Journey of Rosmarinic Acid as Biomedicine to Nano-Biomedicine for Treating Cancer: Current Strategies and Future Perspectives
Abstract
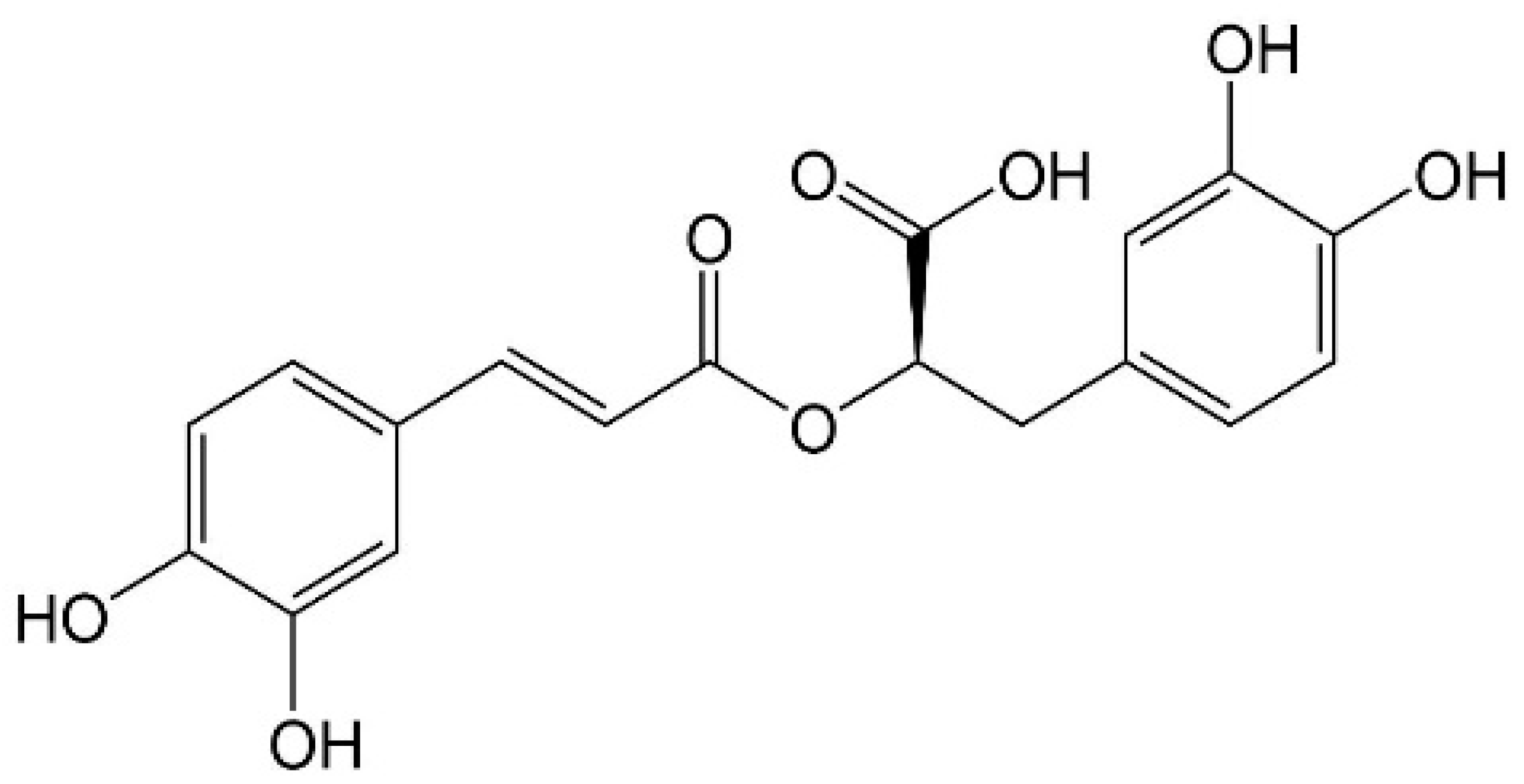
1. Introduction
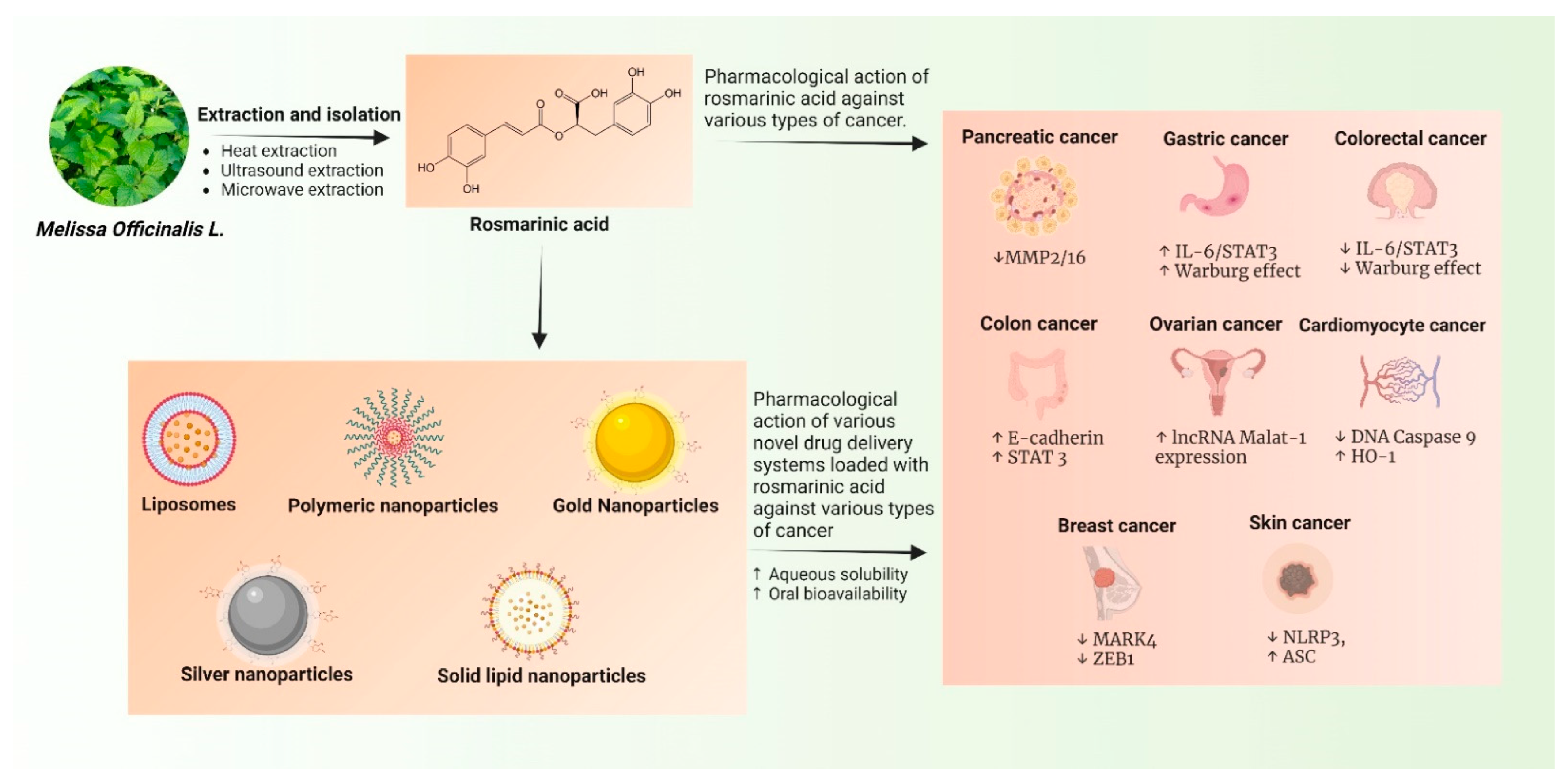
2. Extraction Processes of RA
3. Role of RA in Various Types of Cancer: Emphasis on Their Mechanism of Action
3.1. Oral Cancer
3.2. Colorectal Cancer (CRC)
3.3. Ovarian Cancer
3.4. Cervical Cancer
3.5. Lung Epithelial Cancer
3.6. Non-Small Cell Lung Cancer (NSCLC)
3.7. Breast Cancer
3.8. Brain Tumour
3.9. Osteosarcoma
3.10. Gastric Cancer
3.11. Skin Cancer
