Self−Assembling Anchorage of Hyaluronic Acid on the Nanoparticle Surface Confers Superiority of Triple Negative Breast Cancer Treatment
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis and Characterization of CS-g-PN
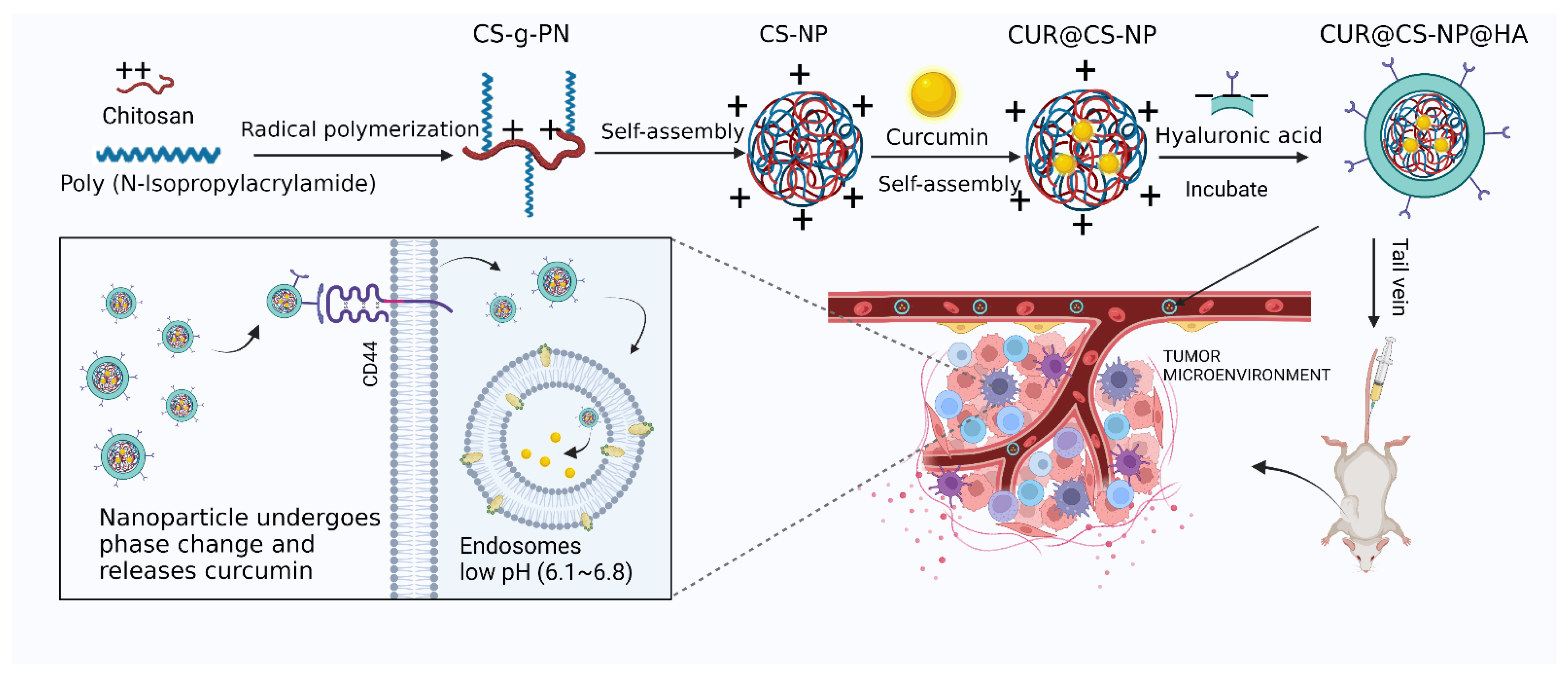
2.3. Preparation of CUR@CS-NP@HA
2.4. Determinations of Drug Encapsulation Efficiency and Drug Loading Capacity
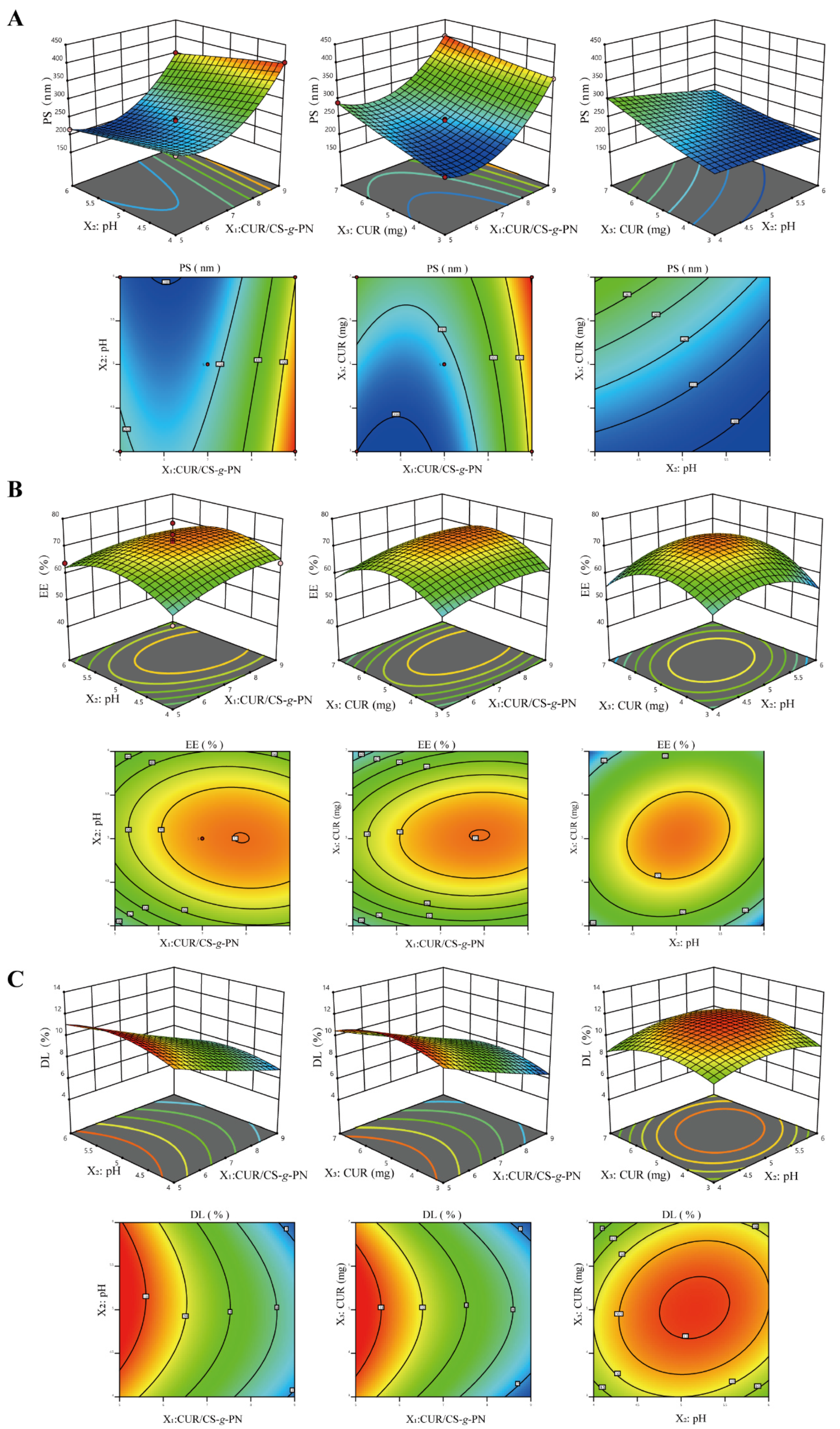
2.5. Formulation Optimization of CUR@CS-NP
2.6. In Vitro Drug Release Test
2.7. In Vitro Cytotoxicity Assay
2.8. Cellular Uptake Study
2.9. Cell Migration Assay
2.10. In Vivo Study
2.10.1. Pharmacokinetic Study
2.10.2. Evaluation of Antitumor Activity
2.10.3. Quantitative Polymerase Chain Reaction (qPCR)
2.10.4. Blood Routine Analysis
2.11. Statistical Analysis
3. Results and Discussion
3.1. Characterization of CS-g-PN
3.2. Optimization and Validation
Y2 = 71.53 + 2.19X1 + 0.55X2 + 0.32X3 − 0.95X1X2 + 2.65X1X3 + 2.65X2X3 − 2.46X12 − 6.11X22 − 8.39X32
Y3 = 9.52 − 2.01X1 + 0.09X2 + 0.03X3 − 0.22X1X2 + 0.04X1X3 + 0.32X2X3 + 0.12X12 − 0.88X22 − 1.11X32
3.3. Characteristics of CUR@CS-NP
3.4. Antitumor Activity In Vitro
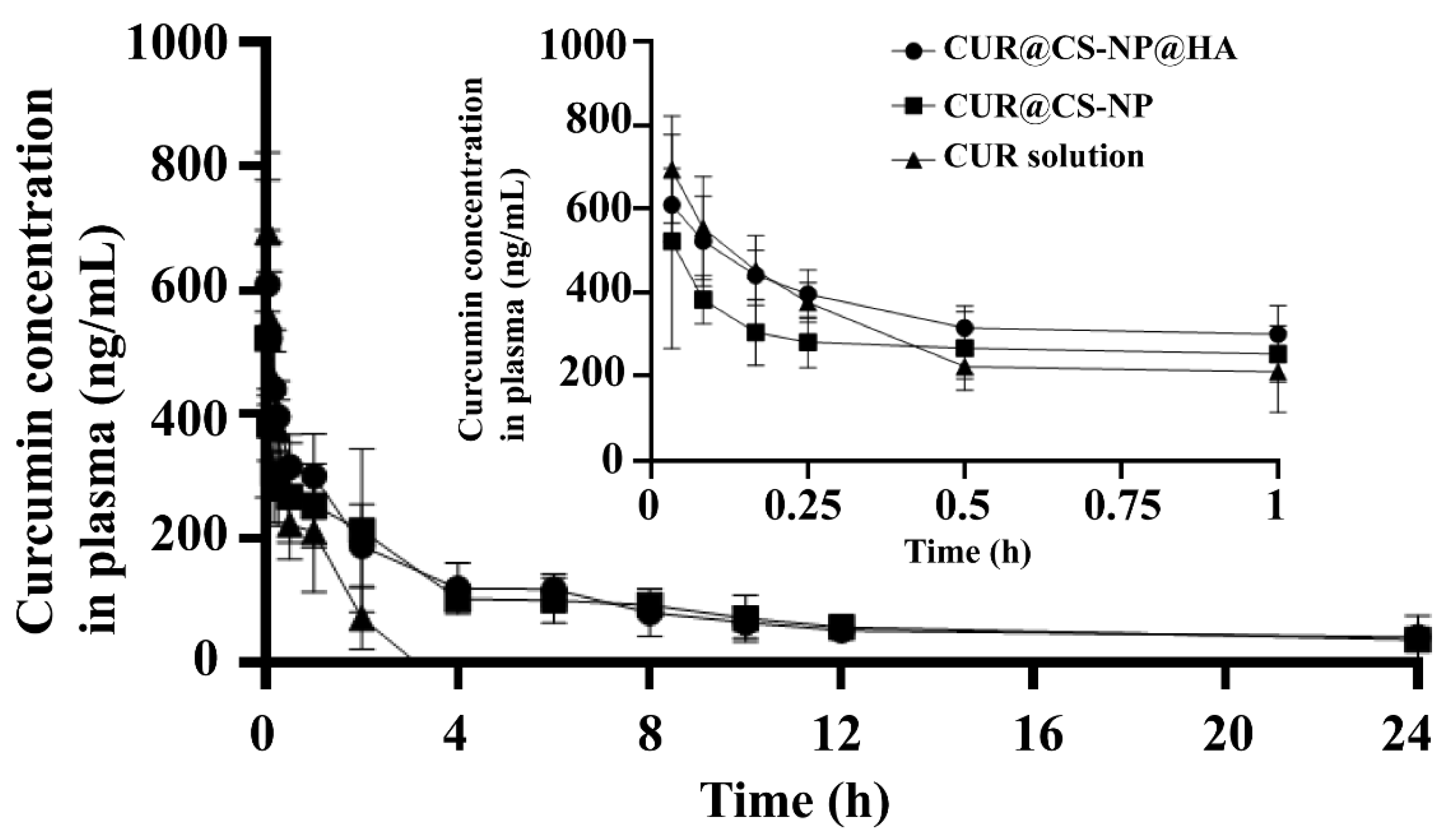
3.5. Pharmacokinetic Studies
3.6. In Vivo Antitumor Studies
3.7. Blood Routine Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Barzaman, K.; Karami, J.; Zarei, Z.; Hosseinzadeh, A.; Kazemi, M.H.; Moradi-Kalbolandi, S.; Safari, E.; Farahmand, L. Breast Cancer: Biology, Biomarkers, and Treatments. Int. Immunopharmacol. 2020, 84, 106535. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Zhao, S.; Karnad, A.; Freeman, J.W. The Biology and Role of CD44 in Cancer Progression: Therapeutic Implications. J. Hematol. Oncol. 2018, 11, 64. [Google Scholar] [CrossRef] [PubMed]
