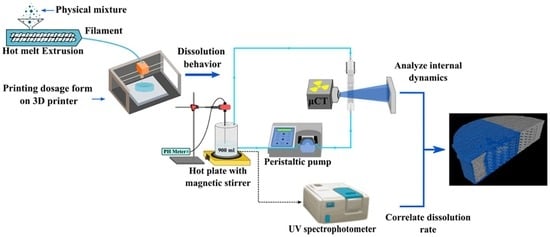
Development of Flow-Through Cell Dissolution Method for In Situ Visualization of Dissolution Processes in Solid Dosage Forms Using X-ray μCT
Abstract
1. Introduction
2. Methods and Materials
2.1. Materials
2.2. Tablet Preparation
2.3. Flow-Through Cell Dissolution Apparatus
2.4. In Vitro Dissolution Experiments
2.4.1. Paddle Dissolution Method
2.4.2. Flow-Through Cell Dissolution Method
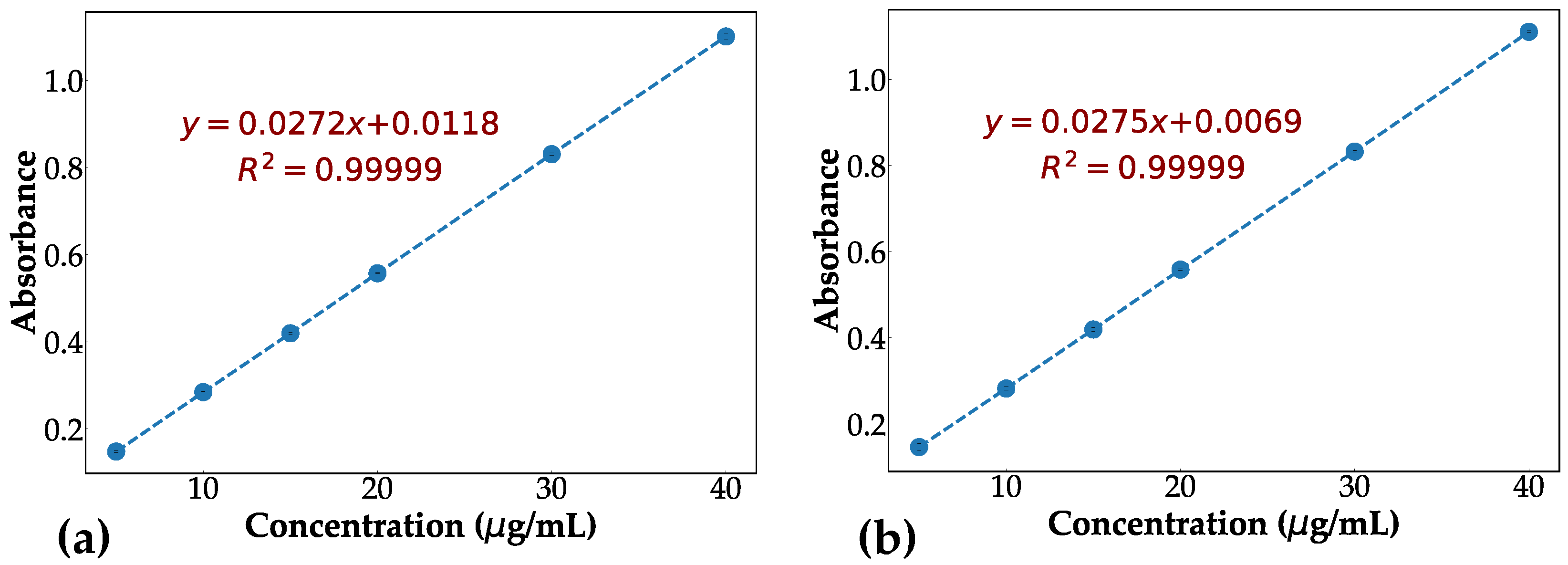
2.4.3. UV Spectrophotometry
2.5. High-Resolution X-Ray Tomography (CT)
2.5.1. CT Set-Up
2.5.2. Contrast Agent
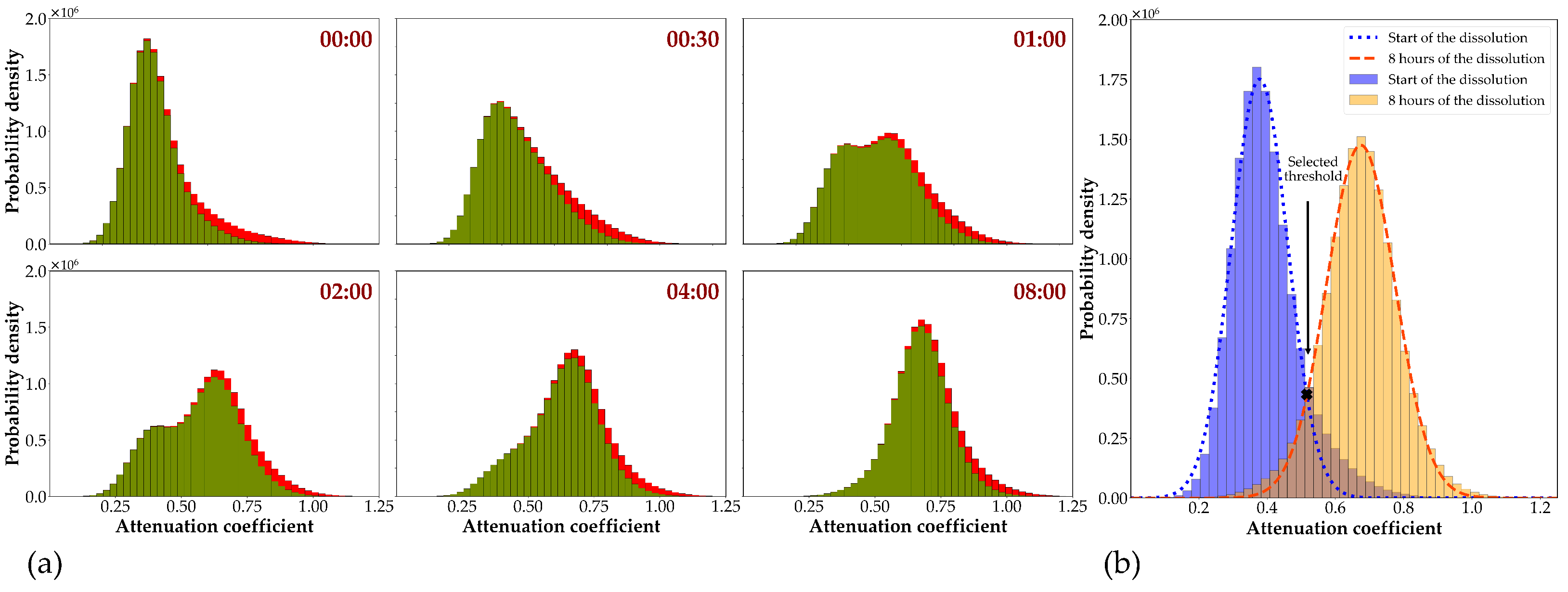
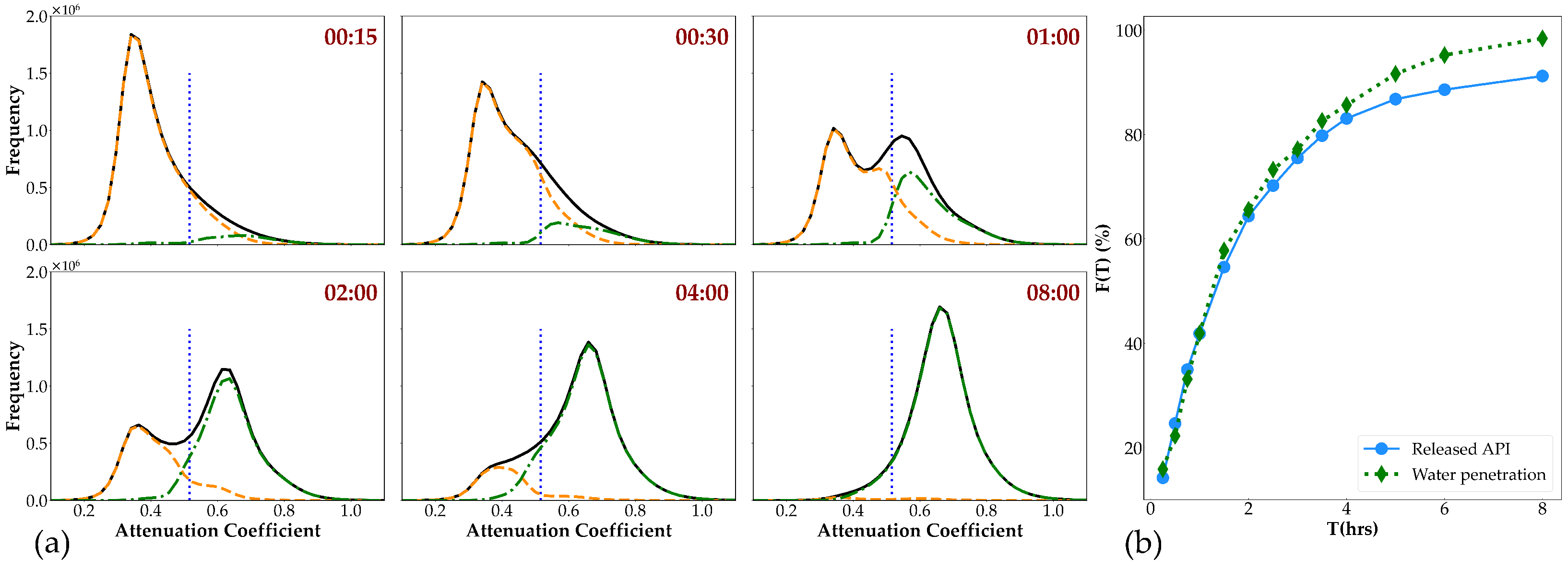
2.5.3. Image Analysis
3. Results and Discussion
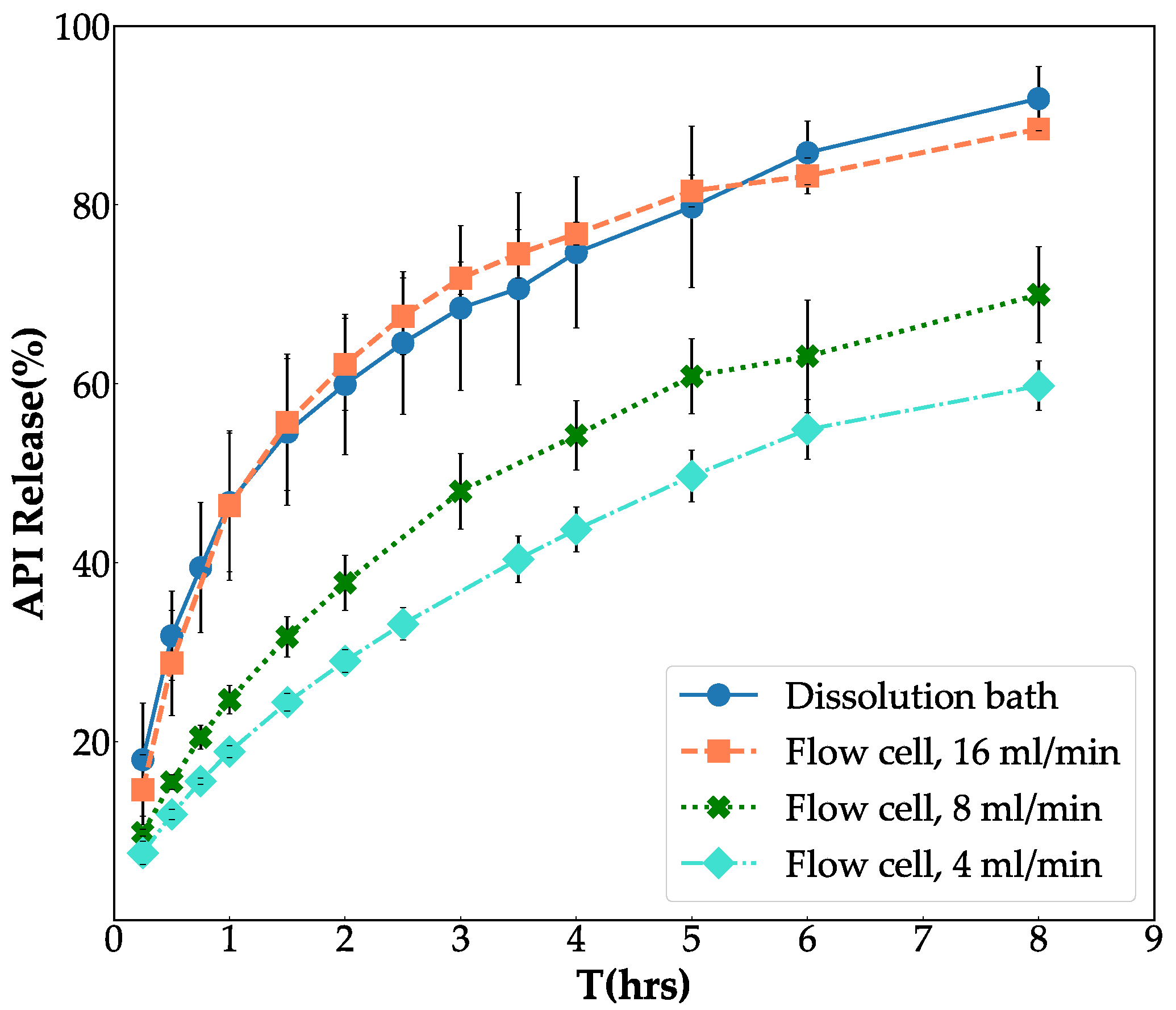
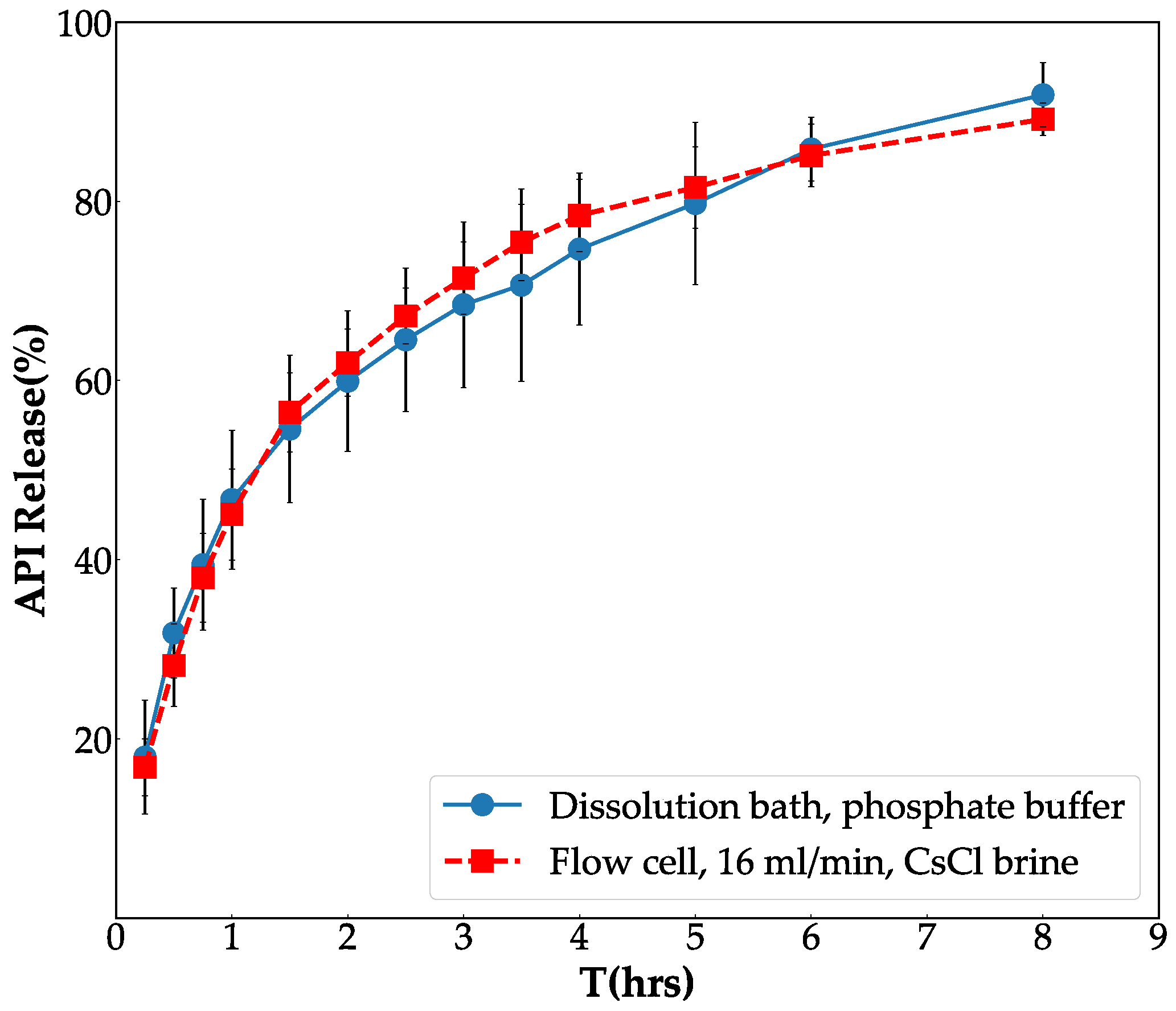
3.1. Fine Tune Flow-Through Cell System
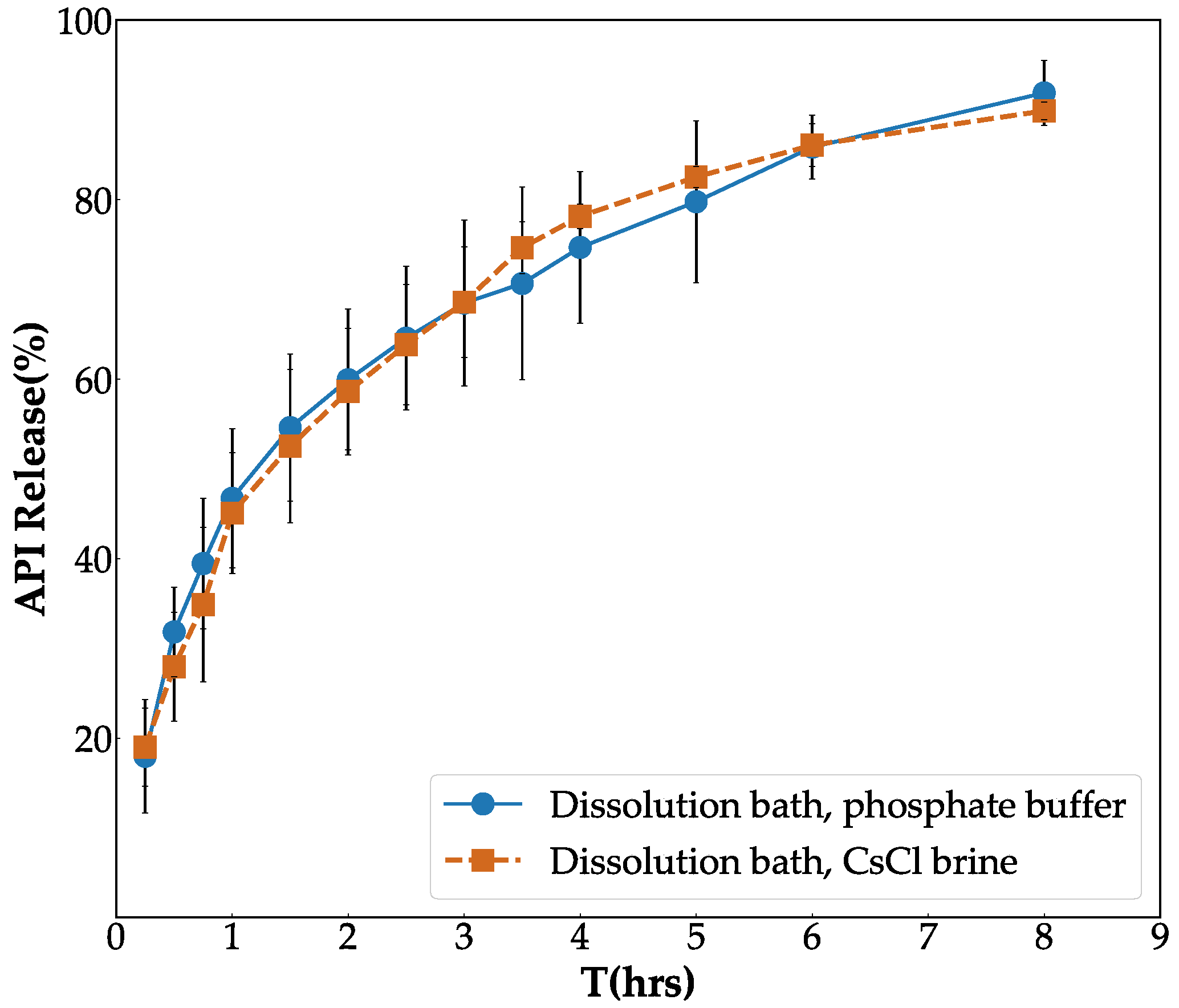
3.2. In Vitro Validation of Contrast Agent
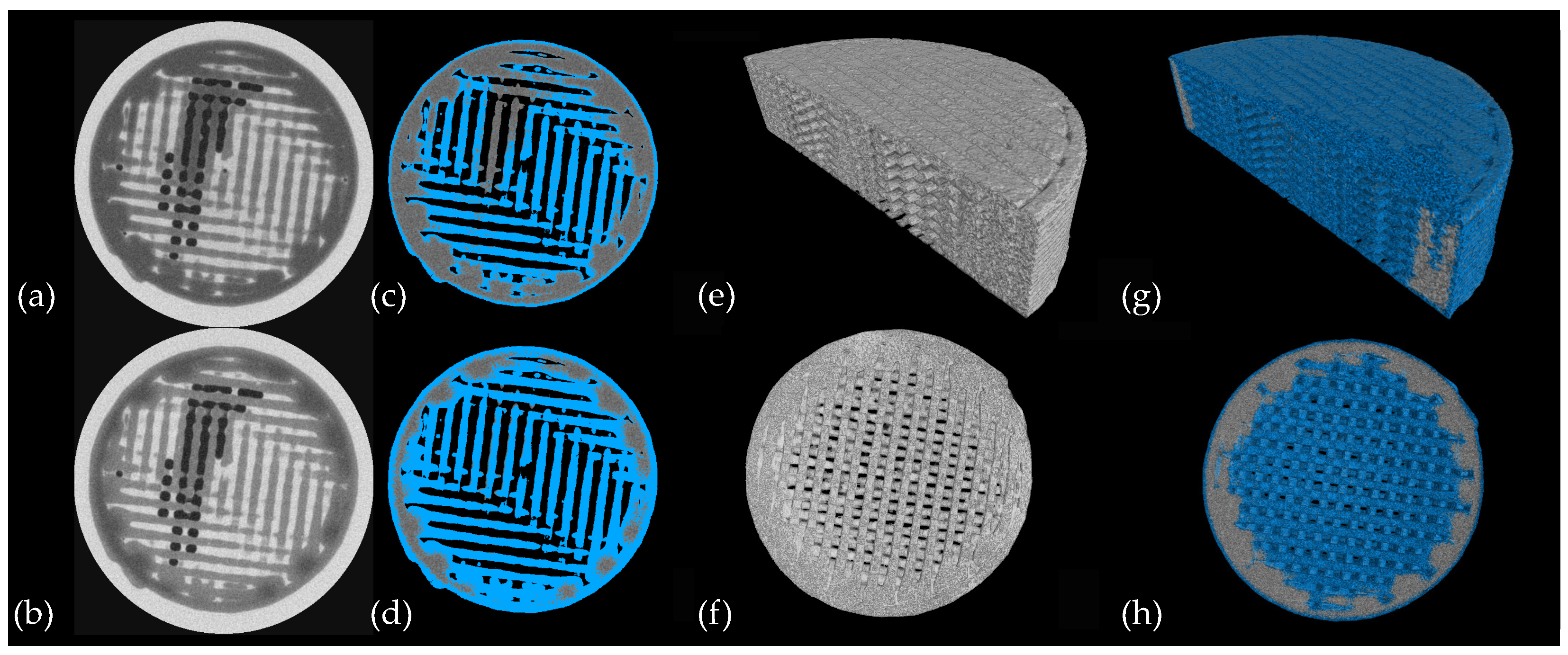
3.3. CT Scanning
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Aulton, M.E.; Taylor, K. Aulton’s Pharmaceutics: The Design and Manufacture of Medicines; Elsevier: Amsterdam, The Netherlands, 2013. [Google Scholar]
- Sever, N.E.; Warman, M.; Mackey, S.; Dziki, W.; Jiang, M. Chapter 35—Process Analytical Technology in Solid Dosage Development and Manufacturing. In Developing Solid Oral Dosage Forms; Qiu, Y., Chen, Y., Zhang, G.G., Liu, L., Porter, W.R., Eds.; Academic Press: San Diego, VA, USA, 2009; pp. 827–841. [Google Scholar] [CrossRef]
- Almeida, A.; Possemiers, S.; Boone, M.; De Beer, T.; Quinten, T.; Van Hoorebeke, L.; Remon, J.; Vervaet, C. Ethylene vinyl acetate as matrix for oral sustained release dosage forms produced via hot-melt extrusion. Eur. J. Pharm. Biopharm. 2011, 77, 297–305. [Google Scholar] [CrossRef] [PubMed]
- Khaled, S.A.; Burley, J.C.; Alexander, M.R.; Yang, J.; Roberts, C.J. 3D printing of five-in-one dose combination polypill with defined immediate and sustained release profiles. J. Control. Release 2015, 217, 308–314. [Google Scholar] [CrossRef] [PubMed]
