Biomaterials for the Prevention of Oral Candidiasis Development
Abstract
1. Introduction
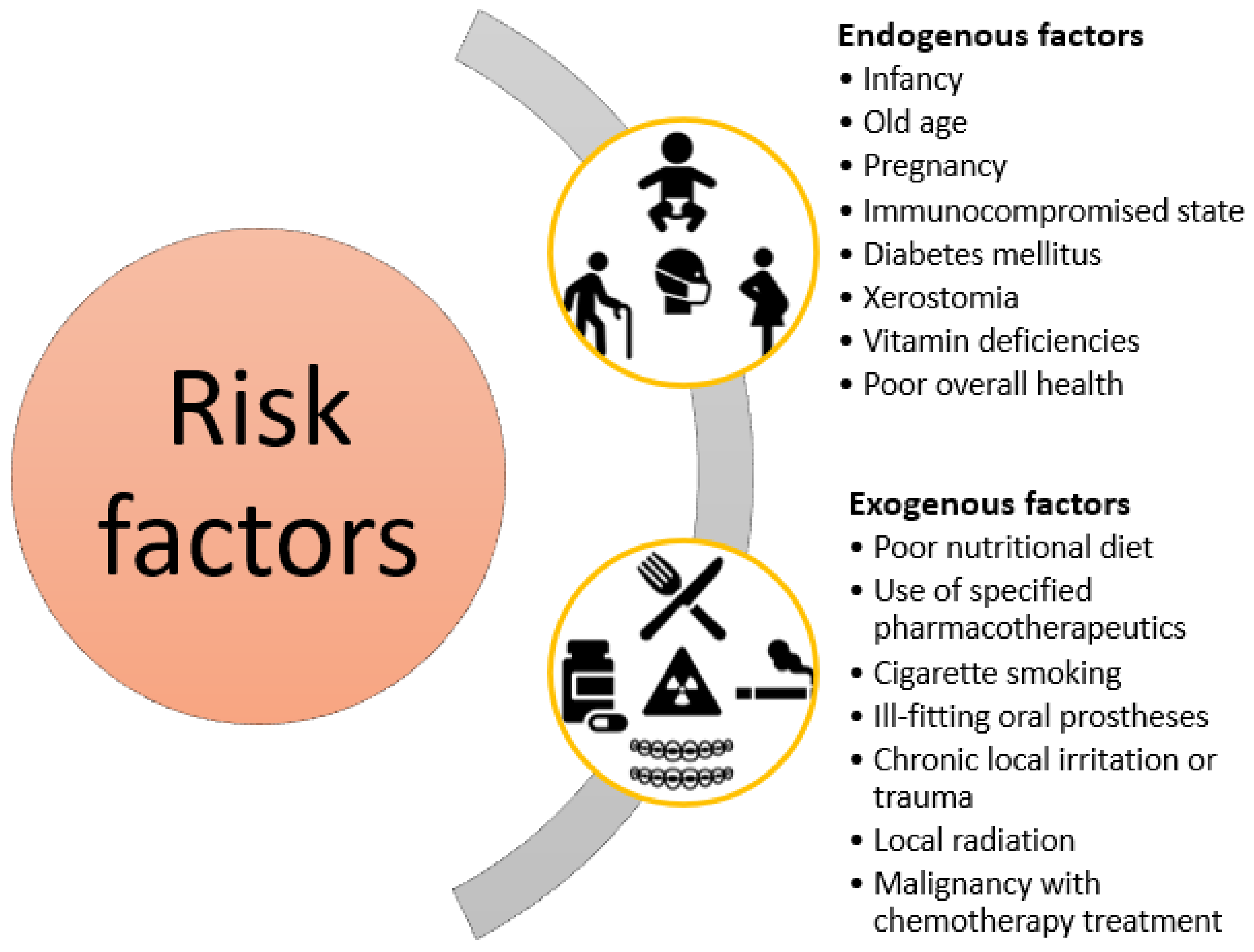
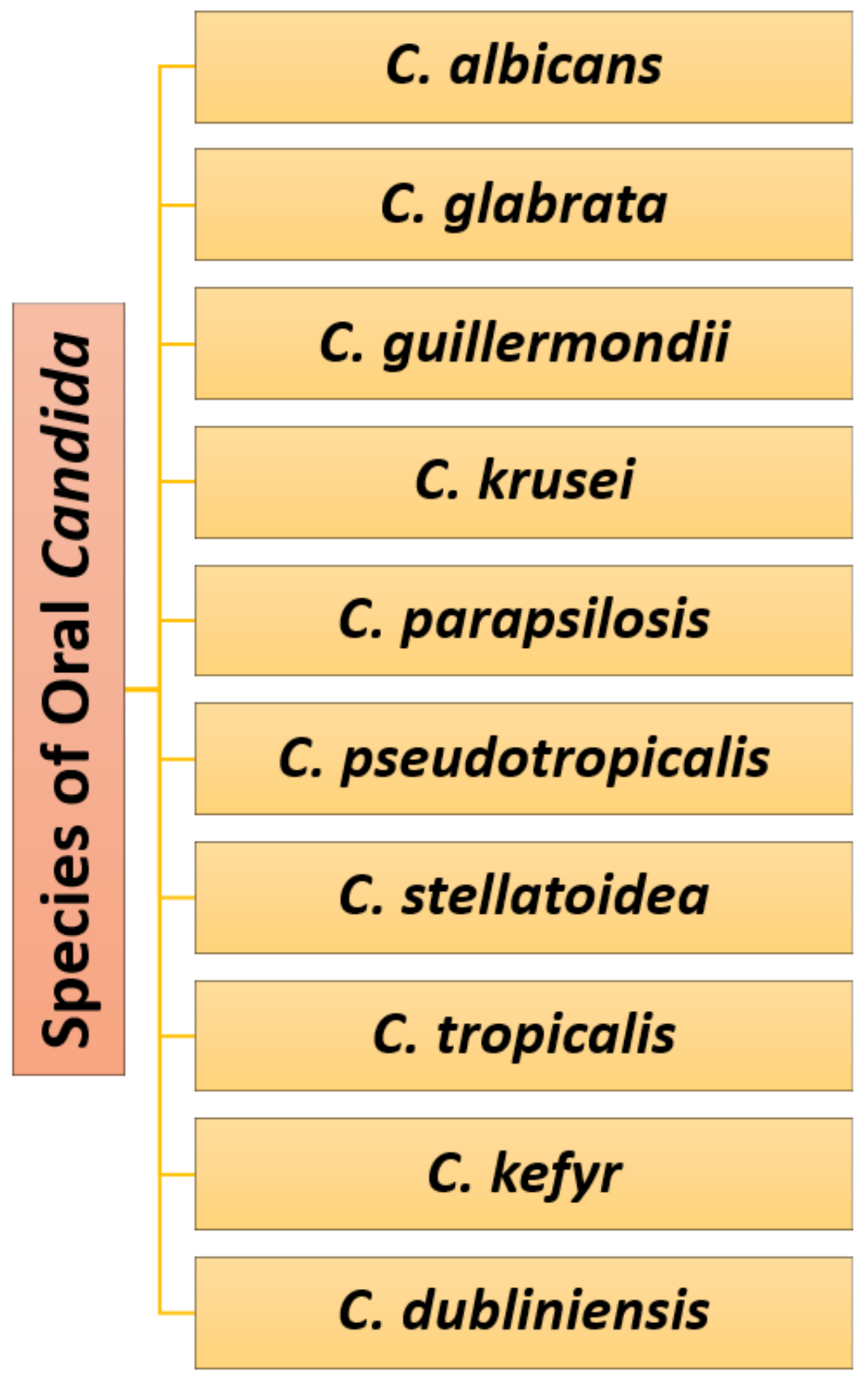
2. Causative Pathogens and Risk Factors
3. Classic Treatment Options
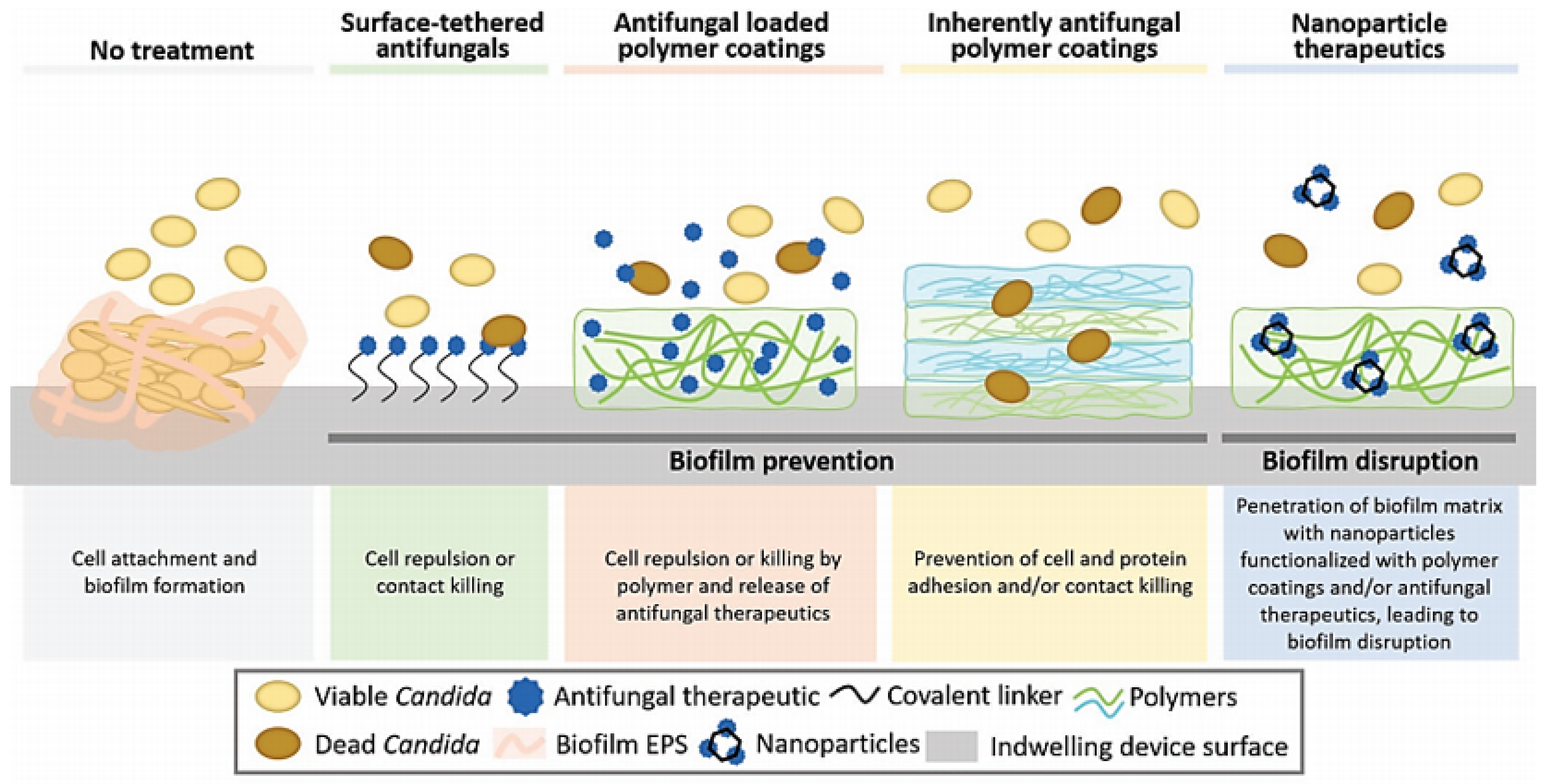
4. Novel Treatment Options
4.1. Intrinsic Anti-Candida Biomaterials/Compounds
4.1.1. Polymeric Materials
4.1.2. Inorganic Nanoparticles
4.1.3. Natural Products
4.2. Biomaterials for Oral Prosthesis and Denture Adhesives
4.3. Drug Delivery Systems
4.4. Combined Approaches
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Arweiler, N.B.; Netuschil, L. The Oral Microbiota. In Microbiota of the Human Body: Implications in Health and Disease; Schwiertz, A., Ed.; Springer International Publishing: Cham, Switzerland, 2016; pp. 45–60. [Google Scholar] [CrossRef]
- Coll, P.P.; Lindsay, A.; Meng, J.; Gopalakrishna, A.; Raghavendra, S.; Bysani, P.; O’Brien, D. The Prevention of Infections in Older Adults: Oral Health. J. Am. Geriatr. Soc. 2020, 68, 411–416. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Kim, J.-G.; Lee, H.; Lee, T.H.; Kim, K.-Y.; Kim, H. Antifungal Activity of 1,4-Dialkoxynaphthalen-2-Acyl Imidazolium Salts by Inducing Apoptosis of Pathogenic Candida spp. Pharmaceutics 2021, 13, 312. [Google Scholar] [CrossRef]
- Volkova, M.; Atamas, A.; Tsarenko, A.; Rogachev, A.; Guskov, A. Cation Transporters of Candida albicans—New Targets to Fight Candidiasis? Biomolecules 2021, 11, 584. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Verma, R.; Murari, A.; Agrawal, A. Oral candidiasis: An overview. J. Oral Maxillofac. Pathol. 2014, 18, S81–S85. [Google Scholar] [CrossRef] [PubMed]
- Sharon, V.; Fazel, N. Oral candidiasis and angular cheilitis. Dermatol. Ther. 2010, 23, 230–242. [Google Scholar] [CrossRef]
- Muñoz, J.E.; Rossi, D.C.P.; Jabes, D.L.; Barbosa, D.A.; Cunha, F.F.M.; Nunes, L.R.; Arruda, D.C.; Pelleschi Taborda, C. In Vitro and In Vivo Inhibitory Activity of Limonene against Different Isolates of Candida spp. J. Fungi 2020, 6, 183. [Google Scholar] [CrossRef]
- Tonglairoum, P.; Ngawhirunpat, T.; Rojanarata, T.; Kaomongkolgit, R.; Opanasopit, P. Fabrication of a novel scaffold of clotrimazole-microemulsion-containing nanofibers using an electrospinning process for oral candidiasis applications. Colloids Surf. B Biointerfaces 2015, 126, 18–25. [Google Scholar] [CrossRef]
- Siqueira, A.B.; Rodriguez, L.R.; Santos, R.K.; Marinho, R.R.; Abreu, S.; Peixoto, R.F.; Gurgel, B.C. Antifungal activity of propolis against Candida species isolated from cases of chronic periodontitis. Braz. Oral Res. 2015, 29. [Google Scholar] [CrossRef]
- Dangi, Y.S.; Soni, M.L.; Namdeo, K.P. Oral candidiasis: A review. Int. J. Pharm. Pharm. Sci. 2010, 2, 36–41. [Google Scholar]
- Kong, E.F.; Tsui, C.; Boyce, H.; Ibrahim, A.; Hoag, S.W.; Karlsson, A.J.; Meiller, T.F.; Jabra-Rizk, M.A. Development and In Vivo Evaluation of a Novel Histatin-5 Bioadhesive Hydrogel Formulation against Oral Candidiasis. Antimicrob. Agents Chemother. 2016, 60, 881. [Google Scholar] [CrossRef]
- Arastehfar, A.; Carvalho, A.; Nguyen, M.H.; Hedayati, M.T.; Netea, M.G.; Perlin, D.S.; Hoenigl, M. COVID-19-Associated Candidiasis (CAC): An Underestimated Complication in the Absence of Immunological Predispositions? J. Fungi 2020, 6, 211. [Google Scholar] [CrossRef]
- Dutta, T.; Ghosh, N.N.; Das, M.; Adhikary, R.; Mandal, V.; Chattopadhyay, A.P. Green synthesis of antibacterial and antifungal silver nanoparticles using Citrus limetta peel extract: Experimental and theoretical studies. J. Environ. Chem. Eng. 2020, 8, 104019. [Google Scholar] [CrossRef]
- Muadcheingka, T.; Tantivitayakul, P. Distribution of Candida albicans and non-albicans Candida species in oral candidiasis patients: Correlation between cell surface hydrophobicity and biofilm forming activities. Arch. Oral Biol. 2015, 60, 894–901. [Google Scholar] [CrossRef]
- Khan, A.A.; Alanazi, A.M.; Alsaif, N.; Algrain, N.; Wani, T.A.; Bhat, M.A. Enhanced Efficacy of Thiosemicarbazone Derivative-Encapsulated Fibrin Liposomes against Candidiasis in Murine Model. Pharmaceutics 2021, 13, 333. [Google Scholar] [CrossRef]
- Ficanha, A.M.M.; Antunes, A.; Oro, C.E.D.; Dallago, R.M.; Mignoni, M.L. Immobilization of Candida antarctica B (CALB) in Silica Aerogel: Morphological Characteristics and Stability. Biointerface Res. Appl. Chem. 2020, 10, 6744–6756. [Google Scholar] [CrossRef]
- Wiench, R.; Skaba, D.; Matys, J.; Grzech-Leśniak, K. Efficacy of Toluidine Blue—Mediated Antimicrobial Photodynamic Therapy on Candida spp. A Systematic Review. Antibiotics 2021, 10, 349. [Google Scholar] [CrossRef]
- Roozbehani, N.; Golfeshan, F.; Pakshir, K.; Doorandishan, M.; Jassbi, A.R.; Mosaddad, S.A. Chemical Composition and Effectiveness of Ocimum basilicum L. Extracts on the Adhesion of Candida albicans and C. dubliniensis on Acrylic Surfaces of Removable Orthodontic Appliances. Biointerface Res. Appl. Chem. 2021, 11, 9477–9489. [Google Scholar] [CrossRef]
- Quindós, G.; Gil-Alonso, S.; Marcos-Arias, C.; Sevillano, E.; Mateo, E.; Jauregizar, N.; Eraso, E. Therapeutic tools for oral candidiasis: Current and new antifungal drugs. Med. Oral Patol. Oral Cir. Bucal 2019, 24, e172–e180. [Google Scholar] [CrossRef]
