Nanofiber Systems as Herbal Bioactive Compounds Carriers: Current Applications in Healthcare
Abstract
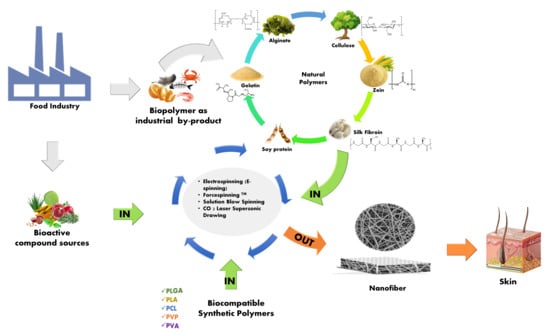
:1. Introduction
2. Methods for Nanofiber Fabrication
2.1. Electrospinning (E-Spinning)
2.2. Centrifugal Spinning (ForcespinningTM)
2.3. Solution Blow Spinning
2.4. Carbon Dioxide (CO2) Laser Supersonic Drawing
3. Polymers in the Nanofiber’s Fabrication
3.1. Natural Polymers
3.1.1. Collagen
3.1.2. Gelatin
3.1.3. Chitin and Chitosan
3.1.4. Silk Fibroin
3.1.5. Zein
3.1.6. Soy Protein
3.1.7. Cellulose
3.2. Biocompatible Synthetic Polymers
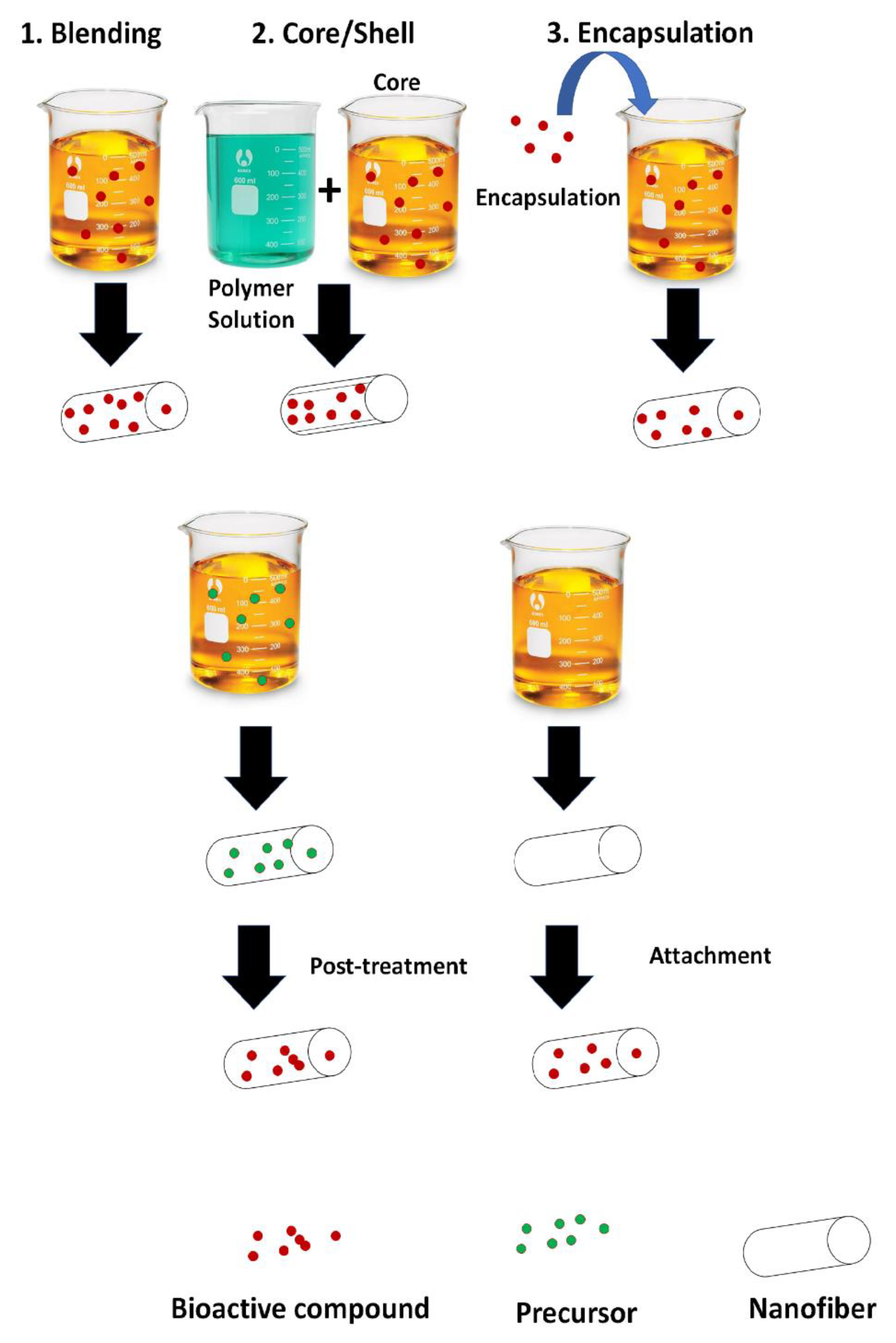
4. Incorporation of Active Compounds
Natural Extracts
5. Applications in Healthcare
5.1. Wound Dressing
5.2. Tissue Engineering
5.3. Drug Delivery
5.4. Food Packaging
5.5. In Vivo Studies
5.6. Commercially Available Scaffolds
6. Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Saka, R.; Chella, N. Nanotechnology for Delivery of Natural Therapeutic Substances: A Review. Environ. Chem. Lett. 2021, 19, 1097–1106. [Google Scholar] [CrossRef]
- Gautam, S.; Dinda, A.K.; Mishra, N.C. Fabrication and Characterization of PCL/Gelatin Composite Nanofibrous Scaffold for Tissue Engineering Applications by Electrospinning Method. Mater. Sci. Eng. C 2013, 33, 1228–1235. [Google Scholar] [CrossRef] [PubMed]
- Jin, G.; Prabhakaran, M.P.; Kai, D.; Annamalai, S.K.; Arunachalam, K.D.; Ramakrishna, S. Tissue Engineered Plant Extracts as Nanofibrous Wound Dressing. Biomaterials 2013, 34, 724–734. [Google Scholar] [CrossRef]
- El-Aassar, M.R.; Ibrahim, O.M.; Al-Oanzi, Z.H. Biotechnological Applications of Polymeric Nanofiber Platforms Loaded with Diverse Bioactive Materials. Polymers 2021, 13, 3734. [Google Scholar] [CrossRef]
- Aswini, E.V.; Vivek, D.; Swathilakshmi, S. Diuretic Activity of Some Known Medicinal Plants: A Review. Am. J. Pharm. Health Res. 2020, 8, 52–65. [Google Scholar] [CrossRef]
- Williamson, E.M.; Liu, X.; Izzo, A.A. Trends in Use, Pharmacology, and Clinical Applications of Emerging Herbal Nutraceuticals. Br. J. Pharmacol. 2020, 177, 1227–1240. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nikmaram, N.; Budaraju, S.; Barba, F.J.; Lorenzo, J.M.; Cox, R.B.; Mallikarjunan, K.; Roohinejad, S. Application of Plant Extracts to Improve the Shelf-Life, Nutritional and Health-Related Properties of Ready-to-Eat Meat Products. Meat Sci. 2018, 145, 245–255. [Google Scholar] [CrossRef]
- Yatoo, M.I.; Gopalakrishnan, A.; Saxena, A.; Parray, O.R.; Tufani, N.A.; Chakraborty, S.; Tiwari, R.; Dhama, K.; Iqbal, H.M.N. Anti-Inflammatory Drugs and Herbs with Special Emphasis on Herbal Medicines for Countering Inflammatory Diseases and Disorders—A Review. Recent Pat. Inflamm. Allergy Drug Discov. 2018, 12, 39–58. [Google Scholar] [CrossRef]
- Farokhi, M.; Mottaghitalab, F.; Reis, R.L.; Ramakrishna, S.; Kundu, S.C. Functionalized Silk Fibroin Nanofibers as Drug Carriers: Advantages and Challenges. J. Control. Release 2020, 321, 324–347. [Google Scholar] [CrossRef]
- Meraz-Dávila, S.; Pérez-García, C.E.; Feregrino-Perez, A.A. Challenges and Advantages of Electrospun Nanofibers in Agriculture: A Review. Mater. Res. Express 2021, 8, 042001. [Google Scholar] [CrossRef]
- Rim, N.G.; Shin, C.S.; Shin, H. Current Approaches to Electrospun Nanofibers for Tissue Engineering. Biomed. Mater. 2013, 8, 014102. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Ding, B.; Li, B. Biomimetic Electrospun Nanofibrous Structures for Tissue Engineering. Mater. Today 2013, 16, 229–241. [Google Scholar] [CrossRef]
- Bhardwaj, N.; Kundu, S.C. Electrospinning: A Fascinating Fiber Fabrication Technique. Biotechnol. Adv. 2010, 28, 325–347. [Google Scholar] [CrossRef] [PubMed]
- Rahmati, M.; Mills, D.K.; Urbanska, A.M.; Saeb, M.R.; Venugopal, J.R.; Ramakrishna, S.; Mozafari, M. Electrospinning for Tissue Engineering Applications. Prog. Mater. Sci. 2021, 117, 100721. [Google Scholar] [CrossRef]
- Xue, J.; Wu, T.; Dai, Y.; Xia, Y. Electrospinning and Electrospun Nanofibers: Methods, Materials, and Applications. Chem. Rev. 2019, 119, 5298–5415. [Google Scholar] [CrossRef] [PubMed]
- Babitha, S.; Rachita, L.; Karthikeyan, K.; Shoba, E.; Janani, I.; Poornima, B.; Purna Sai, K. Electrospun Protein Nanofibers in Healthcare: A Review. Int. J. Pharm. 2017, 523, 52–90. [Google Scholar] [CrossRef] [PubMed]
- Guan, X.; Li, L.; Li, S.; Liu, J.; Huang, K. A Food-Grade Continuous Electrospun Fiber of Hordein/Chitosan with Water Resistance. Food Biosci. 2020, 37, 100687. [Google Scholar] [CrossRef]
- Pant, B.; Park, M.; Park, S.-J. Drug Delivery Applications of Core-Sheath Nanofibers Prepared by Coaxial Electrospinning: A Review. Pharmaceutics 2019, 11, 305. [Google Scholar] [CrossRef] [Green Version]
- Xu, Y.; Li, X.; Xiang, H.-F.; Zhang, Q.-Q.; Wang, X.-X.; Yu, M.; Hao, L.-Y.; Long, Y.-Z. Large-Scale Preparation of Polymer Nanofibers for Air Filtration by a New Multineedle Electrospinning Device. J. Nanomater. 2020, 2020, 4965438. [Google Scholar] [CrossRef]
- Rodriguez, C.; Padilla, V.; Lozano, K.; McDonald, A.; Materon, L.; Chapa, A.; Ahmad, F.; De Leo, C.T.; Gilkerson, R. Fabrication of Forcespinning® Nanofibers Incorporating Nopal Extract. Polym. Int. 2021, 70, 679–686. [Google Scholar] [CrossRef]
- Padilla-Gainza, V.; Morales, G.; Rodríguez-Tobías, H.; Lozano, K. Forcespinning Technique for the Production of Poly( d, l -lactic Acid) Submicrometer Fibers: Process–Morphology–Properties Relationship. J. Appl. Polym. Sci. 2019, 136, 47643. [Google Scholar] [CrossRef]
- Hammami, M.A.; Krifa, M.; Harzallah, O. Centrifugal Force Spinning of PA6 Nanofibers—Processability and Morphology of Solution-Spun Fibers. J. Text. Inst. 2014, 105, 637–647. [Google Scholar] [CrossRef]
- Mellado, P.; McIlwee, H.A.; Badrossamay, M.R.; Goss, J.A.; Mahadevan, L.; Kit Parker, K. A Simple Model for Nanofiber Formation by Rotary Jet-Spinning. Appl. Phys. Lett. 2011, 99, 203107. [Google Scholar] [CrossRef] [Green Version]
