The Potential of Macroporous Silica—Nanocrystalline Cellulose Combination for Formulating Dry Emulsion Systems with Improved Flow Properties: A DoE Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Preparation of Liquid Emulsions
2.2.2. Characterisation of Liquid Emulsions
2.2.3. Atomic Force Microscopy (AFM)
2.2.4. Spray Drying Process
2.2.4.1. Process Parameters
2.2.4.2. Spray Drying Process Yield
2.2.5. Characterisation of Dry Emulsion Powders
2.2.5.1. Drug Content
2.2.5.2. Extent of Released Drug
2.2.5.3. Encapsulation Efficiency
2.2.5.4. Moisture Content
2.2.5.5. Particle Size Analysis
2.2.5.6. Scanning Electron Microscopy (SEM)
2.2.5.7. Dry Emulsion Reconstitution
2.2.5.8. Mercury Intrusion Porosimetry
2.2.5.9. Energy-Dispersive X-ray Spectroscopy (EDS)
2.2.5.10. Flow Properties
2.2.5.11. Dissolution Studies
2.2.5.12. U(H)PLC Analysis
2.3. Experimental Design
3. Results and Discussion
3.1. Liquid Emulsions
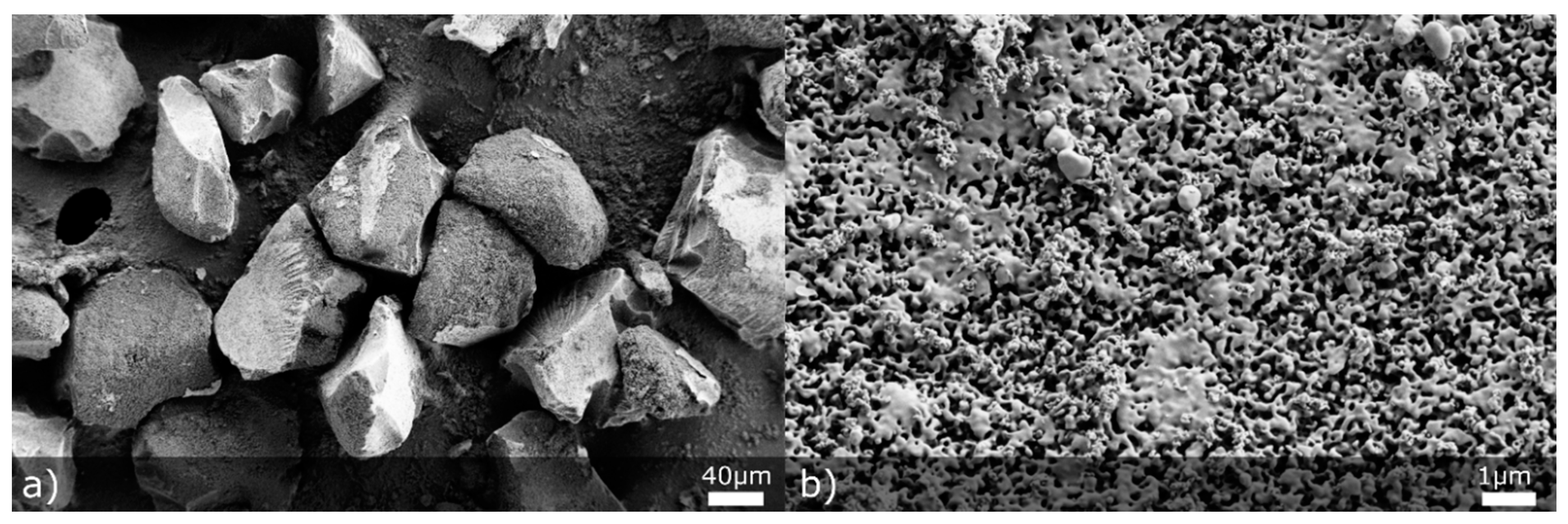
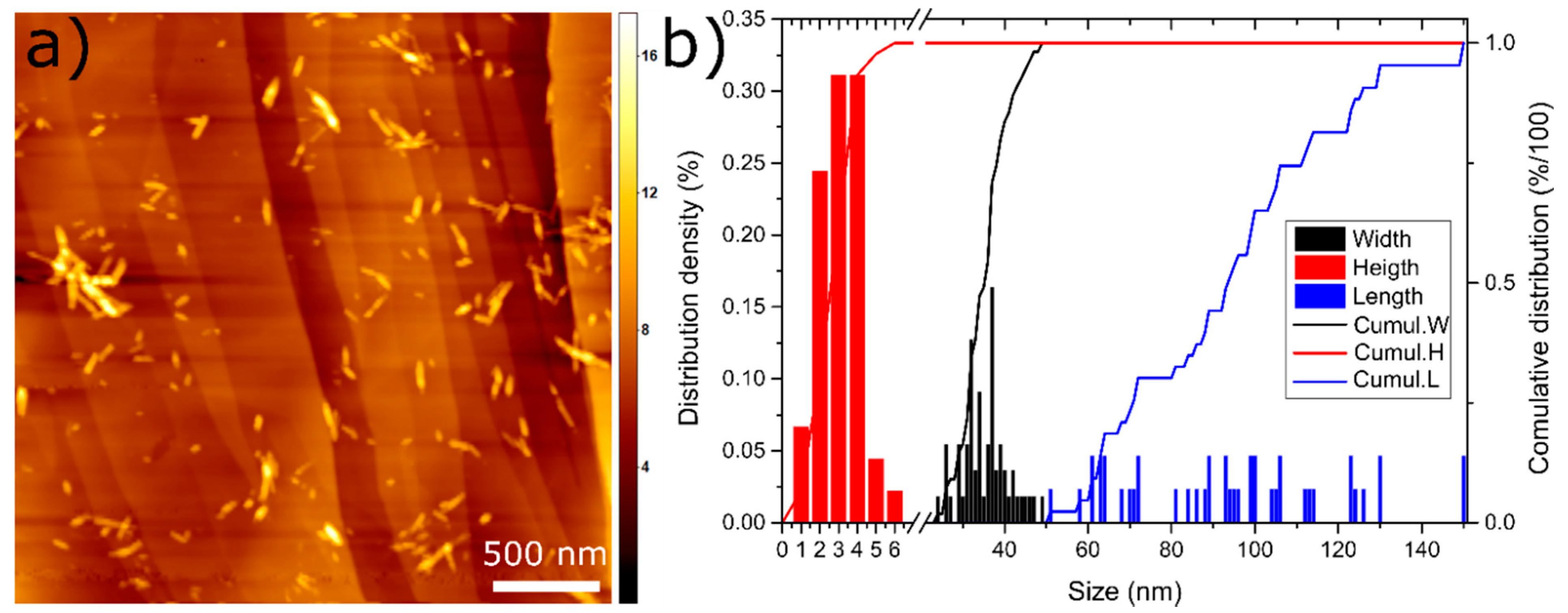
3.2. NCC and MS Characterisation
3.3. Dry Emulsion Powders
3.3.1. Process Yield
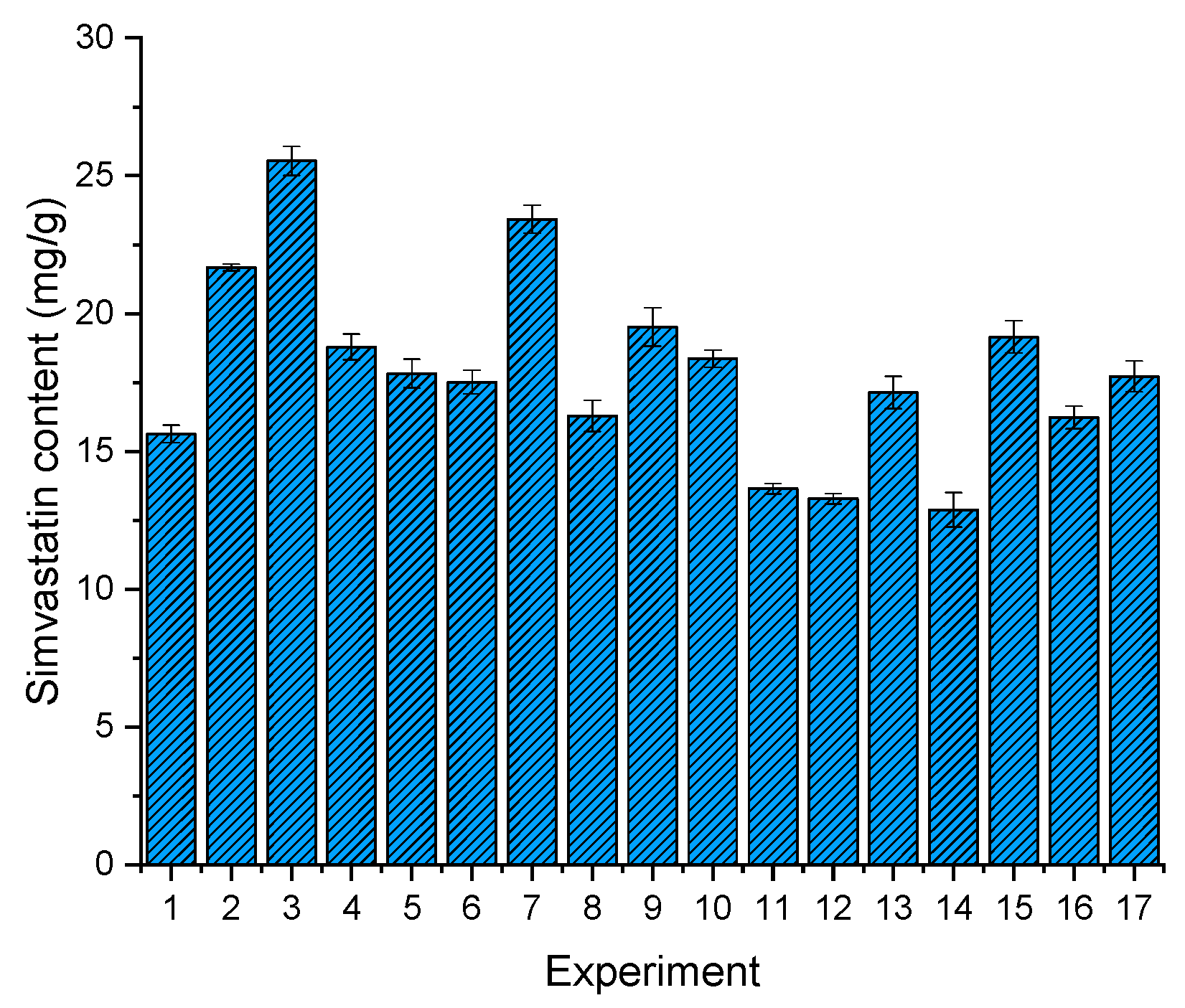
3.3.2. Drug Content
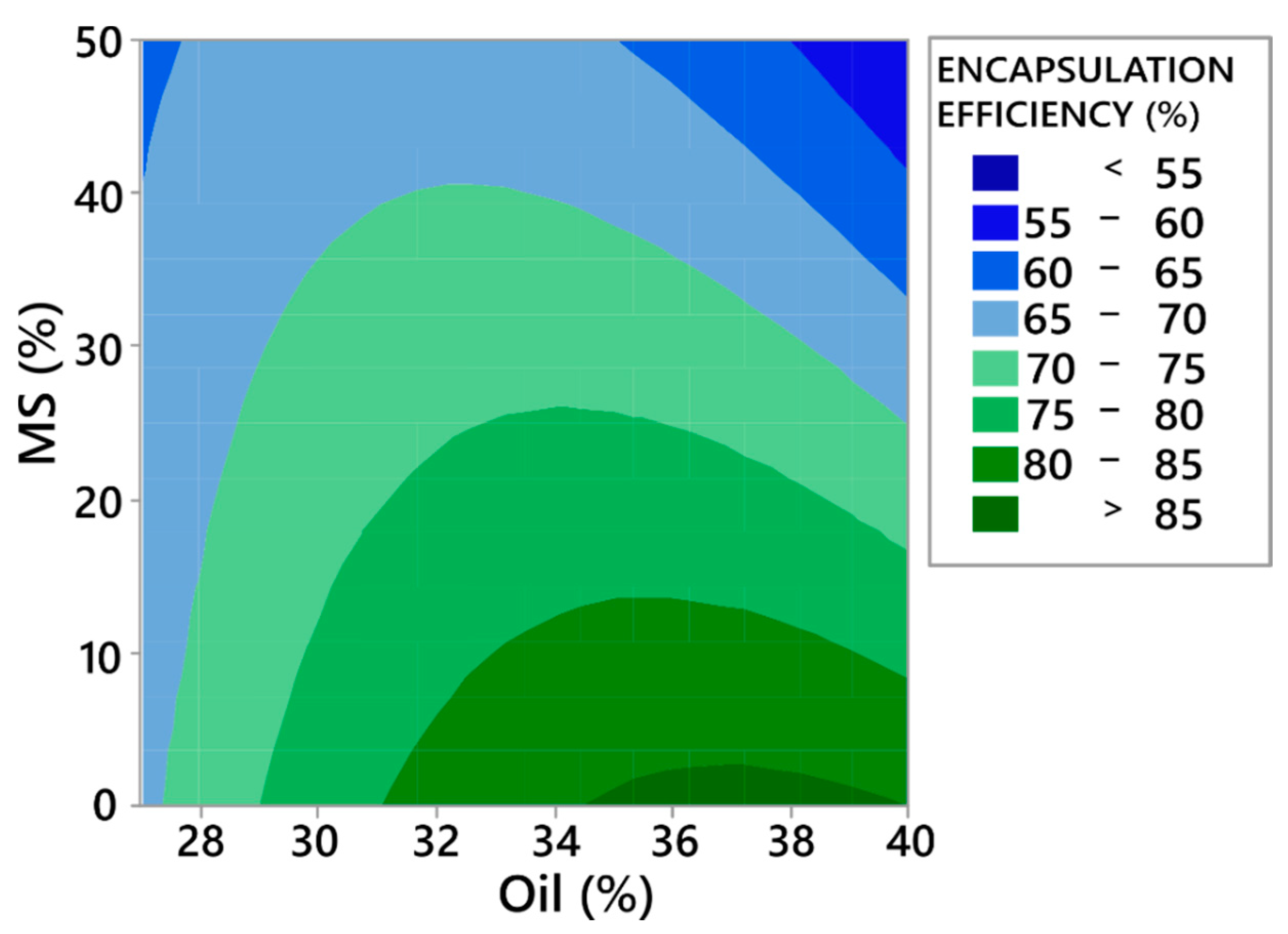
3.3.3. Encapsulation Efficiency
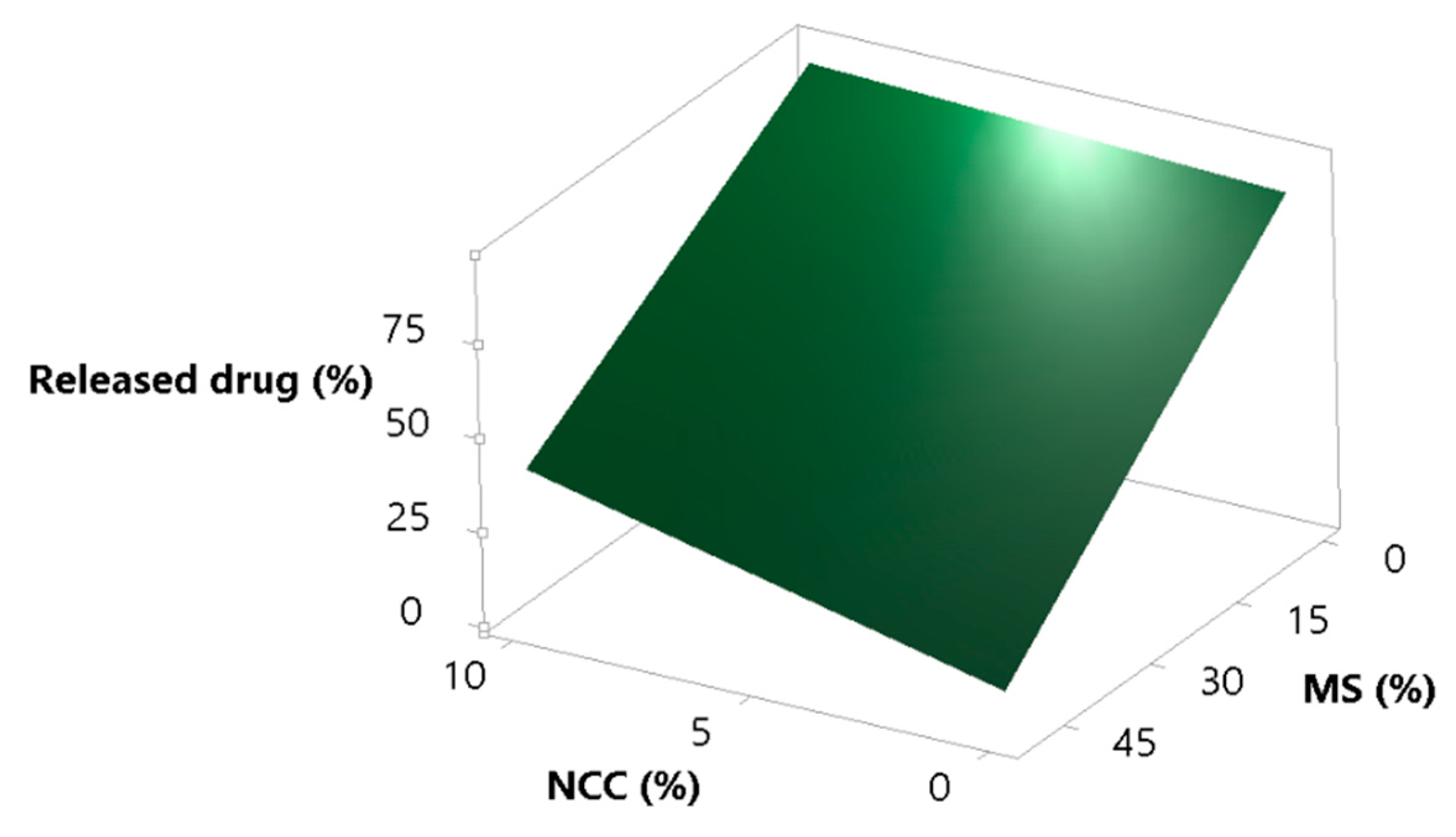
3.3.4. Amount of Released Drug
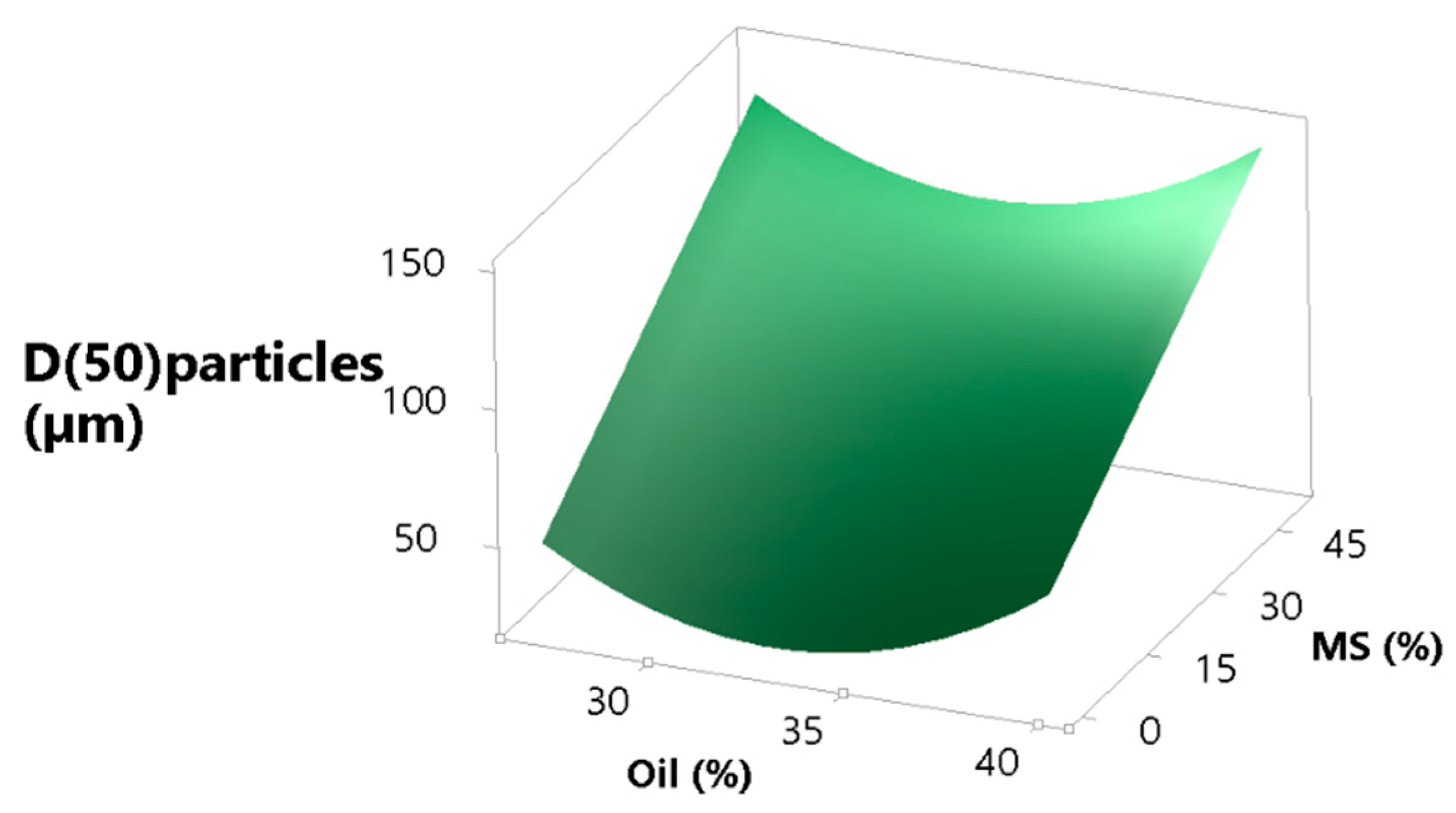
3.3.5. Particle Size and Morphology
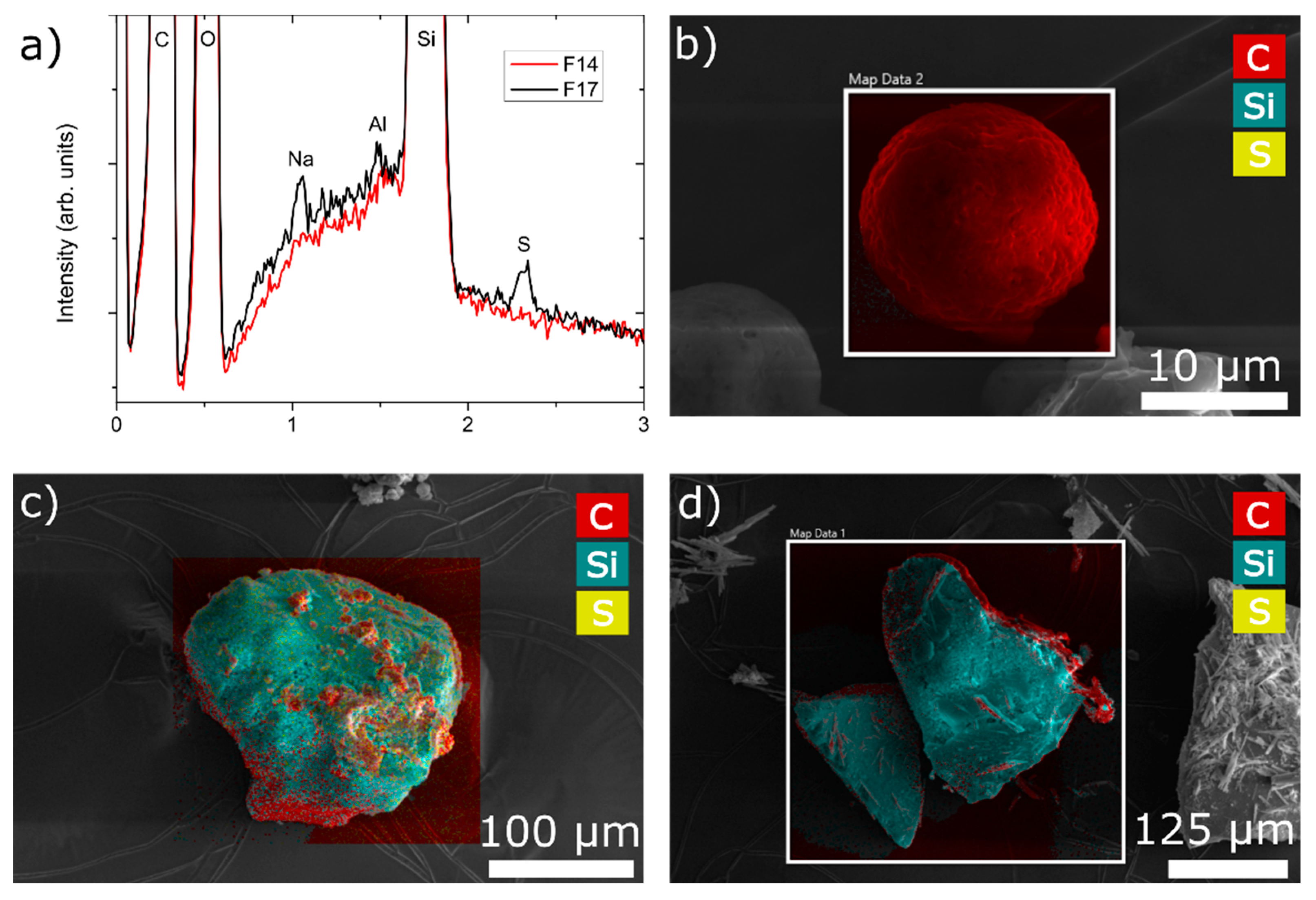
3.3.6. Spatial Distribution of Components
3.3.7. Reconstitution Ability of Dry Emulsion System
3.3.8. Porosity
3.3.9. Flow Properties
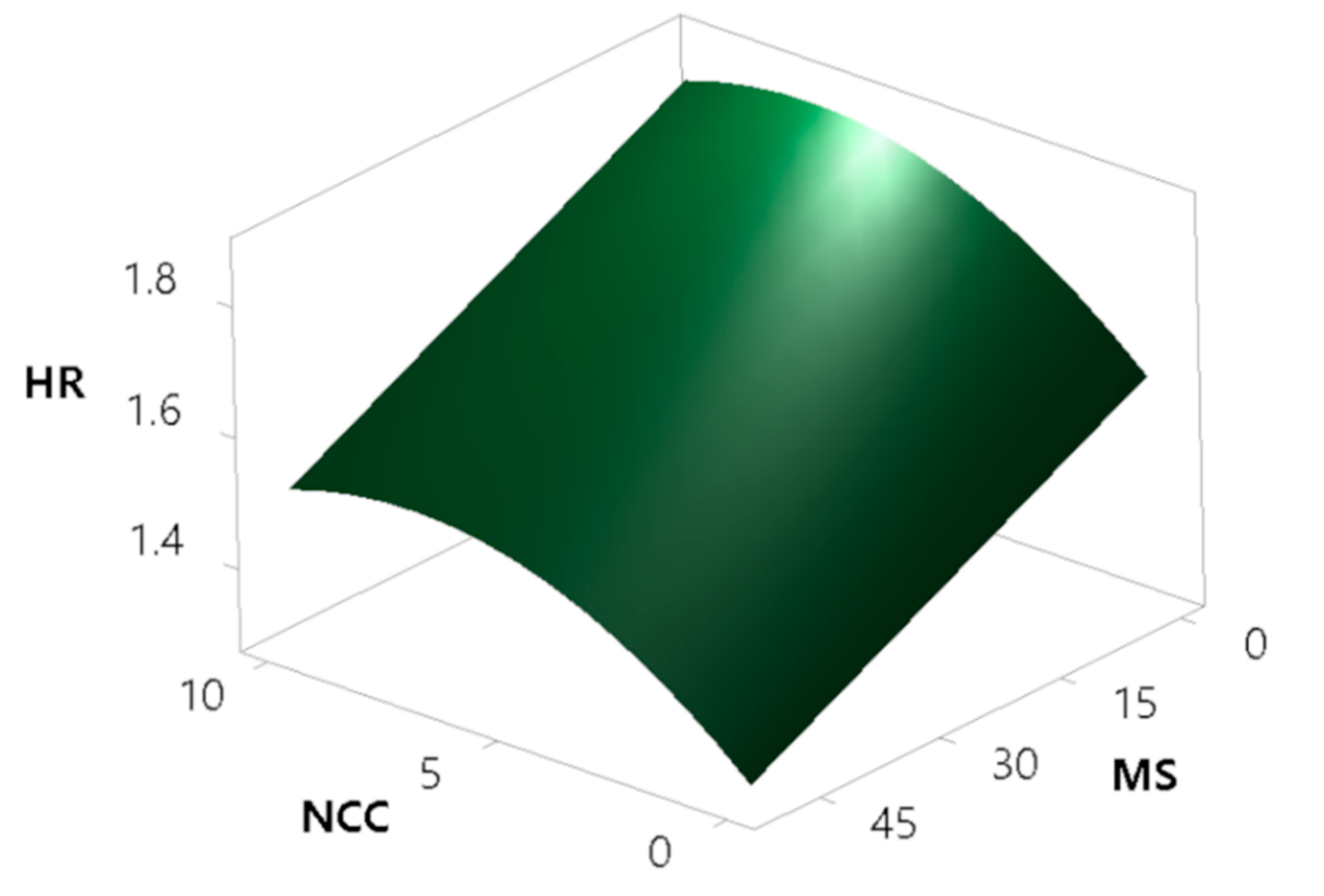
3.3.9.1. Hausner Ratio
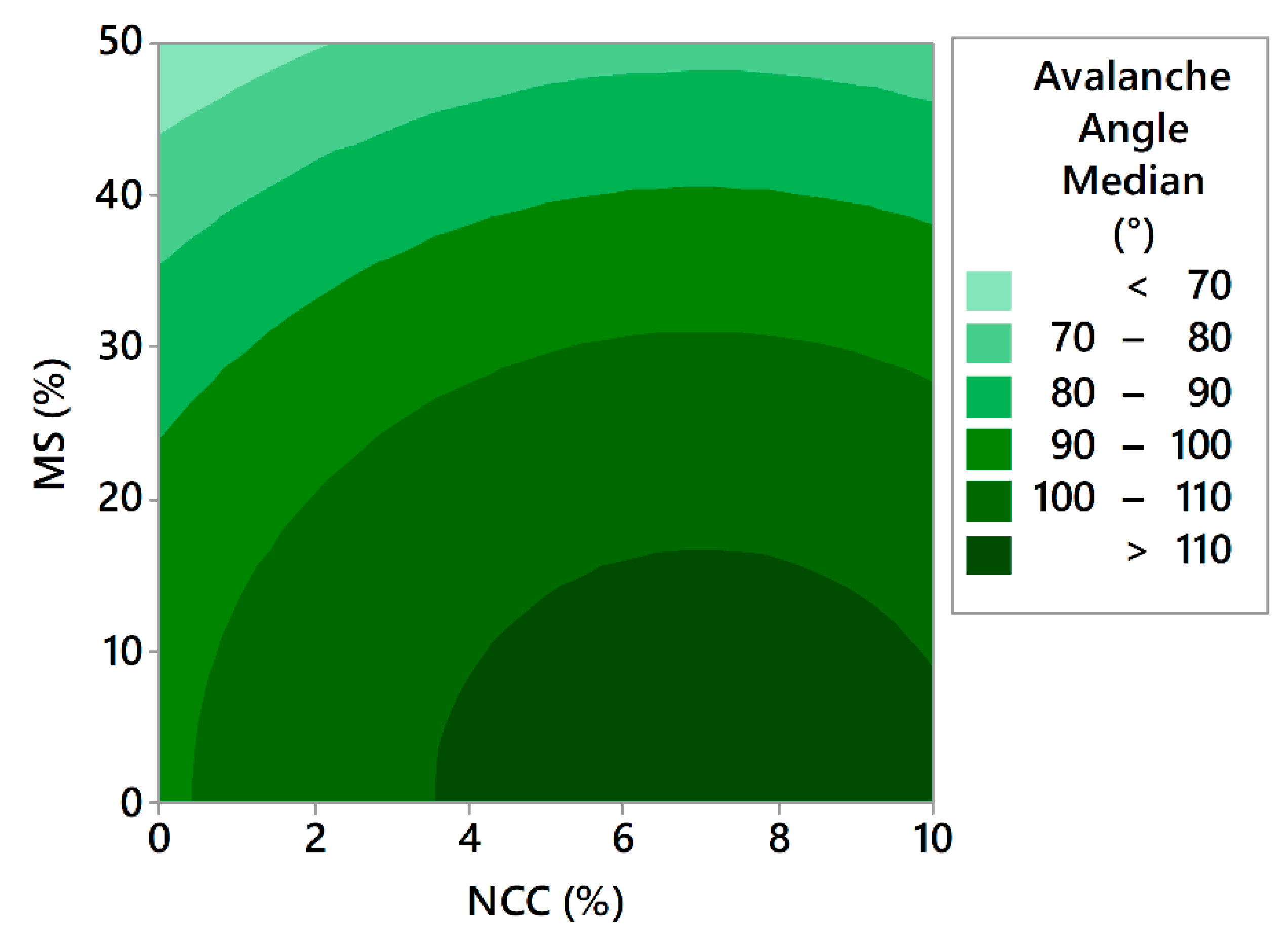
3.3.9.2. Avalanche Testing
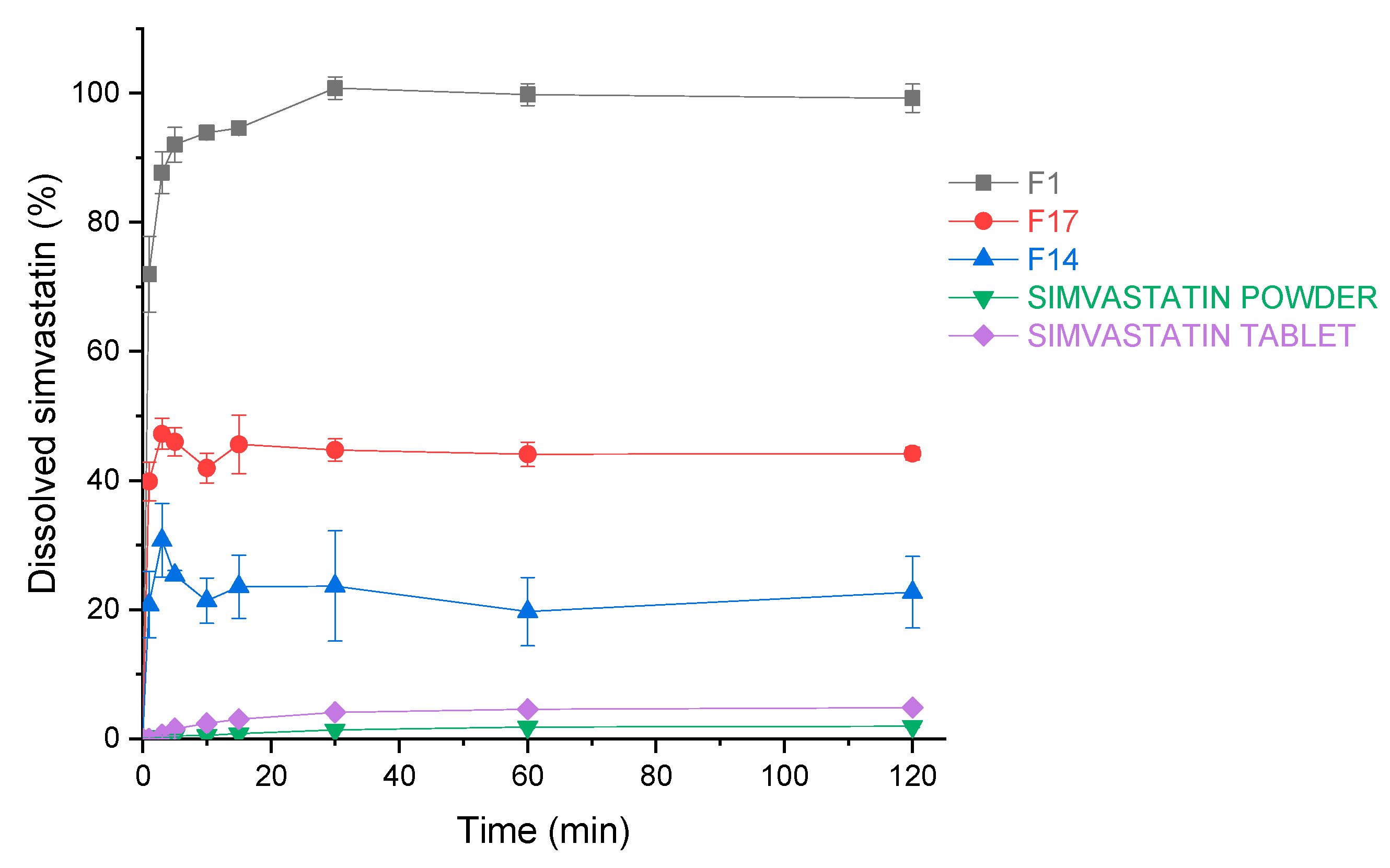
3.3.10. Dissolution
3.3.11. Concurrent Improvement of Flow Properties and Dissolution
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mahmood, A.; Bernkop-Schnürch, A. SEDDS: A game changing approach for the oral administration of hydrophilic macromolecular drugs. Adv. Drug Deliv. Rev. 2019, 142, 91–101. [Google Scholar] [CrossRef]
- Vithani, K.; Jannin, V.; Pouton, C.W.; Boyd, B.J. Colloidal aspects of dispersion and digestion of self-dispersing lipid-based formulations for poorly water-soluble drugs. Adv. Drug Deliv. Rev. 2019, 142, 16–34. [Google Scholar] [CrossRef]
- Pouton, C.W. Formulation of poorly water-soluble drugs for oral administration: Physicochemical and physiological issues and the lipid formulation classification system. Eur. J. Pharm. Sci. 2006, 29, 278–287. [Google Scholar] [CrossRef]
- Fahr, A.; Liu, X. Drug delivery strategies for poorly water-soluble drugs. Expert Opin. Drug Deliv. 2007, 4, 403–416. [Google Scholar] [CrossRef] [PubMed]
