Neuropilin-1 and Integrins as Receptors for Chromogranin A-Derived Peptides
Abstract
1. Introduction
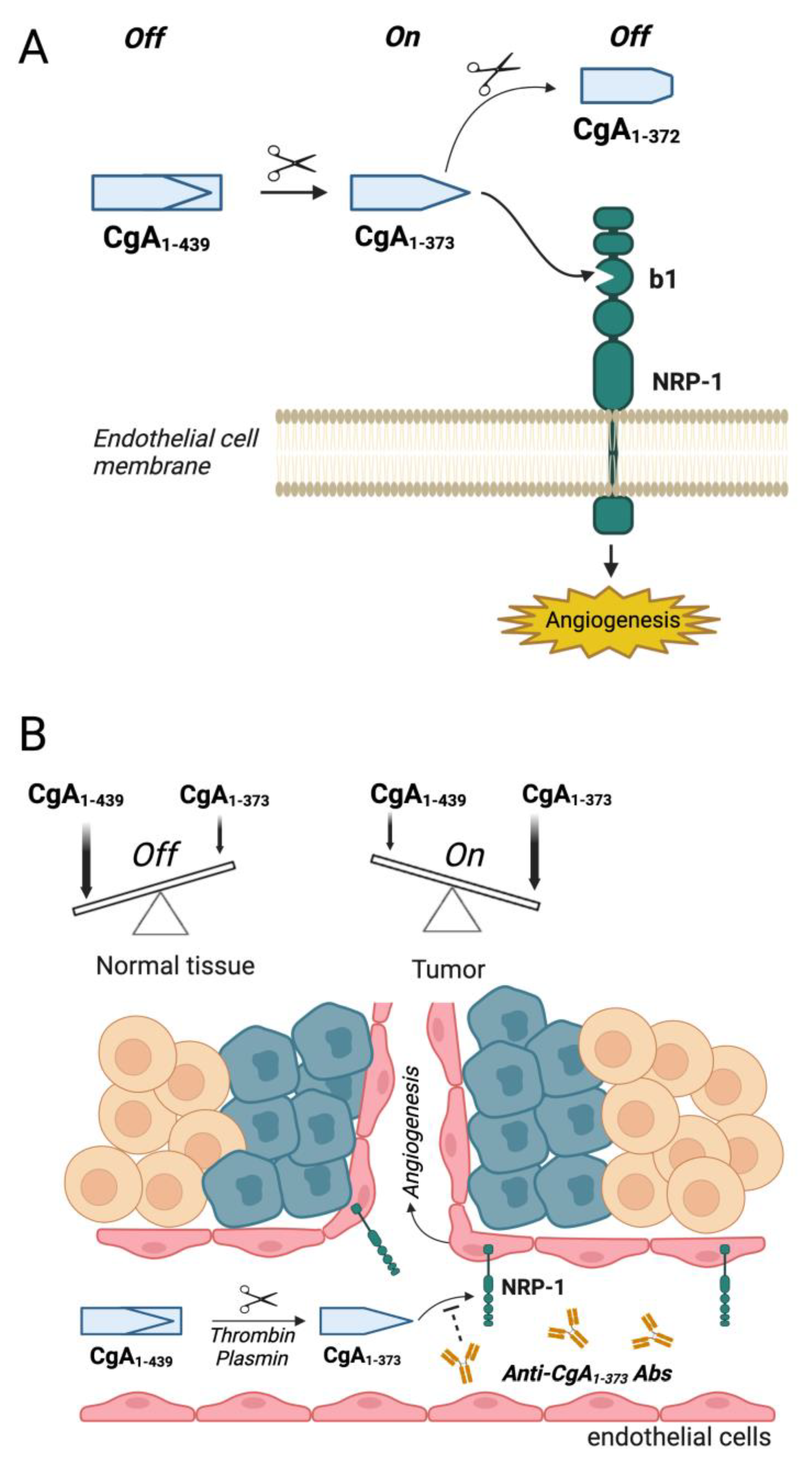
2. Neuropilin-1 as a Receptor for Chromogranin A-Derived Peptides
2.1. Biological Effects of CgA and Its Fragments in Angiogenesis and Tumor Growth
2.2. Mechanisms Underlying the Biological Effects of CgA Fragments in Angiogenesis and Tumor Growth
2.3. Potential Therapeutic Applications of Compounds That Interfere with CgA Fragment/Neuropilin-1 Interaction
2.4. Role of CgA Fragment/Neuropilin-1 Interactions in Cardiovascular Regulation
3. Integrins as Receptors for CgA and CgA-Derived Peptides
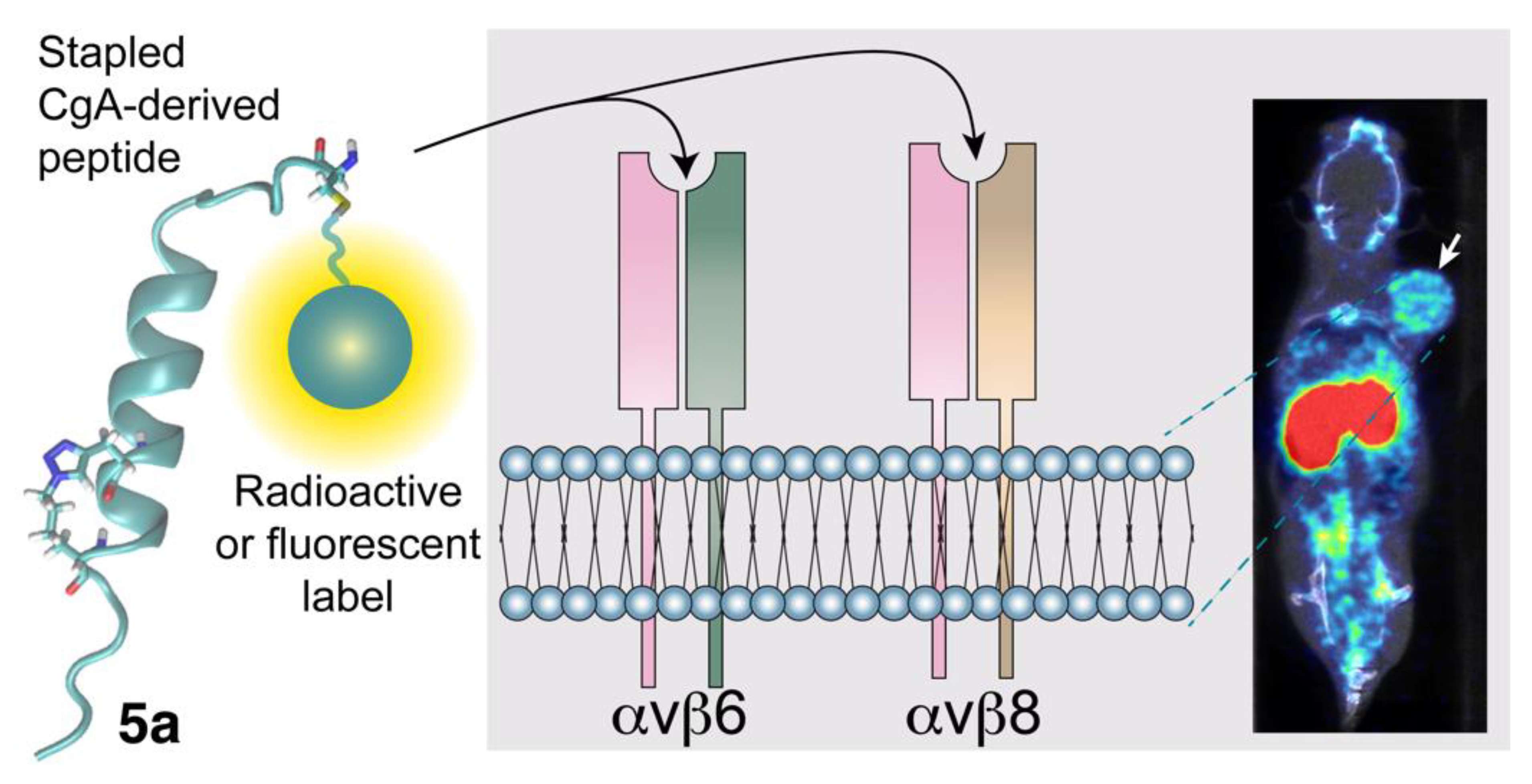
3.1. The Interaction of CgA and CgA Fragments with Integrins
3.2. Role of CgA/Integrin Interactions in Wound Healing
3.3. Potential Diagnostic and Therapeutic Applications of CgA-Derived Peptides That Interact with Integrins in Cancer
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Taupenot, L.; Harper, K.L.; O’Connor, D.T. The chromogranin-secretogranin family. N. Engl. J. Med. 2003, 348, 1134–1149. [Google Scholar] [CrossRef]
- Helle, K.B.; Corti, A.; Metz-Boutigue, M.H.; Tota, B. The endocrine role for chromogranin A: A prohormone for peptides with regulatory properties. Cell Mol. Life Sci. 2007, 64, 2863–2886. [Google Scholar] [CrossRef]
- Corti, A.; Marcucci, F.; Bachetti, T. Circulating chromogranin A and its fragments as diagnostic and prognostic disease markers. Pflugers Arch. 2018, 470, 199–210. [Google Scholar] [CrossRef]
- Gadroy, P.; Stridsberg, M.; Capon, C.; Michalski, J.C.; Strub, J.M.; Van Dorsselaer, A.; Aunis, D.; Metz-Boutigue, M.H. Phosphorylation and O-glycosylation sites of human chromogranin A (CGA79-439) from urine of patients with carcinoid tumors. J. Biol. Chem. 1998, 273, 34087–34097. [Google Scholar] [CrossRef]
- Metz-Boutigue, M.H.; Lugardon, K.; Goumon, Y.; Raffner, R.; Strub, J.M.; Aunis, D. Antibacterial and antifungal peptides derived from chromogranins and proenkephalin-A. From structural to biological aspects. Adv. Exp. Med. Biol. 2000, 482, 299–315. [Google Scholar]
- Shooshtarizadeh, P.; Zhang, D.; Chich, J.F.; Gasnier, C.; Schneider, F.; Haikel, Y.; Aunis, D.; Metz-Boutigue, M.H. The antimicrobial peptides derived from chromogranin/secretogranin family, new actors of innate immunity. Regul. Pept. 2009, 165, 102–110. [Google Scholar] [CrossRef]
- Briolat, J.; Wu, S.D.; Mahata, S.K.; Gonthier, B.; Bagnard, D.; Chasserot-Golaz, S.; Helle, K.B.; Aunis, D.; Metz-Boutigue, M.H. New antimicrobial activity for the catecholamine release-inhibitory peptide from chromogranin A. Cell. Mol. Life Sci. CMLS 2005, 62, 377–385. [Google Scholar] [CrossRef]
- Ioannidis, M.; Mahata, S.K.; van den Bogaart, G. The immunomodulatory functions of chromogranin A-derived peptide pancreastatin. Peptides 2022, 158, 170893. [Google Scholar] [CrossRef]
- Aardal, S.; Helle, K.B. The vasoinhibitory activity of bovine chromogranin A fragment (vasostatin) and its independence of extracellular calcium in isolated segments of human blood vessels. Regul. Pept. 1992, 41, 9–18. [Google Scholar] [CrossRef]
- Helle, K.B. The chromogranin A-derived peptides vasostatin-I and catestatin as regulatory peptides for cardiovascular functions. Cardiovasc. Res. 2010, 85, 9–16. [Google Scholar] [CrossRef]
