Advances in Biomedical Functions of Natural Whitening Substances in the Treatment of Skin Pigmentation Diseases
Abstract
1. Introduction
2. Mechanism in the Development and Progression of Pigmentation
3. Pigmentation Diseases
4. Biomedical Functions of Natural Whitening Substances on Pigmentation Diseases
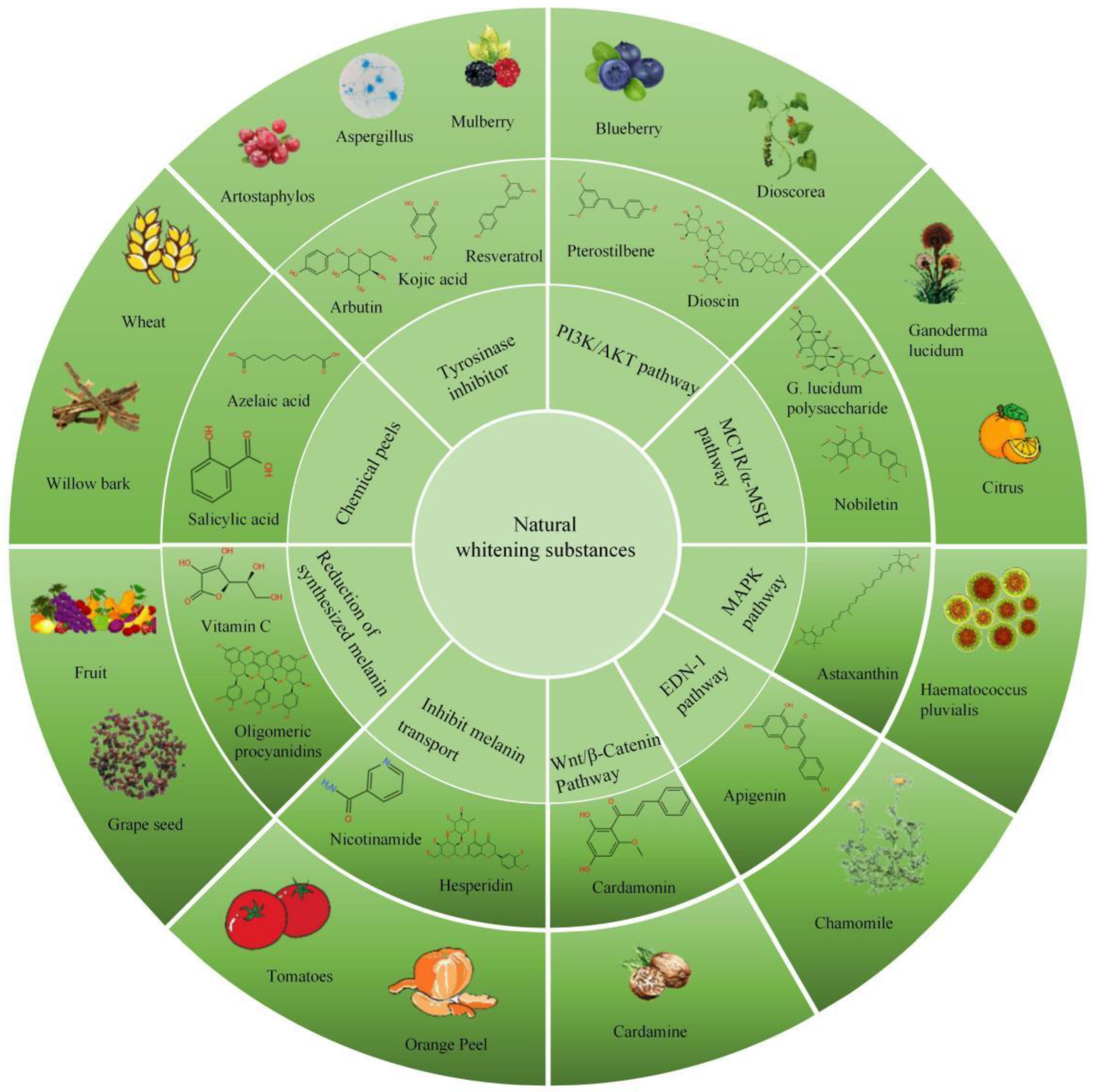
4.1. Affect the Enzymes Needed in the Process of Melanin Production
4.2. Melanin Synthesis Signaling Pathway Inhibitors
4.2.1. PI3K/Akt Signaling Pathway Inhibitors
4.2.2. Melanin Production Is Inhibited by Inhibiting MC1R/α-MSH Signaling Pathway
4.2.3. MAPK Pathway Inhibitors
4.2.4. EDN-1 Mediated Signaling Cascade Inhibitors
4.2.5. Wnt/β-Catenin Signaling Pathway Inhibitors
4.3. Inhibit Melanin Transport Process
4.4. Reduction of Synthesized Melanin
4.5. Accelerate Skin Metabolism and Cuticle Shedding
5. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| UVR | Ultraviolet radiation |
| UV | Ultraviolet |
| UVB | Ultraviolet B |
| UVA | Ultraviolet A |
| VL | Visible light |
| EDN-1 | Endothelin-1 |
| mSCF | Membrane-bound stem cell factor |
| PGE2 | Prostaglandin E2 |
| KGF | Keratinocyte growth factor |
| HGF | Hepatocyte growth factor |
| VitD | Vitamin D |
| ANG | Angiopoietin |
| ET1 | Endothelin 1 |
| TYR | Tyrosinase |
| MITF | Microphthalmia-associated transcription factor |
| TYRP1 | Tyrosinase- related protein 1 |
| TYRP2 | Tyrosinase- related protein 2 |
| L-DOPA | L-3, 4-dihydroxyphenylalanine |
| DHI | 5, 6-dihydroxyindoles |
| DHICA | 5,6-dihydroxyindole-2-carboxylic acid |
| PI3K | Phosphatidylinositol 3-kinase |
| cAMP | Cyclic adenosine monophosphate |
| PKA | protein kinase A |
| MAPK | Mitogen-activated protein kinase |
| MLPH | Melanophilin |
| Rab27a | Ras-related protein |
| Myo Va | Myosin Va |
| ROS | Reactive oxygen species |
| PIH | Post-inflammatory pigmentation |
| NGF | Nerve growth factor |
| TNF-α | Tumor necrosis factor-α |
| IL-1α | Interleukin 1α |
| MC1R | Melanocortin 1 receptor |
| α-MSH | α-melanocyte-stimulating hormone |
| ACTH | Adrenocorticotropic hormone |
| KA | Kojic acid |
| NF-κB | Nuclear factor kappa-B |
| GSK3β | Glycogen synthase kinase 3β |
| Pt | Pterostilbene |
| GLP | G. lucidum polysaccharide |
| CREB | cAMP-response element binding protein |
| ERK1/2 | Extracellular regulated protein kinases |
| JNK | c-Jun NH2-terminal kinase |
| EDN | Endothelin |
| HEE | Human epidermal equivalent |
| QCGG | Quercetin-3-O-β-d-glucopyranosyl-(1 → 6)-β-d-glucopyranosid |
| EDNRB | Endothelin receptor B |
| APC | Argan Press Cake |
| VC | Vitamin C |
| OPC | Oligomeric procyanidins |
References
- Kottner, J.; Beeckman, D.; Vogt, A.; Blume-Peytavi, U. Skin Health and Integrity. In Innovations and Emerging Technologies in Wound Care; Elsevier: Amsterdam, The Netherlands, 2020; pp. 183–196. ISBN 978-0-12-815028-3. [Google Scholar]
- Rossi, A.M.; Perez, M.I. Treatment of Hyperpigmentation. Facial Plast Surg. Clin. N. Am. 2011, 19, 313–324. [Google Scholar] [CrossRef]
- Raveendra, L.; Sidappa, H.; Shree, S. A Study of Quality of Life in Patients with Facial Melanoses. Indian Derm. Online J. 2020, 11, 154–157. [Google Scholar] [CrossRef]
- Gupta, M.; Mahajan, V.K. Clinical Profile of 300 Men with Facial Hypermelanosis. J. Dermatol. Case Rep. 2017, 11, 20–24. [Google Scholar] [CrossRef][Green Version]
- Handel, A.C.; Miot, L.D.B.; Miot, H.A. Melasma: A Clinical and Epidemiological Review. An. Bras. Dermatol. 2014, 89, 771–782. [Google Scholar] [CrossRef]
- Ahmed, I.A.; Mikail, M.A.; Zamakshshari, N.; Abdullah, A.-S.H. Natural Anti-Aging Skincare: Role and Potential. Biogerontology 2020, 21, 293–310. [Google Scholar] [CrossRef]
- Schalka, S. New Data on Hyperpigmentation Disorders. J. Eur. Acad. Dermatol. Venereol. 2017, 31, 18–21. [Google Scholar] [CrossRef]
- Xiaowei, C.; Wei, W.; Hong, G.; Hui, C.; Xiaofei, Z.; Haonan, W.; Yumeng, W.; Xuelan, Z.; Chunchao, H. Review of Polygonatum Sibiricum: A New Natural Cosmetic Ingredient. Pharmazie 2019, 74, 513–519. [Google Scholar] [CrossRef]
- Thiyagarasaiyar, K.; Goh, B.-H.; Jeon, Y.-J.; Yow, Y.-Y. Algae Metabolites in Cosmeceutical: An Overview of Current Applications and Challenges. Mar. Drugs 2020, 18, 323. [Google Scholar] [CrossRef]
- López, S.; Smith-Zubiaga, I.; García de Galdeano, A.; Boyano, M.D.; García, O.; Gardeazábal, J.; Martinez-Cadenas, C.; Izagirre, N.; de la Rúa, C.; Alonso, S. Comparison of the Transcriptional Profiles of Melanocytes from Dark and Light Skinned Individuals under Basal Conditions and Following Ultraviolet-B Irradiation. PLoS ONE 2015, 10, e0134911. [Google Scholar] [CrossRef][Green Version]
- Woo, Y.R.; Park, H.E.; Jeong, S.-W.; Park, H.J. Melanogenic Properties and Expression Profiles of Melanogenic Paracrine Molecules in Riehl’s Melanosis. Int. J. Mol. Sci. 2020, 21, 1695. [Google Scholar] [CrossRef]
- Imokawa, G. Melanocyte Activation Mechanisms and Rational Therapeutic Treatments of Solar Lentigos. Int. J. Mol. Sci. 2019, 20, 3666. [Google Scholar] [CrossRef]
- Flori, E.; Mastrofrancesco, A.; Mosca, S.; Ottaviani, M.; Briganti, S.; Cardinali, G.; Filoni, A.; Cameli, N.; Zaccarini, M.; Zouboulis, C.C.; et al. Sebocytes Contribute to Melasma Onset. iScience 2022, 25, 103871. [Google Scholar] [CrossRef]
