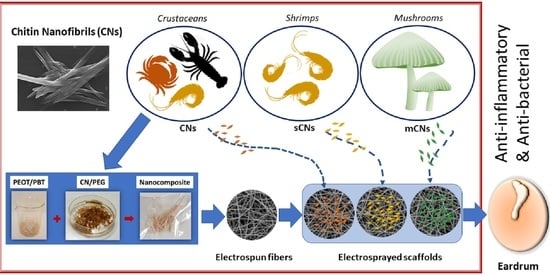
Chitin Nanofibril Application in Tympanic Membrane Scaffolds to Modulate Inflammatory and Immune Response
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Nanocomposite Preparation
2.3. Production of TM Scaffolds
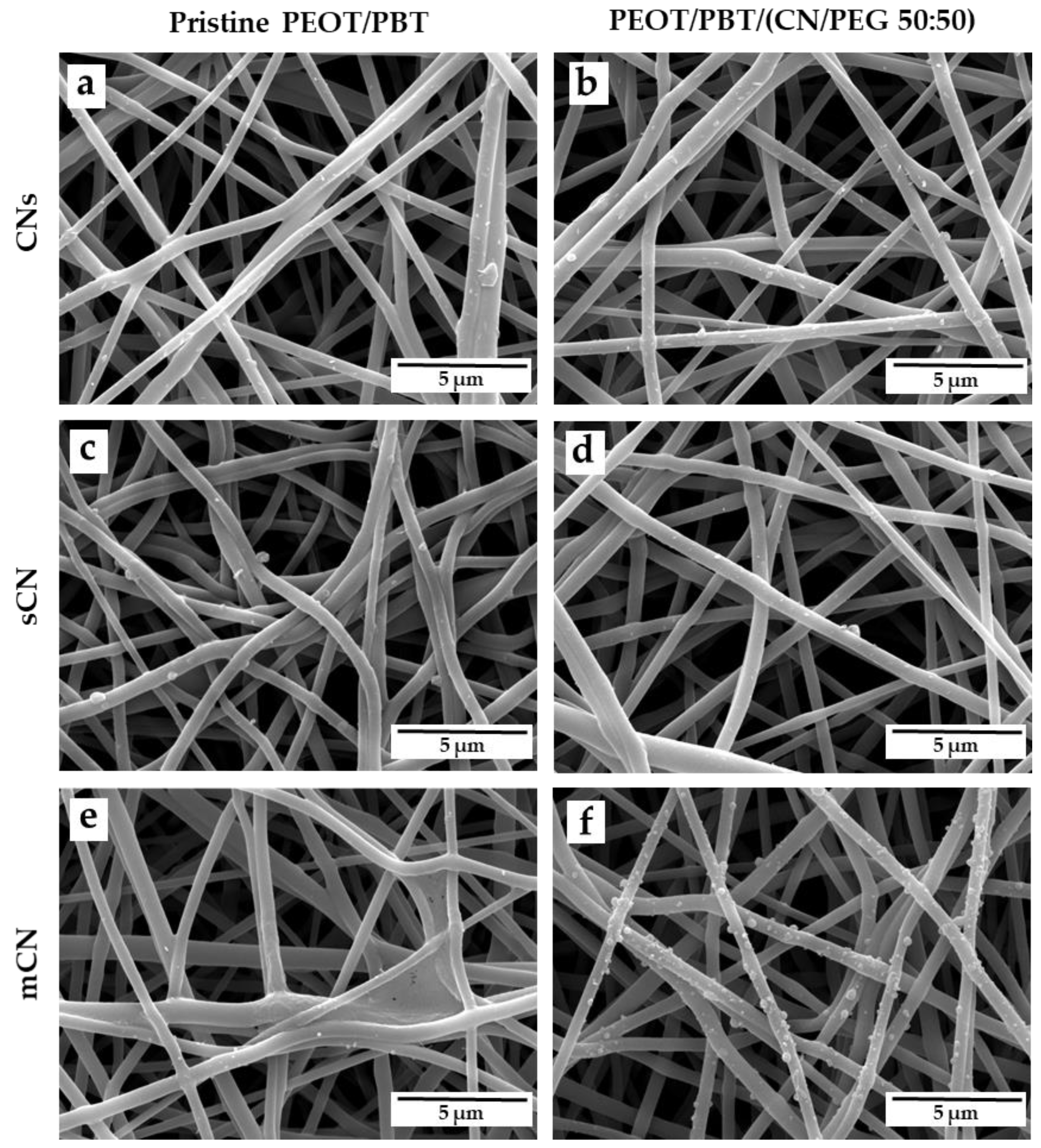
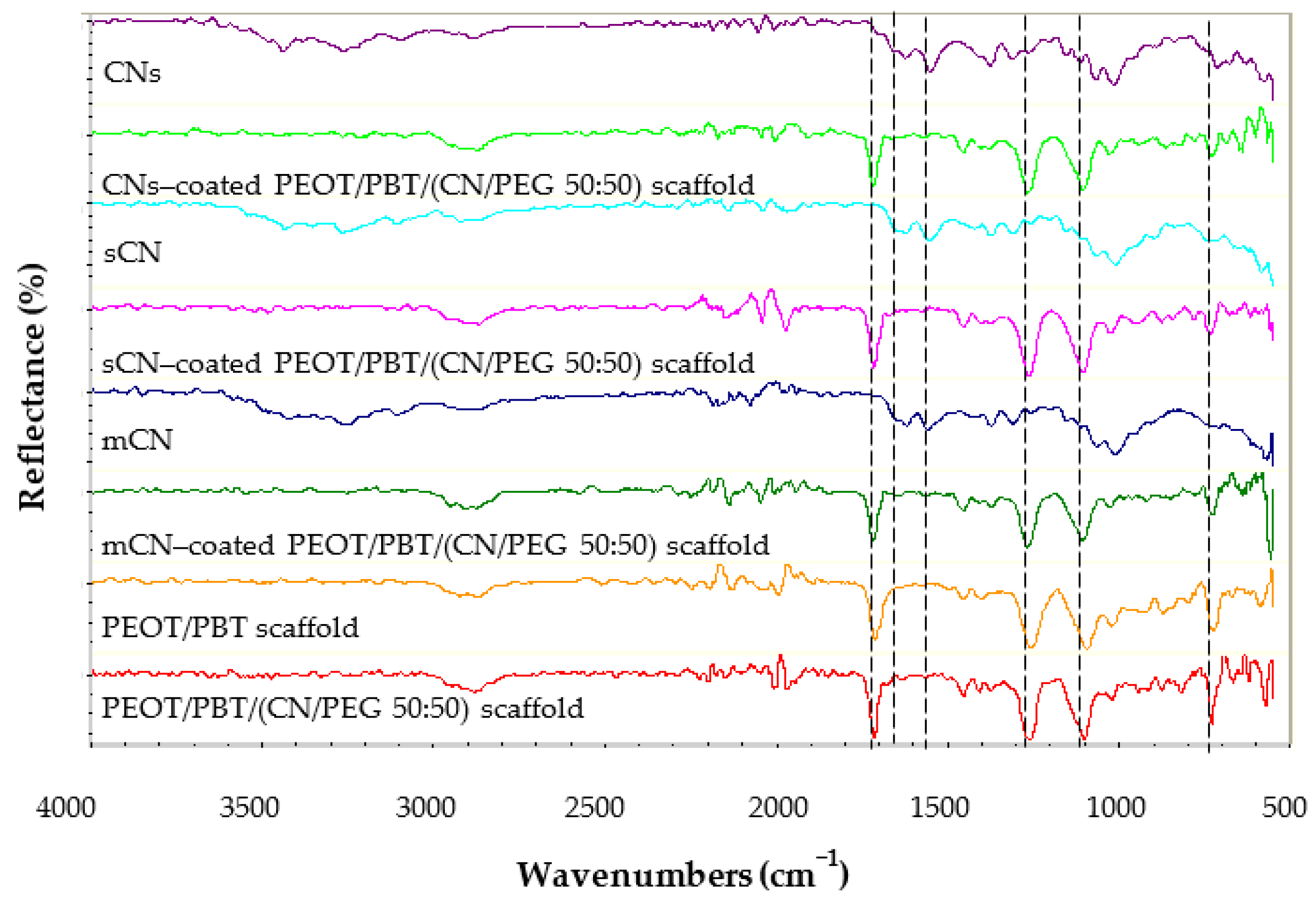
2.4. TM Scaffold Characterization
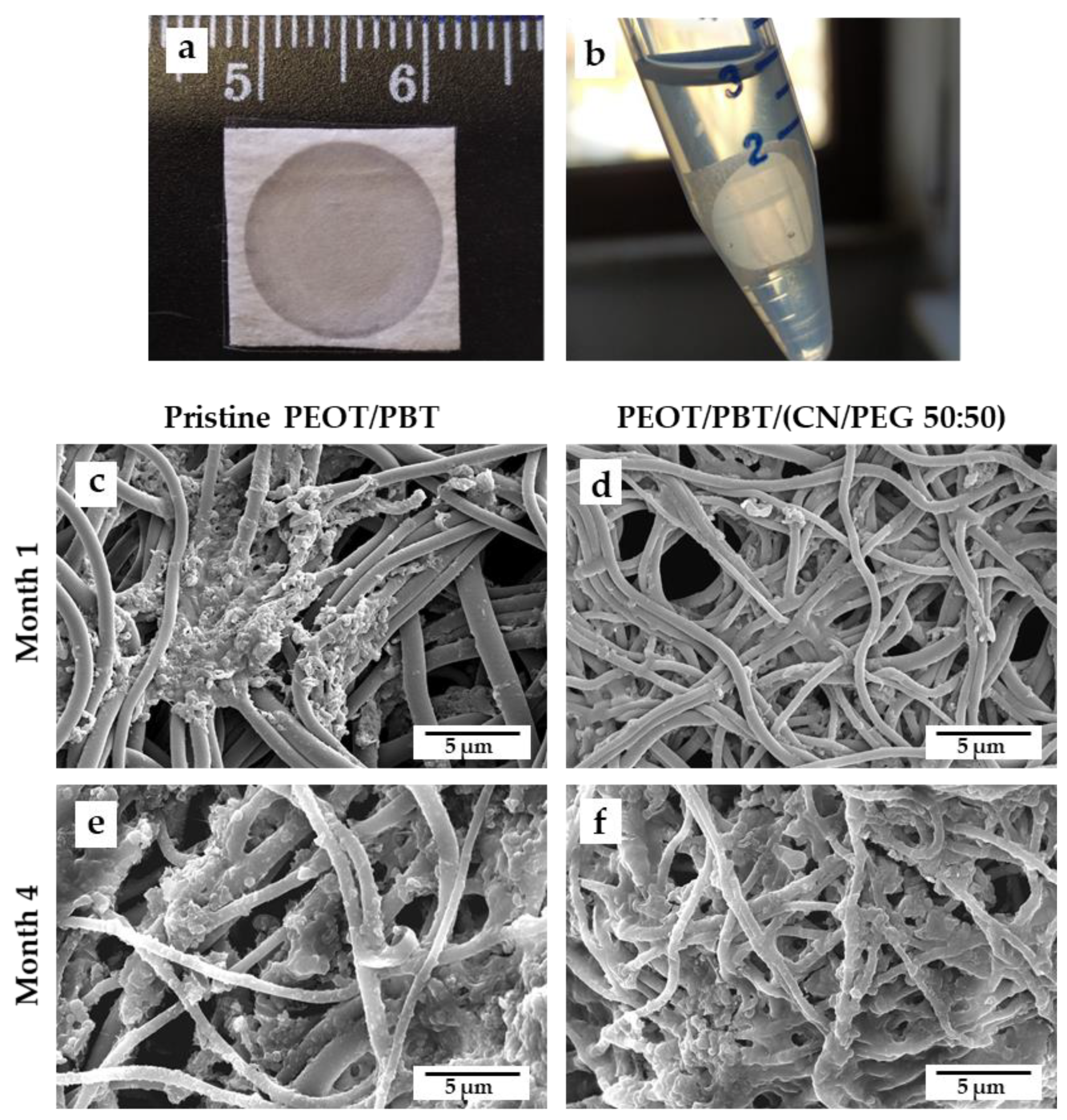
2.5. Degradation Study in Otitis-Simulating Conditions
2.6. Cytocompatibility Evaluation
2.7. Immune and Inflammatory Response Evaluation
2.8. Statistical Analysis
3. Results
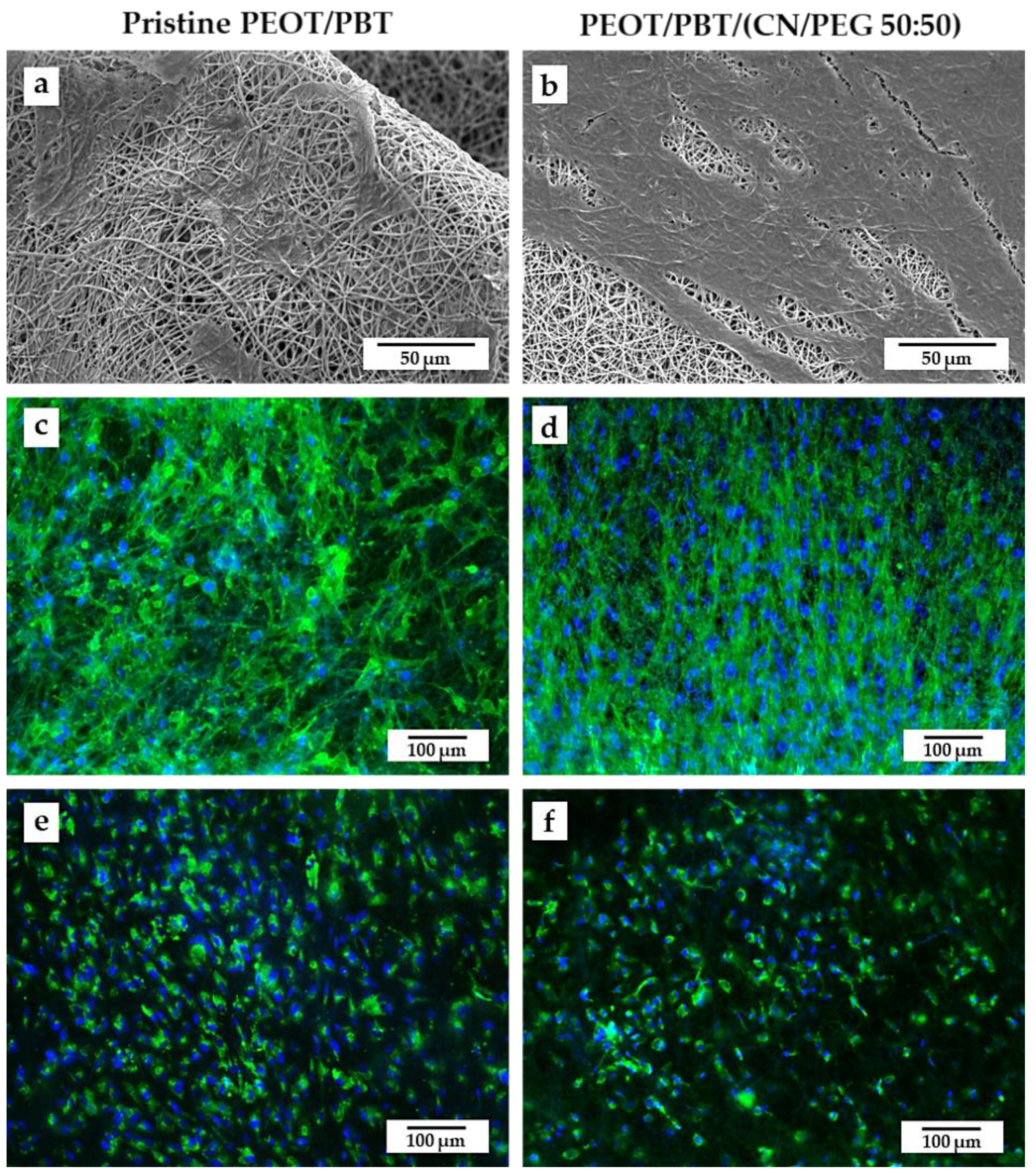
3.1. TM Scaffold Characterization
3.2. Degradation in Otitis-Simulating Solution
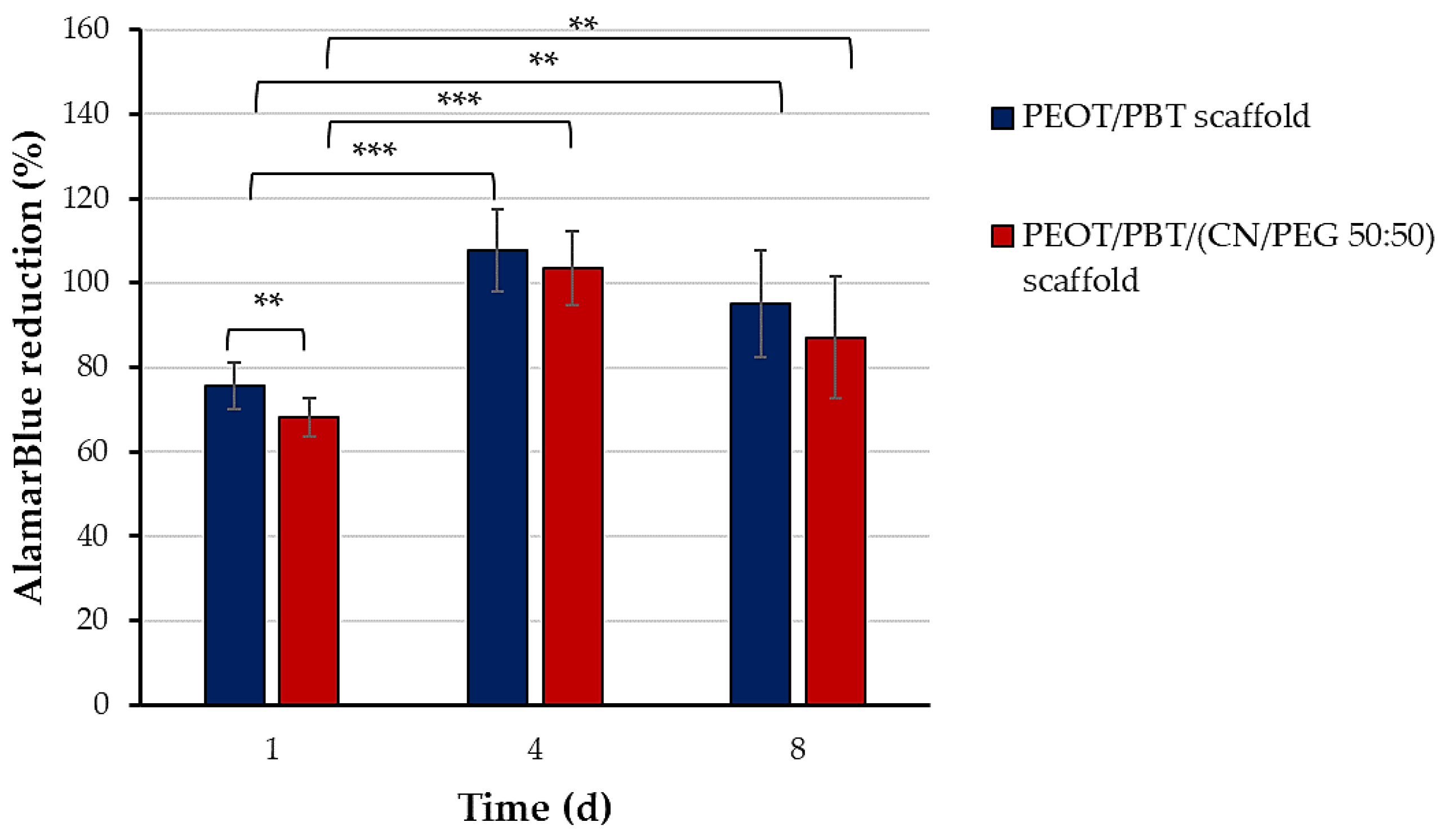
3.3. Interaction of TM Scaffolds with hMSCs
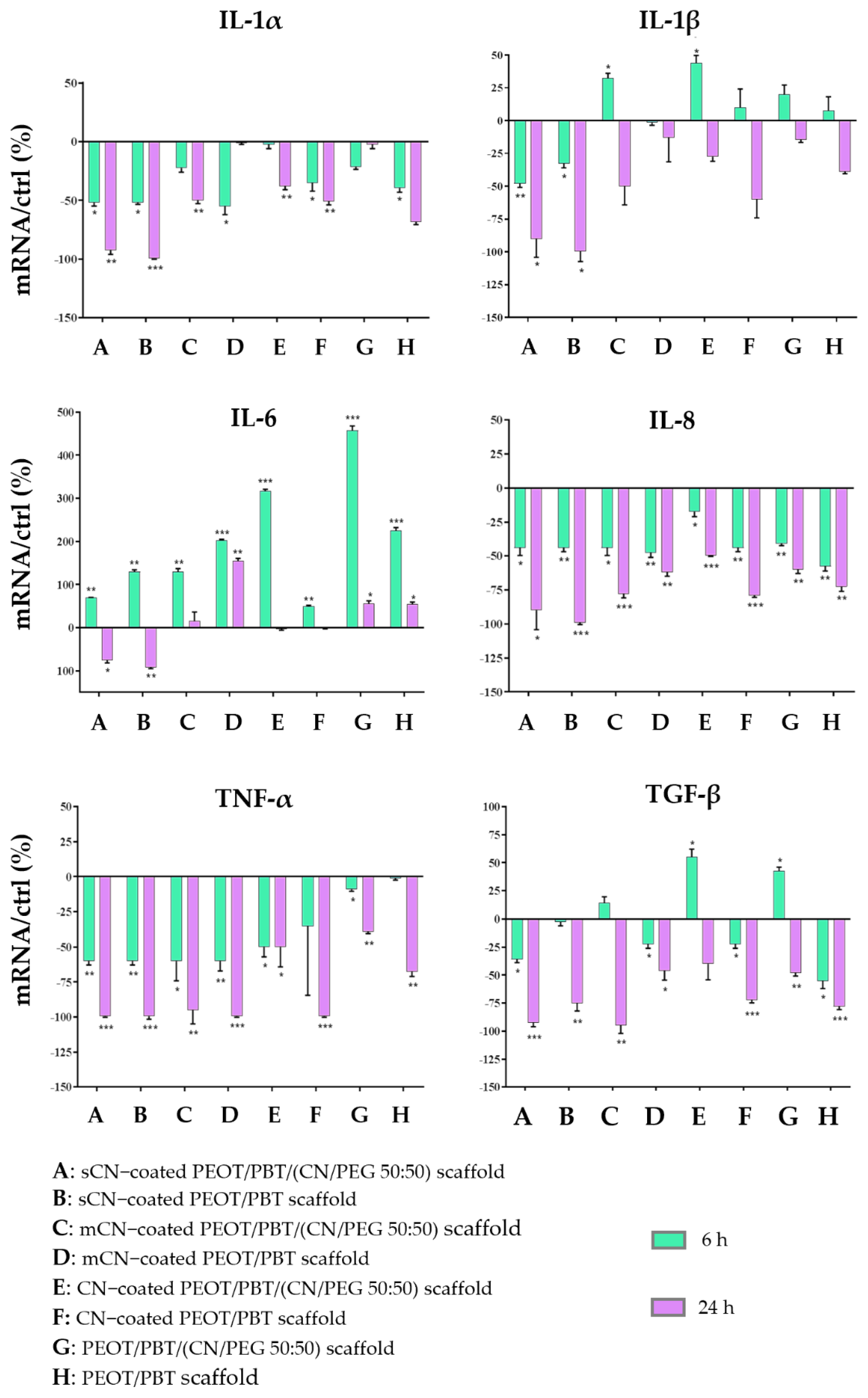
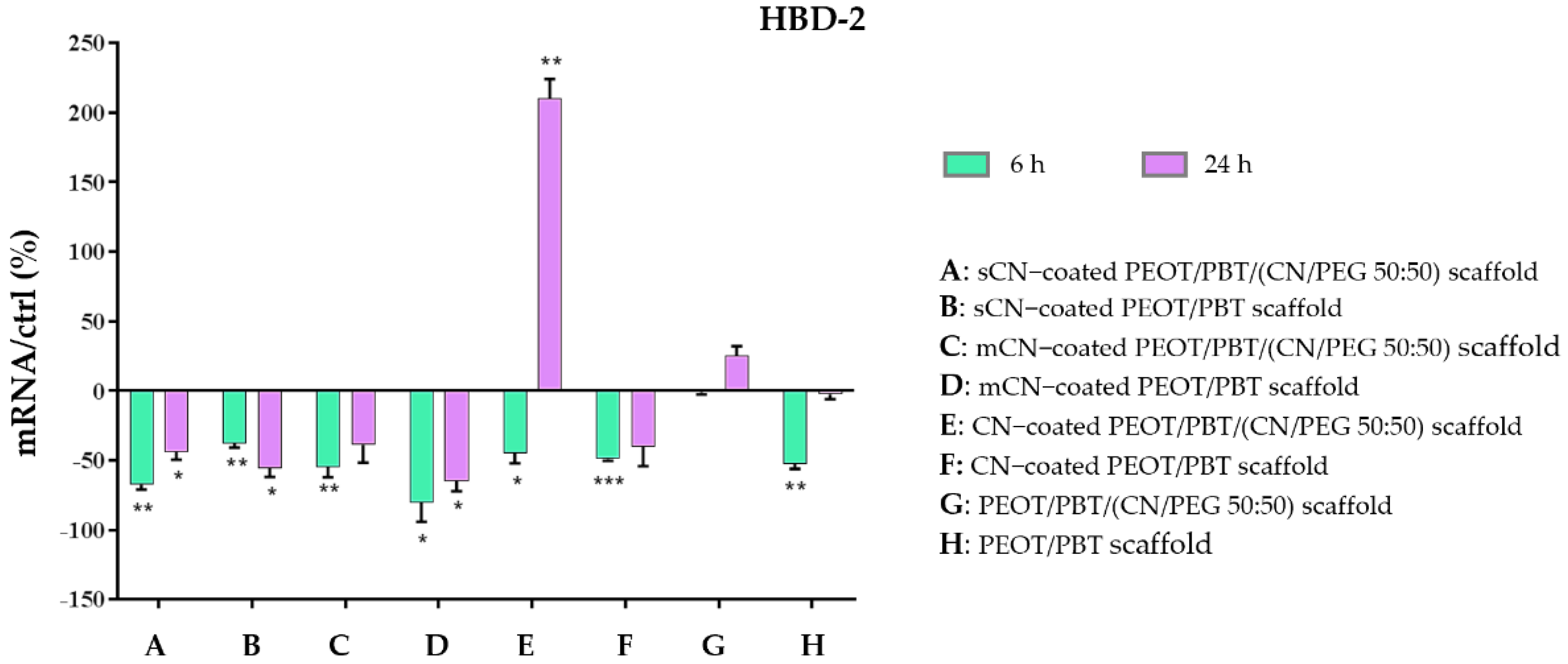
3.4. Anti-Inflammatory and Immune Response of HaCaT Cells
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lim, D.J. Structure and function of the tympanic membrane: A review. Acta Otorhinolaryngol. Belg. 1995, 49, 101–115. [Google Scholar]
- Beutner, D.; Hüttenbrink, K.-B. Passive and active middle ear implants. GMS Curr. Top. Otorhinolaryngol. Head Neck Surg. 2009, 8, Doc09. [Google Scholar]
- Monasta, L.; Ronfani, L.; Marchetti, F.; Montico, M.; Vecchi Brumatti, L.; Bavcar, A.; Grasso, D.; Barbiero, C.; Tamburlini, G. Burden of disease caused by otitis media: Systematic review and global estimates. PLoS ONE 2012, 7, e36226. [Google Scholar] [CrossRef] [PubMed]
- Mehta, R.P.; Rosowski, J.J.; Voss, S.E.; O’Neil, E.; Merchant, S.N. Determinants of hearing loss in perforations of the tympanic membrane. Otol. Neurotol. 2006, 27, 136–143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaftan, H. Tympanic membrane reconstruction with non-autogenous transplants and alloplastic materials. Laryngo-Rhino-Otol. 2010, 89, 562–568. [Google Scholar] [CrossRef] [PubMed]
- Mota, C.; Danti, S.; D’Alessandro, D.; Trombi, L.; Ricci, C.; Puppi, D.; Dinucci, D.; Milazzo, M.; Stefanini, C.; Chiellini, F.; et al. Multiscale fabrication of biomimetic scaffolds for tympanic membrane tissue engineering. Biofabrication 2015, 7, 025005. [Google Scholar] [CrossRef]
