Assisted Extraction with Cyclodextrins as a Way of Improving the Antidiabetic Activity of Actinidia Leaves
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Reagents and Chemicals
2.3. Extract Preparation
2.3.1. Primary Extract from the Raw Material
2.3.2. Extraction Assisted by Cyclodextrins
2.4. Determination of Total Phenolic Content (TPC)
2.5. Chromatographic Determination
2.6. Antioxidant Action
2.6.1. 2,2-Diphenyl-1-picrylhydrazyl (DPPH) Assay
- A0—the absorbance of the control;
- A1—the absorbance of the sample.
2.6.2. Ferric-Reducing Antioxidant Power Assay (FRAP) Assay
2.7. Inhibition of In Vitro Activity of Enzymes
2.7.1. Inhibition of α-Glucosidase
- AC—the absorbance of the control (100% enzyme activity);
- AS—the absorbance of the tested sample (leaves extract or acarbose).
2.7.2. Inhibition of α-Amylase
- AC—the absorbance of the control (100% enzyme activity);
- AS—the absorbance of the tested sample (leaves extract or acarbose).
2.8. Statistical Analysis
3. Results and Discussion
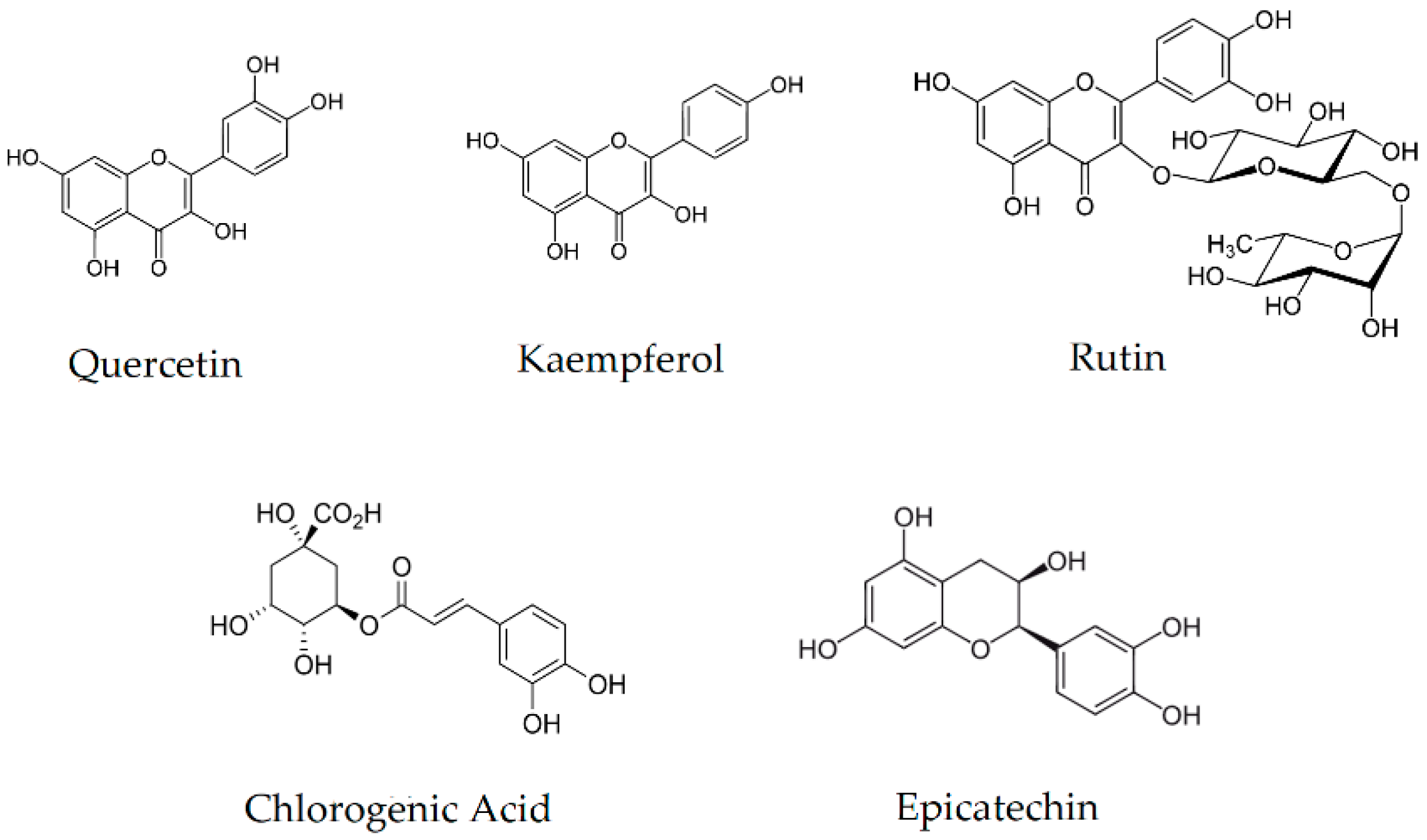
3.1. Analysis of the Content of Active Compounds
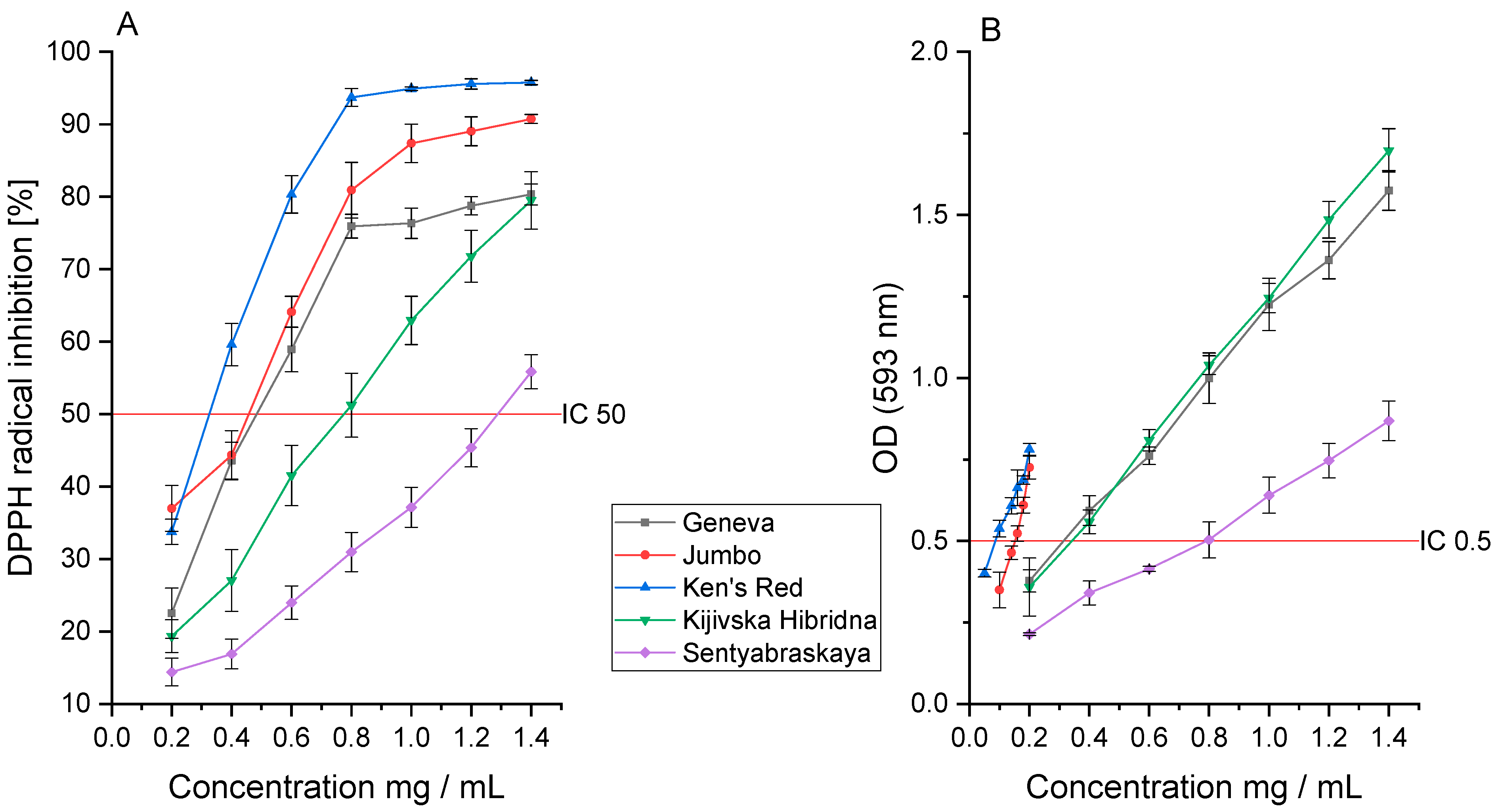
3.2. Antioxidant Activity
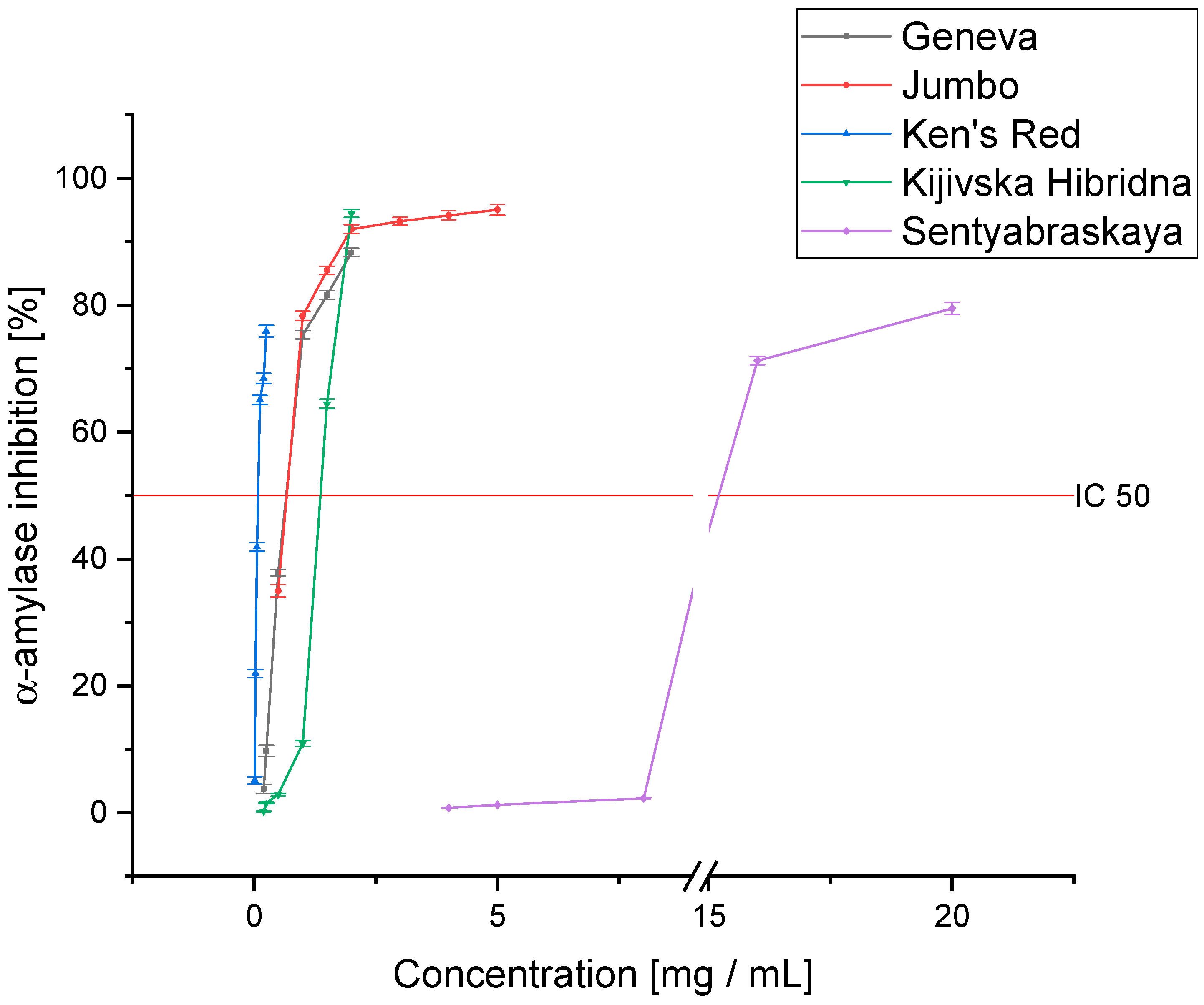
3.3. Antidiabetic Activity
3.4. Cyxdoxetrins Assisted Extraction
3.5. Analysis of the Content of Active Compounds in Cyxdoxetrin Assisted Extraction
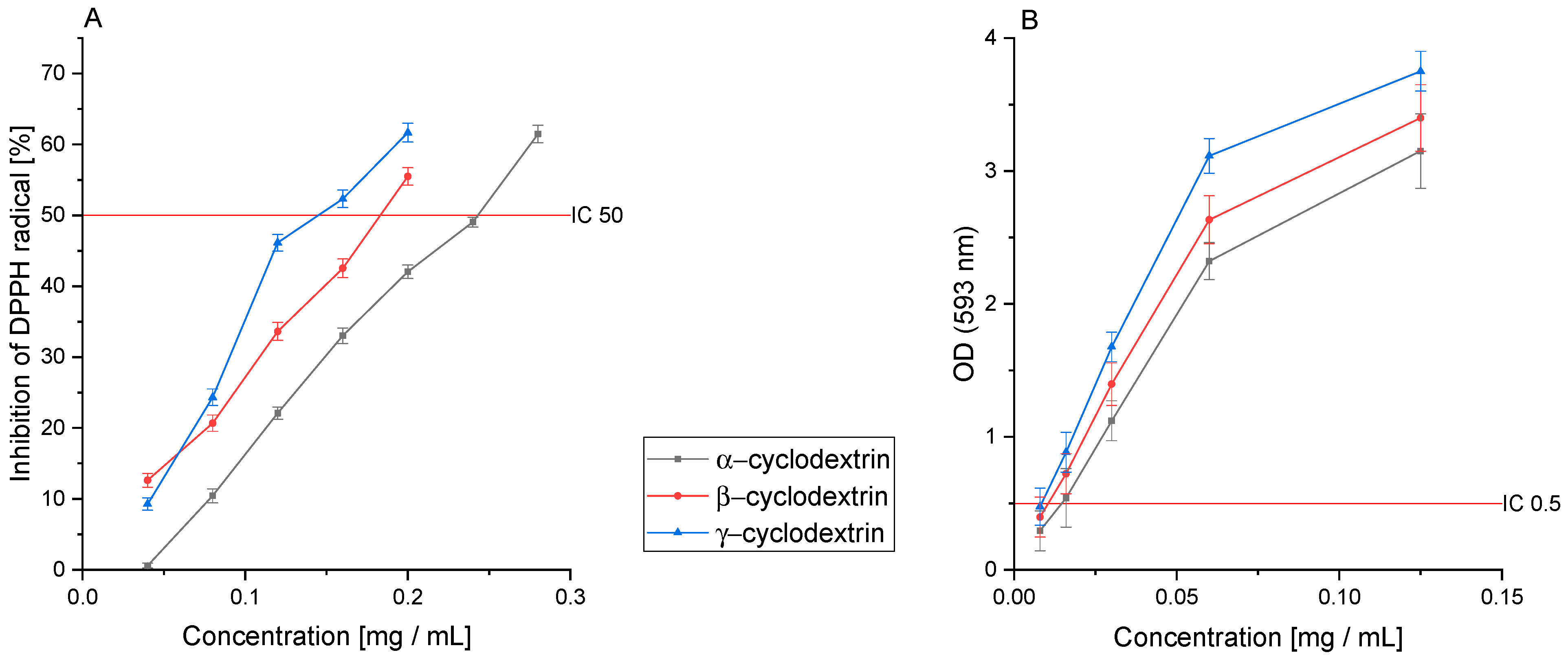
3.6. Antioxidant Activity of CD Extracts
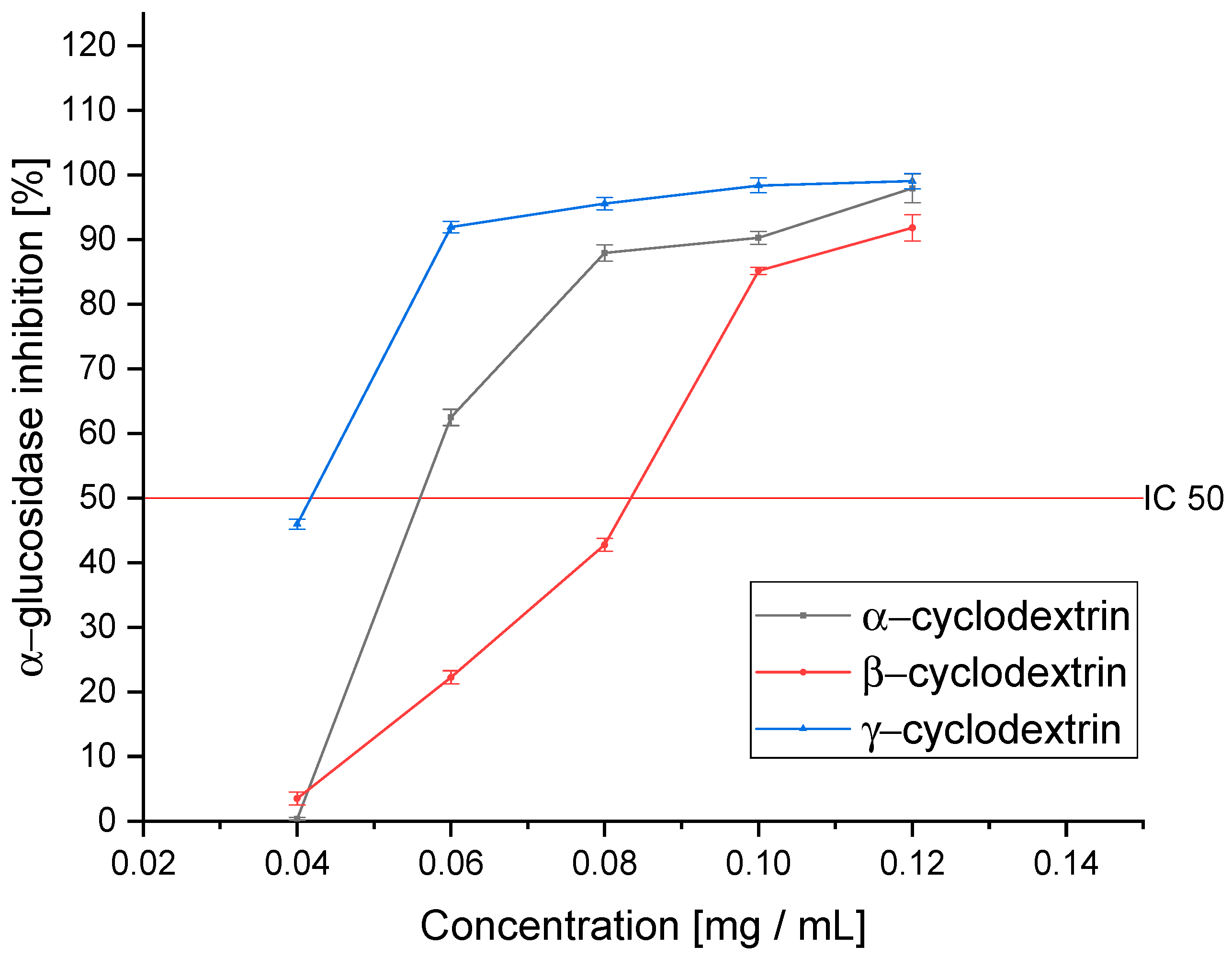
3.7. Antidiabetic Activity of CD Extracts
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Popkin, B.M.; Adair, L.S.; Ng, S.W. NOW AND THEN: The Global Nutrition Transition: The Pandemic of Obesity in Developing Countries. Nutr. Rev. 2012, 70, 3–21. [Google Scholar] [CrossRef] [PubMed]
- Kopp, W. How Western Diet And Lifestyle Drive The Pandemic Of Obesity And Civilization Diseases. Diabetes Metab. Syndr. Obes. 2019, 12, 2221–2236. [Google Scholar] [CrossRef] [PubMed]
- Salehi, B.; Ata, A.; Anil Kumar, N.; Sharopov, F.; Ramírez-Alarcón, K.; Ruiz-Ortega, A.; Abdulmajid Ayatollahi, S.; Tsouh Fokou, P.V.; Kobarfard, F.; Amiruddin Zakaria, Z.; et al. Antidiabetic Potential of Medicinal Plants and Their Active Components. Biomolecules 2019, 9, E551. [Google Scholar] [CrossRef]
- Bors, W.; Michel, C. Chemistry of the Antioxidant Effect of Polyphenols. Ann. New York Acad. Sci. 2002, 957, 57–69. [Google Scholar] [CrossRef]
- Rajendiran, D.; Packirisamy, S.; Gunasekaran, K. A Review on Role of Antioxidants in Diabetes. Asian J. Pharm. Clin. Res. 2018, 11, 48–53. [Google Scholar] [CrossRef]
