Effect of C-terminus Conjugation via Different Conjugation Chemistries on In Vivo Activity of Albumin-Conjugated Recombinant GLP-1
Abstract
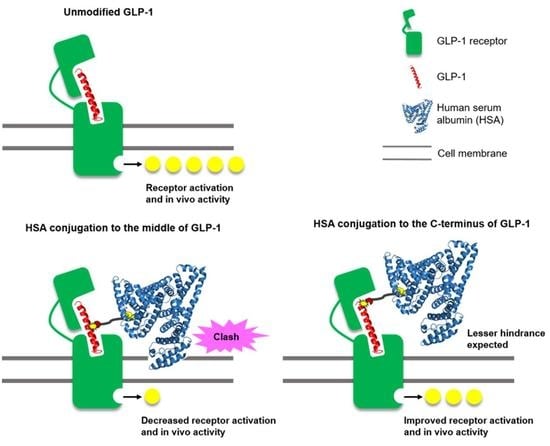
1. Introduction
2. Materials and Methods
2.1. Materials
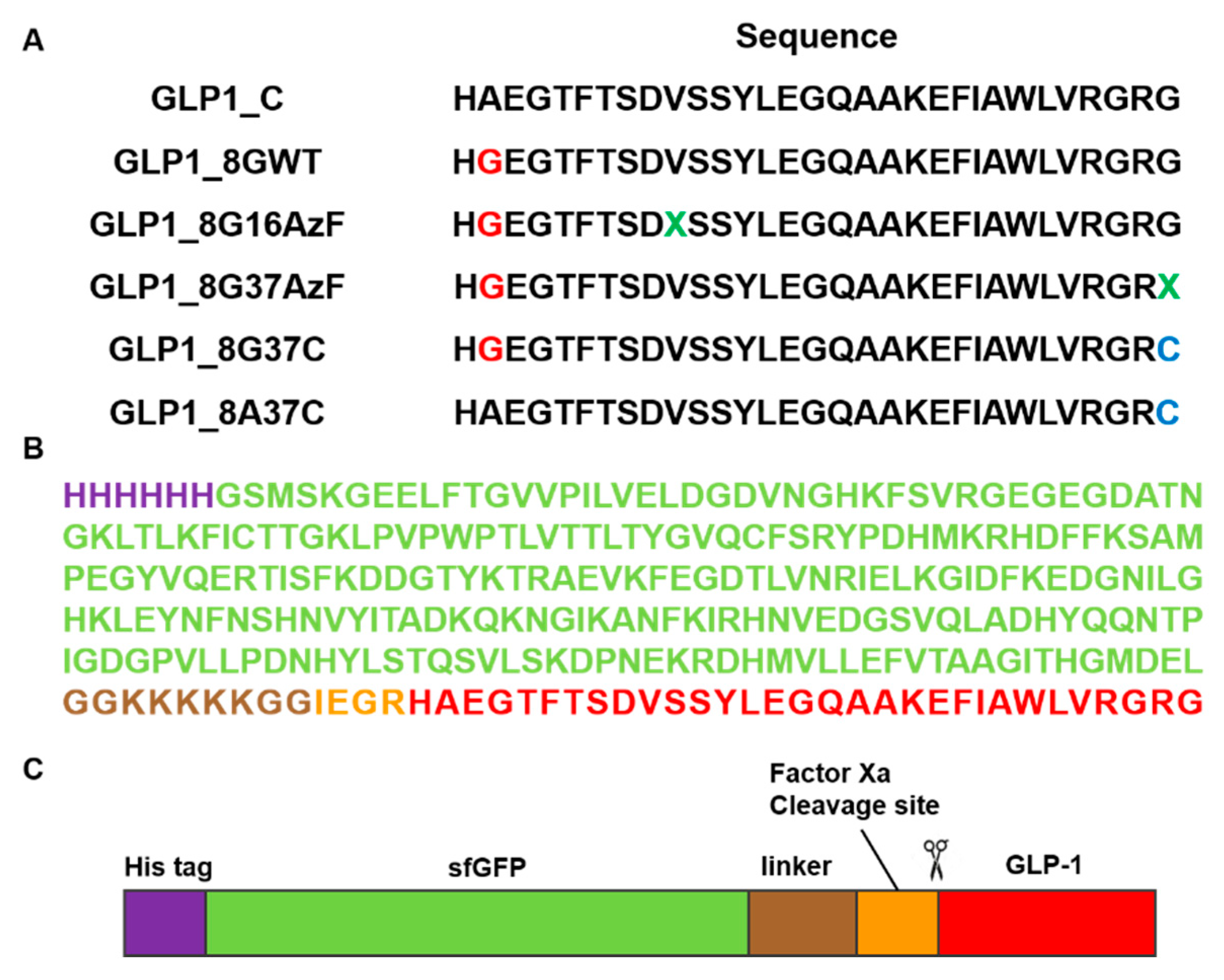
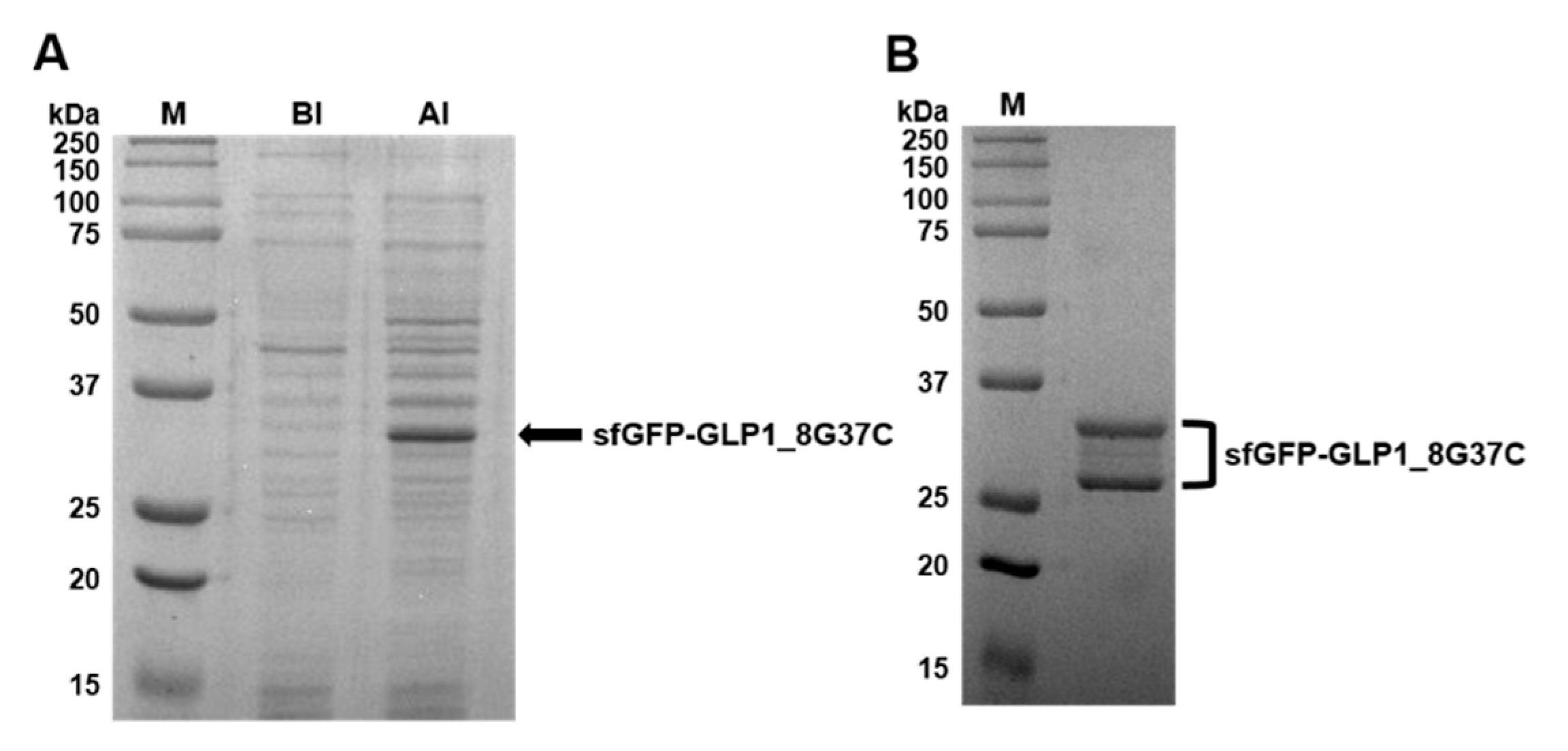
2.2. Preparation of sfGFP and GLP-1 Fusion Protein
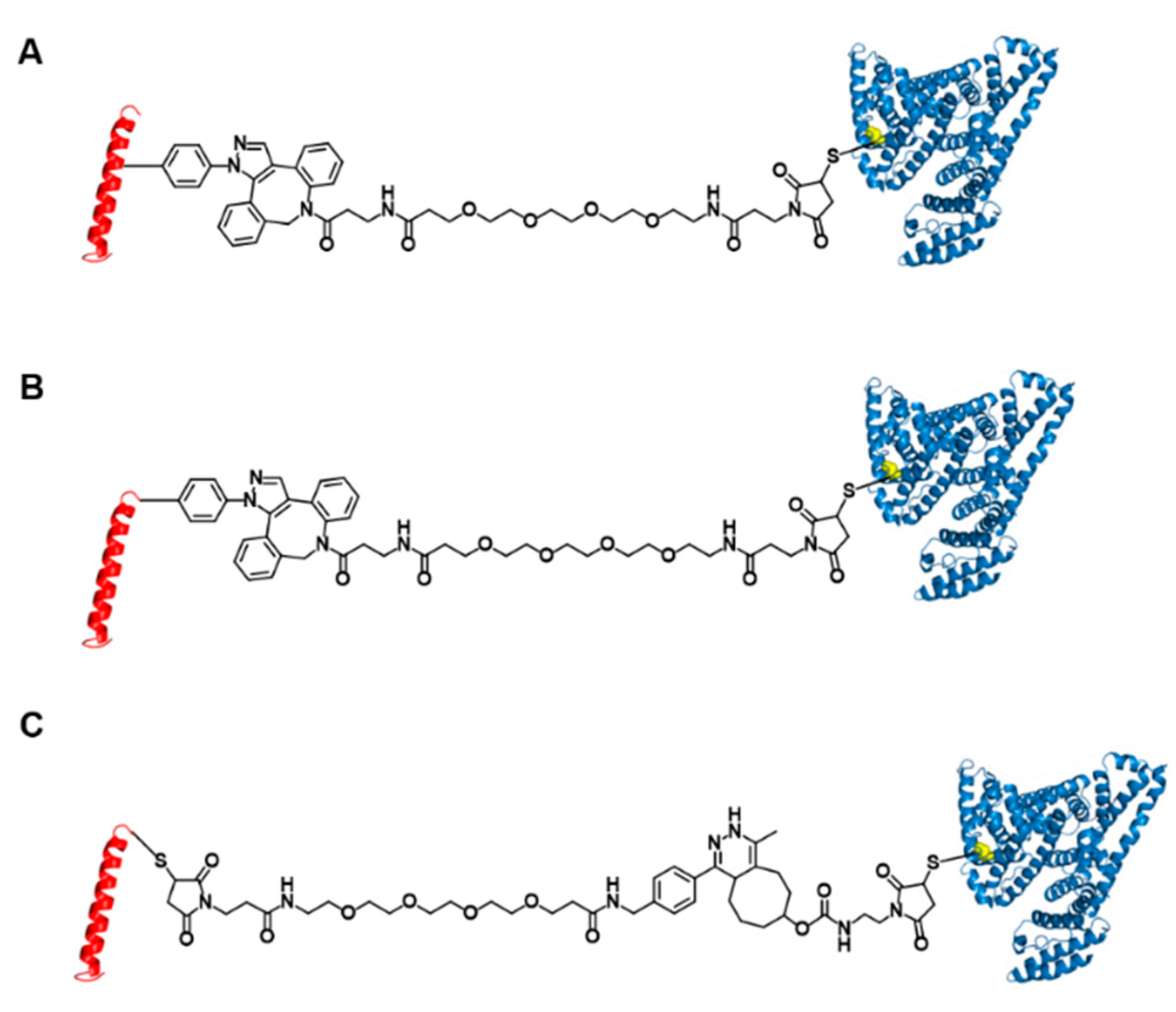
2.3. Preparation of GLP1_8G16AzF-HSA, GLP1_8G37AzF-HSA, GLP1_8G37C-HSA, and GLP1_8A37C-HSA Conjugates
2.4. Labeling of Linker Conjugated sfGFP-GLP1_8G37C by Inverse Electron-Demand Diels-Alder Reaction (IEDDA)
2.5. Mass Spectrometric Analysis
2.6. Enzyme-Linked Immunosorbent Assay (ELISA) of GLP1-HSA Conjugate
2.7. Pharmacokinetic Studies of GLP1-HSA Conjugates
2.8. In Vivo Intraperitoneal Glucose Tolerance Test (IPGTT)
2.9. In Vitro Activity Assay
3. Results and Discussion
3.1. Preparation of C-terminus-Modified GLP-1 Varints
3.2. Preparation of Albumin-Conjugated GLP-1 Variants
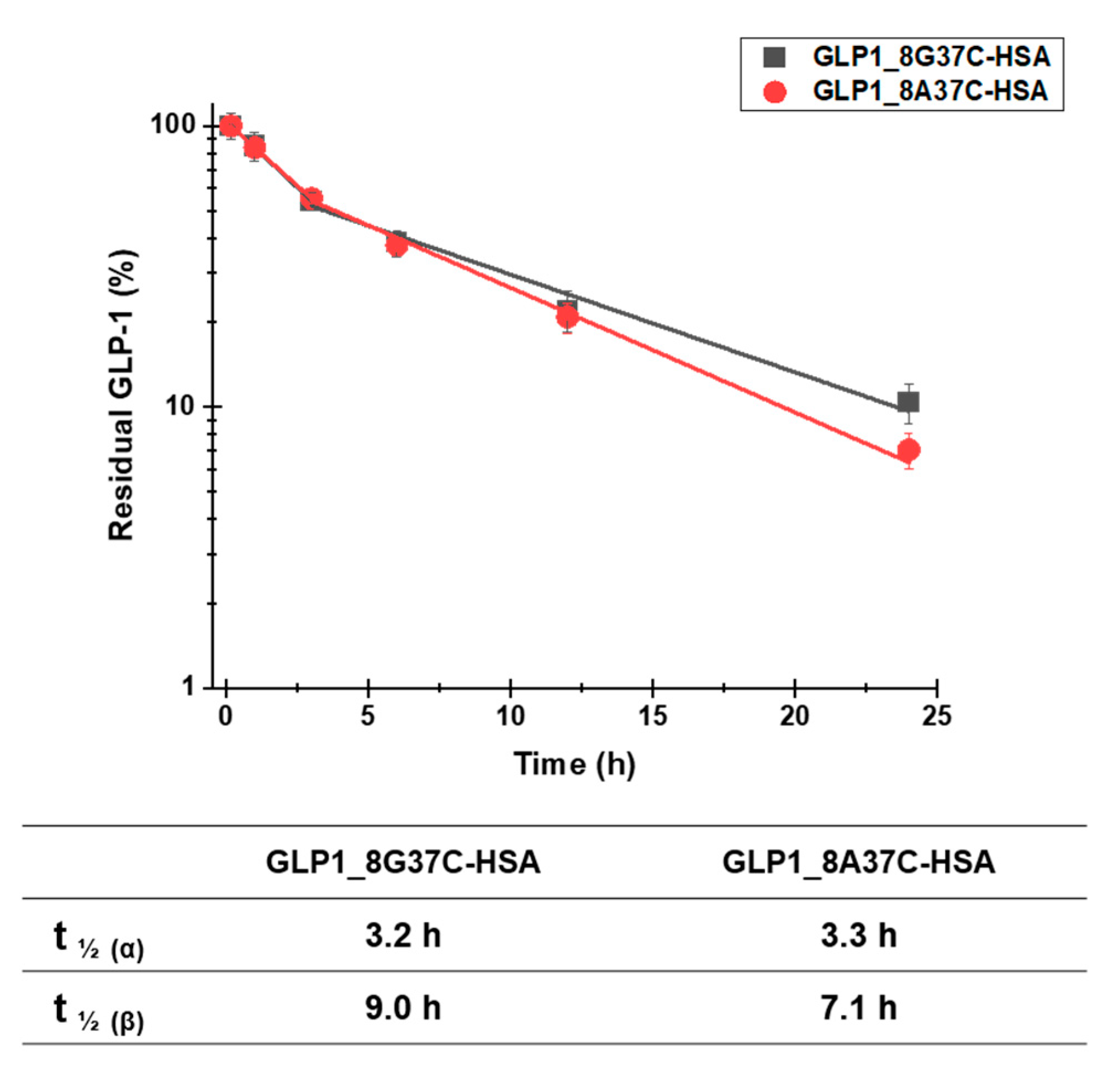
3.3. In Vivo Study
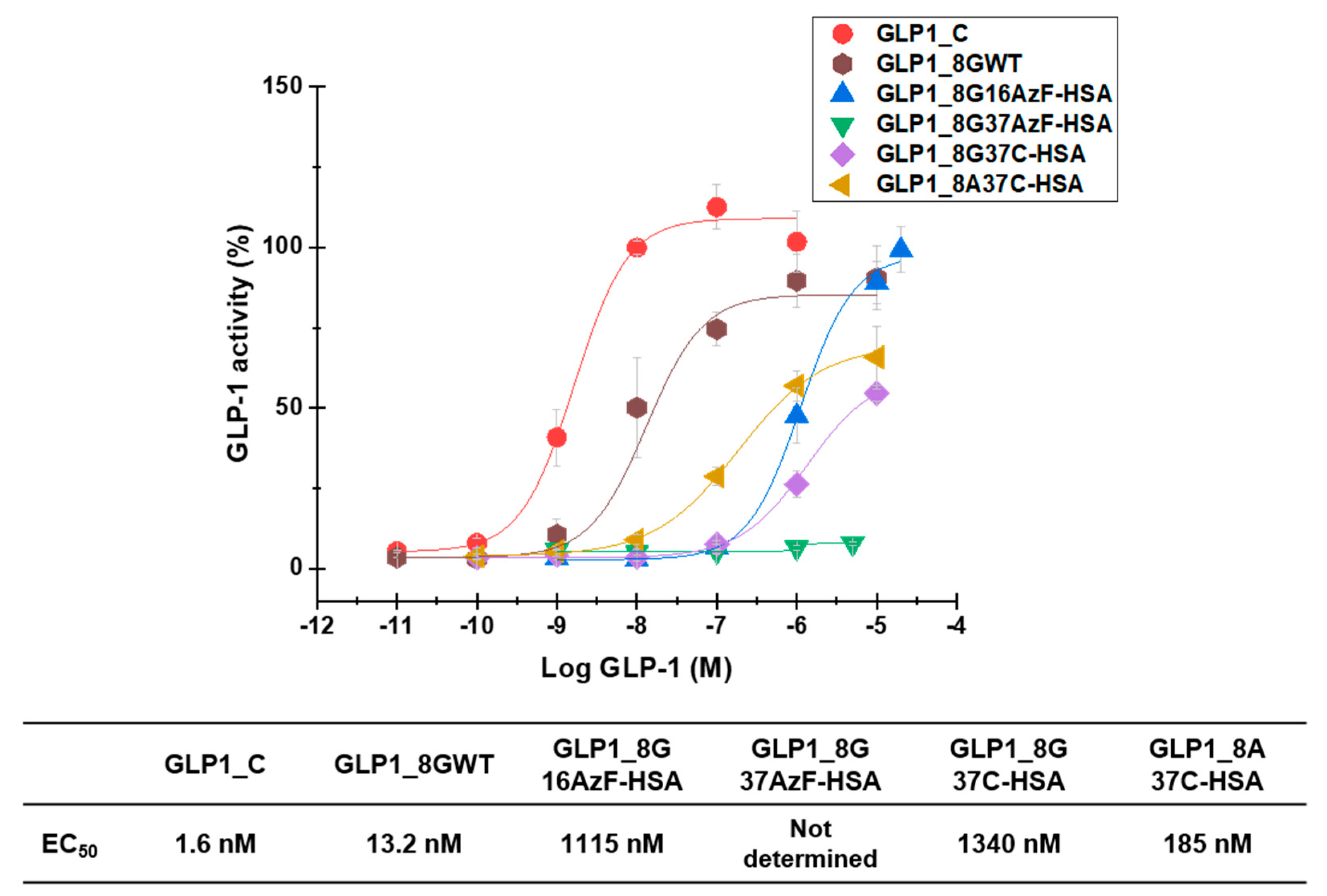
3.4. In Vitro Activity Assay