- Mota, C.; Danti, S. Ear Tissue Engineering. In Comprehensive Biotechnology, 3rd ed.; Moo-Young, M., Ed.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 270–285. [Google Scholar]
- Anand, S.; Stoppe, T.; Lucena, M.; Rademakers, T.; Neudert, M.; Danti, S.; Moroni, L.; Mota, C. Mimicking the Human Tympanic Membrane: The Significance of Scaffold Geometry. Adv. Healthc. Mater. 2021, 2002082, 1–16. [Google Scholar] [CrossRef]
- Moscato, S.; Rocca, A.; D’Alessandro, D.; Puppi, D.; Gramigna, V.; Milazzo, M.; Stefanini, C.; Chiellini, F.; Petrini, M.; Berrettini, S.; et al. Tympanic Membrane Collagen Expression by Dynamically Cultured Human Mesenchymal Stromal Cell/Star-Branched Poly (ε-Caprolactone) Nonwoven Constructs. Appl. Sci. 2020, 10, 3043. [Google Scholar] [CrossRef]
- Danti, S.; Mota, C.; D’alessandro, D.; Trombi, L.; Ricci, C.; Redmond, S.L.; De Vito, A.; Pini, R.; Dilley, R.J.; Moroni, L.; et al. Tissue engineering of the tympanic membrane using electrospun PEOT/PBT copolymer scaffolds: A morphological in vitro study. Hear. Balanc. Commun. 2015, 13, 133–147. [Google Scholar] [CrossRef]
- Günday, C.; Anand, S.; Gencer, H.B.; Munafò, S.; Moroni, L.; Fusco, A.; Donnarumma, G.; Ricci, C.; Hatir, P.C.; Türeli, N.G.; et al. Ciprofloxacin-loaded polymeric nanoparticles incorporated electrospun fibers for drug delivery in tissue engineering applications. Drug Deliv. Transl. Res. 2020, 10, 706–720. [Google Scholar] [CrossRef] [PubMed]
- Dormer, K.J.; Gan, R.Z. Biomaterials for implantable middle ear hearing devices. Otolaryngol. Clin. N. Am. 2001, 34, 289–297. [Google Scholar] [CrossRef]
- Danti, S.; Trombi, L.; Fusco, A.; Azimi, B.; Lazzeri, A.; Morganti, P.; Coltelli, M.-B.; Donnarumma, G. Chitin nanofibrils and nanolignin as functional agents in skin regeneration. Int. J. Mol. Sci. 2019, 20, 2669. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morganti, P. Circular economy: A new horizon for bio-nanocomposites from waste materials. Int. J. Biotechnol. Wellness Ind. 2016, 5, 121–127. [Google Scholar] [CrossRef]
- Morganti, P.; Del Ciotto, P.; Stoller, M.; Chianese, A. Antibacterial and anti-inflammatory green nanocomposites. Chem. Eng. Trans. 2016, 47, 61–66. [Google Scholar]