3.12. Renal Carcinoma
3.13. Cardiomyocyte
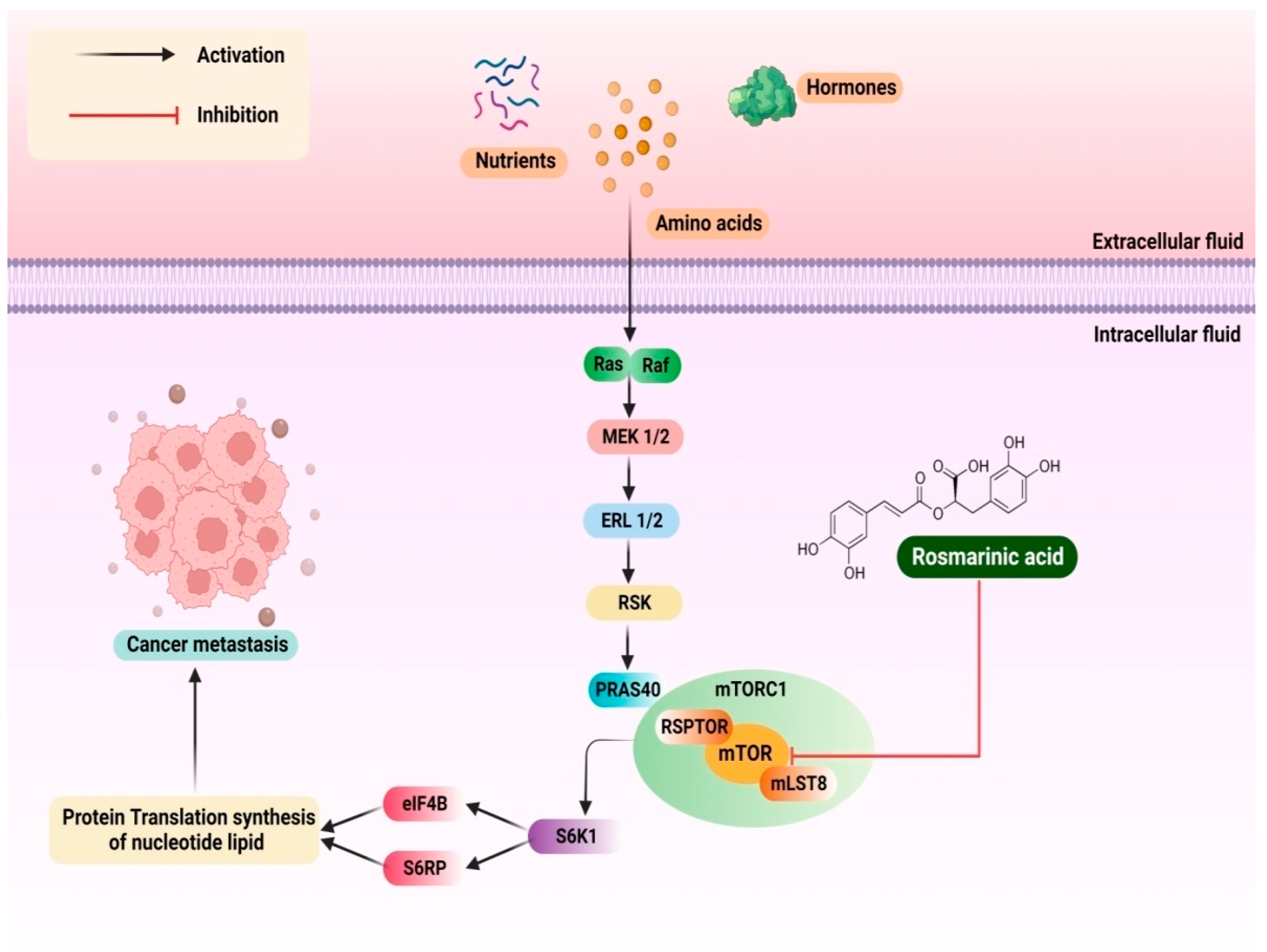
3.14. Pancreatic Cancer
3.15. Prostate Cancer
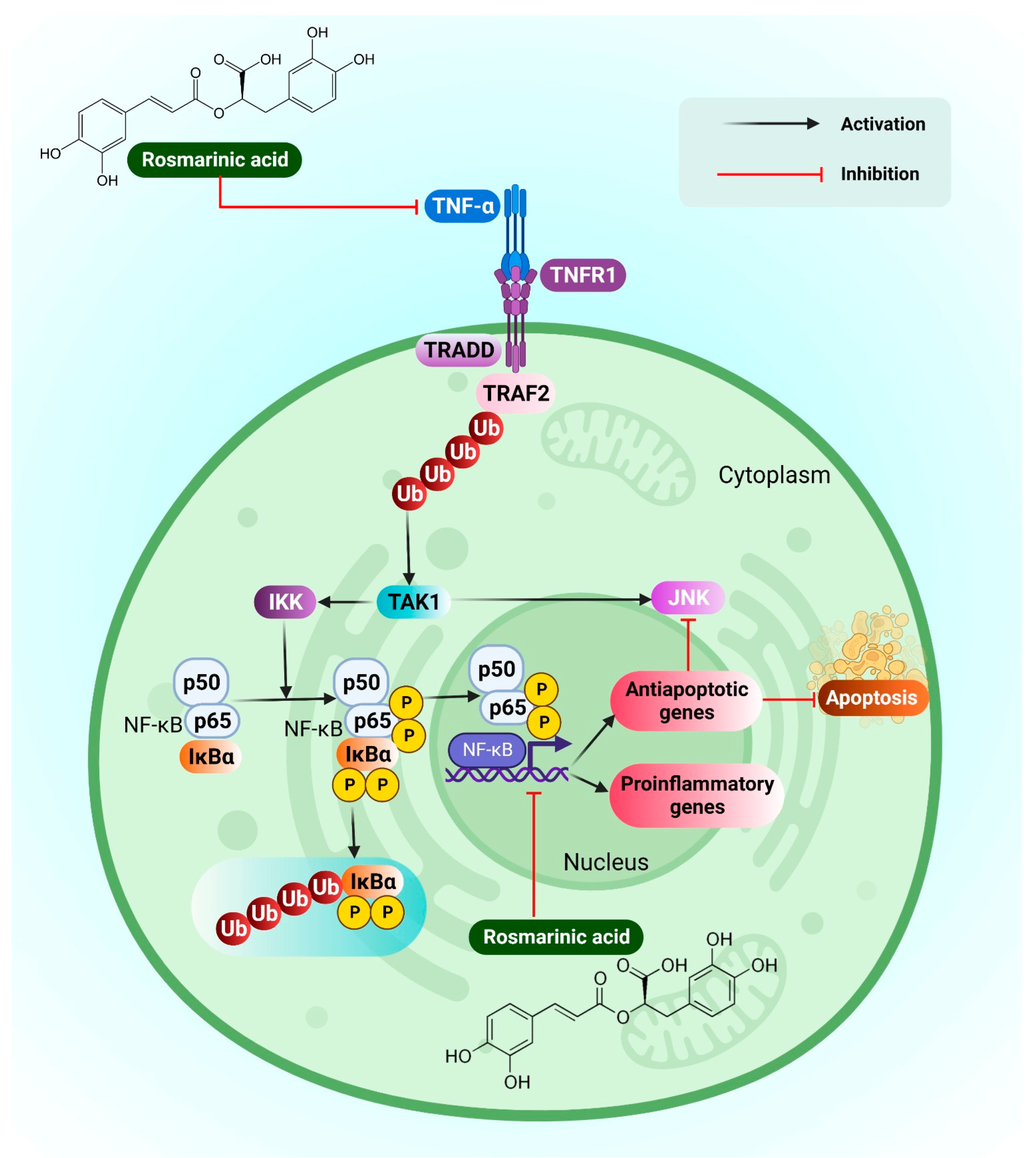
3.16. Hepatocellular Carcinoma
3.17. Leukemia
4. Patents Granted for RA Isolation
5. Challenges in Clinical Translation of RA into Anti-Cancer Therapy
6. Novel Drug Delivery Systems of RA against Cancer
6.1. Liposomes
6.2. Polymeric Nanoparticles
6.3. Gold Nanoparticles
6.4. Solid Lipid Nanoparticles (SLNs)
6.5. Silver Nanoparticles
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics, 2022. CA Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Soerjomataram, I.; Bray, F. Planning for tomorrow: Global cancer incidence and the role of prevention 2020–2070. Nat. Rev. Clin. Oncol. 2021, 18, 663–672. [Google Scholar] [CrossRef] [PubMed]
- GBD 2019 Diseases and Injuries Collaborators. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: A systematic analysis for the global burden of disease study 2019. Lancet 2020, 396, 1204–1222. [Google Scholar] [CrossRef]
- Ferlay, J.; Laversanne, M.E.M. Global Cancer Observatory: Cancer Tomorrow. 2021. Available online: https://gco.iarc.fr/tomorrow/en (accessed on 22 October 2022).
