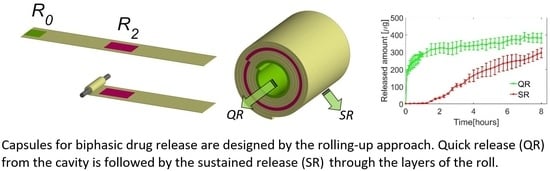
Biphasic Drug Release from Rolled-Up Gelatin Capsules with a Cylindrical Cavity
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Crosslinked Gelatin Films
2.2. Crosslinking Extent Determination Using the 2,4,6-Trinitrobenzene Sulfonic Acid (TNBSa) Method
2.3. Loading the Gelatin Strips Using Fluorescent Probes
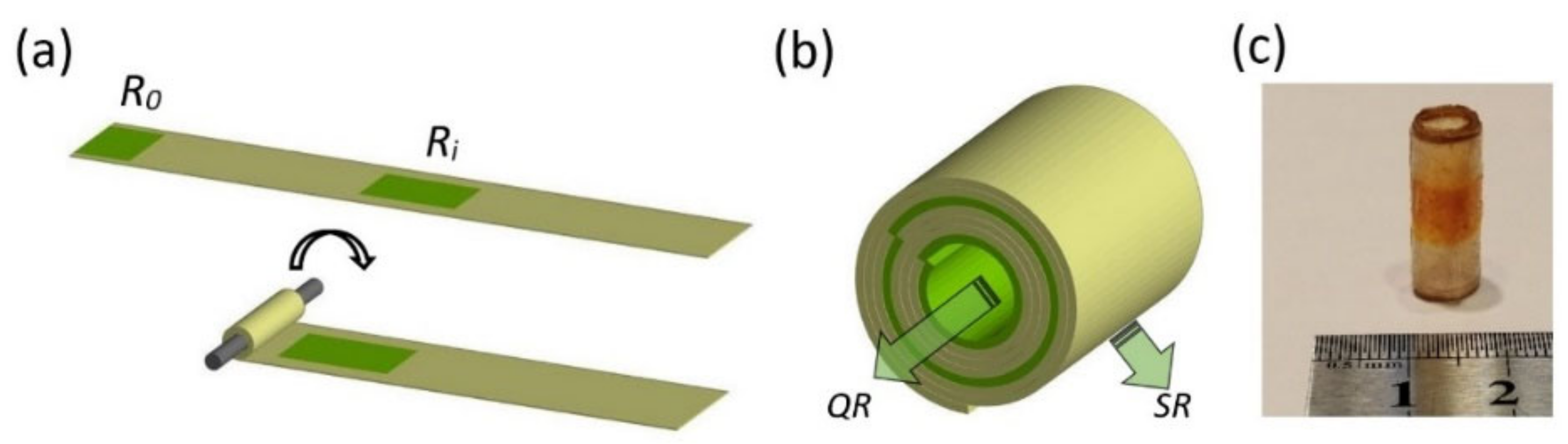
2.4. Fabrication of the Capsules Using the Rolling-Up Approach
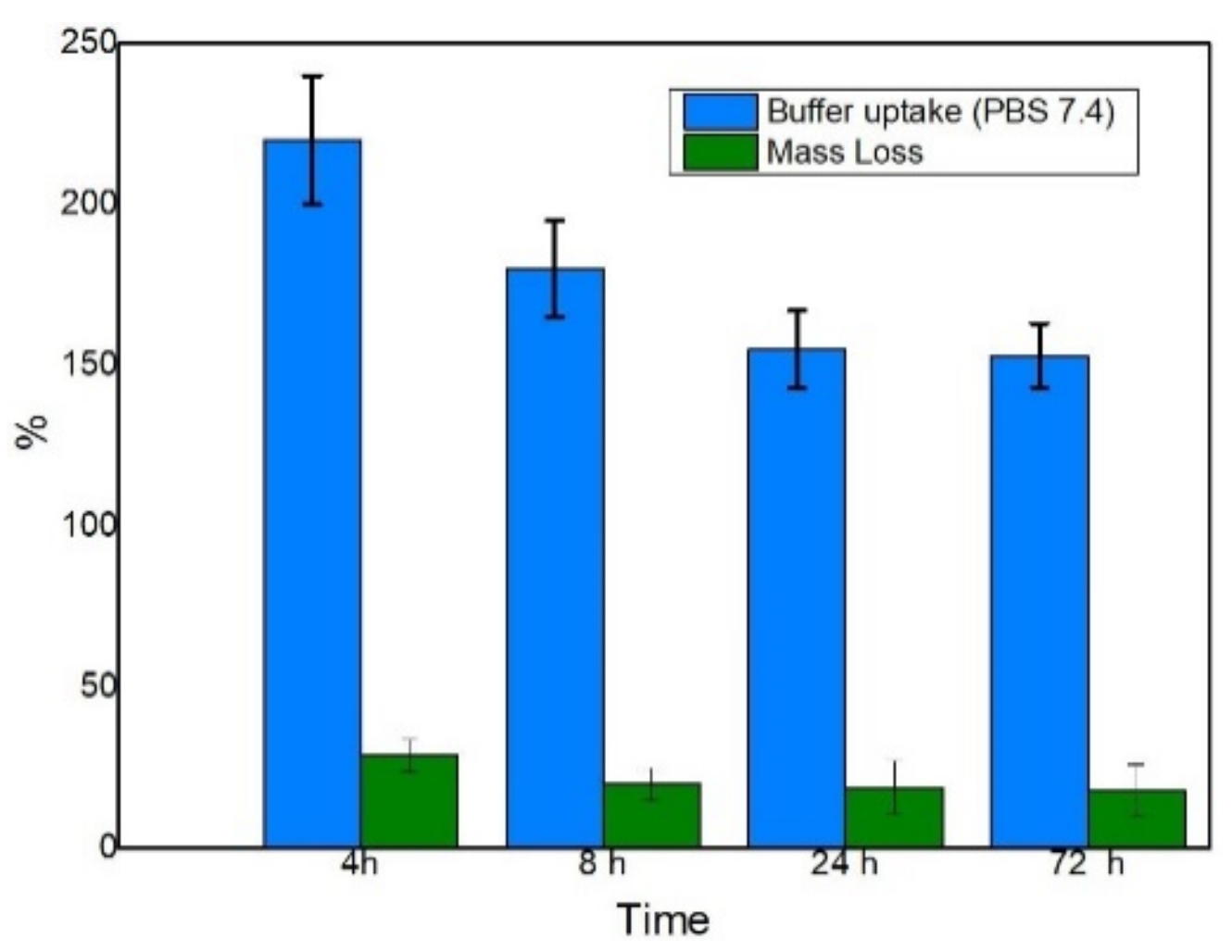
2.5. Swelling Capacity of the Matrix
2.6. In Vitro Release Study
3. Results
3.1. Swelling Behavior of the Capsules
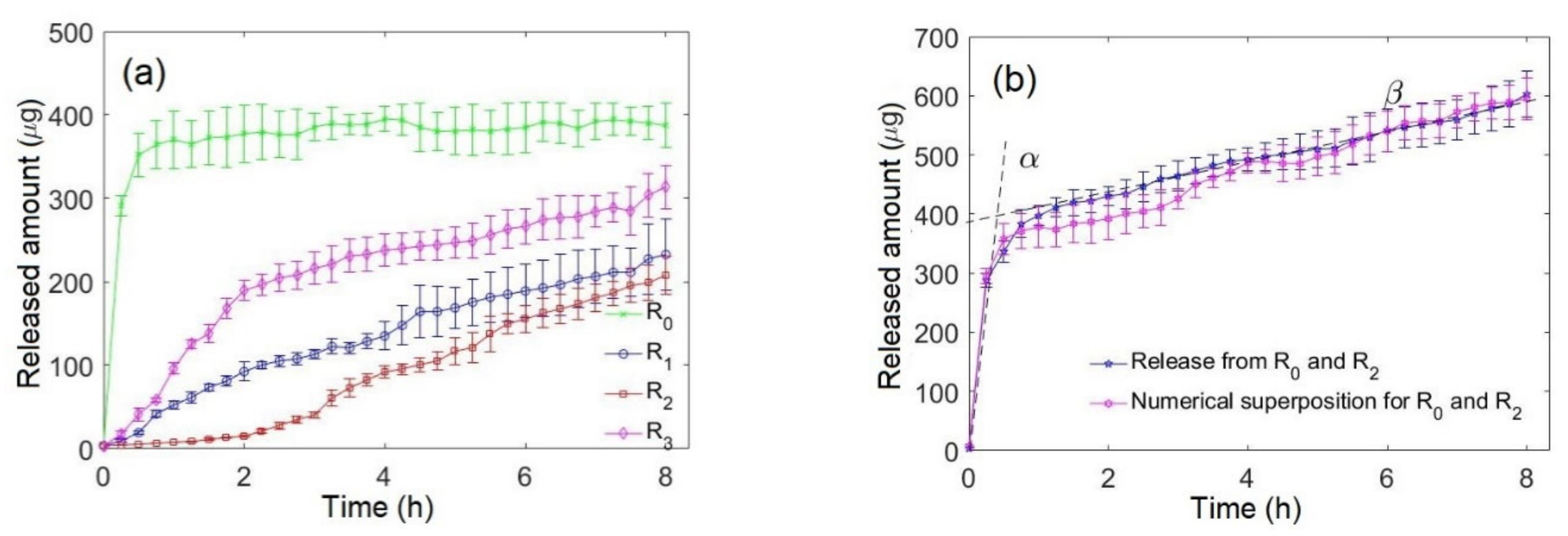
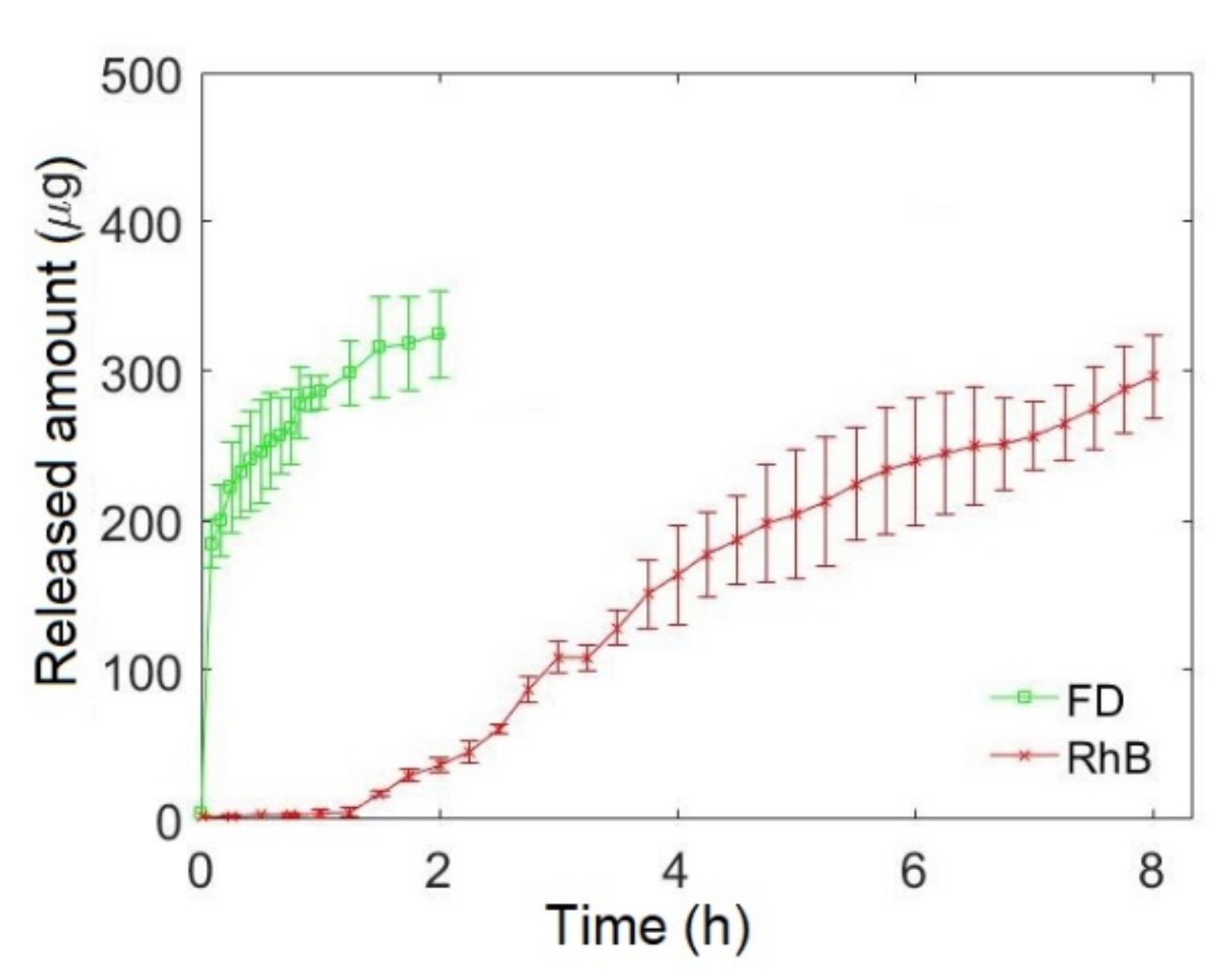
3.2. Monophasic and Biphasic Drug Release
3.3. Biphasic Bidrug Release
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
Appendix B
Appendix C
References
- Martin, C.; De Baerdemaeker, A.; Poelaert, J.; Madder, A.; Hoogenboom, R.; Ballet, S. Controlled-release of opioids for improved pain management. Mater. Today 2016, 19, 491–502. [Google Scholar] [CrossRef]
- Avanapu, S.; Nayeemuddin, M.; Hadi, M. Formulation and evaluation of a novel capsule-in-a-capsule technology for biphasic delivery of lornoxicam in the treatment of migraine. Int. J. Pharm. Biomed. Res. 2013, 4, 170–176. [Google Scholar]
- Barkin, R.L. Zolpidem Extended-Release: A Single Insomnia Treatment Option for Sleep Induction and Sleep Maintenance Symptoms. Am. J. Ther. 2007, 14, 299–305. [Google Scholar] [CrossRef]
