RETRACTED: Development and Optimization of Luliconazole Spanlastics to Augment the Antifungal Activity against Candida albicans
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Experimental Design
2.3. Preparation of Luliconazole Spanlastics
2.4. Particle Size Measurement
2.5. Optimization of Luliconazole Spanlastics
2.6. TEM Investigation of the Optimized Luliconazole Splanlastics Formula
2.7. Assessment of Antifungal Activity of the Optimized Luliconazole Spanlastics
2.7.1. In Vitro Antifungal Susceptibility Testing
2.7.2. Animals
2.7.3. Candida and Culture Conditions
2.7.4. Animal Preparation and Cutaneous Infections
2.7.5. Treatment of the Infection
2.7.6. Evaluation of Infection
3. Results and Discussion
3.1. Particle Size Results and Model Fit Statistical Analysis
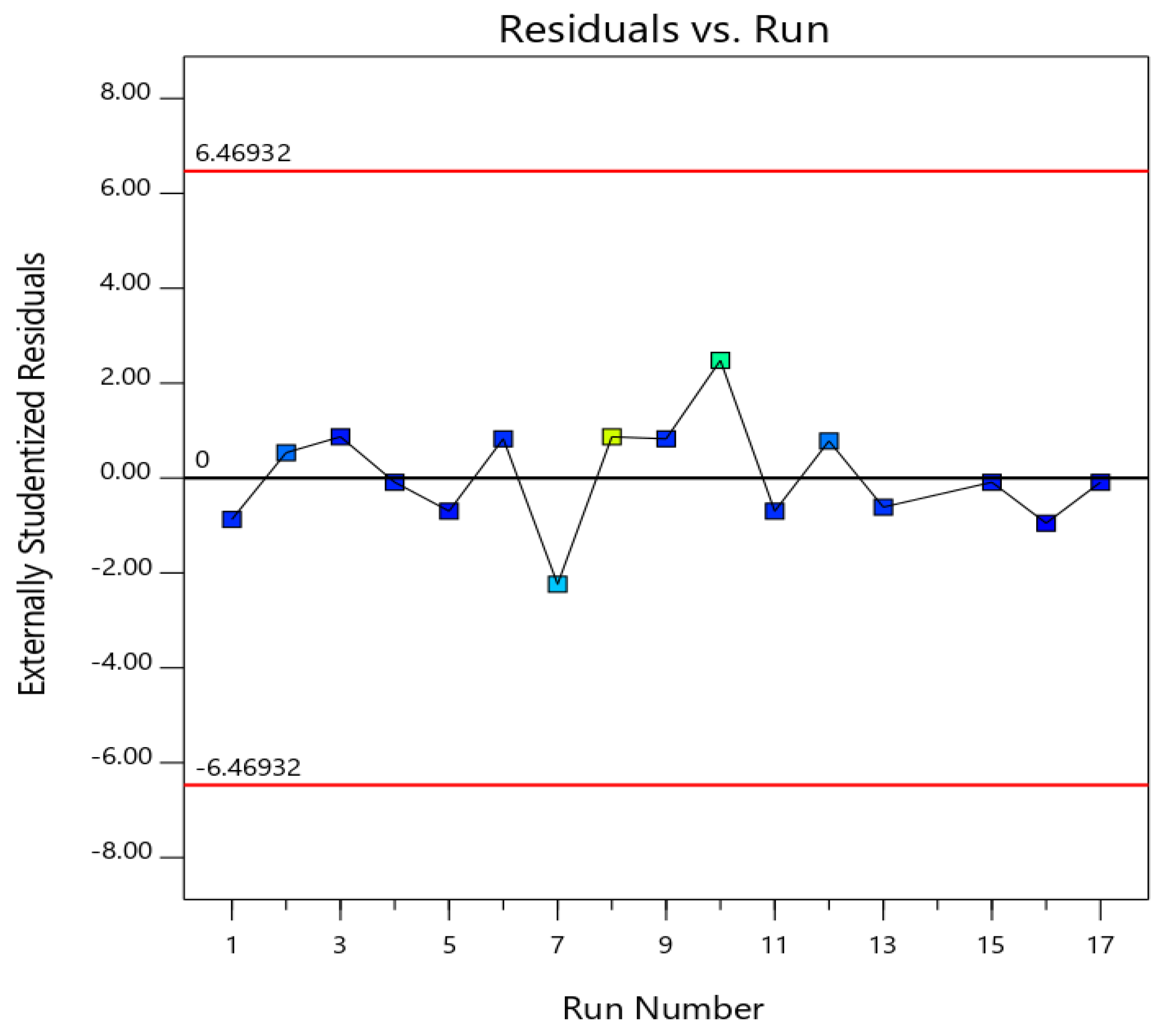
3.2. Diagnostics for the Validity of the Quadratic Model
3.3. Influence of Variables on Particle Size (PS, Y1)
3.4. Optimization Using a Numerical Approach
3.5. Transmission Electron Microscope Investigation of the Optimized Luliconazole Spanlastics
3.6. In Vitro Antifungal Susceptibility Testing
3.7. In Vivo Study
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Khanna, D.; Bharti, S. Luliconazole for the treatment of fungal infections: An evidence-based review. Core Evid. 2014, 9, 113–124. [Google Scholar] [CrossRef] [PubMed]
- Gharaghani, M.; Hivary, S.; Taghipour, S.; Zarei-Mahmoudabadi, A. Luliconazole, a highly effective imidazole, against Fusarium species complexes. Med. Microbiol. Immunol. 2020, 209, 603–612. [Google Scholar] [CrossRef]
- Koga, H.; Nanjoh, Y.; Makimura, K.; Tsuboi, R. In vitro antifungal activities of luliconazole, a new topical imidazole. Med. Mycol. 2009, 47, 640–647. [Google Scholar] [CrossRef]
- FDA. Cder LUZU (luliconazole) Cream, 1%. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/appletter/2013/204153Orig1s000ltr.pdf (accessed on 28 April 2021).
- Kapileshwari, G.R.; Barve, A.R.; Kumar, L.; Bhide, P.J.; Joshi, M.; Shirodkar, R.K. Novel drug delivery system of luliconazole—Formulation and characterisation. J. Drug Deliv. Sci. Technol. 2020, 55, 101302. [Google Scholar] [CrossRef]
- Raz-Pasteur, A.; Ullmann, Y.; Berdicevsky, I. The Pathogenesis of Candida Infections in a Human Skin Model: Scanning Electron Microscope Observations. ISRN Dermatol. 2011, 2011, 150642. [Google Scholar] [CrossRef]