- Hassn Mesrati, M.; Syafruddin, S.E.; Mohtar, M.A.; Syahir, A. CD44: A Multifunctional Mediator of Cancer Progression. Biomolecules 2021, 11, 1850. [Google Scholar] [CrossRef] [PubMed]
- Ma, F.; Li, H.; Wang, H.; Shi, X.; Fan, Y.; Ding, X.; Lin, C.; Zhan, Q.; Qian, H.; Xu, B. Enriched CD44(+)/CD24(−) Population Drives the Aggressive Phenotypes Presented in Triple-Negative Breast Cancer (TNBC). Cancer Lett. 2014, 353, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Mattheolabakis, G.; Milane, L.; Singh, A.; Amiji, M.M. Hyaluronic Acid Targeting of CD44 for Cancer Therapy: From Receptor Biology to Nanomedicine. J. Drug Target. 2015, 23, 605–618. [Google Scholar] [CrossRef]
- Huang, G.; Huang, H. Hyaluronic Acid-Based Biopharmaceutical Delivery and Tumor-Targeted Drug Delivery System. J. Control. Release 2018, 278, 122–126. [Google Scholar] [CrossRef]
- Luo, Z.; Dai, Y.; Gao, H. Development and Application of Hyaluronic Acid in Tumor Targeting Drug Delivery. Acta Pharm. Sin. B 2019, 9, 1099–1112. [Google Scholar] [CrossRef]
- Sakurai, Y.; Harashima, H. Hyaluronan-Modified Nanoparticles for Tumor-Targeting. Expert Opin. Drug Deliv. 2019, 16, 915–936. [Google Scholar] [CrossRef]
- Vafaei, S.Y.; Esmaeili, M.; Amini, M.; Atyabi, F.; Ostad, S.N.; Dinarvand, R. Self Assembled Hyaluronic Acid Nanoparticles as a Potential Carrier for Targeting the Inflamed Intestinal Mucosa. Carbohydr. Polym. 2016, 144, 371–381. [Google Scholar] [CrossRef]