- Hoell, I.A.; Vaaje-Kolstad, G.; Eijsink, V.G.H. Structure and function of enzymes acting on chitin and chitosan. Biotechnol. Genet. Eng. Rev. 2010, 27, 331–366. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yudin, V.E.; Dobrovolskaya, I.P.; Neelov, I.M.; Dresvyanina, E.N.; Popryadukhin, P.V.; Ivan’kova, E.M.; Elokhovskii, V.Y.; Kasatkin, I.A.; Okrugin, B.M.; Morganti, P. Wet spinning of fibers made of chitosan and chitin nanofibrils. Carbohydr. Polym. 2014, 108, 176–182. [Google Scholar] [CrossRef] [PubMed]
- Rizvi, R.; Cochrane, B.; Naguib, H.; Lee, P.C. Fabrication and characterization of melt-blended polylactide-chitin composites and their foams. J. Cell. Plast. 2011, 47, 283–300. [Google Scholar] [CrossRef]
- Herrera, N.; Salaberria, A.M.; Mathew, A.P.; Oksman, K. Plasticized polylactic acid nanocomposite films with cellulose and chitin nanocrystals prepared using extrusion and compression molding with two cooling rates: Effects on mechanical, thermal and optical properties. Compos. Part A Appl. Sci. Manuf. 2016, 83, 89–97. [Google Scholar] [CrossRef] [Green Version]
- Herrera, N.; Roch, H.; Salaberria, A.M.; Pino-Orellana, M.A.; Labidi, J.; Fernandes, S.C.M.; Radic, D.; Leiva, A.; Oksman, K. Functionalized blown films of plasticized polylactic acid/chitin nanocomposite: Preparation and characterization. Mater. Des. 2016, 92, 846–852. [Google Scholar]
- Herrera, N.; Singh, A.A.; Salaberria, A.M.; Labidi, J.; Mathew, A.P.; Oksman, K. Triethyl citrate (TEC) as a dispersing aid in polylactic acid/chitin nanocomposites prepared via liquid-assisted extrusion. Polymers 2017, 9, 406. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Gao, Y.; Zhao, J.; Sun, J.; Li, D. Homogeneous dispersion of chitin nanofibers in polylactic acid with different pretreatment methods. Cellulose 2017, 24, 1705–1715. [Google Scholar] [CrossRef]
- Zhang, Q.; Wei, S.; Huang, J.; Feng, J.; Chang, P.R. Effect of surface acetylated-chitin nanocrystals on structure and mechanical properties of poly (lactic acid). J. Appl. Polym. Sci. 2014, 131, 42–68. [Google Scholar] [CrossRef]