- Riahi, D.N. Nonlinear Rotating Viscoelastic Jets during Forcespinning Process. Proc. R. Soc. A. 2018, 474, 20180346. [Google Scholar] [CrossRef] [Green Version]
- Padron, S.; Fuentes, A.; Caruntu, D.; Lozano, K. Experimental Study of Nanofiber Production through Forcespinning. J. Appl. Phys. 2013, 113, 024318. [Google Scholar] [CrossRef] [Green Version]
- Daristotle, J.L.; Behrens, A.M.; Sandler, A.D.; Kofinas, P. A Review of the Fundamental Principles and Applications of Solution Blow Spinning. ACS Appl. Mater. Interfaces 2016, 8, 34951–34963. [Google Scholar] [CrossRef] [Green Version]
- Oliveira, J.E.; Mattoso, L.H.C.; Orts, W.J.; Medeiros, E.S. Structural and Morphological Characterization of Micro and Nanofibers Produced by Electrospinning and Solution Blow Spinning: A Comparative Study. Adv. Mater. Sci. Eng 2013, 2013, 409572. [Google Scholar] [CrossRef] [Green Version]
- Stojanovska, E.; Canbay, E.; Pampal, E.S.; Calisir, M.D.; Agma, O.; Polat, Y.; Simsek, R.; Gundogdu, N.A.S.; Akgul, Y.; Kilic, A. A Review on Non-Electro Nanofibre Spinning Techniques. RSC Adv. 2016, 6, 83783–83801. [Google Scholar] [CrossRef]
- Suzuki, A.; Arino, K. Polypropylene Nanofiber Sheets Prepared by CO2 Laser Supersonic Multi-Drawing. Eur. Polym. J 2012, 48, 1169–1176. [Google Scholar] [CrossRef]
- Suzuki, A.; Tanizawa, K. Poly(Ethylene Terephthalate) Nanofibers Prepared by CO2 Laser Supersonic Drawing. Polymer 2009, 50, 913–921. [Google Scholar] [CrossRef]
- Suzuki, A.; Ohta, K. Mechanical Properties of Poly(Ethylene Terephthalate) Nanofiber Three-Dimensional Structure Prepared by CO 2 Laser Supersonic Drawing. J. Appl. Polym. Sci. 2018, 135, 45763. [Google Scholar] [CrossRef]
- Suzuki, A.; Shimba, Y. Poly(l-Lactic Acid) Twisted Nanofiber Yarn Prepared by Carbon Dioxide Laser Supersonic Multi-Drawing. Eur. Polym. J. 2019, 110, 145–154. [Google Scholar] [CrossRef]
- Suzuki, A.; Shimizu, R. Biodegradable Poly(Glycolic Acid) Nanofiber Prepared by CO2 Laser Supersonic Drawing. J. Appl. Polym. Sci. 2011, 121, 3078–3084. [Google Scholar] [CrossRef]
- Suzuki, A.; Hayashi, H. Ethylene Tetrafluoroethylene Nanofibers Prepared by CO2 Laser Supersonic Drawing. Express Polym. Lett. 2013, 7, 519–527. [Google Scholar] [CrossRef]
- Koyama, H.; Watanabe, Y.; Suzuki, A. Poly(p-Phenylene Sulfide) Nanofibers Prepared by CO2 Laser Supersonic Drawing. J. Appl. Polym. Sci. 2014, 131. [Google Scholar] [CrossRef]
- Kakoria, A.; Sinha-Ray, S. A Review on Biopolymer-Based Fibers via Electrospinning and Solution Blowing and Their Applications. Fibers 2018, 6, 45. [Google Scholar] [CrossRef] [Green Version]
- Laha, A.; Sharma, C.S.; Majumdar, S. Electrospun Gelatin Nanofibers as Drug Carrier: Effect of Crosslinking on Sustained Release. Mater. Today Proc. 2016, 3, 3484–3491. [Google Scholar] [CrossRef]
- Yen, C.-M.; Shen, C.-C.; Yang, Y.-C.; Liu, B.-S.; Lee, H.-T.; Sheu, M.-L.; Tsai, M.-H.; Cheng, W.-Y. Novel Electrospun Poly(ε-Caprolactone)/Type I Collagen Nanofiber Conduits for Repair of Peripheral Nerve Injury. Neural Regen. Res. 2019, 14, 1617. [Google Scholar] [CrossRef]
- Gokarneshan, N. Application of Natural Polymers and Herbal Extracts in Wound Management. In Advanced Textiles for Wound Care; Elsevier: Sawston, UK, 2019; pp. 541–561. ISBN 978-0-08-102192-7. [Google Scholar]
- Parenteau-Bareil, R.; Gauvin, R.; Cliche, S.; Gariépy, C.; Germain, L.; Berthod, F. Comparative Study of Bovine, Porcine and Avian Collagens for the Production of a Tissue Engineered Dermis. Acta Biomater. 2011, 7, 3757–3765. [Google Scholar] [CrossRef]
- Saghazadeh, S.; Rinoldi, C.; Schot, M.; Kashaf, S.S.; Sharifi, F.; Jalilian, E.; Nuutila, K.; Giatsidis, G.; Mostafalu, P.; Derakhshandeh, H.; et al. Drug Delivery Systems and Materials for Wound Healing Applications. Adv. Drug Deliv. Rev. 2018, 127, 138–166. [Google Scholar] [CrossRef]
- Aldana, A.A.; Abraham, G.A. Current Advances in Electrospun Gelatin-Based Scaffolds for Tissue Engineering Applications. Int. J. Pharm. 2017, 523, 441–453. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moura, L.I.F.; Dias, A.M.A.; Carvalho, E.; de Sousa, H.C. Recent Advances on the Development of Wound Dressings for Diabetic Foot Ulcer Treatment—A Review. Acta Biomater. 2013, 9, 7093–7114. [Google Scholar] [CrossRef] [Green Version]
- Saadat, S.; Emam-Djomeh, Z.; Askari, G. Antibacterial and Antioxidant Gelatin Nanofiber Scaffold Containing Ethanol Extract of Pomegranate Peel: Design, Characterization and In Vitro Assay. Food Bioprocess Technol. 2021, 14, 935–944. [Google Scholar] [CrossRef]
- Mohammadzadehmoghadam, S.; Dong, Y. Fabrication and Characterization of Electrospun Silk Fibroin/Gelatin Scaffolds Crosslinked With Glutaraldehyde Vapor. Front. Mater. 2019, 6, 91. [Google Scholar] [CrossRef]
- Deng, L.; Kang, X.; Liu, Y.; Feng, F.; Zhang, H. Effects of Surfactants on the Formation of Gelatin Nanofibres for Controlled Release of Curcumin. Food Chem. 2017, 231, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Mamidi, N.; Leija Gutiérrez, H.M.; Villela-Castrejón, J.; Isenhart, L.; Barrera, E.V.; Elías-Zúñiga, A. Fabrication of Gelatin-Poly(Epichlorohydrin-Co-Ethylene Oxide) Fiber Scaffolds by Forcespinning® for Tissue Engineering and Drug Release. MRC 2017, 7, 913–921. [Google Scholar] [CrossRef]
- Han, Y.; Xu, Y.; Zhang, S.; Li, T.; Ramakrishna, S.; Liu, Y. Progress of Improving Mechanical Strength of Electrospun Nanofibrous Membranes. Macromol. Mater. Eng. 2020, 305, 2000230. [Google Scholar] [CrossRef]
- Kuijpers, A.J.; Engbers, G.H.M.; Krijgsveld, J.; Zaat, S.A.J.; Dankert, J.; Feijen, J. Cross-Linking and Characterisation of Gelatin Matrices for Biomedical Applications. J. Biomater. Sci. Polym. Ed. 2000, 11, 225–243. [Google Scholar] [CrossRef]
