Prospective Evaluation of Local Sustained Release of Celecoxib in Dogs with Low Back Pain
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of PEA Microparticles
2.2. Study Design
2.3. Inclusion Criteria
2.4. Magnetic Resonance Imaging
- (a)
- Disc protrusion at L7-S1 graded as <25% or ≥25% stenosis of the spinal canal.
- (b)
- Pfirrmann grading [36].
- (c)
- (d)
- Spinal nerve swelling.
- (e)
- Intervertebral foraminal stenosis.
- (f)
- Ventral or lateral spondylosis deformans at the lumbosacral junction.
- (g)
- Degenerative joint disease of the facet joint.
2.5. Intradiscal Injection
2.6. Kinetic Gait Analysis
2.7. Owner Assessment of Pain Related Behaviour
2.8. Statistical Analysis
2.8.1. Primary Outcome Measurements
2.8.2. Secondary Outcome Measurements
3. Results
3.1. Baseline Characteristics
3.2. Owner Assessment of Pain
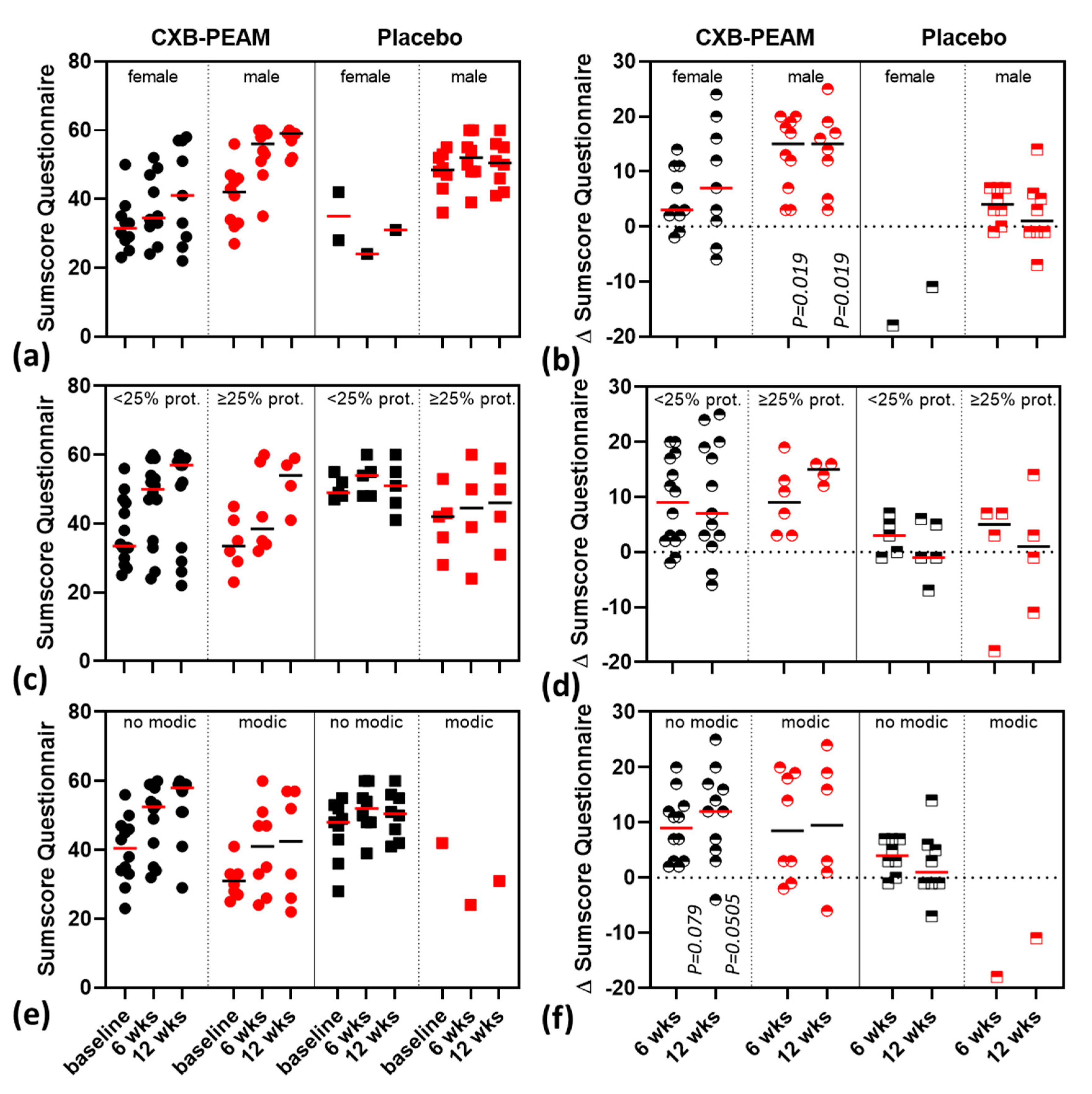
3.3. Subgroup-Analysis
3.4. Kinetic Gait Analysis
3.5. Descriptive MRI Findings, Disc Height Index, and T2-Mapping
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vos, T.; Abajobir, A.A.; Abate, K.H.; Abbafati, C.; Abbas, K.M.; Abd-Allah, F.; Abdulkader, R.S.; Abdulle, A.M.; Abebo, T.A.; Abera, S.F.; et al. Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet 2017, 390, 1211–1259. [Google Scholar] [CrossRef]
- Luoma, K.; Riihimäki, H.; Luukkonen, R.; Raininko, R.; Viikari-Juntura, E.; Lamminen, A. Low Back Pain in Relation to Lumbar Disc Degeneration. Spine 2000, 25, 487–492. [Google Scholar] [CrossRef] [PubMed]
- Cheung, K.M.C.; Samartzis, D.; Karppinen, J.; Luk, K.D.K. Are “Patterns” of Lumbar Disc Degeneration Associated with Low Back Pain? New Insights Based on Skipped Level Disc Pathology. Spine 2012, 37, E430–E438. [Google Scholar] [CrossRef] [PubMed]
- Bergknut, N.; Smolders, L.A.; Grinwis, G.C.M.; Hagman, R.; Lagerstedt, A.-S.; Hazewinkel, H.A.W.; Tryfonidou, M.A.; Meij, B.P. Intervertebral disc degeneration in the dog. Part 1: Anatomy and physiology of the intervertebral disc and characteristics of intervertebral disc degeneration. Vet. J. 2013, 195, 282–291. [Google Scholar] [CrossRef] [PubMed]
- DePalma, M.J.; Ketchum, J.M.; Saullo, T. What Is the Source of Chronic Low Back Pain and Does Age Play a Role? Pain Med. 2011, 12, 224–233. [Google Scholar] [CrossRef]
- Simon, J.; McAuliffe, M.; Shamim, F.; Vuong, N.; Tahaei, A. Discogenic Low Back Pain. Phys. Med. Rehabil. Clin. N. Am. 2014, 25, 305–317. [Google Scholar] [CrossRef]
- Risbud, M.V.; Shapiro, I.M. Role of cytokines in intervertebral disc degeneration: Pain and disc content. Nat. Rev. Rheumatol. 2014, 10, 44–56. [Google Scholar] [CrossRef]
