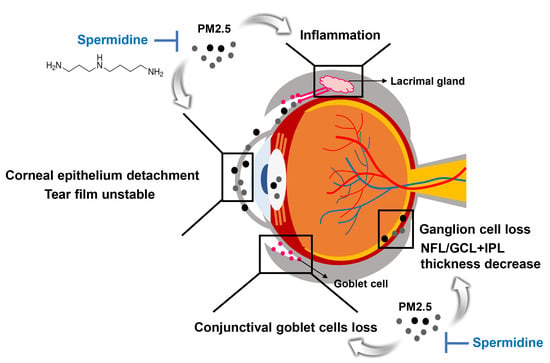
The Protective Effect of Topical Spermidine on Dry Eye Disease with Retinal Damage Induced by Diesel Particulate Matter2.5
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of PM2.5 and Eye Drops
2.2. Animals and Experimental Procedures
2.3. Hematological and Biochemical Analysis
2.4. Tear Production
2.5. Corneal Fluorescein Staining (CFS)
2.6. Hematoxylin and Eosin (H&E) Staining
2.7. Periodic Acid-Schiff (PAS) Staining
2.8. Immunohistochemistry
2.9. Statistical Analysis
3. Results
3.1. Changes in Physiological Condition after 2 Weeks of Treatment in PM2.5-Topical Exposed Sprague-Dawley (SD) Rats