- Salani, D.; Taraboletti, G.; Rosanò, L.; Di Castro, V.; Borsotti, P.; Giavazzi, R.; Bagnato, A. Endothelin-1 Induces an Angiogenic Phenotype in Cultured Endothelial Cells and Stimulates Neovascularization in Vivo. Am. J. Pathol. 2000, 157, 1703–1711. [Google Scholar] [CrossRef]
- Pillaiyar, T.; Manickam, M.; Jung, S.-H. Recent Development of Signaling Pathways Inhibitors of Melanogenesis. Cell. Signal. 2017, 40, 99–115. [Google Scholar] [CrossRef]
- Joly-Tonetti, N.; Wibawa, J.I.D.; Bell, M.; Tobin, D. Melanin Fate in the Human Epidermis: A Reassessment of How Best to Detect and Analyse Histologically. Exp. Dermatol. 2016, 25, 501–504. [Google Scholar] [CrossRef]
- Crawford, M.; Liu, N.; Mahdipour, E.; Barr, K.; Heit, B.; Dagnino, L. Integrin-Linked Kinase Regulates Melanosome Trafficking and Melanin Transfer in Melanocytes. Mol. Biol. Cell 2020, 31, 768–781. [Google Scholar] [CrossRef]
- Robinson, C.L.; Evans, R.D.; Sivarasa, K.; Ramalho, J.S.; Briggs, D.A.; Hume, A.N. The Adaptor Protein Melanophilin Regulates Dynamic Myosin-Va:Cargo Interaction and Dendrite Development in Melanocytes. Mol. Biol. Cell 2019, 30, 742–752. [Google Scholar] [CrossRef]
- Makino-Okamura, C.; Niki, Y.; Takeuchi, S.; Nishigori, C.; Declercq, L.; Yaroch, D.B.; Saito, N. Heparin Inhibits Melanosome Uptake and Inflammatory Response Coupled with Phagocytosis through Blocking PI3k/Akt and MEK/ERK Signaling Pathways in Human Epidermal Keratinocytes. Pigment Cell Melanoma Res. 2014, 27, 1063–1074. [Google Scholar] [CrossRef]
- Hu, S.; Bai, S.; Dai, Y.; Yang, N.; Li, J.; Zhang, X.; Wang, F.; Zhao, B.; Bao, G.; Chen, Y.; et al. Deubiquitination of MITF-M Regulates Melanocytes Proliferation and Apoptosis. Front. Mol. Biosci. 2021, 8, 692724. [Google Scholar] [CrossRef]
- Pillaiyar, T.; Namasivayam, V.; Manickam, M.; Jung, S.-H. Inhibitors of Melanogenesis: An Updated Review. J. Med. Chem. 2018, 61, 7395–7418. [Google Scholar] [CrossRef]
- Lv, J.; Jiang, S.; Yang, Y.; Zhang, X.; Gao, R.; Cao, Y.; Song, G. FGIN-1-27 Inhibits Melanogenesis by Regulating Protein Kinase A/CAMP-Responsive Element-Binding, Protein Kinase C-β, and Mitogen-Activated Protein Kinase Pathways. Front. Pharmacol. 2020, 11, 602889. [Google Scholar] [CrossRef]
- Kim, M.; Lee, C.-S.; Lim, K.-M. Rhododenol Activates Melanocytes and Induces Morphological Alteration at Sub-Cytotoxic Levels. Int. J. Mol. Sci. 2019, 20, 5665. [Google Scholar] [CrossRef]
- Kudo, M.; Kobayashi-Nakamura, K.; Tsuji-Naito, K. Bifunctional Effects of O-Methylated Flavones from Scutellaria Baicalensis Georgi on Melanocytes: Inhibition of Melanin Production and Intracellular Melanosome Transport. PLoS ONE 2017, 12, e0171513. [Google Scholar] [CrossRef]
- Jung, E.; Kim, J.H.; Kim, M.O.; Jang, S.; Kang, M.; Oh, S.W.; Nho, Y.H.; Kang, S.H.; Kim, M.H.; Park, S.-H.; et al. Afzelin Positively Regulates Melanogenesis through the P38 MAPK Pathway. Chem. Biol. Interact. 2016, 254, 167–172. [Google Scholar] [CrossRef]
- Zhu, P.-Y.; Yin, W.-H.; Wang, M.-R.; Dang, Y.-Y.; Ye, X.-Y. Andrographolide Suppresses Melanin Synthesis through Akt/GSK3β/β-Catenin Signal Pathway. J. Dermatol. Sci. 2015, 79, 74–83. [Google Scholar] [CrossRef]
- Sugumaran, M.; Evans, J.; Ito, S.; Wakamatsu, K. Nonenzymatic Spontaneous Oxidative Transformation of 5,6-Dihydroxyindole. Int. J. Mol. Sci. 2020, 21, 7321. [Google Scholar] [CrossRef]
- Zhou, S.; Zeng, H.; Huang, J.; Lei, L.; Tong, X.; Li, S.; Zhou, Y.; Guo, H.; Khan, M.; Luo, L.; et al. Epigenetic Regulation of Melanogenesis. Ageing Res. Rev. 2021, 69, 101349. [Google Scholar] [CrossRef]
- Pavan, W.J.; Sturm, R.A. The Genetics of Human Skin and Hair Pigmentation. Annu. Rev. Genom. Hum. Genet. 2019, 20, 41–72. [Google Scholar] [CrossRef]
- D’Mello, S.A.N.; Finlay, G.J.; Baguley, B.C.; Askarian-Amiri, M.E. Signaling Pathways in Melanogenesis. Int. J. Mol. Sci. 2016, 17, 1144. [Google Scholar] [CrossRef]
- Rachmin, I.; Ostrowski, S.M.; Weng, Q.Y.; Fisher, D.E. Topical Treatment Strategies to Manipulate Human Skin Pigmentation. Adv. Drug Deliv. Rev. 2020, 153, 65–71. [Google Scholar] [CrossRef]
- Bae-Harboe, Y.-S.C.; Park, H.-Y. Tyrosinase: A Central Regulatory Protein for Cutaneous Pigmentation. J. Investig. Dermatol. 2012, 132, 2678–2680. [Google Scholar] [CrossRef] [PubMed]
- Ramsden, C.A.; Riley, P.A. Tyrosinase: The Four Oxidation States of the Active Site and Their Relevance to Enzymatic Activation, Oxidation and Inactivation. Bioorg. Med. Chem. 2014, 22, 2388–2395. [Google Scholar] [CrossRef] [PubMed]
- Whitehead, R.D.; Re, D.; Xiao, D.; Ozakinci, G.; Perrett, D.I. You Are What You Eat: Within-Subject Increases in Fruit and Vegetable Consumption Confer Beneficial Skin-Color Changes. PLoS ONE 2012, 7, e32988. [Google Scholar] [CrossRef]
- de Andrade, B.-A.-B.; Padron-Alvarado, N.-A.; Muñoz-Campos, E.-M.; Morais, T.-M.L.; Martinez-Pedraza, R. Hyperpigmentation of Hard Palate Induced by Chloroquine Therapy. J. Clin. Exp. Dent. 2017, 9, e1487–e1491. [Google Scholar] [CrossRef][Green Version]
- Mishra, K.; Jandial, A.; Prakash, G. Bleomycin Induced Hyperpigmentation of Skin. Hematol. Transfus. Cell Ther. 2018, 40, 90–91. [Google Scholar] [CrossRef]