- Tota, B.; Mazza, R.; Angelone, T.; Nullans, G.; Metz-Boutigue, M.H.; Aunis, D.; Helle, K.B. Peptides from the N-terminal domain of chromogranin A (vasostatins) exert negative inotropic effects in the isolated frog heart. Regul. Pept. 2003, 114, 123–130. [Google Scholar] [CrossRef]
- Tota, B.; Angelone, T.; Cerra, M.C. The surging role of Chromogranin A in cardiovascular homeostasis. Front. Chem. 2014, 2, 64. [Google Scholar] [CrossRef]
- Tatemoto, K.; Efendic, S.; Mutt, V.; Makk, G.; Feistner, G.J.; Barchas, J.D. Pancreastatin, a novel pancreatic peptide that inhibits insulin secretion. Nature 1986, 324, 476–478. [Google Scholar] [CrossRef]
- Mahata, S.K.; Corti, A. Chromogranin A and its fragments in cardiovascular, immunometabolic, and cancer regulation. Ann. N. Y. Acad. Sci. 2019, 1455, 34–58. [Google Scholar] [CrossRef]
- Bandyopadhyay, G.K.; Mahata, S.K. Chromogranin A Regulation of Obesity and Peripheral Insulin Sensitivity. Front. Endocrinol. 2017, 8, 20. [Google Scholar] [CrossRef]
- Theurl, M.; Schgoer, W.; Albrecht, K.; Jeschke, J.; Egger, M.; Beer, A.G.; Vasiljevic, D.; Rong, S.; Wolf, A.M.; Bahlmann, F.H.; et al. The neuropeptide catestatin acts as a novel angiogenic cytokine via a basic fibroblast growth factor-dependent mechanism. Circ. Res. 2010, 107, 1326–1335. [Google Scholar] [CrossRef]
- Crippa, L.; Bianco, M.; Colombo, B.; Gasparri, A.M.; Ferrero, E.; Loh, Y.P.; Curnis, F.; Corti, A. A new chromogranin A-dependent angiogenic switch activated by thrombin. Blood 2013, 121, 392–402. [Google Scholar] [CrossRef]
- Helle, K.B.; Corti, A. Chromogranin A: A paradoxical player in angiogenesis and vascular biology. Cell. Mol. Life Sci. 2015, 78, 339–348. [Google Scholar] [CrossRef]
- Curnis, F.; Gasparri, A.; Longhi, R.; Colombo, B.; D’Alessio, S.; Pastorino, F.; Ponzoni, M.; Corti, A. Chromogranin A Binds to αvβ6-Integrin and Promotes Wound Healing in Mice. Cell. Mol. Life Sci. 2012, 69, 2791–2803. [Google Scholar] [CrossRef]
- Colombo, B.; Curnis, F.; Foglieni, C.; Monno, A.; Arrigoni, G.; Corti, A. Chromogranin a expression in neoplastic cells affects tumor growth and morphogenesis in mouse models. Cancer Res. 2002, 62, 941–946. [Google Scholar]
- Curnis, F.; Dallatomasina, A.; Bianco, M.; Gasparri, A.; Sacchi, A.; Colombo, B.; Fiocchi, M.; Perani, L.; Venturini, M.; Tacchetti, C.; et al. Regulation of tumor growth by circulating full-length chromogranin A. Oncotarget 2016, 7, 72716–72732. [Google Scholar] [CrossRef]
- Salem, S.; Jankowski, V.; Asare, Y.; Liehn, E.; Welker, P.; Raya-Bermudez, A.; Pineda-Martos, C.; Rodriguez, M.; Munoz-Castaneda, J.R.; Bruck, H.; et al. Identification of the Vasoconstriction-Inhibiting Factor (VIF), a Potent Endogenous Cofactor of Angiotensin II Acting on the Angiotensin II Type 2 Receptor. Circulation 2015, 131, 1426–1434. [Google Scholar] [CrossRef]
- Brekke, J.F.; Kirkeleit, J.; Lugardon, K.; Helle, K.B. Vasostatins. Dilators of bovine resistance arteries. Adv. Exp. Med. Biol. 2000, 482, 239–246. [Google Scholar]
- Mahata, S.K.; O’Connor, D.T.; Mahata, M.; Yoo, S.H.; Taupenot, L.; Wu, H.; Gill, B.M.; Parmer, R.J. Novel autocrine feedback control of catecholamine release. A discrete chromogranin a fragment is a noncompetitive nicotinic cholinergic antagonist. J. Clin. Investig. 1997, 100, 1623–1633. [Google Scholar] [CrossRef]
