Development of Guar Gum-Pectin-Based Colon Targeted Solid Self-Nanoemulsifying Drug Delivery System of Xanthohumol
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Equipments
2.2. Methods
2.2.1. Solubility Studies
2.2.2. Formulation of L-SNEDDS
2.2.3. Thermodynamic Stability Studies of L-SNEDDS Prototypes
2.2.4. Formulation of Solid (S) SNEDDS
Determination of Drug Loading Factor (Lf)
Angle of Repose (θ) (AOR)
Adsorption to Solid Carriers and Colon Targeting Formulation of S-SNEDDS
2.2.5. Characterization of SNEEDS
Pre-Compression Properties of S-SNEEDS Powders
Bulk Density ()
Tapped Density ()
Carr’s Compressibility Index (CCI)
Drug Loading (DL)
Droplet Size (DS) and Zeta Potential (ZP) Analysis
Robustness to Dilution and pH Change
Differential Scanning Calorimetry (DSC) and Powder X-ray Diffraction (PXRD) Studies
Microscopic Analysis
- Transmission Electron Microscopy (TEM)
- Scanning Electron Microscopy (SEM)
In Vitro Dissolution Studies Using Rat Caecal Contents
Release Kinetic Study
Cytotoxicity Study Using MTT Assay
Stability Studies
Statistical Analysis
3. Results and Discussion
3.1. Solubility Studies
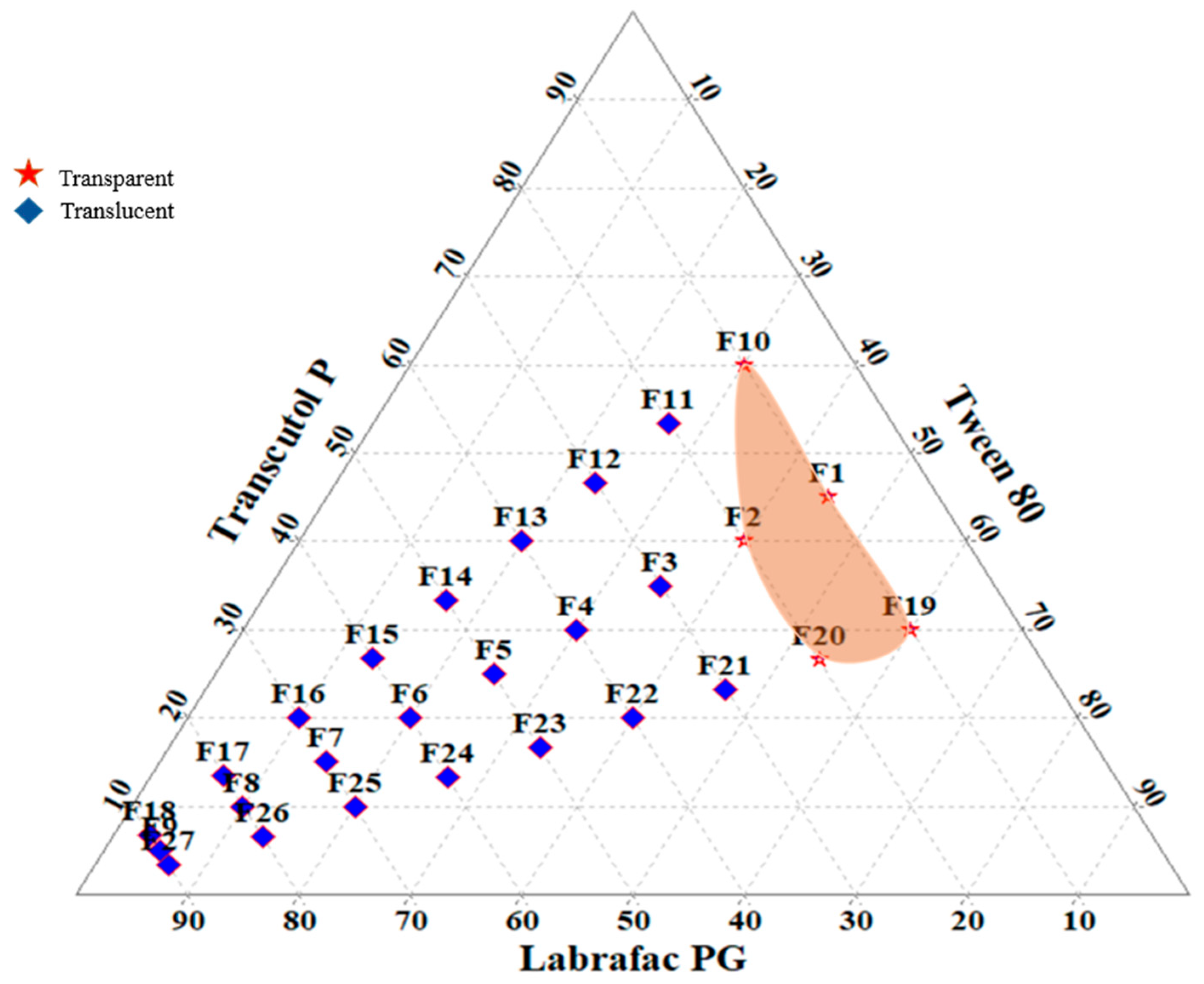
3.2. Construction of Ternary Phase Diagram
3.3. Identification of Droplet Size, Thermodynamic Stability and Drug Loading of Selected L-SNEDDS
3.4. Formulation and Characterization of S-SNEDDS
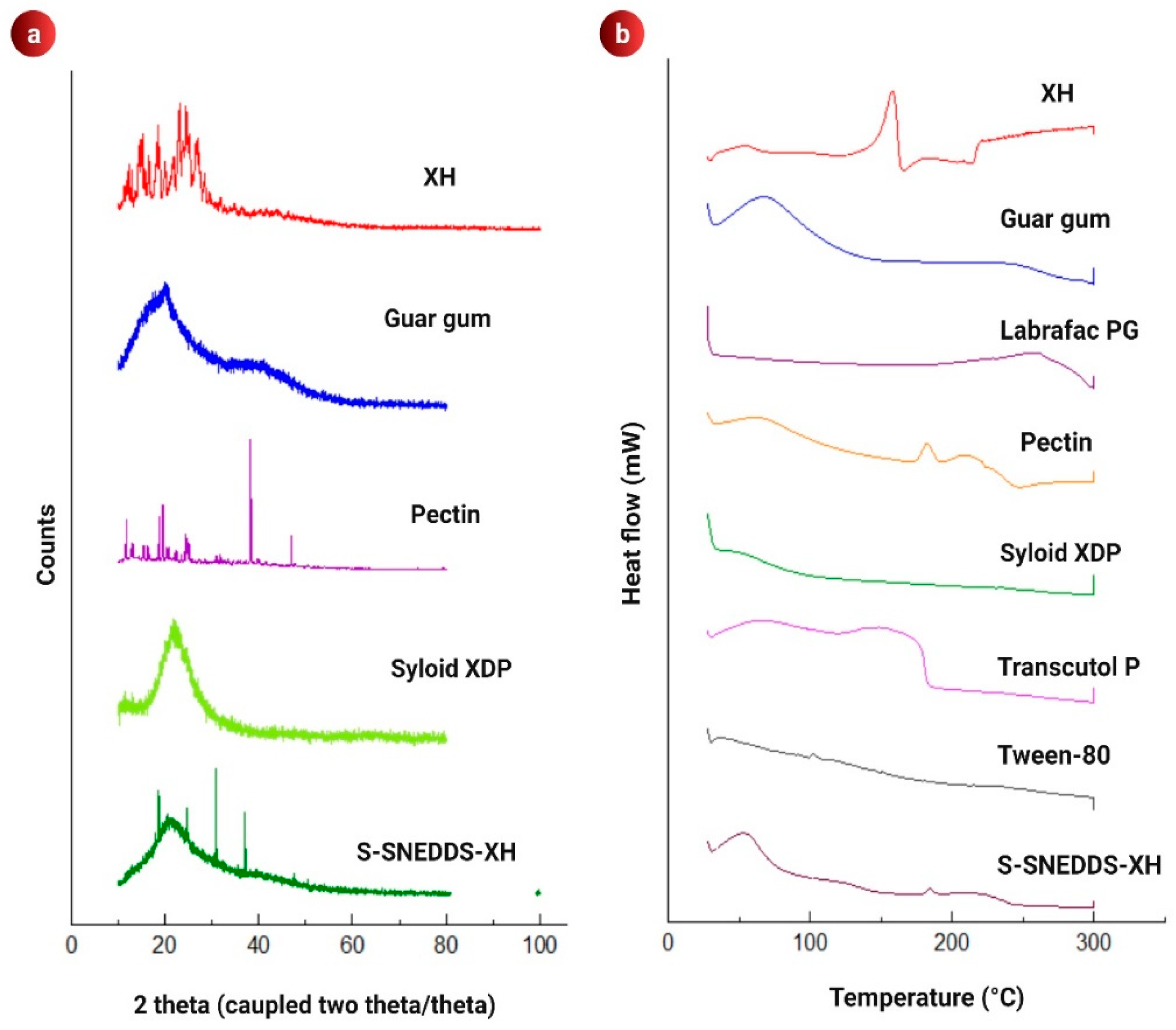
3.5. PXRD Analysis
3.6. DSC Analysis
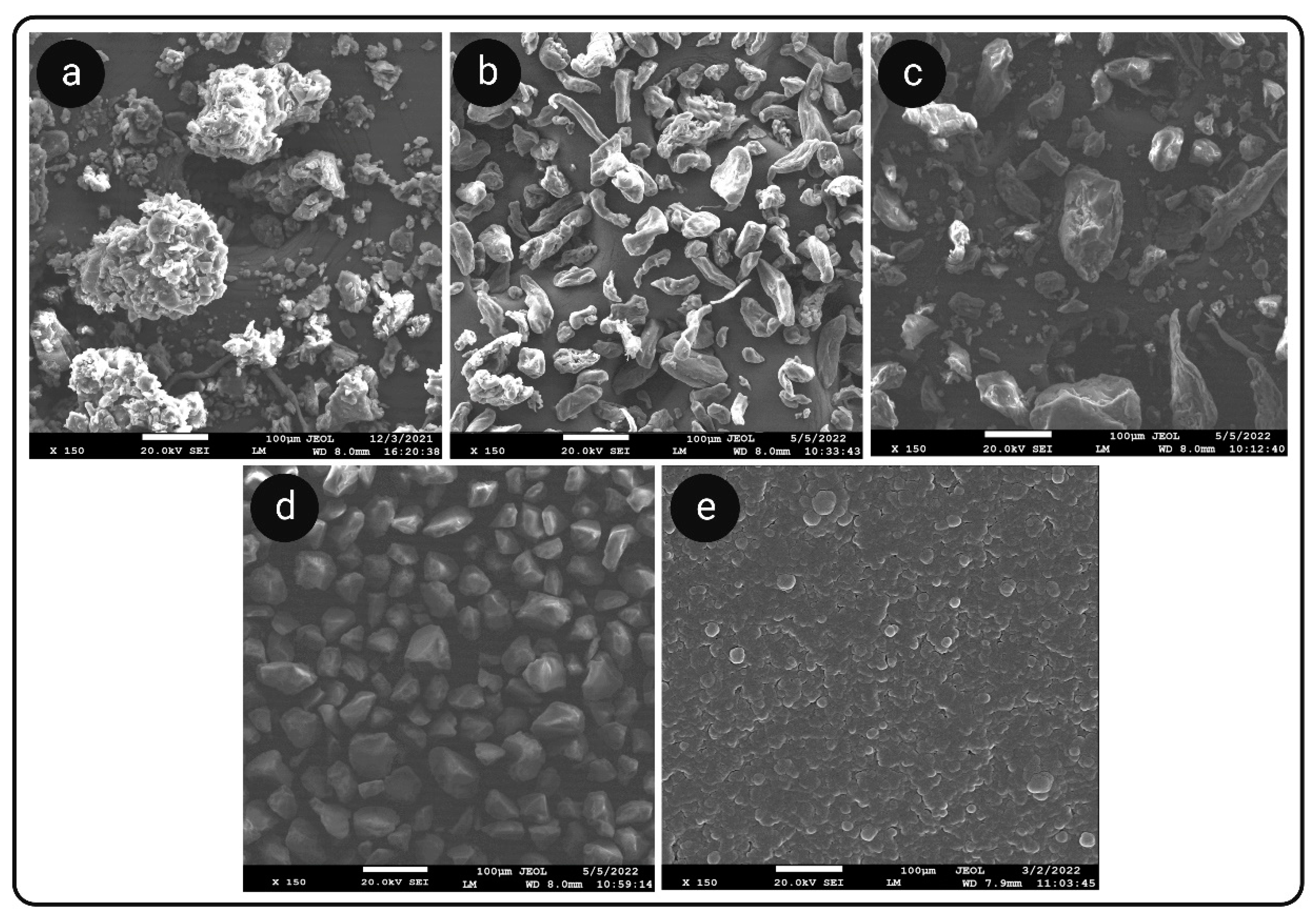
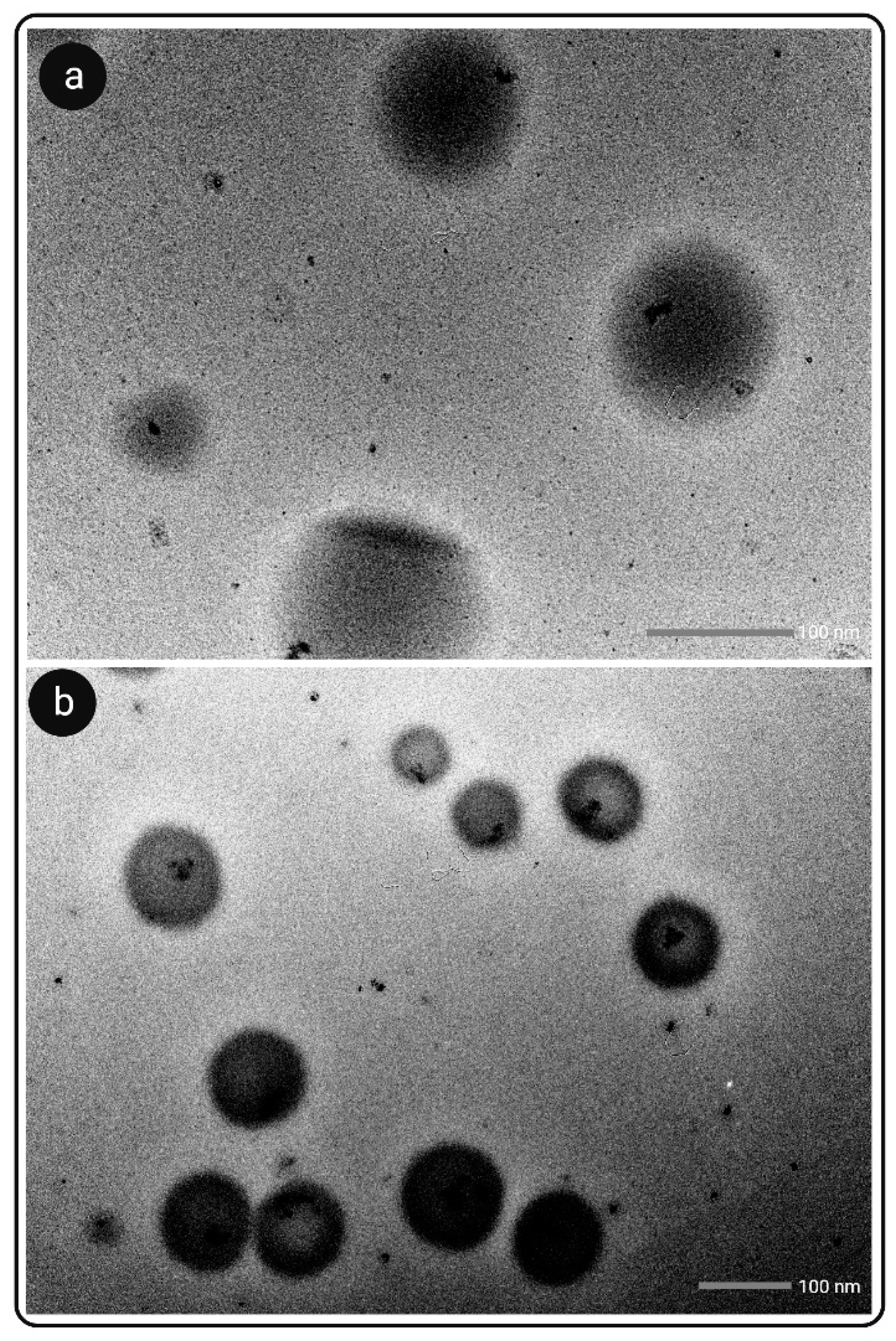
3.7. Microscopic Analysis
3.7.1. SEM Analysis
3.7.2. TEM Analysis
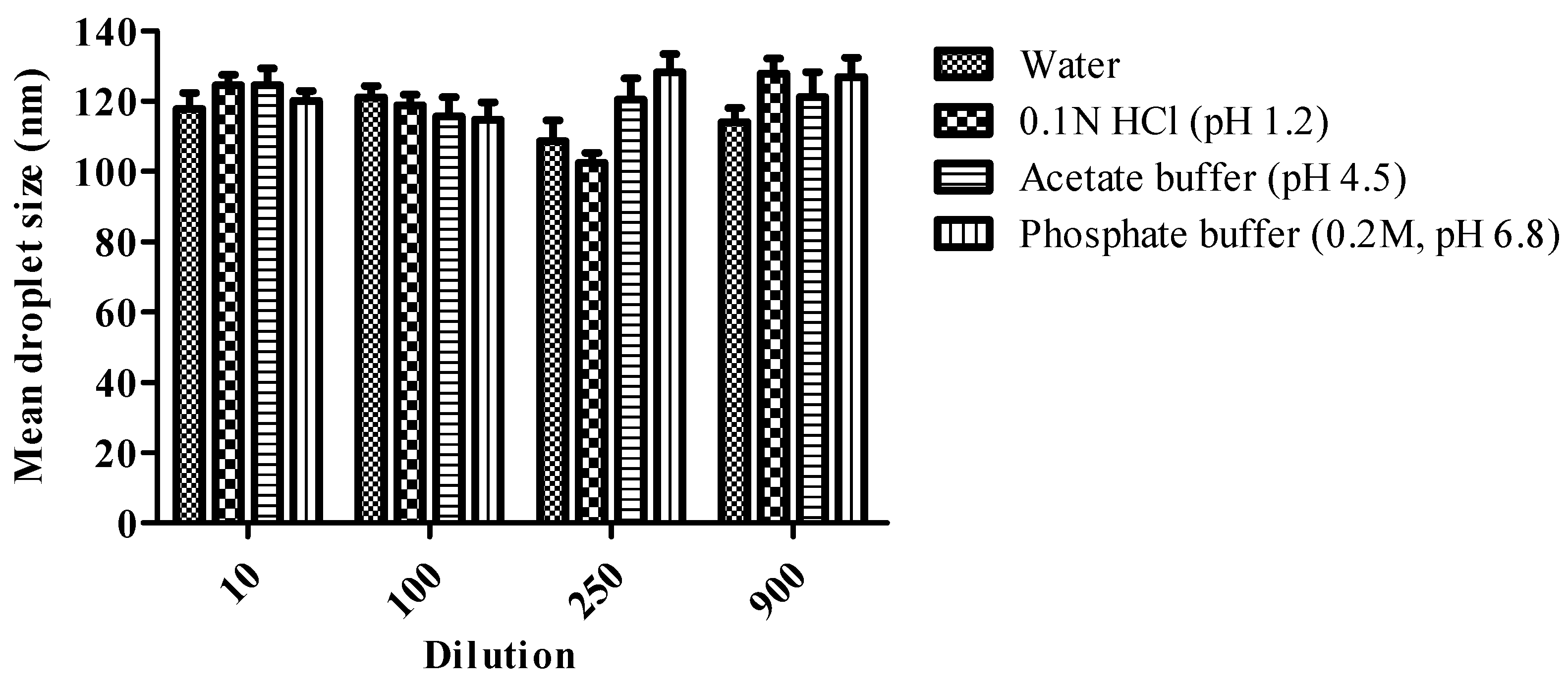
3.8. Effect of Change in Dilution Volume and pH on Droplet Size
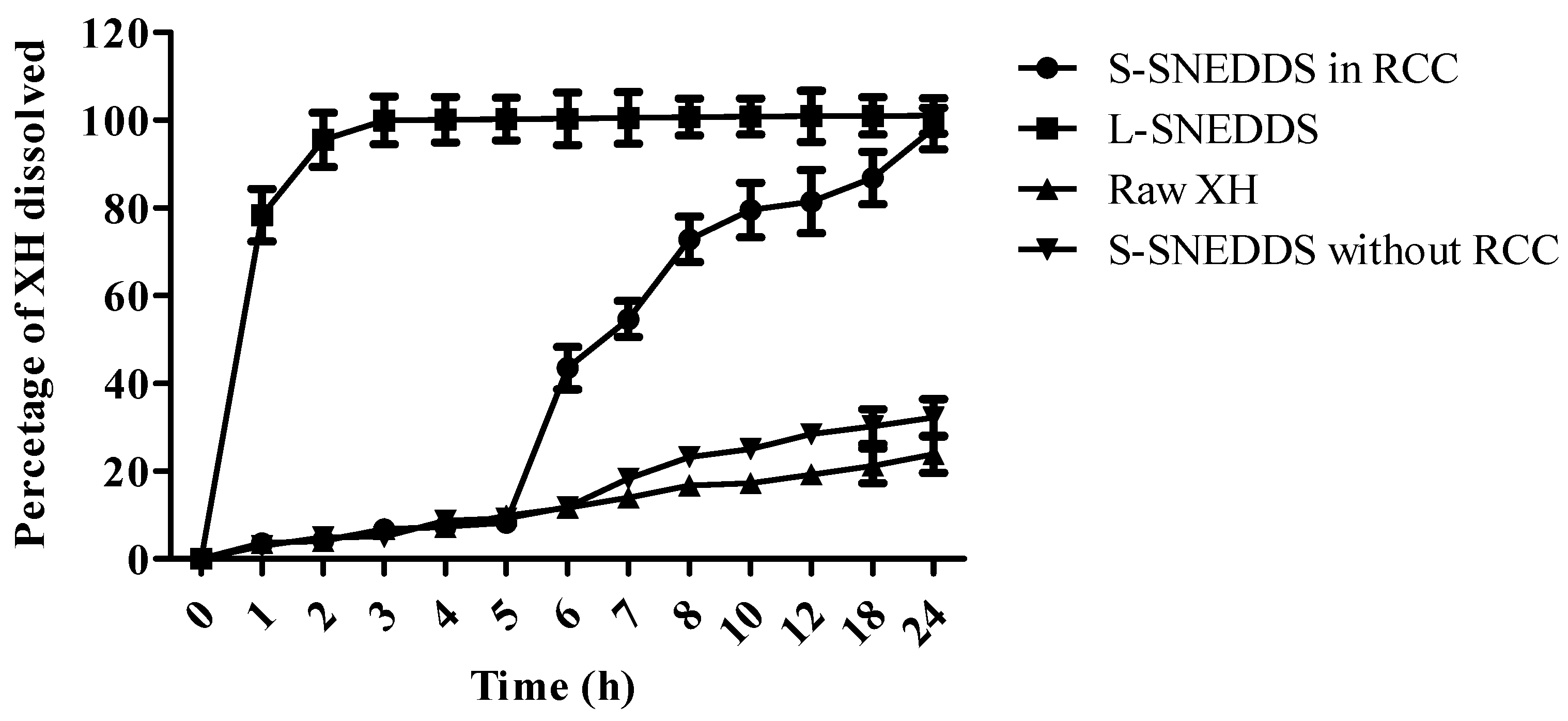
3.9. In Vitro Dissolution Study
3.10. Evaluation of Release Kinetics
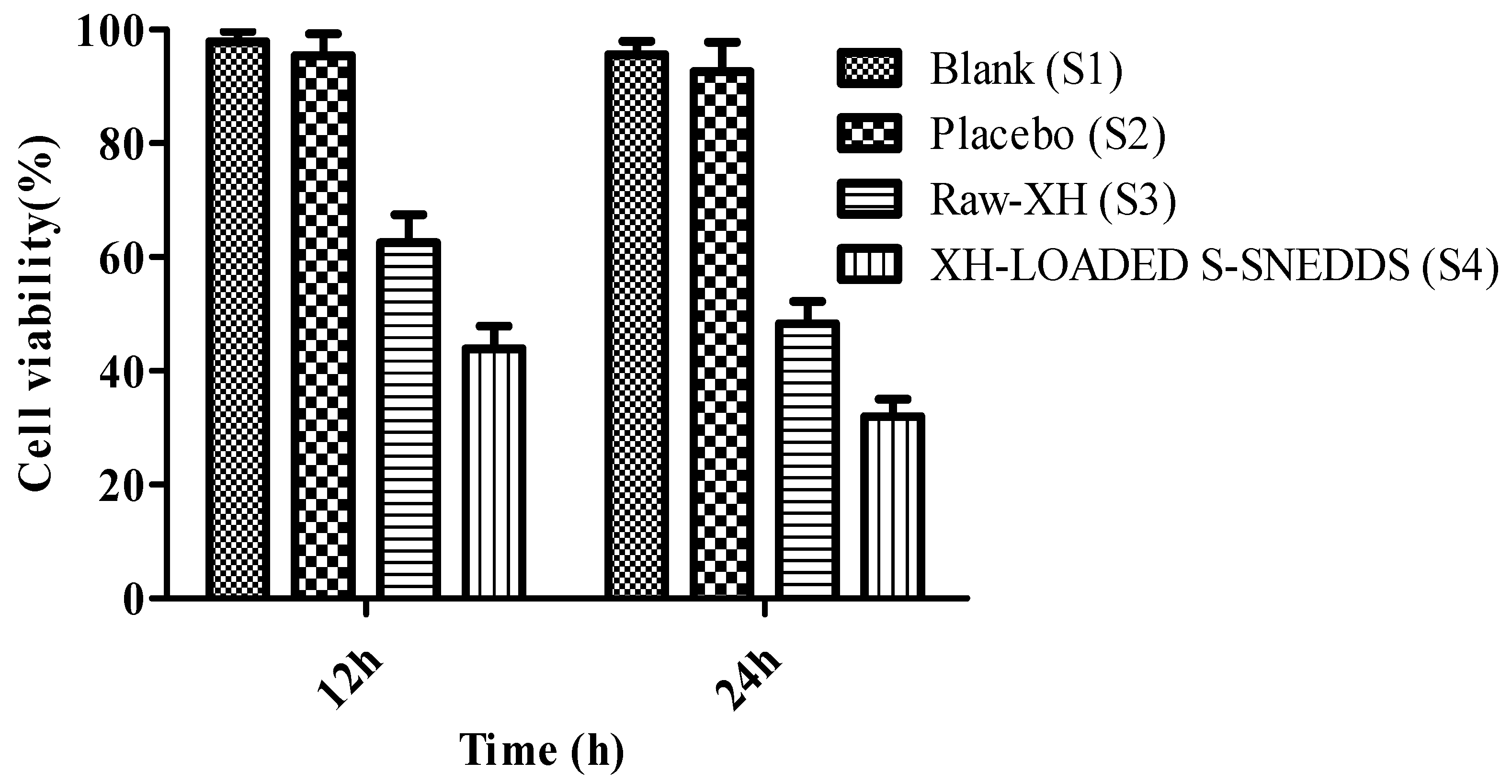
3.11. Cytotoxicity Studies
3.12. Stability Studies
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| MTT | 3-[4,5-dimethylthiazole-2-yl]-2,5-diphenyltetrazolium bromide |
| A-200 | Aerosil 200 |
| AOR | Angle of repose (θ) |
| BD | Bulk density |
| CMCM | Capmul MCM EP/NF |
| CCI | Carr’s compressibility index |
| CRD | Colorectal diseases |
| CST | Cosurfactants |
| DW | Distilled water |
| DL | Drug loading |
| FBS | Fetal bovine serum |
| GIT | Gastrointestinal tract |
| GUG | Guar gum |
| h | Height |
| HPLC | High Performance Liquid Chromatography |
| Caco2 | Human colorectal adenocarcinoma |
| HCl | Hydrochloric acid |
| HLB | Hydrophilic-lipophilic balance |
| LPG; | Labrafac PG |
| LMCS | Labrafil M1944CS |
| LM | Lactose monohydrate |
| Lf | Loading factor |
| MgS | Magnesium stearate |
| MCC | Microcrystalline cellulose PH102 |
| OPA | Ortho-phosphoric acid |
| PTN | Pectin |
| PEG | Polyethylene glycol |
| r | Radius |
