Selective Activation of the Wnt-Signaling Pathway as a Novel Therapy for the Treatment of Diabetic Retinopathy and Other Retinal Vascular Diseases
Abstract
1. Introduction
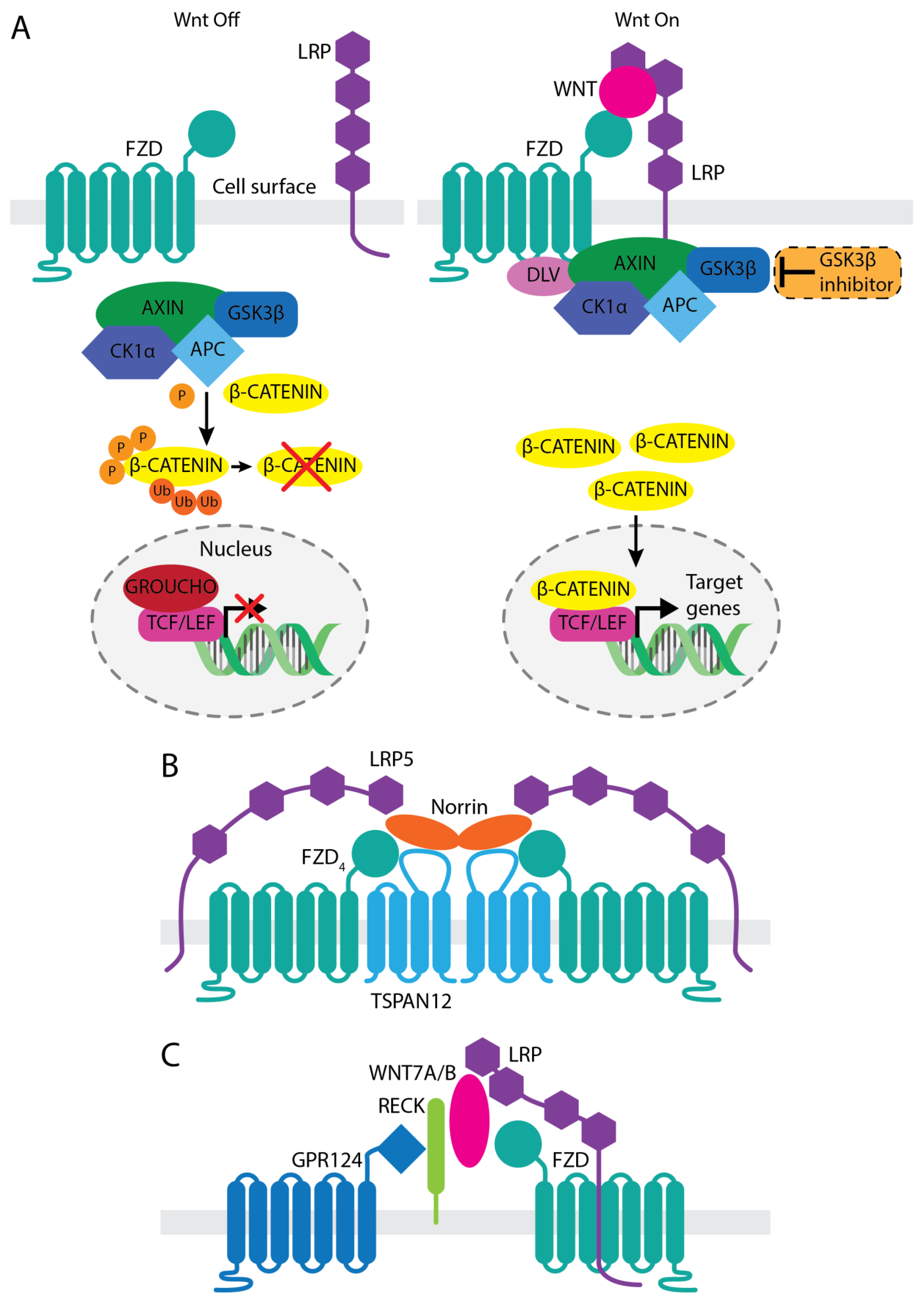
2. Involvement of Wnt/β-Catenin Signaling in Vascular Development and Function
3. Therapeutic Approaches Targeting Wnt Activation
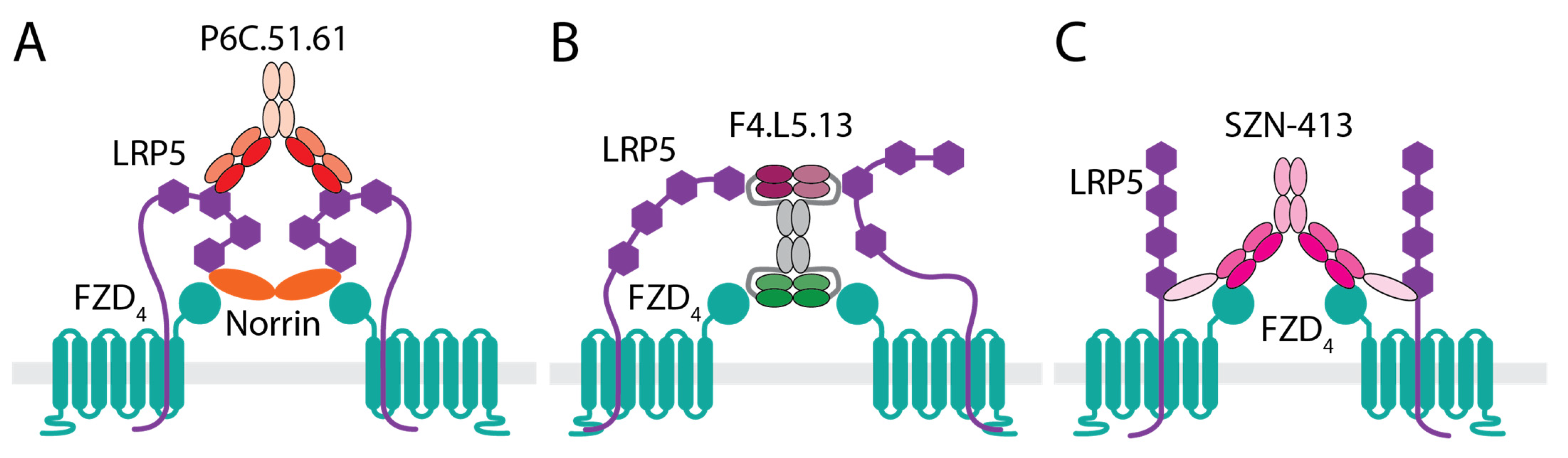
3.1. Anti-LRP5 Antibody That Enhances Norrin Signaling
3.2. WNT Mimetics That Activate FZD4/LRP5
3.3. GSK-3β Inhibitor
4. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Teo, Z.L.; Tham, Y.C.; Yu, M.; Chee, M.L.; Rim, T.H.; Cheung, N.; Bikbov, M.M.; Wang, Y.X.; Tang, Y.; Lu, Y.; et al. Global Prevalence of Diabetic Retinopathy and Projection of Burden through 2045: Systematic Review and Meta-analysis. Ophthalmology 2021, 128, 1580–1591. [Google Scholar] [CrossRef] [PubMed]
- Takkar, B.; Sheemar, A.; Jayasudha, R.; Soni, D.; Narayanan, R.; Venkatesh, P.; Shivaji, S.; Das, T. Unconventional avenues to decelerated diabetic retinopathy. Surv. Ophthalmol. 2022, 67, 1574–1592. [Google Scholar] [CrossRef] [PubMed]
- Yau, J.W.; Rogers, S.L.; Kawasaki, R.; Lamoureux, E.L.; Kowalski, J.W.; Bek, T.; Chen, S.J.; Dekker, J.M.; Fletcher, A.; Grauslund, J.; et al. Global prevalence and major risk factors of diabetic retinopathy. Diabetes Care 2012, 35, 556–564. [Google Scholar] [CrossRef] [PubMed]
- Cheung, N.; Mitchell, P.; Wong, T.Y. Diabetic retinopathy. Lancet 2010, 376, 124–136. [Google Scholar] [CrossRef]
- Horton, W.B.; Barrett, E.J. Microvascular Dysfunction in Diabetes Mellitus and Cardiometabolic Disease. Endocr. Rev. 2021, 42, 29–55. [Google Scholar] [CrossRef]
- Kusuhara, S.; Fukushima, Y.; Ogura, S.; Inoue, N.; Uemura, A. Pathophysiology of Diabetic Retinopathy: The Old and the New. Diabetes Metab. J. 2018, 42, 364–376. [Google Scholar] [CrossRef]
- Das, A. Diabetic Retinopathy: Battling the Global Epidemic. Investig. Ophthalmol. Vis. Sci. 2016, 57, 6669–6682. [Google Scholar] [CrossRef]
- Roy, S.; Kim, D. Retinal capillary basement membrane thickening: Role in the pathogenesis of diabetic retinopathy. Prog. Retin. Eye Res. 2021, 82, 100903. [Google Scholar] [CrossRef]
- Spencer, B.G.; Estevez, J.J.; Liu, E.; Craig, J.E.; Finnie, J.W. Pericytes, inflammation, and diabetic retinopathy. Inflammopharmacology 2020, 28, 697–709. [Google Scholar] [CrossRef]
- Wykoff, C.C.; Yu, H.J.; Avery, R.L.; Ehlers, J.P.; Tadayoni, R.; Sadda, S.R. Retinal non-perfusion in diabetic retinopathy. Eye 2022, 36, 249–256. [Google Scholar] [CrossRef]
- Apte, R.S.; Chen, D.S.; Ferrara, N. VEGF in Signaling and Disease: Beyond Discovery and Development. Cell 2019, 176, 1248–1264. [Google Scholar] [CrossRef] [PubMed]
- Arrigo, A.; Aragona, E.; Bandello, F. VEGF-targeting drugs for the treatment of retinal neovascularization in diabetic retinopathy. Ann. Med. 2022, 54, 1089–1111. [Google Scholar] [CrossRef] [PubMed]
- Penn, J.S.; Madan, A.; Caldwell, R.B.; Bartoli, M.; Caldwell, R.W.; Hartnett, M.E. Vascular endothelial growth factor in eye disease. Prog. Retin. Eye Res. 2008, 27, 331–371. [Google Scholar] [CrossRef]
- Simo, R.; Hernandez, C. Intravitreous anti-VEGF for diabetic retinopathy: Hopes and fears for a new therapeutic strategy. Diabetologia 2008, 51, 1574–1580. [Google Scholar] [CrossRef]
- Naujokaitis, T.; Balciuniene, V.J. Real-World Treatment Patterns and Vision Outcomes with Ranibizumab for Diabetic Macular Edema. J. Ophthalmol. 2021, 2021, 8825082. [Google Scholar] [CrossRef] [PubMed]
