1194–1197. [Google Scholar] [CrossRef]
- Page, K.M. Validation of Early Human Dose Prediction: A Key Metric for Compound Progression in Drug Discovery. Mol. Pharm. 2016, 13, 609–620. [Google Scholar] [CrossRef]
- De Bruyn Kops, C.; Stork, C.; Sicho, M.; Kochev, N.; Svozil, D.; Jeliazkova, N.; Kirchmair, J. GLORY: Generator of the Structures of Likely Cytochrome P450 Metabolites Based on Predicted Sites of Metabolism. Front. Chem. 2019, 7, 402. [Google Scholar] [CrossRef]
- Watanabe, R.; Esaki, T.; Kawashima, H.; Natsume-Kitatani, Y.; Nagao, C.; Ohashi, R.; Mizuguchi, K. Predicting Fraction Unbound in Human Plasma from Chemical Structure: Improved Accuracy in the Low Value Ranges. Mol. Pharm. 2018, 15, 5302–5311. [Google Scholar] [CrossRef] [PubMed]
- Grime, K.; Riley, R.J. The impact of in vitro binding on in vitro-in vivo extrapolations, projections of metabolic clearance and clinical drug-drug interactions. Curr. Drug Metab. 2006, 7, 251–264. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, T.; Kusuhara, H.; Maeda, K.; Shitara, Y.; Sugiyama, Y. Physiologically based pharmacokinetic modeling to predict transporter-mediated clearance and distribution of pravastatin in humans. J. Pharm. Exp. 2009, 328, 652–662. [Google Scholar] [CrossRef] [PubMed]
- Sinz, M.; Wallace, G.; Sahi, J.J.T.A.j. Current industrial practices in assessing CYP450 enzyme induction: Preclinical and clinical. AAPS J. 2008, 10, 391–400. [Google Scholar] [CrossRef] [PubMed]
- Hakkola, J.; Hukkanen, J.; Turpeinen, M.; Pelkonen, O. Inhibition and induction of CYP enzymes in humans: An update. Arch. Toxicol. 2020, 94, 3671–3722. [Google Scholar] [CrossRef] [PubMed]
- Punyawudho, B.; Cloyd, J.C.; Leppik, I.E.; Ramsay, R.E.; Marino, S.E.; Pennell, P.B.; White, J.R.; Birnbaum, A.K. Characterization of the time course of carbamazepine deinduction by an enzyme turnover model. Clin. Pharmacokinet. 2009, 48, 313–320. [Google Scholar] [CrossRef] [PubMed]
- Perdomo, V.G.; Rigalli, J.P.; Villanueva, S.S.; Ruiz, M.L.; Luquita, M.G.; Echenique, C.G.; Catania, V.A. Modulation of biotransformation systems and ABC transporters by benznidazole in rats. Antimicrob. Agents Chemother. 2013, 57, 4894–4902. [Google Scholar] [CrossRef]
- Dixit, V.; Hariparsad, N.; Li, F.; Desai, P.; Thummel, K.E.; Unadkat, J.D. Cytochrome P450 enzymes and transporters induced by anti-human immunodeficiency virus protease inhibitors in human hepatocytes: Implications for predicting clinical drug interactions. Drug Metab. Dispos. 2007, 35, 1853–1859. [Google Scholar] [CrossRef]
- El-Kattan, A.F.; Varma, M.V.S. Navigating Transporter Sciences in Pharmacokinetics Characterization Using the Extended Clearance Classification System. Drug Metab. Dispos. 2018, 46, 729–739. [Google Scholar] [CrossRef]
- Wongwan, T.; Chatsudthipong, V.; Soodvilai, S. Farnesoid X Receptor Activation Stimulates Organic Cations Transport in Human Renal Proximal Tubular Cells. Int. J. Mol. Sci. 2020, 21, 6078. [Google Scholar] [CrossRef]
- Hyrsova, L.; Smutny, T.; Trejtnar, F.; Pavek, P. Expression of organic cation transporter 1 (OCT1): Unique patterns of indirect regulation by nuclear receptors and hepatospecific gene regulation. Drug Metab. Rev. 2016, 48, 139–158. [Google Scholar] [CrossRef] [PubMed]