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics, 2021. CA Cancer J. Clin. 2021, 71, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Wong, R.S.Y. Apoptosis in cancer: From pathogenesis to treatment. J. Exp. Clin. Cancer Res. 2011, 30, 87. [Google Scholar] [CrossRef]
- Zaimy, M.A.; Saffarzadeh, N.; Mohammadi, A.; Pourghadamyari, H.; Izadi, P.; Sarli, A.; Moghaddam, L.K.; Paschepari, S.R.; Azizi, H.; Torkamandi, S.; et al. New methods in the diagnosis of cancer and gene therapy of cancer based on nanoparticles. Cancer Gene Ther. 2017, 24, 233–243. [Google Scholar] [CrossRef]
- Jiang, T.A. Health Benefits of Culinary Herbs and Spices. J. AOAC Int. 2019, 102, 395–411. [Google Scholar] [CrossRef]
- Dreher, M.L. Whole fruits and fruit fiber emerging health effects. Nutrients 2018, 10, 1833. [Google Scholar] [CrossRef]
- Soundararajan, P.; Kim, J.S. Anti-carcinogenic glucosinolates in cruciferous vegetables and their antagonistic effects on prevention of cancers. Molecules 2018, 23, 2983. [Google Scholar] [CrossRef]
- Wang, S.; Long, S.; Deng, Z.; Wu, W. Positive role of chinese herbal medicine in cancer immune regulation. Am. J. Chin. Med. 2020, 48, 1577–1592. [Google Scholar] [CrossRef]
- Brglez Mojzer, E.; Knez Hrnčič, M.; Škerget, M.; Knez, Ž.; Bren, U. Polyphenols: Extraction methods, antioxidative action, bioavailability and anticarcinogenic effects. Molecules 2016, 21, 901. [Google Scholar] [CrossRef] [PubMed]
- Soni, D.; Grover, A. “Picrosides” from picrorhiza kurroa as potential anti-carcinogenic agents. Biomed. Pharmacother. 2019, 109, 1680–1687. [Google Scholar] [CrossRef] [PubMed]
- Vuong, Q.V.; Golding, J.B.; Nguyen, M.; Roach, P.D. Extraction and isolation of catechins from Tea. J. Sep. Sci. 2010, 33, 3415–3428. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-García, C.; Sánchez-Quesada, C.; Gaforio, J.J. Dietary flavonoids as cancer chemopreventive agents: An updated review of human studies. Antioxidants 2019, 8, 137. [Google Scholar] [CrossRef] [PubMed]
- Selvakumar, P.; Badgeley, A.; Murphy, P.; Anwar, H.; Sharma, U.; Lawrence, K.; Lakshmikuttyamma, A. Flavonoids and other polyphenols act as epigenetic modifiers in breast cancer. Nutrients 2020, 12, 761. [Google Scholar] [CrossRef]
- Moore, J.; Yousef, M.; Tsiani, E. Anticancer Effects of Rosemary (Rosmarinus officinalis L.) Extract and rosemary extract polyphenols. Nutrients 2016, 8, 731. [Google Scholar] [CrossRef]
- Oyenihi, O.R.; Oyenihi, A.B.; Erhabor, J.O.; Matsabisa, M.G.; Oguntibeju, O.O. Unravelling the anticancer mechanisms of traditional herbal medicines with metabolomics. Molecules 2021, 26, 6541. [Google Scholar] [CrossRef] [PubMed]
- Jones, R.A.; Taylor, A.G.; Bourguignon, C.; Steeves, R.; Fraser, G.; Lippert, M.; Theodorescu, D.; Mathews, H.; Kilbridge, K.L. Complementary and alternative medicine modality use and beliefs among african american prostate cancer survivors. Oncol. Nurs. Forum 2007, 34, 359–364. [Google Scholar] [CrossRef]
- al-Sereiti, M.R.; Abu-Amer, K.M.; Sen, P. Pharmacology of rosemary (Rosmarinus officinalis linn.) and its therapeutic potentials. Indian J. Exp. Biol. 1999, 37, 124–130. [Google Scholar]
- Hitl, M.; Kladar, N.; Gavarić, N.; Božin, B. Rosmarinic acid-human pharmacokinetics and health benefits. Planta Med. 2021, 87, 273–282. [Google Scholar] [CrossRef]
- Alagawany, M.; Abd El-Hack, M.E.; Farag, M.R.; Gopi, M.; Karthik, K.; Malik, Y.S.; Dhama, K. Rosmarinic Acid: Modes of action, medicinal values and health benefits. Anim. Health Res. Rev. 2017, 18, 167–176. [Google Scholar] [CrossRef] [PubMed]
- Swamy, M.K.; Sinniah, U.R.; Ghasemzadeh, A. Anticancer potential of rosmarinic acid and its improved production through biotechnological interventions and functional genomics. Appl. Microbiol. Biotechnol. 2018, 102, 7775–7793. [Google Scholar] [CrossRef] [PubMed]
- Pantan, R.; Tocharus, J.; Nakaew, A.; Suksamrarn, A.; Tocharus, C. Ethyl rosmarinate prevents the impairment of vascular function and morphological changes in l-name-induced hypertensive rats. Medicina 2019, 55, 777. [Google Scholar] [CrossRef] [PubMed]
- Jordán, M.J.; Lax, V.; Rota, M.C.; Lorán, S.; Sotomayor, J.A. Relevance of carnosic acid, carnosol, and rosmarinic acid concentrations in the in vitro antioxidant and antimicrobial activities of Rosmarinus officinalis (L.) methanolic extracts. J. Agric. Food Chem. 2012, 60, 9603–9608. [Google Scholar] [CrossRef]
- Tsukamoto, Y.; Ikeda, S.; Uwai, K.; Taguchi, R.; Chayama, K.; Sakaguchi, T.; Narita, R.; Yao, W.-L.; Takeuchi, F.; Otakaki, Y.; et al. Rosmarinic acid is a novel inhibitor for hepatitis b virus replication targeting viral epsilon rna-polymerase interaction. PLoS ONE 2018, 13, e0197664. [Google Scholar] [CrossRef]