- Baranowska-Wójcik, E.; Szwajgier, D. Characteristics and Pro-Health Properties of Mini Kiwi (Actinidia Arguta). Hortic.Environ. Biotechnol. 2019, 60, 217–225. [Google Scholar] [CrossRef]
- Latocha, P. The Nutritional and Health Benefits of Kiwiberry (Actinidia Arguta)–a Review. Plant Foods Hum. Nutr. 2017, 72, 325–334. [Google Scholar] [CrossRef]
- Pinto, D.; Delerue-Matos, C.; Rodrigues, F. Bioactivity, Phytochemical Profile and pro-Healthy Properties of Actinidia Arguta: A Review. Food Res. Int. 2020, 136, 109449. [Google Scholar] [CrossRef] [PubMed]
- Hong, Z.; Lu, Y.; Ran, C.; Tang, P.; Huang, J.; Yang, Y.; Duan, X.; Wu, H. The Bioactive Ingredients in Actinidia Chinensis Planch. Inhibit Liver Cancer by Inducing Apoptosis. J. Ethnopharmacol. 2021, 281, 114553. [Google Scholar] [CrossRef]
- Yoo, S.K.; Kang, J.Y.; Lee, U.; Park, S.K.; Kim, J.M.; Han, H.J.; Kim, D.O.; Heo, H.J. Improving Effect of Actinidia Arguta Leaf on Hyperglycemia-Induced Cognitive Dysfunction. J. Funct. Foods 2021, 76, 104315. [Google Scholar] [CrossRef]
- SHIROSAKI, M.; KOYAMA, T.; YAZAWA, K. Anti-Hyperglycemic Activity of Kiwifruit Leaf (Actinidia Deliciosa) in Mice. Biosci. Biotechnol. Biochem. 2008, 72, 1099–1102. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Gao, N.; Shu, C.; Cheng, S.; Sun, X.; Liu, C.; Xin, G.; Li, B.; Tian, J. Phenolics Profile and Antioxidant Activity Analysis of Kiwi Berry (Actinidia Arguta) Flesh and Peel Extracts From Four Regions in China. Front. Plant Sci. 2021, 12, 689038. [Google Scholar] [CrossRef] [PubMed]
- Almeida, D.; Pinto, D.; Santos, J.; Vinha, A.F.; Palmeira, J.; Ferreira, H.N.; Rodrigues, F.; Oliveira, M.B.P.P. Hardy Kiwifruit Leaves (Actinidia Arguta): An Extraordinary Source of Value-Added Compounds for Food Industry. Food Chem. 2018, 259, 113–121. [Google Scholar] [CrossRef]
- Paczkowska-Walendowska, M.; Gościniak, A.; Szymanowska, D.; Szwajgier, D.; Baranowska-Wójcik, E.; Szulc, P.; Dreczka, D.; Simon, M.; Cielecka-Piontek, J. Blackberry Leaves as New Functional Food? Screening Antioxidant, Anti-Inflammatory and Microbiological Activities in Correlation with Phytochemical Analysis. Antioxidants 2021, 10, 1945. [Google Scholar] [CrossRef] [PubMed]
- Insawang, S.; Pripdeevech, P.; Tanapichatsakul, C.; Khruengsai, S.; Monggoot, S.; Nakham, T.; Artrod, A.; D’Souza, P.E.; Panuwet, P. Essential Oil Compositions and Antibacterial and Antioxidant Activities of Five Lavandula Stoechas Cultivars Grown in Thailand. Chem. Biodivers. 2019, 16, e1900371. [Google Scholar] [CrossRef] [PubMed]