- Shute, L.; Walkty, A.; Embil, J.M. Minocycline-Induced Cutaneous Hyperpigmentation. CMAJ 2020, 192, E981. [Google Scholar] [CrossRef] [PubMed]
- Kumari, S.; Thng, S.; Verma, N.; Gautam, H. Melanogenesis Inhibitors. Acta Derm. Venerol. 2018, 98, 924–931. [Google Scholar] [CrossRef]
- França, K.; Keri, J. Psychosocial Impact of Acne and Postinflammatory Hyperpigmentation. An. Bras. Dermatol. 2017, 92, 505–509. [Google Scholar] [CrossRef]
- Kang, H.Y.; Ortonne, J.-P. What Should Be Considered in Treatment of Melasma. Ann. Dermatol. 2010, 22, 373–378. [Google Scholar] [CrossRef]
- Roggenkamp, D.; Dlova, N.; Mann, T.; Batzer, J.; Riedel, J.; Kausch, M.; Zoric, I.; Kolbe, L. Effective Reduction of Post-Inflammatory Hyperpigmentation with the Tyrosinase Inhibitor Isobutylamido-Thiazolyl-Resorcinol (Thiamidol). Int. J. Cosmet. Sci. 2021, 43, 292–301. [Google Scholar] [CrossRef]
- Praetorius, C.; Sturm, R.A.; Steingrimsson, E. Sun-Induced Freckling: Ephelides and Solar Lentigines. Pigment Cell Melanoma Res. 2014, 27, 339–350. [Google Scholar] [CrossRef] [PubMed]
- Bastiaens, M.; ter Huurne, J.; Gruis, N.; Bergman, W.; Westendorp, R.; Vermeer, B.J.; Bouwes Bavinck, J.N. The Melanocortin-1-Receptor Gene Is the Major Freckle Gene. Hum. Mol. Genet. 2001, 10, 1701–1708. [Google Scholar] [CrossRef] [PubMed]
- Ramakrishnan, S.; Hegde, S.P.; Shenoy, M.M.; Pinto, M.; M Iqbal, A.A.; Amin, V.B. A Cross-Sectional Study on Clinico-Dermoscopic Features of Periorbital Melanosis in a Tertiary Care Hospital. J. Cosmet. Dermatol. 2021, 20, 2917–2923. [Google Scholar] [CrossRef] [PubMed]
- Searle, T.; Al-Niaimi, F.; Ali, F.R. The Top 10 Cosmeceuticals for Facial Hyperpigmentation. Dermatol. Ther. 2020, 33, e14095. [Google Scholar] [CrossRef] [PubMed]
- Torres-Álvarez, B.; Mesa-Garza, I.G.; Castanedo-Cázares, J.P.; Fuentes-Ahumada, C.; Oros-Ovalle, C.; Navarrete-Solis, J.; Moncada, B. Histochemical and Immunohistochemical Study in Melasma: Evidence of Damage in the Basal Membrane. Am. J. Dermatopathol. 2011, 33, 291–295. [Google Scholar] [CrossRef]
- Byun, J.W.; Park, I.S.; Choi, G.S.; Shin, J. Role of Fibroblast-Derived Factors in the Pathogenesis of Melasma. Clin. Exp. Dermatol. 2016, 41, 601–609. [Google Scholar] [CrossRef]
- Callender, V.D.; Surin-Lord, S.S.; Davis, E.C.; Maclin, M. Postinflammatory Hyperpigmentation. Am. J. Clin. Dermatol. 2011, 13, 87–99. [Google Scholar] [CrossRef]
- Upadhyay, P.R.; Ho, T.; Abdel-Malek, Z.A. Participation of Keratinocyte- and Fibroblast-Derived Factors in Melanocyte Homeostasis, the Response to UV, and Pigmentary Disorders. Pigment. Cell Melanoma Res. 2021, 34, 762–776. [Google Scholar] [CrossRef]
- Lacz, N.L.; Vafaie, J.; Kihiczak, N.I.; Schwartz, R.A. Postinflammatory Hyperpigmentation: A Common but Troubling Condition. Int. J. Dermatol. 2004, 43, 362–365. [Google Scholar] [CrossRef]
- Shenoy, A.; Madan, R. Post-Inflammatory Hyperpigmentation: A Review of Treatment Strategies. J. Drugs Dermatol. 2020, 19, 763–768. [Google Scholar] [CrossRef]
- Silpa-Archa, N.; Kohli, I.; Chaowattanapanit, S.; Lim, H.W.; Hamzavi, I. Postinflammatory Hyperpigmentation: A Comprehensive Overview: Epidemiology, Pathogenesis, Clinical Presentation, and Noninvasive Assessment Technique. J. Am. Acad. Dermatol. 2017, 77, 591–605. [Google Scholar] [CrossRef] [PubMed]
- Monestier, S.; Gaudy, C.; Gouvernet, J.; Richard, M.A.; Grob, J.J. Multiple Senile Lentigos of the Face, a Skin Ageing Pattern Resulting from a Life Excess of Intermittent Sun Exposure in Dark-Skinned Caucasians: A Case-Control Study. Br. J. Dermatol. 2006, 154, 438–444. [Google Scholar] [CrossRef] [PubMed]
- Praetorius, C.; Grill, C.; Stacey, S.N.; Metcalf, A.M.; Gorkin, D.U.; Robinson, K.C.; Van Otterloo, E.; Kim, R.S.Q.; Bergsteinsdottir, K.; Ogmundsdottir, M.H.; et al. A Polymorphism in IRF4 Affects Human Pigmentation through a Tyrosinase-Dependent MITF/TFAP2A Pathway. Cell 2013, 155, 1022–1033. [Google Scholar] [CrossRef] [PubMed]
- Cario-Andre, M.; Lepreux, S.; Pain, C.; Nizard, C.; Noblesse, E.; Taïeb, A. Perilesional vs. Lesional Skin Changes in Senile Lentigo. J. Cutan. Pathol. 2004, 31, 441–447. [Google Scholar] [CrossRef]
- Krutmann, J.; Moyal, D.; Liu, W.; Kandahari, S.; Lee, G.-S.; Nopadon, N.; Xiang, L.F.; Seité, S. Pollution and Acne: Is There a Link? Clin. Cosmet. Investig. Dermatol. 2017, 10, 199–204. [Google Scholar] [CrossRef]
- Wakabayashi, Y.; Nakajima, H.; Imokawa, G. Abrogating Effect of N-Linked Carbohydrate Modifiers on the Stem Cell Factor and Endothelin-1-Stimulated Epidermal Pigmentation in Human Epidermal Equivalents. J. Dermatol. Sci. 2013, 69, 215–228. [Google Scholar] [CrossRef]
- Kovacs, D.; Cardinali, G.; Aspite, N.; Cota, C.; Luzi, F.; Bellei, B.; Briganti, S.; Amantea, A.; Torrisi, M.R.; Picardo, M. Role of Fibroblast-Derived Growth Factors in Regulating Hyperpigmentation of Solar Lentigo. Br. J. Dermatol. 2010, 163, 1020–1027. [Google Scholar] [CrossRef]
- Shah, V.V.; Bray, F.N.; Aldahan, A.S.; Mlacker, S.; Nouri, K. Lasers and Nevus of Ota: A Comprehensive Review. Lasers Med. Sci. 2016, 31, 179–185. [Google Scholar] [CrossRef]