- Kim, J.; Sudbery, P. Candida albicans, a major human fungal pathogen. J. Microbiol. 2011, 49, 171–177. [Google Scholar] [CrossRef]
- Oxman, D.A.; Chow, J.K.; Frendl, G.; Hadley, S.; Hershkovitz, S.; Ireland, P.; McDermott, L.A.; Tsai, K.; Marty, F.M.; Kontoyiannis, D.P.; et al. Candidaemia associated with decreased in vitro fluconazole susceptibility: Is Candida speciation predictive of the susceptibility pattern? J. Antimicrob. Chemother. 2010, 65, 1460–1465. [Google Scholar] [CrossRef]
- Lortholary, O.; Desnos-Ollivier, M.; Sitbon, K.; Fontanet, A.; Bretagne, S.; Dromer, F.; Bouges-Michel, C.; Poilane, I.; Dunan, J.; Galeazzi, G.; et al. Recent Exposure to Caspofungin or Fluconazole Influences the Epidemiology of Candidemia: A Prospective Multicenter Study Involving 2,441 Patients. Antimicrob. Agents Chemother. 2010, 55, 532–538. [Google Scholar] [CrossRef] [PubMed]
- Marichal, P.; Koymans, L.; Willemsens, S.; Bellens, D.; Verhasselt, P.; Luyten, W.; Borgers, M.; Ramaekers, F.C.S.; Odds, F.C.; Bossche, H. Vanden Contribution of mutations in the cytochrome P450 14α-demethylase (Erg11p, Cyp51p) to azole resistance in Candida albicans. Microbiology 1999, 145, 2701–2713. [Google Scholar] [CrossRef]
- MacPherson, S.; Akache, B.; Weber, S.; De Deken, X.; Raymond, M.; Turcotte, B. Candida albicans Zinc Cluster Protein Upc2p Confers Resistance to Antifungal Drugs and Is an Activator of Ergosterol Biosynthetic Genes. Antimicrob. Agents Chemother. 2005, 49, 2070–2083. [Google Scholar] [CrossRef]
- Coste, A.T.; Karababa, M.; Ischer, F.; Bille, J.; Sanglard, D. TAC1, Transcriptional Activator of CDR Genes, Is a New Transcription Factor Involved in the Regulation of Candida albicans ABC Transporters CDR1 and CDR2. Eukaryot. Cell 2004, 3, 1639–1652. [Google Scholar] [CrossRef] [PubMed]
- Haggag, Y.; Abdel-Wahab, Y.; Ojo, O.; Osman, M.; El-Gizawy, S.; El-Tanani, M.; Faheem, A.; McCarron, P. Preparation and in vivo evaluation of insulin-loaded biodegradable nanoparticles prepared from diblock copolymers of PLGA and PEG. Int. J. Pharm. 2016, 499, 236–246. [Google Scholar] [CrossRef] [PubMed]
- Papageorgiou, D.; Kinloch, I.A.; Young, R.J. Mechanical properties of graphene and graphene-based nanocomposites. Prog. Mater. Sci. 2017, 90, 75–127. [Google Scholar] [CrossRef]