- Sip, S.; Szymanowska, D.; Chanaj-Kaczmarek, J.; Skalicka-Woźniak, K.; Budzyńska, B.; Wronikowska-Denysiuk, O.; Słowik, T.; Szulc, P.; Cielecka-Piontek, J. Potential for Prebiotic Stabilized Cornus Mas L. Lyophilized Extract in the Prophylaxis of Diabetes Mellitus in Streptozotocin Diabetic Rats. Antioxidants 2022, 11, 380. [Google Scholar] [CrossRef] [PubMed]
- Diamanti, A.C.; Igoumenidis, P.E.; Mourtzinos, I.; Yannakopoulou, K.; Karathanos, V.T. Green Extraction of Polyphenols from Whole Pomegranate Fruit Using Cyclodextrins. Food Chem. 2017, 214, 61–66. [Google Scholar] [CrossRef] [PubMed]
- Paczkowska-Walendowska, M.; Szymańska, E.; Winnicka, K.; Szwajgier, D.; Baranowska-Wójcik, E.; Ruchała, M.A.; Simon, M.; Cielecka-Piontek, J. Cyclodextrin as Functional Carrier in Development of Mucoadhesive Tablets Containing Polygoni Cuspidati Extract with Potential for Dental Applications. Pharmaceutics 2021, 13, 1916. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, H.; Xu, Z.; Wu, M.; Xia, W.; Zhang, W. Chimonanthus Praecox Extract/Cyclodextrin Inclusion Complexes: Selective Inclusion, Enhancement of Antioxidant Activity and Thermal Stability. Ind. Crops Prod. 2017, 95, 60–65. [Google Scholar] [CrossRef]
- Blainski, A.; Lopes, G.C.; De Mello, J.C.P. Application and Analysis of the Folin Ciocalteu Method for the Determination of the Total Phenolic Content from Limonium Brasiliense L. Molecules 2013, 18, 6852–6865. [Google Scholar] [CrossRef]
- Studzińska-Sroka, E.; Piotrowska, H.; Kucińska, M.; Murias, M.; Bylka, W. Cytotoxic Activity of Physodic Acid and Acetone Extract from Hypogymnia Physodes against Breast Cancer Cell Lines. Pharm. Biol. 2016, 54, 2480–2485. [Google Scholar] [CrossRef] [PubMed]
- Tiveron, A.P.; Melo, P.S.; Bergamaschi, K.B.; Vieira, T.M.F.S.; Regitano-d’Arce, M.A.B.; Alencar, S.M. Antioxidant Activity of Brazilian Vegetables and Its Relation with Phenolic Composition. Int. J. Mol. Sci. 2012, 13, 8943–8957. [Google Scholar] [CrossRef] [PubMed]
- Telagari, M.; Hullatti, K. In-Vitro α-Amylase and α-Glucosidase Inhibitory Activity of Adiantum Caudatum Linn. and Celosia Argentea Linn. Extracts and Fractions. Indian J. Pharmacol. 2015, 47, 425–429. [Google Scholar] [CrossRef] [PubMed]
- Khromykh, N.O.; Lykholat, Y.V.; Didur, O.O.; Sklyar, T.V.; Davydov, V.R.; Lavrentievа, K.V.; Lykholat, T.Y. Phytochemical Profiles, Antioxidant and Antimicrobial Activity of Actinidia Polygama and A. Arguta Fruits and Leaves. Biosyst. Divers. 2022, 30, 39–45. [Google Scholar] [CrossRef]
- Young, I.S.; Woodside, J.V. Antioxidants in Health and Disease. J. Clin. Pathol. 2001, 54, 176–186. [Google Scholar] [CrossRef]
- Kumar, S.; Narwal, S.; Kumar, V.; Prakash, O. α-Glucosidase Inhibitors from Plants: A Natural Approach to Treat Diabetes. Pharmacogn. Rev. 2011, 5, 19–29. [Google Scholar] [CrossRef]
