In Vitro Photodynamic Treatment Modality for A375 Melanoma Cell Line Using a Sulphonated Aluminum Phthalocyanine Chloride-Photosensitizer-Gold Nanoparticle Conjugate
Abstract
1. Introduction
2. Materials and Methods
2.1. Cells and Culture Conditions
2.2. Isolation of Stem Cells
2.3. Isolated A375 Melanoma Cancer Stem-Cell Characterization
2.3.1. CD133 and CD20 Flow Cytometry
2.3.2. CD133 and C20 Immunofluorescence (IF)
2.4. Subcellular Localization of AlPcS4Cl-AuNP in A375 Cells
2.5. Melanoma and Melanoma CSC AlPcS4Cl-AuNP PDT
2.6. Post-Irradiation Analyses
2.6.1. Light Microscopy
2.6.2. A375 CSC Post-Irradiation Lactate Dehydrogenase (LDH) Assay
2.6.3. A375 CSC Post-Irradiation Adenosine Triphosphate (ATP) Assay
2.6.4. Trypan Blue Dye Viability Exclusion Assay for A375 CSC Post-Irradiation
3. Results and Discussion
3.1. Identification and Analysis of A375 CSCs CD133 and CD20
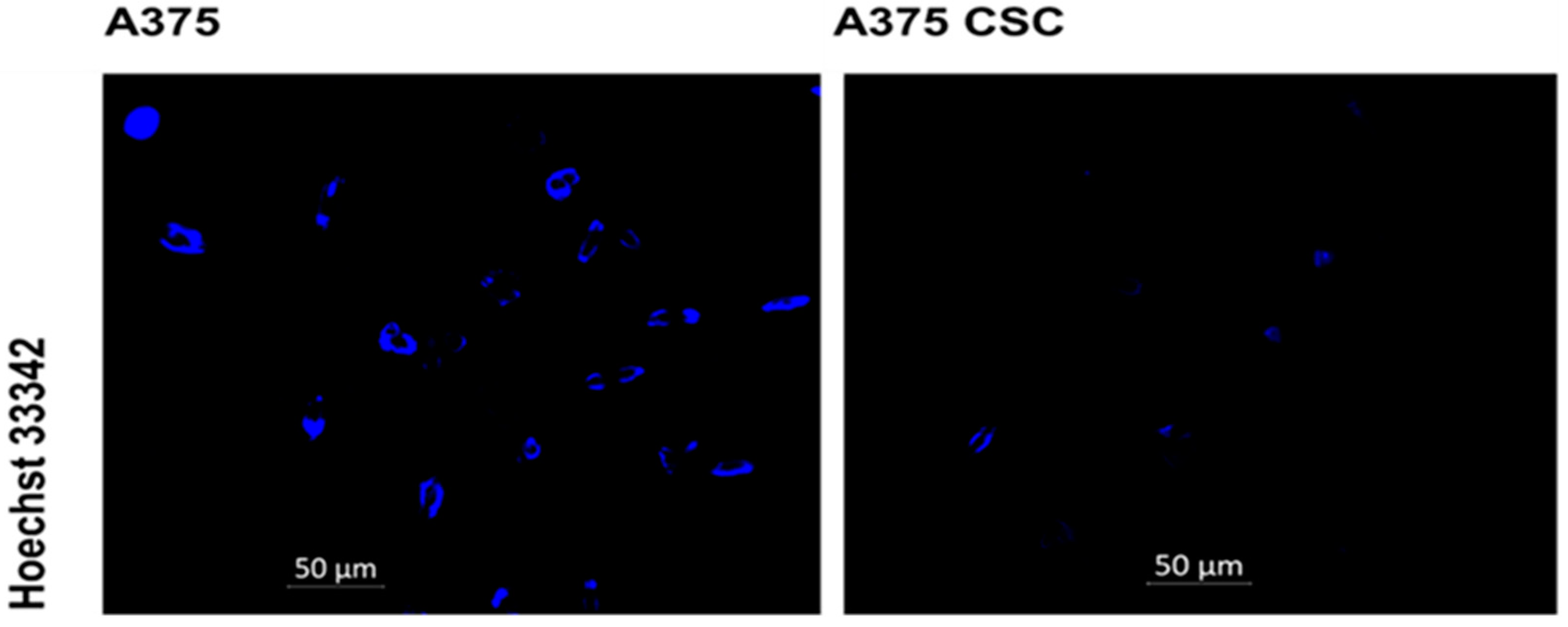
3.2. Hoechst Nuclear Stain
3.3. Subcellular Localization of AlPcS4Cl-AuNP in A375 Cells
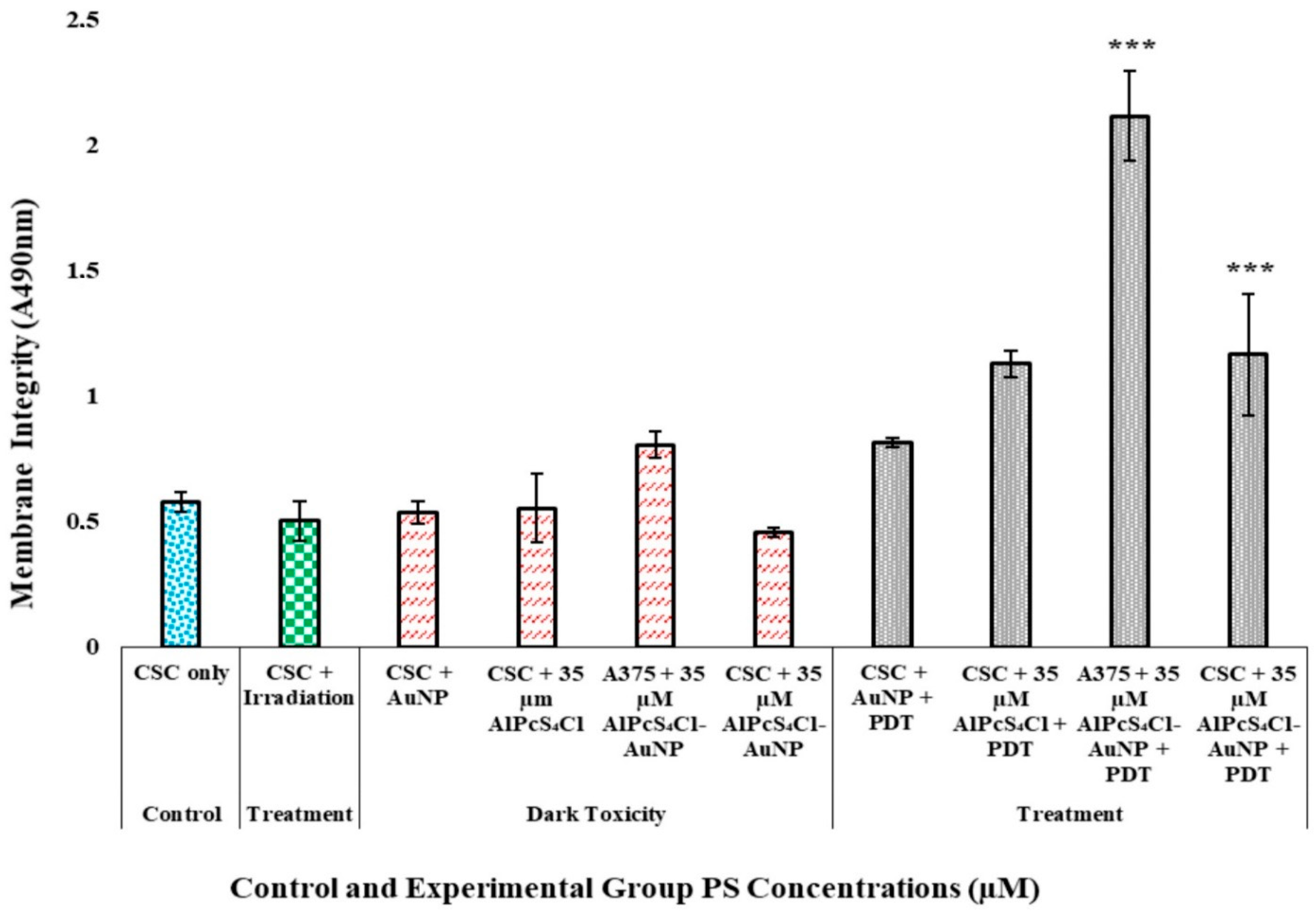
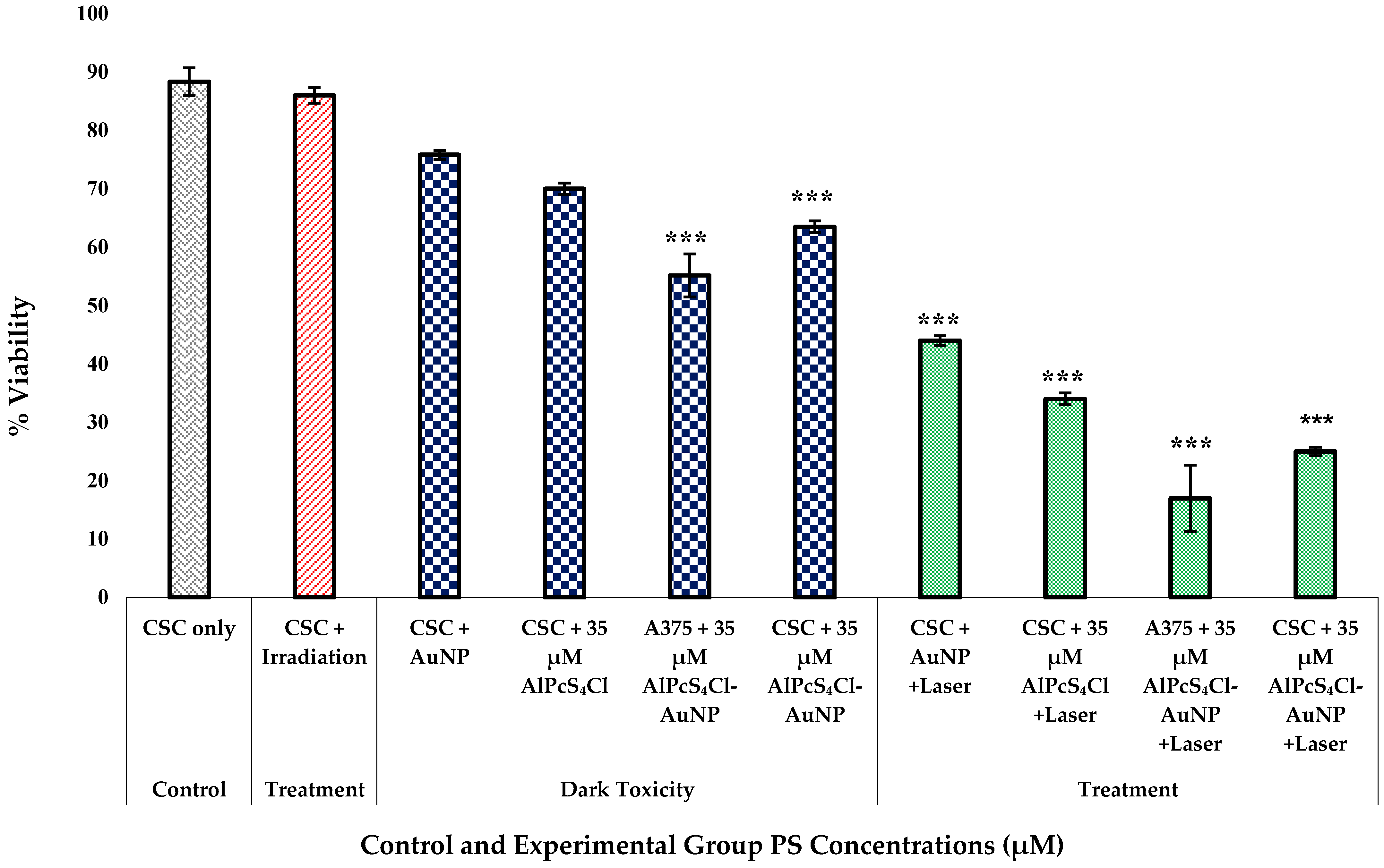
3.4. Post-Irradiation and Biochemical Assay Analysis
3.4.1. A375 CSC Morphology
3.4.2. A375 CSC Post-Irradiation Lactate Dehydrogenase (LDH) Assay
3.4.3. A375 CSC Post-Irradiation Adenosine Triphosphate (ATP) Assay
3.4.4. Trypan Blue Dye Viability Exclusion Assay for A375 CSC Post-Irradiation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nguyen, N.; Couts, K.L.; Luo, Y.; Fujita, M. Understanding Melanoma Stem Cells. Melanoma Manag. 2015, 2, 179–188. [Google Scholar] [CrossRef]
- Shannan, B.; Perego, M.; Somasundaram, R.; Herlyn, M. Heterogeneity in Melanoma. Cancer Treat. Res. 2016, 167, 1–15. [Google Scholar] [CrossRef]
- Takahashi, K.; Yamanaka, S. Induction of Pluripotent Stem Cells from Mouse Embryonic and Adult Fibroblast Cultures by Defined Factors. Cell 2006, 126, 663–676. [Google Scholar] [CrossRef]
