Receptor-Mediated Targeted Delivery of Surface-ModifiedNanomedicine in Breast Cancer: Recent Update and Challenges
Abstract
:1. Introduction
2. Challenges in Breast Cancer Therapy
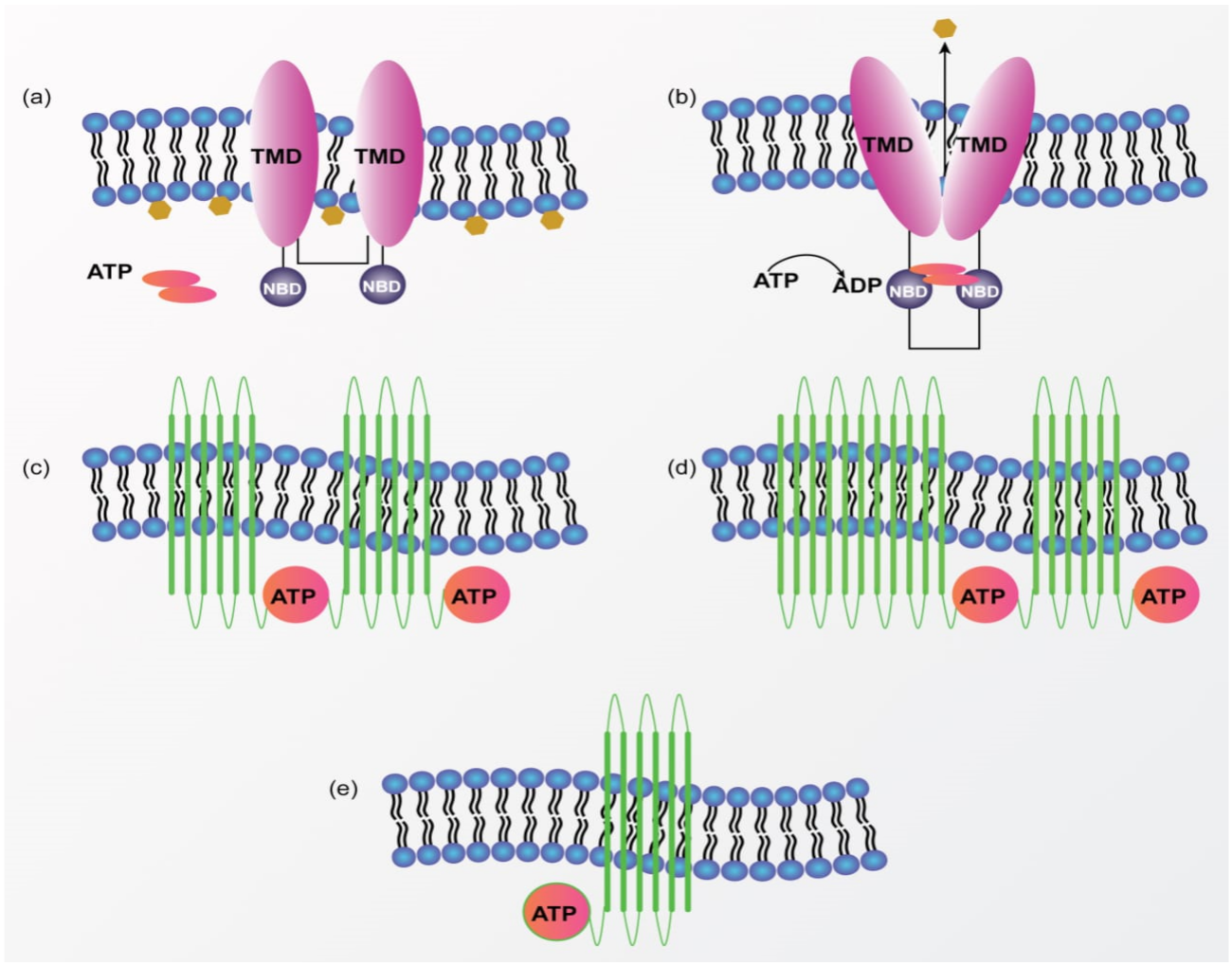
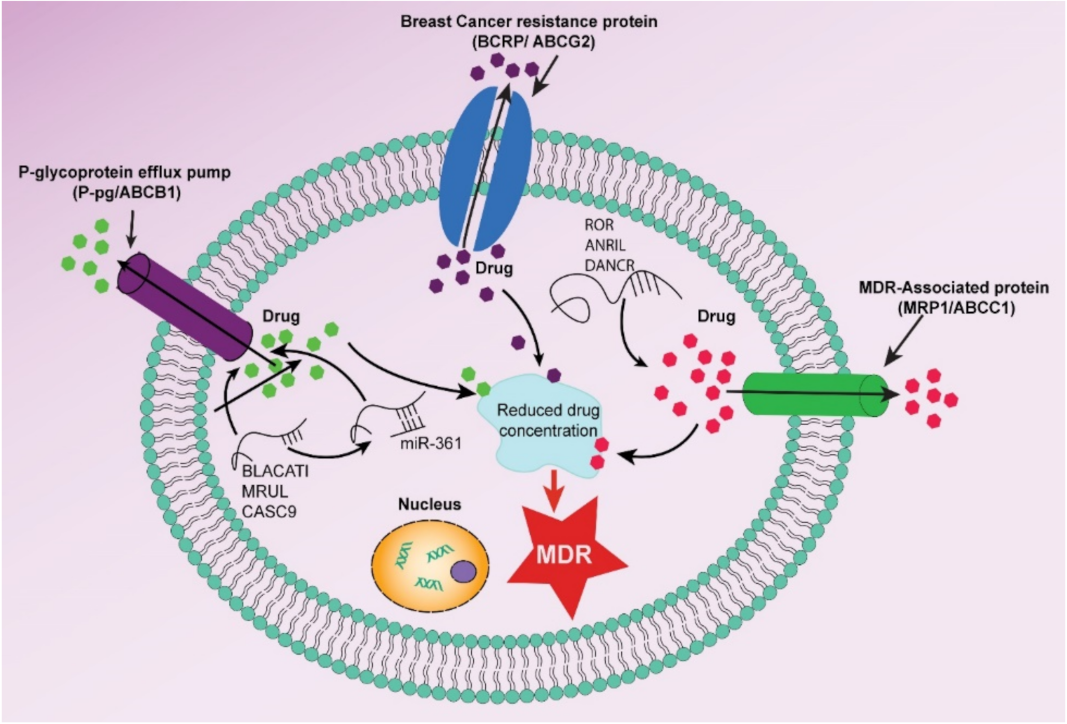
Multidrug Resistance (MDR) in Breast Cancer
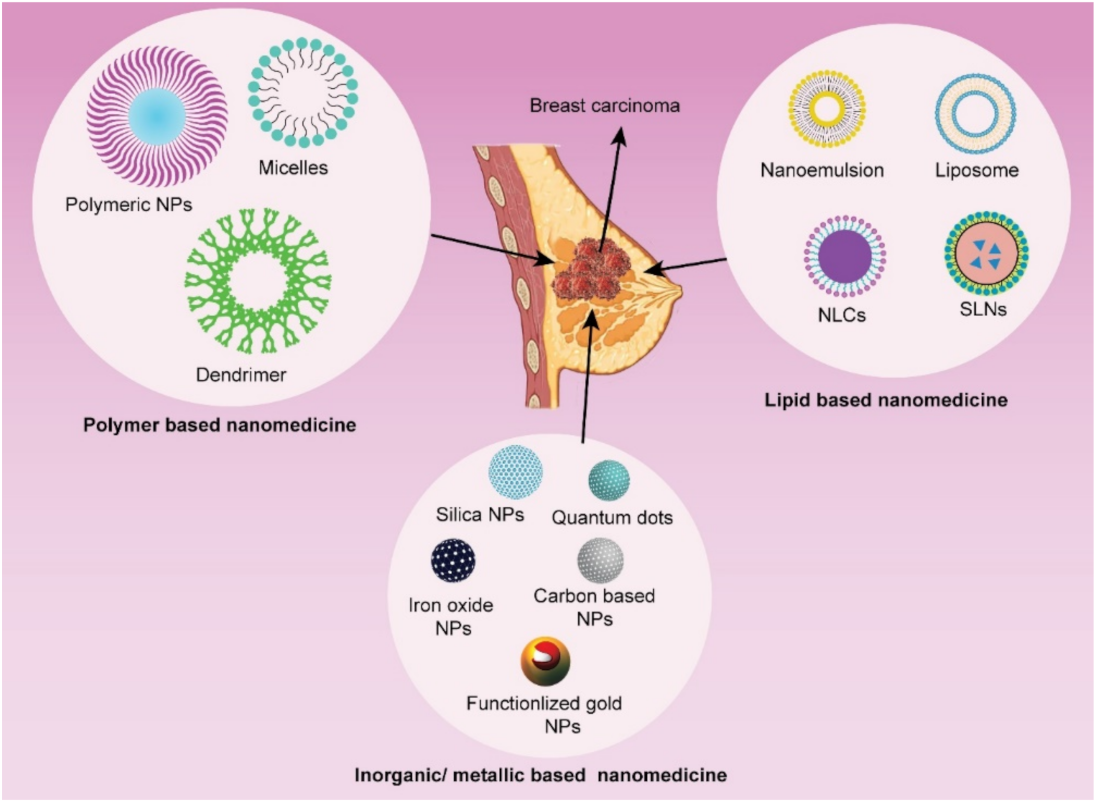
3. Nanotechnology-Mediated Drug Delivery in Breast Cancer
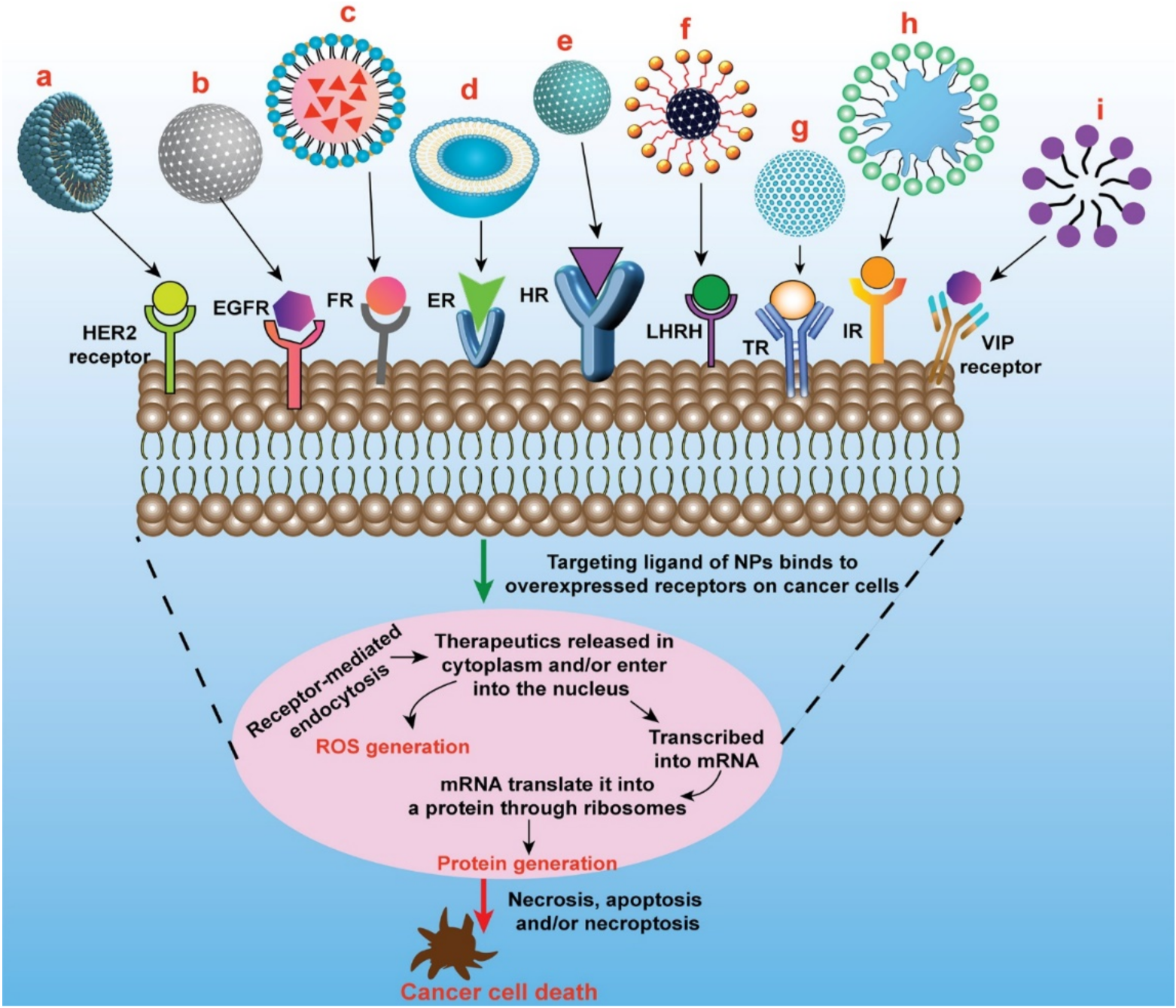
4. Surface-Modified Multifunctional Nanomedicine for Breast Cancer
4.1. Targeting of ErbB Receptor
4.2. Targeting of Folate Receptor
4.3. Targeting of Estrogen Receptor
4.4. Targeting of CD44 Receptor/Hyaluronan Receptor
4.5. Targeting of LHRH Receptor
4.6. Targeting of Transferrin Receptor
4.7. Targeting of Integrin Receptor
4.8. Targeting of Vasoactive Intestinal Peptide (VIP) Receptor
4.9. Other Receptors Targeted for Breast Cancer Therapy
5. Clinical Translation and Future Challenges
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
List of Abbreviations
| ABC | ATP binding cassette |
| BCRP | Breast cancer resistance protein |
| Cur | Curcumin |
| DOX | Doxorubicin |
| EGFR | Epidermal growth factor receptor |
| ER | Estrogen receptor |
| FDA | Food and drug administration |
| FR | Folate receptor |
| HA | Hyaluronic acid |
| HER2 | Human epidermal growth factor receptor 2 |
| HR | Hyaluronan receptor |
| IR | Integrin receptor |
| LHRH | Luteinizing hormone-releasing hormone receptor |
| MDR | Multidrug resistance |
| miRNA | microRNA |
| MNPs | Magnetite nanoparticles |
| MRP1 | MDR associated protein |
| NAG | N-acetyl-D-glucosamine |
| NLC | Nanostructured lipid carriers |
| P-gp | P-glycoprotein |
| PTX | Paclitaxel |
| siRNA | Small interfering RNA |
| SLN | Solid lipid nanoparticles |
| SNEDDS | Self-nano emulsifying drug delivery system |
| TfR | Transferrin receptor |
| TNBC | Triple-negative breast cancer |
| TPGS | D-α-Tocopheryl polyethylene glycol 1000 succinate |
| VIP | Vasoactive intestinal peptide receptor |
| WHO | World Health Organization |
References
- Ebeid, N.I. Egyptian Medicine in the Days of the Pharaohs; General Egyptian Book Organization: Cairo, Egypt, 1999. [Google Scholar]
- Cardoso, D.; Coelho, A.; Fernandes, L.; Matos, L.V.; Serrano, I.; Miranda, H.; Martins, A. Sweet’s Syndrome Induced by Aromatase Inhibitor in the Treatment of Early Breast Cancer. Eur. J. Case Rep. Intern. Med. 2020. [Google Scholar] [CrossRef]
- Rizwanullah, M.; Perwez, A.; Mir, S.R.; Rizvi, M.M.; Amin, S. Exemestane encapsulated polymer-lipid hybrid nanoparticles for improved efficacy against breast cancer: Optimization, in vitro characterization and cell culture studies. Nanotechnology 2021, 32, 415101. [Google Scholar] [CrossRef]
- Sánchez-Jiménez, F.; Pérez, A.P.; de la Cruz-Merino, L.; Sánchez-Margalet, V. Obesity and Breast Cancer: Role of Leptin. Front. Oncol. 2019, 9, 596. [Google Scholar] [CrossRef]
- WHO. Breast Cancer. Available online: https://www.who.int/news-room/fact-sheets/detail/breast-cancer (accessed on 17 September 2021).
