Abstract
The purpose of the present study was to experimentally confirm the thermodynamic correlation between the intrinsic liquid–liquid phase separation (LLPS) concentration () and crystalline solubility () of drug-like molecules. Based on the thermodynamic principles, the crystalline solubility LLPS concentration melting point () equation (CLME) was derived ( for 310 K). The values of 31 drugs were newly measured by simple bulk phase pH-shift or solvent-shift precipitation tests coupled with laser-assisted visual turbidity detection. To ensure the precipitant was not made crystalline at <10 s, the precipitation tests were also performed under the polarized light microscope. The calculated and observed values showed a good correlation (root mean squared error: 0.40 log unit, absolute average error: 0.32 log unit).
1. Introduction
Intrinsic aqueous solubility () is one of the most important physicochemical properties of a crystalline drug. Many prediction methods have been reported in the literature [1,2,3]. In most cases, is directly predicted from the chemical structure by empirical statistic approaches [4,5,6]. However, even though this approach has been investigated for more than several decades, prediction is still challenging [7].
An alternative approach would be to separate the solubilization process into the melting and solvation terms based on the theory of thermodynamics (Figure 1).
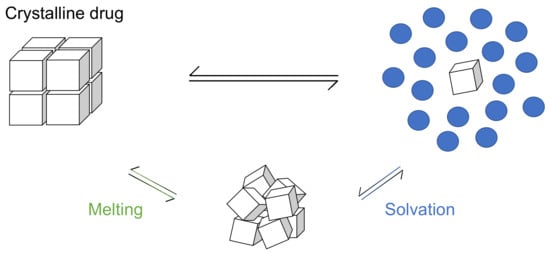
Figure 1.
Thermodynamic scheme of crystalline drug solubilization.
The general solubility equation (GSE) is one of this kind of stepwise prediction approach. Based on the thermodynamic principles, GSE has been formulated as [8],
where is the melting point, T is the temperature, and is the octanol/water partition coefficient. GSE was derived from the thermodynamic principles without parameter fitting. The constant of the GSE (0.5) is attributed to the solubility of a drug in octanol. When a liquid-state drug is completely miscible in octanol, the solubility of the liquid-state drug in octanol is expressed as 0.5 [9].
One merit of this kind of stepwise prediction approach is that it can be used to evaluate the relative contribution of the solvation and crystal lattice energy terms such as and ) in GSE, respectively. Therefore, it is of great help to navigate drug design. In addition, it also helps formulation design because a solubilizing formulation suitable for a drug differs depending on the cause of poor solubility being solvation or crystal lattice energy. Another merit of the stepwise approach is that the intermediate parameters such as and can be directly experimentally measured to confirm the prediction accuracy for each process.
Recently, the liquid–liquid phase separation (LLPS) of a drug attracted a lot of attention in drug research [10,11,12,13,14]. Several methods have been reported to measure the intrinsic LLPS concentration () [15]. As explained in the Theory section, LLPS is synonymous with the solvation phenomena shown in Figure 1. Therefore, in theory, can be approximately described by and melting point () (see Theory section). However, the accuracy of this approximation has not been experimentally confirmed for drug-like molecules. This information is important for clarifying which energy term is responsible for the inaccuracy of prediction, which may lead to breakthroughs in in silico predictions.
The purpose of the present study was to experimentally confirm the thermodynamic correlation between the intrinsic liquid–liquid phase separation (LLPS) concentration () and crystalline solubility () of drug-like molecules. In this study, the values of 31 drugs were newly measured by a simple precipitation test using laser-assisted visual turbidity detection (LAVTD).
2. Theory
The intrinsic solubility ratio of a crystalline drug () and a liquid drug () equals the ideal solubility ratio of the crystal drug () and the liquid drug (),
The ideal solubility is the mole fraction solubility of a drug in an ideal solution (in the liquid drug). By rearranging this,
Assuming that the change in heat capacity upon melting is equal to zero [16], the ideal solubility ratio () can be estimated as,
where is the entropy of melting, and is the melting point.
Approximating by the intrinsic liquid–liquid phase separation concentration (), is expressed as
This formula is hereinafter referred to as the crystalline solubility LLPS concentration melting point equation (CLME). It should be noted that and do not exactly match because water and a liquid phase drug are mutually miscible to some extent. In addition, LLPS and the glass–liquid phase separation were not distinguished in this study [17].
According to Walden’s rule, = 56.5 J/K∙mol. Therefore, at 298 K (25 °C) and 310 K (37 °C), Equation (5) becomes
It should be noted that CLME was derived from the thermodynamic principles without any parameter fitting.
3. Materials and Methods
3.1. Materials
Sodium hydroxide aqueous solution (8 mol/L) (NaOH), 6 mol/L hydrochloric acid (HCl), sodium chloride (NaCl), sodium dihydrogen phosphate dihydrate (NaH2PO4 2H2O), N,N-dimethylacetamide (DMA), boric acid, methanol, 0.1% trifluoroacetic acid-acetonitrile, (S)-(+)-naproxen, diphenhydramine hydrochloride, haloperidol, ibuprofen, indomethacin, ketoprofen, niflumic-acid, papaverine hydrochloride, (±)-propranolol hydrochloride, quinine, and warfarin sodium were purchased from FUJIFILM Wako Pure Chemical Corporation (Osaka, Japan). 2-Naphthoic acid, acemetacin, bifonazole, bupivacaine hydrochloride, carprofen, chlorpromazine hydrochloride, diclofenac sodium salt, dipyridamole, fenofibrate, flufenamic-acid, flumequine, flurbiprofen, furosemide, glipizide, ketoconazole, ketotifen fumarate, losartan potassium, loxoprofen, mefenamic-acid, meloxicam, phenylbutazone, probenecid, procaine hydrochloride, procaine, propafenone hydrochloride, rebamipide, sulfasalazine, sulindac, thioridazine hydrochloride, and verapamil hydrochloride were purchased from Tokyo Chemical Industry (Tokyo, Japan). Meclofenamic-acid sodium salt and phenytoin sodium were purchased from Sigma-Aldrich (Arklow, Ireland). Benzocaine, lidocaine hydrochloride, and terbinafine hydrochloride were purchased from Combi-Blocks (San Diego, CA, USA). Orphenadrine hydrochloride was purchased from Chem Cruz (Huissen, Netherlands). 0.1% trifluoroacetic acid-distilled water was purchased from Kanto Chemical Co., Inc. (Tokyo, Japan). Pramoxine hydrochloride was purchased from Cayman Chemical (Ann Arbor, MI, USA). Warfarin free acid was prepared by adding 1 N HCl to warfarin sodium dissolved in distilled water. Propafenone free base was prepared by adding 1 N NaOH to propafenone hydrochloride dissolved in distilled water.
3.2. Methods
3.2.1. Crystallization Time Measurement
Before the measurements, the crystallization time of 47 drugs was measured by performing the precipitation test under the polarized light microscope (PLM). In the pH-shift precipitation method, an ionizable drug was dissolved in distilled water as a salt form or by adding 1–3 Eq of NaOH (for weak acids) or HCl (for weak bases). A total of 1.0 µL of 1 N HCl (for weak acids) or 1 N NaOH (for weak bases) was dropped onto a glass slide, then the drug solution (9.0 µL) was added and covered with a cover glass. In the solvent-shift precipitation method, an un-dissociable drug was dissolved in N,N-dimethylacetamide. Distilled water (19.8 µL) was added to the drug solution (0.2 µL). The drug concentration was set to 30 mM, except for propafenone (20 mM), lidocaine (200 mM), terbinafine (18 mM), fenofibrate (198 mM), and haloperidol (2 mM). For the measurements at 310 K, the temperature was maintained by a glass plate heater (BLAST Inc., Kanagawa, Japan). The precipitants were monitored under a PLM (crossed-Nicols with a sensitive-tint plate) (Olympus CX-43, Olympus Corporation, Tokyo, Japan). The solid state of the precipitant was diagnosed as crystalline when polarization was observed.
