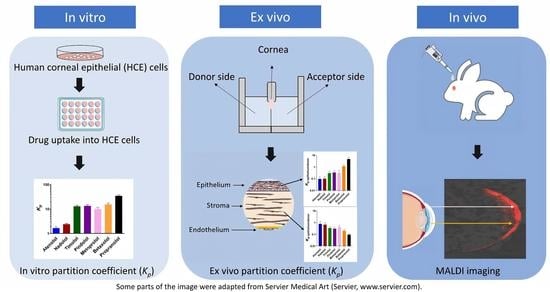
Partitioning and Spatial Distribution of Drugs in Ocular Surface Tissues
Abstract
1. Introduction
2. Materials and Methods
2.1. Drug Molecules
2.2. Drug Distribution in Porcine Ocular Tissues Ex Vivo
2.2.1. Preparation of Porcine Tissues
2.2.2. Drug Distribution into the Ocular Tissues
2.2.3. Calculation of Partition Coefficients
2.3. Cell Uptake in Human Corneal Epithelial (HCE) Cells In Vitro
2.3.1. HCE Cells
2.3.2. Cell Uptake Study
2.3.3. Protein Concentration of the HCE Samples
2.3.4. Determination of Intracellular Drug Concentration
2.3.5. Calculation of Partition Coefficients from Uptake Study
2.4. Analyses of Ex Vivo and In Vitro Samples
2.4.1. Sample Preparation for LC-MS/MS
2.4.2. LC-MS/MS Analyses
2.5. Drug Distribution in Rabbit Cornea In Vivo
2.5.1. Rabbit Tissue Preparation
2.5.2. MALDI-IMS
3. Results
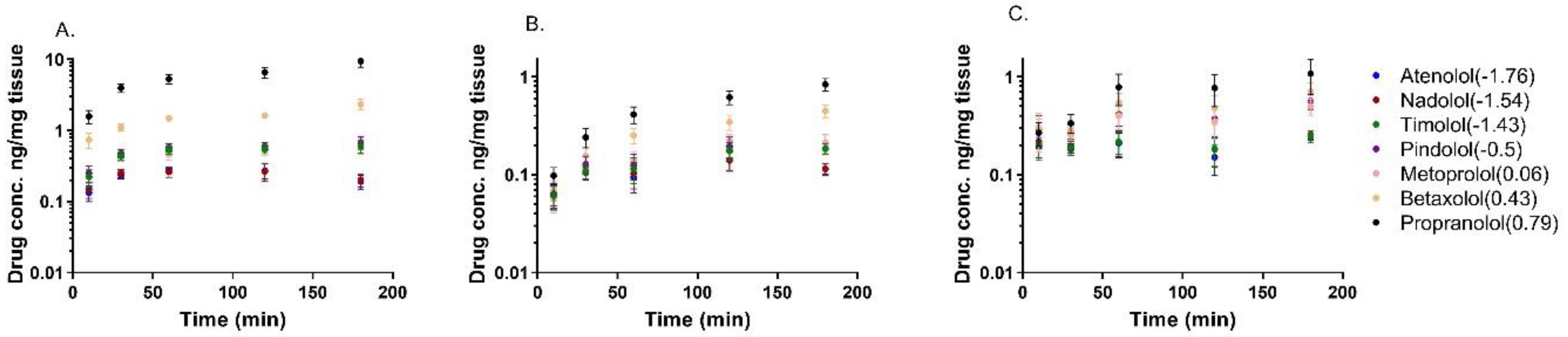
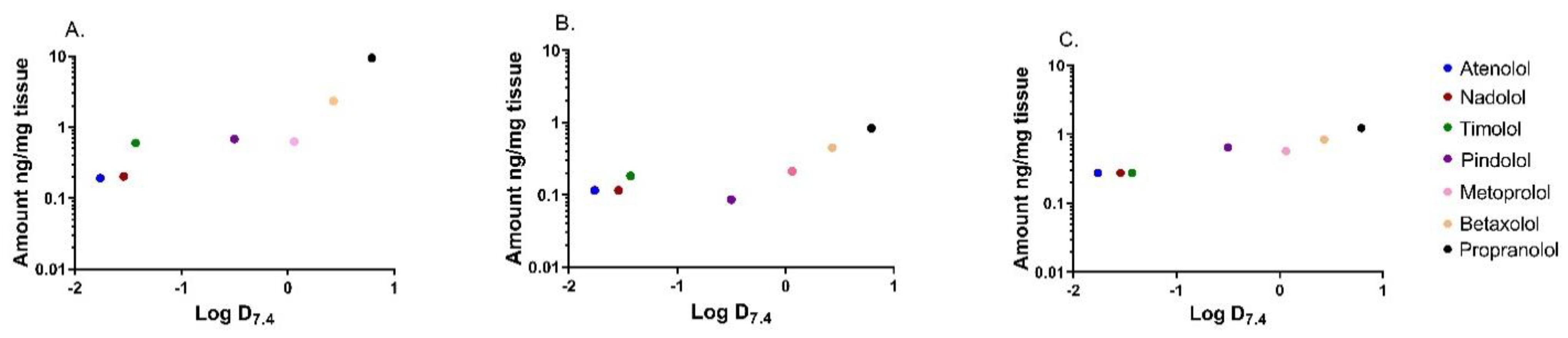
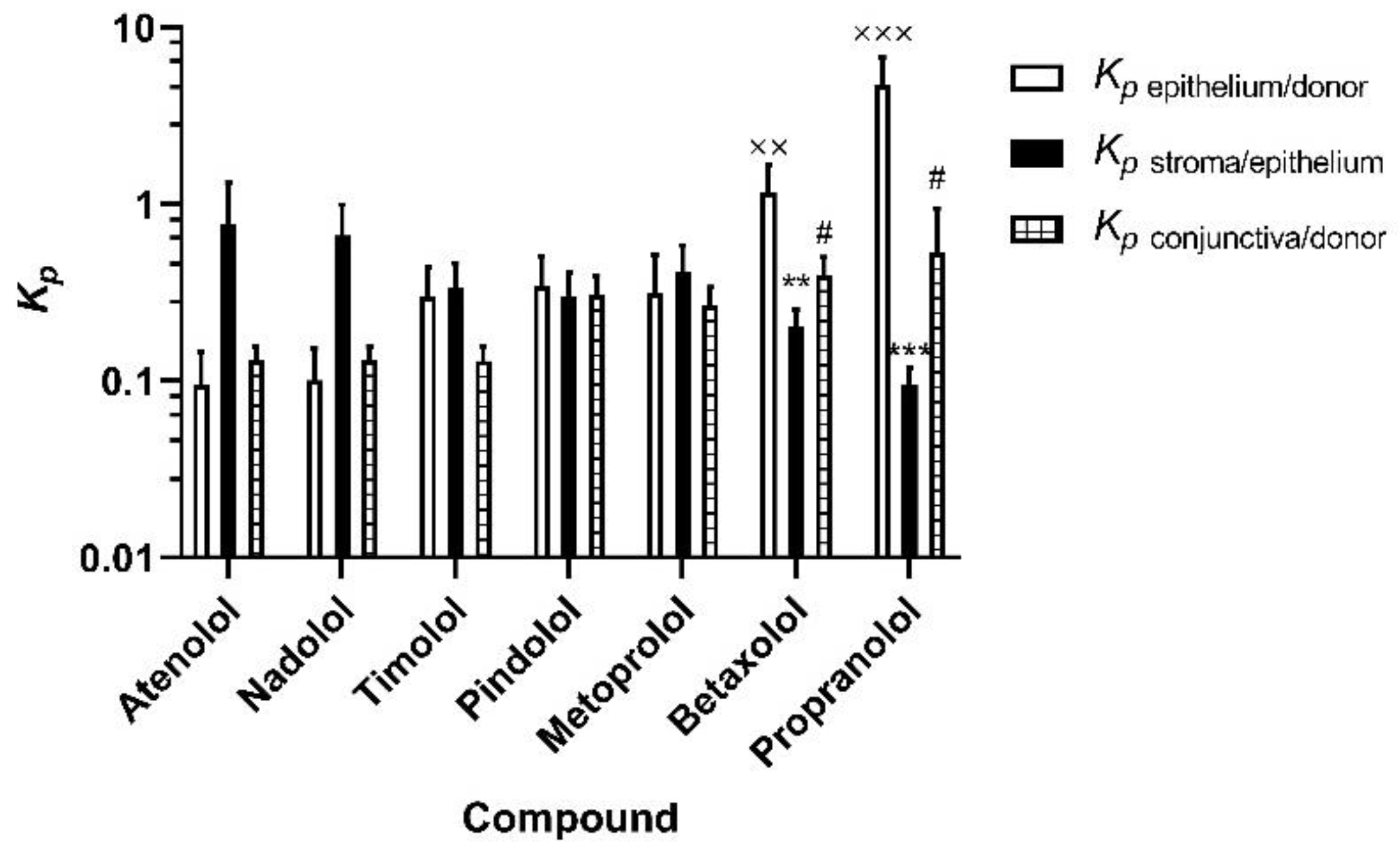
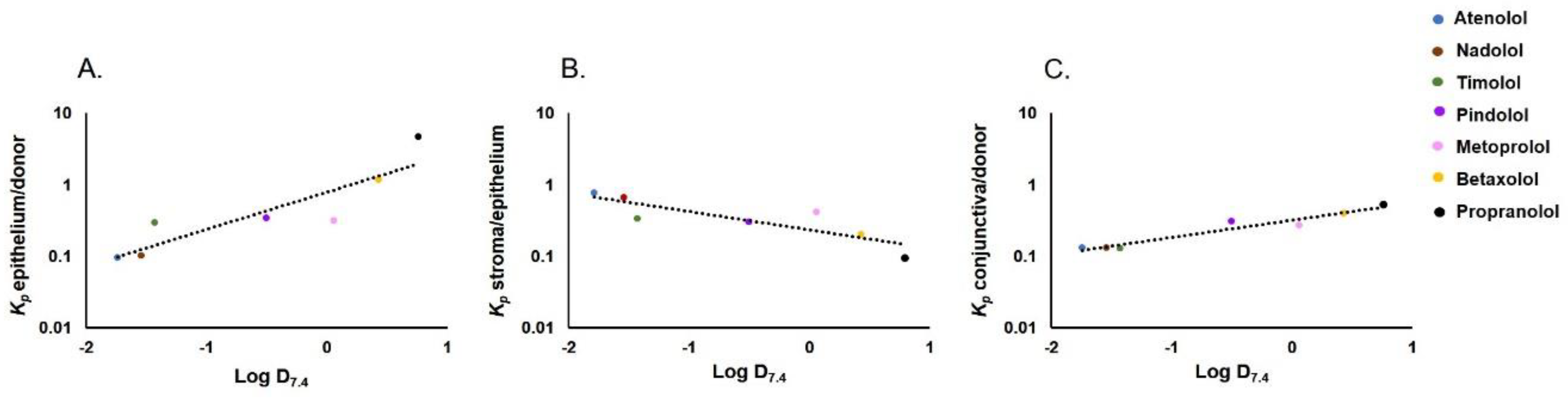
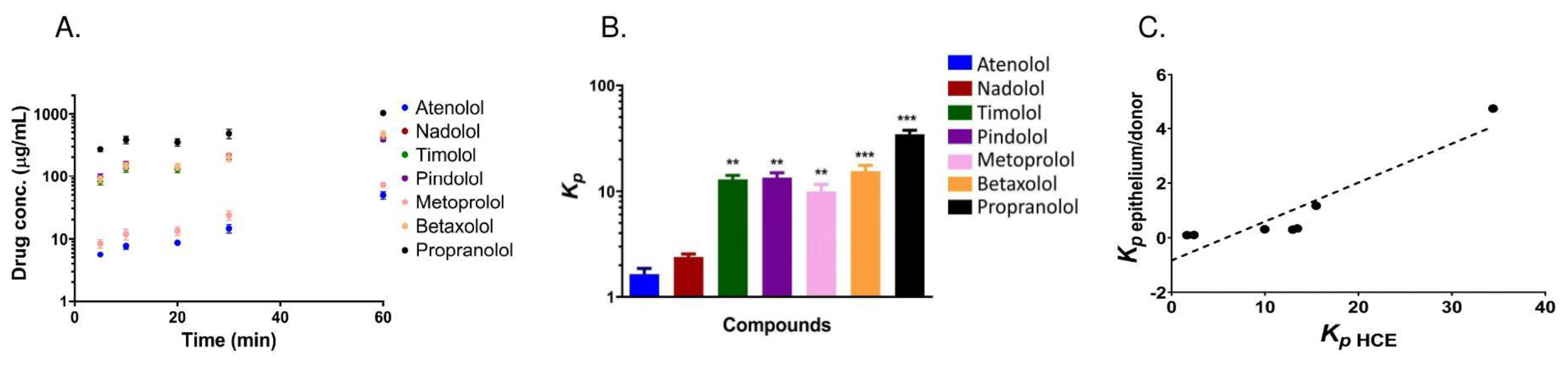
3.1. Drug Distribution in Porcine Ocular Tissues Ex Vivo
3.2. Apparent Distribution Coefficients in Porcine Ocular Tissues Ex Vivo
3.3. Uptake of Beta-Blocking Drugs in HCE Cells
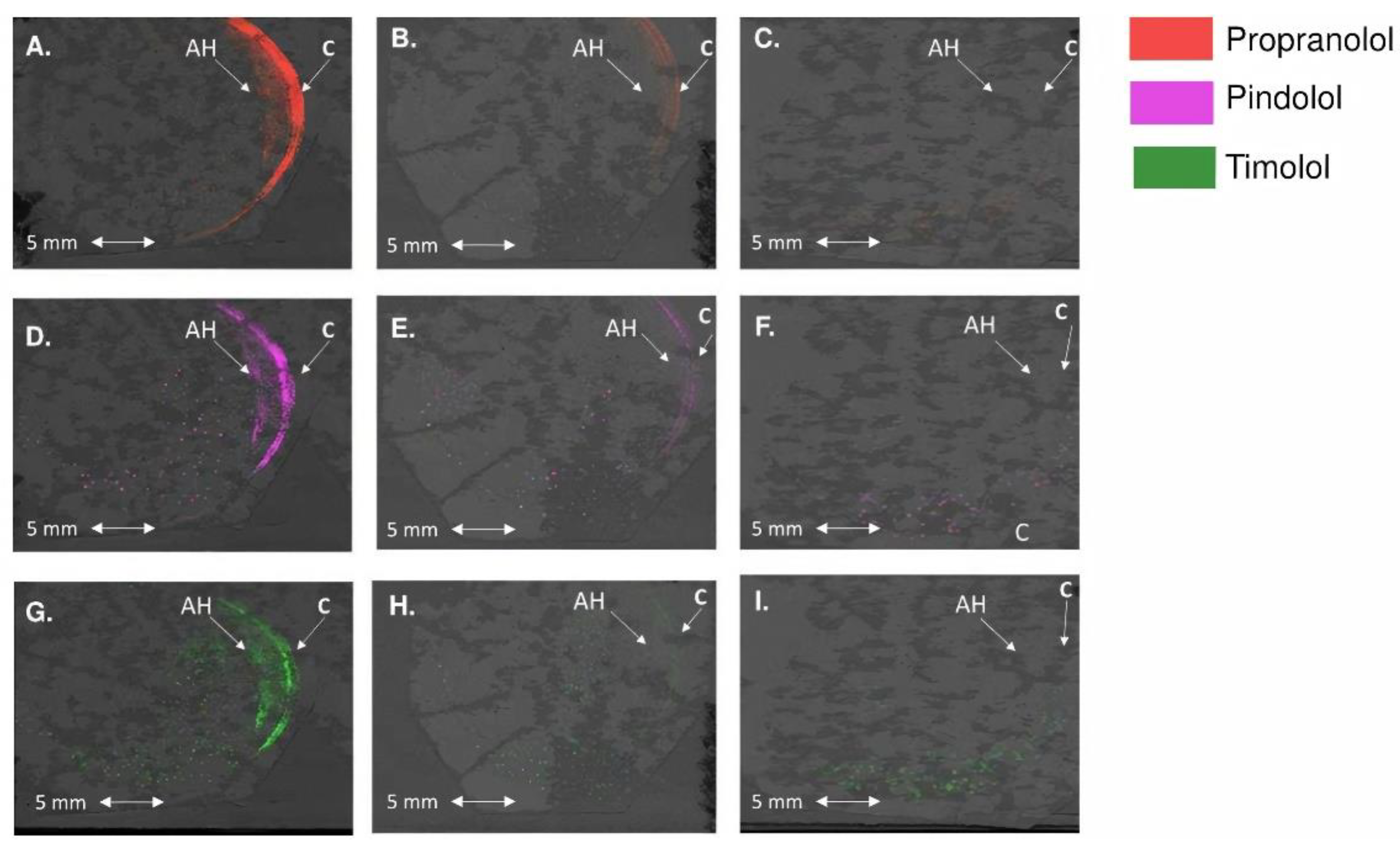
3.4. Distribution Patterns of the Drugs in the Rabbit Cornea
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Patel, A. Ocular drug delivery systems: An overview. World J. Pharmacol. 2013, 2, 47–64. [Google Scholar] [CrossRef]
- Urtti, A. Challenges and obstacles of ocular pharmacokinetics and drug delivery. Adv. Drug Deliv. Rev. 2006, 58, 1131–1135. [Google Scholar] [CrossRef]
- Fayyaz, A.; Ranta, V.-P.; Toropainen, E.; Vellonen, K.-S.; Valtari, A.; Puranen, J.; Ruponen, M.; Gardner, I.; Urtti, A.; Jamei, M.; et al. Topical ocular pharmacokinetics and bioavailability for a cocktail of timolol, betaxolol and atenolol in rabbits. Eur. J. Pharm. Sci. 2020, 155, 105553. [Google Scholar] [CrossRef] [PubMed]