- Eenschooten, C.; Guillaumie, F.; Kontogeorgis, G.M.; Stenby, E.H.; Schwach-Abdellaoui, K. Preparation and Structural Characterisation of Novel and Versatile Amphiphilic Octenyl Succinic Anhydride–Modified Hyaluronic Acid Derivatives. Carbohydr. Polym. 2010, 79, 597–605. [Google Scholar] [CrossRef]
- Duncan, M.B.; Liu, M.; Fox, C.; Liu, J. Characterization of the N-Deacetylase Domain from the Heparan Sulfate N-Deacetylase/N-Sulfotransferase 2. Biochem. Biophys. Res. Commun. 2006, 339, 1232–1237. [Google Scholar] [CrossRef]
- Schanté, C.E.; Zuber, G.; Herlin, C.; Vandamme, T.F. Chemical Modifications of Hyaluronic Acid for the Synthesis of Derivatives for a Broad Range of Biomedical Applications. Carbohydr. Polym. 2011, 85, 469–489. [Google Scholar] [CrossRef]
- Amadi, E.V.; Venkataraman, A.; Papadopoulos, C. Nanoscale Self-Assembly: Concepts, Applications and Challenges. Nanotechnology 2022, 33, 132001. [Google Scholar] [CrossRef]
- Zhang, W.; Xu, W.; Lan, Y.; He, X.; Liu, K.; Liang, Y. Antitumor Effect of Hyaluronic-Acid-Modified Chitosan Nanoparticles Loaded with SiRNA for Targeted Therapy for Non-Small Cell Lung Cancer. Int. J. Nanomed. 2019, 14, 5287–5301. [Google Scholar] [CrossRef]
- Kim, G.H.; Won, J.E.; Byeon, Y.; Kim, M.G.; Wi, T.I.; Lee, J.M.; Park, Y.-Y.; Lee, J.-W.; Kang, T.H.; Jung, I.D.; et al. Selective Delivery of PLXDC1 Small Interfering RNA to Endothelial Cells for Anti-Angiogenesis Tumor Therapy Using CD44-Targeted Chitosan Nanoparticles for Epithelial Ovarian Cancer. Drug Deliv. 2018, 25, 1394–1402. [Google Scholar] [CrossRef]
- Yu, J.; Ruan, Q.; Nie, X. Synthesis and Characterization of Atherosclerotic Target Anti-CD47 Functionalized by Nano- Polyelectrolyte Complexes between Chitosan and Hyaluronic Acid and in Vivo and in Vitro Targeting Experiments. Adv. Clin. Exp. Med. 2020, 29, 1407–1415. [Google Scholar] [CrossRef]