- Miyamoto, H.; Saura, R.; Harada, T.; Doita, M.; Mizuno, K. The role of cyclooxygenase-2 and inflammatory cytokines in pain induction of herniated lumbar intervertebral disc. Kobe J. Med. Sci. 2000, 46, 13–28. [Google Scholar] [PubMed]
- Samad, T.A.; Moore, K.A.; Sapirstein, A.; Billet, S.; Allchorne, A.; Poole, S.; Bonventre, J.V.; Woolf, C.J. Interleukin-1b-mediated induction of Cox-2 in the CNS contributes to in ammatory pain Hypersensitivity. Nature 2001, 410, 5. [Google Scholar] [CrossRef]
- Adams, M.A.; Roughley, P.J. What is Intervertebral Disc Degeneration, and What Causes It? Spine 2006, 31, 2151–2161. [Google Scholar] [CrossRef]
- Navone, S.E.; Marfia, G.; Giannoni, A.; Beretta, M.; Guarnaccia, L.; Gualtierotti, R.; Nicoli, D.; Rampini, P.; Campanella, R. Inflammatory mediators and signalling pathways controlling intervertebral disc degeneration. Histol. Histopathol. 2016, 32, 523–542. [Google Scholar]
- Zhang, Y.-H.; Zhao, C.-Q.; Jiang, L.-S.; Chen, X.-D.; Dai, L.-Y. Modic changes: A systematic review of the literature. Eur. Spine J. 2008, 17, 1289–1299. [Google Scholar] [CrossRef] [PubMed]
- Motaghinasab, S.; Shirazi-Adl, A.; Parnianpour, M.; Urban, J.P.G. Disc size markedly influences concentration profiles of intravenously administered solutes in the intervertebral disc: A computational study on glucosamine as a model solute. Eur. Spine J. 2014, 23, 715–723. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, J.; Feng, X.; Cai, W.; Yang, J.; Zhang, N. Antibiotic penetration into rabbit nucleus pulposus with discitis. Eur. J. Orthop. Surg. Traumatol. 2014, 24, 453–458. [Google Scholar] [CrossRef]
- Enthoven, W.T.M.; Roelofs, P.D.; Koes, B.W. NSAIDs for Chronic Low Back Pain. JAMA 2017, 317, 2327. [Google Scholar] [CrossRef]
- Conaghan, P.G. A turbulent decade for NSAIDs: Update on current concepts of classification, epidemiology, comparative efficacy, and toxicity. Rheumatol. Int. 2012, 32, 1491–1502. [Google Scholar] [CrossRef]
- Bindu, S.; Mazumder, S.; Bandyopadhyay, U. Non-steroidal anti-inflammatory drugs (NSAIDs) and organ damage: A current perspective. Biochem. Pharmacol. 2020, 180, 114147. [Google Scholar] [CrossRef]
- Tryfonidou, M.A.; de Vries, G.; Hennink, W.E.; Creemers, L.B. “Old Drugs, New Tricks”—Local controlled drug release systems for treatment of degenerative joint disease. Adv. Drug Deliv. Rev. 2020, 160, 170–185. [Google Scholar] [CrossRef]
