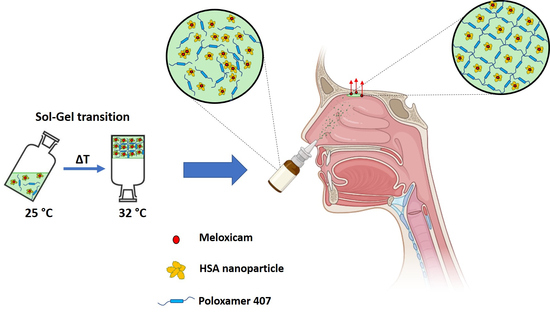
Development of In Situ Gelling Meloxicam-Human Serum Albumin Nanoparticle Formulation for Nose-to-Brain Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
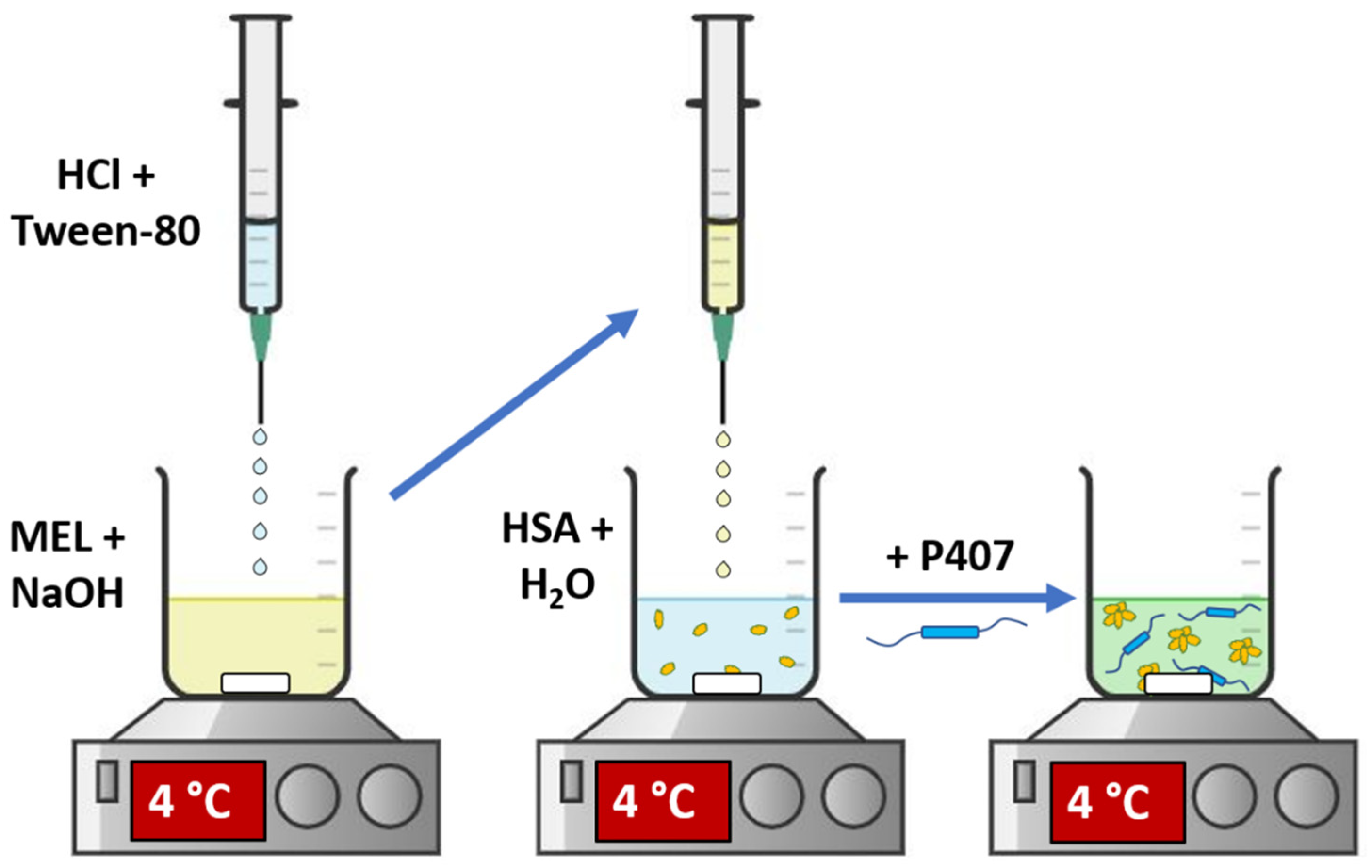
2.2. Preparation of In Situ Gelling MEL-HSA Nanoparticle Formulations
2.3. QbD-Based Comparative Risk Assessment
2.4. Optimization of In Situ Thermogelling Carrier System
2.4.1. Rheological Studies
2.4.2. Muco-Adhesion Measurement
2.4.3. Characterization of Nanoparticles
2.4.4. Rapid Equilibrium Dialysis (RED)
2.4.5. High Performance Liquid Chromatography (HPLC)
2.4.6. In Vitro BBB Permeability Assay
2.5. Cell Line Studies with Optimized Formulation
2.5.1. Cell Cultures
2.5.2. Cell Viability Measurements
2.5.3. Permeability Study on the Co-Cultured Model
2.5.4. Treatments of Cultured Cells
2.5.5. Immunohistochemistry
2.6. Statistical Analysis
3. Results
3.1. Comparative Risk Assessment of MEL-HSA and MEL-HSA-P407
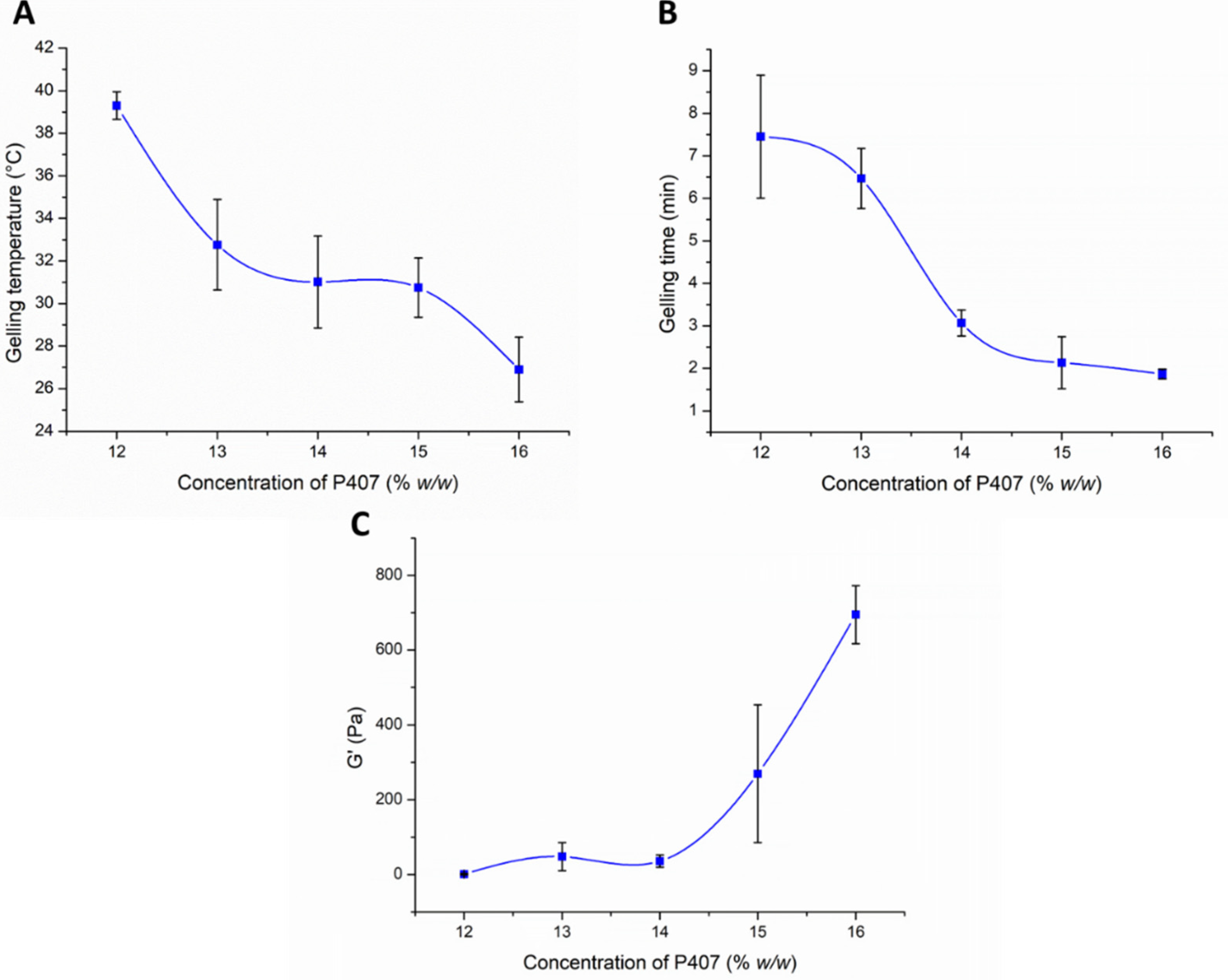
3.2. Optimization of P407 Concentration in the Formulation
3.2.1. Investigation of In Situ Thermo-Gelling Properties
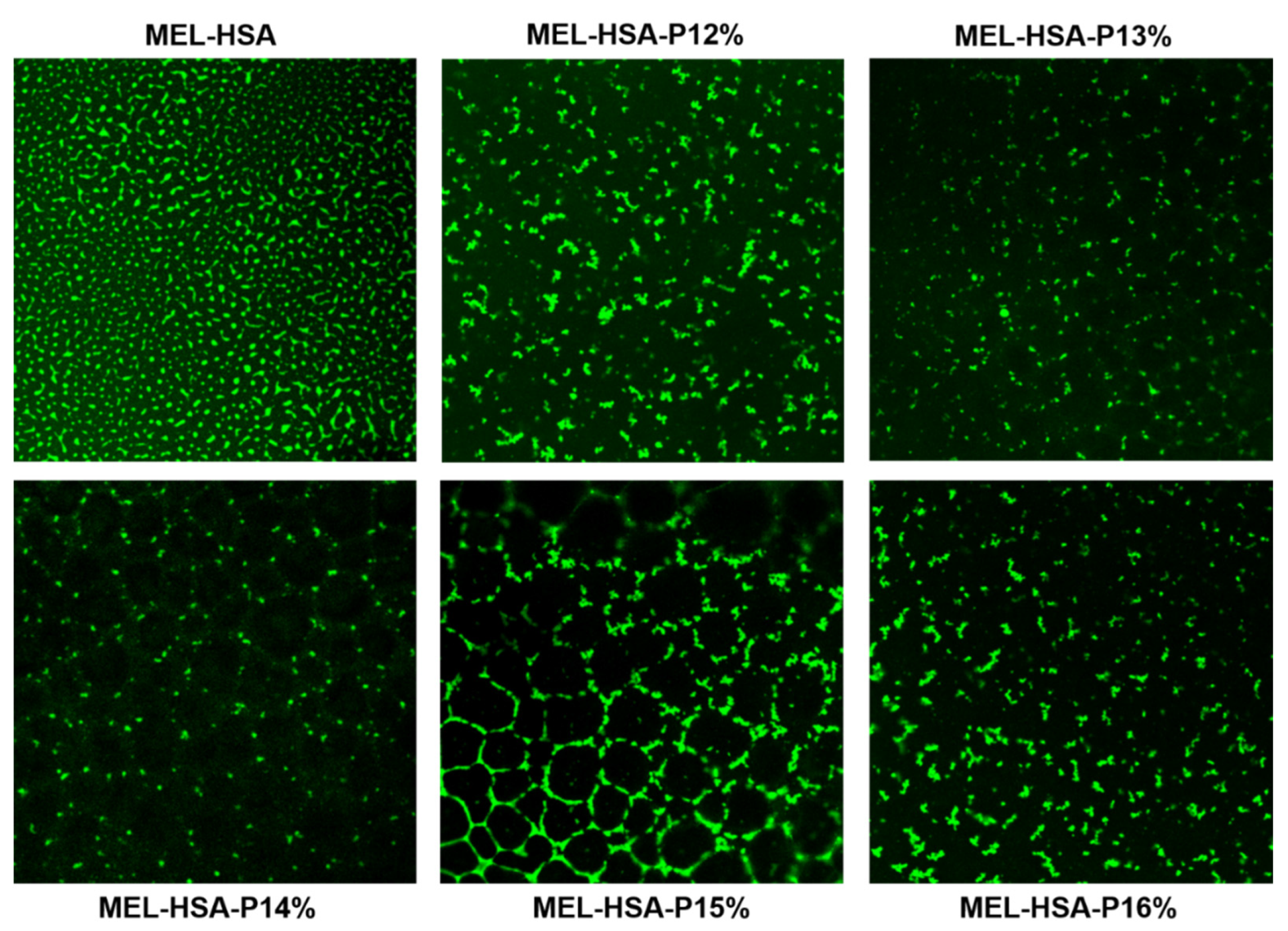
3.2.2. Muco-Adhesion Measurement
3.2.3. Characterization of Carrier Systems
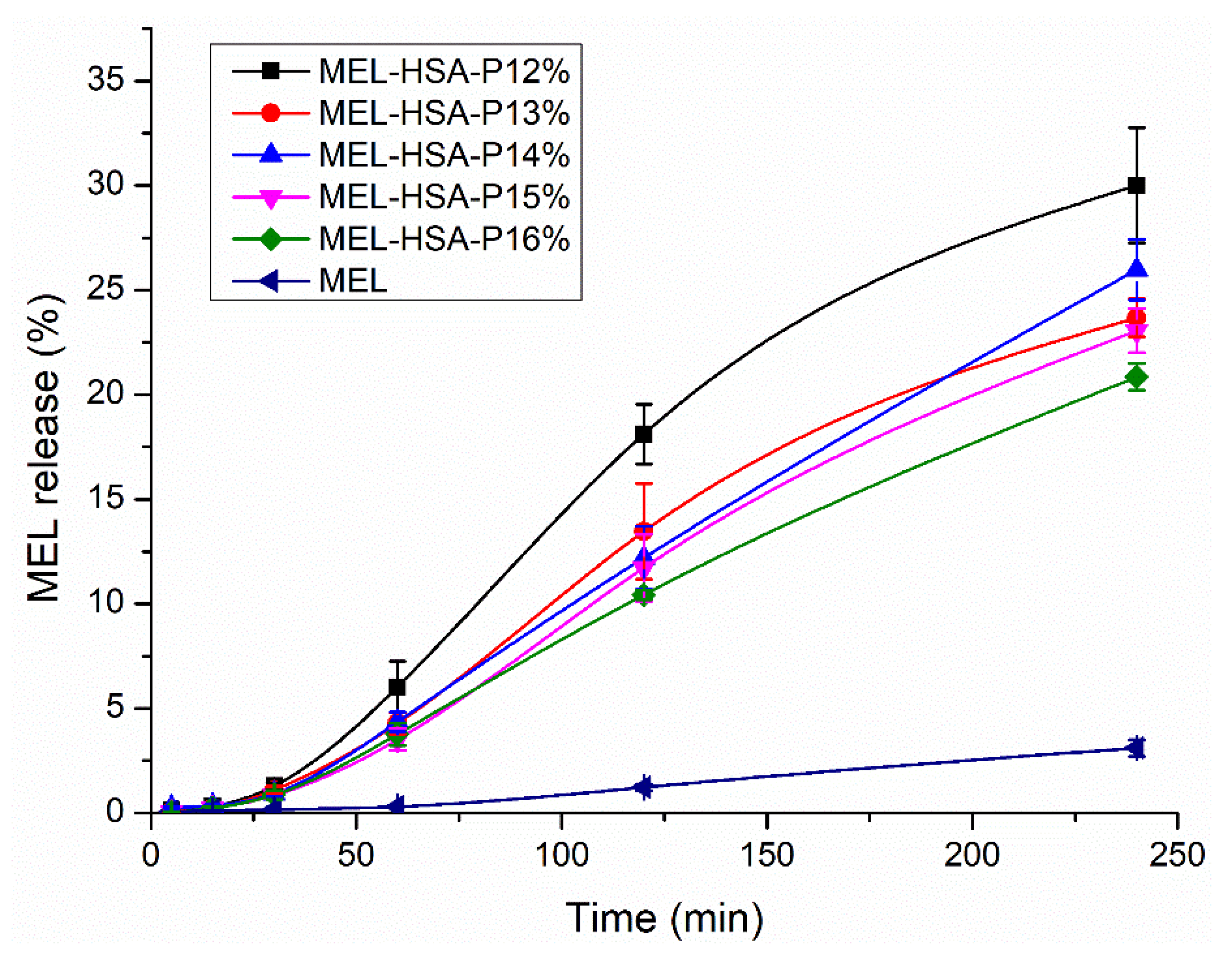
3.2.4. In Vitro Dissolution Profiles (RED)
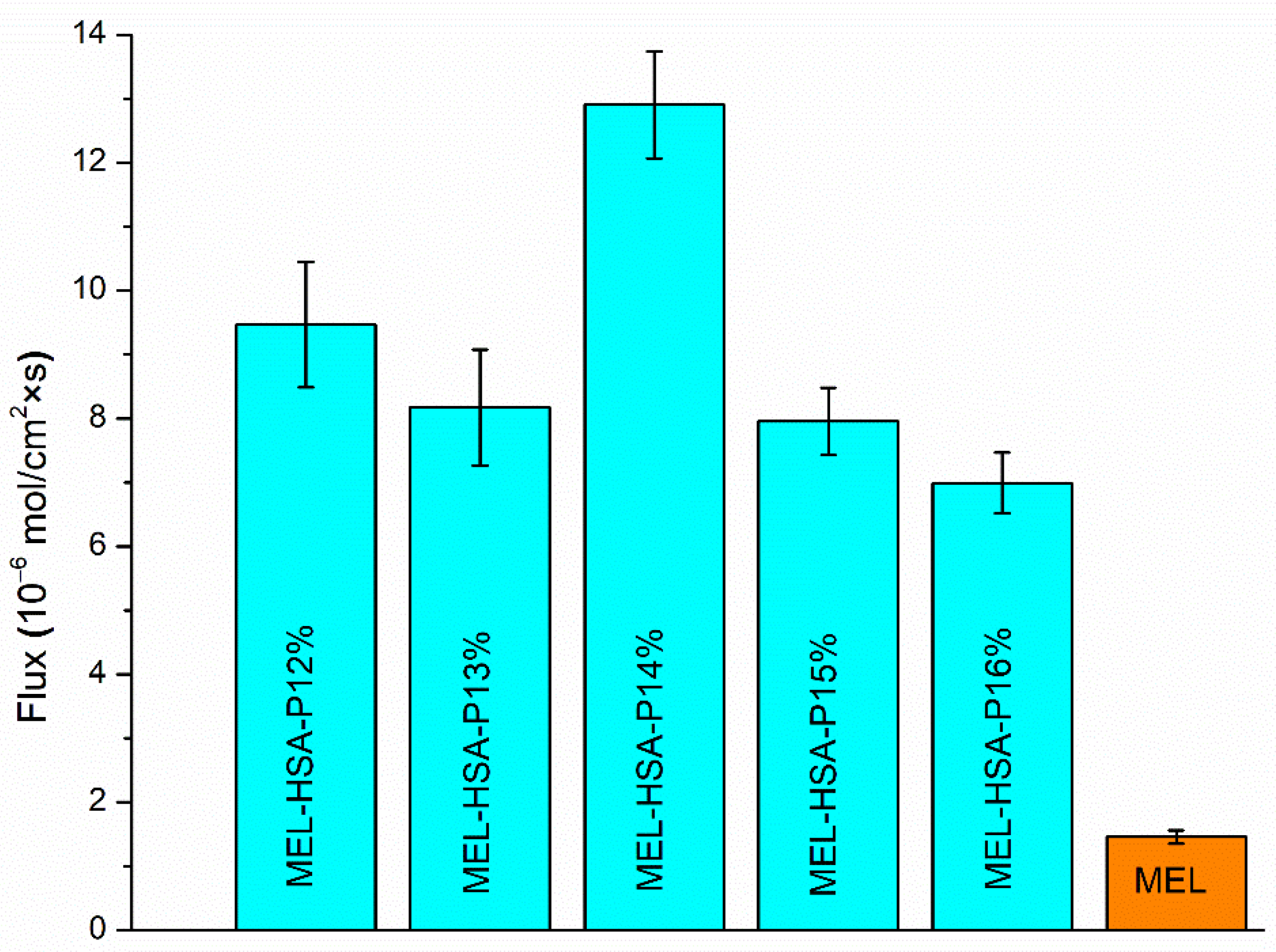
3.2.5. In Vitro BBB Permeability
3.3. Evaluation of Cell Line Studies
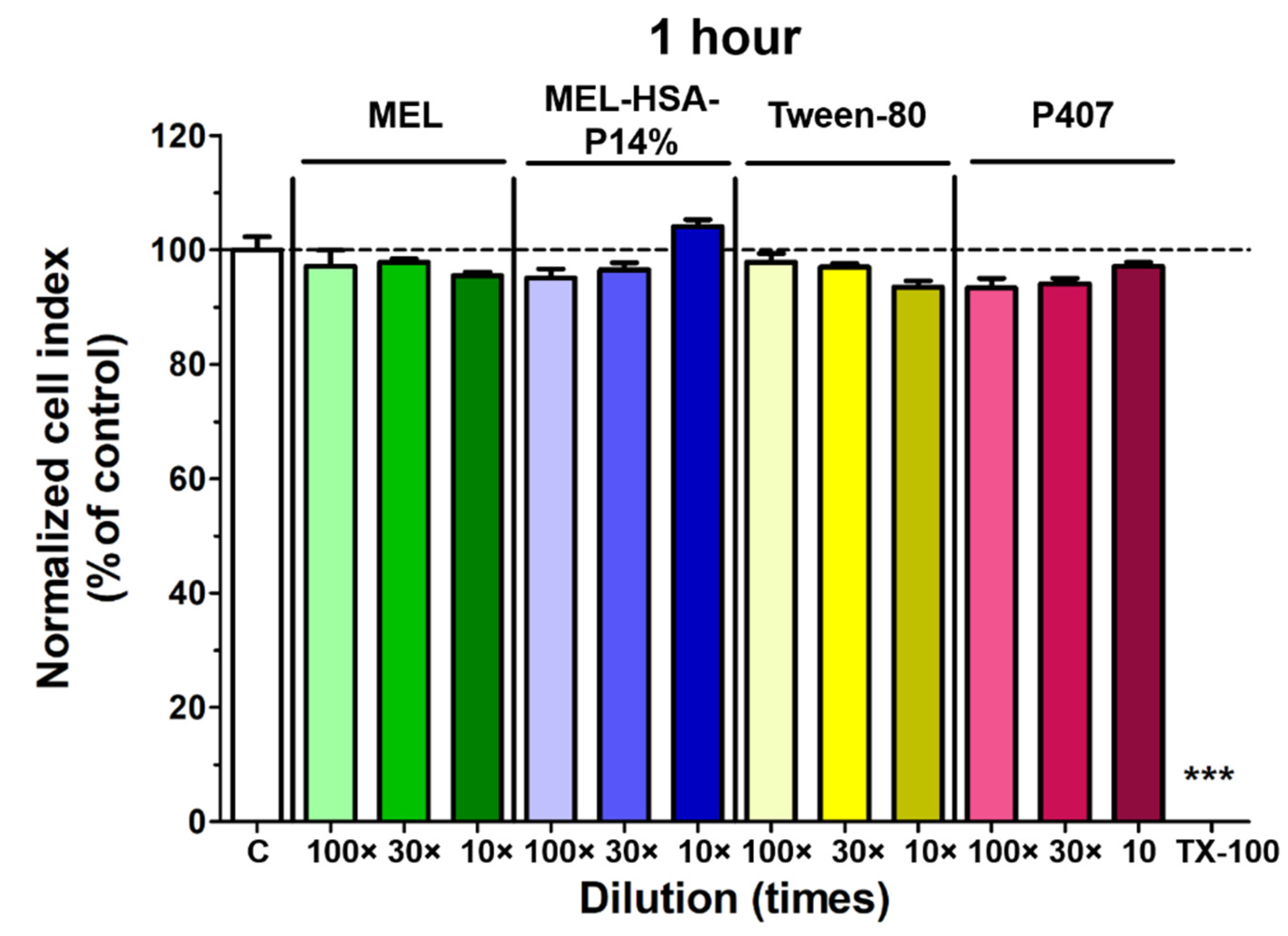
3.3.1. Cell Viability Assay
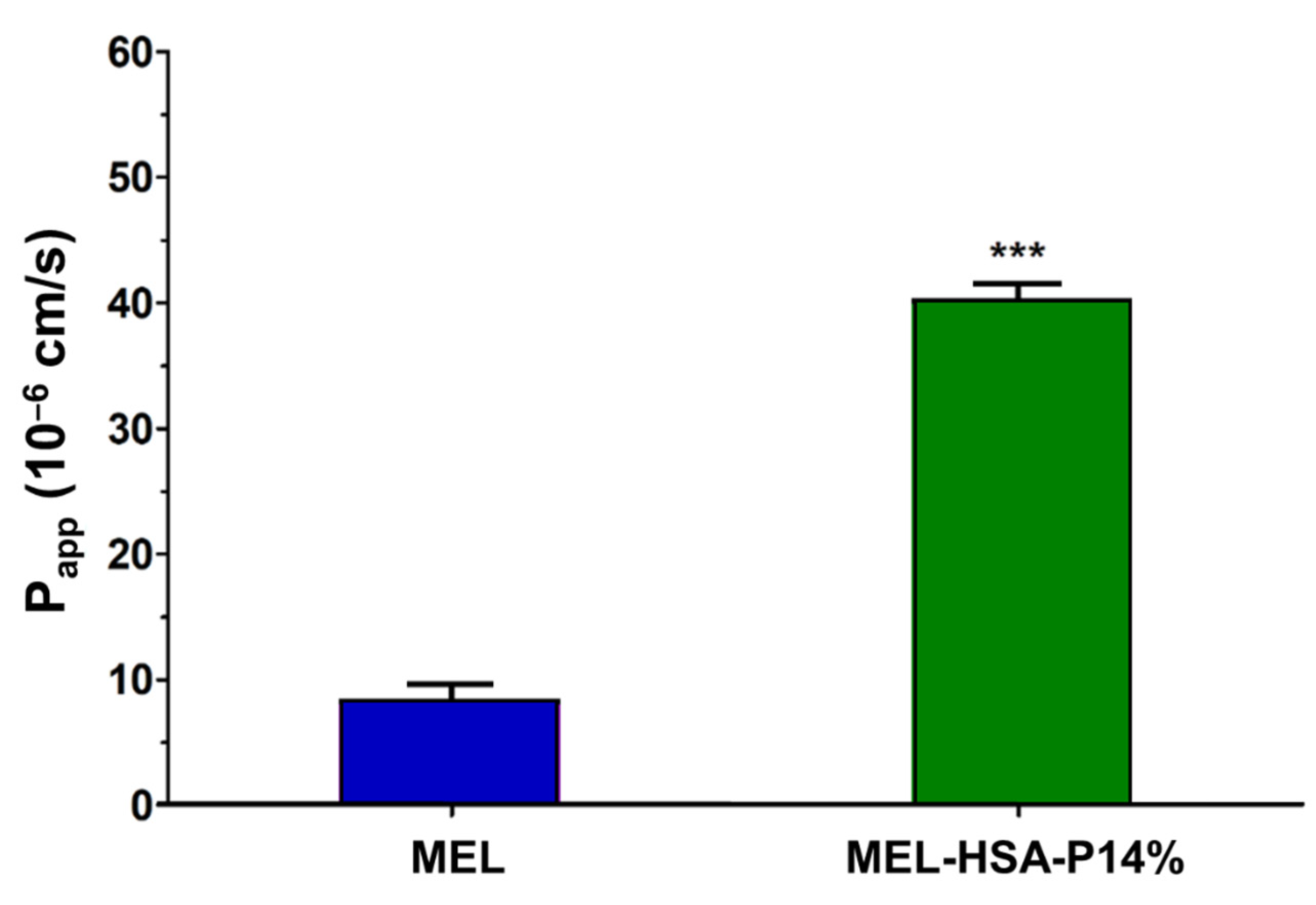
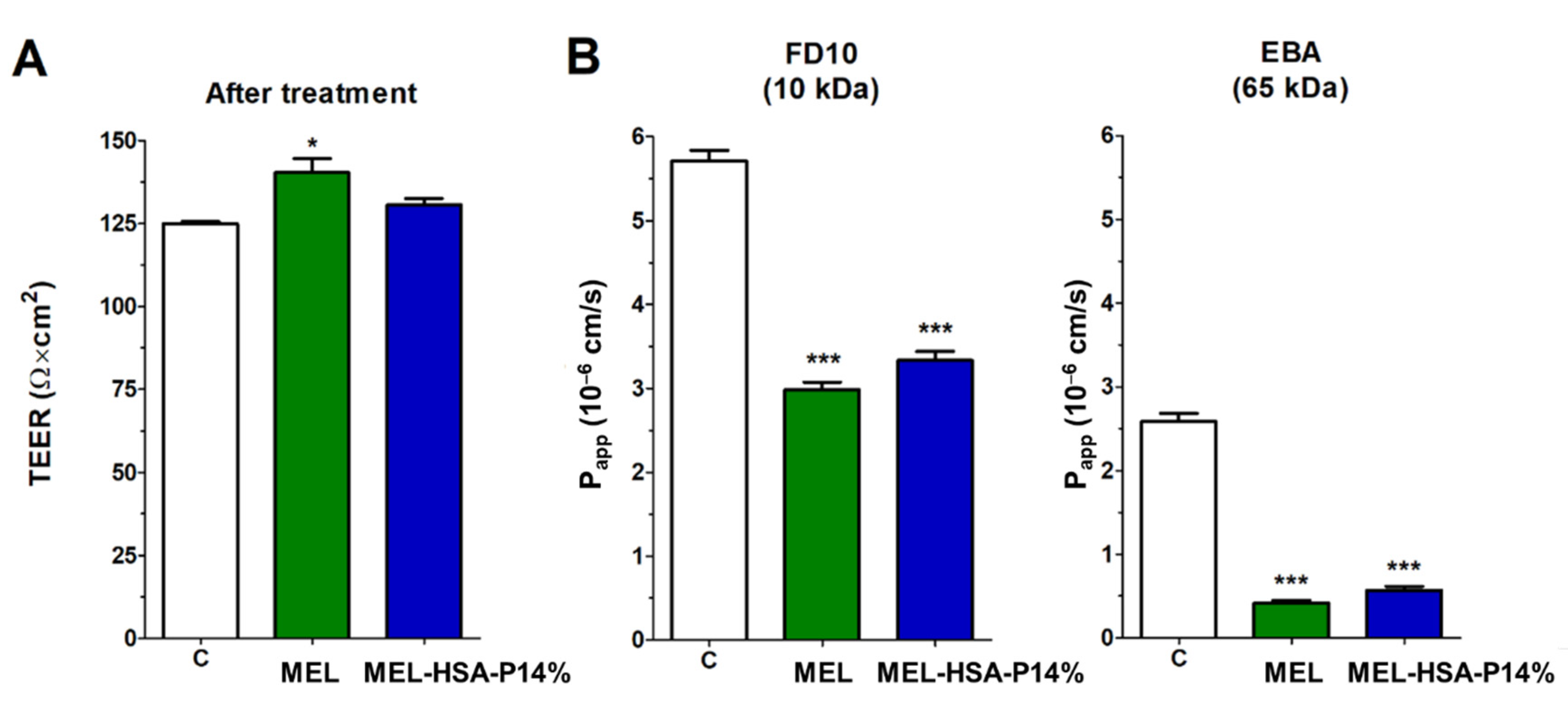
3.3.2. MEL Permeability across the Culture Model of the Nasal Mucosa Barrier
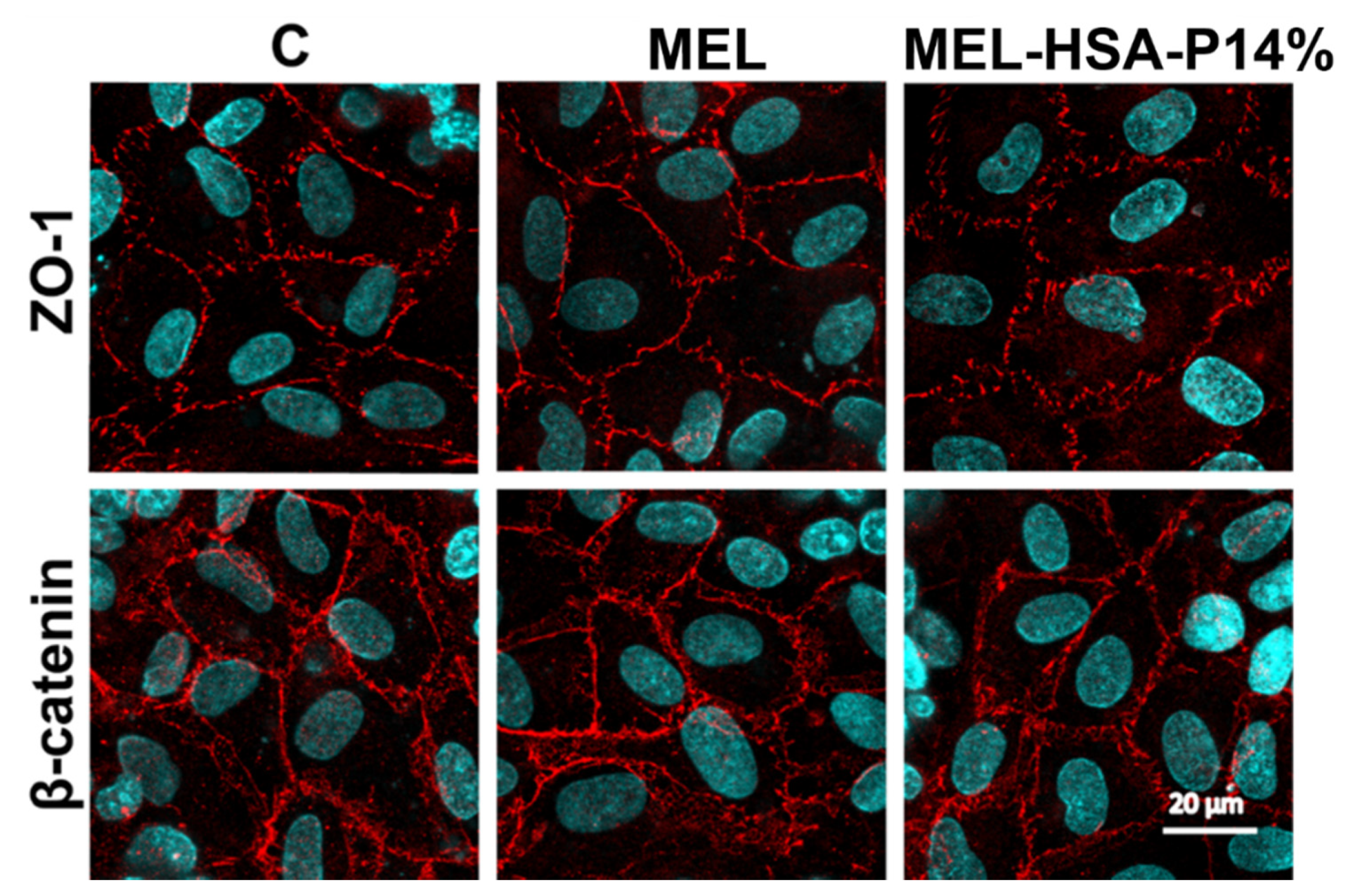
3.3.3. Immunohistochemistry
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hoogenboezem, E.N.; Duvall, C.L. Harnessing albumin as a carrier for cancer therapies. Adv. Drug Deliv. Rev. 2018, 130, 73–89. [Google Scholar] [CrossRef] [PubMed]
- Goverdhan, P.; Sravanthi, A.; Mamatha, T. Neuroprotective effects of Meloxicam and Selegiline in scopolamine-induced cognitive impairment and oxidative stress. Int. J. Alzheimers Dis. 2012, 2012. [Google Scholar] [CrossRef] [PubMed]
- Cimen, M.Y.B.; Cimen, Ö.B.; Eskandari, G.; Sahin, G.; Erdoǧan, C.; Atik, U. In vivo effects of meloxicam, celecoxib, and ibuprofen on free radical metabolism in human erythrocytes. Drug Chem. Toxicol. 2003, 26, 169–176. [Google Scholar] [CrossRef]