- Almalik, A.; Day, P.J.; Tirelli, N. HA-Coated Chitosan Nanoparticles for CD44-Mediated Nucleic Acid Delivery. Macromol. Biosci. 2013, 13, 1671–1680. [Google Scholar] [CrossRef]
- Liu, H.-T.; Ho, Y.-S. Anticancer Effect of Curcumin on Breast Cancer and Stem Cells. Food Sci. Hum. Wellness 2018, 7, 134–137. [Google Scholar] [CrossRef]
- Chenxia, H.; Mengjie, L.; Tingting, G.; Shaoxi, W.; Weiping, H.; Ke, Y.; Zhiwei, L.; Jian, W.; Fengxue, Z.; Hongqi, W. Anti-Metastasis Activity of Curcumin against Breast Cancer via the Inhibition of Stem Cell-like Properties and EMT. Phytomedicine 2019, 58, 152740. [Google Scholar] [CrossRef]
- Duan, C.; Zhang, D.; Wang, F.; Zheng, D.; Jia, L.; Feng, F.; Liu, Y.; Wang, Y.; Tian, K.; Wang, F.; et al. Chitosan-g-Poly(N-Isopropylacrylamide) Based Nanogels for Tumor Extracellular Targeting. Int. J. Pharm. 2011, 409, 252–259. [Google Scholar] [CrossRef]
- Almalik, A.; Donno, R.; Cadman, C.J.; Cellesi, F.; Day, P.J.; Tirelli, N. Hyaluronic Acid-Coated Chitosan Nanoparticles: Molecular Weight-Dependent Effects on Morphology and Hyaluronic Acid Presentation. J. Control. Release 2013, 172, 1142–1150. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, C.; Shang, H.; Hu, R.; Fu, H.; Xiao, X. A High-Throughput and Untargeted Lipidomics Approach Reveals New Mechanistic Insight and the Effects of Salvianolic Acid B on the Metabolic Profiles in Coronary Heart Disease Rats Using Ultra-Performance Liquid Chromatography with Mass Spectrometry. RSC Adv. 2020, 10, 17101–17113. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Qian, D.; Lin, H.-P.; Xie, J.; Yang, P.; Maddy, D.; Xiao, Y.; Huang, X.; Wang, Z.; Yang, C. Nanoparticle-Delivered Miriplatin Ultrasmall Dots Suppress Triple Negative Breast Cancer Lung Metastasis by Targeting Circulating Tumor Cells. J. Control. Release 2021, 329, 833–846. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhong, H.; Zhang, L.; Chen, S.; Zhao, Y.; Zhu, Y. Synthesis and Characterization of Thermosensitive Graft Copolymer of N-Isopropylacrylamide with Biodegradable Carboxymethylchitosan. Carbohydr. Polym. 2009, 77, 785–790. [Google Scholar] [CrossRef]
- Hoare, T.R.; Kohane, D.S. Hydrogels in Drug Delivery: Progress and Challenges. Polymer 2008, 49, 1993–2007. [Google Scholar] [CrossRef]
- Manjula, B.; Varaprasad, K.; Sadilu, R. Raju Preparation and Characterization of Sodium Alginate—Based Hydrogels and Their In Vitro Release Studies. Adv. Polym. Technol. 2013, 32, 21340. [Google Scholar] [CrossRef]
- Shi, J.; Liu, X.; Sun, X.; Cao, S. Hybrid Alginate Beads with Thermal-Responsive Gates for Smart Drug Delivery. Polym. Adv. Technol. 2011, 22, 1539–1546. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, L.; Guo, B.; Ma, P.X. Cytocompatible Injectable Carboxymethyl Chitosan/N-Isopropylacrylamide Hydrogels for Localized Drug Delivery. Carbohydr. Polym. 2014, 103, 110–118. [Google Scholar] [CrossRef]
- Anand, P.; Kunnumakkara, A.B.; Newman, R.A.; Aggarwal, B.B. Bioavailability of Curcumin: Problems and Promises. Mol. Pharm. 2007, 4, 807–818. [Google Scholar] [CrossRef]




| Independent Variables | Levels | ||
|---|---|---|---|
| −1 | 0 | 1 | |
| X1: CUR/CS-g-PN ratio (w/w) | 1:5 | 1:7 | 1:9 |