- Holekamp, N.M.; Campochiaro, P.A.; Chang, M.A.; Miller, D.; Pieramici, D.; Adamis, A.P.; Brittain, C.; Evans, E.; Kaufman, D.; Maass, K.F.; et al. Archway Randomized Phase 3 Trial of the Port Delivery System with Ranibizumab for Neovascular Age-Related Macular Degeneration. Ophthalmology 2022, 129, 295–307. [Google Scholar] [CrossRef] [PubMed]
- Wykoff, C.C.; Abreu, F.; Adamis, A.P.; Basu, K.; Eichenbaum, D.A.; Haskova, Z.; Lin, H.; Loewenstein, A.; Mohan, S.; Pearce, I.A.; et al. Efficacy, durability, and safety of intravitreal faricimab with extended dosing up to every 16 weeks in patients with diabetic macular oedema (YOSEMITE and RHINE): Two randomised, double-masked, phase 3 trials. Lancet 2022, 399, 741–755. [Google Scholar] [CrossRef]
- Heier, J.S.; Khanani, A.M.; Quezada Ruiz, C.; Basu, K.; Ferrone, P.J.; Brittain, C.; Figueroa, M.S.; Lin, H.; Holz, F.G.; Patel, V.; et al. Efficacy, durability, and safety of intravitreal faricimab up to every 16 weeks for neovascular age-related macular degeneration (TENAYA and LUCERNE): Two randomised, double-masked, phase 3, non-inferiority trials. Lancet 2022, 399, 729–740. [Google Scholar] [CrossRef]
- Grishanin, R.; Vuillemenot, B.; Sharma, P.; Keravala, A.; Greengard, J.; Gelfman, C.; Blumenkrantz, M.; Lawrence, M.; Hu, W.; Kiss, S.; et al. Preclinical Evaluation of ADVM-022, A Novel Gene Therapy Approach to Treating Wet Age-Related Macular Degeneration. Mol. Ther. 2019, 27, 118–129. [Google Scholar] [CrossRef]
- Chandrasekaran, P.R.; Madanagopalan, V.G. KSI-301: Antibody biopolymer conjugate in retinal disorders. Ther. Adv. Ophthalmol. 2021, 13, 25158414211027708. [Google Scholar] [CrossRef]
- Kunimoto, D.; Ohji, M.; Maturi, R.K.; Sekiryu, T.; Wang, Y.; Pan, G.; Li, X.Y.; Schneider, S.; Bamboo and Cypress Study Groups. Evaluation of Abicipar Pegol (an Anti-VEGF DARPin Therapeutic) in Patients With Neovascular Age-Related Macular Degeneration: Studies in Japan and the United States. Ophthalmic. Surg. Lasers Imaging Retina 2019, 50, e10–e22. [Google Scholar] [CrossRef] [PubMed]
- Silva, R.L.E.; Kanan, Y.; Mirando, A.C.; Kim, J.; Shmueli, R.B.; Lorenc, V.E.; Fortmann, S.D.; Sciamanna, J.; Pandey, N.B.; Green, J.J.; et al. Tyrosine kinase blocking collagen IV-derived peptide suppresses ocular neovascularization and vascular leakage. Sci. Transl. Med. 2017, 9, eaai8030. [Google Scholar] [CrossRef] [PubMed]
- Brown, D.M.; Boyer, D.S.; Csaky, K.; Vitti, R.; Perlee, L.; Chu, K.W.; Asmus, F.; Leal, S.; Zeitz, O.; Cheng, Y.; et al. Intravitreal Nesvacumab (Antiangiopoietin 2) plus Aflibercept in Diabetic Macular Edema: Phase 2 RUBY Randomized Trial. Retina 2022, 42, 1111–1120. [Google Scholar] [CrossRef] [PubMed]
- Campochiaro, P.A.; Khanani, A.; Singer, M.; Patel, S.; Boyer, D.; Dugel, P.; Kherani, S.; Withers, B.; Gambino, L.; Peters, K.; et al. Enhanced Benefit in Diabetic Macular Edema from AKB-9778 Tie2 Activation Combined with Vascular Endothelial Growth Factor Suppression. Ophthalmology 2016, 123, 1722–1730. [Google Scholar] [CrossRef]
- Shaw, L.T.; Mackin, A.; Shah, R.; Jain, S.; Jain, P.; Nayak, R.; Hariprasad, S.M. Risuteganib—A novel integrin inhibitor for the treatment of non-exudative (dry) age-related macular degeneration and diabetic macular edema. Expert Opin. Investig. Drugs 2020, 29, 547–554. [Google Scholar] [CrossRef]