- Ianiski, F.R.; Alves, C.B.; Ferreira, C.F.; Rech, V.C.; Savegnago, L.; Wilhelm, E.A.; Luchese, C. Meloxicam-loaded nanocapsules as an alternative to improve memory decline in an Alzheimer’s disease model in mice: Involvement of Na+, K+-ATPase. Metab. Brain Dis. 2016, 31, 793–802. [Google Scholar] [CrossRef]
- Szabó-Révész, P. Modifying the physicochemical properties of NSAIDs for nasal and pulmonary administration. Drug Discov. Today Technol. 2018, 27, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Merkus, F.W.H.M.; van den Berg, M.P. Can nasal drug delivery bypass the blood-brain barrier? Drugs R D 2007, 8, 133–144. [Google Scholar] [CrossRef]
- van Hoesen, G.W.; Hyman, B.T.; Damasio, A.R. Entorhinal cortex pathology in Alzheimer’s disease. Hippocampus 1991, 1, 1–8. [Google Scholar] [CrossRef]
- Mistry, A.; Stolnik, S.; Illum, L. Nanoparticles for direct nose-to-brain delivery of drugs. Int. J. Pharm. 2009, 379, 146–157. [Google Scholar] [CrossRef]
- Agrawal, M.; Saraf, S.; Saraf, S.; Antimisiaris, S.G.; Chougule, M.B.; Shoyele, S.A.; Alexander, A. Nose-to-brain drug delivery: An update on clinical challenges and progress towards approval of anti-Alzheimer drugs. J. Control. Release 2018, 281, 139–177. [Google Scholar] [CrossRef]
- Lalatsa, A.; Schatzlein, A.G.; Uchegbu, I.F. Strategies to deliver peptide drugs to the brain. Mol. Pharm. 2014, 11, 1081–1093. [Google Scholar] [CrossRef]
- Novakova, I.; Subileau, E.A.; Toegel, S.; Gruber, D.; Lachmann, B.; Urban, E.; Chesne, C.; Noe, C.R.; Neuhaus, W. Transport rankings of non-steroidal antiinflammatory drugs across blood-brain barrier in vitro models. PLoS ONE 2014, 9, 1–14. [Google Scholar] [CrossRef]
- Sipos, B.; Szabó-Révész, P.; Csóka, I.; Pallagi, E.; Dobó, D.G.; Bélteky, P.; Kónya, Z.; Deák, Á.; Janovák, L.; Katona, G. Quality by design based formulation study of meloxicam-loaded polymeric micelles for intranasal administration. Pharmaceutics 2020, 12, 697. [Google Scholar] [CrossRef] [PubMed]
- Katona, G.; Balogh, G.T.; Dargó, G.; Gáspár, R.; Márki, Á.; Ducza, E.; Sztojkov-Ivanov, A.; Tömösi, F.; Kecskeméti, G.; Janáky, T.; et al. Development of meloxicam-human serum albumin nanoparticles for nose-to-brain delivery via application of a quality by design approach. Pharmaceutics 2020, 12, 97. [Google Scholar] [CrossRef] [PubMed]