| RCC | Rat caecal content |
| S-SNEDDS | Self-nanoemulsifying drug delivery system |
| ST | Surfactants |
| SXDP | Syloid XDP 3150 |
| TPD | Tapped density |
| TP | Transcutol P |
| T80 | Tween 80 |
| XH | Xanthohumol |
References
- Moir, M.; Courage, S.; Limited, B. The Science of Beer Hops—A millennium review hops—A millennium review. J. Am. Soc. Brew. Chem. 2018, 58, 131–146. [Google Scholar]
- Harish, V.; Haque, E.; Śmiech, M.; Taniguchi, H.; Jamieson, S.; Tewari, D.; Bishayee, A. Xanthohumol for human malignancies: Chemistry, pharmacokinetics and molecular targets. Int. J. Mol. Sci. 2021, 22, 4478. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Yin, H.; Liu, G.; Dong, J.; Qian, Z.; Miao, J. Xanthohumol, a prenylated chalcone from beer hops, acts as an α-glucosidase inhibitor in vitro. J. Agric. Food Chem. 2014, 62, 5548–5554. [Google Scholar] [CrossRef] [PubMed]
- Vazquez-Cervantes, G.I.; Ortega, D.R.; Ayala, T.B.; de la Cruz, V.P.; Esquivel, D.F.G.; Salazar, A.; Pineda, B. Redox and anti-inflammatory properties from hop components in beer-related to neuroprotection. Nutrients 2021, 13, 2000. [Google Scholar] [CrossRef]
- Lv, H.; Liu, Q.; Wen, Z.; Feng, H.; Deng, X.; Ci, X. Xanthohumol ameliorates lipopolysaccharide (LPS)-induced acute lung injury via induction of AMPK/GSK3β-Nrf2 signal axis. Redox Biol. 2017, 12, 311–324. [Google Scholar] [CrossRef]
- Girisa, S.; Saikia, Q.; Bordoloi, D.; Banik, K.; Monisha, J.; Daimary, U.D.; Verma, E.; Ahn, K.S.; Kunnumakkara, A.B. Xanthohumol from hop: Hope for cancer prevention and treatment. IUBMB Life 2021, 73, 1016–1044. [Google Scholar] [CrossRef]
- Shreya, A.B.; Raut, S.Y.; Managuli, R.S.; Udupa, N.; Mutalik, S. Active targeting of drugs and bioactive molecules via oral administration by ligand-conjugated lipidic nanocarriers: Recent advances. AAPS PharmSciTech 2019, 20, 15. [Google Scholar] [CrossRef]
- Naeem, M.; Awan, U.A.; Subhan, F.; Cao, J.; Hlaing, S.P.; Lee, J.; Im, E.; Jung, Y.; Yoo, J.W. Advances in colon-targeted nano-drug delivery systems: Challenges and solutions. Arch. Pharm. Res. 2020, 43, 153–169. [Google Scholar] [CrossRef]
- Hamman, J.H.; Enslin, G.M.; Kotzé, A.F. Oral delivery of peptide drugs: Barriers and developments. BioDrugs 2005, 19, 165–177. [Google Scholar] [CrossRef]
- Dubey, S.K.; Parab, S.; Dabholkar, N.; Agrawal, M.; Singhvi, G.; Alexander, A.; Bapat, R.A.; Kesharwani, P. Oral peptide delivery: Challenges and the way ahead. Drug Discov. Today 2021, 26, 931–950. [Google Scholar] [CrossRef]