- Al-Samary, A.; Al Mohizea, S.; Bin-Saif, G.; Al-Balbeesi, A. Pigmentary Demarcation Lines on the Face in Saudi Women. Indian J. Dermatol. Venereol. Leprol. 2010, 76, 378–381. [Google Scholar] [CrossRef]
- Park, K.Y.; Kwon, H.J.; Youn, C.S.; Seo, S.J.; Kim, M.N. Treatments of Infra-Orbital Dark Circles by Various Etiologies. Ann. Dermatol. 2018, 30, 522–528. [Google Scholar] [CrossRef]
- Hwang, K.-S.; Yang, J.Y.; Lee, J.; Lee, Y.-R.; Kim, S.S.; Kim, G.R.; Chae, J.S.; Ahn, J.H.; Shin, D.-S.; Choi, T.-Y.; et al. A Novel Anti-Melanogenic Agent, KDZ-001, Inhibits Tyrosinase Enzymatic Activity. J. Dermatol. Sci. 2018, 89, 165–171. [Google Scholar] [CrossRef] [PubMed]
- Zolghadri, S.; Bahrami, A.; Hassan Khan, M.T.; Munoz-Munoz, J.; Garcia-Molina, F.; Garcia-Canovas, F.; Saboury, A.A. A Comprehensive Review on Tyrosinase Inhibitors. J. Enzyme Inhib. Med. Chem. 2019, 34, 279–309. [Google Scholar] [CrossRef] [PubMed]
- Balakrishna, C.; Payili, N.; Yennam, S.; Uma Devi, P.; Behera, M. Synthesis of New Kojic Acid Based Unnatural α-Amino Acid Derivatives. Bioorg. Med. Chem. Lett. 2015, 25, 4753–4756. [Google Scholar] [CrossRef] [PubMed]
- Tse, T.W. Hydroquinone for Skin Lightening: Safety Profile, Duration of Use and When Should We Stop? J. Dermatol. Treat. 2010, 21, 272–275. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Zhao, J.; Li, A.; Reetz, M.T. Chemical and Biocatalytic Routes to Arbutin. Molecules 2019, 24, 3303. [Google Scholar] [CrossRef]
- Boo, Y.C. Arbutin as a Skin Depigmenting Agent with Antimelanogenic and Antioxidant Properties. Antioxidants 2021, 10, 1129. [Google Scholar] [CrossRef]
- Zhu, X.; Tian, Y.; Zhang, W.; Zhang, T.; Guang, C.; Mu, W. Recent Progress on Biological Production of α-Arbutin. Appl. Microbiol. Biotechnol. 2018, 102, 8145–8152. [Google Scholar] [CrossRef]
- Saeedi, M.; Khezri, K.; Seyed Zakaryaei, A.; Mohammadamini, H. A Comprehensive Review of the Therapeutic Potential of A-arbutin. Phytother. Res. 2021, 35, 4136–4154. [Google Scholar] [CrossRef]
- Avonto, C.; Wang, Y.-H.; Avula, B.; Wang, M.; Rua, D.; Khan, I.A. Comparative Studies on the Chemical and Enzymatic Stability of Alpha- and Beta-Arbutin. Int. J. Cosmet. Sci. 2016, 38, 187–193. [Google Scholar] [CrossRef]
- Suryadi, H.; Irianti, M.I.; Septiarini, T.H. Methods of Random Mutagenesis of Aspergillus Strain for Increasing Kojic Acid Production. Curr. Pharm. Biotechnol. 2022, 23, 486–494. [Google Scholar] [CrossRef]
- Saeedi, M.; Eslamifar, M.; Khezri, K. Kojic Acid Applications in Cosmetic and Pharmaceutical Preparations. Biomed. Pharmacother. 2019, 110, 582–593. [Google Scholar] [CrossRef] [PubMed]
- Sharma, K.; Joshi, N.; Goyal, C. Critical Review of Ayurvedic Varṇya Herbs and Their Tyrosinase Inhibition Effect. Anc. Sci. Life 2015, 35, 18–25. [Google Scholar] [CrossRef]
- Berardesca, E.; Rigoni, C.; Cantù, A.; Cameli, N.; Tedeschi, A.; Laureti, T. Effectiveness of a New Cosmetic Treatment for Melasma. J. Cosmet. Dermatol. 2020, 19, 1684–1690. [Google Scholar] [CrossRef] [PubMed]
- Schurink, M.; van Berkel, W.J.H.; Wichers, H.J.; Boeriu, C.G. Novel Peptides with Tyrosinase Inhibitory Activity. Peptides 2007, 28, 485–495. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.-J.; Hung, C.-C.; Chen, Y.-R.; Lai, S.-T.; Chan, C.-F. Novel Synthetic Kojic Acid-Methimazole Derivatives Inhibit Mushroom Tyrosinase and Melanogenesis. J. Biosci. Bioeng. 2016, 122, 666–672. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-M.; Su, W.-C.; Li, C.; Shi, Y.; Chen, Q.-X.; Zheng, J.; Tang, D.-L.; Chen, S.-M.; Wang, Q. Anti-Melanogenesis of Novel Kojic Acid Derivatives in B16F10 Cells and Zebrafish. Int. J. Biol. Macromol. 2019, 123, 723–731. [Google Scholar] [CrossRef] [PubMed]
- Malaguarnera, L. Influence of Resveratrol on the Immune Response. Nutrients 2019, 11, 946. [Google Scholar] [CrossRef]
- Subbaramaiah, K.; Chung, W.J.; Michaluart, P.; Telang, N.; Tanabe, T.; Inoue, H.; Jang, M.; Pezzuto, J.M.; Dannenberg, A.J. Resveratrol Inhibits Cyclooxygenase-2 Transcription and Activity in Phorbol Ester-Treated Human Mammary Epithelial Cells. J. Biol. Chem. 1998, 273, 21875–21882. [Google Scholar] [CrossRef]
- Ratz-Łyko, A.; Arct, J. Resveratrol as an Active Ingredient for Cosmetic and Dermatological Applications: A Review. J. Cosmet. Laser Ther. 2019, 21, 84–90. [Google Scholar] [CrossRef]
- An, S.M.; Koh, J.-S.; Boo, Y.C. Inhibition of Melanogenesis by Tyrosinase SiRNA in Human Melanocytes. BMB Rep. 2009, 42, 178–183. [Google Scholar] [CrossRef]
- Liu, Q.; Kim, C.; Jo, Y.; Kim, S.; Hwang, B.; Lee, M. Synthesis and Biological Evaluation of Resveratrol Derivatives as Melanogenesis Inhibitors. Molecules 2015, 20, 16933–16945. [Google Scholar] [CrossRef] [PubMed]
- Ryu, J.H.; Seok, J.K.; An, S.M.; Baek, J.H.; Koh, J.S.; Boo, Y.C. A Study of the Human Skin-Whitening Effects of Resveratryl Triacetate. Arch. Dermatol. Res. 2015, 307, 239–247. [Google Scholar] [CrossRef] [PubMed]
- Xing, J.; Yang, J.; Gu, Y.; Yi, J. Research Update on the Anticancer Effects of Buparlisib. Oncol. Lett. 2021, 21. [Google Scholar] [CrossRef] [PubMed]