- Tas, C.; Ozkan, C.K.; Savaser, A.; Ozkan, Y.; Tasdemir, U.; Altunay, H. Nasal absorption of metoclopramide from different Carbopol® 981 based formulations: In vitro, ex vivo and in vivo evaluation. Eur. J. Pharm. Biopharm. 2006, 64, 246–254. [Google Scholar] [CrossRef] [PubMed]
- Casettari, L.; Illum, L. Chitosan in nasal delivery systems for therapeutic drugs. J. Control. Release 2014, 190, 189–200. [Google Scholar] [CrossRef]
- Mahajan, H.S.; Tyagi, V.K.; Patil, R.R.; Dusunge, S.B. Thiolated xyloglucan: Synthesis, characterization and evaluation as mucoadhesive in situ gelling agent. Carbohydr. Polym. 2013, 91, 618–625. [Google Scholar] [CrossRef]
- Dumortier, G.; Grossiord, J.L.; Zuber, M.; Couarraze, G.; Chaumeil, J.C. Rheological study of a thermoreversible morphine gel. Drug Dev. Ind. Pharm. 1991, 17, 1255–1265. [Google Scholar] [CrossRef]
- Dumortier, G.; Grossiord, J.L.; Agnely, F.; Chaumeil, J.C. A review of poloxamer 407 pharmaceutical and pharmacological characteristics. Pharm. Res. 2006, 23, 2709–2728. [Google Scholar] [CrossRef] [PubMed]
- Balakrishnan, P.; Park, E.K.; Song, C.K.; Ko, H.J.; Hahn, T.W.; Song, K.W.; Cho, H.J. Carbopol-Incorporated thermoreversible gel for intranasal drug delivery. Molecules 2015, 20, 4124–4135. [Google Scholar] [CrossRef]
- Fakhari, A.; Corcoran, M.; Schwarz, A. Thermogelling properties of purified poloxamer 407. Heliyon 2017, 3, e00390. [Google Scholar] [CrossRef]
- Csóka, I.; Pallagi, E.; Paál, T.L. Extension of quality-by-design concept to the early development phase of pharmaceutical R&D processes. Drug Discov. Today 2018, 23, 1340–1343. [Google Scholar] [CrossRef]
- Javed, M.N.; Alam, M.S.; Waziri, A.; Pottoo, F.H.; Yadav, A.K.; Hasnain, M.S.; Almalki, F.A. QbD Applications for the Development of Nanopharmaceutical Products. Pharm. Qual. Des. 2019, 229–253. [Google Scholar] [CrossRef]
- Li, Q.; Chen, F.; Liu, Y.; Yu, S.; Gai, X.; Ye, M.; Yang, X.; Pan, W. A novel albumin wrapped nanosuspension of meloxicam to improve inflammation-targeting effects. Int. J. Nanomed. 2018, 13, 4711–4725. [Google Scholar] [CrossRef]
- Rather, M.A.; Amin, S.; Maqbool, M.; Bhat, Z.S.; Gupta, P.N.; Ahmad, Z. Preparation and in vitro characterization of albumin nanoparticles encapsulating an anti-tuberculosis drug-levofloxacin. Adv. Sci. Eng. Med. 2016, 8, 912–917. [Google Scholar] [CrossRef]
- Müller, J.; Martins, A.; Csábi, J.; Fenyvesi, F.; Könczöl, Á.; Hunyadi, A.; Balogh, G.T. BBB penetration-targeting physicochemical lead selection: Ecdysteroids as chemo-sensitizers against CNS tumors. Eur. J. Pharm. Sci. 2017, 96, 571–577. [Google Scholar] [CrossRef]