- Barroca, V.; Lassalle, B.; Coureuil, M.; Louis, J.P.; Le Page, F.; Testart, J.; Allemand, I.; Riou, L.; Fouchet, P. Mouse Differentiating Spermatogonia Can Generate Germinal Stem Cells in Vivo. Nat. Cell Biol. 2009, 11, 190–196. [Google Scholar] [CrossRef]
- Meacham, C.E.; Morrison, S.J. Tumour Heterogeneity and Cancer Cell Plasticity. Nature 2013, 501, 328–337. [Google Scholar] [CrossRef]
- Chen, W.-J.; Ho, C.-C.; Chang, Y.-L.; Chen, H.-Y.; Lin, C.-A.; Ling, T.-Y.; Yu, S.-L.; Yuan, S.-S.; Chen, Y.-J.L.; Lin, C.-Y.; et al. Cancer-Associated Fibroblasts Regulate the Plasticity of Lung Cancer Stemness via Paracrine Signalling. Nat. Commun. 2014, 5, 3472. [Google Scholar] [CrossRef]
- Lengauer, C.; Kinzler, K.W.; Vogelstein, B. Genetic Instabilities in Human Cancers. Nature 1998, 396, 643–649. [Google Scholar] [CrossRef]
- Naidoo, C.; Kruger, C.A.; Abrahamse, H. Photodynamic Therapy for Metastatic Melanoma Treatment: A Review. Technol. Cancer Res. Treat. 2018, 17, 1533033818791795. [Google Scholar] [CrossRef]
- Kolářová, H.; Nevrelová, P.; Bajgar, R.; Jírová, D.; Kejlová, K.; Strnad, M. In Vitro Photodynamic Therapy on Melanoma Cell Lines with Phthalocyanine. Toxicol. Vitr. 2007, 21, 249–253. [Google Scholar] [CrossRef]
- Ravanat, J.-L.; Douki, T.; Cadet, J. Direct and Indirect Effects of UV Radiation on DNA and Its Components. J. Photochem. Photobiol. B 2001, 63, 88–102. [Google Scholar] [CrossRef]
- Weishaupt, K.R.; Gomer, C.J.; Dougherty, T.J. Identification of Singlet Oxygen as the Cytotoxic Agent in Photoinactivation of a Murine Tumor. Cancer Res. 1976, 36, 2326–2329. [Google Scholar] [PubMed]
- Watanabe, K. Photochemistry on Nanoparticles. In Encyclopedia of Interfacial Chemistry; Wandelt, K., Ed.; Elsevier: Oxford, UK, 2018; pp. 563–572. [Google Scholar] [CrossRef]
- Chen, C.-W.; Chan, Y.-C.; Hsiao, M.; Liu, R.-S. Plasmon-Enhanced Photodynamic Cancer Therapy by Upconversion Nanoparticles Conjugated with Au Nanorods. ACS Appl. Mater. Interfaces 2016, 8, 32108–32119. [Google Scholar] [CrossRef]