- Lam, R.Y.Y.; Lin, Z.-X.; Sviderskaya, E.V.; Cheng, C.H.K. Mechanistic Studies of Anti-Hyperpigmentary Compounds: Elucidating Their Inhibitory and Regulatory Actions. Int. J. Mol. Sci. 2014, 15, 14649–14668. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Seo, K.-H.; Yokoyama, W. Chemistry of Pterostilbene and Its Metabolic Effects. J. Agric. Food Chem. 2020, 68, 12836–12841. [Google Scholar] [CrossRef] [PubMed]
- Hseu, Y.-C.; Vudhya Gowrisankar, Y.; Wang, L.-W.; Zhang, Y.-Z.; Chen, X.-Z.; Huang, P.-J.; Yen, H.-R.; Yang, H.-L. The in Vitro and in Vivo Depigmenting Activity of Pterostilbene through Induction of Autophagy in Melanocytes and Inhibition of UVA-Irradiated α-MSH in Keratinocytes via Nrf2-Mediated Antioxidant Pathways. Redox Biol. 2021, 44, 102007. [Google Scholar] [CrossRef]
- Song, X.; Wang, Z.; Liang, H.; Zhang, W.; Ye, Y.; Li, H.; Hu, Y.; Zhang, Y.; Weng, H.; Lu, J.; et al. Dioscin Induces Gallbladder Cancer Apoptosis by Inhibiting ROS-Mediated PI3K/AKT Signalling. Int. J. Biol. Sci. 2017, 13, 782–793. [Google Scholar] [CrossRef]
- Zorina-Lichtenwalter, K.; Lichtenwalter, R.N.; Zaykin, D.V.; Parisien, M.; Gravel, S.; Bortsov, A.; Diatchenko, L. A Study in Scarlet: MC1R as the Main Predictor of Red Hair and Exemplar of the Flip-Flop Effect. Hum. Mol. Genet. 2019, 28, 2093–2106. [Google Scholar] [CrossRef]
- Wolf Horrell, E.M.; Boulanger, M.C.; D’Orazio, J.A. Melanocortin 1 Receptor: Structure, Function, and Regulation. Front. Genet. 2016, 7, 95. [Google Scholar] [CrossRef]
- Chen, S.; Han, C.; Miao, X.; Li, X.; Yin, C.; Zou, J.; Liu, M.; Li, S.; Stawski, L.; Zhu, B.; et al. Targeting MC1R Depalmitoylation to Prevent Melanomagenesis in Redheads. Nat. Commun. 2019, 10, 877. [Google Scholar] [CrossRef]
- Yin, Z.; Yang, B.; Ren, H. Preventive and Therapeutic Effect of Ganoderma (Lingzhi) on Skin Diseases and Care. Adv. Exp. Med. Biol. 2019, 1182, 311–321. [Google Scholar] [CrossRef]
- Hu, S.; Huang, J.; Pei, S.; Ouyang, Y.; Ding, Y.; Jiang, L.; Lu, J.; Kang, L.; Huang, L.; Xiang, H.; et al. Ganoderma Lucidum Polysaccharide Inhibits UVB-Induced Melanogenesis by Antagonizing CAMP/PKA and ROS/MAPK Signaling Pathways. J. Cell. Physiol. 2019, 234, 7330–7340. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Huang, J.; Lu, J.; Hu, S.; Pei, S.; Ouyang, Y.; Ding, Y.; Hu, Y.; Kang, L.; Huang, L.; et al. Ganoderma Lucidum Polysaccharide Reduces Melanogenesis by Inhibiting the Paracrine Effects of Keratinocytes and Fibroblasts via IL-6/STAT3/FGF2 Pathway. J. Cell. Physiol. 2019, 234, 22799–22808. [Google Scholar] [CrossRef] [PubMed]
- Goh, J.X.H.; Tan, L.T.-H.; Goh, J.K.; Chan, K.G.; Pusparajah, P.; Lee, L.-H.; Goh, B.-H. Nobiletin and Derivatives: Functional Compounds from Citrus Fruit Peel for Colon Cancer Chemoprevention. Cancers 2019, 11, 867. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Yonezawa, T.; Teruya, T.; Woo, J.-T.; Cha, B.-Y. Nobiletin, a Polymethoxy Flavonoid, Reduced Endothelin-1 plus SCF-Induced Pigmentation in Human Melanocytes. Photochem. Photobiol. 2015, 91, 379–386. [Google Scholar] [CrossRef] [PubMed]
- Ronkina, N.; Schuster-Gossler, K.; Hansmann, F.; Kunze-Schumacher, H.; Sandrock, I.; Yakovleva, T.; Lafera, J.; Baumgärtner, W.; Krueger, A.; Prinz, I.; et al. Germ Line Deletion Reveals a Nonessential Role of Atypical Mitogen-Activated Protein Kinase 6/Extracellular Signal-Regulated Kinase 3. Mol. Cell. Biol. 2019, 39, e00516–e00518. [Google Scholar] [CrossRef]
- Jiang, W.; Cai, F.; Xu, H.; Lu, Y.; Chen, J.; Liu, J.; Cao, N.; Zhang, X.; Chen, X.; Huang, Q.; et al. Extracellular Signal Regulated Kinase 5 Promotes Cell Migration, Invasion and Lung Metastasis in a FAK-Dependent Manner. Protein Cell 2020, 11, 825–845. [Google Scholar] [CrossRef]
- Huang, L.; Gao, B.; Wu, M.; Wang, F.; Zhang, C. Comparative Transcriptome Analysis of a Long-Time Span Two-Step Culture Process Reveals a Potential Mechanism for Astaxanthin and Biomass Hyper-Accumulation in Haematococcus Pluvialis JNU35. Biotechnol. Biofuels 2019, 12. [Google Scholar] [CrossRef]
- Imokawa, G.; Ishida, K. Inhibitors of Intracellular Signaling Pathways That Lead to Stimulated Epidermal Pigmentation: Perspective of Anti-Pigmenting Agents. Int. J. Mol. Sci. 2014, 15, 8293–8315. [Google Scholar] [CrossRef]
- Jung, H.G.; Kim, H.H.; Paul, S.; Jang, J.Y.; Cho, Y.H.; Kim, H.J.; Yu, J.M.; Lee, E.S.; An, B.J.; Kang, S.C.; et al. Quercetin-3-O-β-d-Glucopyranosyl-(1→6)-β-d-Glucopyranoside Suppresses Melanin Synthesis by Augmenting P38 MAPK and CREB Signaling Pathways and Subsequent CAMP down-Regulation in Murine Melanoma Cells. Saudi J. Biol. Sci. 2015, 22, 706–713. [Google Scholar] [CrossRef]
- Tagashira, H.; Miyamoto, A.; Kitamura, S.-I.; Tsubata, M.; Yamaguchi, K.; Takagaki, K.; Imokawa, G. UVB Stimulates the Expression of Endothelin B Receptor in Human Melanocytes via a Sequential Activation of the P38/MSK1/CREB/MITF Pathway Which Can Be Interrupted by a French Maritime Pine Bark Extract through a Direct Inactivation of MSK1. PLoS ONE 2015, 10, e0128678. [Google Scholar] [CrossRef] [PubMed]
- Zou, L.; Wang, X.; Jiang, L.; Wang, S.; Xiong, X.; Yang, H.; Gao, W.; Gong, M.; Hu, C.-A.A.; Yin, Y. Molecular Cloning, Characterization and Expression Analysis of Frizzled 6 in the Small Intestine of Pigs (Sus Scrofa). PLoS ONE 2017, 12, e0179421. [Google Scholar] [CrossRef] [PubMed]