3.2.2. Measurement by the Precipitation Tests Coupled with Laser-Assisted Visual Turbidity Detection (LAVTD)
The drugs with a crystallization time > 10 s were selected for the measurements. The value was measured by the bulk phase pH-shift precipitation tests or the solvent-shift precipitation tests coupled with laser-assisted visual turbidity detection (LAVTD) as previously reported [18]. Each drug solution was prepared as described above. For the measurements at 310 K, the drug solution, 1 N NaOH, 1 N HCl, and glass test tubes were pre-heated in a water bath. In the case of ionizable drugs, 1 N NaOH or 1 N HCl (100 µL) was added to the glass test tube, then set to the LAVTD device (Figure S1). The drug solution (900 µL) was then added to the glass test tube and immediately vigorously shaken. In the case of un-dissociable drugs, the drug solution (10 µL) was added to the glass tube, then distilled water (990 µL) was added. Turbidity was visually detected within 10 s with the assistance of a red laser (635 nm). The concentration of the drug solution was changed with 0.001 to 0.1 mM increments to give 3 significant digits. The value was defined as the drug concentration at which the solution started to show turbidity. The measurement was performed in triplicate.
To compare with the literature data, was also measured using the same medium conditions as the reference [19]. Diclofenac sodium was dissolved in methanol. The drug solution was added to the glass test tube (10 µL). Phosphate buffer (990 µL, pH 2.0, PO4: 50 mM, NaCl: 128 mM) was added to the glass test tube and immediately vigorously shaken at 298 K. The value was determined as described above.
3.2.3. Measurement by Turbidity Detection Using a UV/VIS Spectrophotometer
Each drug solution was prepared as described above. A 1 N NaOH or 1 N HCl solution (70 µL) was added to the quartz cell and set to a UV/VIS spectrophotometer (UV-1850, Shimadzu Corporation, Kyoto, Japan). A drug solution (630 µL) was rapidly added to the quartz cell and the absorbance was measured at 500 nm within 10 s at 298 K. This wavelength was set to be higher than the absorption wavelength of each drug. The measurement was performed in triplicate.
3.2.4. Intrinsic Solubility Measurement
Crystalline free-form drugs were used for the intrinsic solubility measurement. The intrinsic solubility was measured based on the harmonized protocol as previously reported [20]. Each drug was added to a test solution (10 mL) in a 15 mL tube. The samples were rotated at 40 rpm at 310 K except for procaine (1800 rpm). Before filtration, the sample was allowed to stand still for 1 min. The sample was then filtered (hydrophilic PVDF, 0.22 μm pore size). The first few drops were discarded to avoid filter adsorption [21]. The drug concentration in the filtrate was determined by UV spectroscopy (UV-1850, Shimadzu Corporation, Kyoto, Japan). The residual solid was collected by vacuum filtration and analyzed by differential scanning calorimetry (DSC). The composition of the medium, the amount of the added drug, the incubation time, and the detection wavelength are summarized in Table 1. The achievement of equilibrium was confirmed by time-course measurements up to 48 h.

Table 1.
Experimental conditions of intrinsic solubility measurement.
Procaine showed hydrolysis in alkali conditions [22]. Therefore, the concentration of procaine was determined by HPLC (Shimadzu Prominence LC-20 series; Colum: Zorbax Eclipse Plus C18, 2.1 × 50 mm, particle size: 3.5 μm; mobile phase: acetonitrile/0.1% trifluoroacetic acid (5: 95); flow rate: 0.6 mL/min; temperature: 40 °C; injection volume: 10 μL). The achievement of equilibrium was confirmed by time-course measurements.
3.2.5. Differential Scanning Calorimetry Measurement
The solid form of the residual particles in the intrinsic solubility measurement was determined by differential scanning calorimetry (DSC). The sample was placed in a non-sealed aluminum pan and analyzed by DSC (Shimadzu DSC60 plus, Shimadzu Corporation Kyoto, Japan) under nitrogen gas (50 mL/min). Heat flow was set to 10 °C/min.
3.2.6. Thermodynamic Correlation between the Intrinsic Liquid–Liquid Phase Separation Concentration and Crystalline Solubility
The value was calculated by CLME (Equation (7)). The experimental values were obtained from the literature when available (Table 2). The values at 310 K were also obtained from the literature when available. The correlation between the calculated and observed values was evaluated by the average absolute error (AAE), the root mean square error (RMSE), and the coefficient of determination (r2). The AAE and RMSE were calculated by
where the subscript calc and obs indicate the calculated and observed values.
The value was also calculated by GSE using the experimental values in the literature.
4. Results
4.1. Crystallization Time
Before the precipitation tests, the crystallization time for each compound was determined under PLM. The results are summarized in Table S1. Because LAVTD requires 10 s, the drugs that crystallized within 10 s were excluded from the following studies (31 remained out of 47 drugs).
4.2. Validation of the Precipitation Tests Coupled with Laser-Assisted Visual Turbidity Detection
In LAVTD, the turbidity of a solution was detected by visual inspection. However, visual detection could cause a measurement error. To validate LAVTD, turbidity measurements were also performed by UV/VIS spectrometry for several drugs (diclofenac, ibuprofen, papaverine, propafenone, and warfarin). The photograph of the LAVTD method and the absorbance vs. concentration profiles measured by the UV/VIS spectrometry are summarized in Figure 2. The value was determined as the concentration intercept value. The of diclofenac, ibuprofen, papaverine, propafenone, and warfarin were determined as 0.25 ± 0.00 mM, 0.64 ± 0.01 mM, 0.83 ± 0.00 mM, 0.38 ± 0.00 mM, and 0.56 ± 0.00 mM, respectively by the UV/VIS method. The values measured by LAVTD were almost identical to those measured by the UV/VIS method (Figure 3). The coefficient of determination was 0.997.
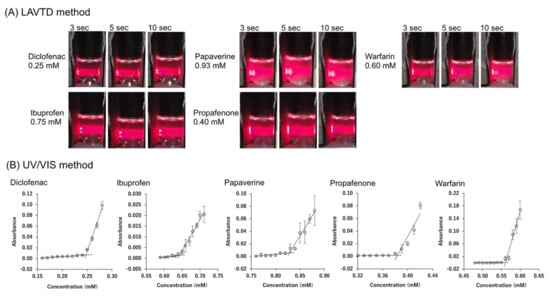
Figure 2.
(A) The photograph of LAVTD method at 298 K. (B) Concentration vs. absorbance profiles in measurement by turbidity detection by UV/VIS spectroscopy at 298 K. The value was determined as the concentration intercept value.
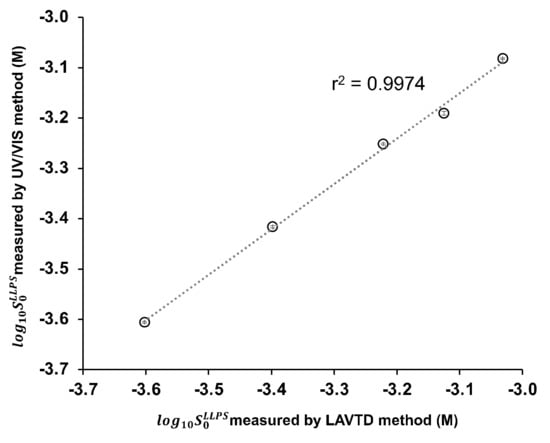
Figure 3.
Comparison of the values measured with the UV/VIS method and the LAVTD method (mean ± S.D., n = 3) (298 K).