3.2. Effect of Spermidine on the Changes of Hematological, Biochemical and Lipid Profiles in PM2.5-Topical Exposed SD Rats
3.3. Effect of Spermidine on Tear Secretion and CFS after Topical Exposure to PM2.5 in SD Rats
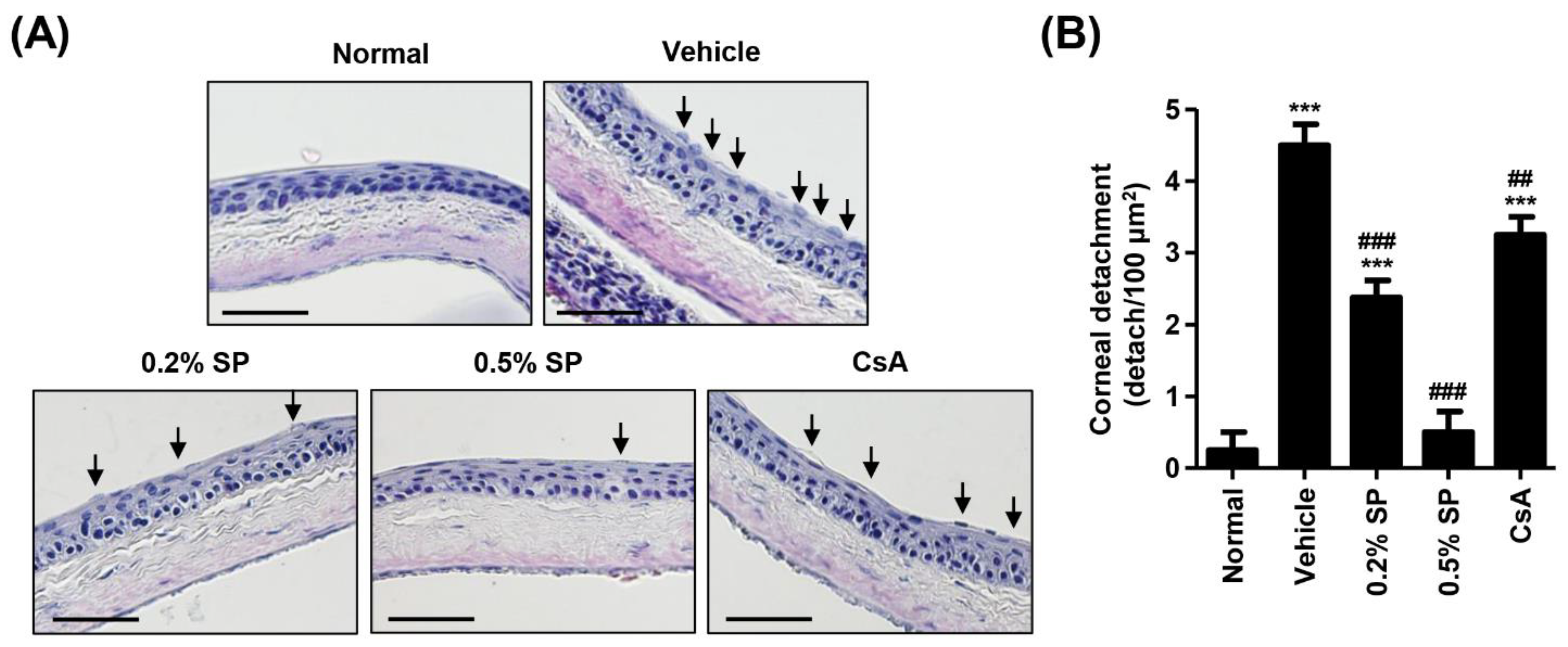
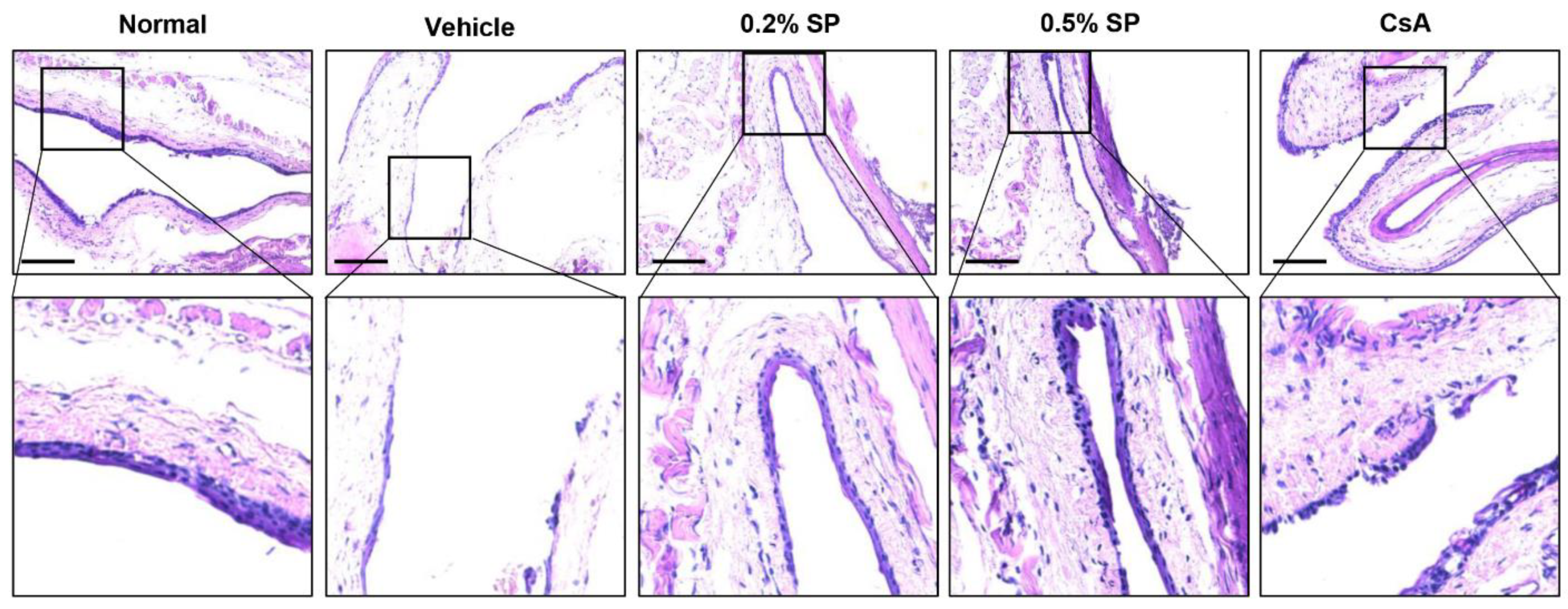
3.4. Effect of Spermidine on Detachment of Corneal Epithelium in a Rat Model of PM2.5-Induced DED
3.5. Effect of Spermidine on Conjunctival Goblet Cell Population in a Rat Model of PM2.5-Induced DED
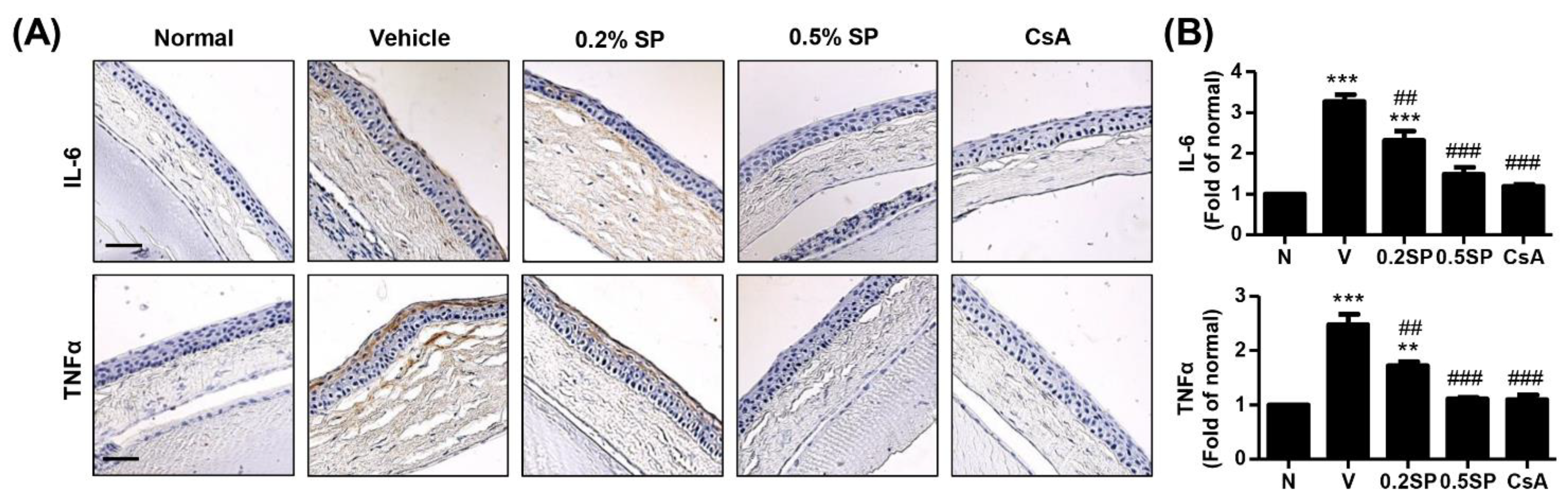
3.6. Effect of Spermidine on Inflammation of Lacrimal Gland and Cornea in a Rat Model of PM2.5-Induced DED
