Stability Enhancement of Freeze-Dried Gelatin/Alginate Coacervates for bFGF Delivery
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Fabrication of Gelatin/Alginate Coacervates with or without Cryoprotectant
2.3. Freeze-Drying Process of Complex Coacervates
2.4. Characterization of Coacervates
2.4.1. Coacervates before Freeze-Drying and after Rehydration of Freeze-Dried Coacervates
2.4.2. Characteristics of Freeze-Dried Coacervates
Scanning Electron Microscope (SEM) Analysis
ATR-FTIR
Thermal Analysis
bFGF Stability Measurement
Release Test
2.4.3. In Vitro Cell Activity Study
HDFs Viability Test
HDFs Procollagen Synthesis
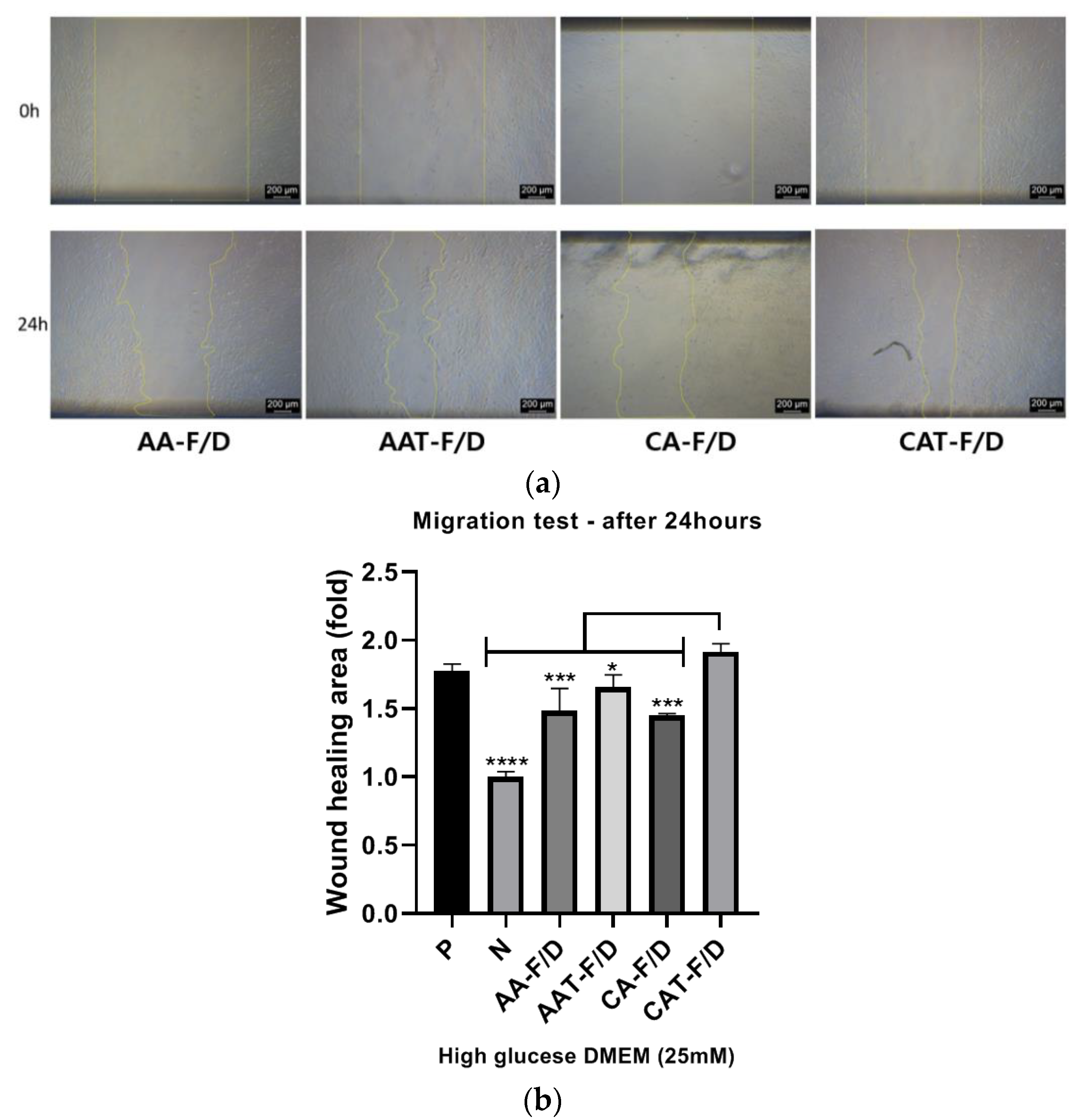
HDFs Scratch Wound Assay
2.5. Statistical Analysis
3. Results
3.1. Characterization of Gelatin/Alginate Coacervates
3.2. Effects of Cryoprotectants on Characteristics of Rehydrated Coacervates
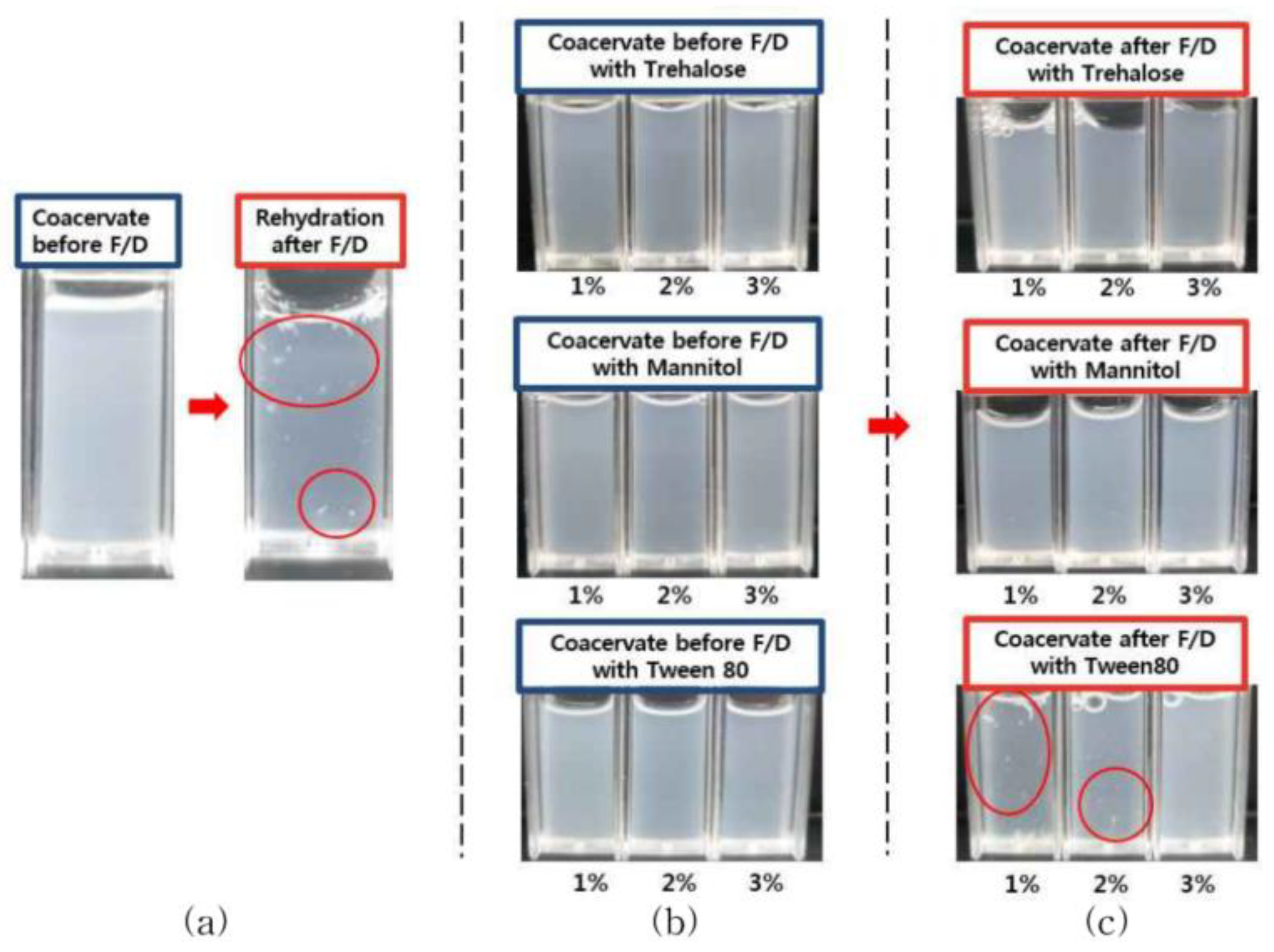
3.2.1. Effect on Aggregation and Turbidity Changes upon Rehydraion after Freeze-Drying
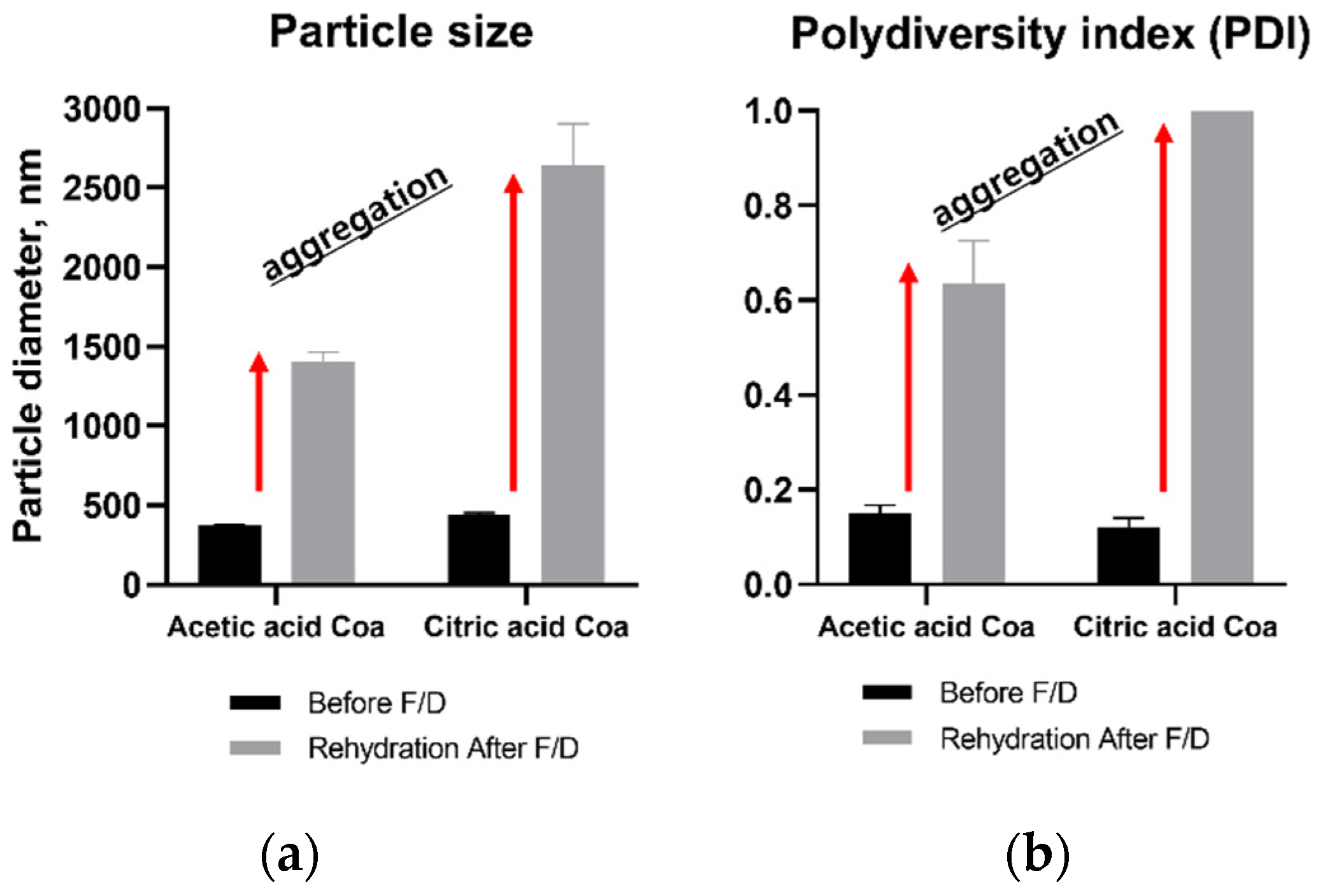
3.2.2. Effects on Particle Size and PDI Value
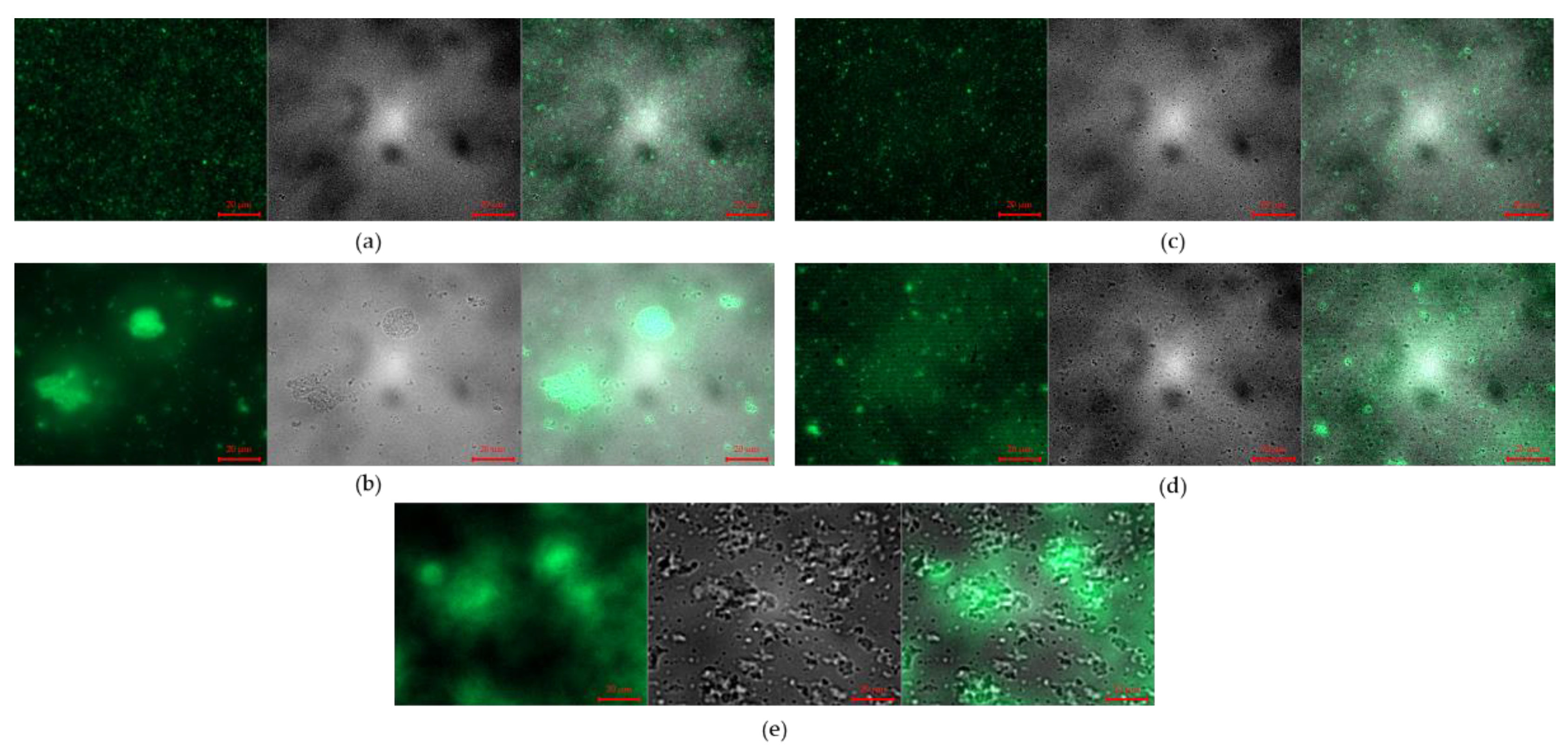
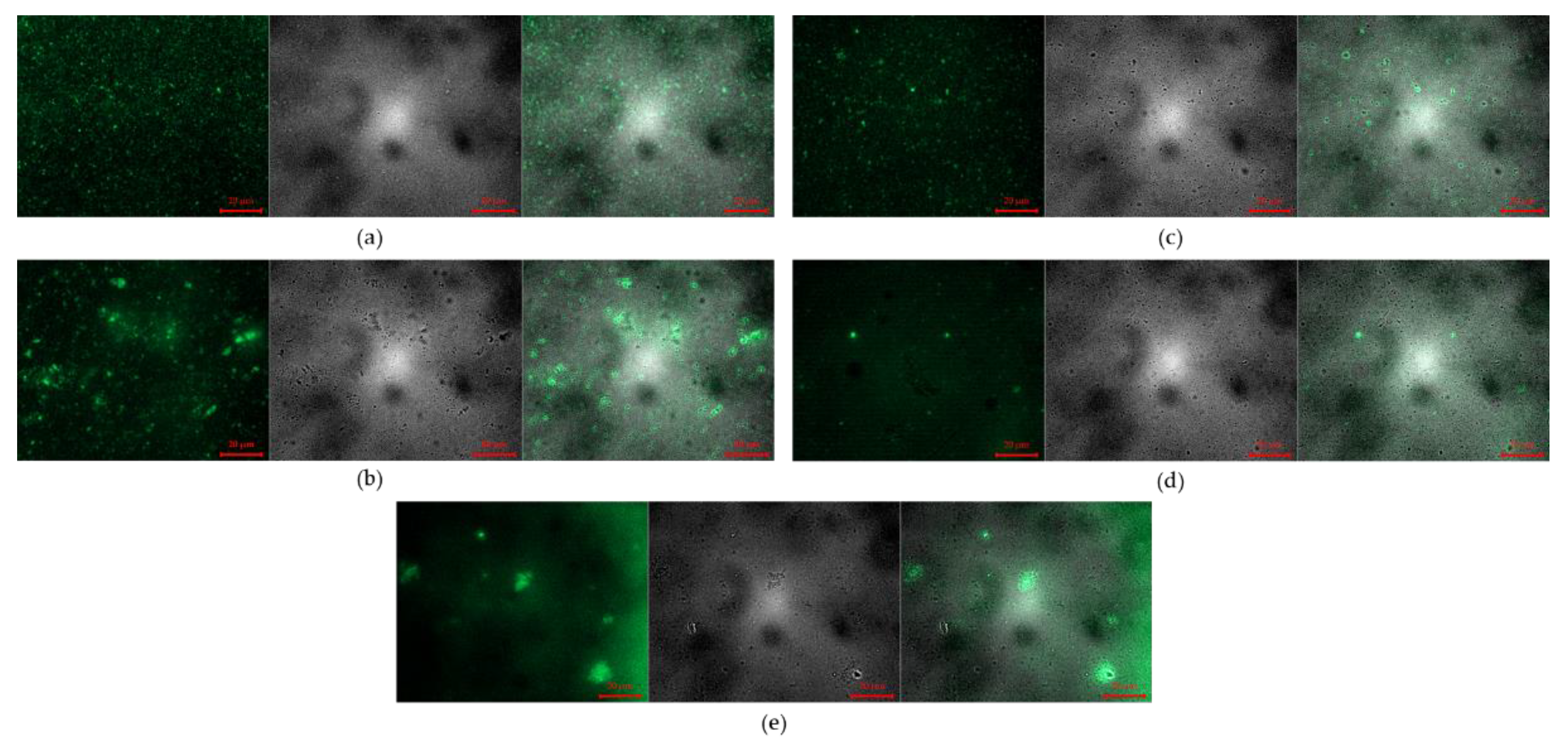
3.2.3. Fluorescence Microscope Images
3.3. Effects of Cryoprotectants on Characteristics of Freeze-Dried Coacervates