- Samaro, A.; Janssens, P.; Vanhoorne, V.; Van Renterghem, J.; Eeckhout, M.; Cardon, L.; De Beer, T.; Vervaet, C. Screening of pharmaceutical polymers for extrusion-Based Additive Manufacturing of patient-tailored tablets. Int. J. Pharm. 2020, 586, 119591. [Google Scholar] [CrossRef] [PubMed]
- Uddin, R.; Saffoon, N.; Sutradhar, K.B. Dissolution and dissolution apparatus: A review. Int. J. Curr. Biomed. Pharm. Res. 2011, 1, 201–207. [Google Scholar]
- Markl, D.; Strobel, A.; Schlossnikl, R.; Bøtker, J.; Bawuah, P.; Ridgway, C.; Rantanen, J.; Rades, T.; Gane, P.; Peiponen, K.E.; et al. Characterisation of pore structures of pharmaceutical tablets: A review. Int. J. Pharm. 2018, 538, 188–214. [Google Scholar] [CrossRef] [PubMed]
- Eckhard, S.; Nebelung, M. Investigations of the correlation between granule structure and deformation behavior. Powder Technol. 2011, 206, 79–87. [Google Scholar] [CrossRef]
- Westermarck, S.; Juppo, A.M.; Kervinen, L.; Yliruusi, J. Pore structure and surface area of mannitol powder, granules and tablets determined with mercury porosimetry and nitrogen adsorption. Eur. J. Pharm. Biopharm. 1998, 46, 61–68. [Google Scholar] [CrossRef]
- Sun, Y.; Østergaard, J. Application of UV imaging in formulation development. Pharm. Res. 2017, 34, 929–940. [Google Scholar] [CrossRef]
- Svanbäck, S.; Ehlers, H.; Yliruusi, J. Optical microscopy as a comparative analytical technique for single-particle dissolution studies. Int. J. Pharm. 2014, 469, 10–16. [Google Scholar] [CrossRef]
- Kulinowski, P.; Dorożyński, P.; Młynarczyk, A.; Węglarz, W.P. Magnetic resonance imaging and image analysis for assessment of HPMC matrix tablets structural evolution in USP apparatus 4. Pharm. Res. 2011, 28, 1065–1073. [Google Scholar] [CrossRef]