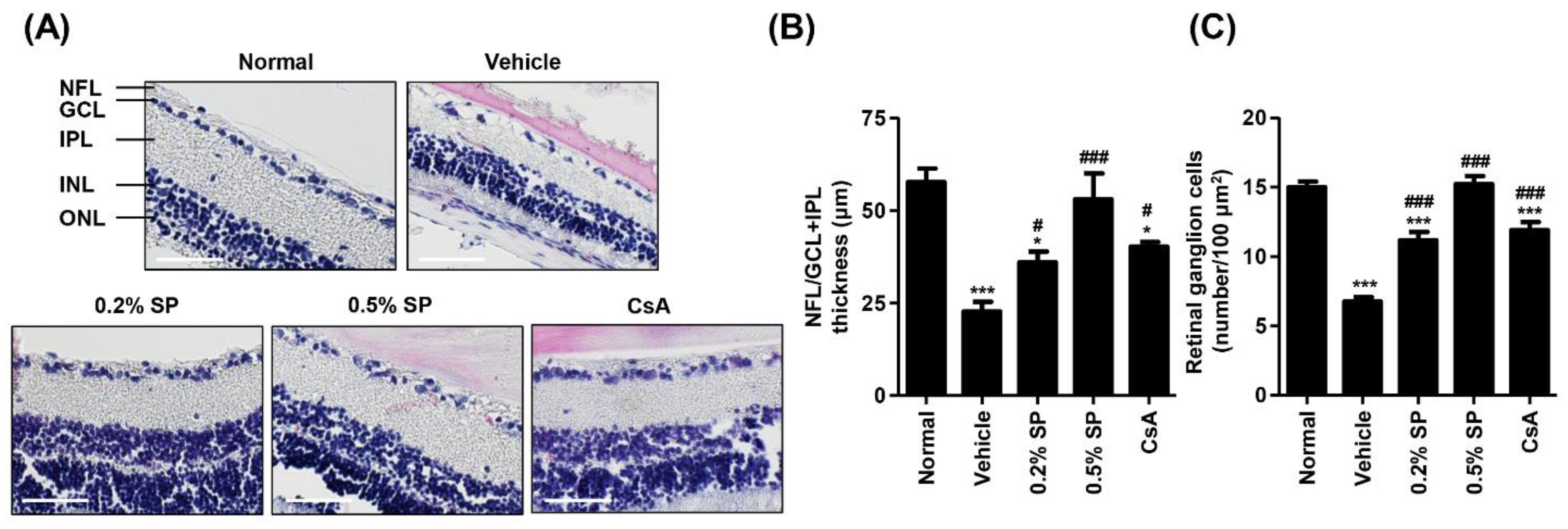
3.7. Effect of Spermidine on Histological Changes of the Retina after Topical Exposure to PM2.5 in SD Rats
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bo, Y.; Chang, L.Y.; Guo, C.; Lin, C.; Lau, A.K.H.; Tam, T.; Lao, X.Q. Reduced ambient PM2.5, better lung function, and decreased risk of chronic obstructive pulmonary disease. Environ. Int. 2021, 156, 106706. [Google Scholar] [CrossRef]
- World Health Organization. Air Pollution. Available online: https://www.who.int/health-topics/air-pollution#tab=tab_1 (accessed on 14 June 2021).