- Avdeef, A. Permeability-PAMPA. In Absorption and Drug Development; John Wiley & Sons: Hoboken, NJ, USA, 2012; pp. 319–498. [Google Scholar]
- Kürti, L.; Veszelka, S.; Bocsik, A.; Ózsvári, B.; Puskás, L.G.; Kittel, Á.; Szabó-Révész, P.; Deli, M.A. Retinoic acid and hydrocortisone strengthen the barrier function of human RPMI 2650 cells, a model for nasal epithelial permeability. Cytotechnology 2013, 65, 395–406. [Google Scholar] [CrossRef] [PubMed]
- Pedroso, D.C.S.; Tellechea, A.; Moura, L.; Fidalgo-Carvalho, I.; Duarte, J.; Carvalho, E.; Ferreira, L. Improved survival, vascular differentiation and wound healing potential of stem cells co-cultured with endothelial cells. PLoS ONE 2011, 6, 1–12. [Google Scholar] [CrossRef]
- Cecchelli, R.; Aday, S.; Sevin, E.; Almeida, C.; Culot, M.; Dehouck, L.; Coisne, C.; Engelhardt, B.; Dehouck, M.P.; Ferreira, L. A stable and reproducible human blood-brain barrier model derived from hematopoietic stem cells. PLoS ONE 2014, 9. [Google Scholar] [CrossRef]
- Harazin, A.; Bocsik, A.; Barna, L.; Kincses, A.; Váradi, J.; Fenyvesi, F.; Tubak, V.; Deli, M.A.; Vecsernyés, M. Protection of cultured brain endothelial cells from cytokine-induced damage by α-melanocyte stimulating hormone. PeerJ 2018, 2018, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Bocsik, A.; Gróf, I.; Kiss, L.; Ötvös, F.; Zsíros, O.; Daruka, L.; Fülöp, L.; Vastag, M.; Kittel, Á.; Imre, N.; et al. Dual action of the PN159/KLAL/MAP peptide: Increase of drug penetration across caco-2 intestinal barrier model by modulation of tight junctions and plasma membrane permeability. Pharmaceutics 2019, 11, 73. [Google Scholar] [CrossRef]
- King, M. The role of mucus viscoelasticity in cough clearance. Biorheology 1987, 24, 589–597. [Google Scholar] [CrossRef]
- Mayol, L.; Biondi, M.; Quaglia, F.; La Rotonda, M.I.; Fusco, S.; Borzacchiello, A.; Ambrosio, L. Injectable thermally responsive mucoadhesive gel for sustained protein delivery. In Proceedings of the 24th European Conference on Biomaterials—Annual Conference of the European Society for Biomaterials, Dublin, Ireland, 4–9 September 2011; pp. 28–33. [Google Scholar]
- Gao, L.; Zhang, D.; Chen, M. Drug nanocrystals for the formulation of poorly soluble drugs and its application as a potential drug delivery system. J. Nanoparticle Res. 2008, 10, 845–862. [Google Scholar] [CrossRef]