3.3.1. Morphology and Texture of Freeze-Dried Coacervates
3.3.2. SEM Images of Freeze-Dried Coacervates
3.3.3. ATR-FTIR Spectra of GA/SA Coacervate with Trehalose
3.3.4. bFGF Stability after Freeze-Drying Process
3.3.5. Controlled Release Rate of Freeze-Dried Coacervates with Trehalose
3.4. In Vitro Cell Activity
3.4.1. In Vitro HDFs Viability Assay
3.4.2. In Vitro HDF Procollagen Synthesis
3.4.3. In Vitro HDF Scratch Wound Assay
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| Abbreviation | Sample composition and history |
| AA | GA/SA coacervates acidified with acetic acid before freeze-drying |
| AA-F/D | freeze-dried GA/SA coacervates acidified with acetic acid |
| AAT-F/D | freeze-dried GA/SA coacervates acidified with acetic acid containing trehalose |
| AAM-F/D | freeze-dried GA/SA coacervates acidified with acetic acid containing mannitol |
| AAW-F/D | freeze-dried GA/SA coacervates acidified with acetic acid containing Tween80 |
| CA | GA/SA coacervates acidified with citric acid before freeze-drying |
| CA-F/D | freeze-dried GA/SA coacervates acidified with citric acid |
| CAT-F/D | freeze-dried GA/SA coacervates acidified with citric acid containing trehalose |
| CAM-F/D | freeze-dried GA/SA coacervates acidified with citric acid containing mannitol |