- Pfeiffer, N.; Voykov, B.; Renieri, G.; Bell, K.; Richter, P.; Weigel, M.; Thieme, H.; Wilhelm, B.; Lorenz, K.; Feindor, M.; et al. First-in-human phase I study of ISTH0036, an antisense oligonucleotide selectively targeting transforming growth factor beta 2 (TGF-beta2), in subjects with open-angle glaucoma undergoing glaucoma filtration surgery. PLoS ONE 2017, 12, e0188899. [Google Scholar] [CrossRef]
- Mundo, L.; Tosi, G.M.; Lazzi, S.; Pertile, G.; Parolini, B.; Neri, G.; Posarelli, M.; De Benedetto, E.; Bacci, T.; Silvestri, E.; et al. LRG1 Expression Is Elevated in the Eyes of Patients with Neovascular Age-Related Macular Degeneration. Int. J. Mol. Sci. 2021, 22, 8879. [Google Scholar] [CrossRef]
- Martowicz, A.; Trusohamn, M.; Jensen, N.; Wisniewska-Kruk, J.; Corada, M.; Ning, F.C.; Kele, J.; Dejana, E.; Nyqvist, D. Endothelial Beta-Catenin Signaling Supports Postnatal Brain and Retinal Angiogenesis by Promoting Sprouting, Tip Cell Formation, and VEGFR (Vascular Endothelial Growth Factor Receptor) 2 Expression. Arterioscler Thromb. Vasc. Biol. 2019, 39, 2273–2288. [Google Scholar] [CrossRef]
- Wang, Y.; Sabbagh, M.F.; Gu, X.; Rattner, A.; Williams, J.; Nathans, J. Beta-catenin signaling regulates barrier-specific gene expression in circumventricular organ and ocular vasculatures. Elife 2019, 8, e43257. [Google Scholar] [CrossRef]
- Chen, Q.; Ma, J.X. Canonical Wnt signaling in diabetic retinopathy. Vision Res. 2017, 139, 47–58. [Google Scholar] [CrossRef]
- Loh, K.M.; van Amerongen, R.; Nusse, R. Generating Cellular Diversity and Spatial Form: Wnt Signaling and the Evolution of Multicellular Animals. Dev. Cell 2016, 38, 643–655. [Google Scholar] [CrossRef] [PubMed]
- Rim, E.Y.; Clevers, H.; Nusse, R. The Wnt Pathway: From Signaling Mechanisms to Synthetic Modulators. Annu. Rev. Biochem. 2022, 91, 571–598. [Google Scholar] [CrossRef] [PubMed]
- Nusse, R.; Clevers, H. Wnt/beta-Catenin Signaling, Disease, and Emerging Therapeutic Modalities. Cell 2017, 169, 985–999. [Google Scholar] [CrossRef] [PubMed]
- Clevers, H.; Nusse, R. Wnt/beta-catenin signaling and disease. Cell 2012, 149, 1192–1205. [Google Scholar] [CrossRef]
- Nusse, R.; Varmus, H. Three decades of Wnts: A personal perspective on how a scientific field developed. EMBO J. 2012, 31, 2670–2684. [Google Scholar] [CrossRef]
- Clevers, H.; Loh, K.M.; Nusse, R. Stem cell signaling. An integral program for tissue renewal and regeneration: Wnt signaling and stem cell control. Science 2014, 346, 1248012. [Google Scholar] [CrossRef]
- Van Amerongen, R. Celebrating Discoveries in Wnt Signaling: How One Man Gave Wings to an Entire Field. Cell 2020, 181, 487–491. [Google Scholar] [CrossRef]
- Niehrs, C. The role of Xenopus developmental biology in unraveling Wnt signalling and antero-posterior axis formation. Dev. Biol. 2022, 482, 1–6. [Google Scholar] [CrossRef]
- Schulte, G.; Wright, S.C. Frizzleds as GPCRs—More Conventional Than We Thought! Trends Pharmacol. Sci. 2018, 39, 828–842. [Google Scholar] [CrossRef]
- Xu, Q.; Wang, Y.; Dabdoub, A.; Smallwood, P.M.; Williams, J.; Woods, C.; Kelley, M.W.; Jiang, L.; Tasman, W.; Zhang, K.; et al. Vascular development in the retina and inner ear: Control by Norrin and Frizzled-4, a high-affinity ligand-receptor pair. Cell 2004, 116, 883–895. [Google Scholar] [CrossRef]