- El-Aassar, M.R.; Ibrahim, O.M.; Fouda, M.M.G.; El-Beheri, N.G.; Agwa, M.M. Wound Healing of Nanofiber Comprising Polygalacturonic/Hyaluronic Acid Embedded Silver Nanoparticles: In-Vitro and In-Vivo Studies. Carbohydr. Polym. 2020, 238, 116175. [Google Scholar] [CrossRef]
- Mogoşanu, G.D.; Grumezescu, A.M. Natural and Synthetic Polymers for Wounds and Burns Dressing. Int. J. Pharm. 2014, 463, 127–136. [Google Scholar] [CrossRef] [PubMed]
- Philibert, T.; Lee, B.H.; Fabien, N. Current Status and New Perspectives on Chitin and Chitosan as Functional Biopolymers. Appl. Biochem. Biotechnol. 2017, 181, 1314–1337. [Google Scholar] [CrossRef] [PubMed]
- Elieh-Ali-Komi, D.; Hamblin, M.R. Chitin and Chitosan: Production and Application of Versatile Biomedical Nanomaterials. Int. J. Adv. Res. 2016, 4, 411–427. [Google Scholar]
- Abdul Khalil, H.P.S.; Saurabh, C.K.; Adnan, A.S.; Nurul Fazita, M.R.; Syakir, M.I.; Davoudpour, Y.; Rafatullah, M.; Abdullah, C.K.; Haafiz, M.K.M.; Dungani, R. A Review on Chitosan-Cellulose Blends and Nanocellulose Reinforced Chitosan Biocomposites: Properties and Their Applications. Carbohydr. Polym. 2016, 150, 216–226. [Google Scholar] [CrossRef]
- Cremar, L.; Gutierrez, J.; Martinez, J.; Materon, L.; Gilkerson, R.; Xu, F.; Lozano, K. Development of Antimicrobial Chitosan Based Nanofiber Dressings for Wound Healing Applications. Nanomed. J. 2018, 5, 6–14. [Google Scholar] [CrossRef]
- Shabunin, A.; Yudin, V.; Dobrovolskaya, I.; Zinovyev, E.; Zubov, V.; Ivan’kova, E.; Morganti, P. Composite Wound Dressing Based on Chitin/Chitosan Nanofibers: Processing and Biomedical Applications. Cosmetics 2019, 6, 16. [Google Scholar] [CrossRef] [Green Version]
- Petrova, V.A.; Chernyakov, D.D.; Poshina, D.N.; Gofman, I.V.; Romanov, D.P.; Mishanin, A.I.; Golovkin, A.S.; Skorik, Y.A. Electrospun Bilayer Chitosan/Hyaluronan Material and Its Compatibility with Mesenchymal Stem Cells. Materials 2019, 12, 2016. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Augustine, R.; Rehman, S.R.U.; Ahmed, R.; Zahid, A.A.; Sharifi, M.; Falahati, M.; Hasan, A. Electrospun Chitosan Membranes Containing Bioactive and Therapeutic Agents for Enhanced Wound Healing. Int. J. Biol. Macromol. 2020, 156, 153–170. [Google Scholar] [CrossRef]
- Bienek, D.R.; Hoffman, K.M.; Tutak, W. Blow-Spun Chitosan/PEG/PLGA Nanofibers as a Novel Tissue Engineering Scaffold with Antibacterial Properties. J. Mater. Sci. Mater. Med. 2016, 27, 146. [Google Scholar] [CrossRef]
- Li, Q.; Wang, X.; Lou, X.; Yuan, H.; Tu, H.; Li, B.; Zhang, Y. Genipin-Crosslinked Electrospun Chitosan Nanofibers: Determination of Crosslinking Conditions and Evaluation of Cytocompatibility. Carbohydr. Polym 2015, 130, 166–174. [Google Scholar] [CrossRef] [PubMed]
- Kohsari, I.; Shariatinia, Z.; Pourmortazavi, S.M. Antibacterial Electrospun Chitosan–Polyethylene Oxide Nanocomposite Mats Containing Bioactive Silver Nanoparticles. Carbohydr. Polym. 2016, 140, 287–298. [Google Scholar] [CrossRef]
- Sedghi, R.; Shaabani, A.; Mohammadi, Z.; Samadi, F.Y.; Isaei, E. Biocompatible Electrospinning Chitosan Nanofibers: A Novel Delivery System with Superior Local Cancer Therapy. Carbohydr. Polym. 2017, 159, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Qin, Z.; Jia, X.; Liu, Q.; Kong, B.; Wang, H. Enhancing Physical Properties of Chitosan/Pullulan Electrospinning Nanofibers via Green Crosslinking Strategies. Carbohydr. Polym. 2020, 247, 116734. [Google Scholar] [CrossRef] [PubMed]
- Grkovic, M.; Stojanovic, D.B.; Pavlovic, V.B.; Rajilic-Stojanovic, M.; Bjelovic, M.; Uskokovic, P.S. Improvement of Mechanical Properties and Antibacterial Activity of Crosslinked Electrospun Chitosan/Poly (Ethylene Oxide) Nanofibers. Compos. B. Eng. 2017, 121, 58–67. [Google Scholar] [CrossRef]
- Pritchard, E.M.; Kaplan, D.L. Silk Fibroin Biomaterials for Controlled Release Drug Delivery. Expert Opin. Drug Deliv. 2011, 8, 797–811. [Google Scholar] [CrossRef] [PubMed]
- Wenk, E.; Merkle, H.P.; Meinel, L. Silk Fibroin as a Vehicle for Drug Delivery Applications. J. Control. Release 2011, 150, 128–141. [Google Scholar] [CrossRef] [PubMed]
- Farokhi, M.; Mottaghitalab, F.; Fatahi, Y.; Khademhosseini, A.; Kaplan, D.L. Overview of Silk Fibroin Use in Wound Dressings. Trends Biotechnol. 2018, 36, 907–922. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Bailey, K.; Wang, S.; Feng, X. Silk Fibroin Films for Potential Applications in Controlled Release. React. Funct. Polym. 2017, 116, 57–68. [Google Scholar] [CrossRef]
- Çalamak, S.; Erdoğdu, C.; Özalp, M.; Ulubayram, K. Silk Fibroin Based Antibacterial Bionanotextiles as Wound Dressing Materials. Mater. Sci. Eng. C 2014, 43, 11–20. [Google Scholar] [CrossRef]
- Koh, L.-D.; Cheng, Y.; Teng, C.-P.; Khin, Y.-W.; Loh, X.-J.; Tee, S.-Y.; Low, M.; Ye, E.; Yu, H.-D.; Zhang, Y.-W.; et al. Structures, Mechanical Properties and Applications of Silk Fibroin Materials. Prog. Polym. Sci. 2015, 46, 86–110. [Google Scholar] [CrossRef]
- Zhang, K.; Qian, Y.; Wang, H.; Fan, L.; Huang, C.; Yin, A.; Mo, X. Genipin-Crosslinked Silk Fibroin/Hydroxybutyl Chitosan Nanofibrous Scaffolds for Tissue-Engineering Application. J. Biomed. Mater. Res. 2010, 95A, 870–881. [Google Scholar] [CrossRef]
- Bae, S.B.; Kim, M.H.; Park, W.H. Electrospinning and Dual Crosslinking of Water-Soluble Silk Fibroin Modified with Glycidyl Methacrylate. Polym. Degrad. Stab. 2020, 179, 109304. [Google Scholar] [CrossRef]
- Deng, L.; Zhang, X.; Li, Y.; Que, F.; Kang, X.; Liu, Y.; Feng, F.; Zhang, H. Characterization of Gelatin/Zein Nanofibers by Hybrid Electrospinning. Food Hydrocoll. 2018, 75, 72–80. [Google Scholar] [CrossRef]
- Vineis, C.; Varesano, A. Natural Polymer-Based Electrospun Fibers for Antibacterial Uses. In Electrofluidodynamic Technologies (EFDTs) for Biomaterials and Medical Devices; Elsevier: Amsterdam, The Netherlands, 2018; pp. 275–294. ISBN 978-0-08-101745-6. [Google Scholar]
- Tanat Uan-On Extraction and Characterization of Zein Protein from Corn for Controlled Drug Release. Curr. Appl. Sci. Technol. 2018, 18, 167. [CrossRef]