| CAW-F/D | freeze-dried GA/SA coacervates acidified with citric acid containing Tween80 |
| FS | bFGF solution |
References
- Burgess, J.L.; Wyant, W.A.; Abdo Abujamra, B.; Kirsner, R.S.; Jozic, I. Diabetic Wound-Healing Science. Medicina 2021, 57, 1072. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Jiang, L.; Zhao, X.; Chen, B.; Shi, W.; Cao, Y.; Chen, Y.; Li, X.; He, Y.; Li, C.; et al. Adipose-Derived Stromal Cell-Sheets Sandwiched, Book-Shaped Acellular Dermal Matrix Capable of Sustained Release of Basic Fibroblast Growth Factor Promote Diabetic Wound Healing. Front. Cell Dev. Biol. 2021, 9, 646967. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.X.; Lin, C.; Lin, B.B.; Wang, Z.G.; Zhang, H.Y.; Wu, F.Z.; Cheng, Y.; Xiang, L.J.; Guo, D.J.; Luo, X.; et al. The anti-scar effects of basic fibroblast growth factor on the wound repair in vitro and in vivo. PLoS ONE 2013, 8, e59966. [Google Scholar] [CrossRef] [PubMed]
- Zubair, M.; Ahmad, J. Role of growth factors and cytokines in diabetic foot ulcer healing: A detailed review. Rev. Endocr. Metab. Disord. 2019, 20, 207–217. [Google Scholar] [CrossRef]
- Liu, Y.; Deng, J.; Li, W.; Nie, X. Fibroblast Growth Factor in Diabetic Foot Ulcer: Progress and Therapeutic Prospects. Front. Endocrinol. 2021, 12, 744868. [Google Scholar] [CrossRef]