- Koshimizu, H.; Cawley, N.X.; Kim, T.; Yergey, A.L.; Loh, Y.P. Serpinin: A novel chromogranin A-derived, secreted peptide up-regulates protease nexin-1 expression and granule biogenesis in endocrine cells. Mol. Endocrinol. 2011, 25, 732–744. [Google Scholar] [CrossRef]
- Loh, Y.P.; Koshimizu, H.; Cawley, N.X.; Tota, B. Serpinins: Role in granule biogenesis, inhibition of cell death and cardiac function. Curr. Med. Chem. 2012, 19, 4086–4092. [Google Scholar] [CrossRef][Green Version]
- Mancino, D.; Kharouf, N.; Scavello, F.; Helle, S.; Salloum-Yared, F.; Mutschler, A.; Mathieu, E.; Lavalle, P.; Metz-Boutigue, M.H.; Haikel, Y. The Catestatin-Derived Peptides Are New Actors to Fight the Development of Oral Candidosis. Int. J. Mol. Sci. 2022, 23, 2066. [Google Scholar] [CrossRef]
- Metz-Boutigue, M.H.; Goumon, Y.; Lugardon, K.; Strub, J.M.; Aunis, D. Antibacterial peptides are present in chromaffin cell secretory granules. Cell. Mol. Neurobiol. 1998, 18, 249–266. [Google Scholar] [CrossRef]
- Fung, M.M.; Salem, R.M.; Mehtani, P.; Thomas, B.; Lu, C.F.; Perez, B.; Rao, F.; Stridsberg, M.; Ziegler, M.G.; Mahata, S.K.; et al. Direct vasoactive effects of the chromogranin A (CHGA) peptide catestatin in humans in vivo. Clin. Exp. Hypertens. 2010, 32, 278–287. [Google Scholar] [CrossRef]
- Kruger, P.G.; Mahata, S.K.; Helle, K.B. Catestatin (CgA344-364) stimulates rat mast cell release of histamine in a manner comparable to mastoparan and other cationic charged neuropeptides. Regul. Pept. 2003, 114, 29–35. [Google Scholar] [CrossRef]
- Pasqua, T.; Corti, A.; Gentile, S.; Pochini, L.; Bianco, M.; Metz-Boutigue, M.H.; Cerra, M.C.; Tota, B.; Angelone, T. Full-length human Chromogranin-A cardioactivity: Myocardial, coronary and stimulus-induced processing evidence in normotensive and hypertensive male rat hearts. Endocrinology 2013, 154, 3353–3365. [Google Scholar] [CrossRef]
- Angelone, T.; Quintieri, A.M.; Brar, B.K.; Limchaiyawat, P.T.; Tota, B.; Mahata, S.K.; Cerra, M.C. The antihypertensive chromogranin a peptide catestatin acts as a novel endocrine/paracrine modulator of cardiac inotropism and lusitropism. Endocrinology 2008, 149, 4780–4793. [Google Scholar] [CrossRef]
- Corti, A.; Mannarino, C.; Mazza, R.; Colombo, B.; Longhi, R.; Tota, B. Vasostatins exert negative inotropism in the working heart of the frog. Ann. N. Y. Acad. Sci. 2002, 971, 362–365. [Google Scholar] [CrossRef]
- Gasparri, A.; Sidoli, A.; Sanchez, L.P.; Longhi, R.; Siccardi, A.G.; Marchisio, P.C.; Corti, A. Chromogranin A fragments modulate cell adhesion. Identification and characterization of a pro-adhesive domain. J. Biol. Chem. 1997, 272, 20835–20843. [Google Scholar] [CrossRef]
- Ferrero, E.; Scabini, S.; Magni, E.; Foglieni, C.; Belloni, D.; Colombo, B.; Curnis, F.; Villa, A.; Ferrero, M.E.; Corti, A. Chromogranin A protects vessels against tumor necrosis factor alpha-induced vascular leakage. FASEB J. 2004, 18, 554–556. [Google Scholar] [CrossRef]
- Di Comite, G.; Rossi, C.M.; Marinosci, A.; Lolmede, K.; Baldissera, E.; Aiello, P.; Mueller, R.B.; Herrmann, M.; Voll, R.E.; Rovere-Querini, P.; et al. Circulating chromogranin A reveals extra-articular involvement in patients with rheumatoid arthritis and curbs TNF-alpha-elicited endothelial activation. J. Leukoc. Biol. 2009, 85, 81–87. [Google Scholar] [CrossRef]