- Bulot, F.; Johnston, S.J.; Basford, P.J.; Easton, N.; Apetroaie-Cristea, M.; Foster, G.L.; Morris, A.; Cox, S.J.; Loxham, M. Long-term field comparison of multiple low-cost particulate matter sensors in an outdoor urban environment. Sci. Rep. 2019, 9, 7497. [Google Scholar] [CrossRef]
- Santibáñez-Andrade, M.; Chirino, Y.I.; González-Ramírez, I.; Sánchez-Pérez, Y.; García-Cuellar, C.M. Deciphering the code between air pollution and disease: The effect of particulate matter on cancer hallmarks. Int. J. Mol. Sci. 2019, 21, 136. [Google Scholar] [CrossRef] [Green Version]
- Crobeddu, B.; Aragao-Santiago, L.; Bui, L.C.; Boland, S.; Baeza Squiban, A. Oxidative potential of particulate matter 2.5 as predictive indicator of cellular stress. Environ. Pollut. 2017, 230, 125–133. [Google Scholar] [CrossRef]
- Hwang, M.; Han, S.; Seo, J.W.; Jeon, K.J.; Lee, H.S. Traffic-related particulate matter aggravates ocular allergic inflammation by mediating dendritic cell maturation. J. Toxicol. Environ. Health Part A 2021, 84, 661–673. [Google Scholar] [CrossRef] [PubMed]
- Kang, W.S.; Choi, H.; Jang, G.; Lee, K.H.; Kim, E.; Kim, K.J.; Jeong, G.Y.; Kim, J.S.; Na, C.S.; Kim, S. Long-term exposure to urban particulate matter on the ocular surface and the incidence of deleterious changes in the cornea, conjunctiva and retina in rats. Int. J. Mol. Sci. 2020, 21, 4976. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Li, K.; Li, D.; Zhang, Y.; Liu, X.; Wu, K. Effects of fine particulate matter on the ocular surface: An in vitro and in vivo study. Biomed. Pharmacother. 2019, 117, 109177. [Google Scholar] [CrossRef] [PubMed]
- Fu, Q.; Mo, Z.; Lyu, D.; Zhang, L.; Qin, Z.; Tang, Q.; Yin, H.; Xu, P.; Wu, L.; Lou, X.; et al. Air pollution and outpatient visits for conjunctivitis: A case-crossover study in Hangzhou, China. Environ. Pollut. 2017, 231, 1344–1350. [Google Scholar] [CrossRef] [PubMed]
- Jung, S.J.; Mehta, J.S.; Tong, L. Effects of environment pollution on the ocular surface. Ocul. Surf. 2018, 16, 198–205. [Google Scholar] [CrossRef]
- Mo, Z.; Fu, Q.; Lyu, D.; Zhang, L.; Qin, Z.; Tang, Q.; Yin, H.; Xu, P.; Wu, L.; Wang, X.; et al. Impacts of air pollution on dry eye disease among residents in Hangzhou, China: A case-crossover study. Environ. Pollut. 2019, 246, 183–189. [Google Scholar] [CrossRef]
- Tan, G.; Li, J.; Yang, Q.; Wu, A.; Qu, D.Y.; Wang, Y.; Ye, L.; Bao, J.; Shao, Y. Air pollutant particulate matter 2.5 induces dry eye syndrome in mice. Sci. Rep. 2018, 8, 17828. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, Q.C.; Bao, J.; Li, C.; Tan, G.; Wu, A.H.; Ye, L.; Ye, L.H.; Zhou, Q.; Shao, Y. A murine model of dry eye induced by topical administration of erlotinib eye drops. Int. J. Mol. Med. 2018, 41, 1427–1436. [Google Scholar] [CrossRef] [PubMed]
- Song, S.J.; Hyun, S.W.; Lee, T.G.; Park, B.; Jo, K.; Kim, C.S. New application for assessment of dry eye syndrome induced by particulate matter exposure. Ecotoxicol. Environ. Saf. 2020, 205, 111125. [Google Scholar] [CrossRef] [PubMed]
- Kashiwagi, K.; Iizuka, Y. Effect and underlying mechanisms of airborne particulate matter 2.5 (PM2.5) on cultured human corneal epithelial cells. Sci. Rep. 2020, 10, 19516. [Google Scholar] [CrossRef]
- Niu, L.; Li, L.; Xing, C.; Luo, B.; Hu, C.; Song, M.; Niu, J.; Ruan, Y.; Sun, X.; Lei, Y. Airborne particulate matter (PM2.5) triggers cornea inflammation and pyroptosis via NLRP3 activation. Ecotoxicol. Environ. Saf. 2021, 207, 111306. [Google Scholar] [CrossRef] [PubMed]
- Hyun, S.W.; Song, S.J.; Park, B.; Lee, T.G.; Kim, C.S. Toxicological effects of urban particulate matter on corneal and conjunctival epithelial cells. Toxicol. Res. 2020, 36, 311–318. [Google Scholar] [CrossRef]
- Igarashi, K.; Kashiwagi, K. Modulation of cellular function by polyamines. Int. J. Biochem. Cell Biol. 2010, 42, 39–51. [Google Scholar] [CrossRef]