- Naageshwaran, V.; Ranta, V.P.; Gum, G.; Bhoopathy, S.; Urtti, A.; del Amo, E.M. Comprehensive Ocular and Systemic Pharmacokinetics of Brinzolamide in Rabbits After Intracameral, Topical, and Intravenous Administration. J. Pharm. Sci. 2021, 110, 529–535. [Google Scholar] [CrossRef]
- Lee, V.H.; Robinson, J.R. Mechanistic and quantitative evaluation of precorneal pilocarpine disposition in albino rabbits. J. Pharm. Sci. 1979, 68, 673–684. [Google Scholar] [CrossRef]
- Zaki, I.; Fitzgerald, P.; Hardy, J.G.; Wilson, C.G. A comparison of the effect of viscosity on the precorneal residence of solutions in rabbit and man. J. Pharm. Pharmacol. 1986, 38, 463–466. [Google Scholar] [CrossRef] [PubMed]
- Meadows, D.L.; Paugh, J.R.; Joshi, A.; Mordaunt, J. A novel method to evaluate residence time in humans using a nonpenetrating fluorescent tracer. Investig. Ophthalmol. Vis. Sci. 2002, 43, 1032–1039. [Google Scholar]
- Maurice, D.M.; Mishima, S. Ocular Pharmacokinetics. In Handbook of Experimental Pharmacology; Springer: Berlin/Heidelberg, Germany, 1984; Volume 69, pp. 19–116. [Google Scholar]
- Järvinen, K.; Järvinen, T.; Urtti, A. Ocular absorption following topical delivery. Adv. Drug Deliv. Rev. 1995, 16, 3–19. [Google Scholar] [CrossRef]
- Ramsay, E.; del Amo, E.M.; Toropainen, E.; Tengvall-Unadike, U.; Ranta, V.P.; Urtti, A.; Ruponen, M. Corneal and conjunctival drug permeability: Systematic comparison and pharmacokinetic impact in the eye. Eur. J. Pharm. Sci. 2018, 119, 83–89. [Google Scholar] [CrossRef]
- Urtti, A.; Salminen, L.; Miinalainen, O. Systemic absorption of ocular pilocarpine is modified by polymer matrices. Int. J. Pharm. 1985, 23, 147–161. [Google Scholar] [CrossRef]
- Chang, S.C.; Lee, V.H. Nasal and Conjunctival Contributions to the Systemic Absorption of Topical Timolol in the Pigmented Rabbit: Implications in the Design of Strategies to Maximize the Ratio of Ocular to Systemic Absorption. J. Ocul. Pharmacol. 1987, 3, 159–169. [Google Scholar] [CrossRef] [PubMed]