- Coltelli, M.-B.; Cinelli, P.; Gigante, V.; Aliotta, L.; Morganti, P.; Panariello, L.; Lazzeri, A. Chitin nanofibrils in poly (lactic acid) (PLA) nanocomposites: Dispersion and thermo-mechanical properties. Int. J. Mol. Sci. 2019, 20, 504. [Google Scholar] [CrossRef] [Green Version]
- Morganti, P.; Fusco, A.; Paoletti, I.; Perfetto, B.; Del Ciotto, P.; Palombo, M.; Chianese, A.; Baroni, A.; Donnarumma, G. Anti-inflammatory, immunomodulatory, and tissue repair activity on human keratinocytes by green innovative nanocomposites. Materials 2017, 10, 843. [Google Scholar] [CrossRef] [Green Version]
- Deschamps, A.A.; Grijpma, D.W.; Feijen, J. Poly (ethylene oxide)/poly (butylene terephthalate) segmented block copolymers: The effect of copolymer composition on physical properties and degradation behavior. Polymer 2001, 42, 9335–9345. [Google Scholar] [CrossRef]
- Grote, J.J.; Bakker, D.; Hesseling, S.C.; van Blitterswijk, C.A. New alloplastic tympanic membrane material. Am. J. Otol. 1991, 12, 329–335. [Google Scholar]
- Deschamps, A.A.; van Apeldoorn, A.A.; Hayen, H.; de Bruijn, J.D.; Karst, U.; Grijpma, D.W.; Feijen, J. In vivo and in vitro degradation of poly (ether ester) block copolymers based on poly (ethylene glycol) and poly (butylene terephthalate). Biomaterials 2004, 25, 247–258. [Google Scholar] [CrossRef]
- Azimi, B.; Ricci, C.; Fusco, A.; Zavagna, L.; Linari, S.; Donnarumma, G.; Hadrich, A.; Cinelli, P.; Coltelli, M.-B.; Danti, S.; et al. Electrosprayed Shrimp and Mushroom Nanochitins on Cellulose Tissue for Skin Contact Application. Molecules 2021, 26, 4374. [Google Scholar] [CrossRef]
- Azimi, B.; Thomas, L.; Fusco, A.; Kalaoglu-Altan, O.I.; Basnett, P.; Cinelli, P.; De Clerck, K.; Roy, I.; Donnarumma, G.; Coltelli, M.-B.; et al. Electrosprayed chitin nanofibril/electrospun polyhydroxyalkanoate fiber mesh as functional nonwoven for skin application. J. Funct. Biomater. 2020, 11, 62. [Google Scholar] [CrossRef]
- Osgood, R.; Salamone, F.; Diaz, A.; Casey, J.R.; Bajorski, P.; Pichichero, M.E. Effect of p H and oxygen on biofilm formation in acute otitis media associated NTH i clinical isolates. Laryngoscope 2015, 125, 2204–2208. [Google Scholar] [CrossRef] [PubMed]
- Thorburn, I.; Smith, G. Long term results of middle ear reconstructive surgery. J. Laryngol. Otol. 1971, 85, 1227–1237. [Google Scholar]