- Laiva, A.L.; O’Brien, F.J.; Keogh, M.B. Innovations in gene and growth factor delivery systems for diabetic wound healing. J. Tissue Eng. Regen. Med. 2018, 12, e296–e312. [Google Scholar] [CrossRef]
- Benington, L.; Rajan, G.; Locher, C.; Lim, L.Y. Fibroblast Growth Factor 2-A Review of Stabilisation Approaches for Clinical Applications. Pharmaceutics 2020, 12, 508. [Google Scholar] [CrossRef]
- Mousavi, S.M.; Nejad, Z.M.; Hashemi, S.A.; Salari, M.; Gholami, A.; Ramakrishna, S.; Chiang, W.H.; Lai, C.W. Bioactive Agent-Loaded Electrospun Nanofiber Membranes for Accelerating Healing Process: A Review. Membranes 2021, 11, 702. [Google Scholar] [CrossRef]
- Jeong, S.; Kim, B.; Park, M.; Ban, E.; Lee, S.H.; Kim, A. Improved Diabetic Wound Healing by EGF Encapsulation in Gelatin-Alginate Coacervates. Pharmaceutics 2020, 12, 334. [Google Scholar] [CrossRef]
- Kim, B.; Ban, E.; Kim, A. Gelatin-Alginate Coacervates Optimized by DOE to Improve Delivery of bFGF for Wound Healing. Pharmaceutics 2021, 13, 2112. [Google Scholar] [CrossRef]
- Maaz Arif, M.; Khan, S.M.; Gull, N.; Tabish, T.A.; Zia, S.; Ullah Khan, R.; Awais, S.M.; Arif Butt, M. Polymer-based biomaterials for chronic wound management: Promises and challenges. Int. J. Pharm. 2021, 598, 120270. [Google Scholar] [CrossRef] [PubMed]