- Wójcik-Pastuszka, D.; Krzak, J.; Macikowski, B.; Berkowski, R.; Osiński, B.; Musiał, W. Evaluation of the release kinetics of a pharmacologically active substance from model intra-articular implants replacing the cruciate ligaments of the knee. Materials 2019, 12, 1202. [Google Scholar] [CrossRef] [PubMed]
- Barichello, J.M.; Morishita, M.; Takayama, K.; Nagai, T. Absorption of insulin from Pluronic F-127 gels following subcutaneous administration in rats. Int. J. Pharm. 1999, 184, 189–198. [Google Scholar] [CrossRef]
- Nie, S.; Hsiao, W.W.; Pan, W.; Yang, Z. Thermoreversible pluronic® F127-based hydrogel containing liposomes for the controlled delivery of paclitaxel: In vitro drug release, cell cytotoxicity, and uptake studies. Int. J. Nanomed. 2011, 6, 151–166. [Google Scholar] [CrossRef]
- Zhang, L.; Parsons, D.L.; Navarre, C.; Kompella, U.B. Development and in-vitro evaluation of sustained release Poloxamer 407 (P407) gel formulations of ceftiofur. J. Control. Release 2002, 85, 73–81. [Google Scholar] [CrossRef]
- Liu, Y.; Lu, W.L.; Wang, J.C.; Zhang, X.; Zhang, H.; Wang, X.Q.; Zhou, T.Y.; Zhang, Q. Controlled delivery of recombinant hirudin based on thermo-sensitive Pluronic® F127 hydrogel for subcutaneous administration: In vitro and in vivo characterization. J. Control. Release 2007, 117, 387–395. [Google Scholar] [CrossRef]
- Biswas, G.; Kim, W.; Kim, K.T.; Cho, J.; Jeong, D.; Song, K.H.; Im, J. Synthesis of Ibuprofen Conjugated Molecular Transporter Capable of Enhanced Brain Penetration. J. Chem. 2017, 2017. [Google Scholar] [CrossRef]
- Wong, L.R.; Ho, P.C. Role of serum albumin as a nanoparticulate carrier for nose-to-brain delivery of R-flurbiprofen: Implications for the treatment of Alzheimer’s disease. J. Pharm. Pharmacol. 2018, 70, 59–69. [Google Scholar] [CrossRef] [PubMed]
- Falcone, J.A.; Salameh, T.S.; Yi, X.; Cordy, B.J.; Mortell, W.G.; Kabanov, A.V.; Banks, W.A. Intranasal administration as a route for drug delivery to the brain: Evidence for a unique pathway for albumin. J. Pharmacol. Exp. Ther. 2014, 351, 54–60. [Google Scholar] [CrossRef]
- Taylor, M.; Tomlins, P.; Sahota, T. Thermoresponsive Gels. Gels 2017, 3, 4. [Google Scholar] [CrossRef]
- Zahir-Jouzdani, F.; Wolf, J.D.; Atyabi, F.; Bernkop-Schnürch, A. In situ gelling and mucoadhesive polymers: Why do they need each other? Expert Opin. Drug Deliv. 2018, 15, 1007–1019. [Google Scholar] [CrossRef]