To further validate LAVTD, the value of diclofenac measured by LAVTD was compared with the literature values measured by the UV/VIS method and the fluorescence spectroscopy method [19]. The value measured by the LAVTD methods, the UV/VIS method, and the fluorescence spectroscopy method were 0.20 mM, 0.18 mM, and 0.17 mM, respectively (at 298 K). Therefore, LAVTD yielded the value close to the previously reported values.
4.3. Thermodynamic Correlation between the Intrinsic Liquid–Liquid Phase Separation Concentration and Crystalline Solubility
The molecular weight (MW), acid–base dissociation constant (), , , , and of each drug are summarized in Table 2. Many of the differed from the values more than 10-fold and up to 158-fold. The correlation between the calculated and observed values is shown in Figure 4. The drugs with a melting point below 310 K were excluded from the analysis. In addition, terbinafine was excluded because it was difficult to prepare the crystalline-free base due to its low melting point (314 K). The AAE, RMSE, and r2 values are summarized in Table 3.
The correlation between and is shown in Figure 5. When the value increased, the value decreased.

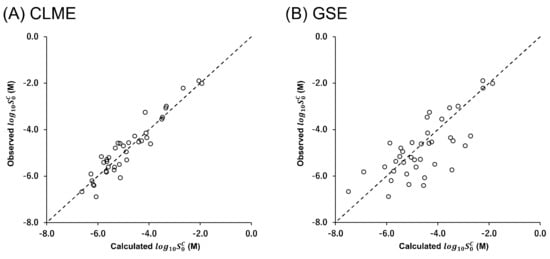
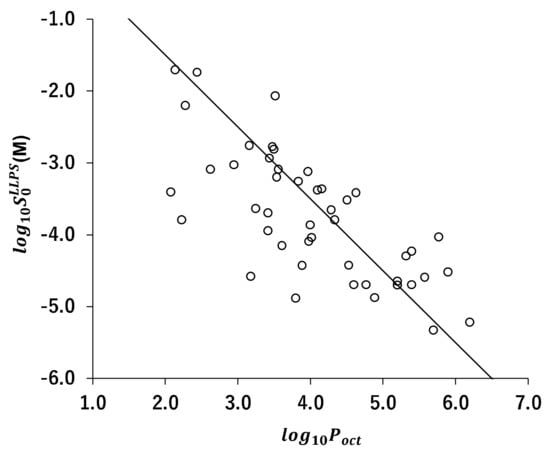


Table 2.
MW, , , , , and of model compounds.
Table 2.
MW, , , , , and of model compounds.
| Drugs | MW | Ref. | |||||
|---|---|---|---|---|---|---|---|
| Atazanavir | 705 | 4.5 (B) | 481 | 5.8 | −4.03 | −5.83 | [23,24] |
| Bifonazole | 310 | 5.7 (B) | 422 2 | 4.8 | −4.70 ± 0.00 2 | −5.42 ± 0.03 2 | [25,26] |
| Carprofen | 274 | 4.2 (A) | 484 2 | 4.3 | −3.66 ± 0.00 2 | −4.80 ± 0.02 2 | [27] |
| Celecoxib | 381 | 11.1 (A) | 437 | 3.4 | −3.95 | −5.50 | [28,29,30] |
| Chlorpromazine 3 | 319 | 9.2 (B) | <298 | 5.4 | −4.70 ± 0.00 2 | - | [27] |
| Cilnidipine | 493 | None | 387 | 5.7 | −5.33 | −6.89 | [31,32,33] |
| Clotrimazole | 345 | 5.9 (B) | 417 | 5.2 | −4.65 | −5.80 | [23,25,34] |
| Clozapine | 327 | 3.8 (B), 7.5 (B) | 458 | 4.1 | −3.38 | −4.57 | [15,25,34] |
| Danazol | 338 | None | 498 | 4.5 | −4.43 | −6.21 | [35,36,37,38] |
| Diclofenac | 296 | 4.0 (A) | 453 | 4.5 | −3.52 ± 0.00 2 | −4.96 | [18,27,39] |
| Diphenhydramine 3 | 255 | 9.1 (B) | <298 | 3.4 | −2.94 ± 0.00 2 | - | [27] |
| Dipyridamole | 505 | 6.4 (B) | 436 | 2.2 | −3.80 ± 0.00 2 | −4.70 | [40,41,42] |
| Efavirenz | 316 | 10.2 (A) | 412 | 5.4 | −4.23 | −4.59 | [15,43,44,45] |
| Enzalutamide | 464 | None between pH 3–11 | 470 | 4.0 | −4.04 | −5.20 | [46,47] |
| Felodipine | 384 | <2 | 415 | 5.6 | −4.59 | −5.61 | [10,15,25,48] |
| Fenofibrate | 361 | None | 354 | 4.6 | −4.70 ± 0.00 2 | −6.08 | [49,50] |
| Flurbiprofen | 244 | 4.0 (A) | 388 2 | 4.2 | −3.37 ± 0.00 2 | −4.15 ± 0.01 2 | [27] |
| Ibuprofen | 206 | 4.4 (A) | 349 | 4.0 | −3.12 ± 0.00 2 | −3.55 | [27,51] |
| Ketoconazole | 531 | 3.3 (B), 6.2 (B) | 423 | 4.3 | −3.80 ± 0.00 2 | −5.31 | [25,52,53,54] |
| Ketoprofen | 254 | 4.2 (A) | 368 | 3.2 | −2.76 ± 0.00 2 | −3.00 | [25,55,56,57] |
| Ketotifen | 309 | 6.7 (B) | 430 | 2.1 | −3.41 ± 0.00 2 | −4.28 | [58,59] |
| Lidocaine | 234 | 8.0 (B) | 342 | 2.4 | −1.74 ± 0.00 2 | −1.90 | [27,60] |
| Loratadine | 383 | 5.3 (B) | 409 | 5.2 | −4.70 | −5.38 | [15,41,61] |
| Losartan | 423 | 3.2 (A) | 457 | 3.5 | −2.07 ± 0.00 2 | −3.47 | [62,63,64,65] |
| Loxoprofen | 246 | 4.2 (A) | 358 2 | 2.3 | −2.21 ± 0.00 2 | −2.22 ± 0.00 2 | [66,67] |
| Meclofenamic-acid | 296 | 4.1 (A) | 530 | 5.9 | −4.52 ± 0.00 2 | −6.68 | [27,68] |
| Miconazole | 416 | 6.1 (B) | 358 | 4.9 | −4.88 | −5.62 | [25,35,69] |
| Orphenadrine 3 | 269 | 9.0 (B) | <298 | 3.8 | −3.26 ± 0.00 2 | - | [27] |
| Paclitaxel | 854 | None | 493 | 3.9 | −4.43 | −6.38 | [35,70,71] |
| Papaverine | 339 | 6.4 (B) | 421 | 3.0 | −3.03 ± 0.00 2 | −4.35 | [27,72] |
| Phenylbutazone | 308 | 4.4 (A) | 379 2 | 3.3 | −3.64 ± 0.00 2 | −4.49 ± 0.01 2 | [27] |
| Posaconazole | 701 | 3.6 (B), 4.6 (B) | 442 | 3.8 | −4.89 | −6.41 | [73,74,75] |
| Pramoxine 3 | 330 | 7.1 (B) | <298 | 3.6 | −3.09 ± 0.00 2 | - | [27] |
| Procaine | 236 | 2.3 (B), 9.0 (B) | 333 2 | 2.1 | −1.71 ± 0.00 2 | −2.02 ± 0.01 2 | [27] |
| Propafenone | 341 | 9.3 (B) | 364 2 | 4.6 | −3.42 ± 0.00 2 | −4.62 ± 0.03 2 | [76,77] |
| Propranolol | 259 | 9.0 (B) | 369 | 3.5 | −2.78 ± 0.00 2 | −3.07 | [27,78] |
| Quinine | 324 | 4.2 (B), 8.6 (B) | 449 2 | 3.5 | −2.82 ± 0.00 2 | −3.26 ± 0.00 2 | [27] |
| Rebamipide | 371 | 3.3 (A) | 579 | 2.6 | −3.09 ± 0.00 2 | −5.29 | [79,80,81] |
| Ritonavir | 721 | 2.4 (B) | 391 | 3.2 | −4.58 | −5.74 | [15,82,83,84] |
| Sulfasalazine | 398 | 2.4 (A), 8.0 (A), 10.9 (A) | 532 2 | 3.6 | −4.15 ± 0.00 2 | −5.92 ± 0.04 2 | [27] |
| Sulindac | 356 | 4.1 (A) | 460 2 | 3.4 | −3.70 ± 0.00 2 | −4.60 ± 0.00 2 | [27] |
| Telaprevir | 680 | 0.3 (B), 11.8 (A) | 519 | 4.0 | −3.87 | −5.17 | [17,85] |
| Terbinafine 3 | 291 | 7.1 (B) | 314 | 6.2 | −5.22 ± 0.00 2 | - | [25,86] |
| Thioridazine 3 | 371 | 8.9 (B) | <298 | 5.3 | −4.30 ± 0.00 2 | - | [87,88,89] |
| Verapamil 3 | 455 | 8.7 (B) | <298 | 4.0 | −4.10 ± 0.00 2 | - | [27] |
| Warfarin | 308 | 4.9 (A) | 436 2 | 3.5 | −3.20 ± 0.00 2 | −4.54 ± 0.01 2 | [27] |
1 A: acid, B: base. 2 Measured values in this study. The and values were measured at 310 K. Detailed information on the measurements (DSC curves of residual particles, the pH values of suspension after reaching equilibrium, and calibration curves) are summarized in Figure S4 and Figure 5, and Table S2. 3 Excluded from the prediction.