- Nardin, I.; Köllner, S. Successful development of oral SEDDS: Screening of excipients from the industrial point of view. Adv. Drug Deliv. Rev. 2019, 142, 128–140. [Google Scholar] [CrossRef] [PubMed]
- Williams, H.D.; Ford, L.; Igonin, A.; Shan, Z.; Botti, P.; Morgen, M.M.; Hu, G.; Pouton, C.W.; Scammells, P.J.; Porter, C.J.H.; et al. Unlocking the full potential of lipid-based formulations using lipophilic salt/ionic liquid forms. Adv. Drug Deliv. Rev. 2019, 142, 75–90. [Google Scholar] [CrossRef] [PubMed]
- Berthelsen, R.; Klitgaard, M.; Rades, T.; Müllertz, A. In vitro digestion models to evaluate lipid based drug delivery systems; present status and current trends. Adv. Drug Deliv. Rev. 2019, 142, 35–49. [Google Scholar] [CrossRef]
- Pouton, C.W. Lipid formulations for oral administration of drugs: Non-emulsifying, self-emulsifying and ‘self-microemulsifying’ drug delivery systems. Eur. J. Pharm. Sci. 2000, 11, S93–S98. [Google Scholar] [CrossRef]
- Alinaghi, A.; Tan, A.; Rao, S.; Prestidge, C.A. Impact of solidification on the performance of lipid-based colloidal carriers: Oil-based versus self-emulsifying systems. Curr. Drug Deliv. 2015, 12, 16–25. [Google Scholar] [CrossRef] [Green Version]
- Joyce, P.; Dening, T.J.; Meola, T.R.; Schultz, H.B.; Holm, R.; Thomas, N.; Prestidge, C.A. Solidification to improve the biopharmaceutical performance of SEDDS: Opportunities and challenges. Adv. Drug Deliv. Rev. 2018, 142, 102–117. [Google Scholar] [CrossRef]
- Tong, Y.; Wang, Y.; Yang, M.; Yang, J.; Chen, L.; Chu, X.; Gao, C.; Jin, Q.; Gong, W.; Gao, C. Systematic development of self-nanoemulsifying liquisolid tablets to improve the dissolution and oral bioavailability of an oily drug, vitamin K1. Pharmaceutics 2018, 10, 96. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pohlen, M.; Pirker, L.; Luštrik, M.; Dreu, R. A Redispersible dry emulsion system with simvastatin prepared via fluid bed layering as a means of dissolution enhancement of a lipophilic drug. Int. J. Pharm. 2018, 549, 325–334. [Google Scholar] [CrossRef] [PubMed]
- Jang, D.-J.; Jeong, E.J.; Lee, H.-M.; Kim, B.-C.; Lim, S.-J.; Kim, C.-K. Improvement of bioavailability and photostability of amlodipine using redispersible dry emulsion. Eur. J. Pharm. Sci. 2006, 28, 405–411. [Google Scholar] [CrossRef]
- Toorisaka, E.; Hashida, M.; Kamiya, N.; Ono, H.; Kokazu, Y.; Goto, M. An enteric-coated dry emulsion formulation for oral insulin delivery. J. Control. Release 2005, 107, 91–96. [Google Scholar] [CrossRef] [PubMed]