- Kulinowski, P.; Młynarczyk, A.; Dorożyński, P.; Jasiński, K.; Gruwel, M.L.; Tomanek, B.; Węglarz, W.P. Magnetic resonance microscopy for assessment of morphological changes in hydrating hydroxypropylmethyl cellulose matrix tablets in situ. Pharm. Res. 2012, 29, 3420–3433. [Google Scholar] [CrossRef] [PubMed]
- Kulinowski, P.; Hudy, W.; Mendyk, A.; Juszczyk, E.; Węglarz, W.P.; Jachowicz, R.; Dorożyński, P. The relationship between the evolution of an internal structure and drug dissolution from controlled-release matrix tablets. AAPS Pharm. Sci. Tech. 2016, 17, 735–742. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Gladden, L.; Avalle, P.; Mantle, M. In vitro quantitative 1H and 19F nuclear magnetic resonance spectroscopy and imaging studies of fluvastatin™ in Lescol® XL tablets in a USP-IV dissolution cell. J. Control. Release 2011, 156, 345–354. [Google Scholar] [CrossRef] [PubMed]
- Dorożyński, P.P.; Kulinowski, P.; Młynarczyk, A.; Stanisz, G.J. MRI as a tool for evaluation of oral controlled release dosage forms. Drug Discov. Today 2012, 17, 110–123. [Google Scholar] [CrossRef] [PubMed]
- Kulinowski, P.; Młynarczyk, A.; Jasiński, K.; Talik, P.; Gruwel, M.L.; Tomanek, B.; Węglarz, W.P.; Dorożyński, P. Magnetic resonance microscopy for assessment of morphological changes in hydrating hydroxypropylmethylcellulose matrix tablets in situ–is it possible to detect phenomena related to drug dissolution within the hydrated matrices? Pharm. Res. 2014, 31, 2383–2392. [Google Scholar] [CrossRef]
- Liguori, C.; Frauenfelder, G.; Massaroni, C.; Saccomandi, P.; Giurazza, F.; Pitocco, F.; Marano, R.; Schena, E. Emerging clinical applications of computed tomography. Med. Devices 2015, 8, 265–278. [Google Scholar] [CrossRef]
- Cnudde, V.; Boone, M. High-resolution X-ray computed tomography in geosciences: A review of the current technology and applications. Earth Sci. Rev. 2013, 123, 1–17. [Google Scholar] [CrossRef]
- Maire, E.; Withers, P.J. Quantitative X-ray tomography. Int. Mater. Rev. 2014, 59, 1–43. [Google Scholar] [CrossRef]