- Jeong, S.; Kim, B.; Lau, H.C.; Kim, A. Gelatin-Alginate Complexes for EGF Encapsulation: Effects of H-Bonding and Electrostatic Interactions. Pharmaceutics 2019, 11, 530. [Google Scholar] [CrossRef]
- Abdelwahed, W.; Degobert, G.; Stainmesse, S.; Fessi, H. Freeze-drying of nanoparticles: Formulation, process and storage considerations. Adv. Drug Deliv. Rev. 2006, 58, 1688–1713. [Google Scholar] [CrossRef] [PubMed]
- Neupane, Y.R.; Huang, C.; Wang, X.; Chng, W.H.; Venkatesan, G.; Zharkova, O.; Wacker, M.G.; Czarny, B.; Storm, G.; Wang, J.W.; et al. Lyophilization Preserves the Intrinsic Cardioprotective Activity of Bioinspired Cell-Derived Nanovesicles. Pharmaceutics 2021, 13, 1052. [Google Scholar] [CrossRef] [PubMed]
- Chung, N.O.; Lee, M.K.; Lee, J. Mechanism of freeze-drying drug nanosuspensions. Int. J. Pharm. 2012, 437, 42–50. [Google Scholar] [CrossRef]
- Chang, T.; Zhao, G. Ice Inhibition for Cryopreservation: Materials, Strategies, and Challenges. Adv Sci 2021, 8, 2002425. [Google Scholar] [CrossRef]
- Fonte, P.; Reis, S.; Sarmento, B. Facts and evidences on the lyophilization of polymeric nanoparticles for drug delivery. J. Control. Release 2016, 225, 75–86. [Google Scholar] [CrossRef]
- Trenkenschuh, E.; Friess, W. Freeze-drying of nanoparticles: How to overcome colloidal instability by formulation and process optimization. Eur. J. Pharm. Biopharm. 2021, 165, 345–360. [Google Scholar] [CrossRef]