- Katsarava, R.; Beridze, V.; Arabuli, N.; Kharadze, D.; Chu, C.C.; Won, C.Y. Amino Acid-Based Bioanalogous Polymers. Synthesis, and Study of Regular Poly(ester amide)s Based on Bis(α-amino acid) α,ω-Alkylene Diesters, and Aliphatic Dicarboxylic Acids. J. Polym. Sci. A Polym. Chem. 1999, 37, 391–407. [Google Scholar] [CrossRef]
- Sun, H.; Meng, F.; Dias, A.A.; Hendriks, M.; Feijen, J.; Zhong, Z. α-Amino Acid Containing Degradable Polymers as Functional Biomaterials: Rational Design, Synthetic Pathway, and Biomedical Applications. Biomacromolecules 2011, 12, 1937–1955. [Google Scholar] [CrossRef]
- Rodriguez-Galan, A.; Franco, L.; Puiggali, J. Degradable Poly(ester amide)s for Biomedical Applications. Polymers 2010, 3, 65–99. [Google Scholar] [CrossRef]
- Herrero-Vanrell, R.; Bravo-Osuna, I.; Andrés-Guerrero, V.; Vicario-de-la-Torre, M.; Molina-Martínez, I.T. The potential of using biodegradable microspheres in retinal diseases and other intraocular pathologies. Prog. Retin. Eye Res. 2014, 42, 27–43. [Google Scholar] [CrossRef]
- Janssen, M.; Timur, U.T.; Woike, N.; Welting, T.J.M.; Draaisma, G.; Gijbels, M.; van Rhijn, L.W.; Mihov, G.; Thies, J.; Emans, P.J. Celecoxib-loaded PEA microspheres as an auto regulatory drug-delivery system after intra-articular injection. J. Control Release 2016, 244, 30–40. [Google Scholar] [CrossRef]
- Willems, N.; Mihov, G.; Grinwis, G.C.M.; van Dijk, M.; Schumann, D.; Bos, C.; Strijkers, G.J.; Dhert, W.J.A.; Meij, B.P.; Creemers, L.B.; et al. Safety of intradiscal injection and biocompatibility of polyester amide microspheres in a canine model predisposed to intervertebral disc degeneration. J. Biomed. Mater. Res. B Appl. Biomater. 2017, 105, 707–714. [Google Scholar] [CrossRef]
- Rudnik-Jansen, I.; Tellegen, A.; Beukers, M.; Öner, F.; Woike, N.; Mihov, G.; Thies, J.; Meij, B.; Tryfonidou, M.; Creemers, L. Safety of intradiscal delivery of triamcinolone acetonide by a poly(esteramide) microsphere platform in a large animal model of intervertebral disc degeneration. Spine J. 2019, 19, 905–919. [Google Scholar] [CrossRef]
- Tellegen, A.R.; Rudnik-Jansen, I.; Beukers, M.; Miranda-Bedate, A.; Bach, F.C.; de Jong, W.; Woike, N.; Mihov, G.; Thies, J.C.; Meij, B.P.; et al. Intradiscal delivery of celecoxib-loaded microspheres restores intervertebral disc integrity in a preclinical canine model. J. Control Release 2018, 286, 439–450. [Google Scholar] [CrossRef] [PubMed]