- Moradkhannejhad, L.; Abdouss, M.; Nikfarjam, N.; Mazinani, S.; Heydari, V. Electrospinning of Zein/Propolis Nanofibers; Antimicrobial Properties and Morphology Investigation. J. Mater. Sci. Mater. Med. 2018, 29, 165. [Google Scholar] [CrossRef]
- Deng, L.; Li, Y.; Feng, F.; Zhang, H. Study on Wettability, Mechanical Property and Biocompatibility of Electrospun Gelatin/Zein Nanofibers Cross-Linked by Glucose. Food Hydrocoll. 2019, 87, 1–10. [Google Scholar] [CrossRef]
- Jiang, Z.; Zhang, H.; Zhu, M.; Lv, D.; Yao, J.; Xiong, R.; Huang, C. Electrospun Soy-Protein-Based Nanofibrous Membranes for Effective Antimicrobial Air Filtration. J. Appl. Polym. Sci. 2018, 135, 45766. [Google Scholar] [CrossRef]
- Ahn, S.; Chantre, C.O.; Gannon, A.R.; Lind, J.U.; Campbell, P.H.; Grevesse, T.; O’Connor, B.B.; Parker, K.K. Soy Protein/Cellulose Nanofiber Scaffolds Mimicking Skin Extracellular Matrix for Enhanced Wound Healing. Adv. Healthc. Mater. 2018, 7, 1701175. [Google Scholar] [CrossRef]
- Tansaz, S.; Boccaccini, A.R. Biomedical Applications of Soy Protein: A Brief Overview: Biomedical Applications of Soy Protein. J. Biomed. Mater. Res. 2016, 104, 553–569. [Google Scholar] [CrossRef]
- Tian, H.; Guo, G.; Fu, X.; Yao, Y.; Yuan, L.; Xiang, A. Fabrication, Properties and Applications of Soy-Protein-Based Materials: A Review. Int. J. Biol. Macromol. 2018, 120, 475–490. [Google Scholar] [CrossRef]
- Ramji, K.; Shah, R.N. Electrospun Soy Protein Nanofiber Scaffolds for Tissue Regeneration. J. Biomater. Appl. 2014, 29, 411–422. [Google Scholar] [CrossRef]
- Vega-Lugo, A.-C.; Lim, L.-T. Electrospinning of Soy Protein Isolate Nanofibers. J. Biobased Mater. Bioenergy 2008, 2, 223–230. [Google Scholar] [CrossRef]
- Nishinari, K.; Fang, Y.; Guo, S.; Phillips, G.O. Soy Proteins: A Review on Composition, Aggregation and Emulsification. Food Hydrocoll. 2014, 39, 301–318. [Google Scholar] [CrossRef]
- Peles, Z.; Zilberman, M. Novel Soy Protein Wound Dressings with Controlled Antibiotic Release: Mechanical and Physical Properties. Acta Biomater. 2012, 8, 209–217. [Google Scholar] [CrossRef]
- Khoshnevisan, K.; Maleki, H.; Samadian, H.; Shahsavari, S.; Sarrafzadeh, M.H.; Larijani, B.; Dorkoosh, F.A.; Haghpanah, V.; Khorramizadeh, M.R. Cellulose Acetate Electrospun Nanofibers for Drug Delivery Systems: Applications and Recent Advances. Carbohydr. Polym. 2018, 198, 131–141. [Google Scholar] [CrossRef]
- Vallejos, M.E.; Peresin, M.S.; Rojas, O.J. All-Cellulose Composite Fibers Obtained by Electrospinning Dispersions of Cellulose Acetate and Cellulose Nanocrystals. J. Polym. Environ. 2012, 20, 1075–1083. [Google Scholar] [CrossRef]
- Zhang, K.; Li, Z.; Kang, W.; Deng, N.; Yan, J.; Ju, J.; Liu, Y.; Cheng, B. Preparation and Characterization of Tree-like Cellulose Nanofiber Membranes via the Electrospinning Method. Carbohydr. Polym. 2018, 183, 62–69. [Google Scholar] [CrossRef]
- Saraiva, S.; Pereira, P.; Paula, C.T.; Rebelo, R.C.; Coelho, J.F.J.; Serra, A.C.; Fonseca, A.C. Development of Electrospun Mats Based on Hydrophobic Hydroxypropyl Cellulose Derivatives. Mater. Sci. Eng. C 2021, 131, 112498. [Google Scholar] [CrossRef]
- Konwarh, R.; Karak, N.; Misra, M. Electrospun Cellulose Acetate Nanofibers: The Present Status and Gamut of Biotechnological Applications. Biotechnol. Adv. 2013, 31, 421–437. [Google Scholar] [CrossRef]
- Ahmed, F.; Ayoub Arbab, A.; Jatoi, A.W.; Khatri, M.; Memon, N.; Khatri, Z.; Kim, I.S. Ultrasonic-Assisted Deacetylation of Cellulose Acetate Nanofibers: A Rapid Method to Produce Cellulose Nanofibers. Ultrason. Sonochem. 2017, 36, 319–325. [Google Scholar] [CrossRef]
- Cai, J.; Zhou, R.; Li, T.; He, J.; Wang, G.; Wang, H.; Xiong, H. Bamboo Cellulose-Derived Cellulose Acetate for Electrospun Nanofibers: Synthesis, Characterization and Kinetics. Cellulose 2018, 25, 391–398. [Google Scholar] [CrossRef]
- Ahmed, F.; Saleemi, S.; Khatri, Z.; Abro, M.I.; Kim, I.-S. Co-Electrospun Poly(ɛ-Caprolactone)/Cellulose Nanofibers-Fabrication and Characterization. Carbohydr. Polym. 2015, 115, 388–393. [Google Scholar] [CrossRef]
- Liao, N.; Unnithan, A.R.; Joshi, M.K.; Tiwari, A.P.; Hong, S.T.; Park, C.-H.; Kim, C.S. Electrospun Bioactive Poly (ɛ-Caprolactone)–Cellulose Acetate–Dextran Antibacterial Composite Mats for Wound Dressing Applications. Colloids Surf. A Physicochem. Eng. Asp. 2015, 469, 194–201. [Google Scholar] [CrossRef]
- Zhang, L.; Hsieh, Y.-L. Ultra-Fine Cellulose Acetate/Poly(Ethylene Oxide) Bicomponent Fibers. Carbohydr. Polym. 2008, 71, 196–207. [Google Scholar] [CrossRef]
- Zhijiang, C.; Yi, X.; Haizheng, Y.; Jia, J.; Liu, Y. Poly(Hydroxybutyrate)/Cellulose Acetate Blend Nanofiber Scaffolds: Preparation, Characterization and Cytocompatibility. Mater. Sci. Eng. C 2016, 58, 757–767. [Google Scholar] [CrossRef]
- Baji, A.; Mai, Y.-W.; Wong, S.-C.; Abtahi, M.; Chen, P. Electrospinning of Polymer Nanofibers: Effects on Oriented Morphology, Structures and Tensile Properties. Compos. Sci. Technol. 2010, 70, 703–718. [Google Scholar] [CrossRef]
- Park, K.E.; Kang, H.K.; Lee, S.J.; Min, B.-M.; Park, W.H. Biomimetic Nanofibrous Scaffolds: Preparation and Characterization of PGA/Chitin Blend Nanofibers. Biomacromolecules 2006, 7, 635–643. [Google Scholar] [CrossRef]
- Perumal, G.; Pappuru, S.; Chakraborty, D.; Maya Nandkumar, A.; Chand, D.K.; Doble, M. Synthesis and Characterization of Curcumin Loaded PLA—Hyperbranched Polyglycerol Electrospun Blend for Wound Dressing Applications. Mater. Sci. Eng. C 2017, 76, 1196–1204. [Google Scholar] [CrossRef]
- Zeng, J.; Haoqing, H.; Schaper, A.; Wendorff, J.H.; Greiner, A. Poly-L-Lactide Nanofibers by Electrospinning—Influence of Solution Viscosity and Electrical Conductivity on Fiber Diameter and Fiber Morphology. e-Polymers 2003, 9, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Zhao, W.; Li, J.; Jin, K.; Liu, W.; Qiu, X.; Li, C. Fabrication of Functional PLGA-Based Electrospun Scaffolds and Their Applications in Biomedical Engineering. Mater. Sci. Eng. C 2016, 59, 1181–1194. [Google Scholar] [CrossRef]