- Swain, R.P.; Pendela, S.; Panda, S. Formulation and Evaluation of Gastro-bilayer Floating Tablets of Simvastatin as Immediate Release Layer and Atenolol as Sustained Release Layer. Indian J. Pharm. Sci. 2016, 78, 458–468. [Google Scholar] [CrossRef]
- Elzayat, E.M.; Abdel-Rahman, A.A.; Ahmed, S.M.; Alanazi, F.K.; Habib, W.A.; Abou-Auda, H.S.; Sakr, A. Formulation and pharmacokinetics of multi-layered matrix tablets: Biphasic delivery of diclofenac. Saudi Pharm. J. 2017, 25, 688–695. [Google Scholar] [CrossRef]
- Akhtar, M.; Jamshaid, M.; Zaman, M.; Mirza, A.Z. Bilayer tablets: A developing novel drug delivery system. J. Drug Deliv. Sci. Technol. 2020, 60, 102079. [Google Scholar] [CrossRef]
- Nagori, S.; Jethwa, B.; Gohel, M.; Parikh, R. Fabrication and evaluation of bi-layer tablet containing conventional paracetamol and modified release diclofenac sodium. Indian J. Pharm. Sci. 2010, 72, 191–196. [Google Scholar] [CrossRef]
- Conceição, J.; Adeoye, O.; Cabral-Marques, H.; Concheiro, A.; Alvarez-Lorenzo, C.; Lobo, J.M.S. Carbamazepine bilayer tablets combining hydrophilic and hydrophobic cyclodextrins as a quick/slow biphasic release system. J. Drug Deliv. Sci. Technol. 2020, 57, 101611. [Google Scholar] [CrossRef]
- Khaled, S.A.; Burley, J.; Alexander, M.; Roberts, C.J. Desktop 3D printing of controlled release pharmaceutical bilayer tablets. Int. J. Pharm. 2014, 461, 105–111. [Google Scholar] [CrossRef]