- Muhoza, B.; Xia, S.; Wang, X.; Zhang, X. The protection effect of trehalose on the multinuclear microcapsules based on gelatin and high methyl pectin coacervate during freeze-drying. Food Hydrocoll. 2020, 105, 105807. [Google Scholar] [CrossRef]
- Mohammady, M.; Mohammadi, Y.; Yousefi, G. Freeze-Drying of Pharmaceutical and Nutraceutical Nanoparticles: The Effects of Formulation and Technique Parameters on Nanoparticles Characteristics. J. Pharm. Sci. 2020, 109, 3235–3247. [Google Scholar] [CrossRef]
- Akao, K.-I.; Okubo, Y.; Asakawa, N.; Inoue, Y.; Sakurai, M. Infrared spectroscopic study on the properties of the anhydrous form II of trehalose. Implications for the functional mechanism of trehalose as a biostabilizer. Carbohydr. Res. 2001, 334, 233–241. [Google Scholar] [CrossRef]
- Raimi-Abraham, B.T.; Moffat, J.G.; Belton, P.S.; Barker, S.A.; Craig, D.Q.M. Generation and Characterization of Standardized Forms of Trehalose Dihydrate and Their Associated Solid-State Behavior. Cryst. Growth Des. 2014, 14, 4955–4967. [Google Scholar] [CrossRef]
- Baheti, A.; Kumar, L.; Bansal, A.K. Excipients used in lyophilization of small molecules. J. Excip. Food Chem. 2010, 1, 41–54. [Google Scholar]