- Shelke, S.; Shahi, S.; Jalalpure, S.; Dhamecha, D.; Shengule, S. Formulation and evaluation of thermoreversible mucoadhesive in-situ gel for intranasal delivery of naratriptan hydrochloride. J. Drug Deliv. Sci. Technol. 2015, 29, 238–244. [Google Scholar] [CrossRef]
- Ravi, P.R.; Aditya, N.; Patil, S.; Cherian, L. Nasal in-situ gels for delivery of rasagiline mesylate: Improvement in bioavailability and brain localization. Drug Deliv. 2015, 22, 903–910. [Google Scholar] [CrossRef]
- Chen, X.; Zhi, F.; Jia, X.; Zhang, X.; Ambardekar, R.; Meng, Z.; Paradkar, A.R.; Hu, Y.; Yang, Y. Enhanced brain targeting of curcumin by intranasal administration of a thermosensitive poloxamer hydrogel. J. Pharm. Pharmacol. 2013, 65, 807–816. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Jiang, S.; Wang, H.; Bie, H. A mucoadhesive, thermoreversible in situ nasal gel of geniposide for neurodegenerative diseases. PLoS ONE 2017, 12, e0189478. [Google Scholar] [CrossRef]
- Salatin, S.; Barar, J.; Barzegar-Jalali, M.; Adibkia, K.; Jelvehgari, M. Thermosensitive in situ nanocomposite of rivastigmine hydrogen tartrate as an intranasal delivery system: Development, characterization, ex vivo permeation and cellular studies. Colloids Surf. B 2017, 159, 629–638. [Google Scholar] [CrossRef]
- Pathan, I.B.; More, B. Formulation and characterization of intra nasal delivery of nortriptyline hydrochloride thermoreversible gelling system in treatment of depression. Acta Pharm. Sci. 2017, 55, 35–44. [Google Scholar] [CrossRef][Green Version]
- Singh, K.; HariKumar, S.L. Injectable in-situ gelling controlled release drug delivery system. Int. J. Drug Dev. Res. 2012, 4, 56–69. [Google Scholar]
- Mistry, A.; Stolnik, S.; Illum, L. Nose-to-Brain Delivery: Investigation of the Transport of Nanoparticles with Different Surface Characteristics and Sizes in Excised Porcine Olfactory Epithelium. Mol. Pharm. 2015, 12, 2755–2766. [Google Scholar] [CrossRef]
- Patel, R.B.; Patel, M.R.; Bhatt, K.K.; Patel, B.G.; Gaikwad, R.V. Evaluation of brain targeting efficiency of intranasal microemulsion containing olanzapine: Pharmacodynamic and pharmacokinetic consideration. Drug Deliv. 2016, 23, 307–315. [Google Scholar] [CrossRef] [PubMed]
- Kapoor, M.; Cloyd, J.C.; Siegel, R.A. A review of intranasal formulations for the treatment of seizure emergencies. J. Control. Release 2016, 237, 147–159. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.S.; Oh, K.T.; Choi, H.G.; Lim, S.J. Liposomal formulations for nose-to-brain delivery: Recent advances and future perspectives. Pharmaceutics 2019, 11, 540. [Google Scholar] [CrossRef] [PubMed]