- Milazzo, M.; Muyshondt, P.G.G.; Carstensen, J.; Dirckx, J.J.J.; Danti, S.; Buehler, M.J. De novo topology optimization of Total Ossicular Replacement Prostheses. J. Mech. Behav. Biomed. Mater. 2020, 103, 103541. [Google Scholar] [CrossRef] [Green Version]
- Danti, S.; D’Alessandro, D.; Pietrabissa, A.; Petrini, M.; Berrettini, S. Development of tissue-engineered substitutes of the ear ossicles: PORP-shaped poly (propylene fumarate)-based scaffolds cultured with human mesenchymal stromal cells. J. Biomed. Mater. Res.-Part A 2010, 92, 1343–1356. [Google Scholar] [CrossRef] [PubMed]
- Morganti, P.; Morganti, G. Chitin nanofibrils for advanced cosmeceuticals. Clin. Dermatol. 2008, 26, 334–340. [Google Scholar] [CrossRef]
- Larsen, S.B.; Cowley, C.J.; Fuchs, E. Epithelial cells: Liaisons of immunity. Curr. Opin. Immunol. 2020, 62, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Pötschke, P.; Paul, D.R. Formation of co-continuous structures in melt-mixed immiscible polymer blends. J. Macromol. Sci.-Polym. Rev. 2003, 43, 87–141. [Google Scholar] [CrossRef]
- Mikešová, J.; Hašek, J.; Tishchenko, G.; Morganti, P. Rheological study of chitosan acetate solutions containing chitin nanofibrils. Carbohydr. Polym. 2014, 112, 753–757. [Google Scholar] [CrossRef] [PubMed]
- Joseph, B.; Mavelil Sam, R.; Balakrishnan, P.; Maria, H.J.; Gopi, S.; Volova, T.; Fernandes, S.C.M.; Thomas, S. Extraction of Nanochitin from Marine Resources and Fabrication of Polymer Nanocomposites: Recent Advances. Polymers 2020, 12, 1664. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Xue, H.; Feng, Z.; Cheng, B.; Yang, H. Increase of tensile strength and toughness of bio-based diglycidyl ether of bisphenol A with chitin nanowhiskers. PLoS ONE 2017, 12, e0177673. [Google Scholar] [CrossRef] [Green Version]
- Irvin, C.W.; Satam, C.C.; Meredith, J.C.; Shofner, M.L. Mechanical reinforcement and thermal properties of PVA tricomponent nanocomposites with chitin nanofibers and cellulose nanocrystals. Compos. Part A Appl. Sci. Manuf. 2019, 116, 147–157. [Google Scholar] [CrossRef]