- Oboh, G.; Isaac, A.T.; Akinyemi, A.J.; Ajani, R.A. Inhibition of Key Enzymes Linked to Type 2 Diabetes and Sodium Nitroprusside Induced Lipid Peroxidation in Rats’ Pancreas by Phenolic Extracts of Avocado Pear Leaves and Fruit. Int. J. Biomed. Sci. 2014, 10, 208–216. [Google Scholar]
- Ghorbani, A. Mechanisms of Antidiabetic Effects of Flavonoid Rutin. Biomed. Pharmacother. 2017, 96, 305–312. [Google Scholar] [CrossRef]
- Abdulkhaleq, L.A.; Assi, M.A.; Noor, M.H.M.; Abdullah, R.; Saad, M.Z.; Taufiq-Yap, Y.H. Therapeutic Uses of Epicatechin in Diabetes and Cancer. Vet. World 2017, 10, 869–872. [Google Scholar] [CrossRef]
- Yan, Y.; Zhou, X.; Guo, K.; Zhou, F.; Yang, H. Use of Chlorogenic Acid against Diabetes Mellitus and Its Complications. J. Immunol. Res. 2020, 2020, 9680508. [Google Scholar] [CrossRef]
- Onal, S.; Timur, S.; Okutucu, B.; Zihnioğlu, F. Inhibition of Alpha-Glucosidase by Aqueous Extracts of Some Potent Antidiabetic Medicinal Herbs. Prep. Biochem. Biotechnol. 2005, 35, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Dhanya, R. Quercetin for Managing Type 2 Diabetes and Its Complications, an Insight into Multitarget Therapy. Biomed. Pharmacother. 2022, 146, 112560. [Google Scholar] [CrossRef] [PubMed]
- Alkhalidy, H.; Moore, W.; Wang, Y.; Luo, J.; McMillan, R.P.; Zhen, W.; Zhou, K.; Liu, D. The Flavonoid Kaempferol Ameliorates Streptozotocin-Induced Diabetes by Suppressing Hepatic Glucose Production. Molecules 2018, 23, 2338. [Google Scholar] [CrossRef] [PubMed]
- Sorriento, D.; De Luca, N.; Trimarco, B.; Iaccarino, G. The Antioxidant Therapy: New Insights in the Treatment of Hypertension. Front. Physiol. 2018, 9, 258. [Google Scholar] [CrossRef] [PubMed]
- Alam, W.; Khan, H.; Shah, M.A.; Cauli, O.; Saso, L. Kaempferol as a Dietary Anti-Inflammatory Agent: Current Therapeutic Standing. Molecules 2020, 25, 4073. [Google Scholar] [CrossRef]
- Lesjak, M.; Beara, I.; Simin, N.; Pintać, D.; Majkić, T.; Bekvalac, K.; Orčić, D.; Mimica-Dukić, N. Antioxidant and Anti-Inflammatory Activities of Quercetin and Its Derivatives. J. Funct. Foods 2018, 40, 68–75. [Google Scholar] [CrossRef]
- Asmat, U.; Abad, K.; Ismail, K. Diabetes Mellitus and Oxidative Stress—A Concise Review. Saudi Pharm. J. 2016, 24, 547–553. [Google Scholar] [CrossRef]
- Ucan, O.; Ovayolu, N. Relationship between Diabetes Mellitus, Hypertension and Obesity, and Health-Related Quality of Life in Gaziantep, a Central South-Eastern City in Turkey: The Quality of Life in Diabetes, Hypertension and Obesity. J. Clin. Nurs. 2010, 19, 2511–2519. [Google Scholar] [CrossRef]