- Wei, L.; Chen, C.; Ding, L.; Mo, M.; Zou, J.; Lu, Z.; Li, H.; Wu, H.; Dai, Y.; Xu, P.; et al. Wnt1 Promotes EAAT2 Expression and Mediates the Protective Effects of Astrocytes on Dopaminergic Cells in Parkinson’s Disease. Neural Plast. 2019, 2019, 1247276. [Google Scholar] [CrossRef] [PubMed]
- Lv, C.; Kong, H.; Dong, G.; Liu, L.; Tong, K.; Sun, H.; Chen, B.; Zhang, C.; Zhou, M. Antitumor Efficacy of α-Solanine against Pancreatic Cancer in Vitro and in Vivo. PLoS ONE 2014, 9, e87868. [Google Scholar] [CrossRef]
- Tokunaga, Y.; Osawa, Y.; Ohtsuki, T.; Hayashi, Y.; Yamaji, K.; Yamane, D.; Hara, M.; Munekata, K.; Tsukiyama-Kohara, K.; Hishima, T.; et al. Selective Inhibitor of Wnt/β-Catenin/CBP Signaling Ameliorates Hepatitis C Virus-Induced Liver Fibrosis in Mouse Model. Sci. Rep. 2017, 7. [Google Scholar] [CrossRef]
- Guo, H.; Yang, K.; Deng, F.; Xing, Y.; Li, Y.; Lian, X.; Yang, T. Wnt3a Inhibits Proliferation but Promotes Melanogenesis of Melan-a Cells. Int. J. Mol. Med. 2012, 30, 636–642. [Google Scholar] [CrossRef]
- Cho, M.; Ryu, M.; Jeong, Y.; Chung, Y.-H.; Kim, D.-E.; Cho, H.-S.; Kang, S.; Han, J.-S.; Chang, M.-Y.; Lee, C.-K.; et al. Cardamonin Suppresses Melanogenesis by Inhibition of Wnt/Beta-Catenin Signaling. Biochem. Biophys. Res. Commun. 2009, 390, 500–505. [Google Scholar] [CrossRef]
- Bourhim, T.; Villareal, M.O.; Gadhi, C.; Isoda, H. Elucidation of Melanogenesis-Associated Signaling Pathways Regulated by Argan Press Cake in B16 Melanoma Cells. Nutrients 2021, 13, 2697. [Google Scholar] [CrossRef]
- Myung, C.H.; Kim, K.; Park, J.I.; Lee, J.E.; Lee, J.A.; Hong, S.C.; Lim, K.-M.; Hwang, J.S. 16-Kauren-2-Beta-18,19-Triol Inhibits Melanosome Transport in Melanocytes by down-Regulation of Melanophilin Expression. J. Dermatol. Sci. 2020, 97, 101–108. [Google Scholar] [CrossRef]
- Rolfe, H.M. A Review of Nicotinamide: Treatment of Skin Diseases and Potential Side Effects. J. Cosmet. Dermatol. 2014, 13, 324–328. [Google Scholar] [CrossRef]
- Snaidr, V.A.; Damian, D.L.; Halliday, G.M. Nicotinamide for Photoprotection and Skin Cancer Chemoprevention: A Review of Efficacy and Safety. Exp. Dermatol. 2019, 28, 15–22. [Google Scholar] [CrossRef]
- Forbat, E.; Al-Niaimi, F.; Ali, F.R. Use of Nicotinamide in Dermatology. Clin. Exp. Dermatol. 2017, 42, 137–144. [Google Scholar] [CrossRef]
- Navarrete-Solís, J.; Castanedo-Cázares, J.P.; Torres-Álvarez, B.; Oros-Ovalle, C.; Fuentes-Ahumada, C.; González, F.J.; Martínez-Ramírez, J.D.; Moncada, B. A Double-Blind, Randomized Clinical Trial of Niacinamide 4% versus Hydroquinone 4% in the Treatment of Melasma. Dermatol. Res. Pract. 2011, 2011. [Google Scholar] [CrossRef]
- Li, C.; Schluesener, H. Health-Promoting Effects of the Citrus Flavanone Hesperidin. Crit. Rev. Food Sci. Nutr. 2017, 57, 613–631. [Google Scholar] [CrossRef]
- Kim, B.; Lee, J.-Y.; Lee, H.-Y.; Nam, K.-Y.; Park, J.; Lee, S.M.; Kim, J.E.; Lee, J.D.; Hwang, J.S. Hesperidin Suppresses Melanosome Transport by Blocking the Interaction of Rab27A-Melanophilin. Biomol. Ther. 2013, 21, 343–348. [Google Scholar] [CrossRef]
- Hou, M.; Man, M.; Man, W.; Zhu, W.; Hupe, M.; Park, K.; Crumrine, D.; Elias, P.M.; Man, M.-Q. Topical Hesperidin Improves Epidermal Permeability Barrier Function and Epidermal Differentiation in Normal Murine Skin. Exp. Dermatol. 2012, 21, 337–340. [Google Scholar] [CrossRef]
- Man, G.; Mauro, T.M.; Kim, P.L.; Hupe, M.; Zhai, Y.; Sun, R.; Crumrine, D.; Cheung, C.; Nuno-Gonzalez, A.; Elias, P.M.; et al. Topical Hesperidin Prevents Glucocorticoid-Induced Abnormalities in Epidermal Barrier Function in Murine Skin. Exp. Dermatol. 2014, 23, 645–651. [Google Scholar] [CrossRef]
- Usach, I.; Taléns-Visconti, R.; Magraner-Pardo, L.; Peris, J.-E. Hesperetin Induces Melanin Production in Adult Human Epidermal Melanocytes. Food Chem. Toxicol. 2015, 80, 80–84. [Google Scholar] [CrossRef]
- Choi, K.-H.; Nam, K.C.; Lee, S.-Y.; Cho, G.; Jung, J.-S.; Kim, H.-J.; Park, B.J. Antioxidant Potential and Antibacterial Efficiency of Caffeic Acid-Functionalized ZnO Nanoparticles. Nanomaterials 2017, 7, 148. [Google Scholar] [CrossRef]
- Padayatty, S.; Levine, M. Vitamin C: The Known and the Unknown and Goldilocks. Oral Dis. 2016, 22, 463–493. [Google Scholar] [CrossRef]
- Miao, F.; Su, M.-Y.; Jiang, S.; Luo, L.-F.; Shi, Y.; Lei, T.-C. Intramelanocytic Acidification Plays a Role in the Antimelanogenic and Antioxidative Properties of Vitamin C and Its Derivatives. Oxid. Med. Cell. Longev. 2019, 2019, 2084805. [Google Scholar] [CrossRef]
- Lykkesfeldt, J.; Michels, A.J.; Frei, B. Vitamin C. Adv. Nutr. 2014, 5, 16–18. [Google Scholar] [CrossRef]
- Choi, S.; Han, J.; Kim, J.H.; Kim, A.; Kim, S.; Lee, W.; Yoon, M.; Kim, G.; Kim, Y. Advances in Dermatology Using DNA Aptamer “Aptamin C” Innovation: Oxidative Stress Prevention and Effect Maximization of Vitamin C through Antioxidation. J. Cosmet. Dermatol. 2020, 19, 970–976. [Google Scholar] [CrossRef]
- Ferreira, D.; Slade, D. Oligomeric Proanthocyanidins: Naturally Occurring O-Heterocycles. Nat. Prod. Rep. 2002, 19, 517–541. [Google Scholar] [CrossRef]
