Monitoring the Effect of Transdermal Drug Delivery Patches on the Skin Using Terahertz Sensing
Abstract
:1. Introduction
2. Materials and Methods
2.1. Skin Patches for Assessment
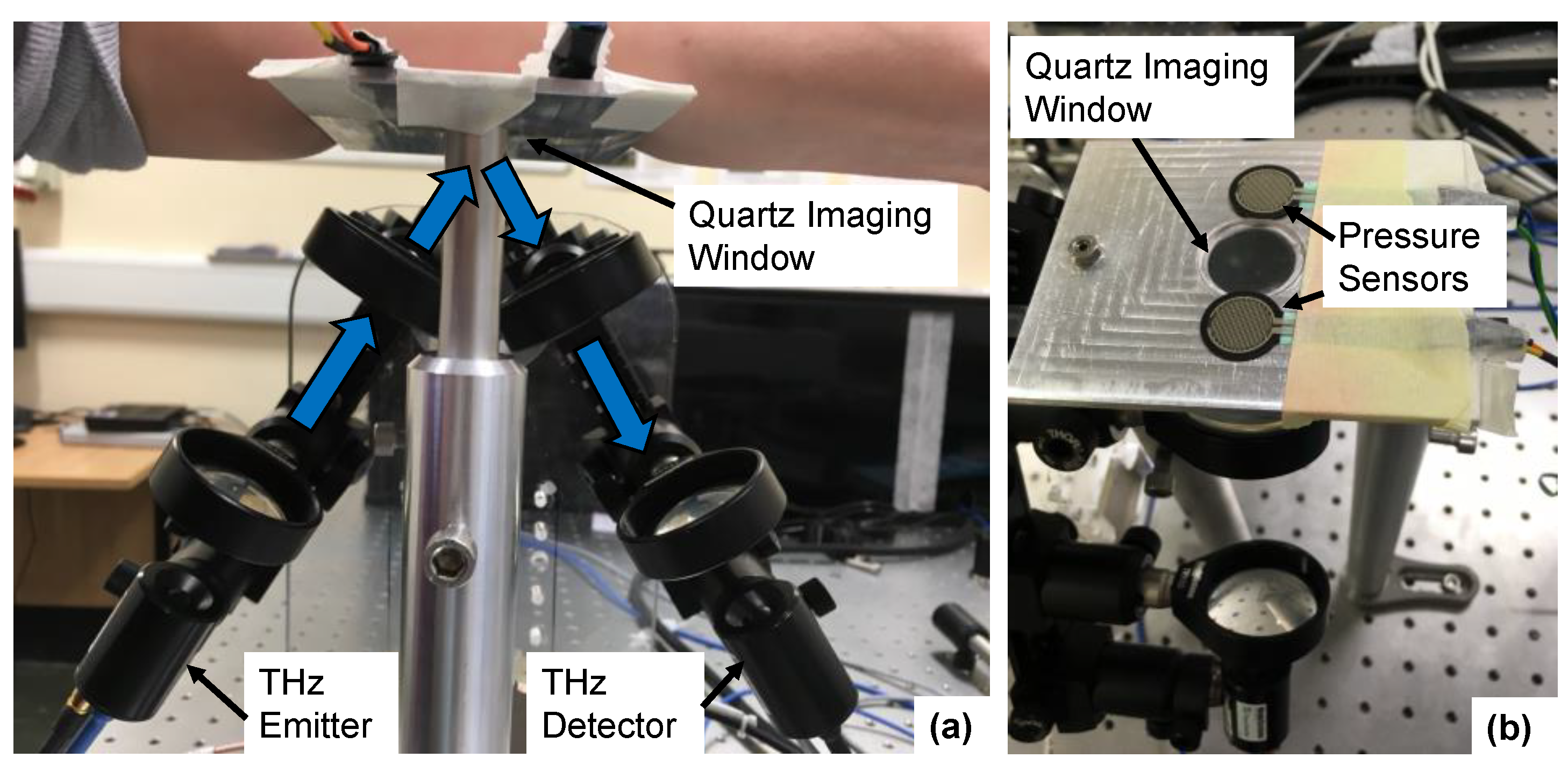
2.2. Terahertz Measurements of the Skin Using a Reflection Geometry
2.3. Protocol for Skin Measurements
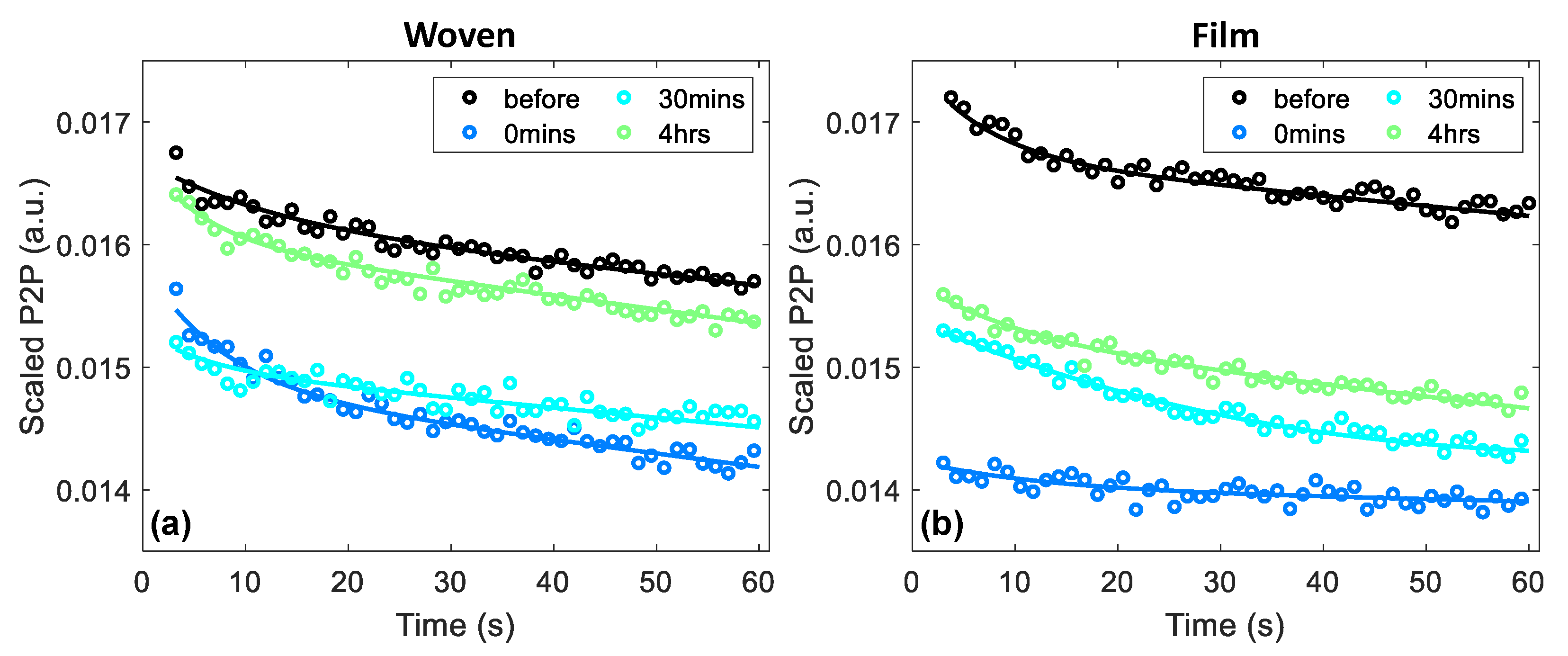
2.4. Approach to Data Analysis
3. Results
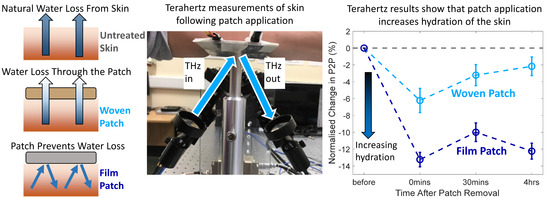
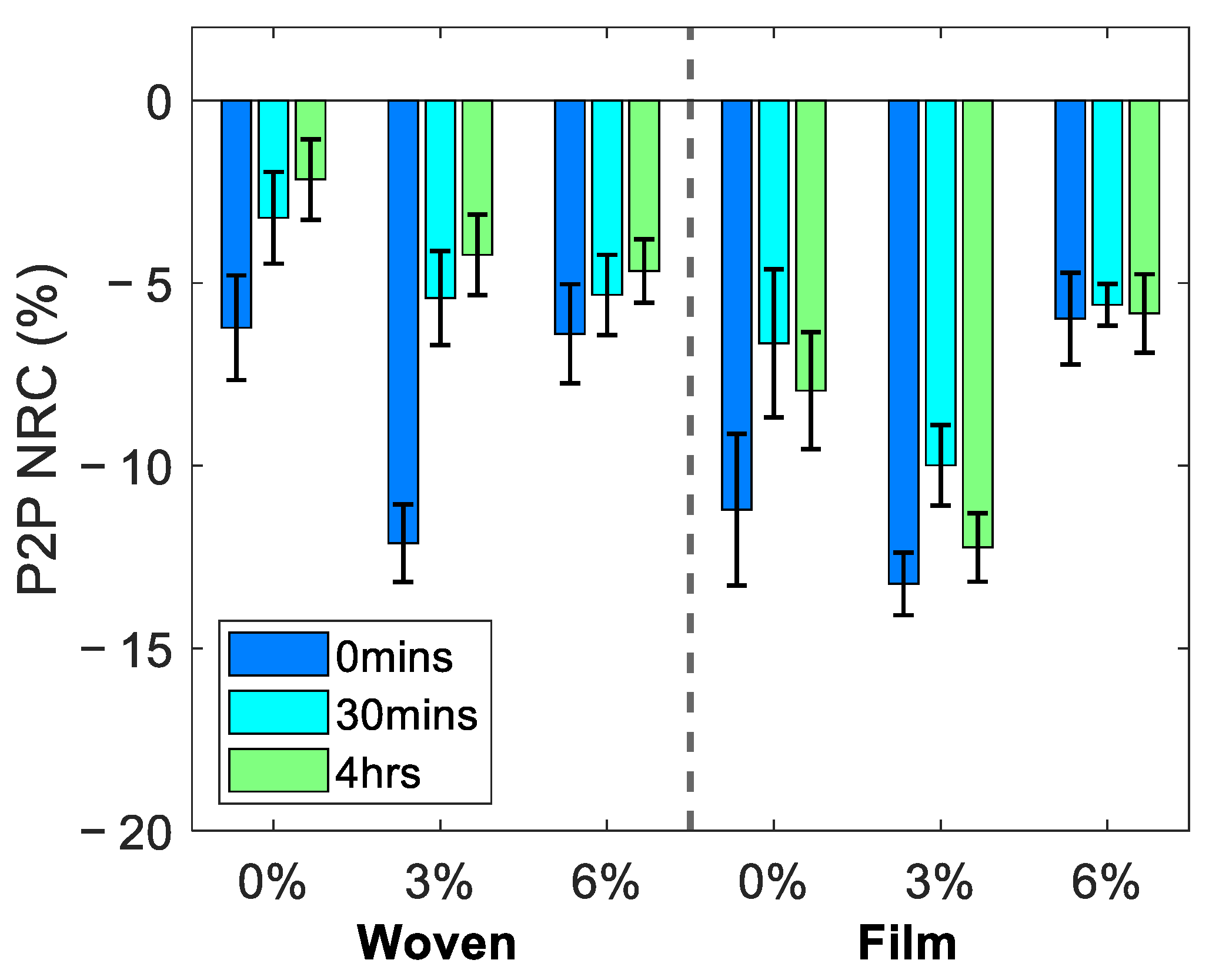
3.1. Average Changes in the Skin following Patch Application
3.2. Effect of Patch Application on the Response of Skin to Occlusion
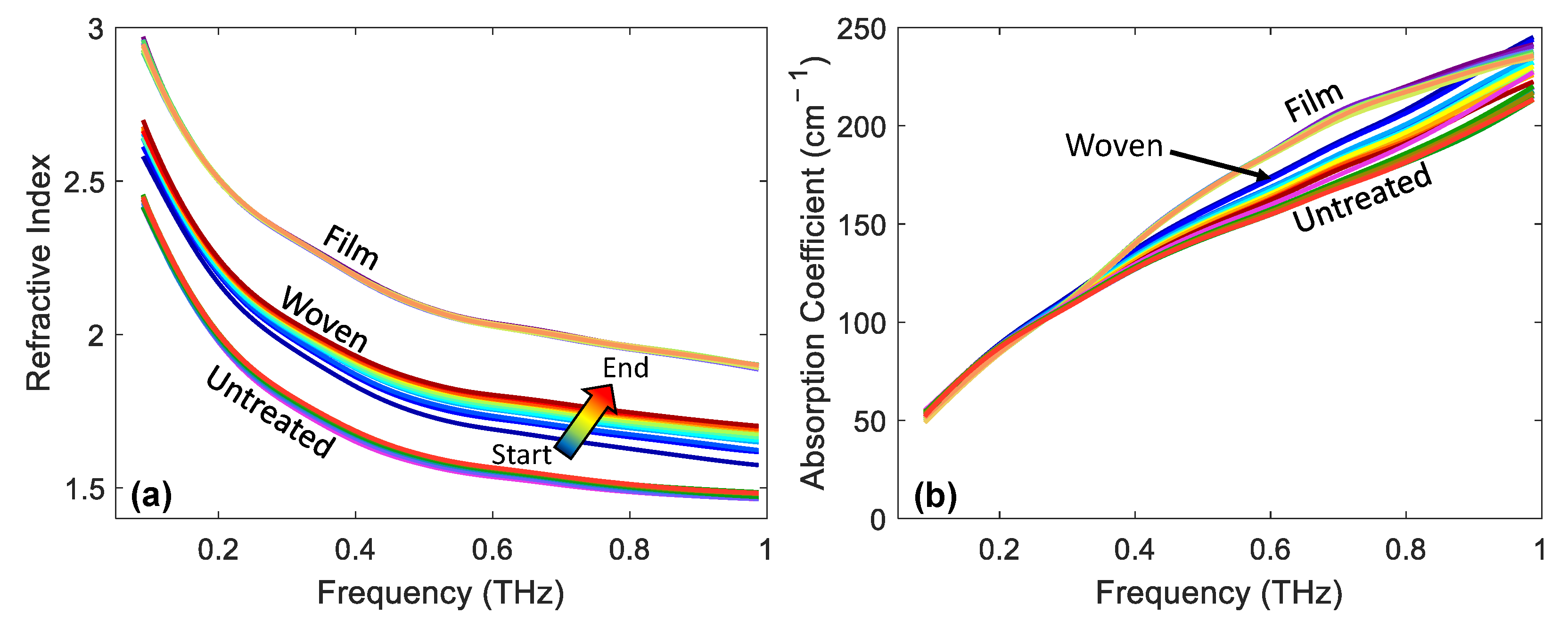
3.3. Changes in Optical Properties
3.4. Statistical Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Prausnitz, M.R.; Langer, R. Transdermal drug delivery. Nat. Biotechnol. 2008, 26, 1261–1268. [Google Scholar] [CrossRef]
- Price, N.M.; Schmitt, L.G.; McGuire, J.; Shaw, J.E.; Trobough, G. Transdermal scopolamine in the prevention of motion sickness at sea. Clin. Pharmacol. Ther. 1981, 29, 414–419. [Google Scholar] [CrossRef] [PubMed]
- Nachum, Z.; Shupak, A.; Gordon, C.R. Transdermal scopolamine for prevention of motion sickness: Clinical pharmacokinetics and therapeutic applications. Clin. Pharmacokinet. 2006, 45, 543–566. [Google Scholar] [CrossRef]
- Buchkremer, G.; Bents, H.; Horstmann, M.; Opitz, K.; Tölle, R. Combination of behavioral smoking cessation with transdermal nicotine substitution. Addict. Behav. 1989, 14, 229–238. [Google Scholar] [CrossRef]