- Jiang, K.; Ma, X.; Guo, S.; Zhang, T.; Zhao, G.; Wu, H.; Wang, X.; Deng, G. Anti-inflammatory effects of rosmarinic acid in lipopolysaccharide-induced mastitis in mice. Inflammation 2018, 41, 437–448. [Google Scholar] [CrossRef]
- Radziejewska, I.; Supruniuk, K.; Bielawska, A. Anti-cancer effect of combined action of anti-muc1 and rosmarinic acid in ags gastric cancer cells. Eur. J. Pharmacol. 2021, 902, 174119. [Google Scholar] [CrossRef]
- de Formiga, R.O.; Alves Júnior, E.B.; Vasconcelos, R.C.; Guerra, G.C.B.; de Araújo, A.A.; de Carvalho, T.G.; Garcia, V.B.; de Araújo Junior, R.F.; Gadelha, F.A.A.F.; Vieira, G.C.; et al. p-cymene and rosmarinic acid ameliorate tnbs-induced intestinal inflammation upkeeping zo-1 and muc-2: Role of antioxidant system and immunomodulation. Int. J. Mol. Sci. 2020, 21, 5870. [Google Scholar] [CrossRef]
- Aldoghachi, F.E.H.; Noor Al-Mousawi, U.M.; Shari, F.H. Antioxidant activity of rosmarinic acid extracted and purified from mentha piperita. Arch. Razi Inst. 2021, 76, 1279–1287. [Google Scholar] [CrossRef]
- Han, Y.-H.; Kee, J.-Y.; Hong, S.-H. Rosmarinic acid activates ampk to inhibit metastasis of colorectal cancer. Front. Pharmacol. 2018, 9, 68. [Google Scholar] [CrossRef]
- Huang, L.; Chen, J.; Quan, J.; Xiang, D. Rosmarinic acid inhibits proliferation and migration, promotes apoptosis and enhances cisplatin sensitivity of melanoma cells through inhibiting ADAM17/EGFR/AKT/GSK3β Axis. Bioengineered 2021, 12, 3065–3076. [Google Scholar] [CrossRef] [PubMed]
- Jin, B.-R.; Chung, K.-S.; Hwang, S.; Hwang, S.N.; Rhee, K.-J.; Lee, M.; An, H.-J. Rosmarinic acid represses colitis-associated colon cancer: A pivotal involvement of the tlr4-mediated NF-ΚB-STAT3 Axis. Neoplasia 2021, 23, 561–573. [Google Scholar] [CrossRef] [PubMed]
- Han, S.; Yang, S.; Cai, Z.; Pan, D.; Li, Z.; Huang, Z.; Zhang, P.; Zhu, H.; Lei, L.; Wang, W. Anti-warburg effect of rosmarinic acid via mir-155 in gastric cancer cells. Drug Des. Devel. Ther. 2015, 9, 2695–2703. [Google Scholar] [CrossRef] [PubMed]
- Gui, H.; Jin, Y.; Lin, A.; Wang, P.; Wang, Y.; Zhu, H. Rosmarinic acid relieves cisplatin-induced ovary toxicity in female mice via suppression of oxidative stress and inflammation. J. Biochem. Mol. Toxicol. 2021, 35, e22839. [Google Scholar] [CrossRef] [PubMed]
- Su, C.-H.; Pham, T.T.T.; Cheng, H.-H. Aqueous enzymatic extraction of rosmarinic acid from salvia officinalis: Optimisation using response surface methodology. Phytochem. Anal. 2020, 31, 575–582. [Google Scholar] [CrossRef]
- Tungmunnithum, D.; Garros, L.; Drouet, S.; Renouard, S.; Lainé, E.; Hano, C. Green Ultrasound assisted extraction of trans rosmarinic acid from Plectranthus scutellarioides (L.) r.br. leaves. Plants 2019, 8, 50. [Google Scholar] [CrossRef]
- Sik, B.; Hanczné, E.L.; Kapcsándi, V.; Ajtony, Z. Conventional and nonconventional extraction techniques for optimal extraction processes of rosmarinic acid from six lamiaceae plants as determined by HPLC-DAD measurement. J. Pharm. Biomed. Anal. 2020, 184, 113173. [Google Scholar] [CrossRef]
- Liu, T.; Sui, X.; Zhang, R.; Yang, L.; Zu, Y.; Zhang, L.; Zhang, Y.; Zhang, Z. Application of ionic liquids based microwave-assisted simultaneous extraction of carnosic acid, rosmarinic acid and essential oil from rosmarinus officinalis. J. Chromatogr. A 2011, 1218, 8480–8489. [Google Scholar] [CrossRef]
- Jafaripour, L.; Naserzadeh, R.; Alizamani, E.; Javad Mashhadi, S.M.; Moghadam, E.R.; Nouryazdan, N.; Ahmadvand, H. Effects of rosmarinic acid on methotrexate-induced nephrotoxicity and hepatotoxicity in wistar rats. Indian J. Nephrol. 2021, 31, 218–224. [Google Scholar] [CrossRef]
- Yang, K.; Shen, Z.; Zou, Y.; Gao, K. Rosmarinic Acid Inhibits Migration, Invasion, and P38/AP-1 signaling via mir-1225-5p in colorectal cancer cells. J. Recept. Signal Transduct. Res. 2021, 41, 284–293. [Google Scholar] [CrossRef]
- Nam, K.H.; Yi, S.A.; Nam, G.; Noh, J.S.; Park, J.W.; Lee, M.G.; Park, J.H.; Oh, H.; Lee, J.; Lee, K.R.; et al. Identification of a Novel S6K1 Inhibitor, Rosmarinic Acid Methyl Ester, for Treating Cisplatin-Resistant Cervical Cancer. BMC Cancer 2019, 19, 773. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Wang, W.; Li, Z.; Chen, L.; Wen, C.; Ruan, Q.; Xu, Z.; Liu, R.; Xu, J.; Bai, Y.; et al. Rosmarinic acid decreases the malignancy of pancreatic cancer through inhibiting gli1 signaling. Phytomedicine 2022, 95, 153861. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Ma, Z.; Xu, X.; Qi, H.; Cheng, Z.; Chen, L. Anticancer effects of rosmarinic acid in human oral cancer cells is mediated via endoplasmic reticulum stress, apoptosis, G2/M Cell Cycle Arrest and Inhibition of Cell Migration. J. BUON 2020, 25, 1245–1250. [Google Scholar] [PubMed]