- Ghosal, K.; Manakhov, A.; Zajíčková, L.; Thomas, S. Structural and Surface Compatibility Study of Modified Electrospun Poly(ε-Caprolactone) (PCL) Composites for Skin Tissue Engineering. AAPS PharmSciTech 2017, 18, 72–81. [Google Scholar] [CrossRef] [Green Version]
- Tsekova, P.B.; Spasova, M.G.; Manolova, N.E.; Markova, N.D.; Rashkov, I.B. Electrospun Curcumin-Loaded Cellulose Acetate/Polyvinylpyrrolidone Fibrous Materials with Complex Architecture and Antibacterial Activity. Mater. Sci. Eng. C 2017, 73, 206–214. [Google Scholar] [CrossRef]
- Chouhan, D.; Janani, G.; Chakraborty, B.; Nandi, S.K.; Mandal, B.B. Functionalized PVA—Silk Blended Nanofibrous Mats Promote Diabetic Wound Healing via Regulation of Extracellular Matrix and Tissue Remodelling. J. Tissue Eng. Regen. Med. 2018, 12, e1559–e1570. [Google Scholar] [CrossRef]
- Surendhiran, D.; Li, C.; Cui, H.; Lin, L. Fabrication of High Stability Active Nanofibers Encapsulated with Pomegranate Peel Extract Using Chitosan/PEO for Meat Preservation. Food Packag. Shelf Life 2020, 23, 100439. [Google Scholar] [CrossRef]
- Hosseinzadeh, S.; Jafarikukhdan, A.; Hosseini, A.; Armand, R. The Application of Medicinal Plants in Traditional and Modern Medicine: A Review of Thymus vulgaris. Int. J. Clin. Med. 2015, 6, 635–642. [Google Scholar] [CrossRef] [Green Version]
- Süntar, I. Importance of Ethnopharmacological Studies in Drug Discovery: Role of Medicinal Plants. Phytochem. Rev. 2020, 19, 1199–1209. [Google Scholar] [CrossRef]
- Chiu, C.-M.; Nootem, J.; Santiwat, T.; Srisuwannaket, C.; Pratumyot, K.; Lin, W.-C.; Mingvanish, W.; Niamnont, N. Enhanced Stability and Bioactivity of Curcuma Comosa Roxb. Extract in Electrospun Gelatin Nanofibers. Fibers 2019, 7, 76. [Google Scholar] [CrossRef] [Green Version]
- Yao, C.-H.; Chen, K.-Y.; Chen, Y.-S.; Li, S.-J.; Huang, C.-H. Lithospermi Radix Extract-Containing Bilayer Nanofiber Scaffold for Promoting Wound Healing in a Rat Model. Mater. Sci. Eng. C 2019, 96, 850–858. [Google Scholar] [CrossRef] [PubMed]
- Pathalamuthu, P.; Siddharthan, A.; Giridev, V.R.; Victoria, V.; Thangam, R.; Sivasubramanian, S.; Savariar, V.; Hemamalini, T. Enhanced Performance of Aloe Vera Incorporated Chitosan-Polyethylene Oxide Electrospun Wound Scaffold Produced Using Novel Spirograph Based Collector Assembly. Int. J. Biol. Macromol. 2019, 140, 808–824. [Google Scholar] [CrossRef]
- Baniasadi, M.; Baniasadi, H.; Azimi, R.; Khosravi Dehaghi, N. Fabrication and Characterization of a Wound Dressing Composed of Polyvinyl Alcohol/Nanochitosan/Artemisia Ciniformis Extract: An RSM Study. Polym. Eng. Sci. 2020, 60, 1459–1473. [Google Scholar] [CrossRef]
- Erbay, E.A.; Dağtekin, B.B.G.; Türe, M.; Yeşilsu, A.F.; Torres-Giner, S. Quality Improvement of Rainbow Trout Fillets by Whey Protein Isolate Coatings Containing Electrospun Poly(ε-Caprolactone) Nanofibers with Urtica dioica L. Extract during Storage. LWT 2017, 78, 340–351. [Google Scholar] [CrossRef]
- Hokmabad, V.R.; Davaran, S.; Aghazadeh, M.; Alizadeh, E.; Salehi, R.; Ramazani, A. Effect of Incorporating Elaeagnus Angustifolia Extract in PCL-PEG-PCL Nanofibers for Bone Tissue Engineering. Front. Chem. Sci. Eng. 2019, 13, 108–119. [Google Scholar] [CrossRef]
- Zadeh, K.M.; Luyt, A.S.; Zarif, L.; Augustine, R.; Hasan, A.; Messori, M.; Hassan, M.K.; Yalcin, H.C. Electrospun Polylactic Acid/Date Palm Polyphenol Extract Nanofibres for Tissue Engineering Applications. Emergent Mater. 2019, 2, 141–151. [Google Scholar] [CrossRef] [Green Version]
- Bonan, R.F.; Bonan, P.R.F.; Batista, A.U.D.; Sampaio, F.C.; Albuquerque, A.J.R.; Moraes, M.C.B.; Mattoso, L.H.C.; Glenn, G.M.; Medeiros, E.S.; Oliveira, J.E. In Vitro Antimicrobial Activity of Solution Blow Spun Poly(Lactic Acid)/Polyvinylpyrrolidone Nanofibers Loaded with Copaiba (Copaifera Sp.) Oil. Mater. Sci. Eng. C 2015, 48, 372–377. [Google Scholar] [CrossRef] [PubMed]
- Rezaeinia, H.; Emadzadeh, B.; Ghorani, B. Electrospun Balangu (Lallemantia royleana) Hydrocolloid Nanofiber Mat as a Fast-Dissolving Carrier for Bergamot Essential Oil. Food Hydrocoll. 2020, 100, 105312. [Google Scholar] [CrossRef]
- Faki, R.; Gursoy, O.; Yilmaz, Y. Effect of Electrospinning Process on Total Antioxidant Activity of Electrospun Nanofibers Containing Grape Seed Extract. Open Chem. 2019, 17, 912–918. [Google Scholar] [CrossRef]
- Kim, J.H.; Lee, H.; Jatoi, A.W.; Im, S.S.; Lee, J.S.; Kim, I.-S. Juniperus chinensis Extracts Loaded PVA Nanofiber: Enhanced Antibacterial Activity. Mater. Lett. 2016, 181, 367–370. [Google Scholar] [CrossRef]
- Andersson, R.; Martínez-Abad, A.; Lagaron, J.; Gedde, U.; Mallon, P.; Olsson, R.; Hedenqvist, M. Antibacterial Properties of Tough and Strong Electrospun PMMA/PEO Fiber Mats Filled with Lanasol—A Naturally Occurring Brominated Substance. Int. J. Mol. Sci. 2014, 15, 15912–15923. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Canbay-Gokce, E.; Akgul, Y.; Gokce, A.Y.; Tasdelen-Yucedag, C.; Kilic, A.; Hassanin, A. Characterization of Solution Blown Thermoplastic Polyurethane Nanofibers Modified with Szygium aromaticum Extract. J. Text. Inst. 2020, 111, 10–15. [Google Scholar] [CrossRef]
- Kalouta, K.; Eleni, P.; Boukouvalas, C.; Vassilatou, K.; Krokida, M. Dynamic Mechanical Analysis of Novel Cosmeceutical Facial Creams Containing Nano-encapsulated Natural Plant and Fruit Extracts. J. Cosmet. Dermatol. 2020, 19, 1146–1154. [Google Scholar] [CrossRef]
- Fayemi, O.E.; Ekennia, A.C.; Katata-Seru, L.; Ebokaiwe, A.P.; Ijomone, O.M.; Onwudiwe, D.C.; Ebenso, E.E. Antimicrobial and Wound Healing Properties of Polyacrylonitrile-Moringa Extract Nanofibers. ACS Omega 2018, 3, 4791–4797. [Google Scholar] [CrossRef]