- Kyyronen, K.; Urtti, A. Improved ocular: Systemic absorption ratio of timolol by viscous vehicle and phenylephrine. Investig. Ophthalmol. Vis. Sci. 1990, 31, 1827–1833. [Google Scholar]
- Urtti, A.; Salminen, L. Minimizing systemic absorption of topically administered ophthalmic drugs. Surv. Ophthalmol. 1993, 37, 435–456. [Google Scholar] [CrossRef]
- Neufeld, A.H. Experimental studies on the mechanism of action of timolol. Surv. Ophthalmol. 1979, 23, 363–370. [Google Scholar] [CrossRef]
- Oh, C.; Apel, A.J.G.; Saville, B.A.; Cheng, Y.L.; Rootman, D.S. Local efficacy of cyclosporine in corneal transplant therapy. Curr. Eye Res. 1994, 13, 337–343. [Google Scholar] [CrossRef]
- Foulks, G.N. Topical cyclosporine for treatment of ocular surface disease. Int. Ophthalmol. Clin. 2006, 46, 105–122. [Google Scholar] [CrossRef]
- Sall, K.; Stevenson, O.D.; Mundorf, T.K.; Reis, B.L. Two multicenter randomized studies of the efficacy and safety of cyclosporine ophthalmic emulsion in moderate to severe dry eye disease. Ophthalmology 2000, 107, 631–639. [Google Scholar] [CrossRef]
- Barber, L.D.; Pflugfelder, S.C.; Tauber, J.; Foulks, G.N. Phase III safety evaluation of cyclosporine 0.1% ophthalmic emulsion administered twice daily to dry eye disease patients for up to 3 years. Ophthalmology 2005, 112, 1790–1794. [Google Scholar] [CrossRef]
- Shen, Y.; Tu, J. Preparation and ocular pharmacokinetics of ganciclovir liposomes. AAPS J. 2007, 9, E371. [Google Scholar] [CrossRef]
- Schoenwald, R.D.; Huang, H.-S. Corneal penetration behavior of β-blocking agents I: Physicochemical factors. J. Pharm. Sci. 1983, 72, 1266–1272. [Google Scholar] [CrossRef]
- Halasa, A.H. Gentamicin in the treatment of bacterial conjunctivitis. Am. J. Ophthalmol. 1967, 63, 1699–1702. [Google Scholar] [CrossRef]
- Parmar, P.; Salman, A.; Kalavathy, C.M.; Kaliamurthy, J.; Prasanth, D.A.; Thomas, P.A.; Jesudasan, C.A.N. Comparison of topical gatifloxacin 0.3% and ciprofloxacin 0.3% for the treatment of bacterial keratitis. Am. J. Ophthalmol. 2006, 141, 282–286. [Google Scholar] [CrossRef]