- Jung, J.; Li, L.; Yeh, C.-K.; Ren, X.; Sun, Y. Amphiphilic quaternary ammonium chitosan/sodium alginate multilayer coatings kill fungal cells and inhibit fungal biofilm on dental biomaterials. Mater. Sci. Eng. C 2019, 104, 109961. [Google Scholar] [CrossRef]
- Vera-González, N.; Shukla, A. Advances in Biomaterials for the Prevention and Disruption of Candida Biofilms. Front. Microbiol. 2020, 11, 2251. [Google Scholar] [CrossRef]
- Namangkalakul, W.; Benjavongkulchai, S.; Pochana, T.; Promchai, A.; Satitviboon, W.; Howattanapanich, S.; Phuprasong, R.; Ungvijanpunya, N.; Supakanjanakanti, D.; Chaitrakoonthong, T.; et al. Activity of chitosan antifungal denture adhesive against common Candida species and Candida albicans adherence on denture base acrylic resin. J. Prosthet. Dent. 2020, 123, 181.e1–181.e7. [Google Scholar] [CrossRef]
- Okonogi, S.; Phumat, P.; Khongkhunthian, S.; Suttiat, K.; Chaijareenont, P. Denture-Soaking Solution Containing Piper betle Extract-Loaded Polymeric Micelles; Inhibition of Candida albicans, Clinical Study, and Effects on Denture Base Resin. Antibiotics 2021, 10, 440. [Google Scholar] [CrossRef]
- Lamfon, H.A. Denture Biofilm and Dentureassociated Stomatitis, A Literature Review. Egypt. Dent. J. 2021, 67, 775–787. [Google Scholar] [CrossRef]
- Takamiya, A.S.; Monteiro, D.R.; Gorup, L.F.; Silva, E.A.; de Camargo, E.R.; Gomes-Filho, J.E.; de Oliveira, S.H.P.; Barbosa, D.B. Biocompatible silver nanoparticles incorporated in acrylic resin for dental application inhibit Candida albicans biofilm. Mater. Sci. Eng. C 2021, 118, 111341. [Google Scholar] [CrossRef]
- Tejada, G.; Barrera, M.G.; García, P.; Sortino, M.; Lamas, M.C.; Lassalle, V.; Alvarez, V.; Leonardi, D. Nanoparticulated Systems Based on Natural Polymers Loaded with Miconazole Nitrate and Lidocaine for the Treatment of Topical Candidiasis. AAPS PharmSciTech 2020, 21, 278. [Google Scholar] [CrossRef]
- Cuéllar-Cruz, M.; Vega-González, A.; Mendoza-Novelo, B.; López-Romero, E.; Ruiz-Baca, E.; Quintanar-Escorza, M.A.; Villagómez-Castro, J.C. The effect of biomaterials and antifungals on biofilm formation by Candida species: A review. Eur. J. Clin. Microbiol. Infect. Dis. 2012, 31, 2513–2527. [Google Scholar] [CrossRef]
- Muhvić-Urek, M.; Saltović, E.; Braut, A.; Kovačević Pavičić, D. Association between Vitamin D and Candida-Associated Denture Stomatitis. Dent. J. 2020, 8, 121. [Google Scholar] [CrossRef]
- Aslani, N.; Janbabaei, G.; Abastabar, M.; Meis, J.F.; Babaeian, M.; Khodavaisy, S.; Boekhout, T.; Badali, H. Identification of uncommon oral yeasts from cancer patients by MALDI-TOF mass spectrometry. BMC Infect. Dis. 2018, 18, 24. [Google Scholar] [CrossRef]
- Coronado-Castellote, L.; Jiménez-Soriano, Y. Clinical and microbiological diagnosis of oral candidiasis. J. Clin. Exp. Dent. 2013, 5, e279–e286. [Google Scholar] [CrossRef]
- Colombo, A.L.; de Almeida Júnior, J.N.; Slavin, M.A.; Chen, S.C.A.; Sorrell, T.C. Candida and invasive mould diseases in non-neutropenic critically ill patients and patients with haematological cancer. Lancet Infect. Dis. 2017, 17, e344–e356. [Google Scholar] [CrossRef]
- Reichart, P.A.; Samaranayake, L.P.; Philipsen, H.P. Pathology and clinical correlates in oral candidiasis and its variants: A review. Oral Dis. 2000, 6, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Araujo, V.H.S.; Duarte, J.L.; Carvalho, G.C.; Silvestre, A.L.P.; Fonseca-Santos, B.; Marena, G.D.; Ribeiro, T.D.C.; dos Santos Ramos, M.A.; Bauab, T.M.; Chorilli, M. Nanosystems against candidiasis: A review of studies performed over the last two decades. Crit. Rev. Microbiol. 2020, 46, 508–547. [Google Scholar] [CrossRef] [PubMed]
- Abraham, C.M. Advances and emerging techniques in the identification, diagnosis and treatment of oral candidiasis. Open Pathol. J. 2011, 5. [Google Scholar] [CrossRef]
- Kofla, G.; Ruhnke, M. Pharmacology and metabolism of anidulafungin, caspofungin and micafungin in the treatment of invasive candidosis—Review of the literature. Eur. J. Med. Res. 2011, 16, 159. [Google Scholar] [CrossRef]
- Garcia-Cuesta, C.; Sarrion-Pérez, M.-G.; Bagán, J.V. Current treatment of oral candidiasis: A literature review. J. Clin. Exp. Dent. 2014, 6, e576–e582. [Google Scholar] [CrossRef]
- Williams, D.; Lewis, M. Pathogenesis and treatment of oral candidosis. J. Oral Microbiol. 2011, 3, 5771. [Google Scholar] [CrossRef]
- Lyu, X.; Zhao, C.; Yan, Z.-M.; Hua, H. Efficacy of nystatin for the treatment of oral candidiasis: A systematic review and meta-analysis. Drug Des. Dev. Ther. 2016, 10, 1161–1171. [Google Scholar] [CrossRef]
- Govindarajan, A.; Bistas, K.G.; Aboeed, A. Fluconazole; StatPearls Publishing: Treasure Island, FL, USA, 2020. [Google Scholar]
- Manik, A.; Bahl, R. A review on oral candidal infection. J. Adv. Med. Dent. Sci. Res. 2017, 5, 54. [Google Scholar]
- Vigneswaran, N.; Muller, S. Pharmacologic Management of Oral Mucosal Inflammatory and Ulcerative Diseases. In Contemporary Dental Pharmacology: Evidence-Based Considerations; Jeske, A.H., Ed.; Springer International Publishing: Cham, Switzerland, 2019; pp. 91–108. [Google Scholar] [CrossRef]
- Hengge, U.R.; Ruzicka, T.; Schwartz, R.A.; Cork, M.J. Adverse effects of topical glucocorticosteroids. J. Am. Acad. Dermatol. 2006, 54, 1–15. [Google Scholar] [CrossRef]