- Maria Leena, M.; Yoha, K.S.; Moses, J.A.; Anandharamakrishnan, C. Edible Coating with Resveratrol Loaded Electrospun Zein Nanofibers with Enhanced Bioaccessibility. Food Biosci. 2020, 36, 100669. [Google Scholar] [CrossRef]
- Torkamani, A.E.; Syahariza, Z.A.; Norziah, M.H.; Wan, A.K.M.; Juliano, P. Encapsulation of Polyphenolic Antioxidants Obtained from Momordica Charantia Fruit within Zein/Gelatin Shell Core Fibers via Coaxial Electrospinning. Food Biosci. 2018, 21, 60–71. [Google Scholar] [CrossRef]
- Asha Krishnan, K.; Thomas, S. Recent Advances on Herb-Derived Constituents-Incorporated Wound-Dressing Materials: A Review. Polym. Adv. Technol. 2019, 30, 823–838. [Google Scholar] [CrossRef]
- Silva, P.; Bonifácio, B.; Ramos, M.; Negri, K.; Maria Bauab, T.; Chorilli, M. Nanotechnology-Based Drug Delivery Systems and Herbal Medicines: A Review. Int. J. Nanomed. 2014, 9, 1–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adeli-Sardou, M.; Yaghoobi, M.M.; Torkzadeh-Mahani, M.; Dodel, M. Controlled Release of Lawsone from Polycaprolactone/Gelatin Electrospun Nano Fibers for Skin Tissue Regeneration. Int. J. Biol. Macromol. 2019, 124, 478–491. [Google Scholar] [CrossRef] [PubMed]
- Yao, C.-H.; Yeh, J.-Y.; Chen, Y.-S.; Li, M.-H.; Huang, C.-H. Wound-Healing Effect of Electrospun Gelatin Nanofibres Containing Centella Asiatica Extract in a Rat Model: Wound Healing Effect of EGC Membrane. J. Tissue Eng. Regen. Med. 2017, 11, 905–915. [Google Scholar] [CrossRef] [PubMed]
- Motealleh, B.; Zahedi, P.; Rezaeian, I.; Moghimi, M.; Abdolghaffari, A.H.; Zarandi, M.A. Morphology, Drug Release, Antibacterial, Cell Proliferation, and Histology Studies of Chamomile-Loaded Wound Dressing Mats Based on Electrospun Nanofibrous Poly(ε-Caprolactone)/Polystyrene Blends: Morphology, Drug Release, Antibacterial, Cell Proliferation, and Histology Studies. J. Biomed. Mater. Res. 2014, 102, 977–987. [Google Scholar] [CrossRef]
- de Oliveira Mori, C.L.S.; dos Passos, N.A.; Oliveira, J.E.; Mattoso, L.H.C.; Mori, F.A.; Carvalho, A.G.; de Souza Fonseca, A.; Tonoli, G.H.D. Electrospinning of Zein/Tannin Bio-Nanofibers. Ind. Crops Prod. 2014, 52, 298–304. [Google Scholar] [CrossRef]
- Hani, N.M.; Torkamani, A.E.; Azarian, M.H.; Mahmood, K.W.; Ngalim, S.H. Characterisation of Electrospun Gelatine Nanofibres Encapsulated with Moringa oleifera Bioactive Extract: Encapsulation of Moringa olieifera Leaf Extract in Nanofibres. J. Sci. Food Agric. 2017, 97, 3348–3358. [Google Scholar] [CrossRef]
- Huang, Y.; Shi, R.; Gong, M.; Zhang, J.; Li, W.; Song, Q.; Wu, C.; Tian, W. Icariin-Loaded Electrospun PCL/Gelatin Sub-Microfiber Mat for Preventing Epidural Adhesions after Laminectomy. Int. J. Nanomed. 2018, 13, 4831–4844. [Google Scholar] [CrossRef] [Green Version]
- Das, S.; Baker, A.B. Biomaterials and Nanotherapeutics for Enhancing Skin Wound Healing. Front. Bioeng. Biotechnol. 2016, 4, 82. [Google Scholar] [CrossRef]
- Tottoli, E.M.; Dorati, R.; Genta, I.; Chiesa, E.; Pisani, S.; Conti, B. Skin Wound Healing Process and New Emerging Technologies for Skin Wound Care and Regeneration. Pharmaceutics 2020, 12, 735. [Google Scholar] [CrossRef]
- Kenry; Lim, C.T. Nanofiber Technology: Current Status and Emerging Developments. Prog. Polym. Sci. 2017, 70, 1–17. [Google Scholar] [CrossRef]
- Liu, M.; Duan, X.-P.; Li, Y.-M.; Yang, D.-P.; Long, Y.-Z. Electrospun Nanofibers for Wound Healing. Mater. Sci. Eng. C 2017, 76, 1413–1423. [Google Scholar] [CrossRef]
- Hadisi, Z.; Nourmohammadi, J.; Nassiri, S.M. The Antibacterial and Anti-Inflammatory Investigation of Lawsonia Inermis-Gelatin-Starch Nano-Fibrous Dressing in Burn Wound. Int. J. Biol. Macromol. 2018, 107, 2008–2019. [Google Scholar] [CrossRef]
- Charernsriwilaiwat, N.; Rojanarata, T.; Ngawhirunpat, T.; Sukma, M.; Opanasopit, P. Electrospun Chitosan-Based Nanofiber Mats Loaded with Garcinia Mangostana Extracts. Int. J. Pharm. 2013, 452, 333–343. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Fan, L.; Ma, L.; Wang, Y.; Lin, S.; Yu, F.; Pan, X.; Luo, G.; Zhang, D.; Wang, H. Green Electrospun Manuka Honey/Silk Fibroin Fibrous Matrices as Potential Wound Dressing. Mater. Des. 2017, 119, 76–84. [Google Scholar] [CrossRef]
- Ranjbar-Mohammadi, M.; Rabbani, S.; Bahrami, S.H.; Joghataei, M.T.; Moayer, F. Antibacterial Performance and in Vivo Diabetic Wound Healing of Curcumin Loaded Gum Tragacanth/Poly(ε-Caprolactone) Electrospun Nanofibers. Mater. Sci. Eng. C 2016, 69, 1183–1191. [Google Scholar] [CrossRef]
- Mishra, D.; Khare, P.; Singh, D.K.; Luqman, S.; Ajaya Kumar, P.V.; Yadav, A.; Das, T.; Saikia, B.K. Retention of Antibacterial and Antioxidant Properties of Lemongrass Oil Loaded on Cellulose Nanofibre-Poly Ethylene Glycol Composite. Ind. Crops Prod. 2018, 114, 68–80. [Google Scholar] [CrossRef]
- Pina, S.; Ribeiro, V.P.; Marques, C.F.; Maia, F.R.; Silva, T.H.; Reis, R.L.; Oliveira, J.M. Scaffolding Strategies for Tissue Engineering and Regenerative Medicine Applications. Materials 2019, 12, 1824. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khorshidi, S.; Solouk, A.; Mirzadeh, H.; Mazinani, S.; Lagaron, J.M.; Sharifi, S.; Ramakrishna, S. A Review of Key Challenges of Electrospun Scaffolds for Tissue-Engineering Applications: Challenges Regarding Electrospun Scaffolds: A Review. J. Tissue Eng. Regen. Med. 2016, 10, 715–738. [Google Scholar] [CrossRef]
- Nemati, S.; Kim, S.; Shin, Y.M.; Shin, H. Current Progress in Application of Polymeric Nanofibers to Tissue Engineering. Nano Converg. 2019, 6, 36. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Selvaraj, S.; Fathima, N.N. Fenugreek Incorporated Silk Fibroin Nanofibers—A Potential Antioxidant Scaffold for Enhanced Wound Healing. ACS Appl. Mater. Interfaces 2017, 9, 5916–5926. [Google Scholar] [CrossRef]