- Giuliano, E.; Paolino, D.; Fresta, M.; Cosco, D. Mucosal applications of poloxamer 407-based hydrogels: An overview. Pharmaceutics 2018, 10, 159. [Google Scholar] [CrossRef] [PubMed]


| QTPP Element | Target |
|---|---|
| Carrier integrity | Nanosized particle size and distribution with uniform API content after gelation at gelation temperature. |
| Drug release in the nasal cavity | The formulation should release more MEL in the dissolution medium compared to initial MEL at pH 5.6. |
| Mucoadhesive properties | The mucoadhesive force and work should be high enough to meet the requirements of the nasal delivery. |
| Residence time on nasal mucosa | Increased residence time compared to non-gel formulations. |
| Nasal epithelial cellular uptake | The formulation should have higher permeability on nasal epithelial cells without damaging the cells forming the nasal barrier compared to raw MEL. |
| Transport in the central nervous system | The flux and permeability value of the gel formulation should be increased across BBB lipids compared to initial MEL |
| Notation of Formulation | Content of P407 (% w/w) | 25 °C | 35 °C | ZP (mV) | EE (%) | LC (%) | pH | Osmolality (mOsmol/kg) | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Z-Average (nm) | PdI | Z-Average (nm) | PdI | |||||||
| MEL-HSA-P12% | 12 | 172 ± 2 | 0.189 ± 0.02 | 180.7 ± 3 | 0.193 ± 0.03 | −9.4 ± 0.7 | 82.34 ± 0.12 | 1.26 ± 0.01 | 5.85 ± 0.08 | 200 ± 2 |
| MEL-HSA-P13% | 13 | 175 ± 2 | 0.211 ± 0.01 | 188.7 ± 2 | 0.282 ± 0.02 | −8.5 ± 0.5 | 82.01 ± 0.19 | 1.17 ± 0.02 | 5.81 ± 0.04 | 220 ± 3 |
| MEL-HSA-P14% | 14 | 176 ± 3 | 0.205 ± 0.02 | 193.7 ± 2 | 0.211 ± 0.02 | −7.9 ± 0.3 | 81.64 ± 0.21 | 1.09 ± 0.02 | 5.61 ± 0.05 | 242 ± 3 |
| MEL-HSA-P15% | 15 | 182 ± 3 | 0.234 ± 0.03 | 262.4 ± 3 | 0.306 ± 0.03 | −7.1 ± 0.4 | 81.19 ± 0.23 | 1.03 ± 0.01 | 5.60 ± 0.02 | 278 ± 2 |
| MEL-HSA-P16% | 16 | 231 ± 3 | 0.268 ± 0.02 | 304.3 ± 5 | 0.328 ± 0.04 | −7.0 ± 0.2 | 79.46 ± 0.24 | 0.97 ± 0.01 | 5.52 ± 0.07 | 311 ± 4 |
| Kinetic Model | Kinetic Parameters | MEL-HSA-P12% | MEL-HSA-P13% | MEL-HSA-P14% | MEL-HSA-P15% | MEL-HSA-P16% |
|---|---|---|---|---|---|---|
| Zero order | k0 (μg min−1) | 0.342 | 0.293 | 0.368 | 0.31 | 0.258 |