- Lee, S.H.; Bajracharya, R.; Min, J.Y.; Han, J.W.; Park, B.J.; Han, H.K. Strategic approaches for colon targeted drug delivery: An overview of recent advancements. Pharmaceutics 2020, 12, 68. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Aguinagalde, O.; Meaurio, E.; Lejardi, A.; Sarasua, J.R. Amorphous solid dispersions in poly(ϵ-caprolactone)/xanthohumol bioactive blends: Physicochemical and mechanical characterization. J. Mater. Chem. B 2021, 9, 4219–4229. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.K.; Srinivasan, K.K.; Singare, D.S.; Gowthamarajan, K.; Prakash, D. Formulation of Ternary Complexes of Glyburide with Hydroxypropyl-β-Cyclodextrin and Other Solubilizing Agents and Their Effect on Release Behavior of Glyburide in Aqueous and Buffered Media at Different Agitation Speeds. Drug Dev. Ind. Pharm. 2012, 38, 1328–1336. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.K.; Srinivasan, K.K.; Gowthamarajan, K.; Singare, D.S.; Prakash, D.; Gaikwad, N.B. Investigation of preparation parameters of nanosuspension by top-down media milling to improve the dissolution of poorly water-soluble glyburide. Eur. J. Pharm. Biopharm. 2011, 78, 441–446. [Google Scholar] [CrossRef] [PubMed]
- Mahesh, K.V.; Singh, S.K.; Gulati, M. A Comparative study of top-down and bottom-up approaches for the preparation of nanosuspensions of glipizide. Powder Technol. 2014, 256, 436–449. [Google Scholar] [CrossRef]
- Alsaidan, O.A.; Pattanayak, P.; Awasthi, A.; Alruwaili, N.K.; Zafar, A.; Almawash, S.; Gulati, M.; Singh, S.K. Quality by design-based optimization of formulation parameters to develop quercetin nanosuspension for improving its biopharmaceutical properties. S. Afr.J. Bot. 2022, 149, 798–806. [Google Scholar] [CrossRef]
- Elgart, A.; Cherniakov, I.; Aldouby, Y.; Domb, A.J.; Hoffman, A. Lipospheres and pro-nano lipospheres for delivery of poorly water soluble compounds. Chem. Phys. Lipids 2012, 165, 438–453. [Google Scholar] [CrossRef]
- Avramoff, A.; Khan, W.; Ezra, A.; Elgart, A.; Hoffman, A.; Domb, A.J. Cyclosporin pro-dispersion liposphere formulation. J. Control. Release 2012, 160, 401–406. [Google Scholar] [CrossRef]
- Atsmon, J.; Cherniakov, I.; Izgelov, D.; Hoffman, A.; Domb, A.J.; Deutsch, L.; Deutsch, F.; Heffetz, D.; Sacks, H. PTL401, a New formulation based on pro-nano dispersion technology, improves oral cannabinoids bioavailability in healthy volunteers. J. Pharm. Sci. 2018, 107, 1423–1429. [Google Scholar] [CrossRef]
- Cherniakov, I.; Izgelov, D.; Barasch, D.; Davidson, E.; Domb, A.J.; Hoffman, A. Piperine-pro-nanolipospheres as a novel oral delivery system of cannabinoids: Pharmacokinetic evaluation in healthy volunteers in comparison to buccal spray administration. J. Control. Release 2017, 266, 1–7. [Google Scholar] [CrossRef]
- Kaur, P.; Singh, S.K.; Garg, V.; Gulati, M.; Vaidya, Y. Optimization of spray drying process for formulation of solid dispersion containing polypeptide-k powder through quality by design approach. Powder Technol. 2015, 284, 1–11. [Google Scholar] [CrossRef]