- Brahatheeswaran, D.; Mathew, A.; Aswathy, R.G.; Nagaoka, Y.; Venugopal, K.; Yoshida, Y.; Maekawa, T.; Sakthikumar, D. Hybrid Fluorescent Curcumin Loaded Zein Electrospun Nanofibrous Scaffold for Biomedical Applications. Biomed. Mater. 2012, 7, 045001. [Google Scholar] [CrossRef] [PubMed]
- Salehi, M.; Farzamfar, S.; Bastami, F.; Tajerian, R. Fabrication and Characterization of Electrospun PLLA/Collagen Nanofibrous Scaffold Coated With Chitosan to Sustain Release of Aloe vera Gel for Skin Tissue Engineering. Biomed. Eng. Appl. Basis Commun. 2016, 28, 1650035. [Google Scholar] [CrossRef] [Green Version]
- Shahbazi, E.; Bahrami, K. Production and Properties Analysis of Honey Nanofibers Enriched with Antibacterial Herbal Extracts for Repair and Regeneration of Skin and Bone Tissues. J. Pharm. Pharmacol. 2019, 7, 37–50. [Google Scholar] [CrossRef] [Green Version]
- Thakkar, S.; Misra, M. Electrospun Polymeric Nanofibers: New Horizons in Drug Delivery. Eur. J. Pharm. Sci. 2017, 107, 148–167. [Google Scholar] [CrossRef] [PubMed]
- Sebe, I.; Szabó, P.; Kállai-Szabó, B.; Zelkó, R. Incorporating Small Molecules or Biologics into Nanofibers for Optimized Drug Release: A Review. Int. J. Pharm. 2015, 494, 516–530. [Google Scholar] [CrossRef]
- Son, Y.J.; Kim, W.J.; Yoo, H.S. Therapeutic Applications of Electrospun Nanofibers for Drug Delivery Systems. Arch. Pharm. Res. 2014, 37, 69–78. [Google Scholar] [CrossRef]
- Avci, H.; Gergeroglu, H. Synergistic Effects of Plant Extracts and Polymers on Structural and Antibacterial Properties for Wound Healing. Polym. Bull. 2019, 76, 3709–3731. [Google Scholar] [CrossRef]
- Taepaiboon, P.; Rungsardthong, U.; Supaphol, P. Vitamin-Loaded Electrospun Cellulose Acetate Nanofiber Mats as Transdermal and Dermal Therapeutic Agents of Vitamin A Acid and Vitamin E. Eur. J. Pharm. Biopharm. 2007, 67, 387–397. [Google Scholar] [CrossRef] [PubMed]
- Fahami, A.; Fathi, M. Development of Cress Seed Mucilage/PVA Nanofibers as a Novel Carrier for Vitamin A Delivery. Food Hydrocoll. 2018, 81, 31–38. [Google Scholar] [CrossRef]
- Tavassoli-Kafrani, E.; Goli, S.A.H.; Fathi, M. Encapsulation of Orange Essential Oil Using Cross-Linked Electrospun Gelatin Nanofibers. Food Bioprocess Technol. 2018, 11, 427–434. [Google Scholar] [CrossRef]
- Chantarodsakun, T.; Vongsetskul, T.; Jangpatarapongsa, K.; Tuchinda, P.; Uamsiri, S.; Bamrungcharoen, C.; Kumkate, S.; Opaprakasit, P.; Tangboriboonrat, P. [6]-Gingerol-Loaded Cellulose Acetate Electrospun Fibers as a Topical Carrier for Controlled Release. Polym. Bull. 2014, 71, 3163–3176. [Google Scholar] [CrossRef]
- Zhu, L.; Liu, X.; Du, L.; Jin, Y. Preparation of Asiaticoside-Loaded Coaxially Electrospinning Nanofibers and Their Effect on Deep Partial-Thickness Burn Injury. Biomed. Pharmacother. 2016, 83, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Ding, F.; Ma, L.; Zhang, Y. Edible Films from Chitosan-Gelatin: Physical Properties and Food Packaging Application. Food Biosci. 2021, 40, 100871. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, D.; Sun, Z.; Liu, F.; Du, L.; Wang, D. Preparation and Characterization of Gelatin/Chitosan/3-Phenylacetic Acid Food-Packaging Nanofiber Antibacterial Films by Electrospinning. Int. J. Biol. Macromol. 2021, 169, 161–170. [Google Scholar] [CrossRef]
- Mahmoudi, N.; Eslahi, N.; Mehdipour, A.; Mohammadi, M.; Akbari, M.; Samadikuchaksaraei, A.; Simchi, A. Temporary Skin Grafts Based on Hybrid Graphene Oxide-Natural Biopolymer Nanofibers as Effective Wound Healing Substitutes: Pre-Clinical and Pathological Studies in Animal Models. J. Mater. Sci. Mater. Med. 2017, 28, 73. [Google Scholar] [CrossRef]
- Uppal, R.; Ramaswamy, G.N.; Arnold, C.; Goodband, R.; Wang, Y. Hyaluronic Acid Nanofiber Wound Dressing-Production, Characterization, and in Vivo Behavior. J. Biomed. Mater. Res. 2011, 97B, 20–29. [Google Scholar] [CrossRef]
- Vieira Batista, S.; Martins da Silva, T.; Brito Salomão, S.; Arêas Bastos, K.; Oliveira Lamas de Souza, S.; Alvarenga Pinto Cotrim, M.; Lambert Oréfice, R.; Faloni de Andrade, S.; de Paula Careta, F.; Aparecida Severi, J.; et al. In Vitro Activity of Pomegranate Peel Extract Alone and in Electrospun Poly(Vinyl Alcohol)/Sodium Alginate Matrix. Biomed. Biopharm. Res. 2020, 17, 294–312. [Google Scholar] [CrossRef]
- Zhang, D.; Li, L.; Shan, Y.; Xiong, J.; Hu, Z.; Zhang, Y.; Gao, J. In Vivo Study of Silk Fibroin/Gelatin Electrospun Nanofiber Dressing Loaded with Astragaloside IV on the Effect of Promoting Wound Healing and Relieving Scar. J. Drug Deliv. Sci. Technol. 2019, 52, 272–281. [Google Scholar] [CrossRef]
- Naeimi, A.; Payandeh, M.; Ghara, A.R.; Ghadi, F.E. In Vivo Evaluation of the Wound Healing Properties of Bio-Nanofiber Chitosan/Polyvinyl Alcohol Incorporating Honey and Nepeta dschuparensis. Carbohydr. Polym. 2020, 240, 116315. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.M.; Lee, B.N.; Ko, J.H.; Kim, G.H.; Kang, K.N.; Kim, D.Y.; Kim, J.H.; Park, Y.H.; Chun, H.J.; Kim, C.H.; et al. In Vivo Biocompatibility Study of Electrospun Chitosan Microfiber for Tissue Engineering. Int. J. Mol. Sci. 2010, 11, 4140–4148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kotroni, E.; Simirioti, E.; Kikionis, S.; Sfiniadakis, I.; Siamidi, A.; Karalis, V.; Vitsos, A.; Vlachou, M.; Ioannou, E.; Roussis, V.; et al. In Vivo Evaluation of the Anti-Inflammatory Activity of Electrospun Micro/Nanofibrous Patches Loaded with Pinus halepensis Bark Extract on Hairless Mice Skin. Materials 2019, 12, 2596. [Google Scholar] [CrossRef] [Green Version]
- Abou Zekry, S.S.; Abdellatif, A.; Azzazy, H.M.E. Fabrication of Pomegranate/Honey Nanofibers for Use as Antibacterial Wound Dressings. Wound Med. 2020, 28, 100181. [Google Scholar] [CrossRef]
- StatNano Products Available in Medicine for Tissue Engineering. Available online: https://product.statnano.com/search?search_title [0]=Medicine&search_title [1]=tissue_engineering&search_in[0 ]=6&search_in[1]=7&i_d2[1]=&and_or[1]=and&advanced=1&lang=2Nanotechnology (accessed on 1 November 2020).