- Tellegen, A.R.; Willems, N.; Beukers, M.; Grinwis, G.C.M.; Plomp, S.G.M.; Bos, C.; Dijk, M.; Leeuw, M.; Creemers, L.B.; Tryfonidou, M.A.; et al. Intradiscal application of a PCLA–PEG–PCLA hydrogel loaded with celecoxib for the treatment of back pain in canines: What’s in it for humans? J. Tissue Eng. Regen. Med. 2018, 12, 642–652. [Google Scholar] [CrossRef]
- Worth, A.; Meij, B.; Jeffery, N. Canine Degenerative Lumbosacral Stenosis: Prevalence, Impact And Management Strategies. Vet. Med. Res. Rep. 2019, 10, 169–183. [Google Scholar] [CrossRef]
- Bergknut, N.; Rutges, J.P.H.J.; Kranenburg, H.-J.C.; Smolders, L.A.; Hagman, R.; Smidt, H.-J.; Lagerstedt, A.-S.; Penning, L.C.; Voorhout, G.; Hazewinkel, H.A.W.; et al. The Dog as an Animal Model for Intervertebral Disc Degeneration? Spine 2012, 37, 351–358. [Google Scholar] [CrossRef] [PubMed]
- Lee, N.N.; Kramer, J.S.; Stoker, A.M.; Bozynski, C.C.; Cook, C.R.; Stannard, J.T.; Choma, T.J.; Cook, J.L. Canine models of spine disorders. JOR Spine 2020, 3, e1109. [Google Scholar] [CrossRef]
- Meij, B.P.; Bergknut, N. Degenerative Lumbosacral Stenosis in Dogs. Vet. Clin. N. Am. Small Anim. Pract. 2010, 40, 983–1009. [Google Scholar] [CrossRef] [PubMed]
- Monteiro-Steagall, B.P.; Steagall, P.V.M.; Lascelles, B.D.X. Systematic Review of Nonsteroidal Anti-Inflammatory Drug-Induced Adverse Effects in Dogs. J. Vet. Int. Med. 2013, 27, 1011–1019. [Google Scholar] [CrossRef] [PubMed]
- Tsitlanadze, G.; Kviria, T.; Katsarava, R.; Chu, C.C. In vitro enzymatic biodegradation of amino acid based poly(ester amide)s biomaterials. J. Mater. Sci. Mater. Med. 2004, 15, 185–190. [Google Scholar] [CrossRef] [PubMed]
- Tellegen, A.; Beukers, M.; Rudnik-Jansen, I. Intra-Articular Slow-Release Triamcinolone Acetonide from Polyesteramide Microspheres as a Treatment for Osteoarthritis. Pharmaceutics 2021, 13, 372. [Google Scholar] [CrossRef]
- Bergknut, N.; Auriemma, E.; Wijsman, S.; Voorhout, G.; Hagman, R.; Lagerstedt, A.-S.; Hazewinkel, H.A.W.; Meij, B.P. Evaluation of intervertebral disk degeneration in chondrodystrophic and nonchondrodystrophic dogs by use of Pfirrmann grading of images obtained with low-field magnetic resonance imaging. Am. J. Vet. Res. 2011, 72, 893–898. [Google Scholar] [CrossRef]
- Lee, N.N.; Salzer, E.; Bach, F.C.; Bonilla, A.F.; Cook, J.L.; Gazit, Z.; Grad, S.; Ito, K.; Smith, L.J.; Vernengo, A.; et al. A comprehensive tool box for large animal studies of intervertebral disc degeneration. JOR Spine 2021, e1162. [Google Scholar] [CrossRef]
- Modic, M.T.; Masaryk, T.J.; Ross, J.S.; Carter, J.R. Imaging of degenerative disk disease. Radiology 1988, 168, 177–186. [Google Scholar] [CrossRef]