- Xu, Y.; Han, S.; Lei, K.; Chang, X.; Wang, K.; Li, Z.; Liu, J. Anti-warburg effect of rosmarinic acid via mir-155 in colorectal carcinoma cells. Eur. J. Cancer Prev. Off. J. Eur. Cancer Prev. Organ. 2016, 25, 481–489. [Google Scholar] [CrossRef] [PubMed]
- Scheckel, K.A.; Degner, S.C.; Romagnolo, D.F. Rosmarinic acid antagonizes activator protein-1-dependent activation of cyclooxygenase-2 expression in human cancer and nonmalignant cell lines. J. Nutr. 2008, 138, 2098–2105. [Google Scholar] [CrossRef]
- Liu, Y.; Xu, X.; Tang, H.; Pan, Y.; Hu, B.; Huang, G. Rosmarinic acid inhibits cell proliferation, migration, and invasion and induces apoptosis in human glioma cells. Int. J. Mol. Med. 2021, 47, 1–11. [Google Scholar] [CrossRef]
- Venkatachalam, K.; Gunasekaran, S.; Jesudoss, V.A.S.; Namasivayam, N. The effect of rosmarinic acid on 1,2-dimethylhydrazine induced colon carcinogenesis. Exp. Toxicol. Pathol. Off. J. Gesellschaft Fur Toxikologische Pathol. 2013, 65, 409–418. [Google Scholar] [CrossRef]
- Zhang, Y.; Hu, M.; Liu, L.; Cheng, X.-L.; Cai, J.; Zhou, J.; Wang, T. Anticancer effects of rosmarinic acid in ovcar-3 ovarian cancer cells are mediated via induction of apoptosis, suppression of cell migration and modulation of LncRNA MALAT-1 Expression. J. BUON 2018, 23, 763–768. [Google Scholar]
- Liao, X.-Z.; Gao, Y.; Sun, L.-L.; Liu, J.-H.; Chen, H.-R.; Yu, L.; Chen, Z.-Z.; Chen, W.-H.; Lin, L.-Z. Rosmarinic acid reverses non-small cell lung cancer cisplatin resistance by activating the mapk signaling pathway. Phytother. Res. 2020, 34, 1142–1153. [Google Scholar] [CrossRef]
- Anwar, S.; Shamsi, A.; Shahbaaz, M.; Queen, A.; Khan, P.; Hasan, G.M.; Islam, A.; Alajmi, M.F.; Hussain, A.; Ahmad, F.; et al. Rosmarinic acid exhibits anticancer effects via mark4 inhibition. Sci. Rep. 2020, 10, 10300. [Google Scholar] [CrossRef]
- Messeha, S.S.; Zarmouh, N.O.; Asiri, A.; Soliman, K.F.A. Rosmarinic acid-induced apoptosis and cell cycle arrest in triple-negative breast cancer cells. Eur. J. Pharmacol. 2020, 885, 173419. [Google Scholar] [CrossRef] [PubMed]
- Juskowiak, B.; Bogacz, A.; Wolek, M.; Kamiński, A.; Uzar, I.; Seremak, M.A.; Czerny, B. Expression profiling of genes modulated by rosmarinic acid (ra) in mcf-7 breast cancer cells. Ginekol. Pol. 2018, 89, 541–545. [Google Scholar] [CrossRef] [PubMed]
- Niforou, K.M.; Anagnostopoulos, A.K.; Vougas, K.; Kittas, C.; Gorgoulis, V.G.; Tsangaris, G.T. The proteome profile of the human osteosarcoma U2OS Cell Line. Cancer Genom. Proteom. 2008, 5, 63–78. [Google Scholar]
- Ma, Z.; Yang, J.; Yang, Y.; Wang, X.; Chen, G.; Shi, A.; Lu, Y.; Jia, S.; Kang, X.; Lu, L. Rosmarinic acid exerts an anticancer effect on osteosarcoma cells by inhibiting DJ-1 via Regulation of the PTEN-PI3K-Akt signaling Pathway. Phytomedicine 2020, 68, 153186. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Gao, W. DJ-1 Expression in cervical carcinoma and its effects on cell viability and apoptosis. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2016, 22, 2943–2949. [Google Scholar] [CrossRef][Green Version]
- Sharmila, R.; Manoharan, S. Anti-tumor activity of rosmarinic acid in 7,12-Dimethylbenz(a)Anthracene (DMBA) induced skin carcinogenesis in swiss albino mice. Indian J. Exp. Biol. 2012, 50, 187–194. [Google Scholar]
- Chou, S.T.; Ho, B.Y.; Tai, Y.T.; Huang, C.J.; Chao, W.W. Bidirect effects from cisplatin combine with rosmarinic acid (ra) or hot water extracts of glechoma hederacea (HWG) on renal cancer cells. Chin. Med. 2020, 15, 77. [Google Scholar] [CrossRef]
- Zhang, Q.; Li, J.; Peng, S.; Zhang, Y.; Qiao, Y. Rosmarinic acid as a candidate in a phenotypic profiling cardio-/cytotoxicity cell model induced by doxorubicin. Molecules 2020, 25, 836. [Google Scholar] [CrossRef]
- Han, Y.; Ma, L.; Zhao, L.; Feng, W.; Zheng, X. Rosmarinic inhibits cell proliferation, invasion and migration via up-regulating mir-506 and suppressing MMP2/16 expression in pancreatic cancer. Biomed. Pharmacother. 2019, 115, 108878. [Google Scholar] [CrossRef]
- Jang, Y.-G.; Hwang, K.-A.; Choi, K.-C. Rosmarinic acid, a component of rosemary tea, induced the cell cycle arrest and apoptosis through modulation of HDAC2 expression in prostate cancer cell lines. Nutrients 2018, 10, 1784. [Google Scholar] [CrossRef]