- Levêque, D.; Nivoix, Y.; Jehl, F.; Herbrecht, R. Clinical pharmacokinetics of voriconazole. Int. J. Antimicrob. Agents 2006, 27, 274–284. [Google Scholar] [CrossRef]
- Levine, M.T.; Chandrasekar, P.H. Adverse effects of voriconazole: Over a decade of use. Clin. Transplant. 2016, 30, 1377–1386. [Google Scholar] [CrossRef]
- Torres, H.A.; Hachem, R.Y.; Chemaly, R.F.; Kontoyiannis, D.P.; Raad, I.I. Posaconazole: A broad-spectrum triazole antifungal. Lancet Infect. Dis. 2005, 5, 775–785. [Google Scholar] [CrossRef]
- Roilides, E.; Carlesse, F.; Tawadrous, M.; Leister-Tebbe, H.; Conte, U.; Raber, S.; Swanson, R.; Yan, J.L.; Aram, J.A.; Queiroz-Telles, F.; et al. Safety, Efficacy and Pharmacokinetics of Anidulafungin in Patients 1 Month to <2 Years of Age with Invasive Candidiasis, Including Candidemia. Pediatr. Infect. Dis. J. 2020, 39, 305–309. [Google Scholar] [CrossRef]
- Glöckner, A. Treatment and prophylaxis of invasive candidiasis with anidulafungin, caspofungin and micafungin—Review of the literature. Eur. J. Med. Res. 2011, 16, 167. [Google Scholar] [CrossRef]
- Ahmad, N.; Jafri, Z.; Khan, Z.H. Evaluation of nanomaterials to prevent oral Candidiasis in PMMA based denture wearing patients. A systematic analysis. J. Oral Biol. Craniofac. Res. 2020, 10, 189–193. [Google Scholar] [CrossRef]
- Antunes, D.P.; Salvia, A.C.R.D.; de Araújo, R.M.; Di Nicoló, R.; Koga Ito, C.Y.; de Araujo, M.A.M. Effect of green tea extract and mouthwash without alcohol on Candida albicans biofilm on acrylic resin. Gerodontology 2015, 32, 291–295. [Google Scholar] [CrossRef]
- Paulone, S.; Malavasi, G.; Ardizzoni, A.; Orsi, C.F.; Peppoloni, S.; Neglia, R.G.; Blasi, E. Candida albicans survival, growth and biofilm formation are differently affected by mouthwashes: An in vitro study. New Microbiol. 2017, 40, 45–52. [Google Scholar]
- Vila, T.; Sultan, A.S.; Montelongo-Jauregui, D.; Jabra-Rizk, M.A. Oral Candidiasis: A Disease of Opportunity. J. Fungi 2020, 6, 15. [Google Scholar] [CrossRef]
- Spirescu, V.A.; Chircov, C.; Grumezescu, A.M.; Andronescu, E. Polymeric Nanoparticles for Antimicrobial Therapies: An up-to-date Overview. Polymers 2021, 13, 724. [Google Scholar] [CrossRef]
- Cheraghipour, K.; Ezatpour, B.; Masoori, L.; Marzban, A.; Sepahvand, A.; Rouzbahani, A.K.; Moridnia, A.; Khanizadeh, S.; Mahmoudvand, H. Anti-Candida activity of Curcumin: A systematic review. Curr. Drug Discov. Technol. 2021, 18, 379–390. [Google Scholar] [CrossRef]
- Meireles, A.B.; Corrêa, D.K.; da Silveira, J.V.W.; Millás, A.L.G.; Bittencourt, E.; de Brito-Melo, G.E.A.; González-Torres, L.A. Trends in polymeric electrospun fibers and their use as oral biomaterials. Exp. Biol. Med. 2018, 243, 665–676. [Google Scholar] [CrossRef]
- Fakhri, E.; Eslami, H.; Maroufi, P.; Pakdel, F.; Taghizadeh, S.; Ganbarov, K.; Yousefi, M.; Tanomand, A.; Yousefi, B.; Mahmoudi, S.; et al. Chitosan biomaterials application in dentistry. Int. J. Biol. Macromol. 2020, 162, 956–974. [Google Scholar] [CrossRef]
- Malviya, R. Exploration of neem gum-chitosan and kheri gum-chitosan polyelectrolyte complex based film for transdermal delivery of protein/peptide. Biointerface Res. Appl. Chem. 2020, 10, 5860–5868. [Google Scholar] [CrossRef]
- Tajdini, K.; Shakeri, A.; Naijian, F. Nanocomposite hydrogel of chitosan-g-poly acrylamide/nanoclay: Effect of degree of cross-linking on their swelling. Lett. Appl. NanoBioSci. 2020, 9, 995–999. [Google Scholar] [CrossRef]
- Srimaneepong, V.; Thanamee, T.; Wattanasirmkit, K.; Muangsawat, S.; Matangkasombut, O. Efficacy of low-molecular weight chitosan against Candida albicans biofilm on polymethyl methacrylate resin. Aust. Dent. J. 2021. [Google Scholar] [CrossRef]
- Ikono, R.; Vibriani, A.; Wibowo, I.; Saputro, K.E.; Muliawan, W.; Bachtiar, B.M.; Mardliyati, E.; Bachtiar, E.W.; Rochman, N.T.; Kagami, H.; et al. Nanochitosan antimicrobial activity against Streptococcus mutans and Candida albicans dual-species biofilms. BMC Res. Notes 2019, 12, 383. [Google Scholar] [CrossRef]
- Liu, R.; Chen, X.; Falk, S.P.; Mowery, B.P.; Karlsson, A.J.; Weisblum, B.; Palecek, S.P.; Masters, K.S.; Gellman, S.H. Structure–Activity Relationships among Antifungal Nylon-3 Polymers: Identification of Materials Active against Drug-Resistant Strains of Candida albicans. J. Am. Chem. Soc. 2014, 136, 4333–4342. [Google Scholar] [CrossRef]
- Velazco-Medel, M.A.; Camacho-Cruz, L.A.; Lugo-Gonzalez, J.C.; Bucio, E. Antifungal polymers for medical applications. Med. Devices Sens. 2020, 4. [Google Scholar] [CrossRef]
- Rank, L.A.; Walsh, N.M.; Liu, R.; Lim, F.Y.; Bok, J.W.; Huang, M.; Keller, N.P.; Gellman, S.H.; Hull, C.M. A Cationic Polymer That Shows High Antifungal Activity against Diverse Human Pathogens. Antimicrob. Agents Chemother. 2017, 61. [Google Scholar] [CrossRef] [PubMed]
- Rank, L.A.; Walsh, N.M.; Lim, F.Y.; Gellman, S.H.; Keller, N.P.; Hull, C.M. Peptide-like nylon-3 polymers with activity against phylogenetically diverse, intrinsically drug-resistant pathogenic fungi. mSphere 2018, 3. [Google Scholar] [CrossRef]
- Martini Garcia, I.; Becker Rodrigues, S.; Rodrigues Gama, M.E.; Branco Leitune, V.C.; Melo, M.A.; Mezzomo Collares, F. Guanidine derivative inhibits C. albicans biofilm growth on denture liner without promote loss of materials’ resistance. Bioact. Mater. 2020, 5. [Google Scholar] [CrossRef]