- Diaz, L.A., Jr.; Williams, R.T.; Wu, J.; Kinde, I.; Hecht, J.R.; Berlin, J.; Allen, B.; Bozic, I.; Reiter, J.G.; Nowak, M.A.; et al. The Molecular Evolution of Acquired Resistance to Targeted EGFR Blockade in Colorectal Cancers. Nature 2012, 486, 537–540. [Google Scholar] [CrossRef] [PubMed]
- Baldea, I.; Filip, A.G. Photodynamic Therapy in Melanoma—An Update. J. Physiol. Pharmacol. 2012, 63, 109–118. [Google Scholar] [PubMed]
- Hong, E.J.; Choi, D.G.; Shim, M.S. Targeted and Effective Photodynamic Therapy for Cancer Using Functionalized Nanomaterials. Acta Pharm. Sin. B 2016, 6, 297–307. [Google Scholar] [CrossRef] [PubMed]
- Jamal, S.M.E.; Alamodi, A.; Wahl, R.U.; Grada, Z.; Shareef, M.A.; Hassan, S.-Y.; Murad, F.; Hassan, S.-L.; Santourlidis, S.; Gomez, C.R.; et al. Melanoma Stem Cell Maintenance and Chemo-Resistance Are Mediated by CD133 Signal to PI3K-Dependent Pathways. Oncogene 2020, 39, 5468–5478. [Google Scholar] [CrossRef]
- Yin, A.H.; Miraglia, S.; Zanjani, E.D.; Almeida-Porada, G.; Ogawa, M.; Leary, A.G.; Olweus, J.; Kearney, J.; Buck, D.W. AC133, a Novel Marker for Human Hematopoietic Stem and Progenitor Cells. Blood 1997, 90, 5002–5012. [Google Scholar] [CrossRef]
- Monzani, E.; Facchetti, F.; Galmozzi, E.; Corsini, E.; Benetti, A.; Cavazzin, C.; Gritti, A.; Piccinini, A.; Porro, D.; Santinami, M.; et al. Melanoma Contains CD133 and ABCG2 Positive Cells with Enhanced Tumourigenic Potential. Eur. J. Cancer 2007, 43, 935–946. [Google Scholar] [CrossRef]
- Fang, D.; Nguyen, T.K.; Leishear, K.; Finko, R.; Kulp, A.N.; Hotz, S.; Van Belle, P.A.; Xu, X.; Elder, D.E.; Herlyn, M. A Tumorigenic Subpopulation with Stem Cell Properties in Melanomas. Cancer Res. 2005, 65, 9328–9337. [Google Scholar] [CrossRef]