- Fahmy, U.A.; Badr-Eldin, S.M.; Ahmed, O.A.A.; Aldawsari, H.M.; Tima, S.; Asfour, H.Z.; Al-Rabia, M.W.; Negm, A.A.; Sultan, M.H.; Madkhali, O.A.A.; et al. Intranasal Niosomal In Situ Gel as a Promising Approach for Enhancing Flibanserin Bioavailability and Brain Delivery: In Vitro Optimization and Ex Vivo/In Vivo Evaluation. Pharmaceutics 2020, 12, 485. [Google Scholar] [CrossRef]
- Fahmy, U.A.; Ahmed, O.A.; Badr-Eldin, S.M.; Aldawsari, H.M.; Okbazghi, S.Z.; Awan, Z.A.; Bakhrebah, M.A.; Alomary, M.N.; Abdulaal, W.H.; Medina, C.; et al. Optimized Nanostructured Lipid Carriers Integrated into In Situ Nasal Gel for Enhancing Brain Delivery of Flibanserin. Int. J. Nanomed. 2020, 15, 5253–5264. [Google Scholar] [CrossRef]
- Alhakamy, N.; Badr-Eldin, S.; Fahmy, U.A.; Alruwaili, N.; Awan, Z.; Caruso, G.; Alfaleh, M.; Alaofi, A.; Arif, F.; Ahmed, O.; et al. Thymoquinone-Loaded Soy-Phospholipid-Based Phytosomes Exhibit Anticancer Potential against Human Lung Cancer Cells. Pharmaceutics 2020, 12, 761. [Google Scholar] [CrossRef]
- Baghel, S.; Nair, V.S.; Pirani, A.; Sravani, A.B.; Bhemisetty, B.; Ananthamurthy, K.; Aranjani, J.M.; Lewis, S.A. Luliconazole-loaded nanostructured lipid carriers for topical treatment of superficial Tinea infections. Dermatol. Ther. 2020, 33, e13959. [Google Scholar] [CrossRef] [PubMed]
- Kaur, M.; Singh, K.; Jain, S.K. Luliconazole vesicular based gel formulations for its enhanced topical delivery. J. Liposome Res. 2020, 30, 388–406. [Google Scholar] [CrossRef]
- Kakkar, S.; Kaur, I.P. Spanlastics—A novel nanovesicular carrier system for ocular delivery. Int. J. Pharm. 2011, 413, 202–210. [Google Scholar] [CrossRef]
- Elsherif, N.I.; Shamma, R.N.; Abdelbary, G. Terbinafine Hydrochloride Trans-ungual Delivery via Nanovesicular Systems: In Vitro Characterization and Ex Vivo Evaluation. AAPS PharmSciTech 2016, 18, 551–562. [Google Scholar] [CrossRef]
- Angulo, I.; Jiménez-Díaz, M.B.; García-Bustos, J.F.; Gargallo, D.; Heras, F.G.D.L.; Muñoz-Fernández, M.Á.; Fresno, M. Candida albicans infection enhances immunosuppression induced by cyclophosphamide by selective priming of suppressive myeloid progenitors for NO production. Cell. Immunol. 2002, 218, 46–58. [Google Scholar] [CrossRef]
- Ahmed, O.A.A.; Badr-Eldin, S.M. In situ misemgel as a multifunctional dual-absorption platform for nasal delivery of raloxifene hydrochloride: Formulation, characterization, and in vivo performance. Int. J. Nanomed. 2018, 13, 6325–6335. [Google Scholar] [CrossRef]