- Salama, A.H.; Basha, M.; El Awdan, S. Experimentally designed lyophilized dry emulsion tablets for enhancing the antihyperlipidemic activity of atorvastatin calcium: Preparation, in-vitro evaluation and in-vivo assessment. Eur. J. Pharm. Sci. 2018, 112, 52–62. [Google Scholar] [CrossRef]
- Benjasirimongkol, P.; Piriyaprasarth, S.; Sriamornsak, P. Improving dissolution and photostability of resveratrol using redispersible dry emulsion: Application of design space for optimizing formulation and spray-drying process. J. Drug Deliv. Sci. Technol. 2019, 51, 411–418. [Google Scholar] [CrossRef]
- Ahmed, I.S.; Aboul-Einien, M.H. In vitro and in vivo evaluation of a fast-disintegrating lyophilized dry emulsion tablet containing griseofulvin. Eur. J. Pharm. Sci. 2007, 32, 58–68. [Google Scholar] [CrossRef]
- Pedersen, G.P.; Fäldt, P.; Bergenståhl, B.; Kristensen, H.G. Solid state characterisation of a dry emulsion: A potential drug delivery system. Int. J. Pharm. 1998, 171, 257–270. [Google Scholar] [CrossRef]
- Adelmann, H.; Binks, B.P.; Mezzenga, R. Oil powders and gels from particle-stabilized emulsions. Langmuir 2012, 28, 1694–1697. [Google Scholar] [CrossRef]
- Onoue, S.; Sato, H.; Ogawa, K.; Kojo, Y.; Aoki, Y.; Kawabata, Y.; Wada, K.; Mizumoto, T.; Yamada, S. Inhalable dry-emulsion formulation of cyclosporine a with improved anti-inflammatory effects in experimental asthma/COPD-model rats. Eur. J. Pharm. Biopharm. 2012, 80, 54–60. [Google Scholar] [CrossRef]
- Wang, S.; Lu, A.; Zhang, L. Recent advances in regenerated cellulose materials. Prog. Polym. Sci. 2016, 53, 169–206. [Google Scholar] [CrossRef]
- Kobayashi, S.; Sakamoto, J.; Kimura, S. In vitro synthesis of cellulose and related polysaccharides. Prog. Polym. Sci. 2001, 26, 1525–1560. [Google Scholar] [CrossRef]
- Ilić, I.; Govedarica, B.; Šibanc, R.; Dreu, R.; Srčič, S. Deformation properties of pharmaceutical excipients determined using an in-die and out-die method. Int. J. Pharm. 2013, 446, 6–15. [Google Scholar] [CrossRef]
- Siepmann, J.; Peppas, N.A. Modeling of drug release from delivery systems based on hydroxypropyl methylcellulose (HPMC). Adv. Drug Deliv. Rev. 2012, 64, 163–174. [Google Scholar] [CrossRef]
- Javanbakht, S.; Shaabani, A. Carboxymethyl cellulose-based oral delivery systems. Int. J. Biol. Macromol. 2019, 133, 21–29. [Google Scholar] [CrossRef]
- Chavan, R.B.; Rathi, S.; Jyothi, V.G.S.S.; Shastri, N.R. Cellulose based polymers in development of amorphous solid dispersions. Asian J. Pharm. Sci. 2018, 14, 248–264. [Google Scholar] [CrossRef] [PubMed]
- Jackson, J.K.; Letchford, K.; Wasserman, B.Z.; Ye, L.; Hamad, W.Y.; Burt, H.M. The use of nanocrystalline cellulose for the binding and controlled release of drugs. Int. J. Nanomed. 2011, 6, 321–330. [Google Scholar] [CrossRef] [Green Version]