- Good, W.R.; Powers, M.S.; Campbell, P.; Schenkel, L. A new transdermal delivery system for estradiol. J. Control. Release 1985, 2, 89–97. [Google Scholar] [CrossRef]
- Arora, P.; Mukherjee, B. Design, development, physicochemical, and in vitro and in vivo evaluation of transdermal patches containing diclofenac diethylammonium salt. J. Pharm. Sci. 2002, 91, 2076–2089. [Google Scholar] [CrossRef] [PubMed]
- Duthie, D.J.R.; Rowbotham, D.J.; Wyld, R.; Henderson, P.D.; Nimmo, W.S. Plasma fentanyl concentrations during transdermal delivery of fentanyl to surgical patients. Br. J. Anaesth. 1988, 60, 614–618. [Google Scholar] [CrossRef]
- Poewe, W.H.; Rascol, O.; Quinn, N.; Tolosa, E.; Oertel, W.H.; Martignoni, E.; Rupp, M.; Boroojerdi, B. Efficacy of pramipexole and transdermal rotigotine in advanced Parkinson’s disease: A double-blind, double-dummy, randomised controlled trial. Lancet Neurol. 2007, 6, 513–520. [Google Scholar] [CrossRef]
- Winblad, B.; Cummings, J.; Andreasen, N.; Grossberg, G.; Onofrj, M.; Sadowsky, C.; Zechner, S.; Nagel, J.; Lane, R. A six-month double-blind, randomized, placebo-controlled study of a transdermal patch in Alzheimer’s disease—Rivastigmine patch versus capsule. Int. J. Geriatr. Psychiatry 2007, 22, 456–467. [Google Scholar] [CrossRef] [PubMed]
- Al Hanbali, O.A.; Khan, H.M.S.; Sarfraz, M.; Arafat, M.; Ijaz, S.; Hameed, A. Transdermal patches: Design and current approaches to painless drug delivery. Acta Pharm. 2019, 69, 197–215. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ryatt, K.S.; Mobayen, M.; Stevenson, J.M.; Maibach, H.I.; Guy, R.H. Methodology to measure the transient effect of occlusion on skin penetration and stratum corneum hydration in vivo. Br. J. Dermatol. 1988, 119, 307–312. [Google Scholar] [CrossRef]