- Wang, Z.; Wang, Z.; Lu, W.W.; Zhen, W.; Yang, D.; Peng, S. Novel biomaterial strategies for controlled growth factor delivery for biomedical applications. NPG Asia Mater. 2017, 9, e435. [Google Scholar] [CrossRef]
- Barrientos, S.; Brem, H.; Stojadinovic, O.; Tomic-Canic, M. Clinical application of growth factors and cytokines in wound healing. Wound Repair Regen. 2014, 22, 569–578. [Google Scholar] [CrossRef] [PubMed]

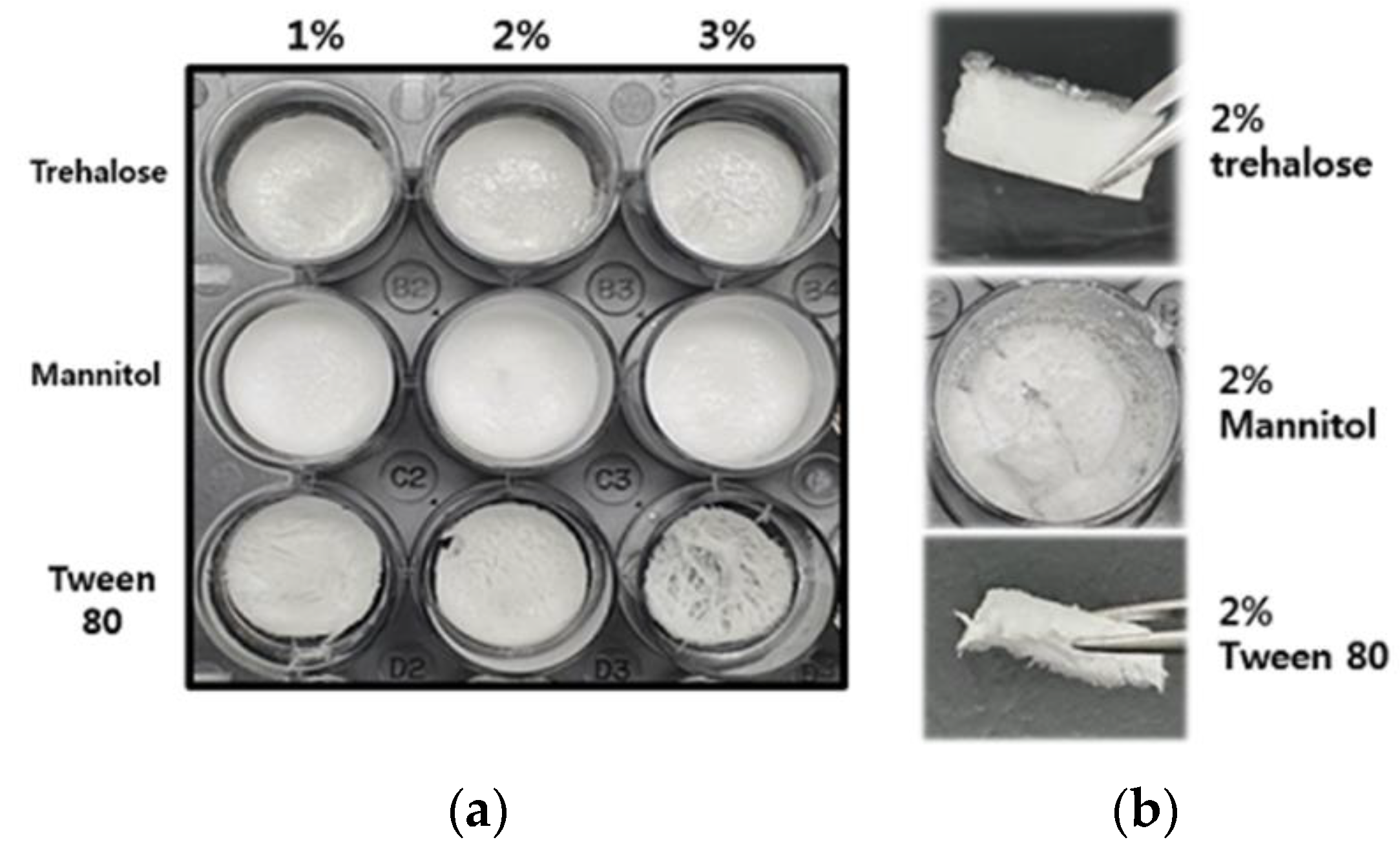
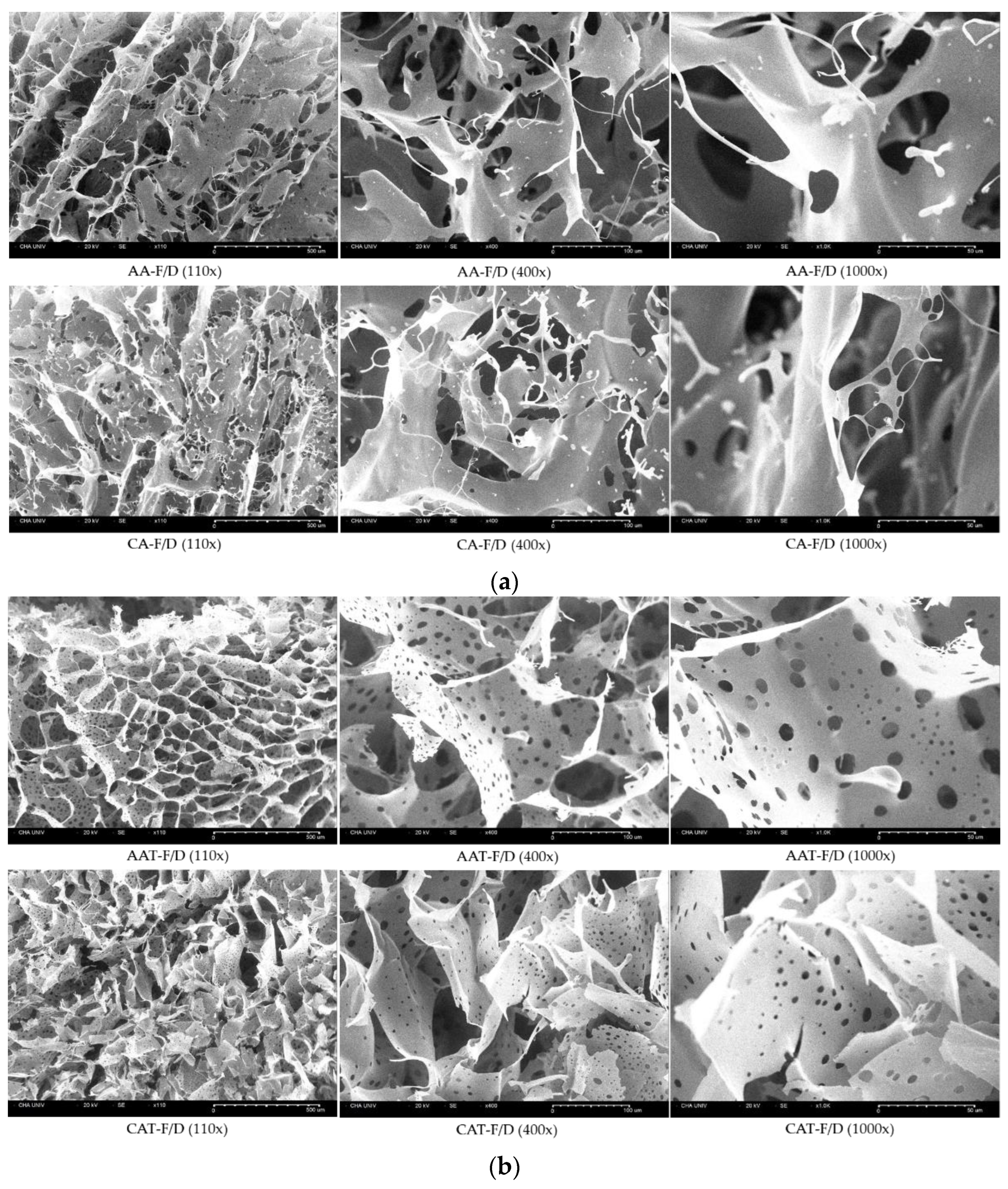

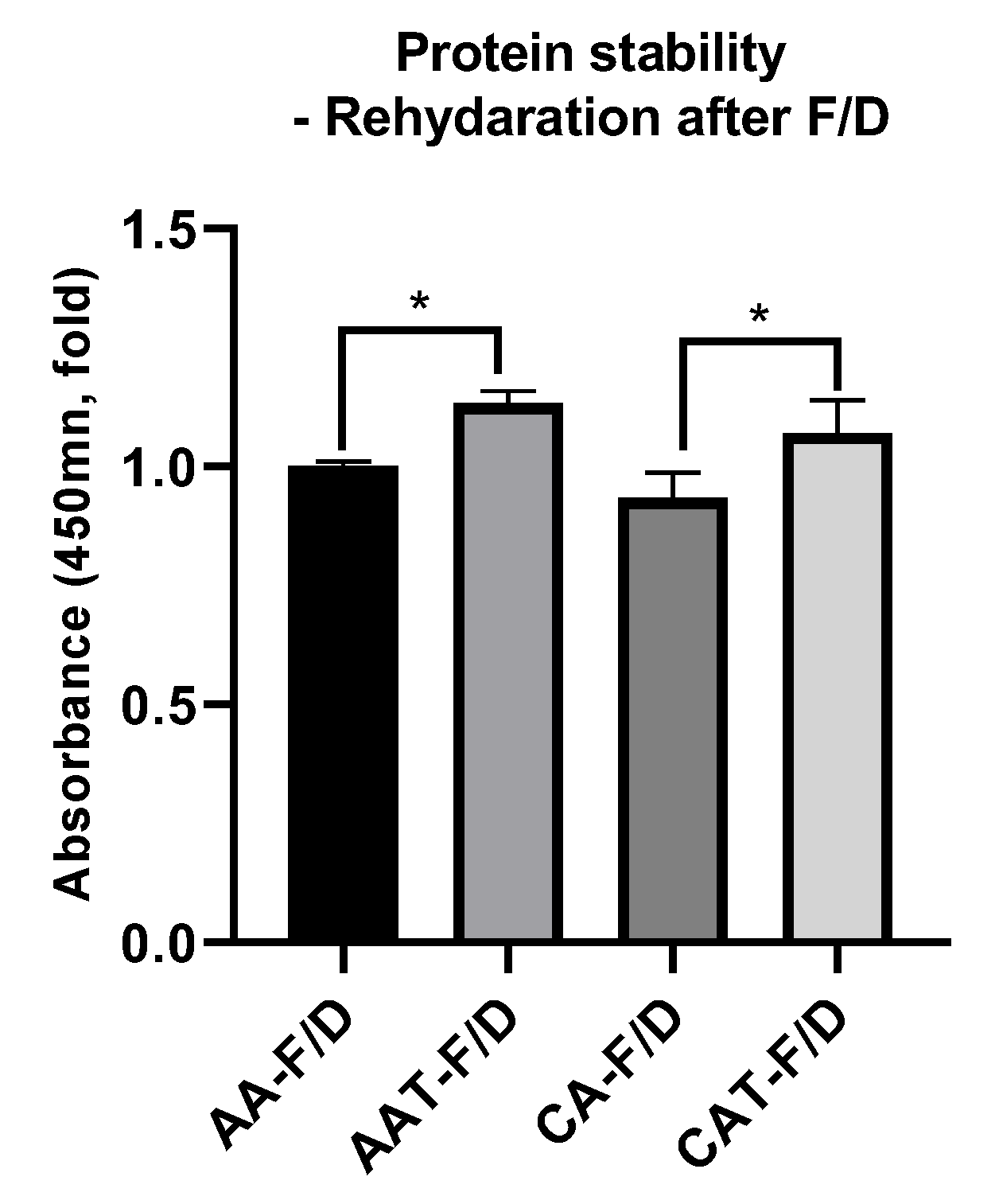
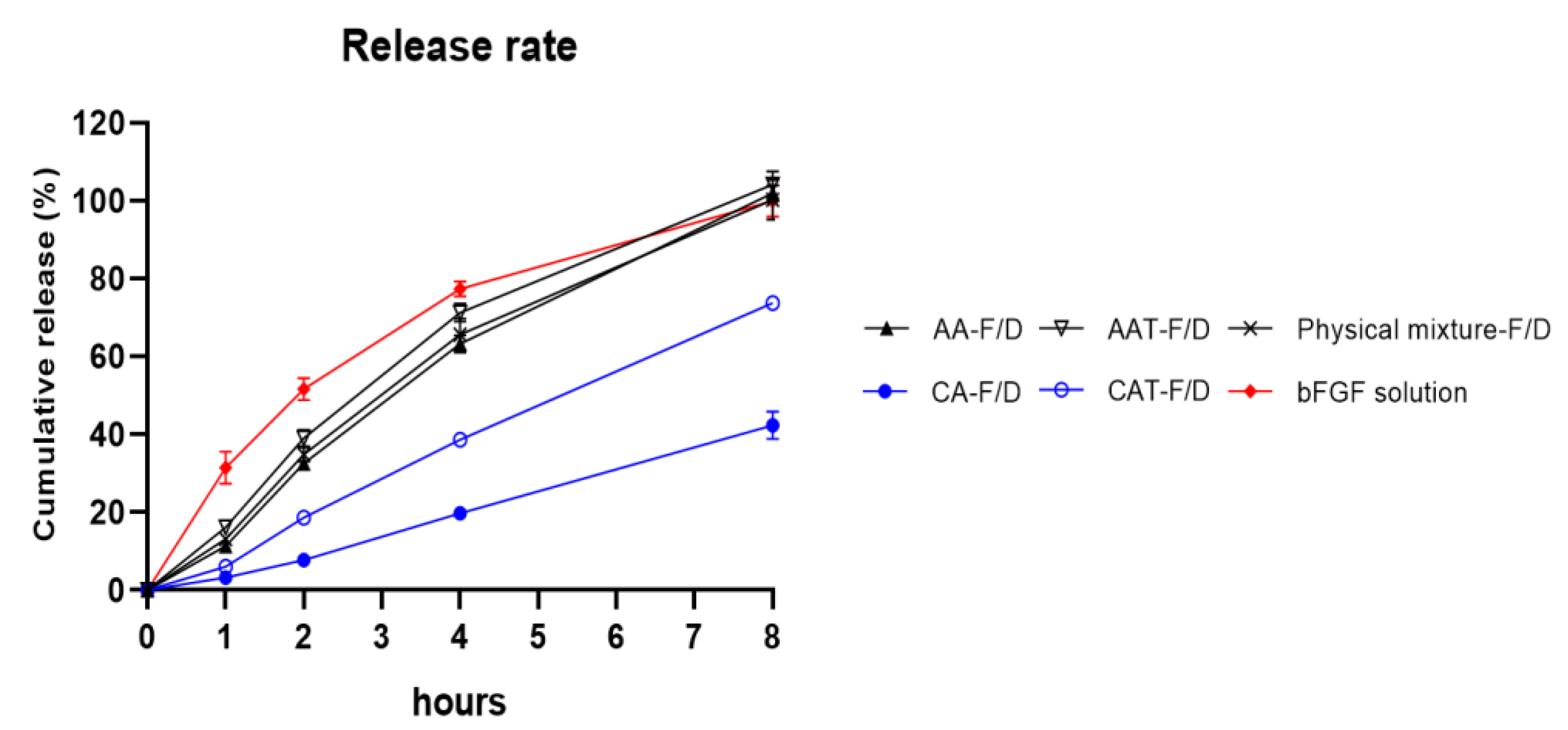


| HWGA/SA Ratio | Total Polymer Weight | pH | Turbidity | Encapsulation Efficacy% | Z-Average (nm) | PDI |
|---|---|---|---|---|---|---|
| 1:1 | 4 mg | 4.34 ± 0.02 | 1.810 ± 0.01 | 89.0 ± 0.8 | 441 ± 8 | 0.121 ± 0.02 |
| Peak Assignment | Trehalose | GA/SA/Trehalose Powder Mixture | CAT-F/D |
|---|---|---|---|
| O-H stretching of trehalose | 3274 | No change | * |
| Asymmetrical and symmetrical stretching of the C-H ring | 2993, 2973, 2950, 2934, 2907, 2881 | No change | * |
| Two vibrational modes (asymmetrical and symmetrical) of the α,α-1↔1-glycosidic bond | 994, 956 | No change | 994→984, 956→941 |
| Coupled bending vibrations of C1−H, CH2 and C−O−H | 910 | No change | disappeared |