- Brinchi, L.; Cotana, F.; Fortunati, E.; Kenny, J.M. Production of nanocrystalline cellulose from lignocellulosic biomass: Technology and applications. Carbohydr. Polym. 2013, 94, 154–169. [Google Scholar] [CrossRef] [PubMed]
- Mohanta, V.; Madras, G.; Patil, S. Layer-by-layer assembled thin films and microcapsules of nanocrystalline cellulose for hydrophobic drug delivery. ACS Appl. Mater. Interfaces 2014, 6, 20093–20101. [Google Scholar] [CrossRef]
- Qing, W.; Wang, Y.; Wang, Y.; Zhao, D.; Liu, X.; Zhu, J. The modified nanocrystalline cellulose for hydrophobic drug delivery. Appl. Surf. Sci. 2016, 366, 404–409. [Google Scholar] [CrossRef] [Green Version]
- Roman, M.; Dong, S.; Hirani, A.; Lee, Y.W. Cellulose nanocrystals for drug delivery. In Polysaccharide Materials: Performance by Design, ACS Symposium Series; American Chemical Society: Washington, DC, USA, 2009; Volume 1017, pp. 81–91. ISBN 978-0-8412-6986-6. [Google Scholar]
- Cha, R.; He, Z.; Ni, Y. Preparation and characterization of thermal/PH-sensitive hydrogel from carboxylated nanocrystalline cellulose. Carbohydr. Polym. 2012, 88, 713–718. [Google Scholar] [CrossRef]
- Kolakovic, R.; Peltonen, L.; Laukkanen, A.; Hirvonen, J.; Laaksonen, T. Nanofibrillar cellulose films for controlled drug delivery. Eur. J. Pharm. Biopharm. 2012, 82, 308–315. [Google Scholar] [CrossRef]
- Wang, C.; Huang, H.; Jia, M.; Jin, S.; Zhao, W.; Cha, R. Formulation and evaluation of nanocrystalline cellulose as a potential disintegrant. Carbohydr. Polym. 2015, 130, 275–279. [Google Scholar] [CrossRef]
- Fujisawa, S.; Togawa, E.; Kuroda, K. Nanocellulose-stabilized pickering emulsions and their applications. Sci. Technol. Adv. Mater. 2017, 18, 959–971. [Google Scholar] [CrossRef]
- Wen, C.; Yuan, Q.; Liang, H.; Vriesekoop, F. Preparation and stabilization of d-limonene pickering emulsions by cellulose nanocrystals. Carbohydr. Polym. 2014, 112, 695–700. [Google Scholar] [CrossRef]
- Wang, W.; Du, G.; Li, C.; Zhang, H.; Long, Y.; Ni, Y. Preparation of cellulose nanocrystals from asparagus (Asparagus officinalis, L.) and their applications to palm oil/water pickering emulsion. Carbohydr. Polym. 2016, 151, 1–8. [Google Scholar] [CrossRef]
- Slowing, I.I.; Vivero-Escoto, J.L.; Wu, C.-W.; Lin, V.S.-Y. Mesoporous silica nanoparticles as controlled release drug delivery and gene transfection carriers. Adv. Drug Deliv. Rev. 2008, 60, 1278–1288. [Google Scholar] [CrossRef]
- Wale, A.; Nalawade, A.; Ponrathnam, S.; Rajan, C.R.; Badiger, M. One-pot synthesis of bimodal (macro-meso, micro-mesoporous) silica by polyHIPE: Parameter studies. J. Porous Mater. 2020, 27, 263–275. [Google Scholar] [CrossRef]