- Dias, F.G.G.; Pereira, L.d.F.; Parreira, R.L.T.; Veneziani, R.C.S.; Bianchi, T.C.; Fontes, V.F.N.D.P.; Galvani, M.D.C.; Cerce, D.D.P.; Martins, C.H.G.; Rinaldi-Neto, F.; et al. Evaluation of the antiseptic and wound healing potential of polyhexamethylene guanidine hydrochloride as well as its toxic effects. Eur. J. Pharm. Sci. 2021, 160, 105739. [Google Scholar] [CrossRef] [PubMed]
- Gama, M.E.R.; Leitune, V.C.B.; Garcia, I.M.; Rodrigues, S.B.; Collares, F.M. Evaluation of guanidine antifungal solutions for denture base resin: An in vitro study. Rev. Fac. Odontol. Porto Alegre 2020, 61. [Google Scholar] [CrossRef]
- Choi, H.; Kim, K.-J.; Lee, D.G. Antifungal activity of the cationic antimicrobial polymer-polyhexamethylene guanidine hydrochloride and its mode of action. Fungal Biol. 2017, 121, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Husain, Q. An overview on the green synthesis of nanoparticles and other nano-materials using enzymes and their potential applications. Biointerface Res. Appl. Chem. 2019, 9, 4255–4271. [Google Scholar] [CrossRef]
- Ratna Geetika, G.; Raji, P.; Bennet Rohan, D.; Divya Kumar, M.; Kripu Sharma, V.; Keerthana, D.; Karishma, S.; Iyappan, P.; Thirumurugan, R.; Samrot, A.V.; et al. Green synthesis and antibacterial activity of silver nanoparticles from the aqueous extracts of Cassia alata. Lett. Appl. NanoBioSci. 2020, 9, 1037–1041. [Google Scholar] [CrossRef]
- Gherasim, O.; Puiu, R.A.; Bîrcă, A.C.; Burdușel, A.-C.; Grumezescu, A.M. An Updated Review on Silver Nanoparticles in Biomedicine. Nanomaterials 2020, 10, 2318. [Google Scholar] [CrossRef]
- Burdușel, A.-C.; Gherasim, O.; Grumezescu, A.M.; Mogoantă, L.; Ficai, A.; Andronescu, E. Biomedical Applications of Silver Nanoparticles: An Up-to-Date Overview. Nanomaterials 2018, 8, 681. [Google Scholar] [CrossRef]
- Fajar, M.N.; Endarko, E.; Rubiyanto, A.; Malek, N.; Hadibarata, T.; Syafiuddin, A. A green deposition method of silver nanoparticles on textiles and their antifungal activity. Biointerface Res. Appl. Chem. 2019, 10, 4902–4907. [Google Scholar]
- Gupta, K.; Chundawat, T.S.; Malek, N.A.N.N. Antibacterial, Antifungal, Photocatalytic Activities and Seed Germination Effect of Mycosynthesized Silver Nanoparticles using Fusarium oxysporum. Biointerface Res. Appl. Chem. 2020, 11, 12082–12091. [Google Scholar] [CrossRef]
- Ratnasari, A.; Endarko, E.; Syafiuddin, A. A Green Method for the Enhancement of Antifungal Properties of Various Textiles Functionalized with Silver Nanoparticles. Biointerface Res. Appl. Chem. 2020, 10, 7284–7294. [Google Scholar] [CrossRef]
- Thiruvengadam, V.; Bansod, A.V. Characterization of Silver Nanoparticles Synthesized using Chemical Method and its Antibacterial Property. Biointerface Res. Appl. Chem. 2020, 10, 7257–7264. [Google Scholar] [CrossRef]
- Geetanjali; Sharma, P.K.; Malviya, R. Toxicity and application of nano-silver in multi-drug resistant therapy. Lett. Appl. NanoBioSci. 2020, 9, 824–829. [Google Scholar] [CrossRef]
- Pathak, J.; Sonker, A.S.; Rajneesh, V.S.; Kumar, D.; Sinha, R.P. Synthesis of silver nanoparticles from extracts of Scytonema geitleri HKAR-12 and their in vitro antibacterial and antitumor potentials. Lett. Appl. NanoBioSci. 2019, 8, 576–585. [Google Scholar] [CrossRef]
- Xue, B.; He, D.; Gao, S.; Wang, D.; Yokoyama, K.; Wang, L. Biosynthesis of silver nanoparticles by the fungus Arthroderma fulvum and its antifungal activity against genera of Candida, Aspergillus and Fusarium. Int. J. Nanomed. 2016, 11, 1899. [Google Scholar]
- Tyagi, P.K.; Mishra, R.; Khan, F.; Gupta, D.; Gola, D. Antifungal Effects of Silver Nanoparticles Against Various Plant Pathogenic Fungi and its Safety Evaluation on Drosophila melanogaster. Biointerface Res. Appl. Chem. 2020, 10, 6587–6596. [Google Scholar] [CrossRef]
- Hashim, T.; Risan, M.H.; Kadhom, M.; Raheem, R.; Yousif, E. Antifungal, Antiviral, and Antibacterial Activities of Silver Nanoparticles Synthesized Using Fungi: A Review. Lett. Appl. NanoBioSci. 2020, 9, 1307–1312. [Google Scholar] [CrossRef]
- Monteiro, D.R.; Gorup, L.F.; Silva, S.; Negri, M.; de Camargo, E.R.; Oliveira, R.; Barbosa, D.B.; Henriques, M. Silver colloidal nanoparticles: Antifungal effect against adhered cells and biofilms of Candida albicans and Candida glabrata. Biofouling 2011, 27, 711–719. [Google Scholar] [CrossRef]
- Kanchi, S.; Inamuddin; Khan, A. Biogenic Synthesis of Selenium Nanoparticles with Edible Mushroom Extract: Evaluation of Cytotoxicity on Prostate Cancer Cell Lines and Their Antioxidant, and Antibacterial Activity. Biointerface Res. Appl. Chem. 2020, 10, 6629–6639. [Google Scholar] [CrossRef]
- Shakibaie, M.; Mohazab, N.S.; Mousavi, S.A.A. Antifungal Activity of Selenium Nanoparticles Synthesized by Bacillus species Msh-1 Against Aspergillus fumigatus and Candida albicans. Jundishapur J. Microbiol. 2015, 8. [Google Scholar] [CrossRef]
- Guisbiers, G.; Lara, H.; Mendoza-Cruz, R.; Naranjo, G.; Vincent, B.A.; Peralta, X.G.; Nash, K.L. Inhibition of Candida albicans biofilm by pure selenium nanoparticles synthesized by pulsed laser ablation in liquids. Nanomedicine 2017, 13, 1095–1103. [Google Scholar] [CrossRef]
- Parveen, S.; Wani, A.H.; Shah, M.A.; Devi, H.S.; Bhat, M.Y.; Koka, J.A. Preparation, characterization and antifungal activity of iron oxide nanoparticles. Microb. Pathog. 2018, 115, 287–292. [Google Scholar] [CrossRef]