| X2: pH | 4 | 5 | 6 |
| X3: CUR dosage (mg) | 3 | 5 | 7 |
| Gene | Primer Sequence | Base Number |
|---|---|---|
| M-GAPDH-F | CAGGAGAGTGTTTCCTCGTCC | 21 |
| M-GAPDH-R | TTCCCATTCTCGGCCTTGAC | 20 |
| M-SOX2-F | CTCGCAGACCTACATGAAC | 19 |
| M-SOX2-R | CTCGGACTTGACCACAGA | 18 |
| M-CD44-F | CACCTTGGCCACCACTCCTAAT | 22 |
| M-CD44-R | CCCTTCTGTCACATGGGAGTC | 21 |
| Parameter | Predicted Value | Measured Value |
|---|---|---|
| PS (nm) | 229.09 | 213.90 ± 6.0 |
| EE% | 67.86 | 70.20 ± 3.90 |
| DL% | 11.65 | 11.40 ± 1.50 |
| NPs | PS (nm) | EE% | DL% |
|---|---|---|---|
| CUR@CSLMW-NP | 245 ± 6.5 | 62.81 ± 2.35 | 10.47 ± 0.39 |
| CUR@CSMMW-NP | 266 ± 12.3 | 51.46 ± 3.40 | 8.58 ± 0.57 |
| CUR@CSHMW-NP | 212 ± 5.0 | 69.10 ± 2.00 | 11.35 ± 0.33 |
| Pharmacokinetics | CUR Solution | CUR@CS-NP | CUR@CS-NP@HA |
|---|---|---|---|
| AUC (0–∞) (h × ng/mL) | 502.30 ± 123.80 | 3133.13 ± 276.90 | 1979.29 ± 373.01 |
| MRT (0–∞) (h) | 0.41 ± 0.07 | 31.28 ± 8.76 | 6.48 ± 1.90 |
| t1/2 (h) | 0.58 ± 0.29 | 12.52 ± 0.89 | 9.88 ± 6.33 |
| CL (mL/h/kg) | 0.004 ± 0.001 | 0.003 ± 0.01 | 0.001 ± 0.00 |
| Tmax (h) | 0.04 ± 0.02 | 0.05 ± 0.02 | 0.04 ± 0.02 |
| Cmax (ng/mL) | 694.11 ± 128.96 | 522.06 ± 256.11 | 609.76 ± 87.54 |
| Index | Saline | Free CUR | CUR@CS-NP | CUR@CS-NP@HA |
|---|---|---|---|---|
| WBC (109/L) | 56.85 ± 21.42 | 67.72 ± 19.78 | 71.90 ± 26.59 | 16.77 ± 12.63 |
| RBC (1012/L) | 9.44 ± 0.32 | 9.91 ± 0.48 | 7.98 ± 0.95 | 8.14 ± 0.65 |
| HGB (g/L) | 144.60 ± 3.71 | 153.60 ± 6.95 | 112.20 ± 13.85 | 114.40 ± 8.44 |
| HCT (%) | 43.54 ± 1.15 | 45.68 ± 2.41 | 34.50 ± 4.31 | 35.06 ± 2.43 |
| MCV (fL) | 46.14 ± 0.34 | 46.14 ± 0.74 | 43.20 ± 0.78 | 43.30 ± 1.70 |