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Emerging Risk Factors Collaboration; Sarwar, N.; Gao, P.; Seshasai, S.R.K.; Gobin, R.; Kaptoge, S.; di Angelantonio, E.; Ingelsson, E.; Lawlor, D.A.; Selvin, E.; et al. The Emerging Risk Factors Collaboration Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: A collaborative meta-analysis of 102 prospective studies. Lancet 2010, 375, 2215–2222. [Google Scholar] [CrossRef] [PubMed]
- Nathan, D.M. For the DCCT/EDIC Research Group the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Study at 30 Years: Overview. Diabetes Care 2014, 37, 9–16. [Google Scholar] [CrossRef]
- Kieffer, T.J.; Habener, J.F. The Glucagon-Like Peptides. Endocr. Rev. 1999, 20, 876–913. [Google Scholar] [CrossRef] [PubMed]
- Sharma, D.; Verma, S.; Vaidya, S.; Kalia, K.; Tiwari, V. Recent updates on GLP-1 agonists: Current advancements & challenges. Biomed. Pharmacother. 2018, 108, 952–962. [Google Scholar] [CrossRef]
- Alvarez, E.; Martínez, M.D.; Roncero, I.; Chowen, J.A.; Garcia-Cuartero, B.; Gispert, J.D.; Sanz, C.; Vazquez, P.; Maldonado, A.; De Cáceres, J.; et al. The expression of GLP-1 receptor mRNA and protein allows the effect of GLP-1 on glucose metabolism in the human hypothalamus and brainstem. J. Neurochem. 2005, 92, 798–806. [Google Scholar] [CrossRef]
- Bullock, B.P. Tissue distribution of messenger ribonucleic acid encoding the rat glucagon-like peptide-1 receptor. Endocrinol. 1996, 137, 2968–2978. [Google Scholar] [CrossRef] [PubMed]