- Tsuchiya, T.; Fukui, Y.; Izumi, R.; Numano, K.; Zeida, M. Effects of Oligomeric Proanthocyanidins (OPCs) of Red Wine to Improve Skin Whitening and Moisturizing in Healthy Women-a Placebo-Controlled Randomized Double-Blind Parallel Group Comparative Study. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 1571–1584. [Google Scholar] [CrossRef]
- Liu, J.; Jiang, R.; Zhou, J.; Xu, X.; Sun, Z.; Li, J.; Chen, X.; Li, Z.; Yan, X.; Zhao, D.; et al. Salicylic Acid in Ginseng Root Alleviates Skin Hyperpigmentation Disorders by Inhibiting Melanogenesis and Melanosome Transport. Eur. J. Pharmacol. 2021, 910, 174458. [Google Scholar] [CrossRef]
- Lee, K.C.; Wambier, C.G.; Soon, S.L.; Sterling, J.B.; Landau, M.; Rullan, P.; Brody, H.J. International Peeling Society Basic Chemical Peeling: Superficial and Medium-Depth Peels. J. Am. Acad. Dermatol. 2019, 81, 313–324. [Google Scholar] [CrossRef]
- Arif, T. Salicylic Acid as a Peeling Agent: A Comprehensive Review. Clin. Cosmet. Investig. Dermatol. 2015, 8, 455–461. [Google Scholar] [CrossRef]
- Imayama, S.; Ueda, S.; Isoda, M. Histologic Changes in the Skin of Hairless Mice Following Peeling with Salicylic Acid. Arch. Dermatol. 2000, 136, 1390–1395. [Google Scholar] [CrossRef]
- Abdel Meguid, A.M.; Elaziz Ahmed Attallah, D.A.; Omar, H. Trichloroacetic Acid Versus Salicylic Acid in the Treatment of Acne Vulgaris in Dark-Skinned Patients. Dermatol. Surg. 2015, 41, 1398–1404. [Google Scholar] [CrossRef]
- Sarkar, R.; Garg, V.; Bansal, S.; Sethi, S.; Gupta, C. Comparative Evaluation of Efficacy and Tolerability of Glycolic Acid, Salicylic Mandelic Acid, and Phytic Acid Combination Peels in Melasma. Dermatol. Surg. 2016, 42, 384–391. [Google Scholar] [CrossRef]
- Searle, T.; Ali, F.R.; Al-Niaimi, F. The Versatility of Azelaic Acid in Dermatology. J. Dermatol. Treat. 2022, 33, 722–732. [Google Scholar] [CrossRef]
- Passeron, T.; Picardo, M. Melasma, a Photoaging Disorder. Pigment Cell Melanoma Res. 2018, 31, 461–465. [Google Scholar] [CrossRef]
- Chilicka, K.; Rogowska, A.M.; Szyguła, R.; Dzieńdziora-Urbińska, I.; Taradaj, J. A Comparison of the Effectiveness of Azelaic and Pyruvic Acid Peels in the Treatment of Female Adult Acne: A Randomized Controlled Trial. Sci. Rep. 2020, 10. [Google Scholar] [CrossRef]
- Omeragic, E.; Dedic, M.; Elezovic, A.; Becic, E.; Imamovic, B.; Kladar, N.; Niksic, H. Application of Direct Peptide Reactivity Assay for Assessing the Skin Sensitization Potential of Essential Oils. Sci. Rep. 2022, 12. [Google Scholar] [CrossRef]
- Roberts, M.S.; Mohammed, Y.; Pastore, M.N.; Namjoshi, S.; Yousef, S.; Alinaghi, A.; Haridass, I.N.; Abd, E.; Leite-Silva, V.R.; Benson, H.; et al. Topical and Cutaneous Delivery Using Nanosystems. J. Control. Release 2017, 247, 86–105. [Google Scholar] [CrossRef]
- Peralta, M.F.; Guzmán, M.L.; Pérez, A.P.; Apezteguia, G.A.; Fórmica, M.L.; Romero, E.L.; Olivera, M.E.; Carrer, D.C. Liposomes Can Both Enhance or Reduce Drugs Penetration through the Skin. Sci. Rep. 2018, 8. [Google Scholar] [CrossRef]
- Dorraj, G.; Carreras, J.J.; Nunez, H.; Abushammala, I.; Melero, A. Lipid Nanoparticles as Potential Gene Therapeutic Delivery Systems for Oral Administration. Curr. Gene Ther. 2017, 17, 89–104. [Google Scholar] [CrossRef]
- Ryu, H.J.; Seo, M.Y.; Jung, S.K.; Maeng, E.H.; Lee, S.-Y.; Jang, D.-H.; Lee, T.-J.; Jo, K.-Y.; Kim, Y.-R.; Cho, K.-B.; et al. Zinc Oxide Nanoparticles: A 90-Day Repeated-Dose Dermal Toxicity Study in Rats. Int. J. Nanomed. 2014, 9, 137–144. [Google Scholar] [CrossRef]
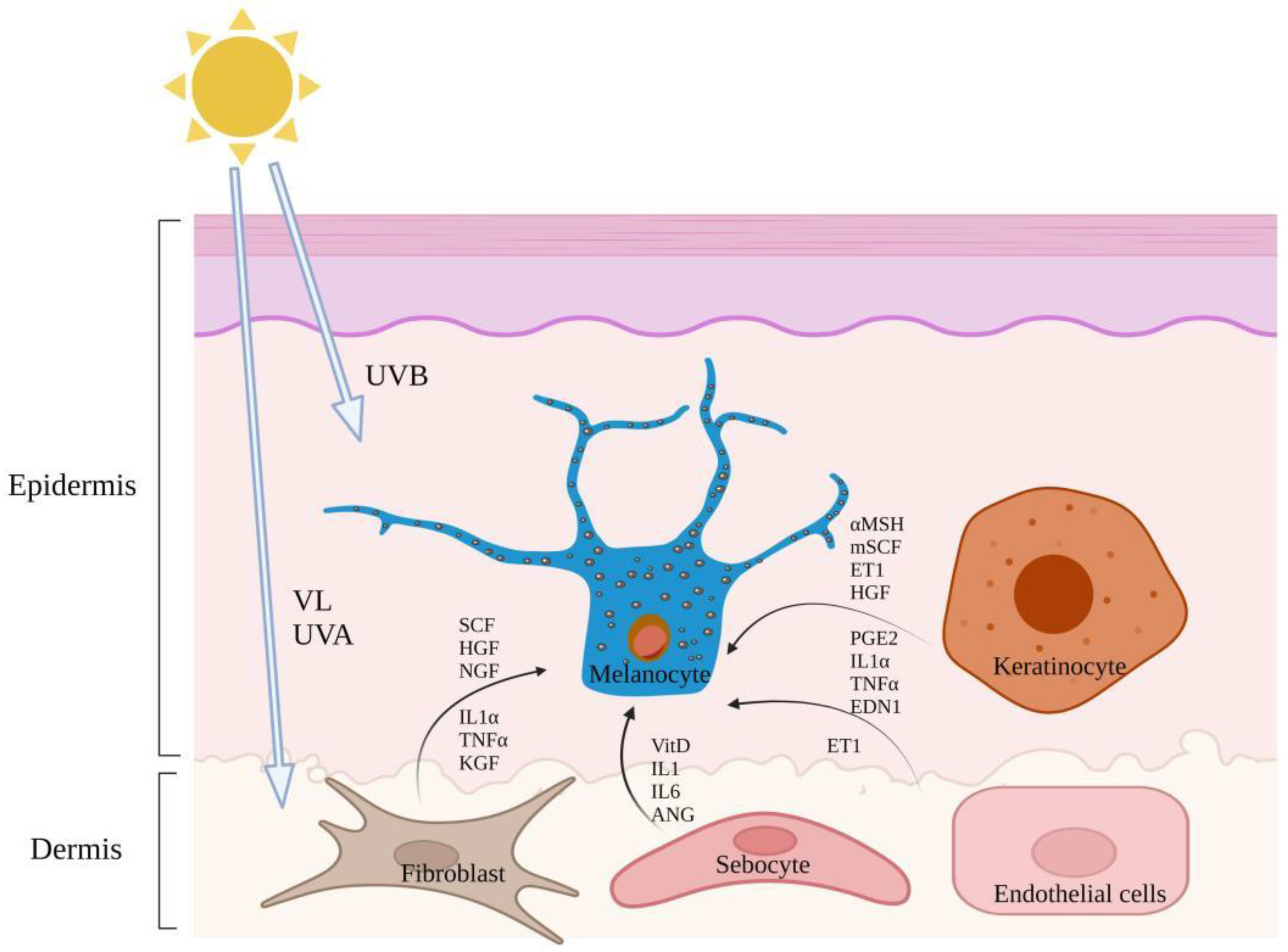

| Conditions That May Lead to Pigmentation Disorders | Major Risk Factors | Action Pathway | References |
|---|---|---|---|
| Sunlight exposure | UVR | Enhanced production of reactive oxygen species (ROS) in keratinocytes and melanocytes, resulting in DNA damage of keratinocytes and further activation of tumor suppressor protein p53. | [30,31,32] |
| Foods with high metal content | Copper, iron, zinc and other metals | Directly or indirectly increase the amount and activity of TYR and the quantity and activity of dopa quinone related to melanin production. | [33,34] |
| Some drugs | Chloroquine, minocycline, bleomycin | The affinity for melanin is particularly strong, which will accentuate skin pigmentation. | [35,36,37] |
| Part of the disease | Malnutrition, urticaria, dermatitis, acne | Oxidation and antioxidant imbalance in the human body; endocrine disorders; microecological imbalance; metabolic disorders; abnormal trace element content in the body. | [38,39] |
| Etiology | Susceptible Group | Clinical Features | Pathologic Features | Associated Causes | Pathogenesis |
|---|---|---|---|---|---|