- Ye, X.; Wang, Y.; Cahill, H.; Yu, M.; Badea, T.C.; Smallwood, P.M.; Peachey, N.S.; Nathans, J. Norrin, frizzled-4, and Lrp5 signaling in endothelial cells controls a genetic program for retinal vascularization. Cell 2009, 139, 285–298. [Google Scholar] [CrossRef] [PubMed]
- Richter, M.; Gottanka, J.; May, C.A.; Welge-Lussen, U.; Berger, W.; Lutjen-Drecoll, E. Retinal vasculature changes in Norrie disease mice. Investig. Ophthalmol. Vis. Sci. 1998, 39, 2450–2457. [Google Scholar]
- Wang, Y.; Cho, C.; Williams, J.; Smallwood, P.M.; Zhang, C.; Junge, H.J.; Nathans, J. Interplay of the Norrin and Wnt7a/Wnt7b signaling systems in blood-brain barrier and blood-retina barrier development and maintenance. Proc. Natl. Acad. Sci. USA 2018, 115, E11827–E11836. [Google Scholar] [CrossRef]
- Agostino, M.; Pohl, S.O. The structural biology of canonical Wnt signalling. Biochem. Soc. Trans. 2020, 48, 1765–1780. [Google Scholar] [CrossRef] [PubMed]
- DeBruine, Z.J.; Xu, H.E.; Melcher, K. Assembly and architecture of the Wnt/beta-catenin signalosome at the membrane. Br. J. Pharmacol. 2017, 174, 4564–4574. [Google Scholar] [CrossRef]
- Junge, H.J.; Yang, S.; Burton, J.B.; Paes, K.; Shu, X.; French, D.M.; Costa, M.; Rice, D.S.; Ye, W. TSPAN12 regulates retinal vascular development by promoting Norrin- but not Wnt-induced FZD4/beta-catenin signaling. Cell 2009, 139, 299–311. [Google Scholar] [CrossRef] [PubMed]
- Lai, M.B.; Zhang, C.; Shi, J.; Johnson, V.; Khandan, L.; McVey, J.; Klymkowsky, M.W.; Chen, Z.; Junge, H.J. TSPAN12 Is a Norrin Co-receptor that Amplifies Frizzled4 Ligand Selectivity and Signaling. Cell Rep. 2017, 19, 2809–2822. [Google Scholar] [CrossRef]
- Wawrzynski, J.; Patel, A.; Badran, A.; Dowell, I.; Henderson, R.; Sowden, J.C. Spectrum of Mutations in NDP Resulting in Ocular Disease; a Systematic Review. Front. Genet. 2022, 13, 884722. [Google Scholar] [CrossRef]
- Hendrickx, M.; Leyns, L. Non-conventional Frizzled ligands and Wnt receptors. Dev. Growth Differ. 2008, 50, 229–243. [Google Scholar] [CrossRef]
- Liebner, S.; Plate, K.H. Differentiation of the brain vasculature: The answer came blowing by the Wnt. J. Angiogenes Res. 2010, 2, 1. [Google Scholar] [CrossRef]
- Rattner, A.; Wang, Y.; Nathans, J. Signaling Pathways in Neurovascular Development. Annu. Rev. Neurosci. 2022, 45, 87–108. [Google Scholar] [CrossRef] [PubMed]
- Noda, M.; Vallon, M.; Kuo, C.J. The Wnt7’s Tale: A story of an orphan who finds her tie to a famous family. Cancer Sci. 2016, 107, 576–582. [Google Scholar] [CrossRef] [PubMed]
- Drenser, K.A. Wnt signaling pathway in retinal vascularization. Eye Brain 2016, 8, 141–146. [Google Scholar] [CrossRef]
- Kato, M.; Patel, M.S.; Levasseur, R.; Lobov, I.; Chang, B.H.; Glass, D.A., 2nd; Hartmann, C.; Li, L.; Hwang, T.H.; Brayton, C.F.; et al. Cbfa1-independent decrease in osteoblast proliferation, osteopenia, and persistent embryonic eye vascularization in mice deficient in Lrp5, a Wnt coreceptor. J. Cell Biol. 2002, 157, 303–314. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Rattner, A.; Zhou, Y.; Williams, J.; Smallwood, P.M.; Nathans, J. Norrin/Frizzled4 signaling in retinal vascular development and blood brain barrier plasticity. Cell 2012, 151, 1332–1344. [Google Scholar] [CrossRef] [PubMed]
- Rehm, H.L.; Zhang, D.S.; Brown, M.C.; Burgess, B.; Halpin, C.; Berger, W.; Morton, C.C.; Corey, D.P.; Chen, Z.Y. Vascular defects and sensorineural deafness in a mouse model of Norrie disease. J. Neurosci. 2002, 22, 4286–4292. [Google Scholar] [CrossRef]