- Campos, R.V.; Lee, Y.C.; Drucke, D.J. Divergent tissue-specific and developmental expression of receptors for glucagon and glucagon-like peptide-1 in the mouse. Endocrinology 1994, 134, 2156–2164. [Google Scholar] [CrossRef]
- Holst, J.J. The Physiology of Glucagon-like Peptide 1. Physiol. Rev. 2007, 87, 1409–1439. [Google Scholar] [CrossRef] [PubMed]
- Pabreja, K.A.; Mohd, M.; Koole, C.; Wootten, D.; Furness, S.G.B. Molecular mechanisms underlying physiological and receptor pleiotropic effects mediated by GLP-1R activation. Br. J. Pharmacol. 2014, 171, 1114–1128. [Google Scholar] [CrossRef] [PubMed]
- Doyle, M.E.; Egan, J.M. Mechanisms of action of glucagon-like peptide 1 in the pancreas. Pharmacol. Ther. 2007, 113, 546–593. [Google Scholar] [CrossRef]
- Buteau, J. GLP-1 receptor signaling: Effects on pancreatic β-cell proliferation and survival. Diabetes Metab. 2008, 34 (Suppl. 2), S73–S77. [Google Scholar] [CrossRef]
- Conover, C.; Lejeune, L.; Linberg, R.; Shum, K.; Shorr, R.G.L. Transitional Vacuole Formation Following a Bolus Infusion of Peg-Hemoglobin in the Rat. Artif. Cells Blood Substit. Biotechnol. 1996, 24, 599–611. [Google Scholar] [CrossRef] [PubMed]