- Schoenwald, R.D.; Ward, R.L. Relationship between steroid permeability across excised rabbit cornea and octanol-water partition coefficients. J. Pharm. Sci. 1978, 67, 786–788. [Google Scholar] [CrossRef] [PubMed]
- Mari Hämäläinen, K.; Kontturi, K.; Auriola, S.; Murtomäki, L.; Urtti, A. Estimation of pore size and pore density of biomembranes from permeability measurements of polyethylene glycols using an effusion-like approach. J. Control. Release 1997, 49, 97–104. [Google Scholar] [CrossRef]
- Barar, J.; Asadi, M.; Mortazavi-Tabatabaei, S.A.; Omidi, Y. Ocular drug delivery; Impact of in vitro cell culture models. J. Ophthalmic Vis. Res. 2014, 4, 238–252. [Google Scholar]
- Ahmed, I.; Gokhale, R.D.; Shah, M.V.; Patton, T.F. Physicochemical Determinants of Drug Diffusion across the Conjunctiva, Sclera, and Cornea. J. Pharm. Sci. 1987, 76, 583–586. [Google Scholar] [CrossRef] [PubMed]
- Huang, A.J.W.; Tseng, S.C.G.; Kenyon, K.R. Paracellular permeability of corneal and conjunctival epithelia. Investig. Ophthalmol. Vis. Sci. 1989, 30, 684–689. [Google Scholar]
- Shih, R.L.; Lee, V.H.L. Rate Limiting Barrier to the Penetration of Ocular Hypotensive Beta Blockers Across the Corneal Epithelium in the Pigmented Rabbit. J. Ocul. Pharmacol. 1990, 6, 329–336. [Google Scholar] [CrossRef]
- Wang, W.; Sasaki, H.; Chien, D.S.; Lee, V.H.L. Lipophilicity influence on conjunctival drug penetration in the pigmented rabbit: A comparison with corneal penetration. Curr. Eye Res. 1991, 10, 571–579. [Google Scholar] [CrossRef]
- Loch, C.; Zakelj, S.; Kristl, A.; Nagel, S.; Guthoff, R.; Weitschies, W.; Seidlitz, A. Determination of permeability coefficients of ophthalmic drugs through different layers of porcine, rabbit and bovine eyes. Eur. J. Pharm. Sci. 2012, 47, 131–138. [Google Scholar] [CrossRef]
- Sieg, J.W.; Robinson, J.R. Vehicle effects on ocular drug bioavailability II: Evaluation of pilocarpine. J. Pharm. Sci. 1977, 66, 1222–1228. [Google Scholar] [CrossRef] [PubMed]