- Laube, G.; Veh, R.W. Astrocytes, not neurons, show most prominent staining for spermidine/spermine-like immunoreactivity in adult rat brain. Glia 1997, 19, 171–179. [Google Scholar] [CrossRef]
- Rider, J.E.; Hacker, A.; Mackintosh, C.A.; Pegg, A.E.; Woster, P.M.; Casero, R.A., Jr. Spermine and spermidine mediate protection against oxidative damage caused by hydrogen peroxide. Amino Acids 2007, 33, 231–240. [Google Scholar] [CrossRef]
- Noro, T.; Namekata, K.; Kimura, A.; Guo, X.; Azuchi, Y.; Harada, C.; Nakano, T.; Tsuneoka, H.; Harada, T. Spermidine promotes retinal ganglion cell survival and optic nerve regeneration in adult mice following optic nerve injury. Cell Death Dis. 2015, 6, e1720. [Google Scholar] [CrossRef] [Green Version]
- Choi, Y.H.; Park, H.Y. Anti-inflammatory effects of spermidine in lipopolysaccharide-stimulated BV2 microglial cells. J. Biomed. Sci. 2012, 19, 31. [Google Scholar] [CrossRef] [Green Version]
- Yang, Q.; Zheng, C.; Cao, J.; Cao, G.; Shou, P.; Lin, L.; Velletri, T.; Jiang, M.; Chen, Q.; Han, Y.; et al. Spermidine alleviates experimental autoimmune encephalomyelitis through inducing inhibitory macrophages. Cell Death Differ. 2016, 23, 1850–1861. [Google Scholar] [CrossRef] [PubMed]
- Noro, T.; Namekata, K.; Azuchi, Y.; Kimura, A.; Guo, X.; Harada, C.; Nakano, T.; Tsuneoka, H.; Harada, T. Spermidine ameliorates neurodegeneration in a mouse model of normal tension glaucoma. Investig. Ophthalmol. Vis. Sci. 2015, 56, 5012–5019. [Google Scholar] [CrossRef] [Green Version]
- Guo, X.; Harada, C.; Namekata, K.; Kimura, A.; Mitamura, Y.; Yoshida, H.; Matsumoto, Y.; Harada, T. Spermidine alleviates severity of murine experimental autoimmune encephalomyelitis. Investig. Ophthalmol. Vis. Sci. 2011, 52, 2696–2703. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, D.H.; Kim, J.H.; Hwangbo, H.; Kim, S.Y.; Ji, S.Y.; Kim, M.Y.; Cha, H.J.; Park, C.; Hong, S.H.; Kim, G.Y.; et al. Spermidine attenuates oxidative stress-induced apoptosis via blocking Ca2+ overload in retinal pigment epithelial cells independently of ROS. Int. J. Mol. Sci. 2021, 22, 1361. [Google Scholar] [CrossRef]
- Kang, M.J.; Gong, J.E.; Kim, J.E.; Choi, H.J.; Bae, S.J.; Choi, Y.J.; Lee, S.J.; Seo, M.S.; Kim, K.S.; Jung, Y.S.; et al. Influence of three BALB/c substrain backgrounds on the skin tumor induction efficacy to DMBA and TPA cotreatment. Lab. Anim. Res. 2020, 36, 30. [Google Scholar] [CrossRef]
- Lee, T.G.; Hyun, S.W.; Jo, K.; Park, B.; Lee, I.S.; Song, S.J.; Kim, C.S. Achyranthis radix extract improves urban particulate matter-induced dry eye disease. Int. J. Environ. Res. Public Health 2019, 16, 3229. [Google Scholar] [CrossRef] [Green Version]
- Lemp, M.A. Report of the National Eye Institute/Industry workshop on Clinical Trials in Dry Eyes. CLAO J. 1995, 21, 221–232. [Google Scholar]
- Sridhar, M.S. Anatomy of cornea and ocular surface. Indian J. Ophthalmol. 2018, 66, 190–194. [Google Scholar] [PubMed]
- Mandell, J.T.; Idarraga, M.; Kumar, N.; Galor, A. Impact of air pollution and weather on dry eye. J. Clin. Med. 2020, 9, 3740. [Google Scholar] [CrossRef]
- Hessen, M.; Akpek, E.K. Dry eye: An inflammatory ocular disease. J. Ophthalmic Vis. Res. 2014, 9, 240–250. [Google Scholar]
- Craig, J.P.; Nichols, K.K.; Akpek, E.K.; Caffery, B.; Dua, H.S.; Joo, C.K.; Liu, Z.; Nelson, J.D.; Nichols, J.J.; Tsubota, K.; et al. TFOS DEWS II definition and classification report. Ocul. Surf. 2017, 15, 276–283. [Google Scholar] [CrossRef] [PubMed]
- Guo, O.D.L.W.; Akpek, E. The negative effects of dry eye disease on quality of life and visual function. Turk. J. Med. Sci. 2020, 50, 1611–1615. [Google Scholar]
- Davidson, H.J.; Kuonen, V.J. The tear film and ocular mucins. Vet. Ophthalmol. 2004, 7, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Cwiklik, L. Tear film lipid layer: A molecular level view. Biochim. Biophys. Acta. 2016, 1858, 2421–2430. [Google Scholar] [CrossRef]