- Cao, W.; Hu, C.; Wu, L.; Xu, L.; Jiang, W. Rosmarinic acid inhibits inflammation and angiogenesis of hepatocellular carcinoma by suppression of NF-ΚB signaling in H22 tumor-bearing mice. J. Pharmacol. Sci. 2016, 132, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Renzulli, C.; Galvano, F.; Pierdomenico, L.; Speroni, E.; Guerra, M.C. Effects of eosmarinic acid against aflatoxin B1 and ochratoxin-A-induced cell damage in a human hepatoma cell line (Hep G2). J. Appl. Toxicol. 2004, 24, 289–296. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.S.; Kuo, C.L.; Wang, J.P.; Cheng, J.S.; Huang, Z.W.; Chen, C.F. Growth inhibitory and apoptosis inducing effect of perilla frutescens extract on human hepatoma hepg2 cells. J. Ethnopharmacol. 2007, 112, 557–567. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Zhu, Y.; Li, F.; Zhang, G.; Shi, J.; Ou, R.; Tong, Y.; Liu, Y.; Liu, L.; Lu, L.; et al. Spica prunellae and its marker compound rosmarinic acid induced the expression of efflux transporters through activation of nrf2-mediated signaling pathway in HepG2 cells. J. Ethnopharmacol. 2016, 193, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Moon, D.-O.; Kim, M.-O.; Lee, J.-D.; Choi, Y.H.; Kim, G.-Y. Rosmarinic acid sensitizes cell death through suppression of tnf-alpha-induced nf-kappab activation and ros generation in human leukemia U937 cells. Cancer Lett. 2010, 288, 183–191. [Google Scholar] [CrossRef]
- Heo, S.-K.; Noh, E.-K.; Yoon, D.-J.; Jo, J.-C.; Koh, S.; Baek, J.H.; Park, J.-H.; Min, Y.J.; Kim, H. Rosmarinic acid potentiates atra-induced macrophage differentiation in acute promyelocytic leukemia NB4 cells. Eur. J. Pharmacol. 2015, 747, 36–44. [Google Scholar] [CrossRef]
- Saiko, P.; Steinmann, M.-T.; Schuster, H.; Graser, G.; Bressler, S.; Giessrigl, B.; Lackner, A.; Grusch, M.; Krupitza, G.; Bago-Horvath, Z.; et al. Epigallocatechin gallate, ellagic acid, and rosmarinic acid perturb dntp pools and inhibit de novo dna synthesis and proliferation of human hl-60 promyelocytic leukemia cells: Synergism with arabinofuranosylcytosine. Phytomedicine 2015, 22, 213–222. [Google Scholar] [CrossRef]
- Wu, C.-F.; Hong, C.; Klauck, S.M.; Lin, Y.-L.; Efferth, T. Molecular mechanisms of rosmarinic acid from salvia miltiorrhiza in acute lymphoblastic leukemia cells. J. Ethnopharmacol. 2015, 176, 55–68. [Google Scholar] [CrossRef]
- Christ, B.; Kurtkesselring, K. Process for Isolating Rosmarinic Acid from Plants. U.S. Patent US4354035A, 12 October 1982. [Google Scholar]
- Kott, L.; Fletcher, R. Production of Rosmarinic Acid from Spearmint and Uses Thereof. Canada Patent CA2676353A1, 23 January 2008. [Google Scholar]
- Wang, J.; Xu, H.; Jiang, H.; Du, X.; Sun, P.; Xie, J. Neurorescue Effect of Rosmarinic acid on 6-hydroxydopamine-lesioned nigral dopamine neurons in rat model of Parkinson’s Disease. J. Mol. Neurosci. 2012, 47, 113–119. [Google Scholar] [CrossRef]
- Shang, A.-J.; Yang, Y.; Wang, H.-Y.; Tao, B.-Z.; Wang, J.; Wang, Z.-F.; Zhou, D.-B. Spinal cord injury effectively ameliorated by neuroprotective effects of rosmarinic acid. Nutr. Neurosci. 2017, 20, 172–179. [Google Scholar] [CrossRef]
- Yang, J.H.; Mao, K.J.; Huang, P.; Ye, Y.J.; Guo, H.S.; Cai, B.C. Effect of piperine on the bioavailability and pharmacokinetics of rosmarinic acid in rat plasma using UPLC-MS/MS. Xenobiotica 2018, 48, 178–185. [Google Scholar] [CrossRef] [PubMed]
- Casanova, F.; Estevinho, B.N.; Santos, L. Preliminary studies of rosmarinic acid microencapsulation with chitosan and modified chitosan for topical delivery. Powder Technol. 2016, 297, 44–49. [Google Scholar] [CrossRef]
- Yang, J.-H.; Zhang, L.; Li, J.-S.; Chen, L.-H.; Zheng, Q.; Chen, T.; Chen, Z.-P.; Fu, T.-M.; Di, L.-Q. Enhanced oral bioavailability and prophylactic effects on oxidative stress and hepatic damage of an oil solution containing a rosmarinic acid–phospholipid complex. J. Funct. Foods 2015, 19, 63–73. [Google Scholar] [CrossRef]
- Lucía, G.; An-Sophie, C.; María, I.C.; Rita, Y.C.; Iciar, A.D. Bioaccessibility of Rutin, Caffeic acid and rosmarinic acid: Influence of the in vitro gastrointestinal digestion models. J. Funct. Foods 2016, 26, 428–438. [Google Scholar]
- Aksamija, A.; Polidori, A.; Plasson, R.; Dangles, O.; Tomao, V. The inclusion complex of rosmarinic acid into beta-cyclodextrin: A thermodynamic and structural analysis by NMR and capillary electrophoresis. Food Chem. 2016, 208, 258–263. [Google Scholar] [CrossRef] [PubMed]
- Flávia, N.S.F.; Luana, R.M.; Juliana, H.A.; Gabriela, S.L. Chitosan-coated rosmarinic acid nanoemulsion nasal administration protects against lps-induced memory deficit, neuroinflammation, and oxidative stress in wistar rats. Neurochem. Int. 2020, 141, 104875. [Google Scholar]
- Huang, J.; Chen, P.X.; Rogers, M.A.; Wettig, S.D. Investigating the phospholipid effect on the bioaccessibility of rosmarinic acid-phospholipid complex through a dynamic gastrointestinal in vitro model. Pharmaceutics 2019, 11, 156. [Google Scholar] [CrossRef]