- Telbisz, Á.; Hegedüs, C.; Özvegy-Laczka, C.; Goda, K.; Várady, G.; Takáts, Z.; Szabó, E.; Sorrentino, B.P.; Váradi, A.; Sarkadi, B. Antibody Binding Shift Assay for Rapid Screening of Drug Interactions with the Human ABCG2 Multidrug Transporter. Eur. J. Pharm. Sci. 2012, 45, 101–109. [Google Scholar] [CrossRef]
- Ozvegy-Laczka, C.; Várady, G.; Köblös, G.; Ujhelly, O.; Cervenak, J.; Schuetz, J.D.; Sorrentino, B.P.; Koomen, G.-J.; Váradi, A.; Német, K.; et al. Function-Dependent Conformational Changes of the ABCG2 Multidrug Transporter Modify Its Interaction with a Monoclonal Antibody on the Cell Surface. J. Biol. Chem. 2005, 280, 4219–4227. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.J.; Aszalos, A. Multidrug Transporters as Drug Targets. Current Drug Targets 2006, 7, 911–921. [Google Scholar] [CrossRef] [PubMed]
- Castelli, V.; Giordano, A.; Benedetti, E.; Giansanti, F.; Quintiliani, M.; Cimini, A.; d’Angelo, M. The Great Escape: The Power of Cancer Stem Cells to Evade Programmed Cell Death. Cancers 2021, 13, 328. [Google Scholar] [CrossRef] [PubMed]
- Tang, M.; Hu, X.; Wang, Y.; Yao, X.; Zhang, W.; Yu, C.; Cheng, F.; Li, J.; Fang, Q. Ivermectin, a Potential Anticancer Drug Derived from an Antiparasitic Drug. Pharmacol. Res. 2021, 163, 105207. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Liu, H.; Yang, H.; Chen, W.; Wu, J.; Feng, X.; Tong, R.; Yu, H.; Chen, Y.; Lv, Z.; et al. Combinatorial Photochemotherapy on Liver Cancer Stem Cells with Organoplatinum(II) Metallacage-Based Nanoparticles. J. Mater. Chem. B 2019, 7, 6476–6487. [Google Scholar] [CrossRef] [PubMed]
- Eid, S.; Boutary, S.; Braych, K.; Sabra, R.; Massaad, C.; Hamdy, A.; Rashid, A.; Moodad, S.; Block, K.; Gorin, Y.; et al. MTORC2 Signaling Regulates Nox4-Induced Podocyte Depletion in Diabetes. Antioxid. Redox Signal. 2016, 25, 703–719. [Google Scholar] [CrossRef]
- Mahalingam, S.M.; Ordaz, J.D.; Low, P.S. Targeting of a Photosensitizer to the Mitochondrion Enhances the Potency of Photodynamic Therapy. ACS Omega 2018, 3, 6066–6074. [Google Scholar] [CrossRef]