- Kadir, D.H. Statistical Evaluation of Main Extraction Parameters in Twenty Plant Extracts for Obtaining Their Optimum Total Phenolic Content and Its Relation to Antioxidant and Antibacterial Activities. Food Sci. Nutr. 2021, 9, 3491–3499. [Google Scholar] [CrossRef]
- Hmamou, A.; Eloutassi, N.; Alshawwa, S.Z.; Al kamaly, O.; Kara, M.; Bendaoud, A.; El-Assri, E.-M.; Tlemcani, S.; El Khomsi, M.; Lahkimi, A. Total Phenolic Content and Antioxidant and Antimicrobial Activities of Papaver Rhoeas L. Organ Extracts Growing in Taounate Region, Morocco. Molecules 2022, 27, 854. [Google Scholar] [CrossRef]
- Xiong, Y.; Ng, K.; Zhang, P.; Warner, R.D.; Shen, S.; Tang, H.-Y.; Liang, Z.; Fang, Z. In Vitro α-Glucosidase and α-Amylase Inhibitory Activities of Free and Bound Phenolic Extracts from the Bran and Kernel Fractions of Five Sorghum Grain Genotypes. Foods 2020, 9, 1301. [Google Scholar] [CrossRef] [PubMed]
- Giacco, F.; Brownlee, M. Oxidative Stress and Diabetic Complications. Circ. Res. 2010, 107, 1058–1070. [Google Scholar] [CrossRef] [PubMed]
- Pratt, D.E. Natural Antioxidants from Plant Material. In Phenolic Compounds in Food and Their Effects on Health II; ACS Symposium Series; American Chemical Society: Washington, DC, USA, 1992; Volume 507, pp. 54–71. ISBN 978-0-8412-2476-6. [Google Scholar]
- Eastwood, M.A. Interaction of Dietary Antioxidants in Vivo: How Fruit and Vegetables Prevent Disease? QJM: Int. J. Med. 1999, 92, 527–530. [Google Scholar] [CrossRef] [PubMed]
- Ferber, S.G.; Namdar, D.; Hen-Shoval, D.; Eger, G.; Koltai, H.; Shoval, G.; Shbiro, L.; Weller, A. The “Entourage Effect”: Terpenes Coupled with Cannabinoids for the Treatment of Mood Disorders and Anxiety Disorders. Curr. Neuropharmacol. 2020, 18, 87–96. [Google Scholar] [CrossRef]
- Rasoanaivo, P.; Wright, C.W.; Willcox, M.L.; Gilbert, B. Whole Plant Extracts versus Single Compounds for the Treatment of Malaria: Synergy and Positive Interactions. Malar. J. 2011, 10, S4. [Google Scholar] [CrossRef]
- Tundis, R.; Loizzo, M.R.; Menichini, F. Natural Products as α-Amylase and α-Glucosidase Inhibitors and Their Hypoglycaemic Potential in the Treatment of Diabetes: An Update. Mini Rev. Med. Chem. 2010, 10, 315–331. [Google Scholar] [CrossRef]
- Si, M.; Lou, J.; Zhou, C.-X.; Shen, J.-N.; Wu, H.-H.; Yang, B.; He, Q.-J.; Wu, H.-S. Insulin Releasing and Alpha-Glucosidase Inhibitory Activity of Ethyl Acetate Fraction of Acorus Calamus in Vitro and in Vivo. J. Ethnopharmacol. 2010, 128, 154–159. [Google Scholar] [CrossRef]