- Palaiokostas, M.; Ding, W.; Shahane, G.; Orsi, M. Effects of lipid composition on membrane permeation. Soft Matter 2018, 14, 8496–8508. [Google Scholar] [CrossRef]
- Riccardi, K.; Ryu, S.; Lin, J.; Yates, P.; Tess, D.; Li, R.; Singh, D.; Holder, B.R.; Kapinos, B.; Chang, G.; et al. Comparison of Species and Cell-Type Differences in Fraction Unbound of Liver Tissues, Hepatocytes, and Cell Lines. Drug Metab. Dispos. 2018, 46, 415–421. [Google Scholar] [CrossRef] [PubMed]
- Xu, E.; Forest, M.P.; Schwab, M.; Avramoglu, R.K.; St-Amand, E.; Caron, A.Z.; Bellmann, K.; Shum, M.; Voisin, G.; Paquet, M.; et al. Hepatocyte-specific Ptpn6 deletion promotes hepatic lipid accretion, but reduces NAFLD in diet-induced obesity: Potential role of PPARgamma. Hepatology 2014, 59, 1803–1815. [Google Scholar] [CrossRef] [PubMed]
- Hirano, M.; Maeda, K.; Shitara, Y.; Sugiyama, Y. Contribution of OATP2 (OATP1B1) and OATP8 (OATP1B3) to the hepatic uptake of pitavastatin in humans. J. Pharm. Exp. 2004, 311, 139–146. [Google Scholar] [CrossRef]
- Giacomini, K.M.; Huang, S.M.; Tweedie, D.J.; Benet, L.Z.; Brouwer, K.L.R.; Chu, X.Y.; Dahlin, A.; Evers, R.; Fischer, V.; Hillgren, K.M.; et al. Membrane transporters in drug development. Nat. Rev. Drug Discov. 2010, 9, 215–236. [Google Scholar] [CrossRef]
- Jones, H.M.; Barton, H.A.; Lai, Y.; Bi, Y.-a.; Kimoto, E.; Kempshall, S.; Tate, S.C.; El-Kattan, A.; Houston, J.B.; Galetin, A.; et al. Mechanistic pharmacokinetic modeling for the prediction of transporter-mediated disposition in humans from sandwich culture human hepatocyte data. Drug Metab. Dispos. 2012, 40, 1007–1017. [Google Scholar] [CrossRef]
- Watanabe, T.; Kusuhara, H.; Maeda, K.; Kanamaru, H.; Saito, Y.; Hu, Z.; Sugiyama, Y. Investigation of the rate-determining process in the hepatic elimination of HMG-CoA reductase inhibitors in rats and humans. Drug Metab. Dispos. 2010, 38, 215–222. [Google Scholar] [CrossRef]
- Baltruschat, M.; Czodrowski, P. Machine learning meets pKa. F1000Res 2020, 9, 113. [Google Scholar] [CrossRef]


| Parameter | Control Group | Vehicle Group | Repeated-Dosing Group |
|---|---|---|---|
| IV (3 mg/kg) | |||
| AUC0→∞ (μg/mL·min) | 6333 ± 1505 (9.7%) | 6319 ± 2854 (18.4%) | 1420 ± 446 ** (12.8%) |
| CL (mL/h/kg) | 30.02 ± 8.29 (11.3%) | 32.99 ± 12.54 (15.4%) | 137.9 ± 43.8 *** (12.9%) |
| Vdss (mL/kg) | 286.4 ± 65 (9.2%) | 316.0 ± 58.7 (7.6%) | 378.4 ± 119.3 (12.8%) |
| CLb (mL/h/kg) | 0.05 ± 0.02 (22%) | 0.061 ± 0.01 (9.8%) | 0.075 ± 0.003 (1.3%) |
| CLr (mL/h/kg) | 0.005 ± 0.002 (20%) | 0.003 ± 0.002 (33%) | 0.007 ± 0.006 (42%) |
| Rb | 3.95 ± 0.64 (9.4%) | 4.58 ± 0.47 (5.9%) | 4.53 ± 0.65 (8.2%) |
| IP (3 mg/kg) | |||
| AUCIP,0→10h (ng/mL·h) | 7604 ± 3110 (16.7%) | 6894 ± 1004 (5.9%) | 951 ± 302 *** (12.9%) |
| Binding properties | |||
| Fraction unbound (fu) | |||
| fu,p a | 0.0078 ± 0.001 (7.7%) | 0.0074 ± 0.002 (13.5%) | 0.0081 ± 0.002 (12.3%) |
| fu,inc,hepa b | 0.032 ± 0.004 (6.2%) | Equal to control | |
| Predicted fu,inc,heap c | 0.024 | 0.024 | |
| Extrapolated fu,inc,hepa d | 0.088 | 0.088 | |