- Gioumouxouzis, C.I.; Baklavaridis, A.; Katsamenis, O.; Markopoulou, C.K.; Bouropoulos, N.; Tzetzis, D.; Fatouros, D.G. A 3D printed bilayer oral solid dosage form combining metformin for prolonged and glimepiride for immediate drug delivery. Eur. J. Pharm. Sci. 2018, 120, 40–52. [Google Scholar] [CrossRef]
- Yu, D.; Wang, X.; Li, X.; Chian, W.; Li, Y.; Liao, Y. Electrospun biphasic drug release polyvinylpyrrolidone/ethyl cellulose core/sheath nanofibers. Acta Biomater. 2013, 9, 5665–5672. [Google Scholar] [CrossRef]
- Huang, Y.-C.; Li, R.-Y.; Chen, J.Y.; Chen, J.K. Biphasic release of gentamicin from chitosan/fucoidan nanoparticles for pulmonary delivery. Carbohydr. Polym. 2016, 138, 114–122. [Google Scholar]
- Higuchi, T. Drug-Delivery Device. United States Brevet. U.S. Patent 3625214A, 7 December 1971. [Google Scholar]
- Egunov, A.I.; Inaba, A.; Gree, S.; Malval, J.-P.; Tamura, K.; Saito, Y.; Luchnikov, V.A. Time-programmed release of fluoroscein isocyanate dextran from micro-pattern-designed polymer scrolls. J. Control. Release 2016, 233, 39–47. [Google Scholar] [CrossRef]
- Pedron, R.; Vandamme, T.; Luchnikov, V.A. Programming of drug release via rolling-up of patterned biopolymer films. Nano Sel. 2021, 2, 948–957. [Google Scholar] [CrossRef]
- Haugh, M.G.; Murphy, C.M.; McKiernan, R.C.; Altenbuchner, C.; O’Brien, F.J. Crosslinking and Mechanical Properties Significantly Influence Cell Attachment, Proliferation, and Migration within Collagen Glycosaminoglycan Scaffolds. Tissue Eng. Part A 2011, 17, 1201–1208. [Google Scholar] [CrossRef]