- U.S. Breast Cancer Statistics|Breastcancer.org. Available online: https://www.breastcancer.org/symptoms/understand_bc/statistics (accessed on 17 September 2021).
- García-Aranda, M.; Redondo, M. Immunotherapy: A Challenge of Breast Cancer Treatment. Cancers 2019, 11, 1822. [Google Scholar] [CrossRef] [Green Version]
- Perou, C.M.; Sørlie, T.; Eisen, M.B.; Van De Rijn, M.; Jeffrey, S.S.; Rees, C.A.; Pollack, J.R.; Ross, D.T.; Johnsen, H.; Akslen, L.A.; et al. Molecular portraits of human breast tumours. Nature 2000, 406, 747–752. [Google Scholar] [CrossRef] [PubMed]
- Fragomeni, S.M.; Sciallis, A.; Jeruss, J.S. Molecular Subtypes and Local-Regional Control of Breast Cancer. Surg. Oncol. Clin. N. Am. 2018, 27, 95–120. [Google Scholar] [CrossRef]
- Ahmad, M.Z.; Akhter, S.; Jain, G.K.; Rahman, M.; Pathan, S.A.; Ahmad, F.J.; Khar, R.K. Metallic nanoparticles: Technology overview & drug delivery applications in oncology. Expert Opin. Drug Deliv. 2010, 7, 927–942. [Google Scholar] [CrossRef]
- Akhter, S.; Ahmad, M.Z.; Singh, A.; Ahmad, I.; Rahman, M.; Anwar, M.; Jain, G.K.; Ahmad, F.; Khar, R.K. Cancer Targeted Metallic Nanoparticle: Targeting Overview, Recent Advancement and Toxicity Concern. Curr. Pharm. Des. 2011, 17, 1834–1850. [Google Scholar] [CrossRef]
- Ahmad, M.Z.; Akhter, S.; Rahman, Z.; Akhter, S.; Anwar, M.; Mallik, N.; Ahmad, F. Nanometric gold in cancer nanotechnology: Current status and future prospect. J. Pharm. Pharmacol. 2012, 65, 634–651. [Google Scholar] [CrossRef]
- Akhter, S.; Ahmad, M.Z.; Ahmad, F.; Storm, G.; Kok, R.J. Gold nanoparticles in theranostic oncology: Current state-of-the-art. Expert Opin. Drug Deliv. 2012, 9, 1225–1243. [Google Scholar] [CrossRef] [PubMed]
- Gadag, S.; Sinha, S.; Nayak, Y.; Garg, S.; Nayak, U.Y. Combination Therapy and Nanoparticulate Systems: Smart Approaches for the Effective Treatment of Breast Cancer. Pharmaceutics 2020, 12, 524. [Google Scholar] [CrossRef]
- Tang, X.; Loc, W.S.; Dong, C.; Matters, G.L.; Butler, P.J.; Kester, M.; Meyers, C.; Jiang, Y.; Adair, J.H. The use of nanoparticulates to treat breast cancer. Nanomedicine 2017, 12, 2367–2388. [Google Scholar] [CrossRef] [PubMed]
- Akhter, M.H.; Rizwanullah, M.; Ahmad, J.; Ahsan, M.J.; Mujtaba, M.A.; Amin, S. Nanocarriers in advanced drug targeting: Setting novel paradigm in cancer therapeutics. Artif. Cells. Nanomed. Biotechnol. 2018, 46, 873–884. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rizwanullah, M.; Ahmad, J.; Amin, S. Nanostructured lipid carriers: A novel platform for chemotherapeutics. Curr. Drug Deliv. 2016, 13, 4–26. [Google Scholar] [CrossRef]
- Ahmad, M.Z.; Ahmad, J.; Alasmary, M.Y.; Akhter, H.; Abdel-Wahab, B.A.; Warsi, M.H.; Haque, A. Progress in nanomedicine-based drug delivery in designing of chitosan nanoparticles for cancer therapy. Int. J. Polym. Mater. 2021, 1–22. [Google Scholar] [CrossRef]
- Ahmad, J.; Akhter, S.; Khan, M.A.; Wahajuddin, M.; Greig, N.H.; Kamal, M.A.; Midoux, P.; Pichon, C. Engineered Nanoparticles Against MDR in Cancer: The State of the Art and its Prospective. Curr. Pharm. Des. 2016, 22, 4360–4373. [Google Scholar] [CrossRef] [Green Version]
- Wind, N.S.; Holen, I. Multidrug Resistance in Breast Cancer: FromIn VitroModels to Clinical Studies. Int. J. Breast Cancer 2011, 2011, 1–12. [Google Scholar] [CrossRef]
- Bukowski, K.; Kciuk, M.; Kontek, R. Mechanisms of Multidrug Resistance in Cancer Chemotherapy. Int. J. Mol. Sci. 2020, 21, 3233. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.-L.; Patel, A.; Kumar, P.; Chen, Z.-S. Role of ABC transporters in cancer chemotherapy. Chin. J. Cancer 2012, 31, 51–57. [Google Scholar] [CrossRef] [Green Version]
- Jones, P.M.; George, A.M. Mechanism of ABC transporters: A molecular dynamics simulation of a well characterized nucleotide-binding subunit. Proc. Natl. Acad. Sci. USA 2002, 99, 12639–12644. [Google Scholar] [CrossRef] [Green Version]
- Xiao, H.; Zheng, Y.; Ma, L.; Tian, L.; Sun, Q. Clinically-Relevant ABC Transporter for Anti-Cancer Drug Resistance. Front. Pharmacol. 2021, 12, 705. [Google Scholar] [CrossRef]
- Vasiliou, V.; Vasiliou, K.; Nebert, D.W. Human ATP-binding cassette (ABC) transporter family. Hum. Genom. 2008, 3, 281–290. [Google Scholar] [CrossRef]
- Juliano, R.; Ling, V. A surface glycoprotein modulating drug permeability in Chinese hamster ovary cell mutants. Biochim. Biophys. Acta (BBA) Biomembr. 1976, 455, 152–162. [Google Scholar] [CrossRef]
- Doyle, L.A.; Yang, W.; Abruzzo, L.V.; Krogmann, T.; Gao, Y.; Rishi, A.K.; Ross, D.D. A multidrug resistance transporter from human MCF-7 breast cancer cells. Proc. Natl. Acad. Sci. USA 1998, 95, 15665–15670. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tomlinson, D.; Martin, H.; Smith, L. Multidrug-resistant breast cancer: Current perspectives. Breast Cancer Targets Ther. 2014, 6, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Grunt, T.W. Novel Approaches for Molecular Targeted Therapy of Breast Cancer: Interfering with PI3K/AKT/mTOR Signaling. Curr. Cancer Drug Targets 2013, 13, 188–204. [Google Scholar] [CrossRef]
- Nahta, R.; O’Regan, R.M. Therapeutic implications of estrogen receptor signaling in HER2-positive breast cancers. Breast Cancer Res. Treat. 2012, 135, 39–48. [Google Scholar] [CrossRef]
- Farazi, T.A.; Hoell, J.I.; Morozov, P.; Tuschl, T. MicroRNAs in Human Cancer. Adv. Exp. Med. Biol. 2012, 774, 1–20. [Google Scholar] [CrossRef] [Green Version]
- Kutanzi, K.R.; Yurchenko, O.V.; Beland, F.A.; Checkhun, V.F.; Pogribny, I.P. MicroRNA-mediated drug resistance in breast cancer. Clin. Epigenetics 2011, 2, 171–185. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jaenisch, R.; Bird, A. Epigenetic regulation of gene expression: How the genome integrates intrinsic and environmental signals. Nat. Genet. 2003, 33, 245–254. [Google Scholar] [CrossRef] [PubMed]
- Sukocheva, O.A.; Lukina, E.; Friedemann, M.; Menschikowski, M.; Hagelgans, A.; Aliev, G. The crucial role of epigenetic regulation in breast cancer anti-estrogen resistance: Current findings and future perspectives. Semin. Cancer Biol. 2020. [Google Scholar] [CrossRef]
- Lubecka, K.; Kurzava, L.; Flower, K.; Buvala, H.; Zhang, H.; Teegarden, D.; Camarillo, I.; Suderman, M.; Kuang, S.; Andrisani, O.; et al. Stilbenoids remodel the DNA methylation patterns in breast cancer cells and inhibit oncogenic NOTCH signaling through epigenetic regulation of MAML2 transcriptional activity. Carcinogenesis 2016, 37, 656–668. [Google Scholar] [CrossRef]
- Shapiro, C.L.; Recht, A. Side Effects of Adjuvant Treatment of Breast Cancer. N. Engl. J. Med. 2001, 344, 1997–2008. [Google Scholar] [CrossRef]
- Early Breast Cancer Trialists’ Collaborative Group (EBCTCG); Peto, R.; Davies, C.; Godwin, J.; Gray, R.; Pan, H.C.; Clarke, M.; Cutter, D.; Darby, S.; McGale, P.; et al. Comparisons between different polychemotherapy regimens for early breast cancer: Meta-analyses of long-term outcome among 100,000 women in 123 randomised trials. Lancet 2012, 379, 432–444. [Google Scholar] [CrossRef] [Green Version]
- Smith, L.A.; Cornelius, V.R.; Plummer, C.J.; Levitt, G.; Verrill, M.; Canney, P.; Jones, A. Cardiotoxicity of anthracycline agents for the treatment of cancer: Systematic review and meta-analysis of randomised controlled trials. BMC Cancer 2010, 10, 337. [Google Scholar] [CrossRef] [Green Version]
- Nurgalieva, Z.; Liu, C.-C.; Du, X.L. Chemotherapy use and risk of bone marrow suppression in a large population-based cohort of older women with breast and ovarian cancer. Med Oncol. 2010, 28, 716–725. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.; Gianos, M.; Klaustermeyer, W.B. Diagnosis and management of hypersensitivity reactions related to common cancer chemotherapy agents. Ann. Allergy Asthma Immunol. 2009, 102, 179–187. [Google Scholar] [CrossRef]
- Hagiwara, H.; Sunada, Y. Mechanism of taxane neurotoxicity. Breast Cancer 2004, 11, 82–85. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Yi, C.; Luo, N.; Gong, C. Nanomedicine to Overcome Cancer Multidrug Resistance. Curr. Drug Metab. 2014, 15, 632–649. [Google Scholar] [CrossRef] [PubMed]
- Rizwanullah, M.; Amin, S.; Mir, S.R.; Fakhri, K.U.; Rizvi, M.M. Phytochemical based nanomedicines against cancer: Current status and future prospects. J. Drug Target. 2018, 26, 731–752. [Google Scholar] [CrossRef]
- Haider, N.; Fatima, S.; Taha, M.; Rizwanullah, M.; Firdous, J.; Ahmad, R.; Mazhar, F.; Khan, M.A. Nanomedicines in diagnosis and treatment of cancer: An update. Curr. Pharm. Des. 2020, 26, 1216–1231. [Google Scholar] [CrossRef] [PubMed]
- Rizwanullah, M.; Alam, M.; Mir, S.R.; Rizvi, M.; Amin, S. Polymer-lipid hybrid nanoparticles: A next-generation nanocarrier for targeted treatment of solid tumors. Curr. Pharm. Des. 2020, 26, 1206–1215. [Google Scholar] [CrossRef]
- O’Brien, M.E.R.; Wigler, N.; Inbar, M.; Rosso, R.; Grischke, E.; Santoro, A.; Catane, R.; Kieback, D.G.; Tomczak, P.; Ackland, S.P.; et al. Reduced cardiotoxicity and comparable efficacy in a phase IIItrial of pegylated liposomal doxorubicin HCl(CAELYX™/Doxil®) versus conventional doxorubicin forfirst-line treatment of metastatic breast cancer. Ann. Oncol. 2004, 15, 440–449. [Google Scholar] [CrossRef]
- Coukos, G.; de Vries, J.; Hawkins, R.E. ESMO Symposium on Immuno-Oncology 2015 Officers and Organisation. Ann. Oncol. 2015, 26. [Google Scholar] [CrossRef]
- Wang, X.; Wang, Q.; Liu, Z.; Zheng, X. Preparation, pharmacokinetics and tumour-suppressive activity of berberine liposomes. J. Pharm. Pharmacol. 2017, 69, 625–632. [Google Scholar] [CrossRef]
- Gregoriou, Y.; Gregoriou, G.; Yilmaz, V.; Kapnisis, K.; Prokopi, M.; Anayiotos, A.; Strati, K.; Dietis, N.; Constantinou, A.I.; Andreou, C. Resveratrol loaded polymeric micelles for theranostic targeting of breast cancer cells. Nanotheranostics 2021, 5, 113–124. [Google Scholar] [CrossRef] [PubMed]
- Abedinpour, N.; Ghanbariasad, A.; Taghinezhad, A.; Osanloo, M. Preparation of Nanoemulsions of Mentha piperita Essential Oil and Investigation of Their Cytotoxic Effect on Human Breast Cancer Lines. BioNanoScience 2021, 11, 428–436. [Google Scholar] [CrossRef]
- Mohanty, C.; Das, M.; Kanwar, J.R.; Sahoo, S.K. Receptor Mediated Tumor Targeting: An Emerging Approach for Cancer Therapy. Curr. Drug Deliv. 2011, 8, 45–58. [Google Scholar] [CrossRef]
- Large, D.E.; Soucy, J.; Hebert, J.; Auguste, D.T. Advances in Receptor-Mediated, Tumor-Targeted Drug Delivery. Adv. Ther. 2018, 2, 1800091. [Google Scholar] [CrossRef] [Green Version]