- Sun, W.-J.; Aburub, A.; Sun, C.C. A mesoporous silica based platform to enable tablet formulations of low dose drugs by direct compression. Int. J. Pharm. 2018, 539, 184–189. [Google Scholar] [CrossRef] [PubMed]
- Bolko Seljak, K.; Ilić, I.G.; Gašperlin, M.; Zvonar Pobirk, A. Self-microemulsifying tablets prepared by direct compression for improved resveratrol delivery. Int. J. Pharm. 2018, 548, 263–275. [Google Scholar] [CrossRef] [PubMed]
- Jadhav, N.R.; Irny, P.V.; Patil, U.S. Solid state behavior of progesterone and its release from neusilin US2 based liquisolid compacts. J. Drug Deliv. Sci. Technol. 2017, 38, 97–106. [Google Scholar] [CrossRef]
- Choudhari, Y.; Hoefer, H.; Libanati, C.; Monsuur, F.; McCarthy, W. Mesoporous silica drug delivery systems. In Amorphous Solid Dispersions: Theory and Practice; Shah, N., Sandhu, H., Choi, D.S., Chokshi, H., Malick, A.W., Eds.; Advances in Delivery Science and Technology; Springer: New York, NY, USA, 2014; pp. 665–693. ISBN 978-1-4939-1598-9. [Google Scholar]
- Hu, Y.; Zhi, Z.; Wang, T.; Jiang, T.; Wang, S. Incorporation of indomethacin nanoparticles into 3-D ordered macroporous silica for enhanced dissolution and reduced gastric irritancy. Eur. J. Pharm. Biopharm. 2011, 79, 544–551. [Google Scholar] [CrossRef]
- Santamaría, E.; Maestro, A.; Porras, M.; Gutiérrez, J.M.; González, C. Controlled release of ibuprofen by meso–macroporous silica. J. Solid State Chem. 2014, 210, 242–250. [Google Scholar] [CrossRef]
- Shi, Z.-G.; Guo, Q.-Z.; Liu, Y.-T.; Xiao, Y.-X.; Xu, L. Drug delivery devices based on macroporous silica spheres. Mater. Chem. Phys. 2011, 126, 826–831. [Google Scholar] [CrossRef]
- Floury, J.; Desrumaux, A.; Axelos, M.A.V.; Legrand, J. Degradation of methylcellulose during ultra-high pressure homogenisation. Food Hydrocoll. 2002, 16, 47–53. [Google Scholar] [CrossRef]
- Yu, J.F.S.; Zakin, J.L.; Patterson, G.K. Mechanical degradation of high molecular weight polymers in dilute solution. J. Appl. Polym. Sci. 1979, 23, 2493–2512. [Google Scholar] [CrossRef]
- Pohlen, M.; Lavrič, Z.; Prestidge, C.; Dreu, R. Preparation, physicochemical characterisation and DoE optimisation of a spray-dried dry emulsion platform for delivery of a poorly soluble drug, simvastatin. AAPS PharmSciTech 2020, 21, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Carr, R.L. Evaluating flow properties of solids. Chem. Eng. 1965, 18, 163–168. [Google Scholar]