- Wei, Q.; Leblon, B.; La Rocque, A. On the use of X-ray computed tomography for determining wood properties: A review. Can. J. For. Res. 2011, 41, 2120–2140. [Google Scholar] [CrossRef]
- Taina, I.; Heck, R.; Elliot, T. Application of X-ray computed tomography to soil science: A literature review. Can. J. Soil. Sci. 2008, 88, 1–19. [Google Scholar] [CrossRef]
- Hopkins, F.F.; Morgan, I.L.; Ellinger, H.D.; Klinksiek, R.V.; Meyer, G.A.; Thompson, J.N. Industrial tomography applications. IEEE Trans. Nucl. Sci. 1981, 28, 1717–1720. [Google Scholar] [CrossRef]
- De Chiffre, L.; Carmignato, S.; Kruth, J.P.; Schmitt, R.; Weckenmann, A. Industrial applications of computed tomography. CIRP Ann. 2014, 63, 655–677. [Google Scholar] [CrossRef]
- Du Plessis, A.; Yadroitsev, I.; Yadroitsava, I.; Le Roux, S.G. X-ray microcomputed tomography in additive manufacturing: A review of the current technology and applications. 3D Print Addit. Manuf. 2018, 5, 227–247. [Google Scholar] [CrossRef]
- Brabant, L.; Vlassenbroeck, J.; De Witte, Y.; Cnudde, V.; Boone, M.N.; Dewanckele, J.; Van Hoorebeke, L. Three-Dimensional Analysis of High-Resolution X-Ray Computed Tomography Data with Morpho. Microsc. Microanal. 2011, 17, 252–263. [Google Scholar] [CrossRef] [PubMed]
- Sinka, I.; Burch, S.; Tweed, J.; Cunningham, J. Measurement of density variations in tablets using X-ray computed tomography. Int. J. Pharm. 2004, 271, 215–224. [Google Scholar] [CrossRef] [PubMed]
- Busignies, V.; Leclerc, B.; Porion, P.; Evesque, P.; Couarraze, G.; Tchoreloff, P. Quantitative measurements of localized density variations in cylindrical tablets using X-ray microtomography. Eur. J. Pharm. Biopharm. 2006, 64, 38–50. [Google Scholar] [CrossRef]
- Wagner-Hattler, L.; Québatte, G.; Keiser, J.; Schoelkopf, J.; Schlepütz, C.M.; Huwyler, J.; Puchkov, M. Study of drug particle distributions within mini-tablets using synchrotron X-ray microtomography and superpixel image clustering. Int. J. Pharm. 2020, 573, 118827. [Google Scholar] [CrossRef]