- Ye, X.; Smallwood, P.; Nathans, J. Expression of the Norrie disease gene (Ndp) in developing and adult mouse eye, ear, and brain. Gene. Expr. Patterns 2011, 11, 151–155. [Google Scholar] [CrossRef] [PubMed]
- Bryant, D.; Pauzuolyte, V.; Ingham, N.J.; Patel, A.; Pagarkar, W.; Anderson, L.A.; Smith, K.E.; Moulding, D.A.; Leong, Y.C.; Jafree, D.J.; et al. The timing of auditory sensory deficits in Norrie disease has implications for therapeutic intervention. JCI Insight 2022, 7, e148586. [Google Scholar] [CrossRef]
- Silbert, M.; Gurwood, A.S. Persistent hyperplastic primary vitreous. Clin. Eye Vis. Care 2000, 12, 131–137. [Google Scholar] [CrossRef]
- Thomas, D.M.; Kannabiran, C.; Balasubramanian, D. Identification of Key Genes and Pathways in Persistent Hyperplastic Primary Vitreous of the Eye Using Bioinformatic Analysis. Front. Med. 2021, 8, 690594. [Google Scholar] [CrossRef]
- Lobov, I.B.; Rao, S.; Carroll, T.J.; Vallance, J.E.; Ito, M.; Ondr, J.K.; Kurup, S.; Glass, D.A.; Patel, M.S.; Shu, W.; et al. WNT7b mediates macrophage-induced programmed cell death in patterning of the vasculature. Nature 2005, 437, 417–421. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Wang, Y.; Tischfield, M.; Williams, J.; Smallwood, P.M.; Rattner, A.; Taketo, M.M.; Nathans, J. Canonical WNT signaling components in vascular development and barrier formation. J. Clin. Investig. 2014, 124, 3825–3846. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Hu, Y.; Zhou, T.; Zhou, K.K.; Mott, R.; Wu, M.; Boulton, M.; Lyons, T.J.; Gao, G.; Ma, J.X. Activation of the Wnt pathway plays a pathogenic role in diabetic retinopathy in humans and animal models. Am. J. Pathol. 2009, 175, 2676–2685. [Google Scholar] [CrossRef] [PubMed]
- Qiu, F.; He, J.; Zhou, Y.; Bai, X.; Wu, G.; Wang, X.; Liu, Z.; Chen, Y.; Ma, J.X.; Liu, Z. Plasma and vitreous fluid levels of Dickkopf-1 in patients with diabetic retinopathy. Eye 2014, 28, 402–409. [Google Scholar] [CrossRef]
- Chen, J.; Stahl, A.; Krah, N.M.; Seaward, M.R.; Dennison, R.J.; Sapieha, P.; Hua, J.; Hatton, C.J.; Juan, A.M.; Aderman, C.M.; et al. Wnt signaling mediates pathological vascular growth in proliferative retinopathy. Circulation 2011, 124, 1871–1881. [Google Scholar] [CrossRef]
- Bucher, F.; Zhang, D.; Aguilar, E.; Sakimoto, S.; Diaz-Aguilar, S.; Rosenfeld, M.; Zha, Z.; Zhang, H.; Friedlander, M.; Yea, K. Antibody-Mediated Inhibition of Tspan12 Ameliorates Vasoproliferative Retinopathy Through Suppression of beta-Catenin Signaling. Circulation 2017, 136, 180–195. [Google Scholar] [CrossRef]
- Bats, M.L.; Bougaran, P.; Peghaire, C.; Gueniot, F.; Abelanet, A.; Chan, H.; Seguy, C.; Jeanningros, S.; Jaspard-Vinassa, B.; Couffinhal, T.; et al. Therapies targeting Frizzled-7/beta-catenin pathway prevent the development of pathological angiogenesis in an ischemic retinopathy model. FASEB J. 2020, 34, 1288–1303. [Google Scholar] [CrossRef]
- Chidiac, R.; Abedin, M.; Macleod, G.; Yang, A.; Thibeault, P.E.; Blazer, L.L.; Adams, J.J.; Zhang, L.; Roehrich, H.; Jo, H.N.; et al. A Norrin/Wnt surrogate antibody stimulates endothelial cell barrier function and rescues retinopathy. EMBO Mol. Med. 2021, 13, e13977. [Google Scholar] [CrossRef]
- Diaz-Coranguez, M.; Lin, C.M.; Liebner, S.; Antonetti, D.A. Norrin restores blood-retinal barrier properties after vascular endothelial growth factor-induced permeability. J. Biol. Chem. 2020, 295, 4647–4660. [Google Scholar] [CrossRef]