| R2 | 0.9265 | 0.9251 | 0.9904 | 0.9968 | 0.9976 | |
| t0.5 (min) | 342.31 | 292.61 | 357.98 | 310.02 | 257.76 | |
| First order | k1 × 10−3 (min−1) | 1.487 | 1.126 | 1.254 | 1.092 | 0.974 |
| R2 | 0.9584 | 0.9586 | 0.9927 | 0.9723 | 0.9972 | |
| t0.5 (min) | 466.15 | 615.43 | 552.71 | 634.49 | 711.49 | |
| Higuchi model | kH (μg min−1/2) | 30.58 | 84.75 | 81.47 | 90.67 | 62.43 |
| R2 | 0.7954 | 0.7855 | 0.8708 | 0.8883 | 0.8684 | |
| t0.5 (min) | 427.58 | 718.23 | 663.67 | 822.44 | 389.75 | |
| Korshmeyer-Peppas model | kK-P × 10−2 (min−n) | 9.427 | 5.684 | 2.998 | 3.118 | 3.292 |
| n | 1.05 | 1.11 | 1.23 | 1.21 | 1.17 | |
| R2 | 0.6105 | 0.5516 | 0.7221 | 0.8396 | 0.7453 | |
| t0.5 (min) | 1043.99 | 1618.39 | 2047.18 | 1740.98 | 1848.54 | |
| Hixon-Crowell model | kH-C (μg 1/3 min−1) | 0.01 | 0.0046 | 0.0062 | 0.0043 | 0.0029 |
| R2 | 0.9838 | 0.987 | 0.9877 | 0.9863 | 0.9974 | |
| t0.5 (min) | 1610.35 | 2055.94 | 1950.68 | 2178.88 | 2466.87 | |
| Best fit | Hixon-Crowell model | Hixon-Crowell model | Zero order | Zero order | Zero order |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Katona, G.; Sipos, B.; Budai-Szűcs, M.; Balogh, G.T.; Veszelka, S.; Gróf, I.; Deli, M.A.; Volk, B.; Szabó-Révész, P.; Csóka, I. Development of In Situ Gelling Meloxicam-Human Serum Albumin Nanoparticle Formulation for Nose-to-Brain Application. Pharmaceutics 2021, 13, 646. https://doi.org/10.3390/pharmaceutics13050646
Katona G, Sipos B, Budai-Szűcs M, Balogh GT, Veszelka S, Gróf I, Deli MA, Volk B, Szabó-Révész P, Csóka I. Development of In Situ Gelling Meloxicam-Human Serum Albumin Nanoparticle Formulation for Nose-to-Brain Application. Pharmaceutics. 2021; 13(5):646. https://doi.org/10.3390/pharmaceutics13050646
Chicago/Turabian StyleKatona, Gábor, Bence Sipos, Mária Budai-Szűcs, György Tibor Balogh, Szilvia Veszelka, Ilona Gróf, Mária A. Deli, Balázs Volk, Piroska Szabó-Révész, and Ildikó Csóka. 2021. "Development of In Situ Gelling Meloxicam-Human Serum Albumin Nanoparticle Formulation for Nose-to-Brain Application" Pharmaceutics 13, no. 5: 646. https://doi.org/10.3390/pharmaceutics13050646
APA StyleKatona, G., Sipos, B., Budai-Szűcs, M., Balogh, G. T., Veszelka, S., Gróf, I., Deli, M. A., Volk, B., Szabó-Révész, P., & Csóka, I. (2021). Development of In Situ Gelling Meloxicam-Human Serum Albumin Nanoparticle Formulation for Nose-to-Brain Application. Pharmaceutics, 13(5), 646. https://doi.org/10.3390/pharmaceutics13050646