- Dallatomasina, A.; Gasparri, A.M.; Colombo, B.; Sacchi, A.; Bianco, M.; Daniele, T.; Esposito, A.; Pastorino, F.; Ponzoni, M.; Marcucci, F.; et al. Spatiotemporal Regulation of Tumor Angiogenesis by Circulating Chromogranin A Cleavage and Neuropilin-1 Engagement. Cancer Res. 2019, 79, 1925–1937. [Google Scholar] [CrossRef]
- Reni, M.; Andreasi, V.; Gasparri, A.M.; Dugnani, E.; Colombo, B.; Macchini, M.; Bianco, M.; Dallatomasina, A.; Citro, A.; Assi, E.; et al. Circulating Chromogranin A Is Cleaved Into Vasoregulatory Fragments in Patients with Pancreatic Ductal Adenocarcinoma. Front. Oncol. 2020, 10, 613582. [Google Scholar] [CrossRef]
- Blois, A.; Holmsen, H.; Martino, G.; Corti, A.; Metz-Boutigue, M.H.; Helle, K.B. Interactions of chromogranin A-derived vasostatins and monolayers of phosphatidylserine, phosphatidylcholine and phosphatidylethanolamine. Regul. Pept. 2006, 134, 30–37. [Google Scholar] [CrossRef]
- Ramella, R.; Boero, O.; Alloatti, G.; Angelone, T.; Levi, R.; Gallo, M.P. Vasostatin 1 activates eNOS in endothelial cells through a proteoglycan-dependent mechanism. J. Cell. Biochem. 2010, 110, 70–79. [Google Scholar] [CrossRef]
- Kiranmayi, M.; Chirasani, V.R.; Allu, P.K.; Subramanian, L.; Martelli, E.E.; Sahu, B.S.; Vishnuprabu, D.; Kumaragurubaran, R.; Sharma, S.; Bodhini, D.; et al. Catestatin Gly364Ser Variant Alters Systemic Blood Pressure and the Risk for Hypertension in Human Populations via Endothelial Nitric Oxide Pathway. Hypertension 2016, 68, 334–347. [Google Scholar] [CrossRef]
- Belloni, D.; Scabini, S.; Foglieni, C.; Veschini, L.; Giazzon, A.; Colombo, B.; Fulgenzi, A.; Helle, K.B.; Ferrero, M.E.; Corti, A.; et al. The vasostatin-I fragment of chromogranin A inhibits VEGF-induced endothelial cell proliferation and migration. FASEB J. 2007, 21, 3052–3062. [Google Scholar] [CrossRef]
- Bianco, M.; Gasparri, A.M.; Colombo, B.; Curnis, F.; Girlanda, S.; Ponzoni, M.; Bertilaccio, M.T.; Calcinotto, A.; Sacchi, A.; Ferrero, E.; et al. Chromogranin A is preferentially cleaved into pro-angiogenic peptides in the bone marrow of multiple myeloma patients. Cancer Res. 2016, 76, 1781–1791. [Google Scholar] [CrossRef]
- Teesalu, T.; Sugahara, K.N.; Kotamraju, V.R.; Ruoslahti, E. C-end rule peptides mediate neuropilin-1-dependent cell, vascular, and tissue penetration. Proc. Natl. Acad. Sci. USA 2009, 106, 16157–16162. [Google Scholar] [CrossRef]
- Teesalu, T.; Sugahara, K.N.; Ruoslahti, E. Tumor-penetrating peptides. Front. Oncol. 2013, 3, 216. [Google Scholar] [CrossRef]
- Rocca, C.; Grande, F.; Granieri, M.C.; Colombo, B.; De Bartolo, A.; Giordano, F.; Rago, V.; Amodio, N.; Tota, B.; Cerra, M.C.; et al. The chromogranin A1-373 fragment reveals how a single change in the protein sequence exerts strong cardioregulatory effects by engaging neuropilin-1. Acta Physiol. 2021, 231, e13570. [Google Scholar] [CrossRef]
- Pena, V.B.; Bonini, I.C.; Antollini, S.S.; Kobayashi, T.; Barrantes, F.J. Alpha 7-type acetylcholine receptor localization and its modulation by nicotine and cholesterol in vascular endothelial cells. J. Cell. Biochem. 2011, 112, 3276–3288. [Google Scholar] [CrossRef]
- Cooke, J.P.; Ghebremariam, Y.T. Endothelial nicotinic acetylcholine receptors and angiogenesis. Trends Cardiovasc. Med. 2008, 18, 247–253. [Google Scholar] [CrossRef]
- Arias, H.R.; Richards, V.E.; Ng, D.; Ghafoori, M.E.; Le, V.; Mousa, S.A. Role of non-neuronal nicotinic acetylcholine receptors in angiogenesis. Int. J. Biochem. Cell. Biol. 2009, 41, 1441–1451. [Google Scholar] [CrossRef]