- Lane, M.E. The transdermal delivery of fentanyl. Eur. J. Pharm. Biopharm. 2013, 84, 449–455. [Google Scholar] [CrossRef]
- Hurkmans, J.F.G.M.; Bodde, H.E.; Van Driel, L.M.J.; Van Doorne, H.; Junginger, H.E. Skin irritation caused by transdermal drug delivery systems during long-term (5 days) application. Br. J. Dermatol. 1985, 112, 461–467. [Google Scholar] [CrossRef] [PubMed]
- Aly, R.; Shirley, C.; Cunico, B.; Maibach, H.I. Effect of prolonged occlusion on the microbial flora, pH, carbon dioxide and transepidermal water loss on human skin. J. Investig. Dermatol. 1978, 71, 378–381. [Google Scholar] [CrossRef] [Green Version]
- Faergemann, J.; Aly, R.; Wilson, D.R.; Maibach, H.I. Skin occlusion: Effect on Pityrosporum orbiculare, skin P_CO2, pH, transepidermal water loss, and water content. Arch. Dermatol. Res. 1983, 275, 383–387. [Google Scholar] [CrossRef] [PubMed]
- Wallace, V.P.; Fitzgerald, A.J.; Shankar, S.; Flanagan, N.; Pye, R.; Cluff, J.; Arnone, D.D. Terahertz pulsed imaging of basal cell carcinoma ex vivo and in vivo. Br. J. Dermatol. 2004, 151, 424–432. [Google Scholar] [CrossRef] [PubMed]
- Bowman, T.C.; El-Shenawee, M.; Campbell, L.K. Terahertz Imaging of Excised Breast Tumor Tissue on Paraffin Sections. IEEE Trans. Antennas Propag. 2015, 63, 2088–2097. [Google Scholar] [CrossRef]
- Ji, Y.B.; Park, C.H.; Kim, H.; Kim, S.-H.; Lee, G.M.; Noh, S.K.; Jeon, T.-I.; Son, J.-H.; Huh, Y.-M.; Haam, S.; et al. Feasibility of terahertz reflectometry for discrimination of human early gastric cancers. Biomed. Opt. Express 2015, 6, 1398. [Google Scholar] [CrossRef] [Green Version]