- Modic, M.T.; Steinberg, P.M.; Ross, J.S.; Masaryk, T.J.; Carter, J.R. Degenerative disk disease: Assessment of changes in vertebral body marrow with MR imaging. Radiology 1988, 166, 193–199. [Google Scholar] [CrossRef]
- Suwankong, N.; Meij, B.P.; Van Klaveren, N.J.; Van Wees, A.M.T.C.; Meijer, E.; Van Den Brom, W.E.; Hazewinkel, H.A.W. Assessment of Decompressive Surgery in Dogs with Degenerative Lumbosacral Stenosis Using Force Plate Analysis and Questionnaires. Vet. Surg. 2007, 36, 423–431. [Google Scholar] [CrossRef]
- Tellegen, A.R.; Willems, N.; Tryfonidou, M.A.; Meij, B.P. Pedicle screw-rod fixation: A feasible treatment for dogs with severe degenerative lumbosacral stenosis. BMC Vet. Res. 2015, 11, 299. [Google Scholar] [CrossRef]
- Klaveren, N.J.; Suwankong, N.; De Boer, S.; Brom, W.E.; Voorhout, G.; Hazewinkel, H.A.W.; Meij, B.P. Force Plate Analysis Before and After Dorsal Decompression for Treatment of Degenerative Lumbosacral Stenosis in Dogs. Vet. Surg. 2005, 34, 450–456. [Google Scholar] [CrossRef]
- Schulz, K.F.; Altman, D.G.; Moher, D. CONSORT 2010 Statement: Updated Guidelines for Reporting Parallel Group Randomized Trials. Ann. Int. Med. 2010, 154, 291–292. [Google Scholar] [CrossRef]
- Brown, D.C.; Bell, M.; Rhodes, L. Power of treatment success definitions when the Canine Brief Pain Inventory is used to evaluate carprofen treatment for the control of pain and inflammation in dogs with Osteoarthritis. Am. J. Vet. Res. 2013, 74, 1467–1473. [Google Scholar] [CrossRef]
- Brown, D.C.; Boston, R.C.; Farrar, J.T. Comparison of Force Plate Gait Analysis and Owner Assessment of Pain Using the Canine Brief Pain Inventory in Dogs with Osteoarthritis. J. Vet. Intern. Med. 2013, 27, 22–30. [Google Scholar] [CrossRef]
- McCormack, P.L. Celecoxib: A Review of its Use for Symptomatic Relief in the Treatment of Osteoarthritis, Rheumatoid Arthritis and Ankylosing Spondylitis. Drugs 2011, 71, 2457–2489. [Google Scholar] [CrossRef] [PubMed]
- Fanchon, L.; Grandjean, D. Accuracy of asymmetry indices of ground reaction forces for diagnosis of hind limb lameness in dogs. Am. J. Vet. Res. 2007, 68, 1089–1094. [Google Scholar] [CrossRef] [PubMed]
- Takashima, H.; Takebayashi, T.; Yoshimoto, M.; Terashima, Y.; Tsuda, H.; Ida, K.; Yamashita, T. Correlation between T2 relaxation time and intervertebral disk degeneration. Skelet. Radiol. 2012, 41, 163–167. [Google Scholar] [CrossRef] [PubMed]