- Mehra, N.K.; Mishra, V.; Jain, N.K. Receptor-based targeting of therapeutics. Ther. Deliv. 2013, 4, 369–394. [Google Scholar] [CrossRef]
- Grobmyer, S.R.; Zhou, G.; Gutwein, L.G.; Iwakuma, N.; Sharma, P.; Hochwald, S.N. Nanoparticle delivery for metastatic breast cancer. Maturitas 2012, 73, 19–26. [Google Scholar] [CrossRef]
- Rizwanullah, M.; Ahmad, M.Z.; Garg, A.; Ahmad, J. Advancement in design of nanostructured lipid carriers for cancer targeting and theranostic application. Biochim. Biophys. Acta Gen. Subj. 2021, 1865, 129936. [Google Scholar] [CrossRef]
- Vhora, I.; Patil, S.; Bhatt, P.; Gandhi, R.; Baradia, D.; Misra, A. Receptor-targeted drug delivery: Current perspective and challenges. Ther. Deliv. 2014, 5, 1007–1024. [Google Scholar] [CrossRef] [PubMed]
- Morales-Cruz, M.; Delgado, Y.; Castillo, B.; Figueroa, C.M.; Molina, A.M.; Torres, A.; Milian, M.; Griebenow, K. Smart Targeting to Improve Cancer Therapeutics. Drug Des. Dev. Ther. 2019, ume 13, 3753–3772. [Google Scholar] [CrossRef] [Green Version]
- Das, M.; Mohanty, C.; Sahoo, S.K. Ligand-based targeted therapy for cancer tissue. Expert Opin. Drug Deliv. 2009, 6, 285–304. [Google Scholar] [CrossRef]
- Rosenblum, D.; Joshi, N.; Tao, W.; Karp, J.M.; Peer, D. Progress and challenges towards targeted delivery of cancer therapeutics. Nat. Commun. 2018, 9, 1410. [Google Scholar] [CrossRef] [Green Version]
- Sigismund, S.; Avanzato, D.; Lanzetti, L. Emerging functions of the EGFR in cancer. Mol. Oncol. 2017, 12, 3–20. [Google Scholar] [CrossRef] [PubMed]
- Pinkas-Kramarski, R.; Alroy, I.; Yarden, Y. ErbB receptors and EGF-like ligands: Cell lineage determination and oncogenesis through combinatorial signaling. J. Mammary Gland. Biol. Neoplasia 1997, 2, 97–107. [Google Scholar] [CrossRef]
- Wee, P.; Wang, Z. Epidermal Growth Factor Receptor Cell Proliferation Signaling Pathways. Cancers 2017, 9, 52. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, X.; Shi, K.; Hao, Y.; Yang, C.; Zha, R.; Yi, C.; Qian, Z. Advances in nanotechnology-based delivery systems for EGFR tyrosine kinases inhibitors in cancer therapy. Asian J. Pharm. Sci. 2019, 15, 26–41. [Google Scholar] [CrossRef]
- Bossuyt, V.; Fadare, O.; Martel, M.; Ocal, I.T.; Burtness, B.; Moinfar, F.; Leibl, S.; Tavassoli, F.A. Remarkably High Frequency of EGFR Expression in Breast Carcinomas with Squamous Differentiation. Int. J. Surg. Pathol. 2005, 13, 319–327. [Google Scholar] [CrossRef] [PubMed]
- Albanell, J.; Baselga, J. The ErbB receptors as targets for breast cancer therapy. J. Mammary Gland. Biol. Neoplasia 1999, 4, 337–351. [Google Scholar] [CrossRef]
- Farasat, A.; Rahbarizadeh, F.; Ahmadvand, D.; Ranjbar, S.; Nikkhoi, S.K. Effective suppression of tumour cells by oligoclonal HER2-targeted delivery of liposomal doxorubicin. J. Liposome Res. 2018, 29, 53–65. [Google Scholar] [CrossRef]
- Duan, D.; Wang, A.; Ni, L.; Zhang, L.; Yan, X.; Jiang, Y.; Mu, H.; Wu, Z.; Sun, K.; Li, Y. Trastuzumab- and Fab′ fragment-modified curcumin PEG-PLGA nanoparticles: Preparation and evaluation in vitro and in vivo. Int. J. Nanomed. 2018, ume 13, 1831–1840. [Google Scholar] [CrossRef] [Green Version]
- Jin, H.; Pi, J.; Zhao, Y.; Jiang, J.; Li, T.; Zeng, X.; Yang, P.; Evans, C.E.; Cai, J. EGFR-targeting PLGA-PEG nanoparticles as a curcumin delivery system for breast cancer therapy. Nanoscale 2017, 9, 16365–16374. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Feng, S.-S. Effects of PEG tethering chain length of vitamin E TPGS with a Herceptin-functionalized nanoparticle formulation for targeted delivery of anticancer drugs. Biomaterials 2014, 35, 3340–3347. [Google Scholar] [CrossRef]
- Kutty, R.V.; Feng, S.-S. Cetuximab conjugated vitamin E TPGS micelles for targeted delivery of docetaxel for treatment of triple negative breast cancers. Biomaterials 2013, 34, 10160–10171. [Google Scholar] [CrossRef] [Green Version]
- Milane, L.; Duan, Z.; Amiji, M. Development of EGFR-Targeted Polymer Blend Nanocarriers for Combination Paclitaxel/Lonidamine Delivery to Treat Multi-Drug Resistance in Human Breast and Ovarian Tumor Cells. Mol. Pharm. 2010, 8, 185–203. [Google Scholar] [CrossRef] [Green Version]
- Dilnawaz, F.; Singh, A.; Mohanty, C.; Sahoo, S.K. Dual drug loaded superparamagnetic iron oxide nanoparticles for targeted cancer therapy. Biomaterials 2010, 31, 3694–3706. [Google Scholar] [CrossRef] [PubMed]
- Acharya, S.; Dilnawaz, F.; Sahoo, S.K. Targeted epidermal growth factor receptor nanoparticle bioconjugates for breast cancer therapy. Biomaterials 2009, 30, 5737–5750. [Google Scholar] [CrossRef]
- Sun, B.; Ranganathan, B.; Feng, S.-S. Multifunctional poly(d,l-lactide-co-glycolide)/montmorillonite (PLGA/MMT) nanoparticles decorated by Trastuzumab for targeted chemotherapy of breast cancer. Biomaterials 2008, 29, 475–486. [Google Scholar] [CrossRef] [PubMed]
- Yu, B.; Tai, H.C.; Xue, W.; Lee, L.J.; Lee, R.J. Receptor-targeted nanocarriers for therapeutic delivery to cancer. Mol. Membr. Biol. 2010, 27, 286–298. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kedar, U.; Phutane, P.; Shidhaye, S.; Kadam, V. Advances in polymeric micelles for drug delivery and tumor targeting. Nanomed. Nanotechnol. Biol. Med. 2010, 6, 714–729. [Google Scholar] [CrossRef]
- Low, P.S.; Kularatne, S.A. Folate-targeted therapeutic and imaging agents for cancer. Curr. Opin. Chem. Biol. 2009, 13, 256–262. [Google Scholar] [CrossRef] [PubMed]
- 102. Fernández, M.; Javaida, F.; Chudasama, V. Advances in targeting the folate receptor in the treatment/imaging of cancers. Chem. Sci. 2018, 9, 790–810. [Google Scholar] [CrossRef] [Green Version]
- Ross, J.F.; Chaudhuri, P.K.; Ratnam, M. Differential regulation of folate receptor isoforms in normal and malignant tissues in vivo and in established cell lines. Physiologic and clinical implications. Cancer 1994, 73, 2432–2443. [Google Scholar] [CrossRef]
- Cheung, A.; Bax, H.J.; Josephs, D.H.; Ilieva, K.M.; Pellizzari, G.; Opzoomer, J.; Bloomfield, J.; Fittall, M.; Grigoriadis, A.; Figini, M.; et al. Targeting folate receptor alpha for cancer treatment. Oncotarget 2016, 7, 52553–52574. [Google Scholar] [CrossRef] [Green Version]
- Elnakat, H. Distribution, functionality and gene regulation of folate receptor isoforms: Implications in targeted therapy. Adv. Drug Deliv. Rev. 2004, 56, 1067–1084. [Google Scholar] [CrossRef]
- Yingchoncharoen, P.; Kalinowski, D.S.; Richardson, D.R. Lipid-Based Drug Delivery Systems in Cancer Therapy: What Is Available and What Is Yet to Come. Pharmacol. Rev. 2016, 68, 701–787. [Google Scholar] [CrossRef] [Green Version]
- Erdoğar, N.; Esendağlı, G.; Nielsen, T.T.; Esendağlı-Yılmaz, G.; Yoyen-Ermis, D.; Erdoğdu, B.; Sargon, M.F.; Eroğlu, H.; Bilensoy, E. Therapeutic efficacy of folate receptor-targeted amphiphilic cyclodextrin nanoparticles as a novel vehicle for paclitaxel delivery in breast cancer. J. Drug Target. 2017, 26, 66–74. [Google Scholar] [CrossRef]
- Thapa, R.K.; Choi, J.Y.; Gupta, B.; Ramasamy, T.; Poudel, B.K.; Ku, S.K.; Youn, Y.S.; Choi, H.G.; Yong, C.S.; Kim, J.O. Liquid crystalline nanoparticles encapsulating cisplatin and docetaxel combination for targeted therapy of breast cancer. Biomater. Sci. 2016, 4, 1340–1350. [Google Scholar] [CrossRef]
- Lin, M.; Teng, L.; Wang, Y.; Zhang, J.; Sun, X. Curcumin-guided nanotherapy: A lipid-based nanomedicine for targeted drug delivery in breast cancer therapy. Drug Deliv. 2015, 23, 1420–1425. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nguyen, D.H.; Lee, J.S.; Bae, J.W.; Choi, J.H.; Lee, Y.; Son, J.Y.; Park, K.D. Targeted doxorubicin nanotherapy strongly suppressing growth of multidrug resistant tumor in mice. Int. J. Pharm. 2015, 495, 329–335. [Google Scholar] [CrossRef] [PubMed]
- Gunduz, U.; Keskin, T.; Tansık, G.; Mutlu, P.; Yalcın, S.; Unsoy, G.; Yakar, A.; Khodadust, R.; Gunduz, G. Idarubicin-loaded folic acid conjugated magnetic nanoparticles as a targetable drug delivery system for breast cancer. Biomed. Pharmacother. 2014, 68, 729–736. [Google Scholar] [CrossRef]
- Zhang, D.; Wang, F.; Chen, Y.; Zhang, Q.; Zheng, D.; Hao, L.; Liu, Y.; Duan, C.; Jia, L.; Liu, G. Folate-mediated targeted and intracellular delivery of paclitaxel using a novel deoxycholic acid-O-carboxymethylated chitosan–folic acid micelles. Int. J. Nanomed. 2012, 7, 325–337. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, J.; Li, S.; Shen, Q.; He, H.; Zhang, Y. Enhanced cellular uptake of folic acid–conjugated PLGA–PEG nanoparticles loaded with vincristine sulfate in human breast cancer. Drug Dev. Ind. Pharm. 2011, 37, 1339–1346. [Google Scholar] [CrossRef]
- Osborne, C.K. Steroid hormone receptors in breast cancer management. Breast Cancer Res. Treat. 1998, 51, 227–238. [Google Scholar] [CrossRef] [PubMed]
- Rai, S.; Paliwal, R.; Vaidya, B.; Khatri, K.; Goyal, A.K.; Gupta, P.N.; Vyas, S.P. Targeted delivery of doxorubicin via estrone-appended liposomes. J. Drug Target. 2008, 16, 455–463. [Google Scholar] [CrossRef]
- Lumachi, F.; Santeufemia, D.; Basso, S.M.M. Current medical treatment of estrogen receptor-positive breast cancer. World J. Biol. Chem. 2015, 6, 231–239. [Google Scholar] [CrossRef]
- Tang, H.; Chen, J.; Wang, L.; Li, Q.; Yang, Y.; Lv, Z.; Bao, H.; Li, Y.; Luan, X.; Li, Y.; et al. Co-delivery of epirubicin and paclitaxel using an estrone-targeted PEGylated liposomal nanoparticle for breast cancer. Int. J. Pharm. 2019, 573, 118806. [Google Scholar] [CrossRef]
- Jain, A.S.; Goel, P.N.; Shah, S.M.; Dhawan, V.V.; Nikam, Y.; Gude, R.P.; Nagarsenker, M.S. Tamoxifen guided liposomes for targeting encapsulated anticancer agent to estrogen receptor positive breast cancer cells: In vitro and in vivo evaluation. Biomed. Pharmacother. 2014, 68, 429–438. [Google Scholar] [CrossRef] [PubMed]
- Paliwal, S.R.; Mishra, N.; Mehta, A.; Vyas, S. A Novel Cancer Targeting Approach Based on Estrone Anchored Stealth Liposome for Site-Specific Breast Cancer Therapy. Curr. Cancer Drug Targets 2010, 10, 343–353. [Google Scholar] [CrossRef] [PubMed]
- Dreaden, E.; Mwakwari, S.C.; Sodji, Q.H.; Oyelere, A.K.; El-Sayed, M.A. Tamoxifen−Poly(ethylene glycol)−Thiol Gold Nanoparticle Conjugates: Enhanced Potency and Selective Delivery for Breast Cancer Treatment. Bioconjugate Chem. 2009, 20, 2247–2253. [Google Scholar] [CrossRef] [Green Version]
- Zöller, M. CD44: Physiological expression of distinct isoforms as evidence for organ-specific metastasis formation. J. Mol. Med. 1995, 73, 425–438. [Google Scholar] [CrossRef]