- Teixeira, C.C.C.; Mendonça, L.M.; Bergamaschi, M.M.; Queiroz, R.H.C.; Souza, G.E.P.; Antunes, L.M.G.; Freitas, L.A.P. Microparticles containing curcumin solid dispersion: Stability, bioavailability and anti-inflammatory activity. AAPS PharmSciTech 2016, 17, 252. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Lee, I.W.; Shin, G.H.; Chen, X.; Park, H.J. Curcumin-eudragit® E PO solid dispersion: A simple and potent method to solve the problems of curcumin. Eur. J. Pharm. Biopharm. 2015, 94, 322–332. [Google Scholar] [CrossRef] [PubMed]
- Thorat, A.A.; Dalvi, S.V. Solid state phase transformations and storage stability of curcumin polymorphs. Cryst. Growth Des. 2015, 15, 1757–1770. [Google Scholar] [CrossRef]
- Sanphui, P.; Goud, N.R.; Khandavilli, U.B.R.; Nangia, A. Fast dissolving curcumin cocrystals. Cryst. Growth Des. 2011, 11, 4135–4145. [Google Scholar] [CrossRef]
- Mohite, R.; Mehta, P.; Arulmozhi, S.; Kamble, R.; Pawar, A.; Bothiraja, C. Synthesis of fisetin co-crystals with caffeine and nicotinamide using the cooling crystallization technique: Biopharmaceutical studies. New J. Chem. 2019, 43, 13471–13479. [Google Scholar] [CrossRef]
- Pandey, N.K.; Sehal, H.R.; Garg, V.; Gaur, T.; Kumar, B.; Singh, S.K.; Gulati, M.; Gowthamarajan, K.; Bawa, P.; Rajesh, S.Y.; et al. Stable co-crystals of glipizide with enhanced dissolution profiles: Preparation and characterization. AAPS PharmSciTech 2017, 18, 2454–2465. [Google Scholar] [CrossRef] [PubMed]
- Awasthi, A.; Kumar, B.; Gulati, M.; Vishwas, S.; Corrie, L.; Kaur, J.; Khursheed, R.; Muhammed, R.A.; Kala, D.; Porwal, O.; et al. Novel nanostructured lipid carriers co-loaded with mesalamine and curcumin: Formulation, optimization and in-vitro evaluation. Pharm. Res. 2022, 1, 1–13. [Google Scholar] [CrossRef]
- Toniazzo, T.; Peres, M.S.; Ramos, A.P.; Pinho, S.C. Encapsulation of quercetin in liposomes by ethanol injection and physicochemical characterization of dispersions and lyophilized vesicles. Food Biosci. 2017, 19, 17–25. [Google Scholar] [CrossRef]
- Gang, W.; Jie, W.J.; Ping, Z.L.; Ming, D.S.; Ying, L.J.; Lei, W.; Fang, Y. Liposomal quercetin: Evaluating drug delivery in vitro and biodistribution in vivo. Expert Opin. Drug Deliv. 2012, 9, 599–613. [Google Scholar] [CrossRef]
- De Leo, V.; Milano, F.; Mancini, E.; Comparelli, R.; Giotta, L.; Nacci, A.; Longobardi, F.; Garbetta, A.; Agostiano, A.; Catucci, L. Encapsulation of curcumin-loaded liposomes for colonic drug delivery in a PH-responsive polymer cluster using a PH-driven and organic solvent-free process. Molecules 2018, 23, 739. [Google Scholar] [CrossRef] [PubMed]