- Lee, J.; Sowndhararajan, K.; Kim, M.; Kim, J.; Kim, D.; Kim, S.; Kim, G.-Y.; Kim, S.; Jhoo, J.-W. Antioxidant, Inhibition of α-Glucosidase and Suppression of Nitric Oxide Production in LPS-Induced Murine Macrophages by Different Fractions of Actinidia Arguta Stem. Saudi J. Biol. Sci. 2014, 21, 532–538. [Google Scholar] [CrossRef]
- Wang, K.; Li, M.; Han, Q.; Fu, R.; Ni, Y. Inhibition of α-Amylase Activity by Insoluble and Soluble Dietary Fibers from Kiwifruit (Actinidia Deliciosa). Food Biosci. 2021, 42, 101057. [Google Scholar] [CrossRef]
- Sudha, P.; Zinjarde, S.S.; Bhargava, S.Y.; Kumar, A.R. Potent α-Amylase Inhibitory Activity of Indian Ayurvedic Medicinal Plants. BMC Complement. Med. Ther. 2011, 11, 5. [Google Scholar] [CrossRef]
- Schreck, K.; Melzig, M.F. Traditionally Used Plants in the Treatment of Diabetes Mellitus: Screening for Uptake Inhibition of Glucose and Fructose in the Caco2-Cell Model. Front. Pharmacol. 2021, 12, 692566. [Google Scholar] [CrossRef] [PubMed]
- Kato, E. Bioactive Compounds in Plant Materials for the Prevention of Diabetesand Obesity. Biosci. Biotechnol. Biochem. 2019, 83, 975–985. [Google Scholar] [CrossRef] [PubMed]
- Fraga, C.G. Plant Polyphenols: How to Translate Their in Vitro Antioxidant Actions to in Vivo Conditions. IUBMB Life 2007, 59, 308–315. [Google Scholar] [CrossRef]
- Kim, J.K.; Park, S.U. Chlorogenic Acid and Its Role in Biological Functions: An up to Date. EXCLI J. 2019, 18, 310–316. [Google Scholar] [CrossRef]
- Yang, D.; Wang, T.; Long, M.; Li, P. Quercetin: Its Main Pharmacological Activity and Potential Application in Clinical Medicine. Oxidative Med. Cell. Longev. 2020, 2020, e8825387. [Google Scholar] [CrossRef] [PubMed]
- Loftsson, T.; Brewster, M.E. Pharmaceutical Applications of Cyclodextrins: Basic Science and Product Development. J. Pharm. Pharmacol. 2010, 62, 1607–1621. [Google Scholar] [CrossRef]
- Fenyvesi, F.; Nguyen, T.L.P.; Haimhoffer, Á.; Rusznyák, Á.; Vasvári, G.; Bácskay, I.; Vecsernyés, M.; Ignat, S.-R.; Dinescu, S.; Costache, M.; et al. Cyclodextrin Complexation Improves the Solubility and Caco-2 Permeability of Chrysin. Materials 2020, 13, 3618. [Google Scholar] [CrossRef]
- Tutunchi, P.; Roufegarinejad, L.; Hamishehkar, H.; Alizadeh, A. Extraction of Red Beet Extract with β-Cyclodextrin-Enhanced Ultrasound Assisted Extraction: A Strategy for Enhancing the Extraction Efficacy of Bioactive Compounds and Their Stability in Food Models. Food Chem. 2019, 297, 124994. [Google Scholar] [CrossRef]
- El Darra, N.; Rajha, H.N.; Debs, E.; Saleh, F.; El-Ghazzawi, I.; Louka, N.; Maroun, R.G. Comparative Study between Ethanolic and β-Cyclodextrin Assisted Extraction of Polyphenols from Peach Pomace. Int. J. Food Sci. 2018, 2018, 9491681. [Google Scholar] [CrossRef]