- Ohene-Abuakwa, Y.; Pignatelli, M. Adhesion Molecules in Cancer Biology. Adv. Exp. Med. Biol. 2002, 465, 115–126. [Google Scholar] [CrossRef]
- Fares, J.; Fares, M.Y.; Khachfe, H.H.; Salhab, H.A.; Fares, Y. Molecular principles of metastasis: A hallmark of cancer revisited. Signal. Transduct. Target. Ther. 2020, 5, 28. [Google Scholar] [CrossRef]
- Gallatin, M.; John, T.P.S.; Siegelman, M.; Reichert, R.; Butcher, E.C.; Weissman, I.L. Lymphocyte homing receptors. Cell 1986, 44, 673–680. [Google Scholar] [CrossRef]
- Nemec, R.E.; Toole, B.P.; Knudson, W. The cell surface hyaluronate binding sites of invasive human bladder carcinoma cells. Biochem. Biophys. Res. Commun. 1987, 149, 249–257. [Google Scholar] [CrossRef]
- Bartolazzi, A.; Peach, R.; Aruffo, A.; Stamenkovic, I. Interaction between CD44 and hyaluronate is directly implicated in the regulation of tumor development. J. Exp. Med. 1994, 180, 53–66. [Google Scholar] [CrossRef] [PubMed]
- Patrawala, L.; Calhoun, T.; Schneiderbroussard, R.; Li, H.; Bhatia, B.; Tang, S.; Reilly, J.; Chandra, D.; Zhou, J.; Claypool, K.; et al. Highly purified CD44+ prostate cancer cells from xenograft human tumors are enriched in tumorigenic and metastatic progenitor cells. Oncogene 2006, 25, 1696–1708. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prince, M.E.; Sivanandan, R.; Kaczorowski, A.; Wolf, G.T.; Kaplan, M.J.; Dalerba, P.; Weissman, I.L.; Clarke, M.F.; Ailles, L.E. Identification of a subpopulation of cells with cancer stem cell properties in head and neck squamous cell carcinoma. Proc. Natl. Acad. Sci. USA 2007, 104, 973–978. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Surace, C.; Arpicco, S.; Dufaÿ-Wojcicki, A.; Marsaud, V.; Bouclier, C.; Clay, D.; Cattel, L.; Renoir, J.-M.; Fattal, E. Lipoplexes Targeting the CD44 Hyaluronic Acid Receptor for Efficient Transfection of Breast Cancer Cells. Mol. Pharm. 2009, 6, 1062–1073. [Google Scholar] [CrossRef]
- Palomeras, S.; Ruiz-Martínez, S.; Puig, T. Targeting Breast Cancer Stem Cells to Overcome Treatment Resistance. Molecules 2018, 23, 2193. [Google Scholar] [CrossRef] [Green Version]
- Rezaei, S.; Kashanian, S.; Bahrami, Y.; Cruz, L.J.; Motiei, M. Redox-Sensitive and Hyaluronic Acid-Functionalized Nanoparticles for Improving Breast Cancer Treatment by Cytoplasmic 17α-Methyltestosterone Delivery. Molecules 2020, 25, 1181. [Google Scholar] [CrossRef] [Green Version]
- Cerqueira, B.B.S.; Lasham, A.; Shelling, A.N.; Al-Kassas, R. Development of biodegradable PLGA nanoparticles surface engineered with hyaluronic acid for targeted delivery of paclitaxel to triple negative breast cancer cells. Mater. Sci. Eng. C 2017, 76, 593–600. [Google Scholar] [CrossRef]
- Liu, Q.; Li, J.; Pu, G.; Zhang, F.; Liu, H.; Zhang, Y. Co-delivery of baicalein and doxorubicin by hyaluronic acid decorated nanostructured lipid carriers for breast cancer therapy. Drug Deliv. 2015, 23, 1364–1368. [Google Scholar] [CrossRef] [Green Version]
- Zhong, Y.; Zhang, J.; Cheng, R.; Deng, C.; Meng, F.; Xie, F.; Zhong, Z. Reversibly crosslinked hyaluronic acid nanoparticles for active targeting and intelligent delivery of doxorubicin to drug resistant CD44+ human breast tumor xenografts. J. Control. Release 2015, 205, 144–154. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, T.; Duan, S.; Davies, N.M.; Forrest, M.L. CD44-tropic polymeric nanocarrier for breast cancer targeted rapamycin chemotherapy. Nanomed. Nanotechnol. Biol. Med. 2014, 10, 1221–1230. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, J.; Zhang, H.; Yu, Y.; Chen, Y.; Wang, D.; Zhang, G.; Zhou, G.; Liu, J.; Sun, Z.; Sun, D.; et al. Biodegradable self-assembled nanoparticles of poly (d,l-lactide-co-glycolide)/hyaluronic acid block copolymers for target delivery of docetaxel to breast cancer. Biomaterials 2013, 35, 550–566. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Liu, Y.; He, Y.; Du, Y.; Wang, W.; Shi, X.; Gao, F. The use of HA oligosaccharide-loaded nanoparticles to breach the endogenous hyaluronan glycocalyx for breast cancer therapy. Biomaterials 2013, 34, 6829–6838. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Taratula, O.; Taratula, O.; Schumann, C.; Minko, T. LHRH-Targeted Drug Delivery Systems for Cancer Therapy. Mini-Rev. Med. Chem. 2017, 17, 258–267. [Google Scholar] [CrossRef] [PubMed]
- Ghanghoria, R.; Kesharwani, P.; Tekade, R.K.; Jain, N.K. Targeting luteinizing hormone-releasing hormone: A potential therapeutics to treat gynecological and other cancers. J. Control. Release 2018, 269, 277–301. [Google Scholar] [CrossRef] [PubMed]
- Obayemi, J.D.; Salifu, A.A.; Eluu, S.C.; Uzonwanne, V.O.; Jusu, S.M.; Nwazojie, C.C.; Onyekanne, C.E.; Ojelabi, O.; Payne, L.; Moore, C.M.; et al. LHRH-Conjugated Drugs as Targeted Therapeutic Agents for the Specific Targeting and Localized Treatment of Triple Negative Breast Cancer. Sci. Rep. 2020, 10, 1–18. [Google Scholar] [CrossRef]
- Hu, J.; Obayemi, J.; Malatesta, K.; Košmrlj, A.; Soboyejo, W. Enhanced cellular uptake of LHRH-conjugated PEG-coated magnetite nanoparticles for specific targeting of triple negative breast cancer cells. Mater. Sci. Eng. C 2018, 88, 32–45. [Google Scholar] [CrossRef] [PubMed]
- Varshosaz, J.; Hassanzadeh, F.; Aliabadi, H.S.; Khoraskani, F.R.; Mirian, M.; Behdadfar, B. Targeted delivery of doxorubicin to breast cancer cells by magnetic LHRH chitosan bioconjugated nanoparticles. Int. J. Biol. Macromol. 2016, 93, 1192–1205. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Tang, Z.; Zhang, Y.; Lv, S.; Li, Q.; Chen, X. Targeted delivery of cisplatin by LHRH-peptide conjugated dextran nanoparticles suppresses breast cancer growth and metastasis. Acta Biomater. 2015, 18, 132–143. [Google Scholar] [CrossRef]
- Taheri, A.; Dinarvand, R.; Ahadi, F.; Khorramizadeh, M.R.; Atyabi, F. The in vivo antitumor activity of LHRH targeted methotrexate–human serum albumin nanoparticles in 4T1 tumor-bearing Balb/c mice. Int. J. Pharm. 2012, 431, 183–189. [Google Scholar] [CrossRef] [PubMed]
- Paliwal, S.R.; Paliwal, R.; Agrawal, G.P.; Vyas, S.P. Liposomal nanomedicine for breast cancer therapy. Nanomedicine 2011, 6, 1085–1100. [Google Scholar] [CrossRef] [PubMed]
- Gocheva, G.; Ivanova, A. A Look at Receptor–Ligand Pairs for Active-Targeting Drug Delivery from Crystallographic and Molecular Dynamics Perspectives. Mol. Pharm. 2019, 16, 3293–3321. [Google Scholar] [CrossRef]
- Ishida, O.; Maruyama, K.; Tanahashi, H.; Iwatsuru, M.; Sasaki, K.; Eriguchi, M.; Yanagie, H. Liposomes Bearing Polyethyleneglycol-Coupled Transferrin with Intracellular Targeting Property to the Solid Tumors In Vivo. Pharm. Res. 2001, 18, 1042–1048. [Google Scholar] [CrossRef]
- Venkatesan, P.; Thirumalaivasan, N.; Yu, H.-P.; Lai, P.-S.; Wu, S.-P. Redox Stimuli Delivery Vehicle Based on Transferrin-Capped MSNPs for Targeted Drug Delivery in Cancer Therapy. ACS Appl. Bio Mater. 2019, 2, 1623–1633. [Google Scholar] [CrossRef]
- Cui, T.; Zhang, S.; Sun, H. Co-delivery of doxorubicin and pH-sensitive curcumin prodrug by transferrin-targeted nanoparticles for breast cancer treatment. Oncol. Rep. 2017, 37, 1253–1260. [Google Scholar] [CrossRef]
- Das, M.; Dilnawaz, F.; Sahoo, S.K. Targeted nutlin-3a loaded nanoparticles inhibiting p53–MDM2 interaction: Novel strategy for breast cancer therapy. Nanomedicine 2011, 6, 489–507. [Google Scholar] [CrossRef]
- Zheng, Y.; Yu, B.; Weecharangsan, W.; Piao, L.; Darby, M.; Mao, Y.; Koynova, R.; Yang, X.; Li, H.; Xu, S.; et al. Transferrin-conjugated lipid-coated PLGA nanoparticles for targeted delivery of aromatase inhibitor 7α-APTADD to breast cancer cells. Int. J. Pharm. 2010, 390, 234–241. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mulik, R.S.; Mönkkönen, J.; Juvonen, R.O.; Mahadik, K.R.; Paradkar, A.R. Transferrin mediated solid lipid nanoparticles containing curcumin: Enhanced in vitro anticancer activity by induction of apoptosis. Int. J. Pharm. 2010, 398, 190–203. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Guo, J.; Tian, Z.; Chen, J.; Gou, G.; Niu, Y.; Li, L.; Yang, J. Piperine-Loaded Glycyrrhizic Acid- and PLGA-Based Nanoparticles Modified with Transferrin for Antitumor. AAPS PharmSciTech 2021, 22, 1–17. [Google Scholar] [CrossRef]
- Desgrosellier, J.S.; Cheresh, D.A. Integrins in cancer: Biological implications and therapeutic opportunities. Nat. Rev. Cancer 2010, 10, 9–22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arihiro, K.; Kaneko, M.; Fujii, S.; Inai, K.; Yokosaki, Y. Significance of α9β1 and αvβ6 integrin expression in breast carcinoma. Breast Cancer 2000, 7, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Bianconi, D.; Unseld, M.; Prager, G.W. Integrins in the Spotlight of Cancer. Int. J. Mol. Sci. 2016, 17, 2037. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Molinaro, R.; Martinez, J.O.; Zinger, A.; De Vita, A.; Storci, G.; Arrighetti, N.; De Rosa, E.; Hartman, K.A.; Basu, N.; Taghipour, N.; et al. Leukocyte-mimicking nanovesicles for effective doxorubicin delivery to treat breast cancer and melanoma. Biomater. Sci. 2019, 8, 333–341. [Google Scholar] [CrossRef]
- Shroff, K.; Kokkoli, E. PEGylated Liposomal Doxorubicin Targeted to α5β1-Expressing MDA-MB-231 Breast Cancer Cells. Langmuir 2012, 28, 4729–4736. [Google Scholar] [CrossRef]
- Graf, N.; Bielenberg, D.R.; Kolishetti, N.; Muus, C.; Banyard, J.; Farokhzad, O.C.; Lippard, S.J. αVβ3 Integrin-Targeted PLGA-PEG Nanoparticles for Enhanced Anti-tumor Efficacy of a Pt(IV) Prodrug. ACS Nano 2012, 6, 4530–4539. [Google Scholar] [CrossRef] [Green Version]
- Ravindranathan, S.; Li, Y.; Wang, S.; Zaidi, M.Y.; Lesinski, G.B.; El-Rayes, B.; Waller, E.K. Abstract 1205: Targeting vasoactive intestinal peptide signaling to enhance pancreatic cancer responsiveness to immunotherapy. Cancer Res. 2019, 79, 1205. [Google Scholar] [CrossRef]
- Akhter, S.; Ahmad, I.; Ahmad, M.Z.; Ramazani, F.; Singh, A.; Rahman, Z.; Ahmad, F.J.; Storm, G.; Kok, R.J. Nanomedicines as cancer therapeutics: Current status. Curr. Cancer Drug Targets 2013, 13, 362–378. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eskandari, Z.; Bahadori, F.; Yapaoz, M.A.; Yenigun, V.B.; Celikten, M.; Kocyigit, A.; Onyuksel, H. Targeting breast cancer using pirarubicin-loaded vasoactive intestinal peptide grafted sterically stabilized micelles. Eur. J. Pharm. Sci. 2021, 162, 105830. [Google Scholar] [CrossRef] [PubMed]
- Gülçür, E.; Thaqi, M.; Khaja, F.; Kuzmis, A.; Önyüksel, H. Curcumin in VIP-targeted sterically stabilized phospholipid nanomicelles: A novel therapeutic approach for breast cancer and breast cancer stem cells. Drug Deliv. Transl. Res. 2013, 3, 562–574. [Google Scholar] [CrossRef] [Green Version]