- Ohlmann, A.; Seitz, R.; Braunger, B.; Seitz, D.; Bosl, M.R.; Tamm, E.R. Norrin promotes vascular regrowth after oxygen-induced retinal vessel loss and suppresses retinopathy in mice. J. Neurosci. 2010, 30, 183–193. [Google Scholar] [CrossRef]
- Ohlmann, A.; Scholz, M.; Goldwich, A.; Chauhan, B.K.; Hudl, K.; Ohlmann, A.V.; Zrenner, E.; Berger, W.; Cvekl, A.; Seeliger, M.W.; et al. Ectopic norrin induces growth of ocular capillaries and restores normal retinal angiogenesis in Norrie disease mutant mice. J. Neurosci. 2005, 25, 1701–1710. [Google Scholar] [CrossRef]
- Tokunaga, C.C.; Chen, Y.H.; Dailey, W.; Cheng, M.; Drenser, K.A. Retinal vascular rescue of oxygen-induced retinopathy in mice by norrin. Investig. Ophthalmol. Vis. Sci. 2013, 54, 222–229. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Liu, C.H.; Huang, S.; Fu, Z.; Tomita, Y.; Britton, W.R.; Cho, S.S.; Chen, C.T.; Sun, Y.; Ma, J.X.; et al. Wnt signaling activates MFSD2A to suppress vascular endothelial transcytosis and maintain blood-retinal barrier. Sci. Adv. 2020, 6, eaba7457. [Google Scholar] [CrossRef] [PubMed]
- Niehrs, C. Norrin and frizzled; a new vein for the eye. Dev. Cell 2004, 6, 453–454. [Google Scholar] [CrossRef]
- Ke, J.; Harikumar, K.G.; Erice, C.; Chen, C.; Gu, X.; Wang, L.; Parker, N.; Cheng, Z.; Xu, W.; Williams, B.O.; et al. Structure and function of Norrin in assembly and activation of a Frizzled 4-Lrp5/6 complex. Genes. Dev. 2013, 27, 2305–2319. [Google Scholar] [CrossRef]
- Chang, T.H.; Hsieh, F.L.; Zebisch, M.; Harlos, K.; Elegheert, J.; Jones, E.Y. Structure and functional properties of Norrin mimic Wnt for signalling with Frizzled4, Lrp5/6, and proteoglycan. Elife 2015, 4, e06554. [Google Scholar] [CrossRef]
- Janda, C.Y.; Waghray, D.; Levin, A.M.; Thomas, C.; Garcia, K.C. Structural basis of Wnt recognition by Frizzled. Science 2012, 337, 59–64. [Google Scholar] [CrossRef]
- Ren, Q.; Chen, J.; Liu, Y. LRP5 and LRP6 in Wnt Signaling: Similarity and Divergence. Front. Cell Dev. Biol. 2021, 9, 670960. [Google Scholar] [CrossRef]
- Gong, Y.; Bourhis, E.; Chiu, C.; Stawicki, S.; DeAlmeida, V.I.; Liu, B.Y.; Phamluong, K.; Cao, T.C.; Carano, R.A.; Ernst, J.A.; et al. Wnt isoform-specific interactions with coreceptor specify inhibition or potentiation of signaling by LRP6 antibodies. PLoS ONE 2010, 5, e12682. [Google Scholar] [CrossRef]
- Ettenberg, S.A.; Charlat, O.; Daley, M.P.; Liu, S.; Vincent, K.J.; Stuart, D.D.; Schuller, A.G.; Yuan, J.; Ospina, B.; Green, J.; et al. Inhibition of tumorigenesis driven by different Wnt proteins requires blockade of distinct ligand-binding regions by LRP6 antibodies. Proc. Natl. Acad. Sci. USA 2010, 107, 15473–15478. [Google Scholar] [CrossRef]
- Poulter, J.A.; Ali, M.; Gilmour, D.F.; Rice, A.; Kondo, H.; Hayashi, K.; Mackey, D.A.; Kearns, L.S.; Ruddle, J.B.; Craig, J.E.; et al. Mutations in TSPAN12 cause autosomal-dominant familial exudative vitreoretinopathy. Am. J. Hum. Genet. 2010, 86, 248–253. [Google Scholar] [CrossRef] [PubMed]
- Janda, C.Y.; Dang, L.T.; You, C.; Chang, J.; de Lau, W.; Zhong, Z.A.; Yan, K.S.; Marecic, O.; Siepe, D.; Li, X.; et al. Surrogate Wnt agonists that phenocopy canonical Wnt and beta-catenin signalling. Nature 2017, 545, 234–237. [Google Scholar] [CrossRef] [PubMed]