- Sondej, F.; Bück, A.; Koslowsky, K.; Bachmann, P.; Jacob, M.; Tsotsas, E. Investigation of coating layer morphology by micro-computed X-ray tomography. Powder Technol. 2015, 273, 165–175. [Google Scholar] [CrossRef]
- Ariyasu, A.; Hattori, Y.; Otsuka, M. Non-destructive prediction of enteric coating layer thickness and drug dissolution rate by near-infrared spectroscopy and X-ray computed tomography. Int. J. Pharm. 2017, 525, 282–290. [Google Scholar] [CrossRef]
- Farber, L.; Tardos, G.; Michaels, J.N. Use of X-ray tomography to study the porosity and morphology of granules. Powder Technol. 2003, 132, 57–63. [Google Scholar] [CrossRef]
- Verstraete, G.; Samaro, A.; Grymonpré, W.; Vanhoorne, V.; Van Snick, B.; Boone, M.; Hellemans, T.; Van Hoorebeke, L.; Remon, J.; Vervaet, C. 3D printing of high drug loaded dosage forms using thermoplastic polyurethanes. Int. J. Pharm. 2018, 536, 318–325. [Google Scholar] [CrossRef] [PubMed]
- Traini, D.; Loreti, G.; Jones, A.; Young, P. X-ray computed microtomography for the study of modified release systems. Micros. Anal. 2008, 22, 13–15. [Google Scholar]
- Li, H.; Yin, X.; Ji, J.; Sun, L.; Shao, Q.; York, P.; Xiao, T.; He, Y.; Zhang, J. Microstructural investigation to the controlled release kinetics of monolith osmotic pump tablets via synchrotron radiation X-ray microtomography. Int. J. Pharm. 2012, 427, 270–275. [Google Scholar] [CrossRef] [PubMed]
- Yin, X.; Li, H.; Guo, Z.; Wu, L.; Chen, F.; de Matas, M.; Shao, Q.; Xiao, T.; York, P.; He, Y.; et al. Quantification of swelling and erosion in the controlled release of a poorly water-soluble drug using synchrotron X-ray computed microtomography. AAPS J. 2013, 15, 1025–1034. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, J.M.; Balcão, V.M.; Vila, M.M.D.C.; Aranha, N.; Yoshida, V.M.H.; Chaud, M.V.; Filho, S.M. Deformulation of a solid pharmaceutical form using computed tomography and X-ray fluorescence. J. Phys. Conf. Ser. 2015, 630, 012002. [Google Scholar] [CrossRef]