- Heeschen, C.; Jang, J.J.; Weis, M.; Pathak, A.; Kaji, S.; Hu, R.S.; Tsao, P.S.; Johnson, F.L.; Cooke, J.P. Nicotine stimulates angiogenesis and promotes tumor growth and atherosclerosis. Nat. Med. 2001, 7, 833–839. [Google Scholar] [CrossRef]
- Taupenot, L.; Mahata, S.K.; Mahata, M.; Parmer, R.J.; O’Connor, D.T. Interaction of the catecholamine release-inhibitory peptide catestatin (human chromogranin A(352–372)) with the chromaffin cell surface and Torpedo electroplax: Implications for nicotinic cholinergic antagonism. Regul. Pept. 2000, 95, 9–17. [Google Scholar] [CrossRef]
- Mahata, S.K.; Mahata, M.; Fung, M.M.; O’Connor, D.T. Catestatin: A multifunctional peptide from chromogranin A. Regul. Pept. 2010, 162, 33–43. [Google Scholar] [CrossRef]
- Sahu, B.S.; Mohan, J.; Sahu, G.; Singh, P.K.; Sonawane, P.J.; Sasi, B.K.; Allu, P.K.; Maji, S.K.; Bera, A.K.; Senapati, S.; et al. Molecular interactions of the physiological anti-hypertensive peptide catestatin with the neuronal nicotinic acetylcholine receptor. J. Cell. Sci. 2012, 125, 2323–2337. [Google Scholar] [CrossRef]
- Folkman, J. Angiogenesis: An organizing principle for drug discovery? Nat. Rev. Drug Discov. 2007, 6, 273–286. [Google Scholar] [CrossRef]
- Italiano, J.E., Jr.; Richardson, J.L.; Patel-Hett, S.; Battinelli, E.; Zaslavsky, A.; Short, S.; Ryeom, S.; Folkman, J.; Klement, G.L. Angiogenesis is regulated by a novel mechanism: Pro- and antiangiogenic proteins are organized into separate platelet alpha granules and differentially released. Blood 2008, 111, 1227–1233. [Google Scholar] [CrossRef]
- Ribatti, D. Endogenous inhibitors of angiogenesis: A historical review. Leuk. Res. 2009, 33, 638–644. [Google Scholar] [CrossRef]
- Kleeff, J.; Korc, M.; Apte, M.; La Vecchia, C.; Johnson, C.D.; Biankin, A.V.; Neale, R.E.; Tempero, M.; Tuveson, D.A.; Hruban, R.H.; et al. Pancreatic cancer. Nat. Rev. Dis. Prim. 2016, 2, 16022. [Google Scholar] [CrossRef]
- Rawla, P.; Sunkara, T.; Gaduputi, V. Epidemiology of Pancreatic Cancer: Global Trends, Etiology and Risk Factors. World J. Oncol. 2019, 10, 10–27. [Google Scholar] [CrossRef]
- Conroy, T.; Desseigne, F.; Ychou, M.; Bouche, O.; Guimbaud, R.; Becouarn, Y.; Adenis, A.; Raoul, J.L.; Gourgou-Bourgade, S.; de la Fouchardiere, C.; et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N. Engl. J. Med. 2011, 364, 1817–1825. [Google Scholar] [CrossRef]
- Kawasaki, T.; Kitsukawa, T.; Bekku, Y.; Matsuda, Y.; Sanbo, M.; Yagi, T.; Fujisawa, H. A requirement for neuropilin-1 in embryonic vessel formation. Development 1999, 126, 4895–4902. [Google Scholar] [CrossRef]
- Wang, Y.; Cao, Y.; Yamada, S.; Thirunavukkarasu, M.; Nin, V.; Joshi, M.; Rishi, M.T.; Bhattacharya, S.; Camacho-Pereira, J.; Sharma, A.K.; et al. Cardiomyopathy and Worsened Ischemic Heart Failure in SM22-alpha Cre-Mediated Neuropilin-1 Null Mice: Dysregulation of PGC1alpha and Mitochondrial Homeostasis. Arterioscler Thromb. Vasc. Biol. 2015, 35, 1401–1412. [Google Scholar] [CrossRef]
- Thomas, G.J.; Nystrom, M.L.; Marshall, J.F. Alphavbeta6 integrin in wound healing and cancer of the oral cavity. J. Oral Pathol. Med. 2006, 35, 1–10. [Google Scholar] [CrossRef]
- Busk, M.; Pytela, R.; Sheppard, D. Characterization of the integrin alpha v beta 6 as a fibronectin-binding protein. J. Biol. Chem. 1992, 267, 5790–5796. [Google Scholar] [CrossRef]