- Dobrovolskaya, I.P.; Yudin, V.E.; Popryadukhin, P.V.; Ivan’kova, E.M.; Shabunin, A.S.; Kasatkin, I.A.; Morgantie, P. Effect of chitin nanofibrils on electrospinning of chitosan-based composite nanofibers. Carbohydr. Polym. 2018, 194, 260–266. [Google Scholar] [CrossRef]
- Ji, Y.; Liang, K.; Shen, X.; Bowlin, G.L. Electrospinning and characterization of chitin nanofibril/polycaprolactone nanocomposite fiber mats. Carbohydr. Polym. 2014, 101, 68–74. [Google Scholar] [CrossRef]
- Azimi, B.; Milazzo, M.; Lazzeri, A.; Berrettini, S.; Uddin, M.J.; Qin, Z.; Buehler, M.J.; Danti, S. Electrospinning piezoelectric fibers for biocompatible devices. Adv. Healthc. Mater. 2020, 9, 1901287. [Google Scholar] [CrossRef]
- Juhn, S.K.; Huff, J.S. Biochemical characteristics of middle ear effusions. Ann. Otol. Rhinol. Laryngol. 1976, 85, 110–116. [Google Scholar] [CrossRef]
- Chung, M.-H.; Choi, J.Y.; Lee, W.-S.; Kim, H.-N.; Yoon, J.-H. Compositional difference in middle ear effusion: Mucous versus serous. Laryngoscope 2002, 112, 152–155. [Google Scholar] [CrossRef] [PubMed]
- Portier, F.; Hsu, W.C.; Herman, P.; Huy, P.T.B. Serous or mucoid effusion in the course of secretory otitis media: Influence of ion transport modulation. Auris Nasus Larynx 2001, 28, 3–7. [Google Scholar] [CrossRef]
- Harada, T.; Juhn, S.K.; Adams, G.L. Lysozyme levels in middle ear effusion and serum in otitis media. Arch. Otolaryngol. Neck Surg. 1990, 116, 54–56. [Google Scholar] [CrossRef]
- Jones, M.; Kujundzic, M.; John, S.; Bismarck, A. Crab vs. Mushroom: A review of crustacean and fungal chitin in wound treatment. Mar. Drugs 2020, 18, 64. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lazzeri, L.; Cascone, M.G.; Danti, S.; Serino, L.P.; Moscato, S.; Bernardini, N. Gelatine/PLLA sponge-like scaffolds: Morphological and biological characterization. J. Mater. Sci. Mater. Med. 2007, 8, 1399–1405. [Google Scholar] [CrossRef] [PubMed]
- Saino, E.; Focarete, M.L.; Gualandi, C.; Emanuele, E.; Cornaglia, A.I.; Imbriani, M.; Visai, L. Effect of electrospun fiber diameter and alignment on macrophage activation and secretion of proinflammatory cytokines and chemokines. Biomacromolecules 2011, 12, 1900–1911. [Google Scholar] [CrossRef]