- Jallo, L.J.; Ghoroi, C.; Gurumurthy, L.; Patel, U.; Davé, R.N. improvement of flow and bulk density of pharmaceutical powders using surface modification. Int. J. Pharm. 2012, 423, 213–225. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, S.; Mukherjee, R.; Chaudhuri, B. On the role of forces governing particulate interactions in pharmaceutical systems: A review. Int. J. Pharm. 2017, 526, 516–537. [Google Scholar] [CrossRef] [PubMed]
- Nalluri, V.R.; Kuentz, M. Flowability characterisation of drug–excipient blends using a novel powder avalanching method. Eur. J. Pharm. Biopharm. 2010, 74, 388–396. [Google Scholar] [CrossRef]
- Zainuddin, N.; Ahmad, I.; Kargarzadeh, H.; Ramli, S. Hydrophobic kenaf nanocrystalline cellulose for the binding of curcumin. Carbohydr. Polym. 2017, 163, 261–269. [Google Scholar] [CrossRef]
- Maury, M.; Murphy, K.; Kumar, S.; Shi, L.; Lee, G. Effects of process variables on the powder yield of spray-dried trehalose on a laboratory spray-dryer. Eur. J. Pharm. Biopharm. 2005, 59, 565–573. [Google Scholar] [CrossRef]
- Tan, A.; Rao, S.; Prestidge, C.A. Transforming lipid-based oral drug delivery systems into solid dosage forms: An overview of solid carriers, physicochemical properties, and biopharmaceutical performance. Pharm. Res. 2013, 30, 2993–3017. [Google Scholar] [CrossRef]
- Kang, B.K.; Lee, J.S.; Chon, S.K.; Jeong, S.Y.; Yuk, S.H.; Khang, G.; Lee, H.B.; Cho, S.H. Development of self-microemulsifying drug delivery systems (SMEDDS) for oral bioavailability enhancement of simvastatin in beagle dogs. Int. J. Pharm. 2004, 274, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Dollo, G.; Le Corre, P.; Guérin, A.; Chevanne, F.; Burgot, J.L.; Leverge, R. Spray-dried redispersible oil-in-water emulsion to improve oral bioavailability of poorly soluble drugs. Eur. J. Pharm. Sci. 2003, 19, 273–280. [Google Scholar] [CrossRef]
- Hansen, T.; Holm, P.; Schultz, K. Process characteristics and compaction of spray-dried emulsions containing a drug dissolved in lipid. Int. J. Pharm. 2004, 287, 55–66. [Google Scholar] [CrossRef]
- Williams, H.D.; Speybroeck, M.V.; Augustijns, P.; Porter, C.J.H. Lipid-based formulations solidified via adsorption onto the mesoporous carrier Neusilin® US2: Effect of drug type and formulation composition on in vitro pharmaceutical performance. J. Pharm. Sci. 2014, 103, 1734–1746. [Google Scholar] [CrossRef] [PubMed]
- Choudhari, Y.; Reddy, U.; Monsuur, F.; Pauly, T.; Hoefer, H.; McCarthy, W. Comparative evaluation of porous silica based carriers for lipids and liquid drug formulations. Open Mater. Sci. 2014, 1, 61–74. [Google Scholar] [CrossRef]
- Van Speybroeck, M.; Williams, H.D.; Nguyen, T.-H.; Anby, M.U.; Porter, C.J.H.; Augustijns, P. Incomplete desorption of liquid excipients reduces the in vitro and in vivo performance of self-emulsifying drug delivery systems solidified by adsorption onto an inorganic mesoporous carrier. Mol. Pharm. 2012, 9, 2750–2760. [Google Scholar] [CrossRef]