- Ameeduzzafar, E.B.I.; Alruwaili, N.K.; Elkomy, M.H.; Ahmad, J.; Afzal, M.; Ahmad, N.; Elmowafy, M.; Alharbi, K.S.; Shoaib, A. Development of novel dapagliflozin loaded solid self-nanoemulsifying oral delivery system: Physiochemical characterization and in vivo antidiabetic activity. J. Drug Deliv. Sci. Technol. 2019, 54, 101279. [Google Scholar] [CrossRef]
- Li, L.; Hui Zhou, C.; Ping Xu, Z. Self-Nanoemulsifying drug delivery system and solidified self nanoemulsifying drug delivery system. Nanocarr. Drug Deliv. 2019, 421–449. [Google Scholar] [CrossRef]
- Udayan, A.; Arumugam, M.; Pandey, A. Nutraceuticals from algae and cyanobacteria. Algal Green Chem. Recent Prog. Biotechnol. 2017, 65–89. [Google Scholar] [CrossRef]
- Wang, X.; Wang, X.; Jiang, H.; Cai, C.; Li, G.; Hao, J.; Yu, G. Marine polysaccharides attenuate metabolic syndrome by fermentation products and altering gut microbiota: An overview. Carbohydr. Polym. 2018, 195, 601–612. [Google Scholar] [CrossRef]
- Yin, C.; Noratto, G.D.; Fan, X.; Chen, Z.; Yao, F.; Shi, D.; Gao, H. The impact of mushroom polysaccharides on gut microbiota and its beneficial effects to host: A review. Carbohydr. Polym. 2020, 250, 116942. [Google Scholar] [CrossRef]
- Khursheed, R.; Singh, S.K.; Wadhwa, S.; Gulati, M.; Awasthi, A.; Kumar, R.; Ramanunny, A.K.; Kapoor, B.; Kumar, P.; Corrie, L. Exploring role of probiotics and Ganoderma lucidum extract powder as solid carriers to solidify liquid self-nanoemulsifying delivery systems loaded with curcumin. Carbohydr. Polym. 2020, 250, 116996. [Google Scholar] [CrossRef]
- Inugala, S.; Eedara, B.B.; Sunkavalli, S.; Dhurke, R.; Kandadi, P.; Jukanti, R.; Bandari, S. Solid self-nanoemulsifying drug delivery system (S-SNEDDS) of darunavir for improved dissolution and oral bioavailability: In vitro and in vivo evaluation. Eur. J. Pharm. Sci. 2015, 74, 1–10. [Google Scholar] [CrossRef]
- Kallakunta, V.R.; Bandari, S.; Jukanti, R.; Veerareddy, P.R. Oral self emulsifying powder of lercanidipine hydrochloride: Formulation and evaluation. Powder Technol. 2012, 221, 375–382. [Google Scholar] [CrossRef]
- Zhang, P.; Liu, Y.; Feng, N.; Xu, J. Preparation and evaluation of self-microemulsifying drug delivery system of oridonin. Int. J. Pharm. 2008, 355, 269–276. [Google Scholar] [CrossRef]
- Jyoti, J.; Anandhakrishnan, N.K.; Singh, S.K.; Kumar, B.; Gulati, M.; Gowthamarajan, K.; Kumar, R.; Yadav, A.K.; Kapoor, B.; Pandey, N.K.; et al. A Three-pronged formulation approach to improve oral bioavailability and therapeutic efficacy of two lipophilic drugs with gastric lability. Drug Deliv. Transl. Res. 2019, 9, 848–865. [Google Scholar] [CrossRef] [PubMed]
- Shah, N.; Shah, T.; Amin, A. Polysaccharides: A targeting strategy for colonic drug delivery. Expert Opin. Drug Deliv. 2011, 8, 779–796. [Google Scholar] [CrossRef] [PubMed]