- Coleman, K.D.; Schmiedt, C.W.; Kirkby, K.A.; Coleman, A.E.; Robertson, S.A.; Hash, J.; Lascelles, B.D.X. Learning Confounds Algometric Assessment of Mechanical Thresholds in Normal Dogs: Learning Affects Algometry in Normal Dogs. Vet. Surg. 2014, 43, 361–367. [Google Scholar] [CrossRef]
- Sanchis-Mora, S.; Chang, Y.-M.; Abeyesinghe, S.; Fisher, A.; Volk, H.A.; Pelligand, L. Development and initial validation of a sensory threshold examination protocol (STEP) for phenotyping canine pain syndromes. Vet. Anaesth. Analg. 2017, 44, 600–614. [Google Scholar] [CrossRef]
- Fillingim, R.B.; King, C.D.; Ribeiro-Dasilva, M.C.; Rahim-Williams, B.; Riley, J.L. Sex, Gender, and Pain: A Review of Recent Clinical and Experimental Findings. J. Pain 2009, 10, 447–485. [Google Scholar] [CrossRef]
- Kanaan, S.F.; Melton, B.L.; Waitman, L.R.; Simpson, M.H.; Sharma, N.K. The effect of age and gender on acute postoperative pain and function following lumbar spine surgeries. Physiother. Res. Int. 2021, 26, e1888. [Google Scholar] [CrossRef]
- Pieretti, S.; Giannuario, A.D.; Giovannandrea, R.D.; Marzoli, F.; Piccaro, G.; Minosi, P.; Aloisi, A.M. Gender differences in pain and its relief. Ann. Dell’ist. Sup. Sanita 2016, 52, 184–189. [Google Scholar]
- Jensen, R.K.; Leboeuf-Yde, C.; Wedderkopp, N.; Sorensen, J.S.; Jensen, T.S.; Manniche, C. Is the development of Modic changes associated with clinical symptoms? A 14-month cohort study with MRI. Eur. Spine J. 2012, 21, 2271–2279. [Google Scholar] [CrossRef]
- Carragee, E.J.; Lincoln, T.; Parmar, V.S.; Alamin, T. A Gold Standard Evaluation of the “Discogenic Pain” Diagnosis as Determined by Provocative Discography. Spine 2006, 31, 2115–2123. [Google Scholar] [CrossRef] [PubMed]
- Iatridis, J.C.; Nicoll, S.B.; Michalek, A.J.; Walter, B.A.; Gupta, M.S. Role of biomechanics in intervertebral disc degeneration and regenerative therapies: What needs repairing in the disc and what are promising biomaterials for its repair? Spine J. 2013, 13, 243–262. [Google Scholar] [CrossRef]
- Cuellar, J.M.; Stauff, M.P.; Herzog, R.J.; Carrino, J.A.; Baker, G.A.; Carragee, E.J. Does provocative discography cause clinically important injury to the lumbar intervertebral disc? A 10-year matched cohort study. Spine J. 2016, 16, 273–280. [Google Scholar] [CrossRef]
- Elliott, D.M.; Yerramalli, C.S.; Beckstein, J.C.; Boxberger, J.I.; Johannessen, W.; Vresilovic, E.J. The Effect of Relative Needle Diameter in Puncture and Sham Injection Animal Models of Degeneration. Spine 2008, 33, 588–596. [Google Scholar] [CrossRef]