Figure 4.
Correlation between the calculated and observed values at 310 K by (A) Equation (7) (CLME with and ) and (B) Equation (1) (GSE using ). The black dotted line indicates the calculated value equals the observed value.

Figure 5.
Correlation between and . The solid line is .

Table 3.
Statistical correlation of CLME and GSE (N = 39).
Table 3.
Statistical correlation of CLME and GSE (N = 39).
| AAE (log unit) | 0.32 | 0.71 |
| RMSE (log unit) | 0.40 | 0.91 |
| r2 | 0.90 | 0.56 |
5. Discussion
In this study, we first validated the bulk phase pH-shift and solvent-shift precipitation tests coupled with laser-assisted visual turbidity detection (LAVTD). This method is simple, rapid, robust, and requires minimum instrumental resources (only a red laser pointer). Because LAVTD requires less than 10 s, it enables the measurements of rapidly crystallizing drugs. However, visual detection could cause a measurement error. Therefore, to validate LAVTD, the values were compared with those measured by the UV/VIS spectrophotometric method and the fluorescence spectroscopy method. There was a good agreement between the values measured by these methods.
In the pH-shift LLPS measurements, in the case of ionizable drugs, the pH value was shifted by adding a small volume of 1 N HCl or 1 N NaOH to a drug solution (1:9). This pH-shift procedure can avoid inducing a local high drug concentration that could facilitate drug crystallization. In the case of un-dissociable drugs, a concentration drug solution in DMA was diluted by adding distilled water (the final DMA concentration was 1.0%). In this case, a high drug concentration may be locally formed at the initial stage of dilution. In this study, the LLPS concentration was sought by changing the initial drug concentration rather than stepwise titration with a concentrated drug solution. Stepwise titration changes the concentration of a rich solvent. In addition, during the stepwise titration process, crystallization could be induced before reaching LLPS concentration.
In this study, to shift the pH value of a drug solution, 1 N HCl or 1 N NaOH was used for weak acids and bases, respectively. Therefore, the measurement was conducted at a pH where a drug is undissociated. In other words, LLPS observed in this study is a phenomenon that undissociated non-electrolytes phases separately to form a drug-rich phase (liquid drug phase). The drug-rich phase is a transient state before crystallization. However, when LLPS was formed, a pseudo-equilibrium state was created between the drug-rich phase and the drug molecules molecularly dissolved in water.
In this study, the values of 31 drugs were newly determined. These values were combined with those reported in the literature to be used for the evaluation of CLME (a total of 39 drugs). CLME appropriately described the values. This result encourages that in silico prediction can be improved by dividing the prediction scheme into two processes, prediction and prediction. The development of an in silico model is currently under investigation. For drug-like molecules, a correlation was observed between and (Figure 5). Therefore, the same parameters for prediction from chemical structure (hydrogen bonds, molecular volumes, etc.) might also be used to predict [90,91]. A large amount of the data set is required to construct an in silico model. The LAVTD-based method is suitable for high throughput (HTS) measurements. The HTS measurement method is also currently under investigation.
Although the number of drugs used in this study was limited, CLME showed a significantly better correlation than GSE (p = 0.0004, comparison of two independent Pearson’s correlation coefficients) (Figure 4). In GSE, is employed to represent the solvation term. In the octanol phase, drug molecules are surrounded by octanol molecules (octanol-drug mixture). On the other hand, in the case of LLPS, in the drug-rich phase, drug molecules are surrounded by themselves (drug-drug mixture). As shown in Figure 1, the solvation process is the same as the partitioning of a drug between the drug-rich phase and the water phase.
There may be three possible ways to further improve the correlation by CLME. First, in this study, the same value (56.5 J/K∙mol) was used for all drugs. However, the values are different for each compound [92]. Second, the activity of the liquid phase (drug-rich phase) saturated with water should be considered. is not exactly the same as because water and a liquid drug phase are mutually miscible to some extent [93,94]. Third, the heat capacity terms should be considered in the ideal solubility ratio calculation [95].
In this study, the values of drugs that crystallized within 10 s were not measured. To measure the value of quickly crystallizing drugs, a crystallization inhibitor such as polymers (e.g., polyvinylpyrrolidone) may be used [96]. However, the value varies depending on the type and concentration of a polymer [97,98]. Therefore, the selection of a polymer for measurements would be important. Alternatively, the results of this study suggest that the value can be calculated from the value and the melting point when the value is difficult to measure.
In conclusion, can be described by CLME with reasonable accuracy. The results of this study are important for a good understanding of drug solubility and shed light on the way to improve in silico prediction. is a drug intrinsic parameter that determines the maximum achievable concentration of molecularly dissolved drugs in aqueous media. The measurement using LAVTD is simple and easy. It would be especially useful for highly lipophilic drugs for which and measurements are often difficult. Therefore, similar to the other drug intrinsic parameters such as pKa and , should be routinely measured in drug discovery.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/pharmaceutics14122560/s1, Figure S1: Illustration of the lase-assisted visual turbidity detection device.; Table S1: Crystallization time of drugs at 310 K; Figure S2: The examples of PLM observation of crystallization time measurement.; Figure S3: The chemical structure and CAS number of model drugs; Figure S4: DSC curves of residual particles in intrinsic solubility measurement.; Table S2: The pH value after reaching the equilibrium.; Figure S5: Calibration curves of intrinsic solubility measurement.