- Sarwar, B.; Katare, O.P.; Sumant, S.; Babita, G.; Khunara, R.K.; Singh, B. Solid self-nanoemulsifying systems of olmesartan medoxomil: Formulation development, micromeritic characterization, in vitro and in vivo evaluation. Powder Technol. 2016, 294, 93–104. [Google Scholar] [CrossRef]
- Ghosh, D.; Singh, S.K.; Khursheed, R.; Pandey, N.K.; Kumar, B.; Kumar, R.; Kumari, Y.; Kaur, G.; Clarisse, A.; Awasthi, A.; et al. Impact of solidification on micromeritic properties and dissolution rate of self-nanoemulsifying delivery system loaded with docosahexaenoic acid. Drug Dev. Ind. Pharm. 2020, 46, 597–605. [Google Scholar] [CrossRef]
- Reddy, M.S.; Swapna, G. Solubility and dissolution enhancement of poorly aqueous soluble drug ibrutinib by self emulsifying drug delivery system. Int. J. Pharm. Biol. Sci. TM 2019, 9, 174–189. [Google Scholar]
- Zhang, N.; Zhang, F.; Xu, S.; Yun, K.; Wu, W.; Pan, W. Formulation and evaluation of luteolin supersaturatable self-nanoemulsifying drug delivery system (S-SNEDDS) for enhanced oral bioavailability. J. Drug Deliv. Sci. Technol. 2020, 58, 101783. [Google Scholar] [CrossRef]
- Khan, A.W.; Kotta, S.; Ansari, S.H.; Sharma, R.K.; Ali, J. Self-nanoemulsifying drug delivery system (SNEDDS) of the poorly water-soluble grapefruit flavonoid naringenin: Design, characterization, in vitro and in vivo evaluation. Drug Deliv. 2015, 22, 552–561. [Google Scholar] [CrossRef]
- Nasr, A.; Gardouh, A.; Ghorab, M. Novel Solid Self-nanoemulsifying drug delivery system (S-SNEDDS) for oral delivery of olmesartan medoxomil: Design, formulation, pharmacokinetic and bioavailability evaluation. Pharmaceutics 2016, 8. [Google Scholar] [CrossRef]
- Yang, L. Biorelevant dissolution testing of colon-specific delivery systems activated by colonic microflora. J. Control. Release 2008, 125, 77–86. [Google Scholar] [CrossRef]
- Corrie, L.; Gulati, M.; Awasthi, A.; Vishwas, S.; Kaur, J.; Khursheed, R.; Kumar, R.; Kumar, A.; Imran, M.; Chellappan, D.K.; et al. Polysaccharide, fecal microbiota, and curcumin-based novel oral colon-targeted solid self-nanoemulsifying delivery system: Formulation, characterization, and in-vitro anticancer evaluation. Mater. Today Chem. 2022, 26, 101165. [Google Scholar] [CrossRef]
- Şueki, F.; Ruhi, M.K.; Gülsoy, M. The effect of curcumin in antitumor photodynamic therapy: In vitro experiments with Caco-2 and PC-3 cancer lines. Photodiagn. Photodyn. Ther. 2019, 27, 95–99. [Google Scholar] [CrossRef] [PubMed]
- Garg, V.; Kaur, P.; Singh, S.K.; Kumar, B.; Bawa, P.; Gulati, M.; Yadav, A.K. Solid self-nanoemulsifying drug delivery systems for oral delivery of polypeptide-k: Formulation, optimization, in-vitro and in-vivo antidiabetic evaluation. Eur. J. Pharm. Sci. 2017, 109, 297–315. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Blevins, W.E.; Park, H.; Park, K. Gastric retention properties of superporous hydrogel composites. J. Control. Release 2000, 64, 39–51. [Google Scholar] [CrossRef]