- Dagar, A.; Kuzmis, A.; Rubinstein, I.; Sekosan, M.; Onyuksel, H. VIP-targeted cytotoxic nanomedicine for breast cancer. Drug Deliv. Transl. Res. 2012, 2, 454–462. [Google Scholar] [CrossRef]
- Önyüksel, H.; Jeon, E.; Rubinstein, I. Nanomicellar paclitaxel increases cytotoxicity of multidrug resistant breast cancer cells. Cancer Lett. 2009, 274, 327–330. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Onyüksel, H.; Mohanty, P.S.; Rubinstein, I. VIP-grafted sterically stabilized phospholipid nanomicellar 17-allylamino-17-demethoxy geldanamycin: A novel targeted nanomedicine for breast cancer. Int. J. Pharm. 2009, 365, 157–161. [Google Scholar] [CrossRef] [Green Version]
- Tian, B.; Ding, Y.; Han, J.; Zhang, J.; Han, Y.; Han, J. N-acetyl-d-glucosamine decorated polymeric nanoparticles for targeted delivery of doxorubicin: Synthesis, characterization and in vitro evaluation. Colloids Surf. B Biointerfaces 2015, 130, 246–254. [Google Scholar] [CrossRef]
- Guo, Z.; He, B.; Yuan, L.; Dai, W.; Zhang, H.; Wang, X.; Wang, J.; Zhang, X.; Zhang, Q. Dual targeting for metastatic breast cancer and tumor neovasculature by EphA2-mediated nanocarriers. Int. J. Pharm. 2015, 493, 380–389. [Google Scholar] [CrossRef] [PubMed]
- Mei, D.; Lin, Z.; Fu, J.; He, B.; Gao, W.; Ma, L.; Dai, W.; Zhang, H.; Wang, X.; Wang, J.; et al. The use of α-conotoxin ImI to actualize the targeted delivery of paclitaxel micelles to α7 nAChR-overexpressing breast cancer. Biomaterials 2015, 42, 52–65. [Google Scholar] [CrossRef]
- Nishikawa, K.; Asai, T.; Shigematsu, H.; Shimizu, K.; Kato, H.; Asano, Y.; Takashima, S.; Mekada, E.; Oku, N.; Minamino, T. Development of anti-HB-EGF immunoliposomes for the treatment of breast cancer. J. Control. Release 2011, 160, 274–280. [Google Scholar] [CrossRef]
- Taheri, A.; Dinarvand, R.; Khorramizadeh, M.; Borougeni, A.T.; Mansoori, P.; Atyabi, F. Use of biotin targeted methotrexate–human serum albumin conjugated nanoparticles to enhance methotrexate antitumor efficacy. Int. J. Nanomed. 2011, 6, 1863–1874. [Google Scholar] [CrossRef] [Green Version]
- Yu, D.-H.; Lu, Q.; Xie, J.; Fang, C.; Chen, H.-Z. Peptide-conjugated biodegradable nanoparticles as a carrier to target paclitaxel to tumor neovasculature. Biomaterials 2010, 31, 2278–2292. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.A.; Jain, V.K.; Ahmad, J.; Jain, K. PI3K/AKT/mTOR pathway inhibitors in triple-negative breast cancer: A review on drug discovery and future challenges. Drug Discov. Today 2019, 24, 2181–2191. [Google Scholar] [CrossRef]
- Mu, Q.; Lin, G.; Jeon, M.; Wang, H.; Chang, F.C.; Revia, R.A.; Yu, J.; Zhang, M. Iron oxide nanoparticle targeted chemo-immunotherapy for triple negative breast cancer. Mater. Today 2021. [Google Scholar] [CrossRef]
- Ko, N.R.; Van, S.Y.; Hong, S.H.; Kim, S.Y.; Kim, M.; Lee, J.S.; Lee, S.J.; Lee, Y.K.; Kwon, I.K.; Oh, S.J. Dual pH-and GSH-responsive degradable PEGylated graphene quantum dot-based nanoparticles for enhanced HER2-positive breast cancer therapy. Nanomaterials 2020, 10, 91. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Naruphontjirakul, P.; Viravaidya-Pasuwat, K. Development of anti-HER2-targeted doxorubicin–core-shell chitosan nanoparticles for the treatment of human breast cancer. Int. J. Nanomed. 2019, ume 14, 4105–4121. [Google Scholar] [CrossRef] [Green Version]
- Dziawer, Ł.; Majkowska-Pilip, A.; Gaweł, D.; Godlewska, M.; Pruszyński, M.; Jastrzębski, J.; Wąs, B.; Bilewicz, A. Trastuzumab-Modified Gold Nanoparticles Labeled with 211At as a Prospective Tool for Local Treatment of HER2-Positive Breast Cancer. Nanomaterials 2019, 9, 632. [Google Scholar] [CrossRef] [Green Version]
- Rong, L.; Zhou, S.; Liu, X.; Li, A.; Jing, T.; Liu, X.; Zhang, Y.; Cai, S.; Tang, X. Trastuzumab-modified DM1-loaded nanoparticles for HER2 + breast cancer treatment: An in vitro and in vivo study. Artif. Cells Nanomed. Biotechnol. 2017, 46, 1708–1718. [Google Scholar] [CrossRef]
- Cui, N.; Zhu, S.-H. Monoclonal antibody-tagged polyethylenimine (PEI)/poly(lactide) (PLA) nanoparticles for the enhanced delivery of doxorubicin in HER-positive breast cancers. RSC Adv. 2016, 6, 79822–79829. [Google Scholar] [CrossRef]
- Nasrollahi, Z.; Mohammadi, S.R.; Mollarazi, E.; Yadegari, M.H.; Hassan, Z.M.; Talaei, F.; Dinarvand, R.; Akbari, H.; Atyabi, F. Functionalized nanoscale β-1,3-glucan to improve Her2+ breast cancer therapy: In vitro and in vivo study. J. Control. Release 2015, 202, 49–56. [Google Scholar] [CrossRef]
- Zhao, J.; Mi, Y.; Feng, S.-S. Targeted co-delivery of docetaxel and siPlk1 by herceptin-conjugated vitamin E TPGS based immunomicelles. Biomaterials 2013, 34, 3411–3421. [Google Scholar] [CrossRef]
- Mattu, C.; Pabari, R.; Boffito, M.; Sartori, S.; Ciardelli, G.; Ramtoola, Z. Comparative evaluation of novel biodegradable nanoparticles for the drug targeting to breast cancer cells. Eur. J. Pharm. Biopharm. 2013, 85, 463–472. [Google Scholar] [CrossRef] [PubMed]
- Dinarvand, R.; Koopaei, M.N.; Amini, M.; Rabbani, H.; Ostad, S.N.; Atyabi, F. Docetaxel immunonanocarriers as targeted delivery systems for HER2-positive tumor cells: Preparation, characterization, and cytotoxicity studies. Int. J. Nanomed. 2011, 6, 1903–1912. [Google Scholar] [CrossRef] [Green Version]
- Gao, J.; Kou, G.; Wang, H.; Chen, H.; Li, B.; Lu, Y.; Zhang, D.; Wang, S.; Hou, S.; Qian, W.; et al. PE38KDEL-loaded anti-HER2 nanoparticles inhibit breast tumor progression with reduced toxicity and immunogenicity. Breast Cancer Res. Treat. 2008, 115, 29–41. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Gao, J.; Lu, Y.; Kou, G.; Zhang, H.; Fan, L.; Sun, Z.; Guo, Y.; Zhong, Y. Preparation and characterization of PE38KDEL-loaded anti-HER2 nanoparticles for targeted cancer therapy. J. Control. Release 2008, 128, 209–216. [Google Scholar] [CrossRef]
- Venugopal, V.; Krishnan, S.; Palanimuthu, V.R.; Sankarankutty, S.; Kalaimani, J.K.; Karupiah, S.; Kit, N.S.; Hock, T.T. Anti-EGFR anchored paclitaxel loaded PLGA nanoparticles for the treatment of triple negative breast cancer. In-vitro and in-vivo anticancer activities. PLoS ONE 2018, 13, e0206109. [Google Scholar] [CrossRef] [PubMed]
- Agnello, L.; Tortorella, S.; d’Argenio, A.; Carbone, C.; Camorani, S.; Locatelli, E.; Auletta, L.; Sorrentino, D.; Fedele, M.; Zannetti, A.; et al. Optimizing Cisplatin Delivery to Triple-negative Breast Cancer through Novel EGFR Aptamer-conjugated Polymeric Nanovectors. J. Exp. Clin. Cancer Res. 2021, 40, 239. [Google Scholar] [CrossRef] [PubMed]
- Wan, X.; Zheng, X.; Pang, X.; Pang, Z.; Zhao, J.; Zhang, Z.; Jiang, T.; Xu, W.; Zhang, Q.; Jiang, X. Lapatinib-loaded human serum albumin nanoparticles for the prevention and treatment of triple-negative breast cancer metastasis to the brain. Oncotarget 2016, 7, 34038–34051. [Google Scholar] [CrossRef]
- Ai, S.; Duan, J.; Liu, X.; Bock, S.; Tian, Y.; Huang, Z. Biological Evaluation of a Novel Doxorubicin−Peptide Conjugate for Targeted Delivery to EGF Receptor-Overexpressing Tumor Cells. Mol. Pharm. 2011, 8, 375–386. [Google Scholar] [CrossRef]
- Yassemi, A.; Kashanian, S.; Zhaleh, H. Folic acid receptor-targeted solid lipid nanoparticles to enhance cytotoxicity of letrozole through induction of caspase-3 dependent-apoptosis for breast cancer treatment. Pharm. Dev. Technol. 2020, 25, 397–407. [Google Scholar] [CrossRef]
- Pan, C.; Liu, Y.; Zhou, M.; Wang, W.; Shi, M.; Xing, M.; Liao, W. Theranostic pH-sensitive nanoparticles for highly efficient targeted delivery of doxorubicin for breast tumor treatment. Int. J. Nanomed. 2018, ume 13, 1119–1137. [Google Scholar] [CrossRef] [Green Version]
- Huang, Y.; Mao, K.; Zhang, B.; Zhao, Y. Superparamagnetic iron oxide nanoparticles conjugated with folic acid for dual target-specific drug delivery and MRI in cancer theranostics. Mater. Sci. Eng. C 2017, 70, 763–771. [Google Scholar] [CrossRef]
- Zhou, X.; Chen, L.; Nie, W.; Wang, W.; Qin, M.; Mo, X.; Wang, H.; He, C. Dual-Responsive Mesoporous Silica Nanoparticles Mediated Codelivery of Doxorubicin and Bcl-2 SiRNA for Targeted Treatment of Breast Cancer. J. Phys. Chem. C 2016, 120, 22375–22387. [Google Scholar] [CrossRef]
- Siafaka, P.I.; Betsiou, M.; Tsolou, A.; Angelou, E.; Agianian, B.; Koffa, M.; Chaitidou, S.; Karavas, E.; Avgoustakis, K.; Bikiaris, D. Synthesis of folate- pegylated polyester nanoparticles encapsulating ixabepilone for targeting folate receptor overexpressing breast cancer cells. J. Mater. Sci. Mater. Electron. 2015, 26, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Zhu, D.; Zhang, L.; Dong, X.; Sun, H.; Song, C.; Wang, C.; Kong, D. Folate-modified lipid–polymer hybrid nanoparticles for targeted paclitaxel delivery. Int. J. Nanomed. 2015, 10, 2101–2114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qian, J.; Xu, M.; Suo, A.; Xu, W.; Liu, T.; Liu, X.; Yao, Y.; Wang, H. Folate-decorated hydrophilic three-arm star-block terpolymer as a novel nanovehicle for targeted co-delivery of doxorubicin and Bcl-2 siRNA in breast cancer therapy. Acta Biomater. 2015, 15, 102–116. [Google Scholar] [CrossRef]
- Saxena, V.; Naguib, Y.; Hussain, M.D. Folate receptor targeted 17-allylamino-17-demethoxygeldanamycin (17-AAG) loaded polymeric nanoparticles for breast cancer. Colloids Surf. B Biointerfaces 2012, 94, 274–280. [Google Scholar] [CrossRef]
- Mohammadi, Z.; Samadi, F.Y.; Rahmani, S.; Mohammadi, Z. Chitosan-Raloxifene nanoparticle containing doxorubicin as a new double-effect targeting vehicle for breast cancer therapy. DARU J. Pharm. Sci. 2020, 28, 433–442. [Google Scholar] [CrossRef]
- Das Kurmi, B.; Paliwal, R.; Paliwal, S.R. Dual cancer targeting using estrogen functionalized chitosan nanoparticles loaded with doxorubicin-estrone conjugate: A quality by design approach. Int. J. Biol. Macromol. 2020, 164, 2881–2894. [Google Scholar] [CrossRef] [PubMed]
- Hao, Q.; Xu, G.; Yang, Y.; Sun, Y.; Cong, D.; Li, H.; Liu, X.; Wang, Z.; Zhang, Z.; Chen, J.; et al. Oestrone-targeted liposomes for mitoxantrone delivery via oestrogen receptor synthesis, physicochemical characterization and in-vitro evaluation. J. Pharm. Pharmacol. 2017, 69, 991–1001. [Google Scholar] [CrossRef] [PubMed]
- Madan, J.; Gundala, S.R.; Kasetti, Y.; Bharatam, P.V.; Aneja, R.; Katyal, A.; Jain, U.K. Enhanced noscapine delivery using estrogen-receptor-targeted nanoparticles for breast cancer therapy. Anti-Cancer Drugs 2014, 25, 704–716. [Google Scholar] [CrossRef]