- Sim, Y.C.; Park, J.Y.; Ahn, K.-M.; Park, C.; Son, J.-H. Terahertz imaging of excised oral cancer at frozen temperature. Biomed. Opt. Express 2013, 4, 1413–1421. [Google Scholar] [CrossRef] [PubMed]
- Taylor, Z.D.; Garritano, J.; Sung, S.; Bajwa, N.; Bennett, D.B.; Nowroozi, B.; Tewari, P.; Sayre, J.W.; Hubschman, J.-P.; Deng, S.X.; et al. THz and mm-Wave Sensing of Corneal Tissue Water Content: In Vivo Sensing and Imaging Results. IEEE Trans. Terahertz Sci. Technol. 2015, 5, 184–196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hernandez-Cardoso, G.G.; Rojas-Landeros, S.C.; Alfaro-Gomez, M.; Hernandez-Serrano, A.I.; Salas-Gutierrez, I.; Lemus-Bedolla, E.; Castillo-Guzman, A.R.; Lopez-Lemus, H.L.; Castro-Camus, E. Terahertz imaging for early screening of diabetic foot syndrome: A proof of concept. Sci. Rep. 2017, 7, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Tewari, P.; Garritano, J.; Bajwa, N.; Sung, S.; Huang, H.; Wang, D.; Grundfest, W.; Ennis, D.B.; Ruan, D.; Brown, E.; et al. Methods for registering and calibrating in vivo terahertz images of cutaneous burn wounds. Biomed. Opt. Express 2019, 10, 322–337. [Google Scholar] [CrossRef] [PubMed]
- Osman, O.B.; Tan, T.J.; Henry, S.; Warsen, A.; Farr, N.; McClintic, A.M.; Wang, Y.-N.; Arbabi, S.; Arbab, M.H. Differentiation of burn wounds in an in vivo porcine model using terahertz spectroscopy. Biomed. Opt. Express 2020, 11, 6528. [Google Scholar] [CrossRef]