- Anderson, M.J.; Whitcomb, P.J. Designing Experiments that Combine Mixture Components with Process Factors Apply powerful statistical tools to optimize your formula while simultaneously finding the peak process parameters. Chem. Eng. Prog. 2000, 96, 27–32. [Google Scholar]
- Piepel, G.; Pasquini, B.; Cooley, S.; Heredia-Langner, A.; Orlandini, S.; Furlanetto, S. Mixture-process variable approach to optimize a microemulsion electrokinetic chromatography method for the quality control of a nutraceutical based on coenzyme Q10. Talanta 2012, 97, 73–82. [Google Scholar] [CrossRef]
- Ahmed, O.A.A.; El-Say, K.M.; Aljaeid, B.M.; Shaimaa, M.B.-E.; Ahmed, T.A. Optimized vinpocetine-loaded vitamin E D-α-tocopherol polyethylene glycol 1000 succinate-alpha lipoic acid micelles as a potential transdermal drug delivery system: In vitro and ex vivo studies. Int. J. Nanomed. 2018, 14, 33–43. [Google Scholar] [CrossRef] [PubMed]
- Elizondo, E.; Moreno, E.; Cabrera, I.; Córdoba, A.; Sala, S.; Veciana, J.; Ventosa, N. Liposomes and other vesicular systems: Structural characteristics, methods of preparation, and use in nanomedicine. In Progress in Molecular Biology and Translational Science; Elsevier B.V.: Amsterdam, The Netherlands, 2011; Volume 104, pp. 1–52. [Google Scholar]
- Dovigo, L.N.; Carmello, J.C.; De Souza Costa, C.A.; Vergani, C.E.; Brunetti, I.L.; Bagnato, V.S.; Pavarina, A.C. Curcumin-mediated photodynamic inactivation of Candida albicans in a murine model of oral candidiasis. Med. Mycol. 2013, 51, 243–251. [Google Scholar] [CrossRef]
- Bhattacharyya, A.; Sinha, M.; Singh, H.; Patel, R.S.; Ghosh, S.; Sardana, K.; Ghosh, S.; Sengupta, S. Mechanistic Insight Into the Antifungal Effects of a Fatty Acid Derivative Against Drug-Resistant Fungal Infections. Front. Microbiol. 2020, 11, 2116. [Google Scholar] [CrossRef]
- Badria, F.; Mazyed, E. Formulation of Nanospanlastics as a Promising Approach for Improving the Topical Delivery of a Natural Leukotriene Inhibitor (3-Acetyl-11-Keto-β-Boswellic Acid): Statistical Optimization, in vitro Characterization, and ex vivo Permeation Study. Drug Des. Dev. Ther. 2020, 14, 3697–3721. [Google Scholar] [CrossRef] [PubMed]




| Mixture Components | Lower Level | Upper Level |
|---|---|---|
| A: Span 20 percentage | 10 | 90 |
| B: Tween 20 percentage | 10 | 90 |
| Process Variable | ||
| C: Sonication time (min) | 0 | 10 |
| Responses | Desirability Constraints | |
| Y: Particle size (PS, nm) | Minimize | |
| Run No. | Mixture Components | Process Variables | Particle Size (nm) | |