- King-Smith, P.E.; Fink, B.A.; Hill, R.M.; Koelling, K.W.; Tiffany, J.M. The thickness of the tear film. Curr. Eye Res. 2004, 29, 357–368. [Google Scholar] [CrossRef]
- Zhou, L.; Beuerman, R.W. Tear analysis in ocular surface diseases. Prog. Retin. Eye Res. 2012, 31, 527–550. [Google Scholar] [CrossRef]
- Zhang, X.; Qu, Y.; He, X.; Ou, S.; Bu, J.; Jia, C.; Wang, J.; Wu, H.; Liu, Z. Dry eye management: Targeting the ocular surface microenvironment. Int. J. Mol. Sci. 2017, 18, 1398. [Google Scholar] [CrossRef] [Green Version]
- Inatomi, T.; Spurr-Michaud, S.; Tisdale, A.S.; Zhan, Q.; Feldman, S.T.; Gipson, I.K. Expression of secretory mucin genes by human conjunctival epithelia. Investig. Ophthalmol. Vis. Sci. 1996, 37, 1684–1692. [Google Scholar]
- Matossian, C.; Trattler, W.; Loh, J. Dry eye treatment with topical cyclosporine 0.1% in chondroitin sulfate ophthalmic emulsion. Clin. Ophthalmol. 2021, 15, 1979–1984. [Google Scholar] [CrossRef]
- Yamaguchi, T. Inflammatory response in dry eye. Investig. Ophthalmol. Vis. Sci. 2018, 59, DES192–DES199. [Google Scholar] [CrossRef] [Green Version]
- Belfort, R., Jr.; Mendes, N.F. Identification of T and B lymphocytes in the human conjunctiva and lacrimal gland in ocular diseases. Br. J. Ophthalmol. 1980, 64, 217–219. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kheirkhah, A.; Rahimi Darabad, R.; Cruzat, A.; Hajrasouliha, A.R.; Witkin, D.; Wong, N.; Dana, R.; Hamrah, P. Corneal epithelial immune dendritic cell alterations in subtypes of dry eye disease: A pilot in vivo confocal microscopic study. Investig. Ophthalmol. Vis. Sci. 2015, 56, 7179–7185. [Google Scholar] [CrossRef] [PubMed]
- Pflugfelder, S.C.; Jones, D.; Ji, Z.; Afonso, A.; Monroy, D. Altered cytokine balance in the tear fluid and conjunctiva of patients with Sjögren’s syndrome keratoconjunctivitis sicca. Curr. Eye Res. 1999, 19, 201–211. [Google Scholar] [CrossRef] [PubMed]
- Dana, M.R.; Hamrah, P. Role of immunity and inflammation in corneal and ocular surface disease associated with dry eye. Adv. Exp. Med. Biol. 2002, 506, 729–738. [Google Scholar] [PubMed]
- Niederkorn, J.Y.; Stern, M.E.; Pflugfelder, S.C.; De Paiva, C.S.; Corrales, R.M.; Gao, J.; Siemasko, K. Desiccating stress induces T cell-mediated Sjögren’s Syndrome-like lacrimal keratoconjunctivitis. J. Immunol. 2006, 176, 3950–3957. [Google Scholar] [CrossRef] [Green Version]
- Chauhan, S.K.; El Annan, J.; Ecoiffier, T.; Goyal, S.; Zhang, Q.; Saban, D.R.; Dana, R. Autoimmunity in dry eye is due to resistance of Th17 to Treg suppression. J. Immunol. 2009, 182, 1247–1252. [Google Scholar] [CrossRef] [Green Version]
- Korn, T.; Reddy, J.; Gao, W.; Bettelli, E.; Awasthi, A.; Petersen, T.R.; Bäckström, B.T.; Sobel, R.A.; Wucherpfennig, K.W.; Strom, T.B.; et al. Myelin-specific regulatory T cells accumulate in the CNS but fail to control autoimmune inflammation. Nat. Med. 2007, 13, 423–431. [Google Scholar] [CrossRef]
- McGeachy, M.J.; Cua, D.J. Th17 cell differentiation: The long and winding road. Immunity 2008, 28, 445–453. [Google Scholar] [CrossRef] [Green Version]
- Lee, H.S.; Han, S.; Seo, J.W.; Jeon, K.J. Exposure to traffic-related particulate matter 2.5 triggers Th2-dominant ocular immune response in a murine model. Int. J. Environ. Res. Public Health 2020, 17, 2965. [Google Scholar] [CrossRef]
- Hyun, S.W.; Kim, J.; Park, B.; Jo, K.; Lee, T.G.; Kim, J.S.; Kim, C.S. Apricot Kernel extract and amygdalin inhibit urban particulate matter-induced keratoconjunctivitis sicca. Molecules 2019, 24, 650. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, Q.Y.; Huang, D.Y.; Zhang, H.J.; Wang, S.; Chen, X.F. Exposure to particulate matter 2.5 (PM2.5) induced macrophage-dependent inflammation, characterized by increased Th1/Th17 cytokine secretion and cytotoxicity. Int. Immunopharmacol. 2017, 50, 139–145. [Google Scholar] [CrossRef]