- Mao, H.; Chen, Q.; Han, B.; Li, F.; Feng, J.; Shi, Z.; Lin, M.; Wang, J. The Effect of Injection Volume on Disc Degeneration in a Rat Tail Model. Spine 2011, 36, 1062–1069. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Shi, R.; Cai, F.; Wang, Y.-T.; Wu, X.-T. Stem Cell Approaches to Intervertebral Disc Regeneration: Obstacles from the Disc Microenvironment. Stem Cells Dev. 2015, 24, 2479–2495. [Google Scholar] [CrossRef]
- Hansen, T.; Smolders, L.A.; Tryfonidou, M.A.; Meij, B.P.; Vernooij, J.C.M.; Bergknut, N.; Grinwis, G.C.M. The Myth of Fibroid Degeneration in the Canine Intervertebral Disc: A Histopathological Comparison of Intervertebral Disc Degeneration in Chondrodystrophic and Nonchondrodystrophic Dogs. Vet. Pathol. 2017, 54, 945–952. [Google Scholar] [CrossRef]

| Types | Questions |
|---|---|
| Questions with a 10-point scale |
|
| |
| |
| |
| |
| |
| |
| |
| |
|
| Baseline Characteristics | CXB-PEAMs (n = 20) | Unloaded PEAMs (Placebo) (n = 10) | |
|---|---|---|---|
| Sex | |||
| Male | n (%) | 10 (50) | 8 (80) |
| Female | n (%) | 10 (50) | 2 (20) |
| Weight (kg) | mean (sd) | 27.7 (7.1) | 29 (9.9) |
| Age (years) | median (IQR) | 5.1 (2.9−6.2) | 4.3 (2.2−6.2) |
| Dose (µL/kg) | median (IQR) | 3.15 (2.9−3.3) | 3.1 (2.8−3.3) |
| Purpose | |||
| companion | n (%) | 13 (65) | 4 (40) |
| sports/service dog | n (%) | 7 (35) | 6 (60) |
| Baseline questionnaire sumscore | median (range) | 33.5 (23−56) | 47.5 (28−55) |
| MRI t = 0 | |||
| Pfirrmann grade | |||
| 2 | n (%) | 10 (50) | 4 (40) |
| 3 | n (%) | 3 (15) | 6 (60) |
| 4 | n (%) | 7 (35) | 0 (0) |
| Disc protrusion | |||
| <25% | n (%) | 14 (70) | 5 (50) |
| ≥25% | n (%) | 6 (30) | 5 (50) |
| Ligamentum flavum bulging | n (%) | 6 (30) | 4 (40) |
| Modic changes | n (%) | 8 (40) | 1 (10) |
| Spinal nerve swelling | n (%) | 4 (20) | 2 (20) |
| Intervertebral foramen stenosis | n (%) | 8 (40) | 4 (40) |
| Facet joint osteoarthrosis | n (%) | 5 (25) | 2 (20) |
| Disc Height Index | median (IQR) | 0.25 (0.24−0.28) | 0.25 (0.24−0.27) |
| FPA t = 0 | |||
| P/T Fz+ | mean (sd) | 0.61 (0.06) | 0.57 (0.06) |
| P/T Fy+ | mean (sd) | 0.55 (0.09) | 0.5 (0.09) |
| P/T Fy− | mean (sd) | 0.78 (0.13) | 0.77 (0.23) |
| CXB-PEAMs | Unloaded PEAMs (Placebo) | |||
|---|---|---|---|---|
| ∆questionnaire Sumscore | Median (Range) | Median (Range) | ||
| 0–6 weeks | + 9 (−2–+20) * | +3 (−18–+7) | ||
| 0–12 weeks | + 12 (−6–+25) # | −1 (−11–+14) | ||
| Dependency on oral pain medication | N, dogs | % | N, dogs | % |
| 0–6 weeks | 5 | 25 | 2 | 20 |
| 0–12 weeks | 4 | 20 | 2 | 20 |
| Subgroup | CXB–PEAMs | Unloaded PEAMS (Placebo) Median (Range) | ||