- Lakka, A.; Lalas, S.; Makris, D.P. Hydroxypropyl-β-Cyclodextrin as a Green Co-Solvent in the Aqueous Extraction of Polyphenols from Waste Orange Peels. Beverages 2020, 6, 50. [Google Scholar] [CrossRef]
- Cid-Samamed, A.; Rakmai, J.; Mejuto, J.C.; Simal-Gandara, J.; Astray, G. Cyclodextrins Inclusion Complex: Preparation Methods, Analytical Techniques and Food Industry Applications. Food Chem. 2022, 384, 132467. [Google Scholar] [CrossRef] [PubMed]
- Poulson, B.G.; Alsulami, Q.A.; Sharfalddin, A.; El Agammy, E.F.; Mouffouk, F.; Emwas, A.-H.; Jaremko, L.; Jaremko, M. Cyclodextrins: Structural, Chemical, and Physical Properties, and Applications. Polysaccharides 2022, 3, 1–31. [Google Scholar] [CrossRef]
- Saokham, P.; Muankaew, C.; Jansook, P.; Loftsson, T. Solubility of Cyclodextrins and Drug/Cyclodextrin Complexes. Molecules 2018, 23, 1161. [Google Scholar] [CrossRef] [PubMed]
- Brewster, M.E.; Loftsson, T. Cyclodextrins as Pharmaceutical Solubilizers. Adv. Drug Deliv. Rev. 2007, 59, 645–666. [Google Scholar] [CrossRef]
- Kim, Y.; Keogh, J.B.; Clifton, P.M. Polyphenols and Glycemic Control. Nutrients 2016, 8, E17. [Google Scholar] [CrossRef]
- Lim, J.; Kim, D.K.; Shin, H.; Hamaker, B.R.; Lee, B.-H. Different Inhibition Properties of Catechins on the Individual Subunits of Mucosal α-Glucosidases as Measured by Partially-Purified Rat Intestinal Extract. Food Funct. 2019, 10, 4407–4413. [Google Scholar] [CrossRef]


| Geneva [mg/g DM **] | Jumbo [mg/g DM **] | Ken’s Red [mg/g DM **] | Kijivska Hibridna [mg/g DM **] | Sentyabraskaya [mg/g DM **] | |
|---|---|---|---|---|---|
| TPC | 21.06 ± 0.11 a | 34.09 ± 0.15 b | 44.23 ± 0.21 c | 24.08 ± 0.18 d | 13.34 ± 0.09 e |
| Quercetin | 0.055 ± 0.002 a | 0.072 ± 0.004 b | 0.169 ± 0.010 c | 0.135 ± 0.009 d | 2.144 ± 0.026 e |
| Kaempferol | 0.135 ± 0.005 a | 0.122 ± 0.007 b | 0.086 ± 0.009 c | 0.124 ± 0.009 b | 0.373 ± 0.024 d |
| Rutin | 0.828 ± 0.009 a | 1.912 ± 0.027 b | 2.447 ± 0.054 c | 1.639 ± 0.027 d | 1.514 ± 0.022 e |
| Chlorogenic Acid | 0.066 ± 0.007 a | 1.553 ± 0.015 b | 2.558 ± 0.043 c | 0.383 ± 0.033 d | n/d *** |
| Epicatechin | 4.469 ± 0.025 a | 5.353 ± 0.041 b | 8.179 ± 0.067 c | 7.150 ± 0.061 d | 4.689 ± 0.037 e |
| Antioxidant Activity [mg DM **/mL] | Ability to Inhibit Enzymes [mg DM **/mL] | |||
|---|---|---|---|---|
| Variety | DPPH IC50 | FRAP IC0.5 | α-glucosidase IC50 | α-amylase IC50 |
| Geneva | 0.497 ± 0.091 a | 0.320 ± 0.009 a | 0.187 ± 0.015 a | 0.692 ± 0.021 a |
| Jumbo | 0.413 ± 0.077 a | 0.149 ± 0.012 b | 0.132 ± 0.011 b | 0.638 ± 0.033 a |
| Ken’s Red | 0.332 ± 0.048 b | 0.064 ± 0.005 c | 0.098 ± 0.007 c | 0.083 ± 0.004 b |