Author Contributions
Conceptualization, K.S.; methodology, T.U., T.W., D.W. and K.S.; validation, T.U., T.W., D.W. and K.S.; investigation, T.U., T.W., D.W. and K.S.; resources, K.S.; data curation, T.U.; writing—original draft preparation, K.S.; writing—review and editing, T.U.; visualization, T.U. and K.S.; supervision, K.S.; project administration, K.S.; funding acquisition, T.U. and K.S. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by JST, the establishment of university fellowships towards the creation of science technology innovation, grant number: JPMJFS2146.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
T.U. thanks the Nagai Memorial Research Scholarship from the Pharmaceutical Society of Japan for their support.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Falcón-Cano, G.; Molina, C.; Cabrera-Pérez, M.A. ADME Prediction with KNIME: A Retrospective Contribution to the Second “Solubility Challenge”. ADMET DMPK 2021, 9, 209–218. [Google Scholar] [CrossRef]
- Salahinejad, M.; Le, T.C.; Winkler, D.A. Aqueous Solubility Prediction: Do Crystal Lattice Interactions Help? Mol. Pharm. 2013, 10, 2757–2766. [Google Scholar] [CrossRef] [PubMed]
- Jain, P.; Yalkowsky, S.H. Prediction of Aqueous Solubility from SCRATCH. Int. J. Pharm. 2010, 385, 1–5. [Google Scholar] [CrossRef]
- Katritzky, A.R.; Wang, Y.; Sild, S.; Tamm, T.; Karelson, M. QSPR Studies on Vapor Pressure, Aqueous Solubility, and the Prediction of Water-Air Partition Coefficients. J. Chem. Inf. Comput. Sci. 1998, 38, 720–725. [Google Scholar] [CrossRef]
- Hewitt, M.; Cronin, M.T.D.; Enoch, S.J.; Madden, J.C.; Roberts, D.W.; Dearden, J.C. In Silico Prediction of Aqueous Solubility: The Solubility Challenge. J. Chem. Inf. Model. 2009, 49, 2572–2587. [Google Scholar] [CrossRef] [PubMed]
- Boobier, S.; Hose, D.R.J.; Blacker, A.J.; Nguyen, B.N. Machine Learning with Physicochemical Relationships: Solubility Prediction in Organic Solvents and Water. Nat. Commun. 2020, 11, 5753. [Google Scholar] [CrossRef]
- Llinas, A.; Avdeef, A. Solubility Challenge Revisited after Ten Years, with Multilab Shake-Flask Data, Using Tight (SD ∼0.17 Log) and Loose (SD ∼0.62 Log) Test Sets. J. Chem. Inf. Model. 2019, 59, 3036–3040. [Google Scholar] [CrossRef] [PubMed]
- Jain, N.; Yalkowsky, S.H. Estimation of the Aqueous Solubility I: Application to Organic Nonelectrolytes. J. Pharm. Sci. 2001, 90, 234–252. [Google Scholar] [CrossRef]
- Ran, Y.; Jain, N.; Yalkowsky, S.H. Prediction of Aqueous Solubility of Organic Compounds by the General Solubility Equation (GSE). J. Chem. Inf. Comput. Sci. 2001, 41, 1208–1217. [Google Scholar] [CrossRef] [PubMed]
- Raina, S.A.; Zhang, G.G.Z.; Alonzo, D.E.; Wu, J.; Zhu, D.; Catron, N.D.; Gao, Y.; Taylor, L.S. Enhancements and Limits in Drug Membrane Transport Using Supersaturated Solutions of Poorly Water Soluble Drugs. J. Pharm. Sci. 2014, 103, 2736–2748. [Google Scholar] [CrossRef]
- Indulkar, A.S.; Gao, Y.; Raina, S.A.; Zhang, G.G.Z.; Taylor, L.S. Exploiting the Phenomenon of Liquid-Liquid Phase Separation for Enhanced and Sustained Membrane Transport of a Poorly Water-Soluble Drug. Mol. Pharm. 2016, 13, 2059–2069. [Google Scholar] [CrossRef]
- Suzuki, K.; Kawakami, K.; Fukiage, M.; Oikawa, M.; Nishida, Y.; Matsuda, M.; Fujita, T. Relevance of Liquid-Liquid Phase Separation of Supersaturated Solution in Oral Absorption of Albendazole from Amorphous Solid Dispersions. Pharmaceutics 2021, 13, 220. [Google Scholar] [CrossRef]
- Van den Mooter, G. The Use of Amorphous Solid Dispersions: A Formulation Strategy to Overcome Poor Solubility and Dissolution Rate. Drug Discov. Today Technol. 2012, 9, e79–e85. [Google Scholar] [CrossRef]
- Ramachandran, G.; Sudheesh, M.S. Role of Permeability on the Biopredictive Dissolution of Amorphous Solid Dispersions. AAPS PharmSciTech 2021, 22, 243. [Google Scholar] [CrossRef]
- Ilevbare, G.A.; Taylor, L.S. Liquid-Liquid Phase Separation in Highly Supersaturated Aqueous Solutions of Poorly Water-Soluble Drugs: Implications for Solubility Enhancing Formulations. Cryst. Growth Des. 2013, 13, 1497–1509. [Google Scholar] [CrossRef]
- Mishra, D.S.; Yalkowsky, S.H. Ideal Solubility of a Solid Solute: Effect of Heat Capacity Assumptions. Pharm. Res. 1992, 9, 958–959. [Google Scholar] [CrossRef] [PubMed]
- Mosquera-Giraldo, L.I.; Taylor, L.S. Glass-Liquid Phase Separation in Highly Supersaturated Aqueous Solutions of Telaprevir. Mol. Pharm. 2015, 12, 496–503. [Google Scholar] [CrossRef]
- Oki, J.; Watanabe, D.; Uekusa, T.; Sugano, K. Mechanism of Supersaturation Suppression in Dissolution Process of Acidic Drug Salt. Mol. Pharm. 2019, 16, 1669–1677. [Google Scholar] [CrossRef] [PubMed]
- Almeida E Sousa, L.; Reutzel-Edens, S.M.; Stephenson, G.A.; Taylor, L.S. Assessment of the Amorphous “Solubility” of a Group of Diverse Drugs Using New Experimental and Theoretical Approaches. Mol. Pharm. 2015, 12, 484–495. [Google Scholar] [CrossRef] [PubMed]
- Ono, A.; Matsumura, N.; Kimoto, T.; Akiyama, Y.; Funaki, S.; Tamura, N.; Hayashi, S.; Kojima, Y.; Fushimi, M.; Sudaki, H.; et al. Harmonizing Solubility Measurement to Lower Inter-Laboratory Variance—Progress of Consortium of Biopharmaceutical Tools (CoBiTo) in Japan. ADMET DMPK 2019, 7, 183–195. [Google Scholar] [CrossRef] [PubMed]
- Völgyi, G.; Csicsák, D.; Takács-Novák, K. Right Filter-Selection for Phase Separation in Equilibrium Solubility Measurement. Eur. J. Pharm. Sci. 2018, 123, 98–105. [Google Scholar] [CrossRef]
- Guerrieri, P.; Jarring, K.; Taylor, L.S. Impact of Counterion on the Chemical Stability of Crystalline Salts of Procaine. J. Pharm. Sci. 2009, 99, 3719–3730. [Google Scholar] [CrossRef]