- Seddighi, N.S.; Salari, S.; Izadi, A.R. Evaluation of antifungal effect of iron-oxide nanoparticles against different Candida species. IET Nanobiotechnol. 2017, 11, 883–888. [Google Scholar] [CrossRef]
- Sangaiya, P.; Jayaprakash, R. A Review on Iron Oxide Nanoparticles and Their Biomedical Applications. J. Supercond. Nov. Magn. 2018, 31, 3397–3413. [Google Scholar] [CrossRef]
- Samrot, A.V.; Sahithya, C.S.; Sruthi, P.D.; Selvarani, A.J.; Raji, P.; Prakash, P.; Ponnaiah, P.; Petchi, I.; Pattammadath, S.; Purayil, S.K.; et al. Itraconazole Coated Super Paramagnetic Iron Oxide Nanoparticles for Antimicrobial Studies. Biointerface Res. Appl. Chem. 2020, 10, 6262–6269. [Google Scholar] [CrossRef]
- Lipovsky, A.; Nitzan, Y.; Gedanken, A.; Lubart, R. Antifungal activity of ZnO nanoparticles—The role of ROS mediated cell injury. Nanotechnology 2011, 22, 105101. [Google Scholar] [CrossRef]
- Pillai, A.M.; Sivasankarapillai, V.S.; Rahdar, A.; Joseph, J.; Sadeghfar, F.; Anuf A, R.; Rajesh, K.; Kyzas, G.Z. Green synthesis and characterization of zinc oxide nanoparticles with antibacterial and antifungal activity. J. Mol. Struct. 2020, 1211, 128107. [Google Scholar] [CrossRef]
- Jamdagni, P.; Khatri, P.; Rana, J.S. Green synthesis of zinc oxide nanoparticles using flower extract of Nyctanthes arbor-tristis and their antifungal activity. J. King Saud Univ. Sci. 2018, 30, 168–175. [Google Scholar] [CrossRef]
- Souza, J.M.T.; de Araújo, A.R.; de Carvalho, A.M.A.; Amorim, A.D.G.N.; Daboit, T.C.; de Almeida, J.R.D.S.; da Silva, D.A.; Eaton, P. Sustainably produced cashew gum-capped zinc oxide nanoparticles show antifungal activity against Candida parapsilosis. J. Clean. Prod. 2020, 247, 119085. [Google Scholar] [CrossRef]
- Spirescu, V.A.; Chircov, C.; Grumezescu, A.M.; Vasile, B.Ș.; Andronescu, E. Inorganic Nanoparticles and Composite Films for Antimicrobial Therapies. Int. J. Mol. Sci. 2021, 22, 4595. [Google Scholar] [CrossRef] [PubMed]
- Sawai, J.; Yoshikawa, T. Quantitative evaluation of antifungal activity of metallic oxide powders (MgO, CaO and ZnO) by an indirect conductimetric assay. J. Appl. Microbiol. 2004, 96, 803–809. [Google Scholar] [CrossRef] [PubMed]
- Kong, F.; Wang, J.; Han, R.; Ji, S.; Yue, J.; Wang, Y.; Ma, L. Antifungal Activity of Magnesium Oxide Nanoparticles: Effect on the Growth and Key Virulence Factors of Candida albicans. Mycopathologia 2020, 185, 485–494. [Google Scholar] [CrossRef] [PubMed]
- Amrulloh, H.; Fatiqin, A.; Simanjuntak, W.; Afriyani, H.; Annissa, A. Bioactivities of nano-scale magnesium oxide prepared using aqueous extract of Moringa oleifera leaves as green agent. Adv. Nat. Sci. Nanosci. Nanotechnol. 2021, 12, 015006. [Google Scholar] [CrossRef]
- Kamboj, A.; Amjad, M.; Ahmad, W.; Singh, A. A general survey on Green synthesis and application of calcium oxide nanoparticles. Int. J. Health Clin. Res. 2020, 3, 41–48. [Google Scholar]
- Amiri, M.; Etemadifar, Z.; Daneshkazemi, A.; Nateghi, M. Antimicrobial Effect of Copper Oxide Nanoparticles on Some Oral Bacteria and Candida Species. J. Dent. Biomater. 2017, 4, 347–352. [Google Scholar]
- Imani, M.M.; Safaei, M.; Moradpoor, H.; Rezaei, R.; Golshah, A.; Rezaei, F.; Jamshidy, L. Optimum synthesis of CuO nanoparticles with the highest antifungal activity against oral pathogen Candida albicans. J. Appl. Pharm. Sci. 2020, 10, 21–25. [Google Scholar]
- Padmavathi, A.R.; Murthy, S.P.; Das, A.; Priya, A.; Sushmitha, T.J.; Pandian, S.K.; Toleti, S.R. Impediment to growth and yeast-to-hyphae transition in Candida albicans by copper oxide nanoparticles. Biofouling 2020, 36, 56–72. [Google Scholar] [CrossRef]
- Dizaj, S.M.; Lotfipour, F.; Barzegar-Jalali, M.; Zarrintan, M.H.; Adibkia, K. Antimicrobial activity of the metals and metal oxide nanoparticles. Mater. Sci. Eng. C 2014, 44, 278–284. [Google Scholar] [CrossRef]
- Haghighi, F.; Mohammadi, S.R.; Mohammadi, P.; Eskandari, M.; Hosseinkhani, S. The evaluation of Candida albicans biofilms formation on silicone catheter, PVC and glass coated with titanium dioxide nanoparticles by XTT method and ATPase assay. Bratisl. Lek. Listy 2012, 113, 707–711. [Google Scholar] [CrossRef]
- Ahmad, N.S.; Abdullah, N.; Yasin, F.M. Antifungal activity of titanium dioxide nanoparticles against Candida albicans. BioResources 2019, 14, 8866–8878. [Google Scholar]
- Haghighi, F.; Roudbar Mohammadi, S.; Mohammadi, P.; Hosseinkhani, S.; Shipour, R. Antifungal activity of TiO2 nanoparticles and EDTA on Candida albicans biofilms. Infect. Epidemiol. Microbiol. 2013, 1, 33–38. [Google Scholar]
- Hernandez-Delgadillo, R.; Velasco-Arias, D.; Martinez-Sanmiguel, J.J.; Diaz, D.; Zumeta-Dube, I.; Arevalo-Niño, K.; Cabral-Romero, C. Bismuth oxide aqueous colloidal nanoparticles inhibit Candida albicans growth and biofilm formation. Int. J. Nanomed. 2013, 8, 1645–1652. [Google Scholar] [CrossRef]
- El-Batal, A.I.; El-Sayyad, G.S.; El-Ghamry, A.; Agaypi, K.M.; Elsayed, M.A.; Gobara, M. Melanin-gamma rays assistants for bismuth oxide nanoparticles synthesis at room temperature for enhancing antimicrobial, and photocatalytic activity. J. Photochem. Photobiol. B Biol. 2017, 173, 120–139. [Google Scholar] [CrossRef]