| Melasma | Most of them are women of childbearing age but also occurs in men [45]. | Light to brown pigmented, symmetrical spots with irregular, serrated shapes commonly found on the forehead, cheeks, upper lips, and chin [40]. | Melanin production and melanocyte increases in the epidermal and dermal skin layers [5] and there are more mast cells in the elastic zone of degenerative melasma [46] and vascular proliferation [14]. Damage to the basement membrane promotes the descent of melanocytes and melanin into the dermis, which is represented by the free melanin or melanocytes often observed in the dermis of skin melasma [46]. | Pregnancy, intake or oral contraceptives and hormone drugs, exposure to sunlight (UVA and UVB wavebands), etc., have an obvious genetic predisposition [5]. | Skin fibroblasts secrete several stimuli including dickkopf-1, SCF, HGF, (nerve growth factor) NGF, (tumor necrosis factor-α) TNF-α and (Interleukin 1α) IL-1α [47], sebaceous glands synthesize Vit D and secrete various cytokines, such as interleukin-6 and growth factors such as angiopoietin and adipokine, which further regulate melanocyte function directly or indirectly [13]. Mast cells induce vascular proliferation through various angiogenic factors and destroy the basal layer through trypsin and granzymes B [46]. Vascular endothelial cells secrete ET1 to induce melanin production [14], most paracrine regulation of melasma involves the Wnt pathway. |
| Post-inflammatory Pigmentation | All skin types, especially those with darker skin colors [41]. | PIH of epidermis was tan, brown, or dark brown; the PIH within the dermis has a bluish-gray appearance [48]. | Melanocyte proliferation, increased the number of melanocyte dendrites and TYR, etc [49]. Elanocyte phagocytes exist in the dermis [50]. | PIH can be caused by skin inflammation, burns, trauma, acne, and other endogenous and external factors that can cause skin tissue and cell damage, including physical factors, chemical factors, biological factors, tissue necrosis, allergies, and foreign bodies [51]. | A variety of endogenous and exogenous factors act on the migration, proliferation, and differentiation of melanocytes. TYR synthesis and activation, melanosome transfer to keratinocytes and other terminal processes through inflammatory mediators, inflammatory cytokines, melanotrophs, NO and other inflammatory regulatory factors, result in the PIH effect [52,53]. |
| Freckles | The onset of Ephelides usually occurs around the age of five, mostly in women and, in some people, it tends to decrease in adulthood [53]. | Yellow brown or brown spots, symmetrical distribution on the face, neck, back, shoulder; mostly 1–2 mm in diameter with small macules and a smooth, clear edge. | Epidermal pigmentation increased but the quantity of melanocytes did not. | Genetic factors mostly: it is an autosomal dominant-inherited disease [53]. | The (melanocortin 1 receptor) MC1R gene is the main gene for the formation of ephelides [43]. MC1R binds to the α-melanocyte-stimulating hormone(α-MSH) and adrenocorticotropic hormone (ACTH) to activate the cAMP pathway, which leads to melanin synthesis. IRF4 cooperates with MITF to activate the expression of TYR and affect the synthesis of melanin [54]. |
| Lentigines tend to be around 50 years. | Color varies from light yellow to dark brown to black; mainly distributed in the forehead, cheek, back of hand and forearm; they are round or oval and irregular in shape [55]. | Epidermal melanocytes proliferate and dendrites increase [42]. | Environmental factors, such as sunlight and air pollution, contribute to the development of lentigines [56]. | IL-1α and TNFα are released from keratinocytes by autocrine and promote the up-regulation of EDN-1 and mSCF [57]. Fibroblast release of KGF acts directly or indirectly through keratinocytes, leading to pigmentation [58]. | |
| Periorbital melanosis | It can occur in both sexes and is more common in dark-skinned individuals. | Dark brown pigmentation may occur on upper and lower eyelids. | Dermal melanocytes increase [59] and blood vessels dilate. | Ocular melanin pigmentation, flabby skin, atopic dermatitis secondary inflammatory pigmentation and genetics. | Increase in dermal melanocytes, excessive pigmentation after inflammation, extension of facial pigment boundary [60], diffusion of superficial position of vascular system [61], edema around eyes when morning or salt intake is too much and lacrimal groove depression [61] may be caused. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, F.; Qu, L.; Li, H.; He, J.; Wang, L.; Fang, Y.; Yan, X.; Yang, Q.; Peng, B.; Wu, W.; et al. Advances in Biomedical Functions of Natural Whitening Substances in the Treatment of Skin Pigmentation Diseases. Pharmaceutics 2022, 14, 2308. https://doi.org/10.3390/pharmaceutics14112308
Liu F, Qu L, Li H, He J, Wang L, Fang Y, Yan X, Yang Q, Peng B, Wu W, et al. Advances in Biomedical Functions of Natural Whitening Substances in the Treatment of Skin Pigmentation Diseases. Pharmaceutics. 2022; 14(11):2308. https://doi.org/10.3390/pharmaceutics14112308
Chicago/Turabian StyleLiu, Fan, Linkai Qu, Hua Li, Jiaxuan He, Lei Wang, Yimeng Fang, Xiaoqing Yan, Qinsi Yang, Bo Peng, Wei Wu, and et al. 2022. "Advances in Biomedical Functions of Natural Whitening Substances in the Treatment of Skin Pigmentation Diseases" Pharmaceutics 14, no. 11: 2308. https://doi.org/10.3390/pharmaceutics14112308
APA StyleLiu, F., Qu, L., Li, H., He, J., Wang, L., Fang, Y., Yan, X., Yang, Q., Peng, B., Wu, W., Jin, L., & Sun, D. (2022). Advances in Biomedical Functions of Natural Whitening Substances in the Treatment of Skin Pigmentation Diseases. Pharmaceutics, 14(11), 2308. https://doi.org/10.3390/pharmaceutics14112308