- Bendele, A.; Seely, J.; Richey, C.; Sennello, G.; Shopp, G. Short Communication: Renal Tubular Vacuolation in Animals Treated with Polyethylene-Glycol-Conjugated Proteins. Toxicol. Sci. 1998, 42, 152–157. [Google Scholar] [CrossRef] [PubMed]
- Verhoef, J.J.F.; Anchordoquy, T.J. Questioning the use of PEGylation for drug delivery. Drug Deliv. Transl. Res. 2013, 3, 499–503. [Google Scholar] [CrossRef] [PubMed]
- Thi, T.T.H.; Pilkington, E.H.; Nguyen, D.H.; Lee, J.S.; Park, K.D.; Truong, N.P. The Importance of Poly(ethylene glycol) Alternatives for Overcoming PEG Immunogenicity in Drug Delivery and Bioconjugation. Polymers 2020, 12, 298. [Google Scholar] [CrossRef]
- Kozma, G.T.; Shimizu, T.; Ishida, T.; Szebeni, J. Anti-PEG antibodies: Properties, formation, testing and role in adverse immune reactions to PEGylated nano-biopharmaceuticals. Adv. Drug Deliv. Rev. 2020, 154–155, 163–175. [Google Scholar] [CrossRef]
- Yang, Q.; Lai, S.K. Anti-PEG immunity: Emergence, characteristics, and unaddressed questions. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2015, 7, 655–677. [Google Scholar] [CrossRef]
- Yang, B.; Kim, J.C.; Seong, J.; Tae, G.; Kwon, I. Comparative studies of the serum half-life extension of a protein via site-specific conjugation to a species-matched or -mismatched albumin. Biomater. Sci. 2018, 6, 2092–2100. [Google Scholar] [CrossRef] [PubMed]