- Babadi, D.; Dadashzadeh, S.; Osouli, M.; Daryabari, M.S.; Haeri, A. Nanoformulation strategies for improving intestinal permeability of drugs: A More precise look at permeability assessment methods and pharmacokinetic properties changes. J. Control. Release 2020, 321, 669–709. [Google Scholar] [CrossRef] [PubMed]
| Vehicle | Solubility of Raw XH (µg/mL) |
|---|---|
| Olive oil | 173.54 ± 10.46 |
| Cottonseed oil | 61.59 ± 3.07 |
| Peanut oil | 67.83 ± 4.75 |
| Eucalyptus oil | 200.02 ± 18.01 |
| Castor oil | 176.16 ± 15.85 |
| Labrafil M1944 CS | 82.56 ± 6.70 |
| Capmul MCM | 16.96 ± 1.17 |
| Labrafac PG | 480.39 ± 38.43 |
| Cremaphor EL | 113.30 ± 10.20 |
| Transcutol P | 525.31 ± 36.7 |
| Tween 80 | 81.18 ± 6.49 |
| PEG 200 | 228.65 ± 20.5 |
| Formulation Code | Composition for L-SNEDDS (1 mL) (LPG:T80:TP) (%) | Droplet Size (nm) (Mean ± s.d.) | Polydispersity Index (PDI) (Mean ± s.d.) | Drug Loading (%) (Mean ± s.d.) | Cloud Point (°C) (Mean ± s.d.) | Appearance |
|---|---|---|---|---|---|---|
| F1 | 10:45:45 | 108.1 ± 5.41 | 0.29 ± 0.014 | 46.42 ± 2.32 | 77.61 ± 3.84 | TP |
| F2 | 20:40:40 | 124.7 ± 6.20 | 0.36 ± 0.018 | 83.31 ± 4.16 | 89.23 ± 4.41 | TL |
| F10 | 10:30:60 | 112.9 ± 5.64 | 0.23 ± 0.011 | 94.20 ± 4.71 | 95.88 ± 4.73 | TP |
| F19 | 10:60:30 | 118.8 ± 5.94 | 0.41 ± 0.020 | 91.01 ± 4.55 | 94.61 ± 4.80 | TP |
| F20 | 20:53:27 | 134.5 ± 6.72 | 0.33 ± 0.016 | 81.21 ± 4.06 | 91.57 ± 4.06 | TL |
| Components | Flow Rate (g/s) | Angle of Repose (θ) | Bulk Density (g/cm3) | Tap Density (g/cm3) | CCI | Drug Loading (%) | Mean Droplet Size (nm) | PDI | Zeta Potential (mv) |
|---|---|---|---|---|---|---|---|---|---|
| L-SNEDDS (F10) | NA | NA | NA | NA | NA | 94.20 ± 4.71 | 112.9 ± 5.64 | 0.23 ± 0.01 | −19.08 ± 0.95 |
| After adsorpion onto solid carriers | |||||||||
| S-SNEDDS (F10) | |||||||||
| MCC | 2.74 ± 0.13 | 53.00 ± 2.65 | 0.20 ± 0.01 | 0.45 ± 0.02 | 55.55 ± 2.77 | 89.47 ± 4.47 | 143.21 ± 7.16 | 0.48 ± 0.02 | −19.43 ± 0.97 |
| GUG | 1.23 ± 0.06 | 28.83 ± 1.44 | 0.44 ± 0.02 | 0.60 ± 0.03 | 26.60 ± 1.33 | 92.14 ± 4.60 | 157.95 ± 7.89 | 0.29 ± 0.01 | −21.98 ± 1.09 |
| PTN | 2.01 ± 0.10 | 40.51 ± 2.02 | 0.43 ± 0.02 | 0.54 ± 0.02 | 20.37 ± 1.01 | 94.78 ± 4.73 | 134.46 ± 6.72 | 0.26 ± 0.01 | −19.87 ± 0.99 |
| SXDP | 3.16 ± 0.15 | 19.50 ± 0.97 | 0.18 ± 0.009 | 0.24 ± 0.01 | 18.18 ± 0.90 | 95.26 ± 4.76 | 124.76 ± 6.23 | 0.22 ± 0.01 | −18.56 ± 0.92 |
| MgS | 1.11 ± 0.05 | 30.31 ± 1.51 | 0.30 ± 0.01 | 0.44 ± 0.02 | 31.81 ± 1.59 | 87.43 ± 4.37 | 148.53 ± 7.42 | 0.36 ± 0.01 | −21.87 ± 1.09 |
| LM | 0.87 ± 0.04 | 29.42 ± 1.47 | 0.42 ± 0.02 | 0.57 ± 0.02 | 26.31 ± 1.31 | 85.41 ± 4.27 | 159.51 ± 7.97 | 0.45 ± 0.02 | −23.76 ± 1.18 |