- Fang, H.; Zhao, X.; Gu, X.; Sun, H.; Cheng, R.; Zhong, Z.; Deng, C. CD44-Targeted Multifunctional Nanomedicines Based on a Single-Component Hyaluronic Acid Conjugate with All-Natural Precursors: Construction and Treatment of Metastatic Breast Tumors in Vivo. Biomacromolecules 2019, 21, 104–113. [Google Scholar] [CrossRef]
- Liu, D.L.D.; Zhang, Q.Z.Q.; Wang, J.W.J.; Fan, L.F.L.; Zhu, W.Z.W.; Cai, D.C.D. Hyaluronic acid-coated single-walled carbon nanotubes loaded with doxorubicin for the treatment of breast cancer. Pharmazie 2019, 74, 83–90. [Google Scholar] [CrossRef]
- Wang, S.; Shao, M.; Zhong, Z.; Wang, A.; Cao, J.; Lu, Y.; Wang, Y.; Zhang, J. Co-delivery of gambogic acid and TRAIL plasmid by hyaluronic acid grafted PEI-PLGA nanoparticles for the treatment of triple negative breast cancer. Drug Deliv. 2017, 24, 1791–1800. [Google Scholar] [CrossRef] [Green Version]
- Tran, B.N.; Nguyen, H.T.; Kim, J.O.; Yong, C.S.; Nguyen, C.N. Combination of a chemopreventive agent and paclitaxel in CD44-targeted hybrid nanoparticles for breast cancer treatment. Arch. Pharmacal Res. 2017, 40, 1420–1432. [Google Scholar] [CrossRef]
- Wang, X.; Liu, Y.; Wang, S.; Shi, D.; Zhou, X.; Wang, C.; Wu, J.; Zeng, Z.; Li, Y.; Sun, J.; et al. CD44-engineered mesoporous silica nanoparticles for overcoming multidrug resistance in breast cancer. Appl. Surf. Sci. 2015, 332, 308–317. [Google Scholar] [CrossRef]
- Yin, T.; Wang, L.; Yin, L.; Zhou, J.; Huo, M. Co-delivery of hydrophobic paclitaxel and hydrophilic AURKA specific siRNA by redox-sensitive micelles for effective treatment of breast cancer. Biomaterials 2015, 61, 10–25. [Google Scholar] [CrossRef]
- Deng, X.; Cao, M.; Zhang, J.; Hu, K.; Yin, Z.; Zhou, Z.; Xiao, X.; Yang, Y.; Sheng, W.; Wu, Y.; et al. Hyaluronic acid-chitosan nanoparticles for co-delivery of MiR-34a and doxorubicin in therapy against triple negative breast cancer. Biomaterials 2014, 35, 4333–4344. [Google Scholar] [CrossRef]
- Han, M.; Lv, Q.; Tang, X.-J.; Hu, Y.-L.; Xu, D.-H.; Li, F.-Z.; Liang, W.-Q.; Gao, J.-Q. Overcoming drug resistance of MCF-7/ADR cells by altering intracellular distribution of doxorubicin via MVP knockdown with a novel siRNA polyamidoamine-hyaluronic acid complex. J. Control. Release 2012, 163, 136–144. [Google Scholar] [CrossRef] [PubMed]
- Jadia, R.; Kydd, J.; Rai, P. Remotely Phototriggered, Transferrin-Targeted Polymeric Nanoparticles for the Treatment of Breast Cancer. Photochem. Photobiol. 2018, 94, 765–774. [Google Scholar] [CrossRef]
- Li, Y.; Xiao, Y.; Lin, H.-P.; Reichel, D.; Bae, Y.; Lee, E.Y.; Jiang, Y.; Huang, X.; Yang, C.; Wang, Z. In vivo β-catenin attenuation by the integrin α5-targeting nano-delivery strategy suppresses triple negative breast cancer stemness and metastasis. Biomaterials 2018, 188, 160–172. [Google Scholar] [CrossRef]
- Hu, G.; Zhang, H.; Zhang, L.; Ruan, S.; He, Q.; Gao, H. Integrin-mediated active tumor targeting and tumor microenvironment response dendrimer-gelatin nanoparticles for drug delivery and tumor treatment. Int. J. Pharm. 2015, 496, 1057–1068. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, A.; Asai, T.; Hirai, Y.; Shimizu, K.; Koide, H.; Minamino, T.; Oku, N. Systemic Administration of siRNA with Anti-HB-EGF Antibody-Modified Lipid Nanoparticles for the Treatment of Triple-Negative Breast Cancer. Mol. Pharm. 2018, 15, 1495–1504. [Google Scholar] [CrossRef]
- Liu, Z.; Tao, Z.; Zhang, Q.; Wan, S.; Zhang, F.; Zhang, Y.; Wu, G.; Wang, J. YSA-conjugated mesoporous silica nanoparticles effectively target EphA2-overexpressing breast cancer cells. Cancer Chemother. Pharmacol. 2018, 81, 687–695. [Google Scholar] [CrossRef] [PubMed]
- Lu, R.; Zhou, L.; Yue, Q.; Liu, Q.; Cai, X.; Xiao, W.; Hai, L.; Guo, L.; Wu, Y. Liposomes modified with double-branched biotin: A novel and effective way to promote breast cancer targeting. Bioorganic Med. Chem. 2019, 27, 3115–3127. [Google Scholar] [CrossRef] [PubMed]
- Lv, L.; Liu, C.; Chen, C.; Yu, X.; Chen, G.; Shi, Y.; Qin, F.; Ou, J.; Qiu, K.; Li, G. Quercetin and doxorubicin co-encapsulated biotin receptor-targeting nanoparticles for minimizing drug resistance in breast cancer. Oncotarget 2016, 7, 32184–32199. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Wang, F.; Wang, Y.; Wang, J.; Yu, Y.; Guo, S.; Chen, R.; Zhou, D. pH and near-infrared light dual-stimuli responsive drug delivery using DNA-conjugated gold nanorods for effective treatment of multidrug resistant cancer cells. J. Control. Release 2016, 232, 9–19. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Luo, Y.; Zhao, X.; Li, X.; Li, K.; Chen, D.; Qiao, M.; Hu, H.; Zhao, X. Co-delivery of doxorubicin and the traditional Chinese medicine quercetin using biotin–PEG2000–DSPE modified liposomes for the treatment of multidrug resistant breast cancer. RSC Adv. 2016, 6, 113173–113184. [Google Scholar] [CrossRef]
- Qian, Q.; Niu, S.; Williams, G.R.; Wu, J.; Zhang, X.; Zhu, L.-M. Peptide functionalized dual-responsive chitosan nanoparticles for controlled drug delivery to breast cancer cells. Colloids Surf. A Physicochem. Eng. Asp. 2018, 564, 122–130. [Google Scholar] [CrossRef]
- Lin, W.; Ma, G.; Kampf, N.; Yuan, Z.; Chen, S. Development of Long-Circulating Zwitterionic Cross-Linked Micelles for Active-Targeted Drug Delivery. Biomacromolecules 2016, 17, 2010–2018. [Google Scholar] [CrossRef]
- Hua, S.; De Matos, M.B.C.; Metselaar, J.M.; Storm, G. Current Trends and Challenges in the Clinical Translation of Nanoparticulate Nanomedicines: Pathways for Translational Development and Commercialization. Front. Pharmacol. 2018, 9, 790. [Google Scholar] [CrossRef] [PubMed]
- Beltrán-Gracia, E.; López-Camacho, A.; Higuera-Ciapara, I.; Velázquez-Fernández, J.B.; Vallejo-Cardona, A.A. Nanomedicine review: Clinical developments in liposomal applications. Cancer Nanotechnol. 2019, 10, 1–40. [Google Scholar] [CrossRef]
- Blair, H.A. Daunorubicin/Cytarabine Liposome: A Review in Acute Myeloid Leukaemia. Drugs 2018, 78, 1903–1910. [Google Scholar] [CrossRef] [Green Version]
- Imam, S.S.; Alshehri, S.; Ghoneim, M.M.; Zafar, A.; Alsaidan, O.A.; Alruwaili, N.K.; Gilani, S.J.; Rizwanullah, M. Recent Advancement in Chitosan-Based Nanoparticles for Improved Oral Bioavailability and Bioactivity of Phytochemicals: Challenges and Perspectives. Polymers 2021, 13, 4036. [Google Scholar] [CrossRef]
- Shi, J.; Kantoff, P.W.; Wooster, R.; Farokhzad, O.C. Cancer nanomedicine: Progress, challenges and opportunities. Nat. Rev. Cancer 2017, 17, 20–37. [Google Scholar] [CrossRef] [PubMed]
- Gu, W.; Meng, F.; Haag, R.; Zhong, Z. Actively targeted nanomedicines for precision cancer therapy: Concept, construction, challenges and clinical translation. J. Control. Release 2021, 329, 676–695. [Google Scholar] [CrossRef]
- Allen, T.M. Drug Delivery Systems: Entering the Mainstream. Science 2004, 303, 1818–1822. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, L.; Gu, F.; Chan, J.; Wang, A.; Langer, R.S.; Farokhzad, O.C. Nanoparticles in Medicine: Therapeutic Applications and Developments. Clin. Pharmacol. Ther. 2007, 83, 761–769. [Google Scholar] [CrossRef] [PubMed]
- Narang, A.; Chang, R.-K.; Hussain, M.A. Pharmaceutical Development and Regulatory Considerations for Nanoparticles and Nanoparticulate Drug Delivery Systems. J. Pharm. Sci. 2013, 102, 3867–3882. [Google Scholar] [CrossRef] [PubMed]
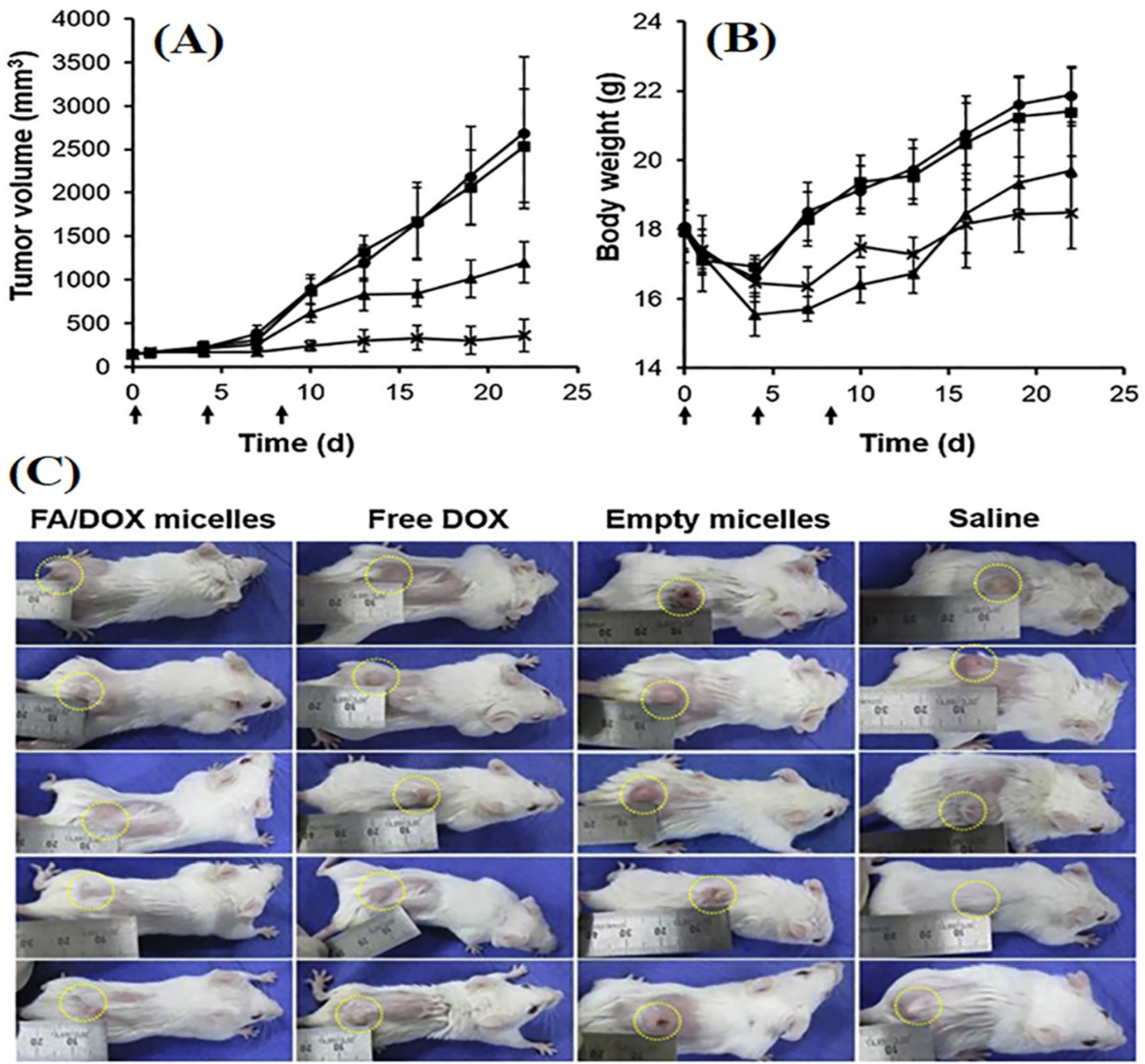
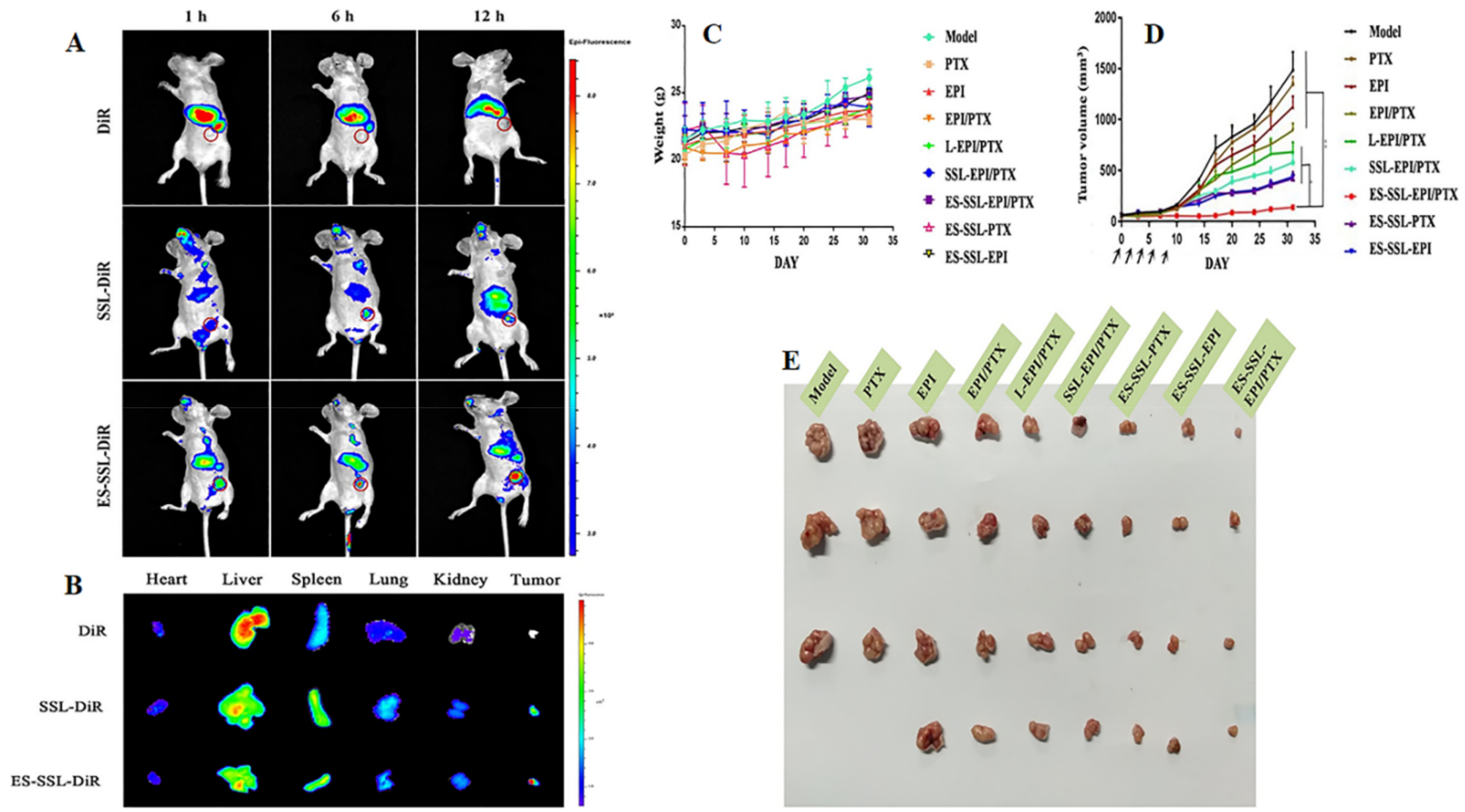
| Receptor Targeted | Ligand | Type of Nanomedicine | Drug Loaded | Breast Cancer Model | Outcome | Ref. |
|---|---|---|---|---|---|---|
| HER2 | Herceptin and PEG | Carbon Based NPs (CNP) | DOX | SK-BR-3 and MDA-MB-231 cells | High cellular uptake with low toxicity Significant inhibition of antitumor activity both in vitro and in vivo | [151] |
| Anti-HER2 monoclonal antibody | Core-shell chitosan NPs | DOX | MCF-7 cells | In vitro cytotoxicity studies showed the lowest IC50, Increased therapeutic efficacy of DOX | [152] | |
| Trastuzumab | Gold NPs | - | HER2 overexpressing human ovarian SKOV-3 cell line | In vitro biological studies indicated higher affinity and cytotoxicity towards the SKOV-3 cells. | [153] | |
| Trastuzumab | Polymeric NPs | Emtansine (DM1) | MDA-MB-453 xenograft bearing mice | Inhibition of tumor growth by 88%, Fewer toxic effects in vivo. | [154] | |
| Herceptin | Polyethyleneimine (PEI)/PLA NPs | DOX | Xenograft nude mice bearing SK-B In vivo R-3 cancer cells Female Balb/c mice | -Superior cytotoxic effect in the cancer cells, Cellular internalization via receptor-mediated endocytosis, and effective targeting ability of the tagged moiety. -Reduced side effects of DOX in the xenograft tumor model. | [155] | |
| Trastuzumab | β-1,3-glucan (Glu) succinate NPs | DOX | In vitro/MCF-7 and HER2 positive murine (4T1) breast cancer cell line In vivo/female Balb/c mice bearing 4T1 breast tumor | Significantly higher cellular uptake Significantly improved in vitro and in vivo cytotoxicity | [156] | |
| Herceptin | PLA NPs | TPGS | SK-BR-3 cells | -Targeted NPs exhibited significantly higher cellular uptake compared to nontargeted NPs, -Targeted NPs exhibited a 9.6-fold lower IC50 value compared to nontargeted NPs | [69] | |
| Herceptin | Micelles | Docetaxel, polo-like kinase 1 siRNA | NIH3T3, MCF-7, and SK-BR-3 breast cancer cells | Significantly enhanced internalization into the cytoplasm of the SK-BR-3 cells, excellent reduction in IC50 value | [157] | |
| Herceptin | poly (ε-caprolactone), PLA, PLGA, polyester urethanes (PURs), and N-Boc-serinol NPs | Paclitaxel | In vitro/HER2 than MCF-7 breast cancer cell lines | 1.2- and 1.3-fold higher cellular uptake compared to nontargeted NPs Significantly higher cytotoxic effect compared to non-HER coated NPs | [158] | |
| Trastuzumab | PEG-PLGA NPs | Docetaxel | In vitro/SK-BR-3 and BT-474 cell lines | Significantly higher cellular uptake Improved in vitro cytotoxicity | [159] | |
| HER2 antibody | Glycerol monooleate coated magnetic NPs (GMO-MNPs) | paclitaxel and/or rapamycin | In vitro/human breast carcinoma MCF-7 cell line | Significantly enhanced cellular uptake Targeted paclitaxel-loaded GMO-MNPs exhibited ~24 folds lower IC50 value than native paclitaxel and ~3 folds lower IC50 value than nontargeted paclitaxel loaded GMO-MNPs. In the case of rapamycin drug, the targeted GMO-MNPs exhibited ~71 folds lower IC50 value than native drug and ~10 folds lower IC50 value than nontargeted GMO-MNPs. In the case of combined drug formulation, the targeted NPs exhibited ~55 folds lower IC50 value than native drugs and ~7 folds lower IC50 value than unconjugated NPs | [72] | |