- Madureira, A.R.; Campos, D.A.; Oliveira, A.; Sarmento, B.; Pintado, M.M.; Gomes, A.M. Insights into the protective role of solid lipid nanoparticles on rosmarinic acid bioactivity during exposure to simulated gastrointestinal conditions. Colloids Surf. B. Biointerfaces 2016, 139, 277–284. [Google Scholar] [CrossRef]
- da Silva, S.B.; Ferreira, D.; Pintado, M.; Sarmento, B. Chitosan-based nanoparticles for rosmarinic acid ocular delivery in vitro tests. Int. J. Biol. Macromol. 2016, 84, 112–120. [Google Scholar] [CrossRef]
- Xue, X.; Ricci, M.; Qu, H.; Lindstrom, A.; Zhang, D.; Wu, H.; Lin, T.-Y.; Li, Y. Iron-crosslinked rososome with robust stability and high drug loading for synergistic cancer therapy. J. Control. Release Off. J. Control. Release Soc. 2021, 329, 794–804. [Google Scholar] [CrossRef]
- Subongkot, T.; Ngawhirunpat, T.; Opanasopit, P. Development of Ultradeformable Liposomes with Fatty Acids for Enhanced Dermal Rosmarinic Acid Delivery. Pharmaceutics 2021, 13, 404. [Google Scholar] [CrossRef] [PubMed]
- Fuster, M.G.; Carissimi, G.; Montalbán, M.G.; Víllora, G. Antitumor activity of rosmarinic acid-loaded silk fibroin nanoparticles on hela and MCF-7 cells. Polymers 2021, 13, 3169. [Google Scholar] [CrossRef] [PubMed]
- Tabatabaeian, K.; Simayee, M.; Fallah, S.A.; Mashayekhi, F. N-Doped Carbon Nanodots@UiO-66-NH(2) as Novel Nanoparticles for Releasing of the Bioactive Drug, Rosmarinic Acid and Fluorescence Imaging. Daru 2019, 27, 307–315. [Google Scholar] [CrossRef] [PubMed]
- Chung, C.H.; Jung, W.; Keum, H.; Kim, T.W.; Jon, S. Nanoparticles derived from the natural antioxidant rosmarinic acid ameliorate acute inflammatory bowel disease. ACS Nano 2020, 14, 6887–6896. [Google Scholar] [CrossRef] [PubMed]
- Ferreira-Gonçalves, T.; Gaspar, M.M.; Coelho, J.M.P.; Marques, V.; Viana, A.S.; Ascensão, L.; Carvalho, L.; Rodrigues, C.M.P.; Ferreira, H.A.; Ferreira, D.; et al. The role of rosmarinic acid on the bioproduction of gold nanoparticles as part of a photothermal approach for breast cancer treatment. Biomolecules 2022, 12, 71. [Google Scholar] [CrossRef]
- Campos, D.A.; Madureira, A.R.; Gomes, A.M.; Sarmento, B.; Pintado, M.M. Optimization of the production of solid witepsol nanoparticles loaded with rosmarinic acid. Colloids Surf. B. Biointerfaces 2014, 115, 109–117. [Google Scholar] [CrossRef]
- Bhatt, S.; Vyas, G.; Paul, P. Rosmarinic acid-capped silver nanoparticles for colorimetric detection of cn- and redox-modulated surface reaction-aided detection of Cr(VI) in water. ACS Omega 2022, 7, 1318–1328. [Google Scholar] [CrossRef]


| S.No. | Type of Cancer | Estimated New Cases | Estimated Deaths |
|---|---|---|---|
| 1 | Breast | 287,850 (Female) | 43,250 |
| 2710 (male) | 530 | ||
| 2. | Colon | 106,180 | 52,580 |
| 3. | Rectum | 44,850 | |
| 4. | Kidney | 79,000 | 13,920 |
| 5. | Leukaemia | 60,650 | 24,000 |
| 6. | Liver | 41,260 | 30,520 |
| 7. | Lung and bronchus | 236,740 | 130,180 |
| 8. | Lymphoma | 89,010 | 21,170 |
| 9. | Pharynx | 54,000 | 11,230 |
| 10. | Ovary | 19,880 | 12,810 |
| 11. | Prostate | 268,490 | 34,500 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chaitanya, M.V.N.L.; Ramanunny, A.K.; Babu, M.R.; Gulati, M.; Vishwas, S.; Singh, T.G.; Chellappan, D.K.; Adams, J.; Dua, K.; Singh, S.K. Journey of Rosmarinic Acid as Biomedicine to Nano-Biomedicine for Treating Cancer: Current Strategies and Future Perspectives. Pharmaceutics 2022, 14, 2401. https://doi.org/10.3390/pharmaceutics14112401
Chaitanya MVNL, Ramanunny AK, Babu MR, Gulati M, Vishwas S, Singh TG, Chellappan DK, Adams J, Dua K, Singh SK. Journey of Rosmarinic Acid as Biomedicine to Nano-Biomedicine for Treating Cancer: Current Strategies and Future Perspectives. Pharmaceutics. 2022; 14(11):2401. https://doi.org/10.3390/pharmaceutics14112401
Chicago/Turabian StyleChaitanya, Motamarri Venkata Naga Lalitha, Arya Kadukkattil Ramanunny, Malakapogu Ravindra Babu, Monica Gulati, Sukriti Vishwas, Thakur Gurjeet Singh, Dinesh Kumar Chellappan, Jon Adams, Kamal Dua, and Sachin Kumar Singh. 2022. "Journey of Rosmarinic Acid as Biomedicine to Nano-Biomedicine for Treating Cancer: Current Strategies and Future Perspectives" Pharmaceutics 14, no. 11: 2401. https://doi.org/10.3390/pharmaceutics14112401
APA StyleChaitanya, M. V. N. L., Ramanunny, A. K., Babu, M. R., Gulati, M., Vishwas, S., Singh, T. G., Chellappan, D. K., Adams, J., Dua, K., & Singh, S. K. (2022). Journey of Rosmarinic Acid as Biomedicine to Nano-Biomedicine for Treating Cancer: Current Strategies and Future Perspectives. Pharmaceutics, 14(11), 2401. https://doi.org/10.3390/pharmaceutics14112401