- Cayot, P.; Tainturier, G. The Quantification of Protein Amino Groups by the Trinitrobenzenesulfonic Acid Method: A Reexamination. Anal. Biochem. 1997, 249, 184–200. [Google Scholar] [CrossRef]
- Kale, R.; Bajaj, A. Ultraviolet Spectrophotometric Method for Determination of Gelatin Crosslinking in the Presence of Amino Groups. J. Young Pharm. 2010, 2, 90–94. [Google Scholar] [CrossRef]
- Kieliszek, M.; Misiewicz, A. Microbial transglutaminase and its application in the food industry. A review. Folia Microbiol. 2014, 59, 241–250. [Google Scholar] [CrossRef]
- ANSM, Solutions Tampons. Pharmacopée Française 2012. Available online: https://ansm.sante.fr/uploads/2020/10/22/solutions-tampons.pdf (accessed on 27 November 2021).
- Xavier, J.R.; Ramana, K.V.; Sharma, R.K. Screening and statistical optimization of media ingredients for production of microbial transglutaminase. Def. Life Sci. J. 2017, 2, 216. [Google Scholar] [CrossRef][Green Version]
- Fallingborg, J. Intraluminal pH of the human gastrointestinal tract. Dan. Med Bull. 1999, 46, 183–196. [Google Scholar]
- Slyusareva, E.A.; Gerasimova, M.A. pH-Dependence of the Absorption and Fluorescent Properties of Fluorone Dyes in Aqueous Solutions. Russ. Phys. J. 2014, 56, 1370–1377. [Google Scholar] [CrossRef]
- ANSM, Taille des Capsules à Envelope Dure (Gélules). Pharmacopée Française 2017. Available online: https://ansm.sante.fr/uploads/2020/10/22/taille-des-capsules-a-enveloppe-dure-gelules.pdf (accessed on 27 November 2021).