- Hamanaka, R.B.; Mutlu, G.M. Particulate matter air pollution: Effects on the cardiovascular system. Front. Endocrinol. 2018, 9, 680. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shou, Y.; Huang, Y.; Zhu, X.; Liu, C.; Hu, Y.; Wang, H. A review of the possible associations between ambient PM2.5 exposures and the development of Alzheimer’s disease. Ecotoxicol. Environ. Saf. 2019, 174, 344–352. [Google Scholar] [CrossRef]
- Boulton, M.; Dayhaw-Barker, P. The role of the retinal pigment epithelium: Topographical variation and ageing changes. Eye 2001, 15 Pt 3, 384–389. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chua, S.Y.L.; Warwick, A.; Peto, T.; Balaskas, K.; Moore, A.T.; Reisman, C.; Desai, P.; Lotery, A.J.; Dhillon, B.; Khaw, P.T.; et al. Association of ambient air pollution with age-related macular degeneration and retinal thickness in UK Biobank. Br. J. Ophthalmol. 2021. [Google Scholar] [CrossRef]
- Kim, S.; Park, H.; Park, H.; Joung, B.; Kim, E. The acute respiratory exposure by intratracheal instillation of Sprague-Dawley rats with diesel particulate matter induces retinal thickening. Cutan. Ocul. Toxicol. 2016, 35, 275–280. [Google Scholar] [CrossRef]
- Louwies, T.; Vuegen, C.; Panis, L.I.; Cox, B.; Vrijens, K.; Nawrot, T.S.; De Boever, P. miRNA expression profiles and retinal blood vessel calibers are associated with short-term particulate matter air pollution exposure. Environ. Res. 2016, 147, 24–31. [Google Scholar] [CrossRef]
- Wang, L.; Cioffi, G.A.; Cull, G.; Dong, J.; Fortune, B. Immunohistologic evidence for retinal glial cell changes in human glaucoma. Investig. Ophthalmol. Vis. Sci. 2002, 3, 1088–1094. [Google Scholar]
- Chang, K.H.; Hsu, P.Y.; Lin, C.J.; Lin, C.L.; Juo, S.H.; Liang, C.L. Traffic-related air pollutants increase the risk for age-related macular degeneration. J. Investig. Med. 2019, 67, 1076–1081. [Google Scholar] [CrossRef]
- Pan, S.C.; Huang, C.C.; Chin, W.S.; Chen, B.Y.; Chan, C.C.; Guo, Y.L. Association between air pollution exposure and diabetic retinopathy among diabetics. Environ. Res. 2020, 181, 108960. [Google Scholar] [CrossRef] [PubMed]
- Provost, E.B.; Panis, L.I.; Saenen, N.D.; Kicinski, M.; Louwies, T.; Vrijens, K.; De Boever, P.; Nawrot, T.S. Recent versus chronic fine particulate air pollution exposure as determinant of the retinal microvasculature in school children. Environ. Res. 2017, 159, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Hwang-Bo, H.; Ji, S.Y.; Kim, M.Y.; Kim, S.Y.; Park, C.; Hong, S.H.; Kim, G.Y.; Song, K.S.; Hyun, J.W.; et al. Diesel particulate matter 2.5 promotes epithelial-mesenchymal transition of human retinal pigment epithelial cells via generation of reactive oxygen species. Environ. Pollut. 2020, 262, 114301. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Kim, D.H.; Kim, J.H.; Park, S.K.; Jeong, J.W.; Kim, M.Y.; Hong, S.H.; Song, K.S.; Kim, G.Y.; Hyun, J.W.; et al. Urban aerosol particulate matter promotes necrosis and autophagy via reactive oxygen species-mediated cellular disorders that are accompanied by cell cycle arrest in retinal pigment epithelial cells. Antioxidants 2021, 10, 149. [Google Scholar] [CrossRef] [PubMed]
- Shin, W.Y.; Kim, J.H.; Lee, G.; Choi, S.; Kim, S.R.; Hong, Y.C.; Park, S.M. Exposure to ambient fine particulate matter is associated with changes in fasting glucose and lipid profiles: A nationwide cohort study. BMC Public Health 2020, 20, 430. [Google Scholar] [CrossRef] [Green Version]
- Mao, S.; Li, S.; Wang, C.; Liu, Y.; Li, N.; Liu, F.; Huang, S.; Liu, S.; Lu, Y.; Mao, Z.; et al. Is long-term PM1 exposure associated with blood lipids and dyslipidemias in a Chinese rural population? Environ. Int. 2020, 138, 105637. [Google Scholar] [CrossRef]