| Bending vibration of equatorial C−H bonds in α anomers | 851, 841 | No change | 851→disappeared 841→no change |
| Statistical Analysis | |||||
|---|---|---|---|---|---|
| AA-F/D | AAT-F/D | CA-F/D | CAT-F/D | Physical | |
| Mixture | |||||
| 1 h | **** | **** | **** | **** | **** |
| 2 h | **** | **** | **** | **** | **** |
| 4 h | **** | * | **** | **** | *** |
| 8 h | ns | ns | **** | **** | ns |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, J.; Ban, E.; Park, H.; Kim, A. Stability Enhancement of Freeze-Dried Gelatin/Alginate Coacervates for bFGF Delivery. Pharmaceutics 2022, 14, 2548. https://doi.org/10.3390/pharmaceutics14122548
Lee J, Ban E, Park H, Kim A. Stability Enhancement of Freeze-Dried Gelatin/Alginate Coacervates for bFGF Delivery. Pharmaceutics. 2022; 14(12):2548. https://doi.org/10.3390/pharmaceutics14122548
Chicago/Turabian StyleLee, JongOk, Eunmi Ban, Heejung Park, and Aeri Kim. 2022. "Stability Enhancement of Freeze-Dried Gelatin/Alginate Coacervates for bFGF Delivery" Pharmaceutics 14, no. 12: 2548. https://doi.org/10.3390/pharmaceutics14122548
APA StyleLee, J., Ban, E., Park, H., & Kim, A. (2022). Stability Enhancement of Freeze-Dried Gelatin/Alginate Coacervates for bFGF Delivery. Pharmaceutics, 14(12), 2548. https://doi.org/10.3390/pharmaceutics14122548