|---|---|---|---|---|
| Median (Range) | ||||
| Sex | Male | Female | Male | Female |
| 0–6 weeks | +15 (4–+20) * | +3 (−2–+14) | 4 (−1–+7) * | −18 (−18) ^ |
| 0–12 weeks | +15 (+3–+25) * | +7(−6–+24) | 1 (−7–+14) * | −11 (−11) ^ |
| Disc Protrusion | <25% | ≥25% | <25% | ≥25% |
| 0–6 weeks | +9 (−2–+20) | +9 (+3–+19) | +3 (−1–+7) | +5 (−18–+7) |
| 0–12 weeks | +7 (−6–+25) | +15 (+12–+16) | −1 (−7–+6) | +1 (−11–+14) |
| Modic Changes | no Modic | Modic I–III | no Modic | Modic I–III |
| 0–6 weeks | +9 (+2–+20) # | +8.5 (−2–+20) | +4 (−1–+7) # | −18 (−18) ^ |
| 0–12 weeks | +12 (−4–+25) # | +9.5 (−6–+24) | +1 (−7–+14) # | −11 (−11) ^ |
| CXB-PEAMs | Unloaded PEAMs (Placebo) | ||||
|---|---|---|---|---|---|
| Mean | SD | Mean | SD | p-Value (*) | |
| Change 0–6 weeks | |||||
| ∆P/T Fz+ | −0.016 | 0.06 | 0.005 | 0.025 | 0.306 |
| ∆P/T Fy+ | −0.027 | 0.082 | 0.001 | 0.062 | 0.366 |
| ∆P/T Fy− | 0.003 | 0.14 | −0.049 | 0.19 | 0.418 |
| Change 0–12 weeks | |||||
| ∆P/T Fz+ | −0.023 | 0.043 | 0.003 | 0.024 | 0.101 |
| ∆P/T Fy+ | −0.017 | 0.084 | −0.004 | 0.032 | 0.671 |
| ∆P/T Fy− | −0.056 | 0.16 | −0.044 | 0.11 | 0.838 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wiersema, T.; Tellegen, A.R.; Beukers, M.; van Stralen, M.; Wouters, E.; van de Vooren, M.; Woike, N.; Mihov, G.; Thies, J.C.; Creemers, L.B.; et al. Prospective Evaluation of Local Sustained Release of Celecoxib in Dogs with Low Back Pain. Pharmaceutics 2021, 13, 1178. https://doi.org/10.3390/pharmaceutics13081178
Wiersema T, Tellegen AR, Beukers M, van Stralen M, Wouters E, van de Vooren M, Woike N, Mihov G, Thies JC, Creemers LB, et al. Prospective Evaluation of Local Sustained Release of Celecoxib in Dogs with Low Back Pain. Pharmaceutics. 2021; 13(8):1178. https://doi.org/10.3390/pharmaceutics13081178
Chicago/Turabian StyleWiersema, Tijn, Anna R. Tellegen, Martijn Beukers, Marijn van Stralen, Erik Wouters, Mandy van de Vooren, Nina Woike, George Mihov, Jens C. Thies, Laura B. Creemers, and et al. 2021. "Prospective Evaluation of Local Sustained Release of Celecoxib in Dogs with Low Back Pain" Pharmaceutics 13, no. 8: 1178. https://doi.org/10.3390/pharmaceutics13081178
APA StyleWiersema, T., Tellegen, A. R., Beukers, M., van Stralen, M., Wouters, E., van de Vooren, M., Woike, N., Mihov, G., Thies, J. C., Creemers, L. B., Tryfonidou, M. A., & Meij, B. P. (2021). Prospective Evaluation of Local Sustained Release of Celecoxib in Dogs with Low Back Pain. Pharmaceutics, 13(8), 1178. https://doi.org/10.3390/pharmaceutics13081178