- Tao, Y.; Mis, M.; Blazer, L.; Ustav, M.J.; Steinhart, Z.; Chidiac, R.; Kubarakos, E.; O’Brien, S.; Wang, X.; Jarvik, N.; et al. Tailored tetravalent antibodies potently and specifically activate Wnt/Frizzled pathways in cells, organoids and mice. Elife 2019, 8, e46134. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Lu, C.; Ouyang, B.; Zhang, H.; Huang, Z.; Bhatia, D.; Lee, S.J.; Shah, D.; Sura, A.; Yeh, W.C.; et al. Development of Potent, Selective Surrogate WNT Molecules and Their Application in Defining Frizzled Requirements. Cell Chem. Biol. 2020, 27, 598–609 e594. [Google Scholar] [CrossRef]
- Miao, Y.; Ha, A.; de Lau, W.; Yuki, K.; Santos, A.J.M.; You, C.; Geurts, M.H.; Puschhof, J.; Pleguezuelos-Manzano, C.; Peng, W.C.; et al. Next-Generation Surrogate Wnts Support Organoid Growth and Deconvolute Frizzled Pleiotropy In Vivo. Cell Stem. Cell 2020, 27, 840–851 e846. [Google Scholar] [CrossRef]
- Fowler, T.W.; Mitchell, T.L.; Janda, C.Y.; Xie, L.; Tu, S.; Chen, H.; Zhang, H.; Ye, J.; Ouyang, B.; Yuan, T.Z.; et al. Development of selective bispecific Wnt mimetics for bone loss and repair. Nat. Commun. 2021, 12, 3247. [Google Scholar] [CrossRef]
- Nguyen, H.; Chen, H.; Vuppalapaty, M.; Whisler, E.; Logas, K.R.; Sampathkumar, P.; Fletcher, R.B.; Sura, A.; Suen, N.; Gupta, S.; et al. SZN-413, a FZD4 Agonist, as a Potential Novel Therapeutic for the Treatment of Diabetic Retinopathy. Transl. Vis. Sci. Technol. 2022, 11, 19. [Google Scholar] [CrossRef]
- Aiello, L.P.; Avery, R.L.; Arrigg, P.G.; Keyt, B.A.; Jampel, H.D.; Shah, S.T.; Pasquale, L.R.; Thieme, H.; Iwamoto, M.A.; Park, J.E.; et al. Vascular endothelial growth factor in ocular fluid of patients with diabetic retinopathy and other retinal disorders. N Engl. J. Med. 1994, 331, 1480–1487. [Google Scholar] [CrossRef]
- Shimada, H.; Akaza, E.; Yuzawa, M.; Kawashima, M. Concentration gradient of vascular endothelial growth factor in the vitreous of eyes with diabetic macular edema. Investig. Ophthalmol. Vis. Sci. 2009, 50, 2953–2955. [Google Scholar] [CrossRef]
- Wang, J.; Chen, S.; Jiang, F.; You, C.; Mao, C.; Yu, J.; Han, J.; Zhang, Z.; Yan, H. Vitreous and plasma VEGF levels as predictive factors in the progression of proliferative diabetic retinopathy after vitrectomy. PLoS ONE 2014, 9, e110531. [Google Scholar] [CrossRef]
- Hoang, M.V.; Smith, L.E.; Senger, D.R. Moderate GSK-3beta inhibition improves neovascular architecture, reduces vascular leakage, and reduces retinal hypoxia in a model of ischemic retinopathy. Angiogenesis 2010, 13, 269–277. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Liu, C.H.; Sun, Y.; Gong, Y.; Favazza, T.L.; Morss, P.C.; Saba, N.J.; Fredrick, T.W.; He, X.; Akula, J.D.; et al. Pharmacologic Activation of Wnt Signaling by Lithium Normalizes Retinal Vasculature in a Murine Model of Familial Exudative Vitreoretinopathy. Am. J. Pathol. 2016, 186, 2588–2600. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Yang, M.; Zhao, R.; Peng, L.; Dai, E.; Huang, L.; Zhao, P.; Li, S.; Yang, Z. Novel truncating variants in CTNNB1 cause familial exudative vitreoretinopathy. J. Med. Genet. 2022. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Li, S.; Huang, L.; Zhao, R.; Dai, E.; Jiang, X.; He, Y.; Lu, J.; Peng, L.; Liu, W.; et al. CTNND1 variants cause familial exudative vitreoretinopathy through the Wnt/cadherin axis. JCI Insight 2022, 7, e158428. [Google Scholar] [CrossRef]
- Zeilbeck, L.F.; Muller, B.; Knobloch, V.; Tamm, E.R.; Ohlmann, A. Differential angiogenic properties of lithium chloride in vitro and in vivo. PLoS ONE 2014, 9, e95546. [Google Scholar] [CrossRef]