- De la Ossa, J.G.; Fusco, A.; Azimi, B.; Esposito Salsano, J.; Digiacomo, M.; Coltelli, M.-B.; De Clerck, K.; Roy, I.; Macchia, M.; Lazzeri, A.; et al. Immunomodulatory Activity of electrospun polyhydroxyalkanoate fiber scaffolds incorporating olive leaf extract. Appl. Sci. 2021, 11, 4006. [Google Scholar] [CrossRef]
- Sugawara, T.; Gallucci, R.M.; Simeonova, P.P.; Luster, M.I. Regulation and role of interleukin 6 in wounded human epithelial keratinocytes. Cytokine 2001, 15, 328–336. [Google Scholar] [CrossRef] [PubMed]
- Himi, T.; Suzuki, T.; Takezawa, H. Immunologic characteristics of cytokines in otitis media with effusion. Ann. Otol. Rhinol. Laryngol. 1992, 101, 21–25. [Google Scholar] [CrossRef]
- Skovbjerg, S.; Roos, K.; Nowrouzian, F.; Lindh, M.; Holm, S.E.; Adlerberth, I.; Olofsson, S.; Wold, A.E. High cytokine levels in perforated acute otitis media exudates containing live bacteria. Clin. Microbiol. Infect. 2010, 16, 1382–1388. [Google Scholar] [CrossRef] [Green Version]
- Aoki, C.A.; Borchers, A.T.; Li, M.; Flavell, R.A.; Bowlus, C.L.; Ansari, A.A.; Gershwin, M.E. Transforming growth factor beta (TGF-beta) and autoimmunity. Autoimmun Rev. 2005, 4, 450–459. [Google Scholar] [CrossRef] [PubMed]
- Schröder, J.-M.; Harder, S. Human beta-defensin-2. Int. J. Biochem. Cell Biol. 1999, 31, 645–651. [Google Scholar] [CrossRef]
- Mahlapuu, M.; Håkansson, J.; Ringstad, L.; Björn, C. Antimicrobial peptides: An emerging category of therapeutic agents. Front. Cell. Infect. Microbiol. 2016, 6, 194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ling, S.; Kaplan, D.; Buehler, M. Nanofibrils in nature and materials engineering. Nat. Rev. Mater. 2018, 3, 18016. [Google Scholar] [CrossRef] [PubMed]

| Operating Parameters | PEOT/PBT/(CN/PEG 50:50) |
|---|---|
| Concentration | 18% (w/v) in CHCl3:HFIP = 70:30 |
| Collector type, rotating speed | Rotating drum covered with PP sheet 1, 150 rpm |
| Needle diameter | 800 μm |
| Ambient parameters | T = 23 °C and H = 40% |
| Voltage applied | 20 kV (grounded collector) |
| Flow rate | 900 μL/h |
| Tip to collector distance | 10 cm |
| Gene | Primer Sequence (Forward and Reverse) | Conditions | Base Pairs |
|---|---|---|---|
| IL−1α | 5′−CATGTCAAATTTCACTGCTTCATCC−3′ 5′−GTCTCTGAATCAGAAATCCTTCTATC−3′ | 5 s at 95 °C, 8 s at 55 °C, 1 s at 72 °C for 45 cycles | 421 |