| MCH (pg) | 15.34 ± 0.40 | 15.52 ± 0.15 | 14.04 ± 0.29 | 14.12 ± 0.61 |
| MCHC (g/L) | 332.20 ± 7.72 | 336.40 ± 6.07 | 325.20 ± 4.76 | 326.20 ± 4.09 |
| RDW (%) | 16.40 ± 0.68 | 16.74 ± 0.87 | 18.60 ± 1.03 | 18.40 ± 1.03 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Liu, L.; Shang, H.; Feng, X.; Fan, N.; Wang, J.; Wu, Y.; Chen, Y.; Chu, X.; Zhong, M.; et al. Self−Assembling Anchorage of Hyaluronic Acid on the Nanoparticle Surface Confers Superiority of Triple Negative Breast Cancer Treatment. Pharmaceutics 2022, 14, 2461. https://doi.org/10.3390/pharmaceutics14112461
Li Y, Liu L, Shang H, Feng X, Fan N, Wang J, Wu Y, Chen Y, Chu X, Zhong M, et al. Self−Assembling Anchorage of Hyaluronic Acid on the Nanoparticle Surface Confers Superiority of Triple Negative Breast Cancer Treatment. Pharmaceutics. 2022; 14(11):2461. https://doi.org/10.3390/pharmaceutics14112461
Chicago/Turabian StyleLi, Yingpeng, Liang Liu, Hongtao Shang, Xuchen Feng, Ni Fan, Jingyu Wang, Yuqi Wu, Yatong Chen, Xinhong Chu, Min Zhong, and et al. 2022. "Self−Assembling Anchorage of Hyaluronic Acid on the Nanoparticle Surface Confers Superiority of Triple Negative Breast Cancer Treatment" Pharmaceutics 14, no. 11: 2461. https://doi.org/10.3390/pharmaceutics14112461
APA StyleLi, Y., Liu, L., Shang, H., Feng, X., Fan, N., Wang, J., Wu, Y., Chen, Y., Chu, X., Zhong, M., Sun, Y., Fu, H., Huang, W., & Li, Y. (2022). Self−Assembling Anchorage of Hyaluronic Acid on the Nanoparticle Surface Confers Superiority of Triple Negative Breast Cancer Treatment. Pharmaceutics, 14(11), 2461. https://doi.org/10.3390/pharmaceutics14112461