- Haq, S.; Rehman, W.; Waseem, M.; Meynen, V.; Awan, S.U.; Saeed, S.; Iqbal, N. Fabrication of pure and moxifloxacin functionalized silver oxide nanoparticles for photocatalytic and antimicrobial activity. J. Photochem. Photobiol. B Biol. 2018, 186, 116–124. [Google Scholar] [CrossRef]
- Mohammed, H.B.; Rayyif, S.M.I.; Curutiu, C.; Birca, A.C.; Oprea, O.-C.; Grumezescu, A.M.; Ditu, L.-M.; Gheorghe, I.; Chifiriuc, M.C.; Mihaescu, G.; et al. Eugenol-Functionalized Magnetite Nanoparticles Modulate Virulence and Persistence in Pseudomonas aeruginosa Clinical Strains. Molecules 2021, 26, 2189. [Google Scholar] [CrossRef]
- Holban, A.M.; Grumezescu, A.M.; Ficai, A.; Chifiriuc, C.M.; Lazar, V.; Radulescu, R. Fe3O4@C18-Carvone to Prevent Candida Tropicalis Biofilm Development. Rev. Romana Mater. 2013, 43, 300. [Google Scholar]
- Nuță, D.C.; Limban, C.; Chiriță, C.; Chifiriuc, M.C.; Costea, T.; Ioniță, P.; Nicolau, I.; Zarafu, I. Contribution of Essential Oils to the Fight against Microbial Biofilms—A Review. Processes 2021, 9, 537. [Google Scholar] [CrossRef]
- Miao, Q.; Zhao, L.; Wang, Y.; Hao, F.; Sun, P.; He, P.; Liu, Y.; Huang, J.; Liu, X.; Liu, X.; et al. Microbial metabolomics and network analysis reveal fungistatic effect of basil (Ocimum basilicum) oil on Candida albicans. J. Ethnopharmacol. 2020, 260, 113002. [Google Scholar] [CrossRef]
- Waskito, H.; Apriasari, M.L.; Utami, J.P. Antifungal Effect of Mauli Banana Stem Extract, Basil Leaf Extract, And their Combination on Candida albicans. Dent. J. Kedokt. Gigi 2020, 5, 179–184. [Google Scholar] [CrossRef]
- Sugio, C.Y.C.; Mengoa, M.G.R.; Gomes, A.C.G.; Garcia, A.A.M.N.; de Oliveira, T.M.; Hermana, K. Use of Natural Products in the Prevention and Treatment of Denture Stomatitis. Open Access J. Biomed. Sci. 2020, 1, 201–206. [Google Scholar]
- Masłowski, M.; Miedzianowska, J.; Czylkowska, A.; Strzelec, K. Horsetail (Equisetum arvense) as a Functional Filler for Natural Rubber Biocomposites. Materials 2020, 13, 2526. [Google Scholar] [CrossRef]
- Briceño-Cardona, K.L.; Romero, C.C.; Delgadillo, R.H.; Galindo-Rodríguez, S.A.; Solís-Soto, J.M. Equisetum extracts are anti-inflammatory and antibacterial, an oral potential therapeutic agent. Int. J. Appl. Dent. Sci. 2021, 7, 480–482. [Google Scholar]
- Martins Almeida, N.L.; Saldanha, L.L.; Alves da Silva, R.; Pinke, K.H.; da Costa, E.F.; Porto, V.C.; Dokkedal, A.L.; Soares Lara, V. Antimicrobial activity of denture adhesive associated with Equisetum giganteum-and Punica granatum-enriched fractions against Candida albicans biofilms on acrylic resin surfaces. Biofouling 2018, 34, 62–73. [Google Scholar] [CrossRef]
- Trifan, A.; Bostănaru, A.-C.; Luca, S.V.; Grădinaru, A.C.; Jităreanu, A.; Aprotosoaie, A.C.; Miron, A.; Cioancă, O.; Hăncianu, M.; Ochiuz, L. Antifungal potential of Pimpinella anisum, Carum carvi and Coriandrum sativum extracts. A comparative study with focus on the phenolic composition. Farmacia 2020, 68, 22–27. [Google Scholar] [CrossRef]
- Silva, F.; Domeño, C.; Domingues, F.C. Chapter 35—Coriandrum sativum L.: Characterization, Biological Activities, and Applications. In Nuts and Seeds in Health and Disease Prevention, 2nd ed.; Preedy, V.R., Watson, R.R., Eds.; Academic Press: Cambridge, MA, USA, 2020; pp. 497–519. [Google Scholar] [CrossRef]
- Furletti, V.F.; Teixeira, I.P.; Obando-Pereda, G.; Mardegan, R.C.; Sartoratto, A.; Figueira, G.M.; Duarte, R.M.T.; Rehder, V.L.G.; Duarte, M.C.T.; Höfling, J.F. Action of Coriandrum sativum L. Essential Oil upon Oral Candida albicans Biofilm Formation. Evid. Based Complement. Altern. Med. 2011, 2011, 985832. [Google Scholar] [CrossRef]
- Ashyuce, S.; Bereli, N.; Topcu, A.; Ramteke, P.W.; Denizli, A. Indian saffron—Turmeric (Curcuma longa) embedded supermacroporous cryogel discs for heavy metal removal. Biointerface Res. Appl. Chem. 2019, 9, 4356–4361. [Google Scholar] [CrossRef]
- Costa Normando, A.G.; Gomes de Meneses, A.; Porto de Toledo, I.; Alvares Borges, G.; Lourenco de Lima, C.; Diniz Dos Reis, P.E.; Silva Guerra, E.N. Effects of turmeric and curcumin on oral mucositis: A systematic review. Phytother. Res. 2019, 33, 1318–1329. [Google Scholar] [CrossRef]
- Adamczak, A.; Ożarowski, M.; Karpiński, T.M. Curcumin, a Natural Antimicrobial Agent with Strain-Specific Activity. Pharmaceuticals 2020, 13, 153. [Google Scholar] [CrossRef] [PubMed]
- Raduly, F.M.; Raditoiu, V.; Raditoiu, A.; Purcar, V. Curcumin: Modern Applications for a Versatile Additive. Coatings 2021, 11, 519. [Google Scholar] [CrossRef]
- Mohammadian, M.; Moghaddam, A.D.; Sharifan, A.; Dabaghi, P.; Hadi, S. Different forms of whey protein aggregates as curcumin delivery systems: Evaluation of free radical scavenging activity and drug release kinetics. Biointerface Res. Appl. Chem. 2020, 10, 5490–5495. [Google Scholar] [CrossRef]
- Narayanan, V.S.; Muddaiah, S.; Shashidara, R.; Sudheendra, U.S.; Deepthi, N.C.; Samaranayake, L. Variable antifungal activity of curcumin against planktonic and biofilm phase of different candida species. Indian J. Dent. Res. 2020, 31, 145. [Google Scholar] [CrossRef]
- Soto-Chilaca, G.A.; Mejia-Garibay, B.; Navarro-Amador, R.; Ramirez-Corona, N.; Palou, E.; Lopez-Malo, A. Cinnamaldehyde-loaded chitosan nanoparticles: Characterization and antimicrobial activity. Biointerface Res. Appl. Chem. 2019, 9, 4060–4065. [Google Scholar] [CrossRef]