| IL−1β | 5′−GCATCCAGCTACGAATCTCC−3′ 5′−CCACATTCAGCACAGGACTC−3′ | 5 s at 95 °C, 14 s at 58 °C, 28 s at 72 °C for 40 cycles | 708 |
| TNF−α | 5′−CAGAGGGAAGAGTTCCCCAG−3′ 5′−CCTTGGTCTGGTAGGAGACG−3′ | 5 s at 95 °C, 6 s at 57 °C, 13 s at 72 °C for 40 cycles | 324 |
| IL−6 | 5′−ATGAACTCCTTCTCCACAAGCGC−3′ 5′−GAAGAGCCCTCAGGCTGGACTG−3′ | 5 s at 95 °C, 13 s at 56 °C, 25 s at 72 °C for 40 cycles | 628 |
| IL−8 | 5−ATGACTTCCAAGCTGGCCGTG−3′ 5−TGAATTCTCAGCCCTCTTCAAAAACTTCTC−3′ | 5 s at 94 °C, 6 s at 55 °C, 12 s at 72 °C for 40 cycles | 297 |
| TGF−β | 5′−CCGACTACTACGCCAAGGAGGTCAC−3′ 5′−AGGCCGGTTCATGCCATGAATGGTG−3′ | 5 s at 94 °C, 9 s at 60 °C, 18 s at 72 °C for 40 cycles | 439 |
| HBD−2 | 5′−GGATCCATGGGTATAGGCGATCCTGTTA−3′ 5′−AAGCTTCTCTGATGAGGGAGCCCTTTCT−3′ | 5 s at 94 °C, 6 s at 63 °C, 10 s at 72 °C for 50 cycles | 198 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Danti, S.; Anand, S.; Azimi, B.; Milazzo, M.; Fusco, A.; Ricci, C.; Zavagna, L.; Linari, S.; Donnarumma, G.; Lazzeri, A.; et al. Chitin Nanofibril Application in Tympanic Membrane Scaffolds to Modulate Inflammatory and Immune Response. Pharmaceutics 2021, 13, 1440. https://doi.org/10.3390/pharmaceutics13091440
Danti S, Anand S, Azimi B, Milazzo M, Fusco A, Ricci C, Zavagna L, Linari S, Donnarumma G, Lazzeri A, et al. Chitin Nanofibril Application in Tympanic Membrane Scaffolds to Modulate Inflammatory and Immune Response. Pharmaceutics. 2021; 13(9):1440. https://doi.org/10.3390/pharmaceutics13091440
Chicago/Turabian StyleDanti, Serena, Shivesh Anand, Bahareh Azimi, Mario Milazzo, Alessandra Fusco, Claudio Ricci, Lorenzo Zavagna, Stefano Linari, Giovanna Donnarumma, Andrea Lazzeri, and et al. 2021. "Chitin Nanofibril Application in Tympanic Membrane Scaffolds to Modulate Inflammatory and Immune Response" Pharmaceutics 13, no. 9: 1440. https://doi.org/10.3390/pharmaceutics13091440
APA StyleDanti, S., Anand, S., Azimi, B., Milazzo, M., Fusco, A., Ricci, C., Zavagna, L., Linari, S., Donnarumma, G., Lazzeri, A., Moroni, L., Mota, C., & Berrettini, S. (2021). Chitin Nanofibril Application in Tympanic Membrane Scaffolds to Modulate Inflammatory and Immune Response. Pharmaceutics, 13(9), 1440. https://doi.org/10.3390/pharmaceutics13091440