- Wang, H.-W.; Zhang, Y.; Tan, P.-P.; Jia, L.-S.; Chen, Y.; Zhou, B.-H. Mitochondrial Respiratory Chain Dysfunction Mediated by ROS Is a Primary Point of Fluoride-Induced Damage in Hepa1-6 Cells. Environ. Pollut. 2019, 255 Pt 3, 113359. [Google Scholar] [CrossRef]
- Sekhejane, P.R.; Houreld, N.N.; Abrahamse, H. Multiorganelle Localization of Metallated Phthalocyanine Photosensitizer in Colorectal Cancer Cells (DLD-1 and CaCo-2) Enhances Efficacy of Photodynamic Therapy. Int. J. Photoenergy 2014, 2014, 383027. [Google Scholar] [CrossRef]
- Emert-Sedlak, L.; Shangary, S.; Rabinovitz, A.; Miranda, M.B.; Delach, S.M.; Johnson, D.E. Involvement of Cathepsin D in Chemotherapy-Induced Cytochrome c Release, Caspase Activation, and Cell Death. Mol. Cancer Ther. 2005, 4, 733–742. [Google Scholar] [CrossRef]
- Marchal, S.; François, A.; Dumas, D.; Guillemin, F.; Bezdetnaya, L. Relationship between Subcellular Localisation of Foscan® and Caspase Activation in Photosensitised MCF-7 Cells. Br. J. Cancer 2007, 96, 944–951. [Google Scholar] [CrossRef] [PubMed]
- Alkilany, A.M.; Murphy, C.J. Toxicity and Cellular Uptake of Gold Nanoparticles: What We Have Learned so Far? J. Nanopart. Res. 2010, 12, 2313–2333. [Google Scholar] [CrossRef] [PubMed]
- Calavia, P.G.; Bruce, G.; Pérez-García, L.; Russell, D.A. Photosensitiser-Gold Nanoparticle Conjugates for Photodynamic Therapy of Cancer. Photochem. Photobiol. Sci. 2018, 17, 1534–1552. [Google Scholar] [CrossRef] [PubMed]
- Marquis, B.J.; Love, S.A.; Braun, K.L.; Haynes, C.L. Analytical Methods to Assess Nanoparticle Toxicity. Analyst 2009, 134, 425–439. [Google Scholar] [CrossRef] [PubMed]
- El-Hussein, A.; Mfouo-Tynga, I.; Abdel-Harith, M.; Abrahamse, H. Comparative Study between the Photodynamic Ability of Gold and Silver Nanoparticles in Mediating Cell Death in Breast and Lung Cancer Cell Lines. J. Photochem. Photobiol. B 2015, 153, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Yi, L.; Wenhui, M.; Jing, W. Theranostics of Gold Nanoparticles with an Emphasis on Photoacoustic Imaging and Photothermal Therapy. Curr. Pharm. Des. 2018, 24, 2719–2728. [Google Scholar]