- Yanakiev, S. Effects of Cinnamon (Cinnamomum spp.) in Dentistry: A Review. Molecules 2020, 25, 4184. [Google Scholar] [CrossRef]
- Ranasinghe, P.; Pigera, S.; Premakumara, G.A.S.; Galappaththy, P.; Constantine, G.R.; Katulanda, P. Medicinal properties of ‘true’ cinnamon (Cinnamomum zeylanicum): A systematic review. BMC Complement. Altern. Med. 2013, 13, 275. [Google Scholar] [CrossRef]
- Jafri, H.; Ansari, F.A.; Ahmad, I. Chapter 9—Prospects of Essential Oils in Controlling Pathogenic Biofilm. In New Look to Phytomedicine; Ahmad Khan, M.S., Ahmad, I., Chattopadhyay, D., Eds.; Academic Press: Cambridge, MA, USA, 2019; pp. 203–236. [Google Scholar] [CrossRef]
- Shahidi Noghabi, M.; Molaveisi, M. The effect of wall formulation on storage stability and physicochemical properties of cinnamon essential oil microencapsulated by spray drying. Chem. Pap. 2020, 74, 3455–3465. [Google Scholar] [CrossRef]
- De Araújo, M.R.C.; Maciel, P.P.; Castellano, L.R.C.; Bonan, P.R.F.; Alves, D.D.N.; de Medeiros, A.C.D.; de Castro, R.D. Efficacy of essential oil of cinnamon for the treatment of oral candidiasis: A randomized trial. Spec. Care Dent. 2021, 41, 349–357. [Google Scholar] [CrossRef]
- Ota, C.; Unterkircher, C.; Fantinato, V.; Shimizu, M.T. Antifungal activity of propolis on different species of Candida. Mycoses 2001, 44, 375–378. [Google Scholar] [CrossRef]
- Kumar, D.; Ayesha, M.J.; Gautam, P.; Joshi, H.; Kumar, N. A Recent Report on ‘Plants with Anti-Candida Properties’. Int. J. Curr. Res. Rev. 2020, 12, 25. [Google Scholar] [CrossRef]
- De Oliveira, S.G.D.; Martos, J.; de Carvalho, R.V.; de Pereira, C.M.P.; Lund, R.G.; Piva, E. Retentive efficacy, antimicrobial and cytotoxicity comparisons between different types of commercial and experimental denture adhesives with antifungal action. Dent. Mater. J. 2021, 2020–2262. [Google Scholar] [CrossRef]
- Karlsson, A.J.; Flessner, R.M.; Gellman, S.H.; Lynn, D.M.; Palecek, S.P. Polyelectrolyte multilayers fabricated from antifungal β-peptides: Design of surfaces that exhibit antifungal activity against Candida albicans. Biomacromolecules 2010, 11, 2321–2328. [Google Scholar] [CrossRef]
- Ramasamy, M.; Lee, J. Recent Nanotechnology Approaches for Prevention and Treatment of Biofilm-Associated Infections on Medical Devices. BioMed Res. Int. 2016, 2016, 1851242. [Google Scholar] [CrossRef]
- Ali Sabri, B.; Satgunam, M.; Abreeza, N.M.; Abed, A.N. A review on enhancements of PMMA Denture Base Material with Different Nano-Fillers. Cogent Eng. 2021, 8, 1875968. [Google Scholar] [CrossRef]
- Cierech, M.; Kolenda, A.; Grudniak, A.M.; Wojnarowicz, J.; Woźniak, B.; Gołaś, M.; Swoboda-Kopeć, E.; Łojkowski, W.; Mierzwińska-Nastalska, E. Significance of polymethylmethacrylate (PMMA) modification by zinc oxide nanoparticles for fungal biofilm formation. Int. J. Pharm. 2016, 510, 323–335. [Google Scholar] [CrossRef]
- Mangal, U.; Kim, J.-Y.; Seo, J.-Y.; Kwon, J.-S.; Choi, S.-H. Novel Poly (Methyl Methacrylate) Containing Nanodiamond to Improve the Mechanical Properties and Fungal Resistance. Materials 2019, 12, 3438. [Google Scholar] [CrossRef] [PubMed]
- Acosta-Torres, L.S.; Mendieta, I.; Nuñez-Anita, R.E.; Cajero-Juárez, M.; Castaño, V.M. Cytocompatible antifungal acrylic resin containing silver nanoparticles for dentures. Int. J. Nanomed. 2012, 7, 4777–4786. [Google Scholar] [CrossRef]
- Kamonkhantikul, K.; Arksornnukit, M.; Takahashi, H. Antifungal, optical, and mechanical properties of polymethylmethacrylate material incorporated with silanized zinc oxide nanoparticles. Int. J. Nanomed. 2017, 12, 2353–2360. [Google Scholar] [CrossRef] [PubMed]
- Gad, M.M.; Al-Thobity, A.M.; Shahin, S.Y.; Alsaqer, B.T.; Ali, A.A. Inhibitory effect of zirconium oxide nanoparticles on Candida albicans adhesion to repaired polymethyl methacrylate denture bases and interim removable prostheses: A new approach for denture stomatitis prevention. Int. J. Nanomed. 2017, 12, 5409. [Google Scholar] [CrossRef]
- Gowri, S.; Rajiv Gandhi, R.; Sundrarajan, M. Structural, Optical, Antibacterial and Antifungal Properties of Zirconia Nanoparticles by Biobased Protocol. J. Mater. Sci. Technol. 2014, 30, 782–790. [Google Scholar] [CrossRef]
- Mahmudi, A.; Varmira, K.; Jamshidy, L. Determining Efficacy and Minimum Inhibitory Concentrations of a Denture Adhesive Containing Particles and Nanoparticles of Zirconium against Candida albicans. J. Evol. Med. Dent. Sci. 2020, 9, 1700–1705. [Google Scholar] [CrossRef]
- Nam, K.-Y.; Lee, C.-H.; Lee, C.-J. Antifungal and physical characteristics of modified denture base acrylic incorporated with silver nanoparticles. Gerodontology 2012, 29, e413–e419. [Google Scholar] [CrossRef]
- Soliman, G.M. Nanoparticles as safe and effective delivery systems of antifungal agents: Achievements and challenges. Int. J. Pharm. 2017, 523, 15–32. [Google Scholar] [CrossRef]
- Renzi, D.F.; Campos, L.D.A.; Miranda, E.H.; Mainardes, R.M.; Abraham, W.-R.; Grigoletto, D.F.; Khalil, N.M. Nanoparticles as a tool for broadening antifungal activities. Curr. Med. Chem. 2020, 28. [Google Scholar] [CrossRef]
- Al-Maghrabi, P.M.; Khafagy, E.-S.; Ghorab, M.M.; Gad, S. Influence of formulation variables on miconazole nitrate–loaded lipid based nanocarrier for topical delivery. Colloids Surf. B Biointerfaces 2020, 193, 111046. [Google Scholar] [CrossRef]