| Fab’ fragments of a humanized anti-HER2 monoclonal antibody | PLGA NPs | PE38KDEL | In vitro/D2F2/E2 and SK-BR-3 cells In vivo/Balb/c mice bearing D2F2/E2 breast tumors | Significantly enhanced in vitro and in vivo cytotoxicity Reduced toxicity and immunogenicity | [160] | |
| Fab’ fragments of a humanized anti-HER2 monoclonal antibody | PLGA NPs | PE38KDEL | In vitro/BT-474, MDA-MB-231 and MCF-7 breast cancer cell lines In vivo/Balb/c nude mice bearing BT-474 human breast cancer cells | Exhibited notably enhanced cytotoxicity against all three cell lines compared to NPs without conjugated with antibody Improved in vivo antitumor activity compared to immunotoxin PE-HER Reduced systemic cytotoxicity | [161] | |
| Trastuzumab | PLGA/montmorillonite NPs | paclitaxel | In vitro/SK-BR-3 cells | Significantly higher cellular uptake 12.74 times higher cytotoxicity than that of the bare NPs and 13.11 times higher than Taxols | [74] | |
| EGFR | Anti-EGFR Protein | Immunonanoparticles (INPs) | Paclitaxel | MDA-MB-468 TNBC cell line. Athymic mice model | INPs showed significantly enhanced cytotoxicity, i.e., remarkable reduction of cell viability NPs penetrated into the cell membrane and showed significantly enhanced cellular uptake. Significant reduction in the expression of EGFR protein | [162] |
| CL4 aptamer | PLGA-b-PEG NPs | Cisplatin | MDA-MB-231 and MDA-MB-231 EGFR-KO cells; Female mice bearing MDA-MB-231 xenografts | High and fast cellular uptake in EGFR-positive TNBC cells as well as high cytotoxicity, i.e., decreased cancer cell viability Significantly high tumor targeting efficiency and therapeutic efficacy without any signs of systemic toxicity | [163] | |
| Anti-SPARC antibody | Human serum albumin NPs | Lapatinib | In vitro/4T1 cells (murine TNBC cells) | Targeted NPs (i.v.) effectively enhanced the accumulation of lapatinib in tumor tissue at 2.38 and 16.6- times the level of LS (i.v.) and Tykerb (p.o.), respectively | [164] | |
| Cetuximab | TPGS Micelles | Docetaxel | In vitro/MDA-MB-468 and MDA-MB 231 cancer cell line | Significantly higher cellular uptake 205.6- and 223.8-folds higher efficiency than Taxotere (free drug solution) for the MDA-MB-468 and MDA-MB-231 cell lines respectively | [70] | |
| NH2- CMYIEALDKYAC-COOH peptide | Peptide nanoconjugate | DOX | In vitro/SW480, SGC-7901, B16 and MCF-7 cancer cell lines In vivo/C57BL/6 mice bearing murine melanoma B16 tumor | Significantly higher cellular uptake Exhibited higher in vitro cytotoxicity in EGFR-overexpressed cancer cells Targeted NPs exhibited a significantly higher reduction in tumor volume compared to nontargeted NPs. | [165] | |
| EGFR-antibody | PLGA NPs | Rapamycin | In vitro/MCF-7 cell line | 13-fold higher cellular uptake compared to nontargeted NPs Significantly higher antiproliferative activity than unconjugated rapamycin loaded NPs and native rapamycin | [73] | |
| Folate | FA | SLNs | Letrozole | MCF-7 cancer cells | Significantly enhanced cytotoxicity Induction of caspase-3 dependent apoptosis | [166] |
| FA | pH-responsive poly(β-thiopropionate) NPs with a magnetic core | DOX | MCF-7, BT549, and MD-MBA-231 cells. MCF-7 cells injected Female Balb/c nude mice | -FA-DOX@IONPs showed the strongest cytotoxicity against breast cancer cells Suppression in in vivo tumor growth in mice -No signs of toxicity in healthy organs | [167] | |
| FA | SPIONs | DOX | MCF-7 cell line Xenograft MCF-7 breast tumor of nude mice | -Internalization by receptor-mediated endocytosis -Inhibition in tumor growth -No significant toxicity of DOX@ FA-SPIONs on mice organs | [168] | |
| Folate | MSNs | DOX and Bcl-2 siRNA | MDA-MB-231 breast cancer cell line | significantly enhanced intracellular uptake Induction of remarkable cell apoptosis | [169] | |
| FA | PEG-poly (propylene succinate) NPs | Ixabepilone | HeLa Kyoto (HeLa K) and MCF-7 cells | Enhanced cellular uptake by receptor-mediated endocytosis | [170] | |
| Folate | Nanostructured lipid carriers | Curcumin | MCF-7 human breast cancer cells Balb/c nude mice | -3.52 and 10.41-fold reductions in IC50 values compared to nontargeted NLC and curcumin solutions, respectively, in vitro -Significantly higher tumor growth inhibition in vivo. | [85] | |
| Folate | Lipid-polymer hybrid NPs | Paclitaxel | EMT6 breast tumor cell line/Balb/c female mice | -Significantly higher cellular uptake, significantly improved antitumor efficacy in vitro and in vivo | [171] | |
| FA | Nanomicelle plexes of hydrophilic cationic star-block terpolymer | DOX and Bcl-2 siRNA | In vitro/MCF-7 breast cancer cell line | -Significantly higher cellular uptake Significantly higher in vitro cytotoxicity compared to nontargeted NPs | [172] | |
| FA | Polymeric micelles | DOX | MCF-7/MDR cells/Balb/c mice | Significantly higher cellular uptake, ~3.3 and 8-fold higher tumor volume reduction in 3 weeks in vivo compared to nontargeted micelles and free DOX solution. | [86] | |
| FA | Magnetic NPs | Idarubicin | MCF-7 cell line | 2-fold higher tumor inhibition in vitro. | [87] | |
| FA | PLGA-PEG NPs | 17-AAG | In vitro/MCF-7 human breast cancer cells | Much higher intracellular uptake 2-fold higher cytotoxicity compared to nontargeted NPs | [173] | |
| FA | PLGA-PEG NPs | Vincristine | In vitro/MCF-7 human breast cancer cells | Significantly higher cellular uptake Targeted NPs exhibited 1.52 and 3.91-folds higher cytotoxicity on MCF-7 cells than that of nontargeted NPs and free vincristine sulfate, respectively | [89] | |
| Estrogen | Estrone | Liposomal NPs | Epirubicin and paclitaxel | MCF-7 cell line | Significantly improved accumulation in tumor cells, Increased systemic circulation time and biodistribution in main organs, Suppression in tumor growth without inducing toxicity. | [93] |
| Raloxifene | Chitosan NPs | DOX | MCF-7 cell line | Significantly higher cytotoxicity Enhanced antitumor efficacy | [174] | |
| Estrone | Chitosan NPs | DOX | MCF-7 cell line Tumor-bearing rat model | Higher potency of developed NPs Significantly improved efficacy least toxic against blood cells and cardiac tissues hence reduction of cardiotoxicity of DOX | [175] | |
| Estrone | Liposomes | Mitoxantrone | HL-60 cells. | Specific cellular uptake via the ligand–receptor-mediated pathway Significant reduction in the growth of HL-60 cells | [176] | |
| Estrone | Gelatin NPs | Noscapine | ER-positive MCF-7 and ER-negative MDA-MB-231 breast cancer cell lines | Cell uptake study displayed higher accumulation of Nos-ES-GN in MCF-7 cells than that of MDA-MB-231 cells, Internalization by receptor-mediated endocytosis | [177] | |
| Tamoxifen | Liposomes | DOX | In vitro/MCF-7 breast cancer cell line In vivo/female Balb/c nude mice bearing MCF-7 breast tumor | -Significantly higher cellular and nuclear uptake -Significantly higher in vitro and in vivo cytotoxicity compared to nontargeted liposomes and free drug solution | [94] | |
| Estrone | Liposomes | DOX | MCF-7 breast cancer cell line, MDA-MB-231 cells/female Balb/c nude mice | 13-fold higher half-life (t1/2) compared to free drug solution, 24.27 and 6.04-fold higher cellular uptake compared to free drug solution and nontargeted liposomes, respectively Significantly higher antitumor efficacy in vivo | [95] | |
| Tamoxifen (ER antagonist) | Gold NPs (AuNPs) | Tamoxifen | In vitro/MCF-7 breast cancer cell line | Significantly higher cellular uptake 2.7-folds improvement in drug potency than nontargeted NPs | [96] | |
| CD44 | HA | Redox-responsive HA–chitosan–lipoic acid NPs | 17α-methyl testosterone | MCF-7 and BT-20 cell lines | Improved cellular internalization, Significantly enhanced cytotoxicity and apoptosis | [107] |
| HA | HA NPs | Docetaxel | 4T1-Luc breast cancer cells Subcutaneous 4T1-Luc tumor-bearing mice | Selective cellular uptake and remarkable cytotoxicity Enhanced growth and metastasis inhibition of 4T1-Luc breast tumors. Better antitumor, antimigration, and anti-invasion activity | [178] | |
| HA | Single-walled carbon nanotubes | DOX | MDA-MB-231 (human breast cancer) cell line | -Improved cellular uptake -Inhibition of migration of MDA-MB-231 cells -Inhibition of the growth of cancer cell spheroids | [179] | |
| HA | Polyethyleneimine (PEI)-PLGA NPs | TRAIL plasmid and Gambogic acid | MCF-7 and MDA-MB-231 breast cancer cell lines, Mouse mammary breast tumor 4T1 cell line Tumor-bearing nude Balb/c mice | -Selective uptake of the drugs in TNBC cells, -Apoptosis of TNBC cells both in vitro and in vivo -Significant inhibition in the growth of tumors | [180] | |
| HA | SLNs | Ibuprofen and Paclitaxel | CD44 negative BT-474 cell line and CD44 positive MDA-MB-231 breast cancer cell line | -Improvement in cellular uptake and induction of apoptosis. -Significantly higher inhibition of the growth of the MDA-MB-231 cells. | [181] | |
| HA | Nanostructured lipid carriers | Baicalein and DOX | MCF-7/ADR breast cancer cells/Kunming mice | -2.25 and 12-fold reduction of IC50 value compared to nontargeted NLCs and mixture of drug solution in vitro, significantly enhanced in vivo antitumor efficacy | [109] | |
| HA | NPs based on HA- L-lysine methyl ester- lipoic acid) conjugates | DOX | In vitro/MCF-7/ADR cancer cell line In vivo/MCF-7/ADR tumor-bearing nude mice | -20-folds higher cellular uptake compared to free drug -Excellent targetability and superior antitumor activity in vitro -Significantly greater survival of mice and low side effects in vivo | [110] | |
| CD44 monoclonal antibody | MSNs | DOX | In vitro/MCF-7/ADR1 cancer cell line In vivo/female nude mice bearing the resistant MCF-7/MDR1 tumors | -Significantly higher cellular uptake -Significantly higher tumor growth inhibition and induced apoptosis | [182] | |
| HA | Micelles | Paclitaxel and AURKA specific siRNA (si-AURKA) | MDA-MB-231 breast cancer cell line, Balb/c nude mice | Significantly enhanced cellular uptake and synergistic cytotoxic effect of drug and siRNA in vitro and in vivo | [183] | |
| HA | Chitosan NPs | DOX and miR-34a | In vitro/MDA-MB-231 cancer cells In vivo/female athymic nude Balb/c mice bearing MDA-MB-231 solid tumor | 1700 folds higher cellular uptake of miR-34a compared to blank NPs Superior in vivo cytotoxicity | [184] | |