- Ndhundhuma, I.M.; Abrahamse, H. Susceptibility of In Vitro Melanoma Skin Cancer to Photoactivated Hypericin versus Aluminium(III) Phthalocyanine Chloride Tetrasulphonate. BioMed Res. Int. 2017, 2017, 5407012. [Google Scholar] [CrossRef]
- Lebeau, P.; Chen, J.; Byun, J.; Platko, K.; Austin, R. The Trypan Blue Cellular Debris Assay: A Novel Low-Cost Method for the Rapid Quantification of Cell Death. MethodsX 2019, 6, 1174–1180. [Google Scholar] [CrossRef]
- Simbulan-Rosenthal, C.M.; Dougherty, R.; Vakili, S.; Ferraro, A.M.; Kuo, L.-W.; Alobaidi, R.; Aljehane, L.; Gaur, A.; Sykora, P.; Glasgow, E.; et al. CRISPR-Cas9 Knockdown and Induced Expression of CD133 Reveal Essential Roles in Melanoma Invasion and Metastasis. Cancers 2019, 11, 1490. [Google Scholar] [CrossRef]
- Simbulan-Rosenthal, C.M.; Gaur, A.; Zhou, H.; AbdusSamad, M.; Qin, Q.; Dougherty, R.; Aljehane, L.; Kuo, L.-W.; Vakili, S.; Karna, K.; et al. CD133 Is Associated with Increased Melanoma Cell Survival after Multikinase Inhibition. J. Oncol. 2019, 2019, e6486173. [Google Scholar] [CrossRef]
- El-Khattouti, A.; Sheehan, N.T.; Monico, J.; Drummond, H.A.; Haikel, Y.; Brodell, R.T.; Megahed, M.; Hassan, M. CD133 + Melanoma Subpopulation Acquired Resistance to Caffeic Acid Phenethyl Ester-Induced Apoptosis Is Attributed to the Elevated Expression of ABCB5: Significance for Melanoma Treatment. Cancer Lett. 2015, 357, 83–104. [Google Scholar] [CrossRef] [PubMed]
- El-Khattouti, A.; Selimović, D.; Haïkel, Y.; Megahed, M.; Gomez, C.R.; Hassan, M. Identification and Analysis of CD133(+) Melanoma Stem-like Cells Conferring Resistance to Taxol: An Insight into the Mechanisms of Their Resistance and Response. Cancer Lett. 2014, 343, 123–133. [Google Scholar] [CrossRef] [PubMed]