- Bandyopadhyay, A.; Raghavan, S. Defining the role of integrin alphavbeta6 in cancer. Curr. Drug Targets 2009, 10, 645–652. [Google Scholar] [CrossRef]
- Ozawa, A.; Sato, Y.; Imabayashi, T.; Uemura, T.; Takagi, J.; Sekiguchi, K. Molecular Basis of the Ligand Binding Specificity of alphavbeta8 Integrin. J. Biol. Chem. 2016, 291, 11551–11565. [Google Scholar] [CrossRef]
- Kraft, S.; Diefenbach, B.; Mehta, R.; Jonczyk, A.; Luckenbach, G.A.; Goodman, S.L. Definition of an unexpected ligand recognition motif for alphav beta6 integrin. J. Biol. Chem. 1999, 274, 1979–1985. [Google Scholar] [CrossRef]
- Dong, X.; Hudson, N.E.; Lu, C.; Springer, T.A. Structural determinants of integrin beta-subunit specificity for latent TGF-beta. Nat. Struct. Mol. Biol. 2014, 21, 1091–1096. [Google Scholar] [CrossRef]
- Dong, X.; Zhao, B.; Iacob, R.E.; Zhu, J.; Koksal, A.C.; Lu, C.; Engen, J.R.; Springer, T.A. Force interacts with macromolecular structure in activation of TGF-beta. Nature 2017, 542, 55–59. [Google Scholar] [CrossRef]
- Kotecha, A.; Wang, Q.; Dong, X.; Ilca, S.L.; Ondiviela, M.; Zihe, R.; Seago, J.; Charleston, B.; Fry, E.E.; Abrescia, N.G.A.; et al. Rules of engagement between alphavbeta6 integrin and foot-and-mouth disease virus. Nat. Commun. 2017, 8, 15408. [Google Scholar] [CrossRef]
- DiCara, D.; Rapisarda, C.; Sutcliffe, J.L.; Violette, S.M.; Weinreb, P.H.; Hart, I.R.; Howard, M.J.; Marshall, J.F. Structure-function analysis of Arg-Gly-Asp helix motifs in alpha v beta 6 integrin ligands. J. Biol. Chem. 2007, 282, 9657–9665. [Google Scholar] [CrossRef]
- Nardelli, F.; Ghitti, M.; Quilici, G.; Gori, A.; Luo, Q.; Berardi, A.; Sacchi, A.; Monieri, M.; Bergamaschi, G.; Bermel, W.; et al. A stapled chromogranin A-derived peptide is a potent dual ligand for integrins alphavbeta6 and alphavbeta8. Chem. Commun. 2019, 55, 14777–14780. [Google Scholar] [CrossRef]
- Lugardon, K.; Chasserot-Golaz, S.; Kieffer, A.E.; Maget-Dana, R.; Nullans, G.; Kieffer, B.; Aunis, D.; Metz-Boutigue, M.H. Structural and biological characterization of chromofungin, the antifungal chromogranin A-(47-66)-derived peptide. J. Biol. Chem. 2001, 276, 35875–35882. [Google Scholar] [CrossRef]
- Koivisto, L.; Bi, J.; Hakkinen, L.; Larjava, H. Integrin alphavbeta6: Structure, function and role in health and disease. Int. J. Biochem. Cell. Biol. 2018, 99, 186–196. [Google Scholar] [CrossRef]
- Liu, H.; Wu, Y.; Wang, F.; Liu, Z. Molecular imaging of integrin alphavbeta6 expression in living subjects. Am. J. Nucl. Med. Mol. Imaging 2014, 4, 333–345. [Google Scholar]
- Koivisto, L.; Larjava, K.; Hakkinen, L.; Uitto, V.J.; Heino, J.; Larjava, H. Different integrins mediate cell spreading, haptotaxis and lateral migration of HaCaT keratinocytes on fibronectin. Cell Adhes. Commun. 1999, 7, 245–257. [Google Scholar] [CrossRef]
- Radek, K.A.; Lopez-Garcia, B.; Hupe, M.; Niesman, I.R.; Elias, P.M.; Taupenot, L.; Mahata, S.K.; O’Connor, D.T.; Gallo, R.L. The neuroendocrine peptide catestatin is a cutaneous antimicrobial and induced in the skin after injury. J. Investig. Dermatol. 2008, 128, 1525–1534. [Google Scholar] [CrossRef]
- Avraamides, C.J.; Garmy-Susini, B.; Varner, J.A. Integrins in angiogenesis and lymphangiogenesis. Nat. Rev. Cancer 2008, 8, 604–617. [Google Scholar] [CrossRef]