- Yin, X.; Li, L.; Gu, X.; Wang, H.; Wu, L.; Qin, W.; Xiao, T.; York, P.; Zhang, J.; Mao, S. Dynamic structure model of polyelectrolyte complex based controlled-release matrix tablets visualized by synchrotron radiation micro-computed tomography. Mater. Sci. Eng. C 2020, 116, 111137. [Google Scholar] [CrossRef]
- Samaro, A.; Shaqour, B.; Goudarzi, N.M.; Ghijs, M.; Cardon, L.; Boone, M.N.; Verleije, B.; Beyers, K.; Vanhoorne, V.; Cos, P.; et al. Can filaments, pellets and powder be used as feedstock to produce highly drug-loaded ethylene-vinyl acetate 3D printed tablets using extrusion-based additive manufacturing? Int. J. Pharm. 2021, 607, 120922. [Google Scholar] [CrossRef]
- Gioumouxouzis, C.I.; Katsamenis, O.L.; Bouropoulos, N.; Fatouros, D.G. 3D printed oral solid dosage forms containing hydrochlorothiazide for controlled drug delivery. J. Drug Deliv. Sci. Technol. 2017, 40, 164–171. [Google Scholar] [CrossRef]
- Young, P.M.; Nguyen, K.; Jones, A.S.; Traini, D. Microstructural analysis of porous composite materials: Dynamic imaging of drug dissolution and diffusion through porous matrices. AAPS J. 2008, 10, 560–564. [Google Scholar] [CrossRef][Green Version]
- Bácskay, I.; Ujhelyi, Z.; Fehér, P.; Arany, P. The Evolution of the 3D-Printed Drug Delivery Systems: A Review. Pharmaceutics 2022, 14, 1312. [Google Scholar] [CrossRef]
- Aquino, R.P.; Barile, S.; Grasso, A.; Saviano, M. Envisioning smart and sustainable healthcare: 3D Printing technologies for personalized medication. Futures 2018, 103, 35–50. [Google Scholar] [CrossRef]
- Norman, J.; Madurawe, R.D.; Moore, C.M.; Khan, M.A.; Khairuzzaman, A. A new chapter in pharmaceutical manufacturing: 3D-printed drug products. Adv. Drug Deliv. Rev. 2017, 108, 39–50. [Google Scholar] [CrossRef] [PubMed]