- Indulkar, A.S.; Box, K.J.; Taylor, R.; Ruiz, R.; Taylor, L.S. PH-Dependent Liquid-Liquid Phase Separation of Highly Supersaturated Solutions of Weakly Basic Drugs. Mol. Pharm. 2015, 12, 2365–2377. [Google Scholar] [CrossRef]
- Duan, J.; Freeling, J.P.; Koehn, J.; Shu, C.; Ho, R.J.Y. Evaluation of Atazanavir and Darunavir Interactions with Lipids for Developing PH-Responsive Anti-HIV Drug Combination Nanoparticles. J. Pharm. Sci. 2014, 103, 2520–2529. [Google Scholar] [CrossRef]
- Avdeef, A. Absorption and Drug Development: Solubility, Permeabiliry, and Charge State, 2nd ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2012. [Google Scholar]
- Popović, G.; Čakar, M. The Effects of β-Cyclodextrin and PH on Bifonazole Hydrosolubility. J. Serb. Chem. Soc. 2004, 69, 225–231. [Google Scholar] [CrossRef]
- Box, K.J.; Comer, J.E.A. Using Measured PKa, LogP and Solubility to Investigate Supersaturation and Predict BCS Class. Curr. Drug Metab. 2008, 9, 869–878. [Google Scholar] [CrossRef]
- Li, N.; Mosquera-Giraldo, L.I.; Borca, C.H.; Ormes, J.D.; Lowinger, M.; Higgins, J.D.; Slipchenko, L.v.; Taylor, L.S. A Comparison of the Crystallization Inhibition Properties of Bile Salts. Cryst. Growth Des. 2016, 16, 7286–7300. [Google Scholar] [CrossRef]
- Iburahim, M.M.; Abd-Elgawad, A.E.H.; Soliman, O.A.E.; Jablonski, M.M. Nanoparticle-Based Topical Ophthalmic Formulations for Sustained Celecoxib Release. Pharm. Nanotechnol. 2012, 102, 1036–1053. [Google Scholar] [CrossRef]
- Paulson, S.K.; Vaughn, M.B.; Jessen, S.M.; Lawal, Y.; Gresk, C.J.; Yan, B.; Maziasz, T.J.; Cook, C.S.; Karim, A. Pharmacokinetics of Celecoxib after Oral Administration in Dogs and Humans: Effect of Food and Site of Absorption. J. Pharmacol. Exp. Ther. 2001, 297, 638–645. [Google Scholar] [PubMed]
- Liu, Q.; Mai, Y.; Gu, X.; Zhao, Y.; Di, X.; Ma, X.; Yang, J. A Wet-Milling Method for the Preparation of Cilnidipine Nanosuspension with Enhanced Dissolution and Oral Bioavailability. J. Drug Deliv. Sci. Technol. 2020, 55, 101371. [Google Scholar] [CrossRef]
- Indulkar, A.S.; Gao, Y.; Raina, S.A.; Zhang, G.G.Z.; Taylor, L.S. Crystallization from Supersaturated Solutions: Role of Lecithin and Composite Simulated Intestinal Fluid. Pharm. Res. 2018, 35, 158. [Google Scholar] [CrossRef] [PubMed]
- Mochida Pharmaceutical Co., Ltd. Cilnidipine Drug Product Information. Available online: https://med.mochida.co.jp/index/atc-h.html (accessed on 22 November 2022).
- Hsieh, Y.L.; Ilevbare, G.A.; van Eerdenbrugh, B.; Box, K.J.; Sanchez-Felix, M.V.; Taylor, L.S. PH-Induced Precipitation Behavior of Weakly Basic Compounds: Determination of Extent and Duration of Supersaturation Using Potentiometric Titration and Correlation to Solid State Properties. Pharm. Res. 2012, 29, 2738–2753. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; Mann, A.K.P.; van Duong, T.; Ormes, J.D.; Okoh, G.A.; Hermans, A.; Taylor, L.S. Drug Release and Nanodroplet Formation from Amorphous Solid Dispersions: Insight into the Roles of Drug Physicochemical Properties and Polymer Selection. Mol. Pharm. 2021, 18, 2066–2081. [Google Scholar] [CrossRef] [PubMed]
- Jackson, M.J.; Toth, S.J.; Kestur, U.S.; Huang, J.; Qian, F.; Hussain, M.A.; Simpson, G.J.; Taylor, L.S. Impact of Polymers on the Precipitation Behavior of Highly Supersaturated Aqueous Danazol Solutions. Mol. Pharm. 2014, 11, 3027–3038. [Google Scholar] [CrossRef] [PubMed]
- Mithani, S.D.; Bakatselou, V.; TenHoor, C.N.; Dressman, J.B. Estimation of the Increase in Solubility of Drugs as a Function of Bile Salt Concentration. Pharm. Res. 1996, 13, 163–167. [Google Scholar] [CrossRef] [PubMed]
- Okazaki, A.; Mano, T.; Sugano, K. Theoretical Dissolution Model of Poly-Disperse Drug Particles in Biorelevant Media. J. Pharm. Sci. 2008, 97, 1843–1852. [Google Scholar] [CrossRef] [PubMed]
- Giordano, F.; Rossi, A.; Pasquali, I.; Bettini, R.; Frigo, E.; Gazzaniga, A.; Sangalli, M.E.; Mileo, V.; Catinella, S. Thermal Degradation and Melting Point Determination of Diclofenac. J. Therm. Anal. Calorim. 2003, 73, 509–518. [Google Scholar] [CrossRef]
- Kostewicz, E.S.; Brauns, U.; Becker, R.; Dressman, J.B. Forecasting the Oral Absorption Behavior of Poorly Soluble Weak Bases Using Solubility and Dissolution Studies in Biorelevant Media. Pharm. Res. 2002, 19, 345–349. [Google Scholar] [CrossRef]
- Sugano, K.; Kato, T.; Suzuki, K.; Keiko, K.; Sujaku, T.; Takashi, M. High Throughput Solubility Measurement with Automated Polarized Light Microscopy Analysis. J. Pharm. Sci. 2006, 95, 2271–2280. [Google Scholar] [CrossRef]
- Xi, Z.; Sharma, N.; Paprikar, A.; Lin, S. Development and Evaluation of Dipyridamole Sustained Release Tablets Containing Micro-Environmental PH Modifiers. J. Drug Deliv. Sci. Technol. 2019, 54, 101231. [Google Scholar] [CrossRef]
- Rabel, S.R.; Michael, M.B.; Susan, R.M.; Hussain, M. Determination of the PKa and PH-Solubility Behavior of an Ionizable Cyclic Carbamate, (S)-6-Chloro-4-(Cyclopropylethynyl)-1,4-Dihydro-4-(Trifluoromethyl)-2H-3,1-Benzoxazin-2-One (DMP 266). Pharm. Dev. Technol. 1996, 1, 91–95. [Google Scholar] [CrossRef]
- Fitriani, L.; Haqi, A.; Zaini, E. Preparation and Characterization of Solid Dispersion Freeze-Dried Efavirenz—Polyvinylpyrrolidone K-30. J. Adv. Pharm. Technol. Res. 2016, 7, 105–109. [Google Scholar] [CrossRef] [PubMed]
- Merck & Co., Inc. Efavirenz Drug Product Information. 2022. Available online: https://www.msdconnect.jp/products/stocrin/ (accessed on 22 November 2022).