| HA | Polymer–drug conjugate | Rapamycin | In vitro/MDA-MB-468 cells In vivo/Balb/c mice bearing CD44-positive 4T1.2neu breast cancer | -3.2-folds higher cellular uptake than free drug -Significantly higher in vitro cell viability reduction -Improved animal survival and suppressed tumor growth in vivo -2.96-fold greater area under the curve (AUC) than that of the free drug -8.82-fold slower total body clearance | [111] | |
| HA | PLGA NPs | Docetaxel | In vitro/MDA-MB-231 cancer cell line In vivo/Balb/c nude mice bearing MDA-MB-231 breast cancer | Significantly higher cellular uptake compared to nontargeted NPs Significantly enhanced tumor targeting and antitumor activity compared to nontargeted NPs | [112] | |
| HA, oligosaccharide (oHA) | Lipid NPs | Paclitaxel | In vitro/BT549, MDA-MB-231, MDA-MB-468, and T47D human breast cancer cell line In vivo/female nude mice bearing MDA-MB-231 breast tumor | Significantly improved antitumor activity | [113] | |
| HA | PAMAM dendrimers | DOX and major vault protein (MVP) targeted small interfering RNA (MVP-siRNA) | MCF-7/ADR breast cancer cells, Female Balb/c nude mice | -Significantly higher AUC and MRT, -Significantly enhanced cellular uptake in vitro ~4-fold reduction in IC50 value, improved gene silencing effect as well as enhanced stability and efficient intracellular delivery of siRNA | [185] | |
| LHRH | LHRH-peptide | Dextran NPs | Cisplatin | In vitro/4T1 breast cancer cell line In vivo/Balb/c mice bearing 4T1 tumor | Significantly higher cellular uptake Significantly higher in vitro and in vivo cytotoxicity and low systemic toxicity | [119] |
| LHRH-peptide | Human serum albumin NPs | Methotrexate | In vivo/Female Balb/c mice bearing 4T1 breast cancer | 7-fold stronger antitumor efficacy 2-fold increase in the life span of mice | [120] | |
| Transferrin | Tf | MSNs | DOX | HT-29 and MCF-7 cells | Receptor-mediated internalization of the drug in cancer cells Drug release is triggered by high GSH concentration in tumor cells. | [124] |
| Tf | Polymeric NPs | Benzoporphyrin derivative monoacid (BPD) | TNBC cell line MDA-MB-231 breast epithelial cell line MCF-12A | -Specificity of the targeted NPs for TNBC cells -Highest photo triggered cytotoxicity in TNBC cells | [186] | |
| Tf | NPs | Curcumin and DOX | MCF-7 breast cancer cells and mice bearing MCF-7 cells | -In vitro cell viability assay exhibited higher cytotoxicity -Inhibition of viability and proliferation of the cancer cell lines with lower IC50 value. -Higher drug concentrations in the tumor tissue owing to EPR effect. | [125] | |
| Tf | Polymeric NPs | Nutlin-3a | MCF-7 breast cancer cells | 22-times and 3-fold higher uptake than native nutlin-3a and unconjugated NPs, superior antiproliferative activity | [126] | |
| Tf | Lipid coated PLGA NPs | 7α-(4′-amino) phenylthio-1,4-androstadiene-3,17-dione (7α-APTADD) | In vitro/SK-BR-3 breast cancer cells | Significantly higher cellular uptake 2.8-folds reduction in IC50 compared to the nontargeted NPs | [127] | |
| Tf | SLNs | Curcumin | In vitro/MCF-7 breast cancer cell line | 2- and 5-fold higher cellular uptake compared to nontargeted NPs and free drug solution 1.5- and 3-fold higher cytotoxicity compared to nontargeted NPs and free drug solution | [128] | |
| Integrin | Leukocytes | Biomimetic nanovesicles (leukosomes) | DOX | 4T1 and B16 cancer cell lines | Significantly higher tumor accumulation for leukosomes More potent anticancer activity in terms of reduction of tumor volume and prolonged survival | [133] |
| RGD motif (Arg-Gly-Asp) | Lipid polymer hybrid NPs | Norcantharidin | Human TNBC cell lines MDA-MB-231, LM2, and SUM159 cells Nude mouse orthotopic mammary TNBC tumor | Specific β-catenin attenuation Significantly enhanced accumulation and longer retention time | [187] | |
| RGD motif (Arg-Gly-Asp) | Polymeric dendritic NPs | DOX | Mouse mammary breast tumor cell line (4T1) HUVEC cells Female BALB/C mice | -Accumulation of drug around the leaky blood vessels, -Shrinkable property of formulation is beneficial for penetration and retention -In vivo, RGD-DOX-DGL-NPs showed a remarkable tumor growth inhibition effect | [188] | |
| Fibronectin-mimetic peptide | PEGylated liposomes | DOX | In vitro/MDA-MB-231 breast cancer cells | Enhanced binding efficacy Enhanced cytotoxicity | [74] | |
| cyclic pentapeptide c(RGDfK) | PLGA-PEG NPs | Cisplatin | In vitro/MCF-7, MCF-7MFP1, DU145, DU145LN2, PC3, PC3MLN4 cell lines In vivo/female nude mice xenograft bearing MCF-7MFP1 breast cancer cell | Significantly higher cellular uptake Significantly higher cytotoxicity in vitro and in vivo | [75] | |
| Vasoactive Intestinal Peptide (VIP) receptor | VIP | Sterically stabilized phospholipid nanomicelles | Curcumin | Breast cancer stem cells MCF-7 human breast cancer cell line | Significantly improved IC50 20% inhibition of tumorsphere formation Significantly enhanced anticancer activity | [139] |
| VIP | Nanomicelles | Paclitaxel | MCF-7 breast cancer cells/Virgin female Sprague-Dawley rats | Significantly higher cellular uptake, 2-fold improvements in ED50 value compared to free paclitaxel, improved in vivo anticancer efficacy | [140] | |
| VIP | Nanomicelles | Paclitaxel | MCF-7 breast cancer cells, BC19/3 breast cancer cells | Significantly enhanced cytotoxicity in vitro | [141] | |
| VIP | Nanomicelles | 17-Allylamino-17-demethoxy geldanamycin | MCF-7 breast cancer cells | Significantly enhanced cytotoxicity in vitro | [142] | |
| Heparin-binding epidermal growth factor (HB-EGF) receptor | Fab’ antibody 8 against heparin-binding EGF growth factor | Lipid NPs | Si-RNA | MDA-MB-231 human TNBC cells | In vivo studies showed long-term blood circulation and accumulation in the tumor tissue Suppression of PLK1 protein expression and tumor growth | [189] |
| Antihuman heparin-binding epidermal growth factor (HB-EGF) monoclonal antibody | Immunoliposomes | DOX | Vero-H cells, MDA-MB-231 human breast cancer cells/Balb/c nude female mice | Significantly higher cellular uptake in vitro, strong suppression, and regression of breast tumor | [146] | |
| N-acetyl-D-glucosamine (NAG) receptor | NAG | Polymeric NPs | DOX | MCF-7 breast cancer cell line | Significantly higher cellular uptake and targeting ability, higher antitumor activity | [143] |
| EphA2 receptor | Homing peptide with a sequence of YSAYPDSVPMMSK | MSNs | DOX | MCF-7 cell lines | -Increased specificity and cytotoxicity of DOX in MCF-7/MDR1 cells in vitro and in vivo -Reduced toxicity and enhanced therapeutic efficacy | [190] |
| Homing peptide with a sequence of YSAYPDSVPMMSK | Liposomes | DOX | MDA-MB-231, HUVEC cells Balb/c nude mice | -Significantly higher cellular uptake In vitro, stronger cytotoxicity in vitro and In vivo, low systemic and cardiac toxicity | [144] | |
| Alpha7 nicotinic acetylcholine receptor (α7 nAChR) | α-conotoxin ImI | Micelles | Paclitaxel | A549 breast cancer cells, MCF-7 breast cancer cells/female Balb/c nude mice | -Significantly higher cellular uptake in vitro, -Significantly higher cytotoxicity in vivo, -Low systemic toxicity | [145] |
| Biotin Receptor | Double branched Biotin | Liposomes | Paclitaxel | MCF-7 cells (Human breast cancer cell line), 4T1 cells (Mouse breast cancer cell line) B16 cells (Mouse skin melanoma cell line) Female Balb/c mice | -Excellent targeting ability to breast cancer. -The relative uptake efficiency (RE) and concentration efficiency (CE) of (Bio2-Chol) Lip were respectively enhanced by 5.61- and 5.06-fold compared to that of naked paclitaxel. | [191] |
| Biotin | PEG-b-poly (ε-caprolactone) NPs | DOX and Quercetin | MCF-7/ADR cell lines | Facilitates the cellular drug uptake and reduces the drug efflux rate Inhibition of both P-gp activity and expression | [192] | |
| Biotin | DNA conjugated gold nanorods (GNR) | DOX | MCF-7/ADR cell lines | -Increased cell uptake and significantly reduced drug efflux, -About 67-fold increased potency than free drug | [193] | |
| Biotin | liposomes | DOX and Quercetin | MCF-7/ADR cell lines | -Higher antitumor activity -Decreased cardiotoxicity of DOX -Downregulation of P-gp expression In vivo. | [194] | |
| Biotin | Human serum albumin NPs | Methotrexate | Balb/c mice bearing 4T1 breast carcinoma | -Significantly stronger anticancer activity and lower toxic effect, -Increased survival and life span of tumor-bearing mice, slight body weight loss | [147] | |
| KDR receptor | K237-peptide | Hybrid chitosan/poly(N-isopropylacrylamide) NPs | Paclitaxel | MDA-MB-231 human breast cancer cells | -MTT assays showed that the K237-conjugated NPs could more effectively inhibit breast cancer cell growth -Enhanced efficacy in preventing cell proliferation | [195] |
| K237- (HTMYYHHYQHHL) peptide | Polymeric NPs | Paclitaxel | HUVEC cells, MDA-MB-231 cells, Female Balb/c nude mice | -Significantly higher cellular uptake, -Significantly stronger antitumor efficacy in vitro and In vivo | [148] | |
| - | Cyclic Arg-Gly-Asp-d-Tyr-Lys [c(RGDyK)] | Polymeric micelles | DOX | BCap-37 cells and Bcap37 cells bearing female BALB/c mice | Developed micelles system prolonged drug half-life in bloodstream, improved therapeutic efficiency, and decreased cardiac toxicity and biotoxicity compared to free drug. | [196] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rizwanullah, M.; Ahmad, M.Z.; Ghoneim, M.M.; Alshehri, S.; Imam, S.S.; Md, S.; Alhakamy, N.A.; Jain, K.; Ahmad, J. Receptor-Mediated Targeted Delivery of Surface-ModifiedNanomedicine in Breast Cancer: Recent Update and Challenges. Pharmaceutics 2021, 13, 2039. https://doi.org/10.3390/pharmaceutics13122039
Rizwanullah M, Ahmad MZ, Ghoneim MM, Alshehri S, Imam SS, Md S, Alhakamy NA, Jain K, Ahmad J. Receptor-Mediated Targeted Delivery of Surface-ModifiedNanomedicine in Breast Cancer: Recent Update and Challenges. Pharmaceutics. 2021; 13(12):2039. https://doi.org/10.3390/pharmaceutics13122039
Chicago/Turabian StyleRizwanullah, Md., Mohammad Zaki Ahmad, Mohammed M. Ghoneim, Sultan Alshehri, Syed Sarim Imam, Shadab Md, Nabil A. Alhakamy, Keerti Jain, and Javed Ahmad. 2021. "Receptor-Mediated Targeted Delivery of Surface-ModifiedNanomedicine in Breast Cancer: Recent Update and Challenges" Pharmaceutics 13, no. 12: 2039. https://doi.org/10.3390/pharmaceutics13122039
APA StyleRizwanullah, M., Ahmad, M. Z., Ghoneim, M. M., Alshehri, S., Imam, S. S., Md, S., Alhakamy, N. A., Jain, K., & Ahmad, J. (2021). Receptor-Mediated Targeted Delivery of Surface-ModifiedNanomedicine in Breast Cancer: Recent Update and Challenges. Pharmaceutics, 13(12), 2039. https://doi.org/10.3390/pharmaceutics13122039