- Cho, J.; Park, J.; Kim, S.; Kim, J.C.; Tae, G.; Jin, M.S.; Kwon, I. Intramolecular distance in the conjugate of urate oxidase and fatty acid governs FcRn binding and serum half-life in vivo. J. Control. Release 2020, 321, 49–58. [Google Scholar] [CrossRef]
- Mentlein, R.; Gallwitz, B.; Schmidt, W.E. Dipeptidyl-peptidase IV hydrolyses gastric inhibitory polypeptide, glucagon-like peptide-1(7-36)amide, peptide histidine methionine and is responsible for their degradation in human serum. Eur. J. Biochem. 1993, 214, 829–835. [Google Scholar] [CrossRef] [PubMed]
- Donnelly, D. The structure and function of the glucagon-like peptide-1 receptor and its ligands. Br. J. Pharmacol. 2012, 166, 27–41. [Google Scholar] [CrossRef]
- Knudsen, L.B.; Lau, J. The Discovery and Development of Liraglutide and Semaglutide. Front. Endocrinol. 2019, 10, 155. [Google Scholar] [CrossRef] [PubMed]
- Deacon, C.F.; Knudsen, L.B.; Madsen, K.; Wiberg, F.C.; Jacobsen, O.; Holst, J.J. Dipeptidyl peptidase IV resistant analogues of glucagon-like peptide-1 which have extended metabolic stability and improved biological activity. Diabetologia 1998, 41, 271–278. [Google Scholar] [CrossRef] [PubMed]