- Wilson, V.; Lou, X.; Osterling, D.J.; Stolarik, D.F.; Jenkins, G.; Gao, W.; Zhang, G.G.Z.; Taylor, L.S. Relationship between Amorphous Solid Dispersion In Vivo Absorption and In Vitro Dissolution: Phase Behavior during Dissolution, Speciation, and Membrane Mass Transport. J. Control. Release 2018, 292, 172–182. [Google Scholar] [CrossRef] [PubMed]
- Volkova, T.V.; Drozd, K.V.; Surov, A.O. Effect of Polymers and Cyclodextrins on Solubility, Permeability and Distribution of Enzalutamide and Apalutamide Antiandrogens. J. Mol. Liq. 2021, 322, 114937. [Google Scholar] [CrossRef]
- Kestur, U.S.; Taylor, L.S. Role of Polymer Chemistry in Influencing Crystal Growth Rates from Amorphous Felodipine. CrystEngComm 2010, 12, 2390–2397. [Google Scholar] [CrossRef]
- Vogt, M.; Kunath, K.; Dressman, J.B. Dissolution Enhancement of Fenofibrate by Micronization, Cogrinding and Spray-Drying: Comparison with Commercial Preparations. Eur. J. Pharm. Biopharm. 2008, 68, 283–288. [Google Scholar] [CrossRef]
- Law, D.; Wang, W.; Schmitt, E.A.; Qiu, Y.; Krill, S.L.; Fort, J.J. Properties of Rapidly Dissolving Eutectic Mixtures of Poly(Ethylene Glycol) and Fenofibrate: The Eutectic Microstructure. J. Pharm. Sci. 2003, 92, 505–515. [Google Scholar] [CrossRef] [PubMed]
- Shaw, L.R.; Irwin, W.J.; Grattan, T.J.; Conway, B.R. The Effect of Selected Water-Soluble Excipients on the Dissolution of Paracetamol and Ibuprofen. Drug Dev. Ind. Pharm. 2005, 31, 515–525. [Google Scholar] [CrossRef]
- Viseras, C.; Salem, I.I.; Galan, I.C.R.; Galan, A.C.; Galindo, A.L. The Effect of Recrystallization on the Crystal Growth, Melting Point and Solubility of Ketoconazole. Thermochim. Acta 1995, 268, 143–151. [Google Scholar] [CrossRef]
- Pathak, S.M.; Ruff, A.; Kostewicz, E.S.; Patel, N.; Turner, D.B.; Jamei, M. Model-Based Analysis of Biopharmaceutic Experiments to Improve Mechanistic Oral Absorption Modeling: An Integrated in Vitro in Vivo Extrapolation Perspective Using Ketoconazole as a Model Drug. Mol. Pharm. 2017, 14, 4305–4320. [Google Scholar] [CrossRef]
- Wan, H.; Holmén, A.G.; Wang, Y.; Lindberg, W.; Englund, M.; Någård, M.B.; Thompson, R.A. High-Throughput Screening of PKa Values of Pharmaceuticals by Pressure-Assisted Capillary Electrophoresis and Mass Spectrometry. Rapid Commun. Mass Spectrom. 2003, 17, 2639–2648. [Google Scholar] [CrossRef] [PubMed]
- Umerska, A.; Zotova, J.; Tajber, L. Formation of Low Melting Point Binary Systems Comprising Ketoprofen and an Amide Local Anaesthetic. Int. J. Pharm. 2021, 607, 120969. [Google Scholar] [CrossRef] [PubMed]
- Sheng, J.J.; Kasim, N.A.; Chandrasekharan, R.; Amidon, G.L. Solubilization and Dissolution of Insoluble Weak Acid, Ketoprofen: Effects of PH Combined with Surfactant. Eur. J. Pharm. Sci. 2006, 29, 306–314. [Google Scholar] [CrossRef] [PubMed]
- Geiser, L.; Henchoz, Y.; Galland, A.; Carrupt, P.A.; Veuthey, J.L. Determination of PKa Values by Capillary Zone Electrophoresis with a Dynamic Coating Procedure. J. Sep. Sci. 2005, 28, 2374–2380. [Google Scholar] [CrossRef] [PubMed]
- Inoue, K.; Ogawa, K.; Okada, J.; Sugibayashi, K. Enhancement of Skin Permeation of Ketotifen by Supersaturation Generated by Amorphous Form of the Drug. J. Control. Release 2005, 108, 306–318. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.-L.; Chiang, C.-H.; Chen, J.-L. In Vitro and in Vivo Percutaneous Absorption Studies of Ketotifen Patches. Drug Dev. Ind. Pharm. 1994, 20, 2965–2976. [Google Scholar] [CrossRef]
- Pedersen, B.T.; Larsen, S.W.; Østergaard, J.; Larsen, C. In Vitro Assessment of Lidocaine Release from Aqueous and Oil Solutions and from Preformed and in Situ Formed Aqueous and Oil Suspensions. Parenteral Depots for Intra-Articular Administration. Drug Deliv. 2008, 15, 23–30. [Google Scholar] [CrossRef]
- Popović, G.; Čakar, M.; Agbaba, D. Acid-Base Equilibria and Solubility of Loratadine and Desloratadine in Water and Micellar Media. J. Pharm. Biomed. Anal. 2009, 49, 42–47. [Google Scholar] [CrossRef] [PubMed]
- Hafez, H.M.; Abdullah, A.E.; Abdelaziz, L.M.; Kamal, M.M. Quantitative Determination of Amlodipine Besylate, Losartan Potassium, Valsartan and Atorvastatin Calcium by HPLC in Their Pharmaceutical Formulations. J. Chromatogr. Sep. Tech. 2014, 5, 1000226. [Google Scholar] [CrossRef]
- Madasu, S.B.; Vekariya, N.A.; Koteswaramma, C.; Islam, A.; Sanasi, P.D.; Korupolu, R.B. An Efficient, Commercially Viable, and Safe Process for Preparation of Losartan Potassium, an Angiotensin II Receptor Antagonist. Org. Process Res. Dev. 2012, 16, 2025–2030. [Google Scholar] [CrossRef]
- Winiwarter, S.; Ax, F.; Lennernäs, H.; Hallberg, A.; Pettersson, C.; Karlén, A. Hydrogen Bonding Descriptors in the Prediction of Human in Vivo Intestinal Permeability. J. Mol. Graph. Model. 2003, 21, 273–287. [Google Scholar] [CrossRef]
- De Souza, J.B.; de Souza, J.; de Castro, L.M.L.; Siqueira, M.F.; Savedra, R.M.L.; Silva-Barcellos, N.M. Evaluation of the Losartan Solubility in the Biowaiver Context by Shake-Flask Method and Intrinsic Dissolution. Pharm. Dev. Technol. 2019, 24, 283–292. [Google Scholar] [CrossRef] [PubMed]
- Narumi, K.; Kobayashi, M.; Kondo, A.; Furugen, A.; Yamada, T.; Takahashi, N.; Iseki, K. Characterization of Loxoprofen Transport in Caco-2 Cells: The Involvement of a Proton-Dependent Transport System in the Intestinal Transport of Loxoprofen. Biopharm. Drug Dispos. 2016, 37, 447–455. [Google Scholar] [CrossRef] [PubMed]
- Daiichi Sankyo Company, Limited. Loxoprofen Drug Product Information. Available online: https://www.medicalcommunity.jp/member/certification?destination=/products/druginfo/loxonin_tablets_60mg& (accessed on 22 November 2022).
- Pobudkowska, A.; DomańSka, U. Study of PH-Dependent Drugs Solubility in Water. Chem. Ind. Chem. Eng. Q. 2014, 20, 115–126. [Google Scholar] [CrossRef]
- Ribeiro, A.; Figueiras, A.; Santos, D.; Veiga, F. Preparation and Solid-State Characterization of Inclusion Complexes Formed between Miconazole and Methyl-β-Cyclodextrin. AAPS PharmSciTech 2008, 9, 1102–1109. [Google Scholar] [CrossRef]
- Martins, K.F.; Messias, A.D.; Leite, F.L.; Duek, E.A.R. Preparation and Characterization of Paclitaxel-Loaded PLDLA Microspheres. Mater. Res. 2014, 17, 650–656. [Google Scholar] [CrossRef]
- Xavier Junior, F.H.; Gueutin, C.; do Vale Morais, A.R.; do Nascimento Alencar, E.; do Egito, E.S.T.; Vauthier, C. HPLC Method for the Dosage of Paclitaxel in Copaiba Oil: Development, Validation, Application to the Determination of the Solubility and Partition Coefficients. Chromatographia 2016, 79, 405–412. [Google Scholar] [CrossRef]
- Serajuddin, A.T.M.; Mufson, D. PH-Solubility Profiles of Organic Bases and Their Hydrochloride Salts. Pharm. Res. Off. J. Am. Assoc. Pharm. Sci. 1985, 2, 65–68. [Google Scholar]
- Courtney, R.; Wexler, D.; Radwanski, E.; Lim, J.; Laughlin, M. Effect of Food on the Relative Bioavailability of Two Oral Formulations of Posaconazole in Healthy Adults. Br. J. Clin. Pharmacol. 2004, 57, 218–222. [Google Scholar] [CrossRef]
- Van Duong, T.; Ni, Z.; Taylor, L.S. Phase Behavior and Crystallization Kinetics of a Poorly Water-Soluble Weakly Basic Drug as a Function of Supersaturation and Media Composition. Mol. Pharm. 2022, 19, 1146–1159. [Google Scholar] [CrossRef]
- Merck & Co., Inc. Posaconzaole Drug Product Information. Available online: https://www.msdconnect.jp/products/noxafil/download/ (accessed on 22 November 2022).