| Kijivska Hibridna | 0.780 ± 0.067 c | 0.333 ± 0.023 a | 0.155 ± 0.014 b | 1.800 ± 0.066 c |
| Sentyabraskaya | 1.314 ± 0.098 d | 0.753 ± 0.015 d | 0.826 ± 0.045 d | 14.264 ± 0.098 d |
| Reference substance | Trolox | Acarbose | ||
| 0.092 ± 0.007 | 0.041 ± 0.004 | 1.251 ± 0.071 | 0.218 ± 0.015 | |
| Ken’s Red [mg/g DM **] | α–CD [mg/g DM **] | β-CD [mg/g DM **] | γ-CD [mg/g DM **] | |
|---|---|---|---|---|
| TPC | 41.23 ± 0.21a | 93.39 ± 0.28 b | 82.22 ± 0.17 c | 114.05 ± 0.04 d |
| Quercetin | 0.169 ± 0.010a | 0.642 ± 0.016 b | 0.264 ± 0.015 c | 0.718 ± 0.021 d |
| Kaempferol | 0.086 ± 0.009a | 0.162 ± 0.011 b | 7.682 ± 0.033 c | 0.283 ± 0.025 d |
| Rutin | 2.447 ± 0.054a | 8.328 ± 0.065 b | 7.831 ± 0.027 c | 8.133 ± 0.018 d |
| Chlorogenic Acid | 2.558 ± 0.043a | 8.091 ± 0.048 b | 23.353 ± 0.031 c | 7.718 ± 0.037 d |
| Epicatechin | 8.179 ± 0.067a | 3.077 ± 0.010 b | 23.358 ± 0.078 c | 21.76 ± 0.064 d |
| Antioxidant Activity [mg DM **/mL] | Ability to Inhibit Enzymes [mg DM **/mL] | |||
|---|---|---|---|---|
| Variety | DPPH IC50 | FRAP IC0.5 | α-glucosidase IC50 | α-amylase IC50 |
| Ken’s Red | 0.332 ± 0.048a | 0.064 ± 0.005a | 0.098 ± 0.007a | 0.083 ± 0.004a |
| α–CD | 0.240 ± 0.031 b | 0.012 ± 0.001 b | 0.059 ± 0.005 b | 0.030 ± 0.003 b |
| β-CD | 0.180 ± 0.018 c | 0.010 ± 0.001 bc | 0.084 ± 0.007 c | 0.008 ± 0.002 c |
| γ–CD | 0.160 ± 0.019 c | 0.008 ± 0.001 c | 0.040 ± 0.002 d | 0.012 ± 0.003 c |
| Reference substance | Trolox | Acarbose | ||
| 0.092 ± 0.007 | 0.041 ± 0.004 | 1.251 ± 0.071 | 0.218 ± 0.015 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sip, S.; Gościniak, A.; Szulc, P.; Walkowiak, J.; Cielecka-Piontek, J. Assisted Extraction with Cyclodextrins as a Way of Improving the Antidiabetic Activity of Actinidia Leaves. Pharmaceutics 2022, 14, 2473. https://doi.org/10.3390/pharmaceutics14112473
Sip S, Gościniak A, Szulc P, Walkowiak J, Cielecka-Piontek J. Assisted Extraction with Cyclodextrins as a Way of Improving the Antidiabetic Activity of Actinidia Leaves. Pharmaceutics. 2022; 14(11):2473. https://doi.org/10.3390/pharmaceutics14112473
Chicago/Turabian StyleSip, Szymon, Anna Gościniak, Piotr Szulc, Jarosław Walkowiak, and Judyta Cielecka-Piontek. 2022. "Assisted Extraction with Cyclodextrins as a Way of Improving the Antidiabetic Activity of Actinidia Leaves" Pharmaceutics 14, no. 11: 2473. https://doi.org/10.3390/pharmaceutics14112473
APA StyleSip, S., Gościniak, A., Szulc, P., Walkowiak, J., & Cielecka-Piontek, J. (2022). Assisted Extraction with Cyclodextrins as a Way of Improving the Antidiabetic Activity of Actinidia Leaves. Pharmaceutics, 14(11), 2473. https://doi.org/10.3390/pharmaceutics14112473