- Vraníková, B.; Niederquell, A.; Ditzinger, F.; Šklubalová, Z.; Kuentz, M. Mechanistic aspects of drug loading in liquisolid systems with hydrophilic lipid-based mixtures. Int. J. Pharm. 2020, 578, 119099. [Google Scholar] [CrossRef]
- Liu, L.X.; Marziano, I.; Bentham, A.C.; Litster, J.D.; White, E.T.; Howes, T. Effect of particle properties on the flowability of ibuprofen powders. Int. J. Pharm. 2008, 362, 109–117. [Google Scholar] [CrossRef]
- Brika, S.E.; Letenneur, M.; Dion, C.A.; Brailovski, V. Influence of particle morphology and size distribution on the powder flowability and laser powder bed fusion manufacturability of Ti-6Al-4V alloy. Addit. Manuf. 2020, 31, 100929. [Google Scholar] [CrossRef]
- Crouter, A.; Briens, L. The effect of moisture on the flowability of pharmaceutical excipients. AAPS PharmSciTech 2013, 15, 65–74. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Smidt, P.C.; Campanero, M.A.; Trocóniz, I.F. Intestinal absorption of penclomedine from lipid vehicles in the conscious rat: Contribution of emulsification versus digestibility. Int. J. Pharm. 2004, 270, 109–118. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Cheng, M.; Gabriel, M.S.; Teixeira Neto, Â.A.; da Silva Bernardes, J.; Berry, R.; Tam, K.C. Polymeric hollow microcapsules (PHM) via cellulose nanocrystal stabilized pickering emulsion polymerization. J. Colloid Interface Sci. 2019, 555, 489–497. [Google Scholar] [CrossRef]
- Jager, P.D.; Bramante, T.; Luner, P.E. Assessment of pharmaceutical powder flowability using shear cell-based methods and application of Jenike’s methodology. J. Pharm. Sci. 2015, 104, 3804–3813. [Google Scholar] [CrossRef]
- Fassihi, A.R.; Kanfer, I. Effect of compressibility and powder flow properties on tablet weight variation. Drug Dev. Ind. Pharm. 1986, 12, 1947–1966. [Google Scholar] [CrossRef]
- Tan, S.B.; Newton, J.M. Powder flowability as an indication of capsule filling performance. Int. J. Pharm. 1990, 61, 145–155. [Google Scholar] [CrossRef]
- Davis, M.T.; Potter, C.B.; Walker, G.M. Downstream processing of a ternary amorphous solid dispersion: The impacts of spray drying and hot melt extrusion on powder flow, compression and dissolution. Int. J. Pharm. 2018, 544, 242–253. [Google Scholar] [CrossRef] [Green Version]
- Hickey, A.J.; Mansour, H.M.; Telko, M.J.; Xu, Z.; Smyth, H.D.C.; Mulder, T.; McLean, R.; Langridge, J.; Papadopoulos, D. Physical characterization of component particles included in dry powder inhalers. II. Dynamic characteristics. J. Pharm. Sci. 2007, 96, 1302–1319. [Google Scholar] [CrossRef] [PubMed]