- Desgrosellier, J.S.; Cheresh, D.A. Integrins in cancer: Biological implications and therapeutic opportunities. Nat. Rev. Cancer 2010, 10, 9–22. [Google Scholar] [CrossRef]
- Elayadi, A.N.; Samli, K.N.; Prudkin, L.; Liu, Y.H.; Bian, A.; Xie, X.J.; Wistuba, I.I.; Roth, J.A.; McGuire, M.J.; Brown, K.C. A peptide selected by biopanning identifies the integrin alphavbeta6 as a prognostic biomarker for nonsmall cell lung cancer. Cancer Res. 2007, 67, 5889–5895. [Google Scholar] [CrossRef]
- Moore, K.M.; Thomas, G.J.; Duffy, S.W.; Warwick, J.; Gabe, R.; Chou, P.; Ellis, I.O.; Green, A.R.; Haider, S.; Brouilette, K.; et al. Therapeutic targeting of integrin alphavbeta6 in breast cancer. J. Natl. Cancer Inst. 2014, 106. [Google Scholar] [CrossRef]
- Sipos, B.; Hahn, D.; Carceller, A.; Piulats, J.; Hedderich, J.; Kalthoff, H.; Goodman, S.L.; Kosmahl, M.; Kloppel, G. Immunohistochemical screening for beta6-integrin subunit expression in adenocarcinomas using a novel monoclonal antibody reveals strong up-regulation in pancreatic ductal adenocarcinomas in vivo and in vitro. Histopathology 2004, 45, 226–236. [Google Scholar] [CrossRef]
- Reader, C.S.; Vallath, S.; Steele, C.W.; Haider, S.; Brentnall, A.; Desai, A.; Moore, K.M.; Jamieson, N.B.; Chang, D.; Bailey, P.; et al. The integrin alphavbeta6 drives pancreatic cancer through diverse mechanisms and represents an effective target for therapy. J. Pathol. 2019, 249, 332–342. [Google Scholar] [CrossRef]
- Niu, J.; Li, Z. The roles of integrin alphavbeta6 in cancer. Cancer Lett. 2017, 403, 128–137. [Google Scholar] [CrossRef]
- Hazelbag, S.; Kenter, G.G.; Gorter, A.; Dreef, E.J.; Koopman, L.A.; Violette, S.M.; Weinreb, P.H.; Fleuren, G.J. Overexpression of the alpha v beta 6 integrin in cervical squamous cell carcinoma is a prognostic factor for decreased survival. J. Pathol. 2007, 212, 316–324. [Google Scholar] [CrossRef]
- Zhang, Z.Y.; Xu, K.S.; Wang, J.S.; Yang, G.Y.; Wang, W.; Wang, J.Y.; Niu, W.B.; Liu, E.Y.; Mi, Y.T.; Niu, J. Integrin alphanvbeta6 acts as a prognostic indicator in gastric carcinoma. Clin. Oncol. 2008, 20, 61–66. [Google Scholar] [CrossRef]
- Bates, R.C.; Bellovin, D.I.; Brown, C.; Maynard, E.; Wu, B.; Kawakatsu, H.; Sheppard, D.; Oettgen, P.; Mercurio, A.M. Transcriptional activation of integrin beta6 during the epithelial-mesenchymal transition defines a novel prognostic indicator of aggressive colon carcinoma. J. Clin. Investig. 2005, 115, 339–347. [Google Scholar] [CrossRef]
- Altmann, A.; Sauter, M.; Roesch, S.; Mier, W.; Warta, R.; Debus, J.; Dyckhoff, G.; Herold-Mende, C.; Haberkorn, U. Identification of a Novel ITGalphavbeta6-Binding Peptide Using Protein Separation and Phage Display. Clin. Cancer Res. 2017, 23, 4170–4180. [Google Scholar] [CrossRef]
- Roesch, S.; Lindner, T.; Sauter, M.; Loktev, A.; Flechsig, P.; Muller, M.; Mier, W.; Warta, R.; Dyckhoff, G.; Herold-Mende, C.; et al. Comparison of the RGD Motif-Containing alphavbeta6 Integrin-Binding Peptides SFLAP3 and SFITGv6 for Diagnostic Application in HNSCC. J. Nucl. Med. 2018, 59, 1679–1685. [Google Scholar] [CrossRef]
- Quigley, N.G.; Czech, N.; Sendt, W.; Notni, J. PET/CT imaging of pancreatic carcinoma targeting the “cancer integrin” alphavbeta6. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 4107–4108. [Google Scholar] [CrossRef]