- Grass, G.M.; Robinson, J.R. Mechanisms of corneal drug penetration II: Ultrastructural analysis of potential pathways for drug movement. J. Pharm. Sci. 1988, 77, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Brechue, W.F.; Maren, T.H. pH and drug ionization affects ocular pressure lowering of topical carbonic anhydrase inhibitors. Investig. Ophthalmol. Vis. Sci. 1993, 34, 2581–2587. [Google Scholar]
- Kidron, H.; Vellonen, K.S.; Del Amo, E.M.; Tissari, A.; Urtti, A. Prediction of the corneal permeability of drug-like compounds. Pharm. Res. 2010, 27, 1398–1407. [Google Scholar] [CrossRef]
- Nichols, B.A. Conjunctiva. Microsc. Res. Tech. 1996, 33, 296–319. [Google Scholar] [CrossRef]
- Dartt, D.A. Regulation of mucin and fluid secretion by conjunctival epithelial cells. Prog. Retin. Eye Res. 2002, 21, 555–576. [Google Scholar] [CrossRef]
- Ramsay, E.; Ruponen, M.; Picardat, T.; Tengvall, U.; Tuomainen, M.; Auriola, S.; Toropainen, E.; Urtti, A.; del Amo, E.M. Impact of Chemical Structure on Conjunctival Drug Permeability: Adopting Porcine Conjunctiva and Cassette Dosing for Construction of In Silico Model. J. Pharm. Sci. 2017, 106, 2463–2471. [Google Scholar] [CrossRef]
- Watsky, M.A.; Jablonski, M.M.; Edelhauser, H.F. Comparison of conjunctival and corneal surface areas in rabbit and human. Curr. Eye Res. 1988, 7, 483–486. [Google Scholar] [CrossRef]
- Ahmed, I.; Patton, T.F. Effect of pH and buffer on the precorneal disposition and ocular penetration of pilocarpine in rabbits. Int. J. Pharm. 1984, 19, 215–227. [Google Scholar] [CrossRef]
- Prausnitz, M.R. Permeability of cornea, sciera, and conjunctiva: A literature analysis for drug delivery to the eye. J. Pharm. Sci. 1998, 87, 1479–1488. [Google Scholar] [CrossRef]
- Ranta, V.P.; Laavola, M.; Toropainen, E.; Vellonen, K.S.; Talvitie, A.; Urtti, A. Ocular pharmacokinetic modeling using corneal absorption and desorption rates from in vitro permeation experiments with cultured corneal epithelial cells. Pharm. Res. 2003, 20, 1409–1416. [Google Scholar] [CrossRef] [PubMed]