| Organ Weight (g) | Group | ||||
|---|---|---|---|---|---|
| Normal | Vehicle | 0.2% SP | 0.5% SP | CsA | |
| Body weight gain | 32.53 ± 11.91 | 26.20 ± 8.23 | 28.34 ± 8.37 | 31.35 ± 9.09 | 36.17 ± 14.19 |
| Thymus | 0.41 ± 0.06 | 0.38 ± 0.10 | 0.37 ± 0.09 | 0.43 ± 0.10 | 0.39 ± 0.10 |
| Heart | 0.72 ± 0.01 | 0.68 ± 0.02 | 0.67 ± 0.03 | 0.70 ± 0.04 | 0.68 ± 0.07 |
| Lung | 1.10 ± 0.07 | 1.04 ± 0.06 | 1.00 ±0.04 | 1.02 ± 0.07 | 1.02 ± 0.06 |
| Liver | 6.58 ± 0.53 | 6.57 ± 0.24 | 6.17 ± 0.72 | 6.57 ± 0.36 | 6.59 ± 0.52 |
| Kidney | 1.57 ± 0.08 | 1.50 ± 0.03 | 1.46 ± 0.20 | 1.45 ± 0.11 | 1.51 ± 0.16 |
| Spleen | 0.53 ± 0.08 | 0.52 ± 0.06 | 0.58 ± 0.08 | 0.51 ± 0.05 | 0.55 ± 0.05 |
| Parameter (Units) | Group | ||||
|---|---|---|---|---|---|
| Normal | Vehicle | 0.2% SP | 0.5% SP | CsA | |
| RBC (106/μL) | 8.11 ± 0.32 | 8.25 ± 0.14 | 8.09 ± 0.27 | 7.96 ± 0.21 | 7.79 ± 0.36 |
| WBC (103/μL) | 5.17 ± 0.64 | 4.98 ± 1.84 | 4.30 ± 0.80 | 4.13 ± 1.49 | 4.12 ± 1.73 |
| Hematocrit (%) | 49.86 ± 1.83 | 49.60 ± 0.75 | 48.26 ± 1.54 | 48.94 ± 2.02 | 48.48 ± 1.44 |
| Hemoglobin (g/dL) | 15.52 ± 0.18 | 15.42 ± 0.25 | 15.32 ± 0.48 | 14.92 ± 0.53 | 14.86 ± 0.36 |
| MCV (fL) | 61.54 ± 1.43 | 60.13 ± 0.63 | 59.66 ± 0.83 | 61.48 ± 1.39 | 62.34 ± 1.14 |
| MCH (pg) | 19.18 ± 0.62 | 18.68 ± 0.16 | 18.94 ± 0.28 | 18.72 ± 0.22 | 19.14 ± 0.67 |
| Platelet (103/μL) | 791.40 ± 128.32 | 935.67 ± 88.50 | 899.20 ± 68.88 | 955.40 ± 79.64 | 943.80 ± 81.62 |
| AST (U/L) | 138.96 ± 27.49 | 152.58 ± 24.23 | 167.06 ± 34.21 | 157.98 ± 22.57 | 134.18 ± 25.11 |
| ALT (U/L) | 20.60 ± 3.18 | 23.50 ± 4.66 | 23.90 ± 3.80 | 24.50 ± 4.72 | 19.78 ± 3.20 |
| ALP (U/L) | 420.30 ± 41.41 | 422.85 ± 78.25 | 432.38 ± 66.61 | 454.80 ± 76.49 | 457.36 ± 117.58 |
| BUN (mg/dL) | 13.15 ± 1.89 | 13.81 ± 1.28 | 14.58 ± 0.04 | 14.69 ± 1.91 | 15.43 ± 3.13 |
| Creatinine (mg/dL) | 0.46 ± 0.02 | 0.47 ± 0.05 | 0.50 ± 0.04 | 0.50 ± 0.04 | 0.51 ± 0.04 |
| TC (mg/dL) | 58.25 ± 3.85 | 74.42 ± 6.77 * | 65.43 ± 12.06 | 57.16 ± 6.71 # | 58.15 ± 6.88 # |
| TG (mg/dL) | 48.68 ± 18.05 | 46.43 ± 10.01 | 43.24 ± 9.17 | 36.36 ± 14.75 | 44.70 ± 9.98 |
| HDL-C (mg/dL) | 29.92 ± 5.47 | 30.58 ± 2.03 | 26.00 ± 4.68 | 25.12 ± 3.53 | 25.98 ± 1.19 |
| LDL-C (mg/dL) | 6.03 ± 1.36 | 9.62 ± 1.64 * | 7.63 ± 2.26 | 6.75 ± 1.09 | 7.30 ± 1.71 |
| FFA (uEq/L) | 654.20 ± 46.55 | 725.50 ± 102.97 | 662.40 ± 21.17 | 612.20 ± 89.71 | 672.40 ± 101.21 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, H.; Kim, D.H.; Hwangbo, H.; Kim, S.Y.; Ji, S.Y.; Kim, M.Y.; Shim, J.-H.; Leem, S.-H.; Hyun, J.W.; Kim, G.-Y.; et al. The Protective Effect of Topical Spermidine on Dry Eye Disease with Retinal Damage Induced by Diesel Particulate Matter2.5. Pharmaceutics 2021, 13, 1439. https://doi.org/10.3390/pharmaceutics13091439
Lee H, Kim DH, Hwangbo H, Kim SY, Ji SY, Kim MY, Shim J-H, Leem S-H, Hyun JW, Kim G-Y, et al. The Protective Effect of Topical Spermidine on Dry Eye Disease with Retinal Damage Induced by Diesel Particulate Matter2.5. Pharmaceutics. 2021; 13(9):1439. https://doi.org/10.3390/pharmaceutics13091439
Chicago/Turabian StyleLee, Hyesook, Da Hye Kim, Hyun Hwangbo, So Young Kim, Seon Yeong Ji, Min Yeong Kim, Jung-Hyun Shim, Sun-Hee Leem, Jin Won Hyun, Gi-Young Kim, and et al. 2021. "The Protective Effect of Topical Spermidine on Dry Eye Disease with Retinal Damage Induced by Diesel Particulate Matter2.5" Pharmaceutics 13, no. 9: 1439. https://doi.org/10.3390/pharmaceutics13091439
APA StyleLee, H., Kim, D. H., Hwangbo, H., Kim, S. Y., Ji, S. Y., Kim, M. Y., Shim, J.-H., Leem, S.-H., Hyun, J. W., Kim, G.-Y., & Choi, Y. H. (2021). The Protective Effect of Topical Spermidine on Dry Eye Disease with Retinal Damage Induced by Diesel Particulate Matter2.5. Pharmaceutics, 13(9), 1439. https://doi.org/10.3390/pharmaceutics13091439