- Sun, Q.; Parrott, E.P.J.; He, Y.; Pickwell-MacPherson, E. In vivo THz imaging of human skin: Accounting for occlusion effects. J. Biophotonics 2018, 11, e201700111. [Google Scholar] [CrossRef]
- Wang, J.; Sun, Q.; Stantchev, R.I.; Chiu, T.-W.; Ahuja, A.T.; Pickwell-Macpherson, E. In vivo terahertz imaging to evaluate scar treatment strategies: Silicone gel sheeting. Biomed. Opt. Express 2019, 10, 3584–3590. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Stantchev, R.I.; Wang, J.; Parrott, E.P.J.; Cottenden, A.; Chiu, T.-W.; Ahuja, A.T.; Pickwell-MacPherson, E. In vivo estimation of water diffusivity in occluded human skin using terahertz reflection spectroscopy. J. Biophotonics 2019, 12, e201800145. [Google Scholar] [CrossRef]
- Kim, K.W.; Kim, K.-S.; Kim, H.; Lee, S.H.; Park, J.-H.; Han, J.-H.; Seok, S.-H.; Park, J.; Choi, Y.; Kim, Y.I.; et al. Terahertz dynamic imaging of skin drug absorption. Opt. Express 2012, 20, 9476. [Google Scholar] [CrossRef]
- Wang, J.; Lindley-Hatcher, H.; Liu, K.; Pickwell-MacPherson, E. Evaluation of transdermal drug delivery using terahertz pulsed imaging. Biomed. Opt. Express 2020, 11, 4484–4490. [Google Scholar] [CrossRef] [PubMed]
- Lee, G.; Namkung, H.; Do, Y.; Lee, S.; Kang, H.; Kim, J.W.; Han, H. Quantitative label-free terahertz sensing of transdermal nicotine delivered to human skin. Curr. Opt. Photonics 2020, 4, 368–372. [Google Scholar] [CrossRef]
- Carrer, V.; Alonso, C.; Pont, M.; Zanuy, M.; Córdoba, M.; Espinosa, S.; Barba, C.; Oliver, M.A.; Martí, M.; Coderch, L. Effect of propylene glycol on the skin penetration of drugs. Arch. Dermatol. Res. 2020, 312, 337–352. [Google Scholar] [CrossRef]