- Patton, T.F.; Robinson, J.R. Quantitative Precorneal Disposition of Topically Applied Pilocarpine Nitrate in Rabbit Eyes. J. Pharm. Sci. 1976, 65. [Google Scholar] [CrossRef]
- Northfield, S.E.; Manallack, D.T. Lipophilicity of beta-adrenocepter antagonists: A new classification scheme for clinical use. J. Pharm. Pract. Res. 2007, 37, 98–101. [Google Scholar] [CrossRef]
- Ramsay, E.; Hagström, M.; Vellonen, K.S.; Boman, S.; Toropainen, E.; del Amo, E.M.; Kidron, H.; Urtti, A.; Ruponen, M. Role of retinal pigment epithelium permeability in drug transfer between posterior eye segment and systemic blood circulation. Eur. J. Pharm. Biopharm. 2019, 143, 18–23. [Google Scholar] [CrossRef] [PubMed]
- Kawamoto, T. Use of a new adhesive film for the preparation of multi-purpose fresh-frozen sections from hard tissues, whole-animals, insects and plants. Arch. Histol. Cytol. 2003, 66, 123–143. [Google Scholar] [CrossRef]
- Boughton, B.A.; Thomas, O.R.B.; Demarais, N.J.; Trede, D.; Swearer, S.E.; Grey, A.C. Detection of small molecule concentration gradients in ocular tissues and humours. J. Mass Spectrom. 2020, 55, e4460. [Google Scholar] [CrossRef]
- Toropainen, E.; Ranta, V.P.; Vellonen, K.S.; Palmgrén, J.; Talvitie, A.; Laavola, M.; Suhonen, P.; Hämäläinen, K.M.; Auriola, S.; Urtti, A. Paracellular and passive transcellular permeability in immortalized human corneal epithelial cell culture model. Eur. J. Pharm. Sci. 2003, 20, 99–106. [Google Scholar] [CrossRef]
- Sieg, J.W.; Robinson, J.R. Mechanistic studies on transcorneal permeation of fluorometholone. J. Pharm. Sci. 1981, 70, 1026–1029. [Google Scholar] [CrossRef]
- Makoid, M.C.; Robinson, J.R. Pharmacokinetics of Topically Applied Pilocarpine in the Albino Rabbit Eye. J. Pharm. Sci. 1979, 68, 435–443. [Google Scholar] [CrossRef]
- Ahmed, I.; Patton, T.F. Importance of the noncorneal absorption route in topical ophthalmic drug delivery. Investig. Ophthalmol. Vis. Sci. 1985, 26, 584–587. [Google Scholar]