- Kimura, R.H.; Wang, L.; Shen, B.; Huo, L.; Tummers, W.; Filipp, F.V.; Guo, H.H.; Haywood, T.; Abou-Elkacem, L.; Baratto, L.; et al. Evaluation of integrin alphavbeta6 cystine knot PET tracers to detect cancer and idiopathic pulmonary fibrosis. Nat. Commun. 2019, 10, 4673. [Google Scholar] [CrossRef]
- Hausner, S.H.; Bold, R.J.; Cheuy, L.Y.; Chew, H.K.; Daly, M.E.; Davis, R.A.; Foster, C.C.; Kim, E.J.; Sutcliffe, J.L. Preclinical Development and First-in-Human Imaging of the Integrin αvβ6 with [18F]αvβ6-Binding Peptide in Metastatic Carcinoma. Clin. Cancer Res. 2019, 25, 1206–1215. [Google Scholar] [CrossRef]
- McCarty, J.H. alphavbeta8 integrin adhesion and signaling pathways in development, physiology and disease. J. Cell Sci. 2020, 133. [Google Scholar] [CrossRef]
- Takasaka, N.; Seed, R.I.; Cormier, A.; Bondesson, A.J.; Lou, J.; Elattma, A.; Ito, S.; Yanagisawa, H.; Hashimoto, M.; Ma, R.; et al. Integrin alphavbeta8-expressing tumor cells evade host immunity by regulating TGF-beta activation in immune cells. JCI Insight 2018, 3. [Google Scholar] [CrossRef]
- Jin, S.; Lee, W.C.; Aust, D.; Pilarsky, C.; Cordes, N. beta8 Integrin Mediates Pancreatic Cancer Cell Radiochemoresistance. Mol. Cancer Res. 2019, 17, 2126–2138. [Google Scholar] [CrossRef]
- Ahmedah, H.T.; Patterson, L.H.; Shnyder, S.D.; Sheldrake, H.M. RGD-Binding Integrins in Head and Neck Cancers. Cancers 2017, 9, 56. [Google Scholar] [CrossRef]
- Monieri, M.; Rainone, P.; Sacchi, A.; Gori, A.; Gasparri, A.M.; Coliva, A.; Citro, A.; Ferrara, B.; Policardi, M.; Valtorta, S.; et al. A stapled chromogranin A-derived peptide homes in on tumors that express αvβ6 or αvβ8 integrins. Int. J. Biol. Sci. 2022, 19, 156–166. [Google Scholar]
- Conroy, K.P.; Kitto, L.J.; Henderson, N.C. Alphav integrins: Key regulators of tissue fibrosis. Cell Tissue Res. 2016, 365, 511–519. [Google Scholar] [CrossRef]
| Competitor | Competitive Binding Assay to Integrins (Ki, nM) a | Ref. | ||||
|---|---|---|---|---|---|---|
| αvβ6 | αvβ8 | αvβ3 | αvβ5 | α5β1 | ||
| CgA1-439 | 105 ± 34 | >2000 | >2000 | >2000 | >2000 | [19] |
| Vasostatin-1 | 74 ± 30 | >10,000 | >10,000 | >10,000 | >10,000 | [19] |
| CgA39-63 | 15.5 ± 3.2 | 7663 ± 1704 | 2192 ± 690 | 3600 ± 525 | 9206 ± 1810 | [71] |
| CgA39-63 (RGE) | >50,000 | >50,000 | >50,000 | >50,000 | >50,000 | [71] |
| CgA39-63 (RGDL) | 1.6 ± 0.3 | 8.5 ± 3.7 | 1928 ± 226 | 2405 ± 592 | 924 ± 198 | [71] |
| CgA39-63 (RGDL)-Stapled | 0.6 ± 0.1 | 3.2 ± 1.2 | 2453 ± 426 | 2741 ± 615 | 1310 ± 389 | [71] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Corti, A.; Anderluzzi, G.; Curnis, F. Neuropilin-1 and Integrins as Receptors for Chromogranin A-Derived Peptides. Pharmaceutics 2022, 14, 2555. https://doi.org/10.3390/pharmaceutics14122555
Corti A, Anderluzzi G, Curnis F. Neuropilin-1 and Integrins as Receptors for Chromogranin A-Derived Peptides. Pharmaceutics. 2022; 14(12):2555. https://doi.org/10.3390/pharmaceutics14122555
Chicago/Turabian StyleCorti, Angelo, Giulia Anderluzzi, and Flavio Curnis. 2022. "Neuropilin-1 and Integrins as Receptors for Chromogranin A-Derived Peptides" Pharmaceutics 14, no. 12: 2555. https://doi.org/10.3390/pharmaceutics14122555
APA StyleCorti, A., Anderluzzi, G., & Curnis, F. (2022). Neuropilin-1 and Integrins as Receptors for Chromogranin A-Derived Peptides. Pharmaceutics, 14(12), 2555. https://doi.org/10.3390/pharmaceutics14122555