- Bak, M.; Park, J.; Min, K.; Cho, J.; Seong, J.; Hahn, Y.S.; Tae, G.; Kwon, I. Recombinant Peptide Production Platform Coupled with Site-Specific Albumin Conjugation Enables a Convenient Production of Long-Acting Therapeutic Peptide. Pharmaceutics 2020, 12, 364. [Google Scholar] [CrossRef] [PubMed]
- Alavi, S.E.; Cabot, P.J.; Yap, G.Y.; Moyle, P.M. Optimized Methods for the Production and Bioconjugation of Site-Specific, Alkyne-Modified Glucagon-like Peptide-1 (GLP-1) Analogs to Azide-Modified Delivery Platforms Using Copper-Catalyzed Alkyne–Azide Cycloaddition. Bioconjugate Chem. 2020, 31, 1820–1834. [Google Scholar] [CrossRef] [PubMed]
- Knudsen, L.B.; Nielsen, P.F.; Huusfeldt, P.O.; Johansen, N.L.; Madsen, K.; Pedersen, F.Z.; Thøgersen, H.; Wilken, M.; Agersø, H. Potent Derivatives of Glucagon-like Peptide-1 with Pharmacokinetic Properties Suitable for Once Daily Administration. J. Med. Chem. 2000, 43, 1664–1669. [Google Scholar] [CrossRef] [PubMed]
- Chin, J.W.; Santoro, S.W.; Martin, A.B.; King, D.S.; Wang, L.; Schultz, P.G. Addition ofp-Azido-l-phenylalanine to the Genetic Code ofEscherichiacoli. J. Am. Chem. Soc. 2002, 124, 9026–9027. [Google Scholar] [CrossRef] [PubMed]
- Lajoie, M.J.; Rovner, A.J.; Goodman, D.B.; Aerni, H.; Haimovich, A.D.; Kuznetsov, G.; Mercer, J.A.; Wang, H.H.; Carr, P.A.; Mosberg, J.A.; et al. Genomically recoded organisms expand Biological Functions. Science 2013, 342, 357–360. [Google Scholar] [CrossRef]