- Zakowiecki, D.; Frankiewicz, M.; Hess, T.; Cal, K.; Gajda, M.; Dabrowska, J.; Kubiak, B.; Paszkowska, J.; Wiater, M.; Hoc, D.; et al. Development of a Biphasic-Release Multiple-Unit Pellet System with Diclofenac Sodium Using Novel Calcium Phosphate-Based Starter Pellets. Pharmaceutics 2021, 13, 805. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, A.; Bhatia, M. Development and evaluation of regioselective bilayer floating tablets of Atenolol and Lovastatin for biphasic release profile. Iran. J. Pharm. Sci. 2009, 8, 15–25. [Google Scholar]

| Reservoir | Turn | x1 [mm] | x2 [mm] |
|---|---|---|---|
| R0 | 1 | 0 | 17 |
| R1 | 3 | 35 | 53 |
| R2 | 6 | 91 | 110 |
| R3 | 8.5 | 140 | 160 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mzoughi, J.; Vandamme, T.; Luchnikov, V. Biphasic Drug Release from Rolled-Up Gelatin Capsules with a Cylindrical Cavity. Pharmaceutics 2021, 13, 2040. https://doi.org/10.3390/pharmaceutics13122040
Mzoughi J, Vandamme T, Luchnikov V. Biphasic Drug Release from Rolled-Up Gelatin Capsules with a Cylindrical Cavity. Pharmaceutics. 2021; 13(12):2040. https://doi.org/10.3390/pharmaceutics13122040
Chicago/Turabian StyleMzoughi, Jihane, Thierry Vandamme, and Valeriy Luchnikov. 2021. "Biphasic Drug Release from Rolled-Up Gelatin Capsules with a Cylindrical Cavity" Pharmaceutics 13, no. 12: 2040. https://doi.org/10.3390/pharmaceutics13122040
APA StyleMzoughi, J., Vandamme, T., & Luchnikov, V. (2021). Biphasic Drug Release from Rolled-Up Gelatin Capsules with a Cylindrical Cavity. Pharmaceutics, 13(12), 2040. https://doi.org/10.3390/pharmaceutics13122040