- Rayasam, G.V.; Tulasi, V.K.; Sodhi, R.; Davis, J.A.; Ray, A. Glycogen synthase kinase 3: More than a namesake. Br. J. Pharmacol. 2009, 156, 885–898. [Google Scholar] [CrossRef]
- Zhang, Z.; Broderick, C.; Nishimoto, M.; Yamaguchi, T.; Lee, S.J.; Zhang, H.; Chen, H.; Patel, M.; Ye, J.; Ponce, A.; et al. Tissue-targeted R-spondin mimetics for liver regeneration. Sci. Rep. 2020, 10, 13951. [Google Scholar] [CrossRef]
- Xie, L.; Fletcher, R.B.; Bhatia, D.; Shah, D.; Phipps, J.; Deshmukh, S.; Zhang, H.; Ye, J.; Lee, S.; Le, L.; et al. Robust Colonic Epithelial Regeneration and Amelioration of Colitis via FZD-Specific Activation of Wnt Signaling. Cell Mol. Gastroenterol. Hepatol. 2022, 14, 435–464. [Google Scholar] [CrossRef]
- Xiao, H.; Tong, Y.; Zhu, Y.; Peng, M. Familial Exudative Vitreoretinopathy-Related Disease-Causing Genes and Norrin/beta-Catenin Signal Pathway: Structure, Function, and Mutation Spectrums. J. Ophthalmol. 2019, 2019, 5782536. [Google Scholar] [CrossRef]
- Orozco, L.D.; Chen, H.H.; Cox, C.; Katschke, K.J., Jr.; Arceo, R.; Espiritu, C.; Caplazi, P.; Nghiem, S.S.; Chen, Y.J.; Modrusan, Z.; et al. Integration of eQTL and a Single-Cell Atlas in the Human Eye Identifies Causal Genes for Age-Related Macular Degeneration. Cell Rep. 2020, 30, 1246–1259 e1246. [Google Scholar] [CrossRef]

| Signaling Pathway | Drug | Target | Drug Type | Reference |
|---|---|---|---|---|
| VEGF-binding | Port Delivery System with ranibizumab | VEGF-A | Monoclonal antibody Fab | [16] |
| ADVM-022 | VEGF-A, B, Placenta growth factor | AAV-7m8 vector coding aflibercept | [19] | |
| KSI-301 | VEGF-A | Antibody biopolymer conjugate | [20] | |
| Abicipar pegol | VEGF-A | Small proteins that contain engineered ankyrin repeat domain | [21] | |
| Faricimab | VEGF-A, Ang-2 | Humanized full-length bispecific IgG1 antibody that selectively neutralizes VEGF-A and Ang-2 | [17,18] | |
| Non VEGF-binding | AXT107 | Integrin | Peptide | [22] |
| Nesvacumab | Ang-2 | Monoclonal antibody | [23] | |
| Razuprotafib | Tie2 | Small molecule | [24] | |
| Risuteganib | Integrin | Peptide | [25] | |
| Anti-LRG1 | Leucine-rich alpha-2-glycoprotein 1 (LRG1) | Antibody | [26] | |
| THR-149 | Plasma kallikrein | Peptide | [27] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nguyen, H.; Lee, S.-J.; Li, Y. Selective Activation of the Wnt-Signaling Pathway as a Novel Therapy for the Treatment of Diabetic Retinopathy and Other Retinal Vascular Diseases. Pharmaceutics 2022, 14, 2476. https://doi.org/10.3390/pharmaceutics14112476
Nguyen H, Lee S-J, Li Y. Selective Activation of the Wnt-Signaling Pathway as a Novel Therapy for the Treatment of Diabetic Retinopathy and Other Retinal Vascular Diseases. Pharmaceutics. 2022; 14(11):2476. https://doi.org/10.3390/pharmaceutics14112476
Chicago/Turabian StyleNguyen, Huy, Sung-Jin Lee, and Yang Li. 2022. "Selective Activation of the Wnt-Signaling Pathway as a Novel Therapy for the Treatment of Diabetic Retinopathy and Other Retinal Vascular Diseases" Pharmaceutics 14, no. 11: 2476. https://doi.org/10.3390/pharmaceutics14112476
APA StyleNguyen, H., Lee, S.-J., & Li, Y. (2022). Selective Activation of the Wnt-Signaling Pathway as a Novel Therapy for the Treatment of Diabetic Retinopathy and Other Retinal Vascular Diseases. Pharmaceutics, 14(11), 2476. https://doi.org/10.3390/pharmaceutics14112476