- Soh, J.L.P.; Liew, C.V.; Heng, P.W.S. New indices to characterize powder flow based on their avalanching behavior. Pharm. Dev. Technol. 2006, 11, 93–102. [Google Scholar] [CrossRef] [PubMed]
- Nalluri, V.R.; Puchkov, M.; Kuentz, M. Toward better understanding of powder avalanching and shear cell parameters of drug–excipient blends to design minimal weight variability into pharmaceutical capsules. Int. J. Pharm. 2013, 442, 49–56. [Google Scholar] [CrossRef]
- Spierings, A.B.; Voegtlin, M.; Bauer, T.; Wegener, K. Powder flowability characterisation methodology for powder-bed-based metal additive manufacturing. Prog. Addit. Manuf. 2016, 1, 9–20. [Google Scholar] [CrossRef] [Green Version]




| Experiment | Oil Phase (%) X1 | NCC (%) X2 | MS (%) X3 | Soluble Matrix Formers (%) |
|---|---|---|---|---|
| F1 | 27 | 0 | 0 | 73 |
| F2 | 40 | 5 | 25 | 30 |
| F3 | 40 | 10 | 0 | 50 |
| F4 | 33.5 | 10 | 25 | 31.5 |
| F5 | 33.5 | 5 | 25 | 36.5 |
| F6 | 33.5 | 0 | 25 | 41.5 |
| F7 | 40 | 0 | 0 | 60 |
| F8 | 33.5 | 5 | 50 | 11.5 |
| F9 | 33.5 | 5 | 0 | 61.5 |
| F10 | 33.5 | 5 | 25 | 36.5 |
| F11 | 27 | 5 | 25 | 43 |
| F12 | 27 | 10 | 50 | 13 |
| F13 | 40 | 0 | 50 | 10 |
| F14 | 27 | 0 | 50 | 23 |
| F15 | 33.5 | 5 | 25 | 36.5 |
| F16 | 27 | 10 | 0 | 63 |
| F17 | 40 | 10 | 50 | 0 |
| Formulation | MS | NCC | Oil Phase | Released Drug (%) |
|---|---|---|---|---|
| S1 | 2 | 1 | 0.68 | 46.4% |
| S2 | 1 | 1 | 0.68 | 72.3% |
| S3 | 1 | 2 | 0.34 | 84.5% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pohlen, M.; Pirker, L.; Dreu, R. The Potential of Macroporous Silica—Nanocrystalline Cellulose Combination for Formulating Dry Emulsion Systems with Improved Flow Properties: A DoE Study. Pharmaceutics 2021, 13, 1177. https://doi.org/10.3390/pharmaceutics13081177
Pohlen M, Pirker L, Dreu R. The Potential of Macroporous Silica—Nanocrystalline Cellulose Combination for Formulating Dry Emulsion Systems with Improved Flow Properties: A DoE Study. Pharmaceutics. 2021; 13(8):1177. https://doi.org/10.3390/pharmaceutics13081177
Chicago/Turabian StylePohlen, Mitja, Luka Pirker, and Rok Dreu. 2021. "The Potential of Macroporous Silica—Nanocrystalline Cellulose Combination for Formulating Dry Emulsion Systems with Improved Flow Properties: A DoE Study" Pharmaceutics 13, no. 8: 1177. https://doi.org/10.3390/pharmaceutics13081177
APA StylePohlen, M., Pirker, L., & Dreu, R. (2021). The Potential of Macroporous Silica—Nanocrystalline Cellulose Combination for Formulating Dry Emulsion Systems with Improved Flow Properties: A DoE Study. Pharmaceutics, 13(8), 1177. https://doi.org/10.3390/pharmaceutics13081177