|---|---|---|---|---|
| A | B | C | ||
| 1 | 90 | 10 | 10 | 307.6 |
| 2 | 10 | 90 | 10 | 701.9 |
| 3 | 50 | 50 | 0 | 198.0 |
| 4 | 10 | 90 | 5 | 256.2 |
| 5 | 30 | 70 | 2.5 | 166.1 |
| 6 | 30 | 70 | 7.5 | 330.7 |
| 7 | 90 | 10 | 0 | 1204.0 |
| 8 | 10 | 90 | 0 | 4174.0 |
| 9 | 70 | 30 | 7.5 | 324.1 |
| 10 | 90 | 10 | 0 | 2130.0 |
| 11 | 70 | 30 | 2.5 | 327.5 |
| 12 | 90 | 10 | 10 | 771.5 |
| 13 | 10 | 90 | 10 | 367.6 |
| 14 | 30 | 70 | 0 | 5891.0 |
| 15 | 90 | 10 | 5 | 218.2 |
| 16 | 50 | 50 | 10 | 60.8 |
| 17 | 50 | 50 | 5 | 143.6 |
| Sample No. | Treatment (groups) | Number of Animals | Log CFU Infected Site |
|---|---|---|---|
| 1 | Control | 5 | 0 |
| 2 | Untreated | 5 | 14 ± 2.2 |
| 3 | Luliconazole | 5 | 3.1 ± 0.2 |
| 4 | Luliconazole spanlastics | 5 | 0.2 ± 0.05 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alhakamy, N.A.; Al-Rabia, M.W.; Md, S.; Sirwi, A.; Khayat, S.S.; AlOtaibi, S.S.; Hakami, R.A.; Al Sadoun, H.; Eldakhakhny, B.M.; Abdulaal, W.H.; et al. RETRACTED: Development and Optimization of Luliconazole Spanlastics to Augment the Antifungal Activity against Candida albicans. Pharmaceutics 2021, 13, 977. https://doi.org/10.3390/pharmaceutics13070977
Alhakamy NA, Al-Rabia MW, Md S, Sirwi A, Khayat SS, AlOtaibi SS, Hakami RA, Al Sadoun H, Eldakhakhny BM, Abdulaal WH, et al. RETRACTED: Development and Optimization of Luliconazole Spanlastics to Augment the Antifungal Activity against Candida albicans. Pharmaceutics. 2021; 13(7):977. https://doi.org/10.3390/pharmaceutics13070977
Chicago/Turabian StyleAlhakamy, Nabil A., Mohammed W. Al-Rabia, Shadab Md, Alaa Sirwi, Selwan Saud Khayat, Sahar Saad AlOtaibi, Raghad Abkar Hakami, Hadeel Al Sadoun, Basmah Medhat Eldakhakhny, Wesam H. Abdulaal, and et al. 2021. "RETRACTED: Development and Optimization of Luliconazole Spanlastics to Augment the Antifungal Activity against Candida albicans" Pharmaceutics 13, no. 7: 977. https://doi.org/10.3390/pharmaceutics13070977
APA StyleAlhakamy, N. A., Al-Rabia, M. W., Md, S., Sirwi, A., Khayat, S. S., AlOtaibi, S. S., Hakami, R. A., Al Sadoun, H., Eldakhakhny, B. M., Abdulaal, W. H., Aldawsari, H. M., Badr-Eldin, S. M., & Elfaky, M. A. (2021). RETRACTED: Development and Optimization of Luliconazole Spanlastics to Augment the Antifungal Activity against Candida albicans. Pharmaceutics, 13(7), 977. https://doi.org/10.3390/pharmaceutics13070977