| Polymeric Solution Parameter | Technical Parameters | Environmental Parameters |
|---|---|---|
| Polymer viscoelasticity Polymer–solvent compatibility | Spinneret angular velocity Needle diameter Spinneret distance to the collector | Temperature Humidity |
| Polymer | Classification | Description | Reference |
|---|---|---|---|
| Poly (Glycolic Acid) (PGA) | Polyester | Thermoplastic polymer with high crystallinity (46–50%). | Park et al. [98] |
| Transition and melting temperatures of 36 °C and 225 °C | |||
| Degradation product: glycolic acid | |||
| Poly (Lactic Acid) (PLA) | Polyester | Semi crystalline polymer | Perumal et al. [99]; Zeng et al. [100] |
| Hydrophobic | |||
| Degradation product: lactic acid | |||
| Poly (Lactic-Glycolic Acid) (PLGA) | Polyester | Amorphous and crystalline polymer | Zhao et al. [101] |
| Transition and melting temperature: 37 °C and 225 °C | |||
| Poly (Ε-Caprolactone) (PCL) | Polylactone | Semi crystalline polymer | Ghosal et al. [102] |
| Glass transition and melting temperature of −60 °C and 59 °C | |||
| Polyvinyl Pyrrolidone (PVP) | Polyamide | Water-soluble polymer | Tsekova et al. [103] |
| Glass transition temperature of 173 °C | |||
| Poly (Vinyl Alcohol) (PVA) | Polyvinyl ester | Hydrophilic polymer | Chouhan et al. [104] |
| Melting temperature of 300 °C |
| Herbal Component | Polymer | Properties | Technique | Reference |
| Curcuma comosa Roxb. Extract | Gelatin | Antioxidant, anti-tyrosinase, and anti-bacterial activities | Electrospinning | Chiu et al. [108] |
| Lithospermi radix extract | Gelatin/chitosan/PVA | Non-immunogenicity, antibacterial, tissue regeneration, anti-inflammatory, anti-apoptosis | Electrospinning | Yao et al. [109] |
| Pomegranate (Punica granatum) peel extract | Chitosan/polyethylene oxide (PEO) | Antioxidant, anti-diabetic, anti-hypersensitive, anti-inflammatory, antiviral, anti-bacterial | Electrospinning | Surendhiran et al. [105] |
| Aloe vera extract | Chitosan/polyethylene oxide (PEO) | Wound healing, anti-inflammatory, strengthening of the immune system, anti-carcinogenic, anti-diabetic, antioxidant | Electrospinning | Pathalamuthu et al. [110] |
| Artemisia ciniformis extract | PVA/chitosan | Antimicrobial | Electrospinning | Baniasadi et al. [111] |
| Urtica dioica L. extract | PCL | Antimicrobial | Electrospinning | Erbay et al. [112] |
| Eleaeagnus angustifolia extract | PEG-PCL-PEG | Antinociceptive, anti-inflammatory, antibacterial, antioxidant | Electrospinning | Hokmabad et al. [113] |
| Date palm fruit extract | PLA | Polyphenolic activity, antioxidant, anti-diabetic, anti-carcinogenic, antibacterial | Electrospinning | Zadeh et al. [114] |
| Copaiba (Copaifera sp.) oil | PLA/polyvinylpyrrolidone (PVP) | Anti-inflammatory, bactericidal | Solution blow spinning | Bonan, et al. [115] |
| Lallemantia royleana extract | PVA | Antioxidant, polyphenolic, and antimicrobial activities | Electrospinning | Rezaeinia et al. [116] |
| Grape Seed (Vitis vinifera L.) extract | PVA | Antioxidant | Electrospinning | Faki et al. [117] |
| Juniperus chinensis | PVA | Antibacterial, antifungal, antioxidant | Electrospinning | Kim et al. [118] |
| Lanasol from Rhodomela confervoides | PMMA/PEO | Antimicrobial | Electrospinning | Andersson et al. [119] |
| Szygium aromaticum extract | Thermoplastic polyurethane | Antibacterial, antiseptic, antifungal, analgesic, anticarcinogenic | Forcespinning | Canbay-Gokce et al. [120] |
| Tea tree oil extract (Melaleuca alternifolia)/Pomegranate peel extract | HP-ß-Cyclodextrin | Antioxidant, anti-inflammatory, antiseptic, and antimicrobial | Electrospinning | Kalouta et al. [121] |
| Moringa oleifera leaf extract | Polyacrylonitrile | Antimicrobial, antiproliferative, antioxidant, polyphenolic activity | Electrospinning | Fayemi et al. [122] |
| Resveratrol Veri-TeTM | Zein from maize | Antioxidant, anti-cancer, tissue engineering, barrier | Electrospinning | Leena et al. [123] |
| Momordica charantia fruit extract | Zein/gelatin | Antioxidant | Electrospinning | Torkamani, A. et al. [124] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huesca-Urióstegui, K.; García-Valderrama, E.J.; Gutierrez-Uribe, J.A.; Antunes-Ricardo, M.; Guajardo-Flores, D. Nanofiber Systems as Herbal Bioactive Compounds Carriers: Current Applications in Healthcare. Pharmaceutics 2022, 14, 191. https://doi.org/10.3390/pharmaceutics14010191
Huesca-Urióstegui K, García-Valderrama EJ, Gutierrez-Uribe JA, Antunes-Ricardo M, Guajardo-Flores D. Nanofiber Systems as Herbal Bioactive Compounds Carriers: Current Applications in Healthcare. Pharmaceutics. 2022; 14(1):191. https://doi.org/10.3390/pharmaceutics14010191
Chicago/Turabian StyleHuesca-Urióstegui, Kathya, Elsy J. García-Valderrama, Janet A. Gutierrez-Uribe, Marilena Antunes-Ricardo, and Daniel Guajardo-Flores. 2022. "Nanofiber Systems as Herbal Bioactive Compounds Carriers: Current Applications in Healthcare" Pharmaceutics 14, no. 1: 191. https://doi.org/10.3390/pharmaceutics14010191
APA StyleHuesca-Urióstegui, K., García-Valderrama, E. J., Gutierrez-Uribe, J. A., Antunes-Ricardo, M., & Guajardo-Flores, D. (2022). Nanofiber Systems as Herbal Bioactive Compounds Carriers: Current Applications in Healthcare. Pharmaceutics, 14(1), 191. https://doi.org/10.3390/pharmaceutics14010191