| fu,hepa e | 0.004 ± 0.002 (25%) | 0.01 ± 0.008 (40%) | |
| Tissue:Plasma Partition Coefficients (Kp) | |||
| Kp_liver | 2.06 ± 0.06 (1.4%) | 1.27 ± 0.15 (6.3%) | 1.96 ± 1.09 (32.1%) |
| Kp_kidney | 1.20 ± 0.1 (5%) | 1.37 ± 0.15 (5.8%) | 5.50 ± 0.82 * (8.5%) |
| Unit | Control | Vehicle | Repeated-Dosing | |
|---|---|---|---|---|
| Method 1 | ||||
| in vitro intrinsic metabolic clearance (CLint,met,vitro) | μL/min per 106 | 1.32 | 1.21 | 1.69 |
| in vivo overall intrinsic clearance (CLint,all) | mL/min per kg | 160.4 | 147.0 | 205.3 |
| Predicted hepatic clearance (CLh) | mL/h per kg | 74.8 | 68.6 | 95.7 |
| Method 2 | ||||
| in vitro hepatocyte uptake clearance (PSinf,hep) | μL/min per 106 | 11.9 | 43.2 | |
| in vivo overall intrinsic clearance (CLint,all) | mL/min per kg | 46.2 | 168.0 | |
| Predicted hepatic clearance (CLh) | mL/h per kg | 21.6 | 78.4 | |
| Method 3 | ||||
| intrinsic metabolic clearance (CLint,met) | mL/min per kg | 160.4 | 205.3 | |
| liver-to-plasma unbound drug concentration ratio at steady state (Kpuu,ss) | 0.11 | 1.7 | ||
| in vivo overall intrinsic clearance (CLint,all) | mL/min per kg | 17.3 | 344.3 | |
| Predicted hepatic clearance (CLh) | mL/h per kg | 8.1 | 160 | |
| Observed in vivo clearance (CL) | mL/h per kg | 30.02 | 32.99 | 137.9 |
| Parameters | Comment | ||
|---|---|---|---|
| Control | Repeated-Dosing | ||
| Single-adjustment compartment | |||
| kin (hr−1) | 2.7 | Equal to Control | Fitted from observed data |
| kout (hr−1) | 1.0 | Equal to Control | Fitted from observed data |
| Tissue volume (L/kg) | |||
| Central | 0.018 | Equal to Control | Fitted from observed data |
| Liver | 0.037 | Equal to Control | [28] |
| Extracellular space in the liver | 0.01 | Equal to Control | [44] |
| Hepatocytes | 0.027 | Equal to Control | [44] |
| Blood flow (L/h/kg) | |||
| Liver | 3.69 | Equal to Control | [28] |
| Monte Carlo simulation parameters | |||
| PSinf,act (L/h/kg) | (2.5, 0.8) | (9.2, 0.6) | (Mean, SD) |
| PSdiff (L/h/kg) | (0.3, 0.09) | (0.86, 0.4) | (Mean, SD) |
| CLint,met (L/h/kg) | (9.8, 1.0) | (12.3, 0.08) | (Mean, SD) |
| fu,hepa | (0.004, 0.002) | (0.01, 0.008) | (Mean, SD) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, M.-C.; Lee, Y.-J. Analysis of Time-Dependent Pharmacokinetics Using In Vitro–In Vivo Extrapolation and Physiologically Based Pharmacokinetic Modeling. Pharmaceutics 2022, 14, 2562. https://doi.org/10.3390/pharmaceutics14122562
Kim M-C, Lee Y-J. Analysis of Time-Dependent Pharmacokinetics Using In Vitro–In Vivo Extrapolation and Physiologically Based Pharmacokinetic Modeling. Pharmaceutics. 2022; 14(12):2562. https://doi.org/10.3390/pharmaceutics14122562
Chicago/Turabian StyleKim, Min-Chang, and Young-Joo Lee. 2022. "Analysis of Time-Dependent Pharmacokinetics Using In Vitro–In Vivo Extrapolation and Physiologically Based Pharmacokinetic Modeling" Pharmaceutics 14, no. 12: 2562. https://doi.org/10.3390/pharmaceutics14122562
APA StyleKim, M.-C., & Lee, Y.-J. (2022). Analysis of Time-Dependent Pharmacokinetics Using In Vitro–In Vivo Extrapolation and Physiologically Based Pharmacokinetic Modeling. Pharmaceutics, 14(12), 2562. https://doi.org/10.3390/pharmaceutics14122562