- Tombs, E.L.; Nikolaou, V.; Nurumbetov, G.; Haddleton, D.M. Transdermal Delivery of Ibuprofen Utilizing a Novel Solvent-Free Pressure-sensitive Adhesive (PSA): TEPI® Technology. J. Pharm. Innov. 2018, 13, 48–57. [Google Scholar] [CrossRef] [Green Version]
- Haddleton, D.M.; Nurumbetov, G.; Ross, A.; Nikolaou, V. PATCH. WO2020016582A1, 23 January 2020. [Google Scholar]
- Lindley-Hatcher, H.; Hernandez-Serrano, A.I.; Sun, Q.; Wang, J.; Cebrian, J.; Blasco, L.; Pickwell-MacPherson, E. A Robust Protocol for In Vivo THz Skin Measurements. J. Infrared Millim. Terahertz Waves 2019, 40, 980–989. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Stantchev, R.I.; Sun, Q.; Chiu, T.-W.; Ahuja, A.T.; MacPherson, E.P. THz in vivo measurements: The effects of pressure on skin reflectivity. Biomed. Opt. Express 2018, 9, 6467. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, X.; Parrott, E.P.J.; Ung, B.S.Y.; Pickwell-Macpherson, E. A Robust Baseline and Reference Modification and Acquisition Algorithm for Accurate THz Imaging. IEEE Trans. Terahertz Sci. Technol. 2017, 7, 493–501. [Google Scholar] [CrossRef]
- Lindley-Hatcher, H.; Hernandez-Serrano, A.I.; Wang, J.; Cebrian, J.; Hardwicke, J.; Pickwell-MacPherson, E. Evaluation of in vivo THz sensing for assessing human skin hydration. J. Phys. Photonics 2021, 3, 014001. [Google Scholar] [CrossRef]
- Tukey, J.W. Comparing Individual Means in the Analysis of Variance. Biometrics 1949, 5, 99. [Google Scholar] [CrossRef] [PubMed]