| Groups (n = 6) |
|---|
| 1. CSCs Only |
| 2. CSCs + Laser |
| 3. CSCs + AuNPs |
| 4. CSCs + 35 µm AlPcS4Cl |
| 5. A375 Cells + 35 µM AlPcS4Cl-AuNP |
| 6. CSCs + 35 µM AlPcS4Cl-AuNP |
| 7. CSCs + AuNPs + Laser |
| 8. CSCs + 35 µM AlPcS4Cl + Laser |
| 9. A375 Cells + 35 µM AlPcS4Cl-AuNP + Laser |
| 10. CSCs + 35 µM AlPcS4Cl-AuNP + Laser |
| Name | Parameter |
|---|---|
| Laser Type | Semiconductor (Diode) |
| Laser Average Output | 75 mW |
| Wavelength | 673.2 nm |
| Wave Emission | Continuous Wave (CW) |
| Spectrum | Red (Visible) |
| Radiant Exposure | 5 J/cm2 |
| Photosensitizer (PS) | AlPcS4Cl-AuNP |
| PS Concentrations | 35 µM (IC50 Standardized Dose) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mkhobongo, B.; Chandran, R.; Abrahamse, H. In Vitro Photodynamic Treatment Modality for A375 Melanoma Cell Line Using a Sulphonated Aluminum Phthalocyanine Chloride-Photosensitizer-Gold Nanoparticle Conjugate. Pharmaceutics 2022, 14, 2474. https://doi.org/10.3390/pharmaceutics14112474
Mkhobongo B, Chandran R, Abrahamse H. In Vitro Photodynamic Treatment Modality for A375 Melanoma Cell Line Using a Sulphonated Aluminum Phthalocyanine Chloride-Photosensitizer-Gold Nanoparticle Conjugate. Pharmaceutics. 2022; 14(11):2474. https://doi.org/10.3390/pharmaceutics14112474
Chicago/Turabian StyleMkhobongo, Bridgette, Rahul Chandran, and Heidi Abrahamse. 2022. "In Vitro Photodynamic Treatment Modality for A375 Melanoma Cell Line Using a Sulphonated Aluminum Phthalocyanine Chloride-Photosensitizer-Gold Nanoparticle Conjugate" Pharmaceutics 14, no. 11: 2474. https://doi.org/10.3390/pharmaceutics14112474
APA StyleMkhobongo, B., Chandran, R., & Abrahamse, H. (2022). In Vitro Photodynamic Treatment Modality for A375 Melanoma Cell Line Using a Sulphonated Aluminum Phthalocyanine Chloride-Photosensitizer-Gold Nanoparticle Conjugate. Pharmaceutics, 14(11), 2474. https://doi.org/10.3390/pharmaceutics14112474