- Macedo, J.; Samaro, A.; Vanhoorne, V.; Vervaet, C.; Pinto, J.F. Processability of poly(vinyl alcohol) Based Filaments With Paracetamol Prepared by Hot-Melt Extrusion for Additive Manufacturing. J. Pharm. Sci. 2020, 109, 3636–3644. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, H.; Teruya, K.; Abe, Y.; Furuishi, T.; Fukuzawa, K.; Yonemochi, E.; Izutsu, K.i. Altered Media Flow and Tablet Position as Factors of How Air Bubbles Affect Dissolution of Disintegrating and Non-disintegrating Tablets Using a USP 4 Flow-Through Cell Apparatus. AAPS PharmSciTech 2021, 22, 1–11. [Google Scholar] [CrossRef]
- Rohrs, B.; Steiner, D. Deaeration techniques for dissolution media. Dissolution Technol. 1995, 2, 7–8. [Google Scholar] [CrossRef]
- Kang, Y.J.; Yeom, E.; Seo, E.; Lee, S.J. Bubble-free and pulse-free fluid delivery into microfluidic devices. Biomicrofluidics 2014, 8, 014102. [Google Scholar] [CrossRef]
- Dierick, M.; Van Loo, D.; Masschaele, B.; Van den Bulcke, J.; Van Acker, J.; Cnudde, V.; Van Hoorebeke, L. Recent micro-CT scanner developments at UGCT. Nucl. Instrum. Methods. Phys. Res. B 2014, 324, 35–40. [Google Scholar] [CrossRef]
- Van Offenwert, S.; Cnudde, V.; Boone, M.; Bultreys, T. Fast micro-computed tomography data of solute transport in porous media with different heterogeneity levels. Sci. Data 2021, 8, 1–8. [Google Scholar] [CrossRef]
- Karakosta, E.; Jenneson, P.M.; Sear, R.P.; McDonald, P.J. Observations of coarsening of air voids in a polymer–highly-soluble crystalline matrix during dissolution. Phys. Rev. E 2006, 74, 011504. [Google Scholar] [CrossRef]
- Vlassenbroeck, J.; Dierick, M.; Masschaele, B.; Cnudde, V.; Van Hoorebeke, L.; Jacobs, P. Software tools for quantification of X-ray microtomography at the UGCT. Nucl. Instrum. Methods. Phys. Res. A 2007, 580, 442–445. [Google Scholar] [CrossRef]
- Krämer, J.; Stippler, E. Experiences with USP apparatus 4 calibration. Dissolution Technol. 2005, 12, 33–39. [Google Scholar] [CrossRef]
- James, G.; Witten, D.; Hastie, T.; Tibshirani, R. An Introduction to Statistical Learning; Springer: New York, NY, USA, 2013; Volume 112. [Google Scholar]