- Wang, L.Z.; Syn, N.; Li, S.; Barathi, V.A.; Tong, L.; Neo, J.; Beuerman, R.W.; Zhou, L. The penetration and distribution of topical atropine in animal ocular tissues. Acta Ophthalmol. 2019, 97, e238–e247. [Google Scholar] [CrossRef] [PubMed]
- Mori, N.; Mochizuki, T.; Yamazaki, F.; Takei, S.; Mano, H.; Matsugi, T.; Setou, M. MALDI imaging mass spectrometry revealed atropine distribution in the ocular tissues and its transit from anterior to posterior regions in the whole-eye of rabbit after topical administration. PLoS ONE 2019, 14, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Grove, K.J.; Kansara, V.; Prentiss, M.; Long, D.; Mogi, M.; Kim, S.; Rudewicz, P.J. Application of Imaging Mass Spectrometry to Assess Ocular Drug Transit. SLAS Discov. 2017, 22, 1239–1245. [Google Scholar] [CrossRef] [PubMed]
- Heikkinen, E.M.; Auriola, S.; Ranta, V.P.; Demarais, N.J.; Grey, A.C.; Del Amo, E.M.; Toropainen, E.; Vellonen, K.S.; Urtti, A.; Ruponen, M. Distribution of small molecular weight drugs into the porcine lens: studies on imaging mass spectrometry, partition coefficients, and implications in ocular pharmacokinetics. Mol. Pharm. 2019, 16, 3968–3976. [Google Scholar] [CrossRef] [PubMed]
- Urtti, A.; Sendo, T.; Pipkin, J.D.; Rork, G.; Repta, A.J. Application Site Dependent Ocular Absorption of Timolol. J. Ocul. Pharmacol. Ther. 1988, 4, 335–343. [Google Scholar] [CrossRef]
- Feizi, S.; Jafarinasab, M.R.; Karimian, F.; Hasanpour, H.; Masudi, A. Central and Peripheral Corneal Thickness Measurement in Normal and Keratoconic Eyes Using Three Corneal Pachymeters. J. Ophthalmic Vis. Res. 2014, 9, 296–304. [Google Scholar] [CrossRef]
- Mann, I.D.A. A Study of Epithelial Regeneration in the living EYE. Br. J. Ophthalmol. 1941, 28, 26–40. [Google Scholar] [CrossRef] [PubMed]
- Davanger, M.; Evensen, A. Role of the Pericorneal Papillary Structure in Renewal of Corneal Epithelium. Nature 1971, 229, 560–561. [Google Scholar] [CrossRef] [PubMed]
| Beta-Blocking Drugs | Log D7.4 * | Molecular Weight (g/mol) | Manufacturer |