- Bartosova, L.; Bajgar, J. Transdermal Drug Delivery In Vitro Using Diffusion Cells. Curr. Med. Chem. 2012, 19, 4671–4677. [Google Scholar] [CrossRef]
- Wang, J.; Lindley-Hatcher, H.; Chen, X.; Pickwell-MacPherson, E. THz Sensing of Human Skin: A Review of Skin Modeling Approaches. Sensors 2021, 21, 3624. [Google Scholar] [CrossRef]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lindley-Hatcher, H.; Wang, J.; Hernandez-Serrano, A.I.; Hardwicke, J.; Nurumbetov, G.; Haddleton, D.M.; Pickwell-MacPherson, E. Monitoring the Effect of Transdermal Drug Delivery Patches on the Skin Using Terahertz Sensing. Pharmaceutics 2021, 13, 2052. https://doi.org/10.3390/pharmaceutics13122052
Lindley-Hatcher H, Wang J, Hernandez-Serrano AI, Hardwicke J, Nurumbetov G, Haddleton DM, Pickwell-MacPherson E. Monitoring the Effect of Transdermal Drug Delivery Patches on the Skin Using Terahertz Sensing. Pharmaceutics. 2021; 13(12):2052. https://doi.org/10.3390/pharmaceutics13122052
Chicago/Turabian StyleLindley-Hatcher, Hannah, Jiarui Wang, Arturo I. Hernandez-Serrano, Joseph Hardwicke, Gabit Nurumbetov, David M. Haddleton, and Emma Pickwell-MacPherson. 2021. "Monitoring the Effect of Transdermal Drug Delivery Patches on the Skin Using Terahertz Sensing" Pharmaceutics 13, no. 12: 2052. https://doi.org/10.3390/pharmaceutics13122052
APA StyleLindley-Hatcher, H., Wang, J., Hernandez-Serrano, A. I., Hardwicke, J., Nurumbetov, G., Haddleton, D. M., & Pickwell-MacPherson, E. (2021). Monitoring the Effect of Transdermal Drug Delivery Patches on the Skin Using Terahertz Sensing. Pharmaceutics, 13(12), 2052. https://doi.org/10.3390/pharmaceutics13122052