- Kroeze, W.K.; Sassano, M.F.; Huang, X.-P.; Lansu, K.; McCorvy, J.D.; Giguère, P.M.; Sciaky, N.; Roth, B.L. PRESTO-Tango as an open-source resource for interrogation of the druggable human GPCRome. Nat. Struct. Mol. Biol. 2015, 22, 362–369. [Google Scholar] [CrossRef]
- Mørtz, E.; Sareneva, T.; Haebel, S.; Julkunen, I.; Roepstorff, P. Mass spectrometric characterization of glycosylated interferon-γ variants separated by gel electrophoresis. Electrophoresis 1996, 17, 925–931. [Google Scholar] [CrossRef]
- Yang, B.; Kwon, K.; Jana, S.; Kim, S.; Avila-Crump, S.; Tae, G.; Mehl, R.A.; Kwon, I. Temporal Control of Efficient In Vivo Bioconjugation Using a Genetically Encoded Tetrazine-Mediated Inverse-Electron-Demand Diels–Alder Reaction. Bioconjugate Chem. 2020, 31, 2456–2464. [Google Scholar] [CrossRef] [PubMed]
- Kieffer, T.J.; McIntosh, C.H.; Pederson, R.A. Degradation of glucose-dependent insulinotropic polypeptide and truncated glucagon-like peptide 1 in vitro and in vivo by dipeptidyl peptidase IV. Endocrinology 1995, 136, 3585–3596. [Google Scholar] [CrossRef] [PubMed]