| A-200 | 2.89 ± 0.14 | 20.76 ± 1.03 | 0.13 ± 0.006 | 0.29 ± 0.01 | 55.17 ± 2.75 | 92.97 ± 4.64 | 139.24 ± 6.96 | 0.39 ± 0.01 | −21.48 ± 1.05 |
| GUG:PTN:SXDP (1:1:1) | 3.03 ± 0.15 | 22.62 ± 1.23 | 0.17 ± 0.008 | 0.22 ± 0.01 | 22.74 ± 1.13 | 96.84 ± 4.81 | 118.96 ± 5.94 | 0.19 ± 0.009 | −22.78 ± 1.13 |
| Model | S-SNEDDS in RCC | L-SNEDDS | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| R2 | K | RMSE | AIC | BIC | R2 | K | RMSE | AIC | BIC | |
| Zero order | 0.6108 | 3.43 | 1.6952 | 6.5922 | 6.6081 | 0.1646 | 1.62 | 2.4401 | 1.204 | 1.2153 |
| First order | 0.3625 | 7.36 | 2.5069 | 7.2182 | 7.2341 | 0.1129 | 3.7 | 1.3423 | 2.246 | 2.2573 |
| Higuchi | 0.4995 | 1.74 | 1.9223 | 6.7933 | 6.8092 | 4.5899 | 1.23 | 6.312 | 1.4511 | 1.4624 |
| Krosmeyer Peppas | 0.5233 | 4.64 | 2.4185 | 7.1607 | 7.1766 | 0.9857 | 1.33 | 1.2026 | 1.6187 | 1.63 |
| Model | Raw XH | S-SNEDDS without RCC | ||||||||
| R2 | K | RMSE | AIC | BIC | R2 | K | RMSE | AIC | BIC | |
| Zero order | 0.8576 | 9.9 | 2.6917 | 6.3089 | 6.4219 | 0.8357 | 1.49 | 4.4091 | 7.592 | 7.705 |
| First order | 0.1591 | 4.06 | 3.2298 | 1.8756 | 1.8869 | 0.1732 | 4.27 | 5.2706 | 2.0029 | 2.0142 |
| Higuchi | 0.7273 | 1.66 | 9.3761 | 9.5536 | 9.6666 | 0.5941 | 1.91 | 1.3733 | 1.0545 | 1.0658 |
| Krosmeyer Peppas | 0.9978 | 1.76 | 6.9537 | 8.7765 | 8.8895 | 0.9988 | 2.03 | 6.8756 | 8.7472 | 8.8602 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hanmantrao, M.; Chaterjee, S.; Kumar, R.; Vishwas, S.; Harish, V.; Porwal, O.; Alrouji, M.; Alomeir, O.; Alhajlah, S.; Gulati, M.; et al. Development of Guar Gum-Pectin-Based Colon Targeted Solid Self-Nanoemulsifying Drug Delivery System of Xanthohumol. Pharmaceutics 2022, 14, 2384. https://doi.org/10.3390/pharmaceutics14112384
Hanmantrao M, Chaterjee S, Kumar R, Vishwas S, Harish V, Porwal O, Alrouji M, Alomeir O, Alhajlah S, Gulati M, et al. Development of Guar Gum-Pectin-Based Colon Targeted Solid Self-Nanoemulsifying Drug Delivery System of Xanthohumol. Pharmaceutics. 2022; 14(11):2384. https://doi.org/10.3390/pharmaceutics14112384
Chicago/Turabian StyleHanmantrao, Mahesh, Sourabh Chaterjee, Rajan Kumar, Sukriti Vishwas, Vancha Harish, Omji Porwal, Mohammed Alrouji, Othman Alomeir, Sharif Alhajlah, Monica Gulati, and et al. 2022. "Development of Guar Gum-Pectin-Based Colon Targeted Solid Self-Nanoemulsifying Drug Delivery System of Xanthohumol" Pharmaceutics 14, no. 11: 2384. https://doi.org/10.3390/pharmaceutics14112384
APA StyleHanmantrao, M., Chaterjee, S., Kumar, R., Vishwas, S., Harish, V., Porwal, O., Alrouji, M., Alomeir, O., Alhajlah, S., Gulati, M., Gupta, G., Dua, K., & Singh, S. K. (2022). Development of Guar Gum-Pectin-Based Colon Targeted Solid Self-Nanoemulsifying Drug Delivery System of Xanthohumol. Pharmaceutics, 14(11), 2384. https://doi.org/10.3390/pharmaceutics14112384