- Völgyi, G.; Baka, E.; Box, K.J.; Comer, J.E.A.; Takács-Novák, K. Study of PH-Dependent Solubility of Organic Bases. Revisit of Henderson-Hasselbalch Relationship. Anal. Chim. Acta 2010, 673, 40–46. [Google Scholar] [CrossRef]
- Moriguchi, I.; Hirono, S.; Nakagome, I.; Hirano, H. Comparision of Reliability of LogP Values for Drugs Calculated by Several Metods. Chem. Pharm. Bull. 1994, 42, 976–978. [Google Scholar] [CrossRef]
- Maitani, Y.; Coutel-Egros, A.; Obata, Y.; Nagai, T. Prediction of Skin Permeabilities of Diclofenac and Propranolol from Theoretical Partition Coefficients Determined from Cohesion Parameters. J. Pharm. Sci. 1993, 82, 416–420. [Google Scholar] [CrossRef] [PubMed]
- Jindal, A.; Singh, R.; Tomar, S.; Dureja, J.; Karan, M.; Chadha, R. Engineering a Remedy to Modulate and Optimize Biopharmaceutical Properties of Rebamipide by Synthesizing New Cocrystal: In Silico and Experimental Studies. Pharm. Res. 2021, 38, 2129–2145. [Google Scholar] [CrossRef]
- Pradhan, R.; Tran, T.H.; Choi, J.Y.; Choi, I.S.; Choi, H.G.; Yong, C.S.; Kim, J.O. Development of a Rebamipide Solid Dispersion System with Improved Dissolution and Oral Bioavailability. Arch. Pharm. Res. 2015, 38, 522–533. [Google Scholar] [CrossRef]
- Otsuka Pharmaceutical, Rebamipide Drug Product Information. Available online: https://www.otsuka-elibrary.jp/check/ (accessed on 22 November 2022).
- Tsinman, O.; Tsinman, K.; Sun, N.; Avdeef, A. Physicochemical Selectivity of the BBB Microenvironment Governing Passive Diffusion—Matching with a Porcine Brain Lipid Extract Artificial Membrane Permeability Model. Pharm. Res. 2011, 28, 337–363. [Google Scholar] [CrossRef] [PubMed]
- Grizić, D.; Lamprecht, A. Predictability of Drug Encapsulation and Release from Propylene Carbonate/PLGA Microparticles. Int. J. Pharm. 2020, 586, 119601. [Google Scholar] [CrossRef] [PubMed]
- Law, D.; Krill, S.L.; Schmitt, E.A.; Fort, J.J.; Qiu, Y.; Wang, W.; Porter, W.R. Physicochemical Considerations in the Preparation of Amorphous Ritonavir-Poly(Ethylene Glycol) 8000 Solid Dispersions. J. Pharm. Sci. 2001, 90, 1015–1025. [Google Scholar] [CrossRef] [PubMed]
- Kwong, A.D.; Kauffman, R.S.; Hurter, P.; Mueller, P. Discovery and Development of Telaprevir: An NS3-4A Protease Inhibitor for Treating Genotype 1 Chronic Hepatitis C Virus. Nat. Biotechnol. 2011, 29, 993–1003. [Google Scholar] [CrossRef] [PubMed]
- Al Hossain, A.S.M.M.; Sil, B.C.; Iliopoulos, F.; Lever, R.; Hadgraft, J.; Lane, M.E. Preparation, Characterisation, and Topical Delivery of Terbinafine. Pharmaceutics 2019, 11, 548. [Google Scholar] [CrossRef] [PubMed]
- Hopkała, H. Preparation and Potentiometric Determination of Quaternary N-Methiodides of Phenothiazine Derivatives. Anal. Lett. 1990, 23, 159–167. [Google Scholar] [CrossRef]
- Franke, U.; Munk, A.; Wiese, M. Ionization Constants and Distribution Coefficients of Phenothiazines and Calcium Channel Antagonists Determined by a PH-Metric Method and Correlation with Calculated Partition Coefficients. J. Pharm. Sci. 1999, 88, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Domańska, U.; Pelczarska, A.; Pobudkowska, A. Solubility and PK a Determination of Six Structurally Related Phenothiazines. Int. J. Pharm. 2011, 421, 135–144. [Google Scholar] [CrossRef]
- Moriguchi, I.; Hirono, S.; Liu, Q.; Nakagome, I.; Matsushita, Y. Simple Method of Calculating Octanol/Water Partition Coefficient. Chem. Pharm. Bull. 1992, 40, 127–130. [Google Scholar] [CrossRef]
- Mannhold, R.; Rekker, R.F. The Hydrophobic Fragmental Constant Approach for Calculating Log P in Octanol/Water and Aliphatic Hydrocarbon/Water Systems. Perspect. Drug Discov. Des. 2000, 18, 1–18. [Google Scholar] [CrossRef]
- Dannenfelser, R.M.; Yalkowsky, S.H. Estimation of Entropy of Melting from Molecular Structure: A Non-Group Contribution Method. Ind. Eng. Chem. Res. 1996, 35, 1483–1486. [Google Scholar] [CrossRef]
- Bogner, R.H.; Murdande, S.B.; Pikal, M.J.; Shanker, R.M. Solubility Advantage of Amorphous Pharmaceuticals: II. Application of Quantitative Thermodynamic Relationships for Prediction of Solubility Enhancement in Structurally Diverse Insoluble Pharmaceuticals. Pharm. Res. 2010, 27, 2704–2714. [Google Scholar] [CrossRef]
- Murdande, S.B.; Pikal, M.J.; Shanker, R.M.; Bogner, R.H. Solubility Advantage of Amorphous Pharmaceuticals: I. A Thermodynamic Analysis. J. Pharm. Sci. 2009, 99, 1254–1264. [Google Scholar] [CrossRef] [PubMed]
- Ozaki, S.; Kushida, I.; Yamashita, T.; Hasebe, T.; Shirai, O.; Kano, K. Evaluation of Drug Supersaturation by Thermodynamic and Kinetic Approaches for the Prediction of Oral Absorbability in Amorphous Pharmaceuticals. J. Pharm. Sci. 2012, 101, 4220–4230. [Google Scholar] [CrossRef]
- Brouwers, J.; Marcus, E.; Brewster, P.A. Supersaturating Drug Delivery Systems: The Answer to Solubility-Limited Oral Bioavailability? J. Pharm. Sci. 2009, 98, 2549–2568. [Google Scholar] [CrossRef]
- Ueda, K.; Higashi, K.; Moribe, K. Unusual Correlation between the Apparent Amorphous Solubility of a Drug and Solubilizer Concentration Revealed by NMR Analysis. Mol. Pharm. 2022, 19, 3336–3349. [Google Scholar] [CrossRef] [PubMed]
- Ueda, K.; Moseson, D.E.; Pathak, V.; Taylor, L.S. Effect of Polymer Species on Maximum Aqueous Phase Supersaturation Revealed by Quantitative Nuclear Magnetic Resonance Spectroscopy. Mol. Pharm. 2021, 18, 1344–1355. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).