- Vilsbøll, T.; Agersø, H.; Krarup, T.; Holst, J.J. Similar Elimination Rates of Glucagon-Like Peptide-1 in Obese Type 2 Diabetic Patients and Healthy Subjects. J. Clin. Endocrinol. Metab. 2003, 88, 220–224. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, J.; Bak, M.; Min, K.; Kim, H.-W.; Cho, J.-H.; Tae, G.; Kwon, I. Effect of C-terminus Conjugation via Different Conjugation Chemistries on In Vivo Activity of Albumin-Conjugated Recombinant GLP-1. Pharmaceutics 2021, 13, 263. https://doi.org/10.3390/pharmaceutics13020263
Park J, Bak M, Min K, Kim H-W, Cho J-H, Tae G, Kwon I. Effect of C-terminus Conjugation via Different Conjugation Chemistries on In Vivo Activity of Albumin-Conjugated Recombinant GLP-1. Pharmaceutics. 2021; 13(2):263. https://doi.org/10.3390/pharmaceutics13020263
Chicago/Turabian StylePark, Junyong, Mijeong Bak, Kiyoon Min, Hyun-Woo Kim, Jeong-Haeng Cho, Giyoong Tae, and Inchan Kwon. 2021. "Effect of C-terminus Conjugation via Different Conjugation Chemistries on In Vivo Activity of Albumin-Conjugated Recombinant GLP-1" Pharmaceutics 13, no. 2: 263. https://doi.org/10.3390/pharmaceutics13020263
APA StylePark, J., Bak, M., Min, K., Kim, H.-W., Cho, J.-H., Tae, G., & Kwon, I. (2021). Effect of C-terminus Conjugation via Different Conjugation Chemistries on In Vivo Activity of Albumin-Conjugated Recombinant GLP-1. Pharmaceutics, 13(2), 263. https://doi.org/10.3390/pharmaceutics13020263