- FDA. Guidance for Industry: Dissolution Testing of Immediate-Release Solid Oral Dosage Forms. Available online: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/dissolution-testing-immediate-release-solid-oral-dosage-forms (accessed on 9 November 2022).



| Scan Setting | High Quality Scans | Fast Scans |
|---|---|---|
| Voxels size (m) | 10.09 | 20.18 |
| Number of projection (-) | 1800 | 600 |
| Number of averages (-) | 3 | 2 |
| Acquisition time (min) | 8 | 2 |
| In vitro Dissolution Experiment | Rate | |
|---|---|---|
| Paddle dissolution method | 100 rpm | reference |
| Flow-through cell method | 16 mL/min | 77% |
| Flow-through cell method | 8 mL/min | 35% |
| Flow-through cell method | 4 mL/min | 28% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moazami Goudarzi, N.; Samaro, A.; Vervaet, C.; Boone, M.N. Development of Flow-Through Cell Dissolution Method for In Situ Visualization of Dissolution Processes in Solid Dosage Forms Using X-ray μCT. Pharmaceutics 2022, 14, 2475. https://doi.org/10.3390/pharmaceutics14112475
Moazami Goudarzi N, Samaro A, Vervaet C, Boone MN. Development of Flow-Through Cell Dissolution Method for In Situ Visualization of Dissolution Processes in Solid Dosage Forms Using X-ray μCT. Pharmaceutics. 2022; 14(11):2475. https://doi.org/10.3390/pharmaceutics14112475
Chicago/Turabian StyleMoazami Goudarzi, Niloofar, Aseel Samaro, Chris Vervaet, and Matthieu N. Boone. 2022. "Development of Flow-Through Cell Dissolution Method for In Situ Visualization of Dissolution Processes in Solid Dosage Forms Using X-ray μCT" Pharmaceutics 14, no. 11: 2475. https://doi.org/10.3390/pharmaceutics14112475
APA StyleMoazami Goudarzi, N., Samaro, A., Vervaet, C., & Boone, M. N. (2022). Development of Flow-Through Cell Dissolution Method for In Situ Visualization of Dissolution Processes in Solid Dosage Forms Using X-ray μCT. Pharmaceutics, 14(11), 2475. https://doi.org/10.3390/pharmaceutics14112475