- Nagrath, M.; Sikora, A.; Graca, J.; Chinnici, J.L.; Rahman, S.U.; Reddy, S.G.; Ponnusamy, S.; Maddi, A.; Arany, P.R. Functionalized prosthetic interfaces using 3D printing: Generating infection-neutralizing prosthesis in dentistry. Mater. Today Commun. 2018, 15, 114–119. [Google Scholar] [CrossRef]

| Antifungal Agent | Amphotericin | Fluconazole | Anidulafungin | Caspofungin | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Candida Species | MIC Range | MIC50 | MIC90 | MIC Range | MIC50 | MIC90 | MIC Range | MIC50 | MIC90 | MIC Range | MIC50 | MIC90 | |
| C. albicans | 0.016–16 | 1 | 4 | 0.063–64 | 0.5 | 8 | 0.008–0.25 | 0.031 | 0.125 | 0.008–8 | 0.25 | 1 | |
| C. dubliniensis | 0.063–0.125 | 0.031 | 2 | 0.063–0.125 | 0.125 | 0.125 | 0.008–0.125 | 0.125 | 0.25 | 0.25–2 | 0.5 | 2 | |
| C. glabrata | 0.016–4 | 1 | 2 | 0.25–64 | 8 | 64 | 0.016–1 | 0.063 | 1 | 0.008–2 | 0.5 | 2 | |
| C. krusei | 0.063–2 | 0.5 | 1 | 0.25–64 | 8 | 64 | 0.016–0.25 | 0.125 | 0.25 | 0.063–4 | 2 | 4 | |
| C. tropicalis | 0.031–2 | 1 | 2 | 0.063–8 | 4 | 8 | 0.008–0.063 | 0.063 | 0.063 | 0.031–8 | 0.5 | 8 | |
| C. keyfr | 0.016–1 | 0.5 | 1 | 0.25–32 | 4 | 32 | 0.031–0.063 | 0.063 | 0.5 | 0.125–0.05 | 0.25 | 0.5 | |
| Drug | Form | Dose | Indication | Adverse Effects | Refs. |
|---|---|---|---|---|---|
| Amphotericin B | Infusion | 100–200 mg/6 h | Intraoral candidiasis, chronic erythematous candidiasis | Renal, cardiovascular, spinal and neurological effects | [10,36,37] |
| Nystatin | Suspension | 4–6 mL/6 h | Intraoral candidiasis | Well tolerated | [10,36] |
| Ointment | 2–4 applications/day | Angular cheilitis | Well tolerated | [10,36] | |
| Tablets/Pastilles | 2 every 8 h | Denture stomatitis | Uncommon effects: nausea, vomiting, gastrointestinal effects | [36,38] | |
| Fluconazole | Tablets | 50–100 mg/day | Pseudomembranous candidiasis, acute erythematous candidiasis, chronic hyperplastic candidiasis | Nausea, vomiting, diarrhea, abdominal pain | [36,37] |
| Suspension | 10 mg/mL | Oropharyngeal candidiasis | Nausea, vomiting, diarrhea, abdominal pain | [36,37,39] | |
| Miconazole | Gel/cream | 100 mg/6 h | Angular cheilitis, chronic erythematous candidiasis | Uncommon effects: burning, irritation, nausea, diarrhea | [10,36,37] |
| Ketoconazole | Gel/cream | 3 times/day | Angular cheilitis | Nausea, vomiting | [10,36] |
| Tablets | 200 mg, 2–2/day | Pseudomembranous candidiasis, acute erythematous candidiasis, chronic hyperplastic candidiasis | Abdominal pain | [36,37] | |
| Clotrimazole | Gel/cream | 3 times/day | Angular cheilitis | Occasional effects: skin irritation, burning sensation | [10,36] |
| Tablets/troches | 5 times/day | Intraoral candidiasis | Occasional effects: skin irritation, burning sensation | [10,36] | |
| Betamethasone dipropionate clotrimazole | Cream | 4 times/day | Chronic angular cheilitis | Local irritation | [10,40,41,42] |
| Itraconazole | Capsules | 100–200 mg/day | Pseudomembranous candidiasis, acute erythematous candidiasis, chronic hyperplastic candidiasis | Nausea, vomiting, diarrhea, abdominal pain | [36,37] |
| Voriconazole | Infusion | First day: 6 mg/kg once every 12 h Rest of the treatment: 4 mg/kg once every 12 h | Intraoral candidiasis | Neuropsychiatric and gastrointestinal effects | [37,40,43,44] |
| Tablets | First day: 200–400 mg once every 12 h Rest of the treatment: 100–200 mg once every 12 h | Intraoral candidiasis | Neuropsychiatric and gastrointestinal effects | [37,40,43,44] | |
| Posaconazole | Oral suspension/Tablets | First week: 200 mg, 4 times/day Rest of the treatment: 400 mg, 2 times/day | Oropharyngeal candidiasis | Headaches, gastrointestinal effects | [37,40,45] |
| Anidulafungin | Infusion | First day: 3 mg/kg/day (max 200 mg) Rest of the treatment: 1.5 mg/kg/day (max 100 mg) | Invasive candidiasis | Occasional effects: anemia, diarrhea, pyrexia, vomiting, hypokalemia | [19,46,47] |
| Caspofungin | Infusion | First day: 70 mg/day Rest of the treatment: 50 mg/day | Invasive candidiasis | Occasional effects: phlebitis, fever, abdominal pain, nausea, diarrhea, headache, rash, leukopenia, hypokalemia | [19,47] |
| Micafungin | Infusion | 1–2 mg/kg/day (max 100 mg/day) | Invasive candidiasis | Occasional effects: fever, nausea, headache, rash | [19,47] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gheorghe, D.C.; Niculescu, A.-G.; Bîrcă, A.C.; Grumezescu, A.M. Biomaterials for the Prevention of Oral Candidiasis Development. Pharmaceutics 2021, 13, 803. https://doi.org/10.3390/pharmaceutics13060803
Gheorghe DC, Niculescu A-G, Bîrcă AC, Grumezescu AM. Biomaterials for the Prevention of Oral Candidiasis Development. Pharmaceutics. 2021; 13(6):803. https://doi.org/10.3390/pharmaceutics13060803
Chicago/Turabian StyleGheorghe, Dan Cristian, Adelina-Gabriela Niculescu, Alexandra Cătălina Bîrcă, and Alexandru Mihai Grumezescu. 2021. "Biomaterials for the Prevention of Oral Candidiasis Development" Pharmaceutics 13, no. 6: 803. https://doi.org/10.3390/pharmaceutics13060803
APA StyleGheorghe, D. C., Niculescu, A.-G., Bîrcă, A. C., & Grumezescu, A. M. (2021). Biomaterials for the Prevention of Oral Candidiasis Development. Pharmaceutics, 13(6), 803. https://doi.org/10.3390/pharmaceutics13060803