|---|---|---|---|
| Atenolol | −1.76 | 266.34 | Sigma-Aldrich, St. Louis, MO, USA |
| Nadolol | −1.54 | 309.40 | Sigma-Aldrich, St. Louis, MO, USA |
| Timolol | −1.43 | 316.42 | Sigma-Aldrich, St. Louis, MO, USA |
| Pindolol | −0.5 | 248.23 | Sigma-Aldrich, St. Louis, MO, USA |
| Metoprolol | 0.06 | 267.36 | Sigma-Aldrich, St. Louis, MO, USA |
| Betaxolol | 0.43 | 307.43 | Alcon, Fort Worth, TX, USA |
| Propranolol | 0.79 | 259.34 | Sigma-Aldrich, St. Louis, MO, USA |
| Beta-Blocking Drugs | Epithelium | Stroma | ||
|---|---|---|---|---|
| 10 min | 60 min | 10 min | 60 min | |
| Pindolol | 0.21 ± 0.01 | 0.356± 0.08 | 0.57 ± 0.00 | 0.83 ± 0.13 |
| Timolol | 0.19 ± 0.02 | 0.25 ± 0.08 | 0.36 ± 0.01 | 0.45 ± 0.17 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Balla, A.; Auriola, S.; Grey, A.C.; Demarais, N.J.; Valtari, A.; Heikkinen, E.M.; Toropainen, E.; Urtti, A.; Vellonen, K.-S.; Ruponen, M. Partitioning and Spatial Distribution of Drugs in Ocular Surface Tissues. Pharmaceutics 2021, 13, 658. https://doi.org/10.3390/pharmaceutics13050658
Balla A, Auriola S, Grey AC, Demarais NJ, Valtari A, Heikkinen EM, Toropainen E, Urtti A, Vellonen K-S, Ruponen M. Partitioning and Spatial Distribution of Drugs in Ocular Surface Tissues. Pharmaceutics. 2021; 13(5):658. https://doi.org/10.3390/pharmaceutics13050658
Chicago/Turabian StyleBalla, Anusha, Seppo Auriola, Angus C. Grey, Nicholas J. Demarais, Annika Valtari, Emma M. Heikkinen, Elisa Toropainen, Arto Urtti, Kati-Sisko Vellonen, and Marika Ruponen. 2021. "Partitioning and Spatial Distribution of Drugs in Ocular Surface Tissues" Pharmaceutics 13, no. 5: 658. https://doi.org/10.3390/pharmaceutics13050658
APA StyleBalla, A., Auriola, S., Grey, A. C., Demarais, N. J., Valtari, A., Heikkinen, E. M., Toropainen, E., Urtti, A., Vellonen, K.-S., & Ruponen, M. (2021). Partitioning and Spatial Distribution of Drugs in Ocular Surface Tissues. Pharmaceutics, 13(5), 658. https://doi.org/10.3390/pharmaceutics13050658